Abstract
Background:
Vitis vinifera (black grape) is cultivated worldwide and has numerous oral and therapeutic applications. It has proven anti-inflammatory, antioxidant, antimicrobial, and wound healing properties. The aim of this study was to investigate the effect of black grape seed (hydroalcoholic) extract (BGSE) and black grape seed oil (BGSO) on experimental colitis.
Methods:
BGSE (50, 100, and 200 mg/kg) and BGSO (2, 4, and 8 mL/kg) were administered orally (p.o.) in groups of six male Wistar rats, 2 h before induction of colitis and continued further for 4 days. Prednisolone (4 mg/kg) and mesalamine (100 mg/kg) were used as reference drugs. Weight/length of colons, macroscopic and histopathologic indices, and biochemical parameters including myeloperoxidase (MPO) and malondialdehyde (MDA) were evaluated.
Results:
All doses of BGSE and BGSO significantly decreased the colon weight, ulcer index, and total colitis index in comparison with the control group, although greater doses of both fractions had more significant protection. Data of MPO activity revealed that all treated groups with the exception of BGSE (50 mg/kg) and BGSO (2 mL/kg) showed a meaningful decline in comparison with the control group. Concerning the MDA values in colonic tissue, it was demonstrated that BGSE (100, 200 mg/kg) and BGSO (8 mL/kg) caused a significant dip in this oxidative stress parameter.
Conclusions:
Oral administration of BGSE and BGSO had an appropriate anti-inflammatory effect and so could be considered as a suitable candidate for treating or preventing ulcerative colitis. Furthermore, detailed studies are warranted to explore the exact mechanism of action and clinical preference of these compounds.
Keywords: Animal model, colitis, plant extract, rats, seeds oil, Vitis vinifera
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are two types of idiopathic inflammatory bowel diseases (IBD) which potentially affects the gastrointestinal tract. UC and CD cause diffuse and superficial inflammation mostly in the colon and rectum area.[1,2] Some factors such as genetics, bacterial infections, environmental, and immune system abnormalities are known as risk factors for UC. Medical treatments in UC may induce and maintain remission periods; however, none of them is curative.[3,4] Aminosalicylates (5-ASA), corticosteroids, azathioprine, mercaptopurine, cyclosporine A, adalimumab, and infliximab are evaluated in this regard. These agents have specific adverse effects that can restrict their application. Headache, visual disturbances, hepatotoxicity, osteoporosis, hypertension, infection, leukopenia, rheumatoid arthritis, and life-threatening effects are among the common adverse effects.[5,6,7] Based on the mentioned problems and growing incidence rate of UC, it is necessary to try natural and traditional products to find better medications.[8] Vitis vinifera (grape, black royal cultivar, Vitaceae) is one of the oldest fruits indigenous to Asia, Europe, and North America. This plant, botanically a berry, grows in a climbing inosculated pattern.[9] Grapes have different types and species and are cultivated for various uses. The most important ingredients in the grapes are anthocyanins (malvidin, cyanidin, and peonidin), flavonols (quercetin, catechin, and epicatechin), polyphenols, stilbenes (resveratrol), carbohydrates, salts (calcium, magnesium, potassium, and iron), various vitamins (A, D, C, and E), and saturated or unsaturated fats (in seeds).[10,11,12,13,14] The fruit has high nutritional value and all parts of this plant such as fruits, stems, leaves, and seeds have numerous pharmacological effects.[15] For example, the extract of its stems has antimicrobial and antioxidant properties.[16] Its fruit extract has a cytotoxic effect on colon cancer cells because of the presence of proanthocyanidin and resveratrol.[17,18] In addition, it is effective in preventing chronic nontransmissible diseases such as diabetes and cardiovascular disorders.[19,20,21] Grape seeds contain about 8 to 20% oil, which varies in different species along with compounds such as phenolic compounds, flavonoids, phenolic acids, resveratrol, unsaturated fatty acids, phytosterols, and vitamin E, almost all which could be responsible for the nutritional benefits and therapeutic effects of this oil.[22] The results of previous studies have indicated the anti-inflammatory effect of grape seeds through the presence of the abovementioned compounds.[23] Some other pharmacological activities including neuroprotection, hepatoprotection, wound healing, and anti-seizure activity, especially due to its seeds have been reported.[24,25,26,27,28,29] According to these properties, grape seeds might be useful in the treatment of ulcerative colitis. Hence, the aim of this study was to evaluate the anti-inflammatory and anti-ulcerative effects of Vitis vinifera (black grape) seed extracts (BGSE) and oil (BGSO) in experimental colitis in rats.
Methods
Preparation of grape seed extract and oil
Grapefruits (black royal cultivar) were purchased from a trusted fruit shop in Tehran, Iran and its genus, variety, and cultivar were authenticated by one of the botanists in Isfahan University of Medical Sciences. Grape seeds were dried and completely powdered. Later, the hydroalcoholic extract was prepared by macerating 135 g of finely powdered seeds mixed in 374 mL of EtOH/H2O (80:20) and the mixture was shaken and filtered thrice during 3 consecutive days to make full extraction. On the fourth day, the final extract was dried in a rotary evaporator and then freeze-dried to achieve fully dried extract. The dried extract was weighed and the yield of the hydroalcoholic extract was determined about 8.15% w/w. The total polyphenolic content (TPC) was determined using the Folin-Ciocalteu method as described by Postescu et al.[30] TPC for the grape seeds extract was determined as 5.8 mg gallic acid equivalent (GAEq)/g. Alternatively, pure grape seeds oil (Black or red Shiraz cultivar, cultivated in Fars Province) was procured from a local traditional market in Isfahan. It was absolutely natural and without water or cholesterol impurity as indicated.
Drugs and chemicals
Prednisolone and mesalamine powders were purchased from Iran Hormone Company (Tehran, Iran). o-dianisidine dihydrochloride (ODD) and hexadecyl trimethyl ammonium bromide (HTAB) were purchased from Sigma Company (St. Louis, USA). Ethanol, diethyl ether oxide, glacial acetic acid, and formaldehyde were purchased from Merck Company (Darmstadt, Germany). Normal saline was purchased from Shahid-Ghazi Company (Tabriz, Iran).
Animals
In this study, 60 male Wistar rats weighing 200 ± 20 g and grown up in the animal house of School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran were used. All animals were treated in a controlled area under appropriate light, humidity, temperature, and nutrition conditions. They were kept in standard cages and fed with special pelleted diet and tap water. They were acclimatized to the laboratory conditions for a week before the start of the experiment. The Ethics Committee of Isfahan University of Medical Sciences approved the protocol of the current study (IR.MUI.RESEARCH.REC.1397.201).
Experimental design
All 60 rats were randomly divided into ten groups (six rats in each group) as below:
Group 1: Sham (normal) received oral normal saline/Tween as a vehicle that was given 2 h prior to instillation of normal saline in the rectum.
Group 2: Colitis induced (negative control), normal saline/ Tween as vehicle administered by gavage 2 h prior to instillation of acetic acid for induction of colitis in the rectum and continued for 4 days on a daily basis.[31]
Groups 3, 4, and 5: Three doses of BGSE (50, 100, and 200 mg/kg) administered by gavage 2 h prior to instillation of acetic acid for induction of colitis in the rectum and continued for 4 days on a daily basis.[32]
Groups 6, 7, and 8: Three doses of BGSO (2, 4, and 8 mL/kg) administered by gavage 2 h prior to instillation of acetic acid for induction of colitis in the rectum and continued for 4 days on a daily basis.
Group 9 and 10: Reference (positive control), prednisolone (4 mg/kg), or masalamine (100 mg/kg) administered by gavage 2 h prior to instillation of acetic acid for induction of colitis in the rectum and continued for 4 days on a daily basis.
For conserving the uniformity of prepared dosage, 2–3 drops of Tween 80 (dispersing agent) was added to all serving doses of extracts.
Before the induction of colitis, animals have fasted for 24 h. Rats were kept under CO2 anesthesia and a catheter with 2 mm diameter and 8 cm length was used for enemas of 2 mL acetic acid 3%.[33]
Evaluation of macroscopic features of colonic tissue
The animals were sacrificed 24 h after the last day of treatment (day 5) via inhaled CO2 overdose. The last 8 cm of each colon (3 cm apart from the anus) was removed, opened longitudinally, washed with normal saline, and the wet weight of the specimen was measured. The tissue was fixed on a white surface and a photo required for macroscopic evaluations was captured with a camera and ulcer area assessed by Fiji-win 32 software. Ulcer severity was scored as 0: no ulcer; 1: inflammation, edema, thickness, and superficial erosion; 2: bleeding, hemorrhage, and definite ulcer; 3: deep ulcers, necrosis, and/or perforation. Ulcer index was also calculated by summing the ulcer area and ulcer severity.[34] After the macroscopic assessment, tissue specimens were divided into two equal parts. One of them was preserved in formalin 10% for histopathologic tests and the other specimen was stored in the freezer (-70°C) for two biochemical tests.[35]
For histopathologic studies, prepared colon tissues were fixed, paraffin-embedded, processed, and sectioned in 4 mm thick layers. Then, they were sequentially deparaffinized with xylene, hydrated with ethanol, and stained with hematoxylin and eosin (H and E). Inflammation severity (0: none, 1: slight, 2: moderate, 3: severe), inflammation extent (0: none, 1: mucosa, 2: mucosa and submucosa, 3: transmural), crypt damage (0: none, 1: basal 1/3 damaged, 2: basal 2/3 damaged, 3: surface epithelium intact only), and leukocyte infiltration (0: trace, 1: mild, 2: moderated, 3: severe) were evaluated in H and E stained samples. The total colitis index was calculated by summing the abovementioned scores. Histopathological evaluations were carried out by using a Zeiss microscope equipped with a color video camera (Sony, Japan) for digital imaging.[35]
Evaluation of myeloperoxidase (MPO) activity
MPO activity was determined as an index for leukocyte migration and infiltration within the tissue was measured according to the method described by Motavallian et al.[36] About 0.1 g of colon specimens were homogenized with 5 mL of potassium buffer (pH, 6) and 0.5% HTAB. Then, the homogenate was sonicated for 10 s in an ice bath and the suspension was centrifuged (4000 rpm). The supernatant in the volume of 0.1 mL and 2.9 mL of 50 mM phosphate buffer (pH = 6) was mixed containing 0.167 mg/mL ODD and 0.0005% hydrogen peroxide. After incubation, the absorbance was measured at 450 nm using UV-Vis spectrophotometer (LSI Model Alfa-1502) at 0 and 3 min interval. MPO activity was reported as U/g for the weight of wet colon tissue.[36]
Evaluation of malondialdehyde (MDA) content of colonic tissue
To measure the amount of MDA as an index of lipid peroxidation, 1 mL of potassium chloride 1.15% w/v was added to 0.1 g of colonic tissue, and then samples were homogenized and centrifuged (1200 rpm for 10 min). The supernatant was separated, centrifuged (3000 rpm for 15 min), and the absorbance was measured at 532 nm. All the process for assessing the activity of MDA was done with Navand assay kit (Navandsalamat, Iran) according to the company package insert instruction.[37]
Statistical analysis
Statistical analyses were performed using SPSS (Version 23, Chicago. IL, USA). All parametric data were expressed as mean ± SEM and group differences were measured by parametric one-way ANOVA with Tukey’s HSD Post hoc. Nonparametric (scoring) data were expressed as median (range) and were analyzed using the Mann–Whitney U-test. For all tests, P < 0.05 was considered significant.
Results
Effect of RGSE and RGSO on macroscopic parameters
On the basis of analyzed data, there were no changes in the Sham (normal) group and it was understood that handling and surgical procedure had no effect on the results of this experiment. In all groups which were treated with BGSE and BGSO; ulcer index and wet weight of colon (mg) diminished significantly in comparison with the control group (P < 0.05). The exception was BGSO (2 mL/kg) which represented a significant effect on colon weight only (P < 0.05). Ulcer area, ulcer score, ulcer index, and wet weight of colon of reference groups (prednisolone and mesalamine) were also attenuated significantly (P < 0.001) [Table 1 and Figure 1].
Table 1.
Effect of BGSE and BGSO on macroscopic parameters of colitis induced by acetic acid in rats
| Groups/Dose | Ulcer severity (0-3) | Ulcer area cm2 | Ulcer index (0-11) | Colon weight (mg) |
|---|---|---|---|---|
| Sham | 0.0 (0-0) | 0.0±0.0 | 0.0±0.0 | 79.8±5.1 |
| Control (colitis) | 3.0 (3-3) | 5.95±0.23 | 8.95±0.23 | 250.8±7.4 |
| BGSE 50 | 3.0 (2-3) | 4.60±0.55** | 7.10±0.87* | 181.4±18.9*** |
| BGSE 100 | 2.0 (2-3)* | 1.13±0.16***# | 2.80±0.4***# | 139.2±8.1***# |
| BGSE 200 | 1.0 (0-2)*** | 0.37±0.08*** | 1.20±0.34*** | 110.0±10.9*** |
| BGSO 2 | 3.0 (2-3) | 5.16±0.57 | 8.00±0.67 | 189.2±29.7* |
| BGSO 4 | 2.0 (2-3)* | 3.28±0.44*** | 5.45±0.7***# | 122.3±17.8***# |
| BGSO 8 | 2.0 (1-3)*** | 1.72±0.0.18*** | 3.05±0.3*** | 118.1±11.4*** |
| Prednisolone 4 | 1.0 (1-3)*** | 0.24±0.02*** | 0.74±0.2*** | 89.6±2.2*** |
| Mesalamine 100 | 1.0 (1-2)*** | 0.31±0.02*** | 0.98±0.2*** | 98.8±6.0*** |
Sham: Normal rats received normal saline/Tween (5 mL/kg/day), Control: Rats with colitis received normal saline/Tween (5 mL/kg/day), BGSE: Black grape seeds extract (50, 100, 200 mg/kg), BGSO: Black grape seeds oil (2, 4, 8 mL/kg). Data are expressed as mean±SEM or median (range) for scoring parameters, n=6. *P<0.05, **P<0.01, ***P<0.001, indicate significant difference versus control. #P<0.05, indicate significant difference versus previous lower dose
Figure 1.
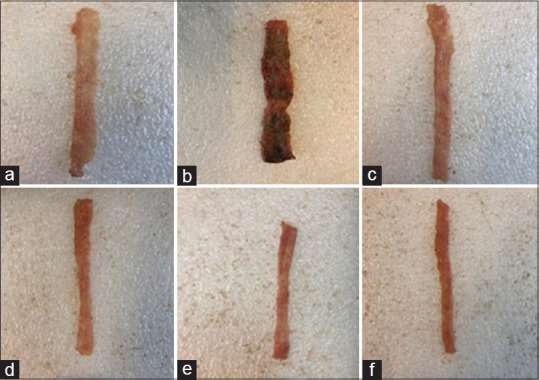
Photos of colon tissue, 5 days after acetic acid induced colitis in rats: (a) Normal colon treated with normal saline/Tween (5 mL/kg); (b) Control colitis treated with normal saline/Tween (5 mL/kg); (c) Colitis treated with BGSE (200 mg/kg); (d) Colitis treated with BGSO (8 mg/kg); (e) colitis treated with prednisolone (4 mg/kg); (f) colitis treated with mesalamine (100 mg/kg)
Effect of BGSE and BGSO on microscopic parameters
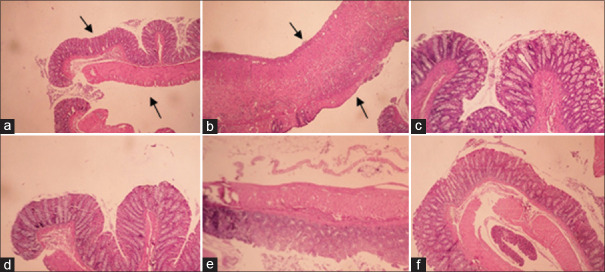
In the control group, edema, hemorrhage, inflammation, crypt damage, and leukocyte infiltration in colon tissue were at the maximum level due to the induction of colitis by acetic acid and no treatment [Table 2]. In all groups treated with BGSE and BGSO with the exception of BGSE (50 mg/kg) and BGSO (2 mL/kg), total colitis index was significantly reduced compared to control group (at least P < 0.01). In groups treated with reference drugs (prednisolone and mesalamine), pathologic parameters and total colitis index were significantly reduced in comparison with the control group (P < 0.001) [Table 2 and Figure 2].
Table 2.
Effect of BGSE and BGSO on macroscopic parameters of colitis induced by acetic acid in rats
| Groups/Dose | Inflammation severity (0-3) | Inflammation extent (0-3) | Leukocyte infiltration (0-3) | Crypt damage (0-4) | Total colitis index (0-10) |
|---|---|---|---|---|---|
| Sham | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Control (colitis) | 3.0 (3-3) | 3.0 (2-3) | 3.0 (2-3) | 4.0 (3-4) | 12.0 (11-13) |
| BGSE 50 | 3.0 (1-3) | 3.0 (1-3) | 2 (2-3) | 2 (2-3) | 10 (6-13) |
| BGSE 100 | 2.0 (1-2)** | 1.0 (1-3)** | 2.0 (1-3)** | 2.0 (1-3)** | 7.0 (5-11)*** |
| BGSE 200 | 1.5 (0-2)** | 1.0 (0-1)*** | 1.0 (0-2)*** | 1.0 (0-2)*** | 4.5 (2-5)***# |
| BGSO 2 | 2.0 (2-3)* | 3 (2-3) | 3 (2-3) | 3 (2-4) | 11.0 (8-13) |
| BGSO4 | 1.5 (1-3)*** | 2.0 (1-2)* | 2.0 (1-3) | 2.5 (2-4)* | 8.0 (6-12)**# |
| BGSO 8 | 1.5 (12)*** | 2.0 (1-2)** | 1.0 (1-2)*** | 1 (1-2)*** | 5.5 (4-6)***# |
| Prednisolone 4 | 1.0 (0-1)*** | 1.0 (0-1)*** | 1.0 (0-1)*** | 1.0 (0-2)*** | 4.0 (2-3)*** |
| Mesalamine 100 | 0.5 (0-1)*** | 1.0 (0-1)*** | 1.0 (0-1)*** | 1.0 (0-2)*** | 3.5 (0-5)*** |
Sham: Normal rats received normal saline/Tween (5 mL/kg/day), Control: rats with colitis received normal saline/Tween (5 mL/kg/day), BGSE: Black grape seeds extract (50,100, 200 mg/kg), BGSO: Black grape seeds oil (2, 4, and 8 mL/kg). ). Data are expressed as median (range) for scoring parameters, n=6. *P<0.05, **P<0.01, ***P<0.001, indicate significant difference versus control. #P<0.05 indicate significant difference versus previous lower dose
Figure 2.
Macroscopic illustration of colonic tissue in rats. (a) Normal colon treated with normal saline/Tween (5 mL/kg); (b) Control colitis treated with normal saline/Tween (5 mL/kg); black arrows presents damaged crypts, mucosal and submucosal layers, and leucocyte infiltration (c) Colitis treated with BGSE (200 mg/kg); (d) Colitis treated with BGSO (8 mg/kg); (e) colitis treated with prednisolone (4 mg/kg); (f) colitis treated with mesalamine (100 mg/kg)
MPO activity measurements
Results of this experiment revealed that MPO activity in groups treated with BGSE (100, 200 mg/kg) and BGSO (4, 8 mL/kg) decreased significantly (P < 0.01). The groups treated with BGSO (2 mL/kg) and BGSE (50 mg/kg) did not show a significant dip in MPO activity in a significant manner (P > 0.05). Prednisolone and mesalamine as expected were effective in reducing MPO activity in related tissues (P < 0.001) [Figure 3].
Figure 3.
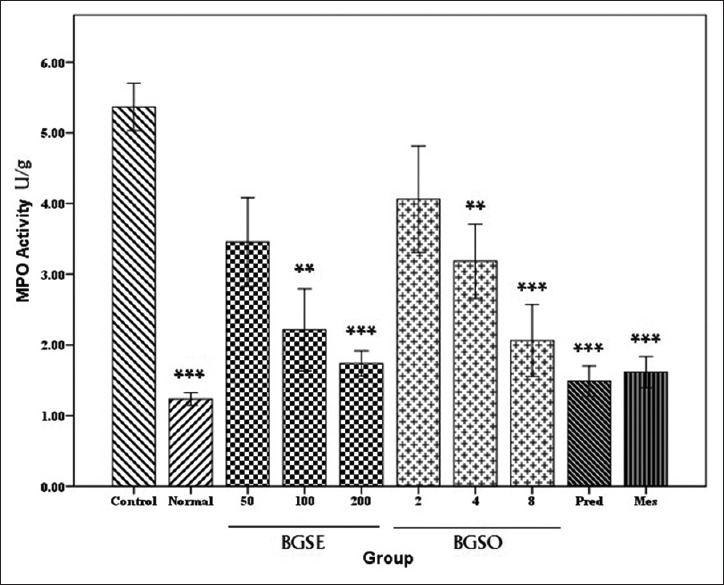
Myeloperoxidase (MPO) activity (U/g) in colonic tissue of rats treated with normal saline/Tween (5 mL/kg), Black grape seeds hydroalcoholic extract (BGSE), Black grape seeds oil (BGSO), Prednisolone (Pred. 4 mg/kg), Mesalamine (Mes. 100 mg/kg). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, indicate significant difference versus control
MDA content measurements
MDA content of colonic tissues treated with BGSE (100, 200 mg/kg) and BGSO (8 mL/kg) decreased significantly (at least P < 0.01) but decline in MDA content in BGSE (50 mg/kg) and BGSO (2, 4 mL/kg) groups was not meaningful (P > 0.05). MDA values in reference groups represented a significant reduction in both groups though attenuation in meslamine group was somewhat more prominent than prednisolone [Figure 4].
Figure 4.
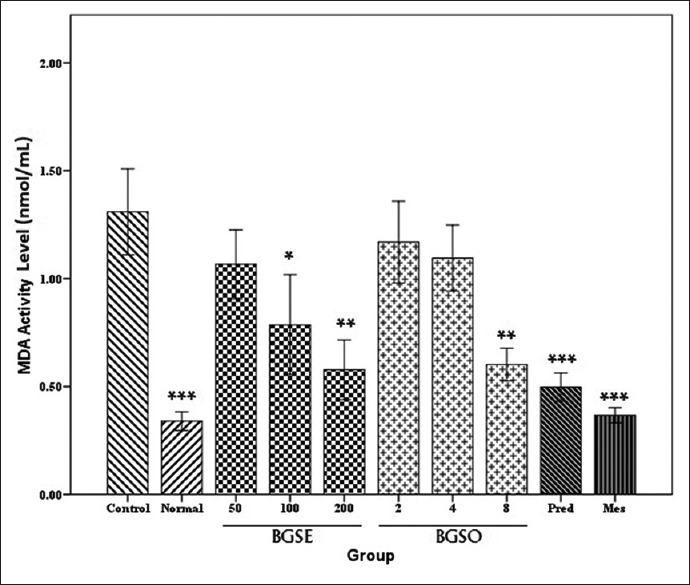
Malondialdehyde (MDA) content (nmol/mL) in colonic tissue of rats treated with normal saline/Tween (5 mL/kg), black grape seeds hydroalcoholic extract (BGSE, 50, 100, and 200 mg/kg), black grape seeds oil (BGSO, 2, 4, and 8 mg/kg), Prednisolone (Pred. 4 mg/kg), Mesalamine (Mes. 100 mg/kg). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, indicate significant difference versus control
Discussion
The macroscopic, histopathological, and biochemical results of this experiment specified that BGSE and BGSO had protective action on acute acetic acid-induced colitis in rats. Our findings indicated an increase in colon weight, edema, mucosa and submucosa inflammation, tissue necrosis, and leukocyte infiltration in the control group as a result of rectal induction of acute colitis by acetic acid.[3] Besides, biochemical results such as MPO and MDA activity supported macroscopic and microscopic data of control and treated groups which can be caused by oxidative stress and lipid peroxidation.[38,39] Data evaluation revealed that almost all doses of BGSE and BGSO with the exception of BGSO (2 mL/kg) could meaningfully decrease ulcer index and weight of colon as indices of the macroscopic parameter. On the other hand, total colitis index in all groups treated with BGSE and BGSO except BGSE (50 mg/kg) and BGSO (2 mL/kg) showed a significant reduction. In biochemical investigations, MPO activity in all groups of GSE and GSO except GSE (50 mg/kg) was reduced significantly in comparison with control group and MDA activity was decreased in GSE (100, 200 mg/kg) and GSO (8 mg/kg); however, GSE (50 mg/kg) and GSO (2, 4 mL/kg) groups could not decrease MDA content meaningfully. Concerning all assessed indices of colitis, it was revealed that GSE (200 mg/kg) and GSO (8 mL/kg) were more effective than other test doses so active components of both black grape seed extract and oil are more active in higher doses and a dose-related effect could be considered for both fractions. The treatments were made orally and the results were promising in colitis alleviation so it could be deduced that active ingredients of BGSE and BGSO were likely similar and had reasonable bioavailability in this route of administration. Though it is likely that some of the active compounds in examined extracts have poor bioavailability they could reach into the colon through the gut lumen and exert a kind of targeted local delivery like Pentasa or Asacol.[5] As mentioned earlier, the high similarity of the compounds present in the hydroalcoholic extract and grape seed oil may explain the relatively similar effects of these two fractions on ulcerative colitis.
Anti-inflammatory and antioxidant effect of black grape seeds was proved in different studies which were due to a reduction in inflammatory mediators like IL6, TNFα, and NF-κB.[11,25] The most likely compounds responsible for these effects are phenolic compounds that are among the most highlighted secondary metabolites found in many plants. Grape seed extracts had a significant effect on surgery wound healing because of the presence of proanthocyanidins and flavonoids that have antioxidant and anti-inflammatory properties.[26] In a study by Filocamo et al., the anti-microbial effect of grape juice extract was proved on gram-positive and gram-negative bacteria and phenolic compounds were introduced as responsible ingredients. This effect is valuable because harmful microorganisms could be one of ulcerative colitis risk factors especially when abscesses and fistula are suppurative complications.[40] Resveratrol which is responsible for many beneficial effects of grape is reported to have an anti-inflammatory effect in acute small intestinal inflammation in a murine model by reducing inflammatory cytokines such as TNF-α, IL-6, IL-8, and COX-2 which has a key role in ulcerative colitis pathogenesis.[3,41] As mentioned above, flavonols (rutin, quercetin, myricetin, catechin, and epicatechin) found in grape had anti-inflammatory, antimicrobial, neuroprotectant, and hepatoprotectant properties.[9] Malvidin, one of the grape anthocyanidins, inhibited human macrophage-derived inflammation in arthritic rats which was further reported to have an inhibitory effect on the secretion of TNFα, IL1, IL6, and TNFα and iNOS.[13] Phenolic compounds such as flavonoids, resveratrol, and anthocyanidins are the most likely components of black grape seed extracts which might have beneficial effects on colitis remission.
Conclusions
In conclusion, black grape seed oil and hydroalcoholic extract had protective and preventive effects on the acute model of experimental ulcerative colitis after oral administration and this activity was greatly dose-related. Although Phenolic compounds with anti-inflammatory, antioxidant, antiulcer, and antimicrobial actions are most likely the accountable agents for this activity, further detailed experiments are required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kasper DL, Fauci AS, Haucer SL, Longo DL, Jameson JL, Loscalzo J. Inflammatory bowel disease. In: Freidman S, Blumberg RS, editors. Harrison’s Principles of Internal Medicine. New York: Mc Graw Hill Education; 2015. pp. 1947–8. [Google Scholar]
- 2.Solberg IC, Lygren I, Jahnsen J, Aadland E, Hoie O, Cvancarova M, et al. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study) Scand J Gasteroentrol. 2009;44:431–40. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 3.Head KA, Jurenka JS. Inflammatory bowel disease Part 1: Ulcerative colitis--pathophysiology and conventional and alternative treatment options. Alt Med Rev. 2003;8:247–83. [PubMed] [Google Scholar]
- 4.Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Ther Adv Gastroenterol. 2015;8:66–82. doi: 10.1177/1756283X14558193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mcquaid KR. Drugs used in the treatment of gastrointestinal diseases. In: Katzung BG, Trevor AJ, editors. Basic and Clinical Pharmacology. New York: Mc Graw Hill Education; 2015. pp. 1071–6. [Google Scholar]
- 6.Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD) Pharmacol Rep. 2011;63:629–42. doi: 10.1016/s1734-1140(11)70575-8. [DOI] [PubMed] [Google Scholar]
- 7.Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;18:Cd000478. doi: 10.1002/14651858.CD000478.pub4. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira FV, Vilela EG, Damiao A, Vieira A, Albuquerque IC, Parente JML, et al. Ulcerative colitis-treatment with biologicals. Rev Assoc Med Bras (1992) 2019;65:547–53. doi: 10.1590/1806-9282.65.4.547. [DOI] [PubMed] [Google Scholar]
- 9.Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis Vinifera (Grape) and its bioactive constituents: An update. Phythoter Res. 2016;30:1392–403. doi: 10.1002/ptr.5644. [DOI] [PubMed] [Google Scholar]
- 10.Georgiev V, Ananga A, Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6:391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negro C, Tommasi L, Miceli A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour Technol. 2003;87:41–4. doi: 10.1016/s0960-8524(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 12.Woerdeman J, Van-Poelgeest E, Ket JCF, Eringa EC, Serne EH, Smulders YM. Do grape polyphenols improve metabolic syndrome components? A systematic review. Eur J Clin Nut. 2017;71:1381–92. doi: 10.1038/ejcn.2016.227. [DOI] [PubMed] [Google Scholar]
- 13.Decendit A, Mamani-Matsuda M, Aumont V, Waffo-Teguo P, Moynet D, Boniface K, et al. Malvidin-3-O-beta glucoside, major grape anthocyanin, inhibits human macrophage-derived inflammatory mediators and decreases clinical scores in arthritic rats. Biochem Pharmacol. 2013;86:1461–7. doi: 10.1016/j.bcp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro . Res Commun Mol Pathol Pharmacol. 1997;95:179–89. [PubMed] [Google Scholar]
- 15.Myles S, Boyko AR, Owens CL, Brown PJ, Grassi F, Aradhya MK, et al. Genetic structure and domestication history of the grape. Proc Natl Acad Sci U S A. 2011;108:3530–5. doi: 10.1073/pnas.1009363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez-Armenta FJ, Silva-Espinoza BA, Cruz-Valenzuela MR, Gonzalez-Aguilar GA, Nazzaro F, Fratianni F, et al. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J Food Sci Technol. 2017;54:3192–200. doi: 10.1007/s13197-017-2759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelbrecht AM, Mattheyse M, Ellis B, Loos B, Thomas M, Smith R, et al. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007;258:144–53. doi: 10.1016/j.canlet.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 19.Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009;26:526–31. doi: 10.1111/j.1464-5491.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 20.Vislocky LM, Fernandez ML. Biomedical effects of grape products. Nutr Rev. 2010;68:656–70. doi: 10.1111/j.1753-4887.2010.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Lekakis J, Rallidis LS, Andreadou I, Vamvakou G, Kazantzoglou G, Magiatis P, et al. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Garavaglia J, Markoski MM, Oliveira A, Marcadenti A. Grape seed oil compounds: Biological and chemical actions for health. Nutr Metab Insights. 2016;9:59–64. doi: 10.4137/NMI.S32910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cianciosi D, Varela-Lopez A, Forbes-Hernandez TY, Gasparrini M, Afrin S, Reboredo-Rodriguez P, et al. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol Res. 2018;135:150–65. doi: 10.1016/j.phrs.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues AD, Scheffel TB, Scola G, Santos MT, Fank B, de-Freitas SC, et al. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem Int. 2012;60:799–805. doi: 10.1016/j.neuint.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Ismail AF, Salem AA, Eassawy MM. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of gamma-irradiated rat. J Photochem Photobiol. 2016;160:1–10. doi: 10.1016/j.jphotobiol.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Hemmati AA, Foroozan M, Houshmand G, Moosavi ZB, Bahadoram M, Maram NS. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob J Health Sci. 2014;7:52–8. doi: 10.5539/gjhs.v7n3p52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna S, Venojarvi M, Roy S, Sharma N, Trikha P, Bagchi D, et al. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic Biol Med. 2002;33:1089–96. doi: 10.1016/s0891-5849(02)00999-1. [DOI] [PubMed] [Google Scholar]
- 28.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–82. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 29.Bashmakov YK, Assaad-Khalil SH, Abou Seif M, Udumyan R, Megallaa M, Rohoma KH, et al. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014;2014:816307. doi: 10.1155/2014/816307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postescu ID, Tatomir C, Chereches G, Brie I, Damian G, Petrisor D, et al. Spectroscopic characterization of some grape extracts with potential role in tumor growth inhibition. J Optoelec Adv Mater. 2007;9:564–7. [Google Scholar]
- 31.Mascolo N, Izzo A, Autore G, Maiello F, Dicarlo G, Capsso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272:469–75. [PubMed] [Google Scholar]
- 32.Zolfaghari B, Kazemi M, Nematbakhsh M. The effects of unripe grape extract on systemic blood pressure and serum levels of superoxide dismutase, malondialdehyde and nitric oxide in rat. Adv Biomed Res. 2015;4:109. doi: 10.4103/2277-9175.157822. doi: 10.4103/2277-9175.157822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidari B, Sajjadi SE, Minaiyan M. Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid- induced acute colitis in rats. Avicenna J Phytomed. 2016;6:205–14. [PMC free article] [PubMed] [Google Scholar]
- 34.Minaiyan M, Mostaghel E, Mahzouni P. Preventive therapy of experimental colitis with selected iron chelators and anti-oxidants. Int J Prev Med. 2012;3(1):S162–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 36.Motavallian-Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J. 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Mazraati P, Minaiyan M. Hepatoprotective effect of metadoxine on acetaminophen-induced liver toxicity in mice. Adv Biomed Res. 2018;24:7–67. doi: 10.4103/abr.abr_142_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khani M, Motamedi P, Dehkhoda MR, Dabagh Nikukheslat S, Karimi P. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J Int Soc Sports Nutr. 2017;21:14–11. doi: 10.1186/s12970-017-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology. 2012;51:1796–803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 40.Filocamo A, Bisignano C, Mandalari G, Navarra M. In vitro antimicrobial activity and effect on biofilm production of a white grape juice (Vitis Vinifera) extract. Evid Bas Compl Alt Med 2015. 2015 doi: 10.1155/2015/856243. doi: 101155/2015/856243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, et al. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;3;5:e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]