Abstract
Maintenance antiretroviral therapy with combination of two injectable long-acting drugs, cabotegravir and rilpivirine, is a new strategy addressing the challenges of daily adherence to oral pills that has shown non-inferior efficacy to standard of care therapy in patients with suppressed HIV-infection. Patients co-infected with hepatitis B virus (HBV) are not eligible for this dual therapy since it has no activity against HBV, but this strategy should also be restricted to patients with anti-HBs antibodies since people with HIV are still at risk of HBV acquisition due to high risk behavior and since HBV vaccination does not always elicit anti-HBs antibodies, as highlighted in the case report below.
Keywords: Acute hepatitis B, Long-acting antiretroviral HIV, cabotegravir, rilpivirine, HBV vaccination
Maintenance antiretroviral therapy (ART) with injectable long-acting (LA) drugs is a new strategy that addresses the challenges of daily adherence to oral pills that have shown noninferior efficacy to standard-of-care therapy in patients with suppressed human immunodeficiency virus (HIV) infection [1, 2]. Patients have reported that the convenience of monthly or bimonthly injections is a significant benefit, in addition to minimizing the potential for HIV disclosure and eliminating the “daily reminder of living with HIV” [3]. This strategy relies on the combination of 2 LA drugs, cabotegravir and rilpivirine. People with HIV (PWH) who develop active hepatitis B virus (HBV) coinfection are not eligible for this dual therapy while coinfected because this 2-drug regimen lacks activity against HBV. Moreover, as this case report highlights, not all individuals who receive the full series of HBV vaccination develop protective antibody responses (anti-hepatitis B surface [HBs] antibodies), reinforcing the need for important preventative measures such as condom use and risk reduction techniques for individuals at higher risk of HBV infection who either have not completed a full HBV vaccination series or lack serologic evidence of immunity [4, 5].
A 31-year-old man who has sex with men was diagnosed with HIV-1 infection in November 2016 and was enrolled in the FLAIR study—an HIV clinical trial—and started induction ART in February 2017 with dolutegravir, lamivudine, and abacavir. Laboratory tests obtained at the screening visit showed no markers of prior HBV infection (negative HBs antigen, negative HBs antibodies, and negative hepatitis B core [HBc] antibodies) and no history of HBV vaccination. Human immunodeficiency virus-1 plasma viral load became undetectable 2 months after induction therapy. In September 2017, this patient was randomized to receive LA therapy with cabotegravir and rilpivirine and was started on 4 weeks of an oral lead with cabotegravir and rilpivirine before monthly LA injections of cabotegravir and rilpivirine.
He received 3 intramuscular injections of HBV vaccine in July 2017, August 2017, and April 2018 combined with hepatitis A virus vaccination. He was treated for multiple sexually transmitted infections between December 2016 and April 2018: syphilis, urethral and anal chlamydia, and shigellosis.
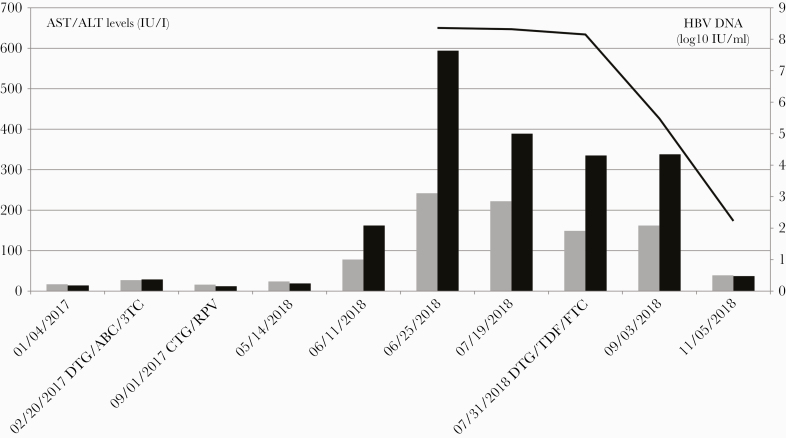
In June 2018, at a monthly follow-up visit, increased aspartate aminotransferase (78 IU/L) and alanine aminotransferase (ALT) levels (162 IU/L) were documented for the first time in an otherwise asymptomatic individual who reported no injectable drug use. Bilirubin levels remained in the normal range, but ALT levels continued to increase up to 594 IU/L. Acute HBV infection was diagnosed based on positive HBs antigen, negative HBs antibodies, positive HBc immunoglobulin M antibodies, positive HBe antigen, negative HBe antibodies, and high HBV deoxyribonucleic acid (DNA) levels 229 000 000 IU/mL (8.36 log10). Serologic tests for other viral hepatitides (A, C, D, E) remained negative with plasma HIV viral ribonucleic acid levels still below 50 c/mL.
Retrospective testing of serum drawn in January 2018, 5 months after the second injection of HBV vaccine, did not show detectable anti-HBs antibodies. In July 2018, because of persistent ALT elevation, the patient left the FLAIR study with the ART switched to tenofovir disoproxil fumarate, emtricitabine, and dolutegravir. Three months postswitch, the ALT level returned to normal with a significant decrease in HBV-DNA levels to 170 IUI/mL (2.23 log10) (Figure 1).
Figure 1.
Time course of serum hepatitis B virus (HBV) markers over time. Solid line, HBV deoxyribonucleic acid (DNA) levels in plasma (log10 IU/L); black boxes, alanine aminotransferase (ALT) levels (IU/L); gray boxes, aspartate aminotransferase (AST) levels (IU/L). ABC, abacavir; CTG, cabotegravir; DTG, dolutegravir; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine.
This case-report illustrates the risk of acute HBV infection in PWH when switching to an ART with no activity against HBV. Moreover, this case report also highlights the need to ensure HBV vaccinations have generated protective titers of anti-HBs antibodies. In cases in which protective immunity has not developed, revaccination should be considered [6–10]. Clinical judgment in addition to preventative measures for high-risk individual continues to be of paramount importance for PWH at increased risk for HBV acquisition. (The patient’s written informed consent was obtained, and the work was approved by ethics committee [the patient was a participant of the Flair study [2]].)
Acknowledgments
We thank Dr. Ronald D’Amico (Medical Director, Clinical Research and Development, ViiV Healthcare) for reviewing the manuscript and giving us permission to proceed with the publication of our case report in Open Forum Infectious Diseases.
Financial support. The FLAIR study was funded by ViiV Healthcare.
Potential conflicts of interest. C. P. reports personal fees and nonfinancial support from Janssen-Cilag, outside the submitted work. C. D. reports grants and personal fees from ViiV, Gilead Sciences, and Merck, outside the submitted work. J.-M. M. has received honoraria for advisory boards with Gilead, ViiV, Merck, and Sanofi and a grant from Gilead, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 2. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 3. Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Launay O, van der Vliet D, Rosenberg AR, et al. ; ANRS HB03 VIHVAC-B Trial Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011; 305:1432–40. [DOI] [PubMed] [Google Scholar]
- 5. Binka M, Butt ZA, Wong S, et al. Differing profiles of people diagnosed with acute and chronic hepatitis B virus infection in British Columbia, Canada. World J Gastroenterol 2018; 24:1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdullahi A, Fopoussi OM, Torimiro J, et al. Hepatitis B virus (HBV) infection and re-activation during nucleos(t)ide reverse transcriptase inhibitor-sparing antiretroviral therapy in a high-HBV endemicity setting. Open Forum Infect Dis 2018; 5:ofy251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014; 28:999–1005. [DOI] [PubMed] [Google Scholar]
- 8. Shilaih M, Marzel A, Scherrer AU, et al. ; Swiss HIV Cohort Study a; Swiss HIV Cohort Study Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016; 214:599–606. [DOI] [PubMed] [Google Scholar]
- 9. Nicolini LA, Magne F, Signori A, et al. Hepatitis B virus vaccination in HIV: immunogenicity and persistence of seroprotection up to 7 years following a primary immunization course. AIDS Res Hum Retroviruses 2018; 34:922–8. [DOI] [PubMed] [Google Scholar]
- 10. Rey D, Piroth L, Wendling MJ, et al. ; ANRS HB04 B-BOOST study group Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): a multicentre, open-label, randomised controlled trial. Lancet Infect Dis 2015; 15:1283–91. [DOI] [PubMed] [Google Scholar]