Abstract
Depressive rumination is considered a prominent risk factor for the occurrence, severity, and duration of depressive episodes. A variety of treatment options have been developed to treat depressive rumination of which mindfulness based programs are especially promising. In the current study, we investigated the neural underpinnings of a short mindfulness intervention and mindful emotion regulation in high and low trait ruminators in an ecologically valid environment using functional near-infrared spectroscopy (fNIRS). Participants were randomly assigned to a mindfulness instruction (MT) group or an instructed thinking (IT) group. Participants in the MT group were trained to either focus their attention mindfully on their breath or their emotions, while the IT group focused their attention on the past or future. Afterwards, all participants underwent an emotion regulation paradigm in which they either watched negative or neutral movie clips. During both paradigms cortical hemodynamic changes were assessed by means of fNIRS. Participants in the MT group showed lower activity in the cognitive control network (CCN) during the focus on breath condition in comparison to the focus on emotion condition. Additionally, oxygenated hemoglobin in the MT group tended to be lower than in the IT group. Further, self-reports of emotional distress during the instruction paradigm were reduced in the MT group. During the emotion regulation paradigm, we observed reduced emotional reactivity in terms of emotional distress and avoidance in the MT group in comparison to the IT group. Furthermore, on a neural level, we observed higher CCN activity in the MT group in comparison to the IT group. We did not find any effect of rumination, neither on the intervention nor on the emotion regulation task. The results of this pilot study are discussed in light of the present literature on the neural correlates of mindfulness based interventions in rumination and emphasize the use of fNIRS to track neural changes in situ over the course of therapy.
Subject terms: Human behaviour, Emotion, Social neuroscience
Introduction
Major Depressive Disorder (MDD) is considered a leading contributor to the global burden of disease1 and has a serious impact on social functioning and everyday routines. Despite the abundance of readily available treatment options, the prevalence and burden of MDD has not decreased, most probably due to moderate recovery rates and reduced stability of treatment effects2. Between one to two thirds of patients successfully treated with cognitive behavioral therapy (CBT) relapse within two years of initial treatment3,4. Relapse rates after discontinuation of pharmacotherapy are even higher5–7. One of the factors that increase the risk for relapse is habitual depressive rumination8,9, which is often defined as a pessimistic self-referential repetitive thinking style about problems with little or no goal- and change-orientation10. On a conceptual level, rumination lines up with and is correlated to other maladaptive perseverative phenomena such as worry and intrusive thought8. In addition to worsening relapse rates, depressive rumination is associated with further clinical outcomes, such as higher symptom severity, longer duration of depressive episodes and higher risk of suicide8,11–15. Further, rumination is associated with cognitive malfunctioning16,17, reduced emotional wellbeing, emotion dysregulation18,19, and higher physiological stress parameters20. On a neural level, rumination has been shown to be related to increased activity in areas of the cognitive control network (CCN) and default mode network (DMN) during induction paradigms21–25, decreased activity in cognitive tasks26,27 as well as aberrant functional connectivity between these networks26,28,29. The CCN is typically involved in tasks that require effortful control and top-down regulation and comprises areas such as the dorsolateral prefrontal cortex and posterior parietal cortex30,31. In contrast, the DMN involves areas such as the medial prefrontal cortex, posterior midline, precuneus and lateral temporal cortex, and is most active during rest26,32,33. With respect to emotion regulation, high trait ruminators show a pattern of decreased prefrontal activity during the downregulation of negative affect in prefrontal areas of cognitive control27,34 and increased activity in subcortical areas34,35.
In light of the increasing evidence indicating how depressive rumination complicates the severity and treatment efficacy of conditions such as MDD36, interventions for the acute treatment and relapse prevention have been developed that specifically target processes such as negative self-referentiality including depressive rumination37–42. With respect to acute treatment, interventions such as Emotion Regulation Therapy (ERT) incorporate techniques from CBT and mindfulness-based intervention to tackle pathological processes such as worrying and rumination. First empirical evidence suggests that ERT is indeed highly effective in reducing pathological self-referential thinking such as worry and rumination and symptoms in generalized anxiety disorder (GAD) and MDD43–45. Furthermore, a recent study by O’Toole et al.46 showed that ERT-related increase in cognitive reframing (CBT skill) and decentering (mindfulness skill) preceded decreased worry in GAD46. In terms of relapse prevention, a recent meta-analysis suggests that Mindfulness-based Cognitive Therapy (MBCT) decreases the risk for relapse by a hazard-ratio of 0.7 in comparison to active treatment37. In this meta-analysis, participants that received MBCT had an 11% reduced relapse risk as compared to subjects that did not receive MBCT (38% vs. 49%) within 60 weeks. Similarly, findings indicate that Rumination Focused Cognitive Behavioral Therapy as compared to usual care lowers the rate of relapses within a 6 months follow-up (9.5% vs. 53%)38.
As outlined above, mindfulness—the ability to direct one’s attention to the present moment and cultivate a non-judgmental awareness—is often a component of successful treatment of depressive rumination47–50. With respect to neural correlates, it is important to distinguish between studies of advanced meditators and novices as the latter usually activate more areas of the CCN during meditation51,52. Most likely, this difference is attributable to effects of acquiring and learning the skill of mindfulness in novices, e.g. needing more effortful control to achieve and maintain the meditative state. In general, mindfulness has been associated with activity in brain areas that are involved in emotion regulation (e.g., areas in the CCN and limbic regions), attentional control [e.g., anterior cingulate cortex (ACC)] and self-awareness (e.g., areas in the DMN). Meta-analytic data on different forms of meditation showed further overlapping activation in the limbic system, basal ganglia and medial prefrontal cortex for different forms of meditation53. In a study on the neural correlates of Mindfulness-Based Stress Reduction (MBSR) in GAD, Hölzel et al.54 showed that an 8-week MBSR program reduced amygdala activation and increased ventrolateral prefrontal activation during an affect labeling task. Further, frontolimbic connectivity increased in the treatment group and changes in activation and functional connectivity were associated with changes in clinical assessments54. These findings were replicated recently in a healthy cohort by Doll et al.55 who found that attention-to-breath mindfulness practice was associated with lateral and medial prefrontal activation and with fronto-limbic coupling during an emotion-regulation task in an fMRI investigation55. Aside from the reported long term intervention studies, first evidence from short-term laboratory manipulations suggests that mindfulness reduces induced negative mood56,57, as well as perceived stress during social evaluative situations58 and increases attentional control and immune reactivity59.
The current pilot study aimed to investigate the neural correlates of mindful emotion regulation in high and low trait ruminators. To this end, we employed the optical imaging method of functional near-infrared spectroscopy (fNIRS) during two paradigms in high and low trait ruminators. By using fNIRS we were able to assess cortical blood oxygenation in an ecologically valid environment, as fNIRS is able to be applied in various body positions (sitting, staying, walking) and is relatively subtle in terms of measurement related noises or hardware.
In the first phase of the study, 34 high trait ruminators and 33 low trait ruminators were randomized to either a mindfulness (MT) or instructive thinking (IT) group after a baseline assessment. In the mindfulness group, subjects were asked to cultivate either a mindfulness focus on the breath or mindfulness focus on experiences and emotions during 14 trials of 40 s duration. In the instructive thinking group, subjects were asked to direct their attention either to a situation in the past or in the future and think about this situation over and over again during 14 trials of 40 s duration. After each block, subjectively rated success, effort, emotional distress and attentional lapses were assessed. In the second study phase, all subjects completed an emotion regulation task during which subjects watched video clips with neutral and negative valence. After each trial, subjectively rated emotional distress and avoidance were assessed. Furthermore, emotion ratings, state rumination and state decentering were measured over the course of the experiment with questionnaires (baseline, after the short instruction paradigms, directly after the emotion regulation paradigm and 10 min following the completion of the emotion regulation paradigm).
Based on our prior work on rumination, we hypothesized that high ruminators would show generally elevated levels (main effect rumination) of state rumination, negative affect, emotional distress and avoidance, as well as higher reactivity following the emotion regulation task in comparison to baseline (interaction rumination*time). Further, as even brief mindfulness trainings have been shown to be effective on short-term emotion regulation, we expected that the mindfulness instruction would decrease the emotional reactivity in general (main effect) and within the high ruminators more strongly than in the low ruminators (ordinal interaction: instruction group*rumination*time).
With respect to cortical oxygenation (O2Hb) as measured with fNIRS, we expected that the MT group would show higher CCN activity than the control group, especially during mindful non-judgmental focus (interaction: intervention*condition). We investigated the effect of rumination in an exploratory manner during the instruction paradigm.
Additionally, we expected that the high ruminators would show reduced cortical oxygenation during the emotion regulation task in the CCN and that the trained instruction would normalize this reduction (interaction: instruction group*rumination*condition). For a detailed description of the hypotheses per paradigm and used post-hoc tests see Supplementary Table S1.
Materials and methods
Participants
Sixty-seven participants were recruited via email and flyers for this study. Exclusion criteria were acute physical illness, neurological disorders, substance abuse, chronic or acute diseases that affect brain functioning such as diabetes or kidney failure, cardiac arrhythmia as well as other cardiac diseases. The ethics committee of the University Hospital and University of Tuebingen approved this project and all subjects gave written informed consent. 34 high ruminators and 33 low ruminators were recruited for this study based on their response in the Ruminative Response Scale60,61. In total, 193 subjects completed the RRS online questionnaire. High ruminators were defined by an RRS mean score higher than 2.25 (percentile > 65), low ruminators by a score lower than 1.8 (percentile < 34). Cut-off criteria were defined as in previous studies in our group62, based on comparisons between depressed patients and non-clinical controls26 (see Supplemental Material Fig. S1).
Out of the 193 completers, 131 subjects that fulfilled our criteria were contacted. Out of those, 74 subjects were randomized. However, 7 subjects dropped out of the study during participation, in 6 cases before the measurement, and in 1 case during the measurement due to overly high emotional arousal during the emotion regulation task. Subjects were randomized to two different treatment groups: Mindfulness Training (MT) or Instructed Thinking (IT). Sociodemographic and clinical characteristics are shown in Table 1. In total, 10 subjects had some kind of experience with relaxation training (n = 2), autogenous training (n = 3) and/or meditation (n = 8). Eight of the 10 subjects just started with practicing within the last year, and 7 subjects reported to practice less than once per week or not at all on a regular basis. As no subject reported to practice mindfulness meditation for a substantial amount of time on a regular basis, we did not exclude subjects due to mindfulness practice. The treatment groups did not differ significantly in sex ratio. However, the low ruminators in the MT group showed a significantly higher mean age than the other three groups (all t-values > 2.17, p < 0.05, d > 0.7). As expected, low ruminators showed reduced levels in depressive symptomatology (BDI: F(1,62) = 22.161, p < 0.001, partial η2 = 0.15), habitual rumination (RRS: F(1,62) = 303.709, p < 0.001, partial η2 = 0.83), state rumination (state-rumination: F(1,62) = 18.120, p < 0.001, partial η2 = 0.23), negative affect (PANAS-NA: F(1,62) = 7.777, p < 0.01, partial η2 = 0.11) and increased levels of baseline measured habitual mindful acting (KIMS-act: F(1,62) = 8.552, p < 0.01, partial η2 = 0.12), acceptance (KIMS-acceptance: F(1,62) = 38.202, p < 0.001, partial η2 = 0.37) and decentering (EQ(1,62) ) = 5.292, p < 0.05, partial η2 = 0.08) (see Supplementary Table S3). As the study groups differed significantly in age, we repeated the reported analysis using age, sex and experience with relaxation/mediation as covariates within MANCOVAs (see Supplemental Material Table S5 and results).
Table 1.
Demographic and clinical variables of the experimental groups at baseline.
| Variable | MT group | IT group | ||||||
|---|---|---|---|---|---|---|---|---|
| Low ruminators | High ruminators | Low ruminators | High ruminators | |||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Age (years) | 28.6 | (9.59) | 23.2 | (3.15) | 22.9 | (3.46) | 22.3 | (3.46) |
| Sex ratio (f/m) | 12/4 | 15/3 | 12/5 | 9/7 | ||||
| Prior knowledge (yes/no) | 4/10 | 4/14 | 1/16 | 1/15 | ||||
| BDI | 4.4 | (4.0) | 9.8 | (7.7) | 3.3 | (4.7) | 11.0 | (5.3) |
| RRS | 1.5 | (.21) | 2.6 | (.32) | 1.6 | (.17) | 2.5 | (.23) |
| EQ-trait | 2.4 | (.32) | 2.3 | (.57) | 2.5 | (.39) | 2.1 | (.32) |
| Baseline NA | 1.1 | (.14) | 1.3 | (.35) | 1.2 | (.20) | 1.3 | (.30) |
| Baseline SR | 1.4 | (.33) | 1.9 | (.64) | 1.4 | (.31) | 1.9 | (.75) |
| KIMS—observing | 3.2 | (.66) | 3.7 | (.55) | 3.4 | (.50) | 3.5 | (.50) |
| KIMS—describing | 3.6 | (.41) | 3.7 | (.60) | 3.5 | (.36) | 3.3 | (.55) |
| KIMS—acting | 3.3 | (.50) | 2.9 | (.48) | 3.3 | (.33) | 2.9 | (.75) |
| KIMS—acceptance | 4.1 | (.50) | 3.3 | (.63) | 4.0 | (.52) | 3.0 | (.62) |
Prior knowledge = Number of subjects with experiences in meditation or relaxation training, RRS = Rumination Response Scale, EQ = Experiences Questionnaire, Baseline NA = Negative emotions as rated by the Positive and Negative Affect Schedule, Baseline SR = Baseline state rumination, KIMS = Kentucky Inventory of Mindfulness Skills.
Procedures
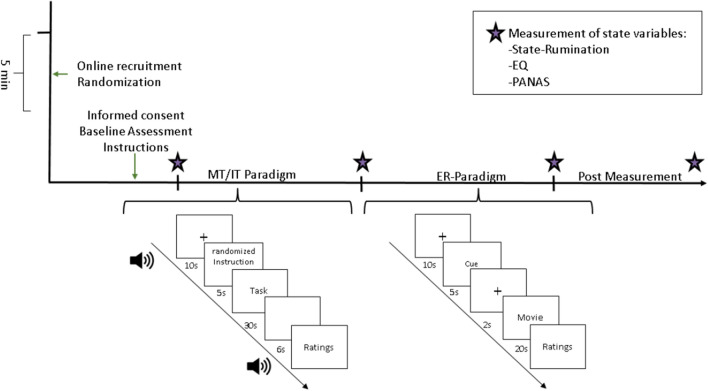
On the day of the measurement, participants completed baseline questionnaires while the fNIRS measurement was prepared (see Fig. 1). The baseline questionnaires consisted of sociodemographic data, the Beck Depression Inventory (BDI)63, Kentucky Inventory of Mindfulness Skills (KIMS)64, Experiences Questionnaire (EQ)65,66 in the trait form, the state form of the Positive and Negative Affect Schedule (PANAS)67 and a state rumination questionnaire26,27. Afterwards, subjects were given written information about the first task of the study: either the MT or IT. After reading the instruction (see supplemental material), subjects were asked to summarize their task and were instructed again verbally by the examiner. The MT group was instructed to either focus on their breath in the present moment while being non-judgmental (condition one: focus) or focus on their current feelings and emotions in a non-judgmental way while being in the present moment (condition two: equanimity). The two conditions were presented in randomized order and used to implement the two core elements of mindfulness: (1) focusing the attention in the present moment and (2) being non-judgmental about the present experience68. Furthermore, both of these aspects are present during mindfulness practice. While the main task during mindfulness meditation is, e.g., being focused on the breath non-judgmentally, this focus often becomes disturbed by emotionally loaded thoughts. In this case, meditators are instructed to recognize that they are not mindful anymore, and redirect their attention back to the breath while being non-judgmental about their feelings and thoughts.
Figure 1.
Experimental workflow and used paradigms. MT mindfulness instruction, IT instructed thinking, ER emotion regulation.
The IT group was instructed to direct their attention towards a past (condition one: past) or future event (condition two: future) and to think about this event over and over again. The IT was designed as a control paradigm to the MT group, as thinking about the future is a non-mindful task (at least in non-meditators). Furthermore, we sought to create a control condition for the MT aside from baseline comparisons.
Both of the short interventions were similar in their design. First, subjects were asked if they had any questions about the task. Afterwards, subjects were instructed via audio to close their eyes. A 10 s baseline measurement followed before a peep-tone marked the start of the trial. During the first 10 s of the trial, subjects were instructed about their focus during this trial. Afterwards, subjects performed the task for 30 s. A second peep-tone marked the end of the task, followed by a 6 s inter-trial interval before subjects were instructed to open their eyes again. At the end of each trial, subjects gave ratings on perceived success, effort, emotional distress and attentional shifts during the trial. Subjects performed 14 blocks with randomized ordering of conditions. At the end of the completion of the short intervention, the MT group was instructed to stay in the mindful focus during the following emotion regulation task, while the IT group was left without instruction. We chose not to give specific instructions to the IT group to lower the risk for artificial neural activity (e.g. while subjects are actively trying to not regulate). Therefore, the emotion regulation task measures the mindful emotion regulation in the MT group and the emotional reactance in the IT group.
After this first phase of the study, subjects completed state measurements of decentering, negative emotion ratings and state-rumination. In the second study phase, all subjects participated in an emotion regulation task. During the paradigm subjects were asked to watch 28 different movie clips of 20 s length each with strongly negative or neutral valence. During the experiment, movie clips were presented in blocks, each consisting of 7 trials of a given valence. Movie clips were presented in an ABAB and BABA version in randomized order of movie blocks within each version. This procedure was used as some movie clips were in relation to each other, e.g. a suicide scene was cut to be used in 4 clips starting from the beginning of the suicide to the finding of the corpse by the parents of the suicide victim. Each trial consisted of a 10 s baseline followed by a 5 s prompt, instructing which trial would follow (anticipation phase). Next, a 2 s fixation cross was presented before the 20 s video clip started (watching phase). At the end of each clip, subjects rated their emotional distress and their avoidance during the movie clip on a 1 (not at all) to 9 (very much) Likert-scale. Avoidance was defined as the urge or act of fleeing the situation and withdrawing from the emotional experience. Negative clips included holocaust scenes (movie “The pianist”), suicide (“13 Reasons why”), murder (“American History X”), crying girl (“The fault is in our stars”), a dead baby (“Trainspotting”); neutral clips included informational film clips about sunflowers, gardening, chess, paper plane folding and knitting (for descriptive statistics per video see Supplementary Table S2).
After the emotion regulation task, subjects completed the state questionnaires two times: directly and 10 min after the task.
fNIRS
Cortical activation was measured with a continuous wave, multichannel NIRS system (ETG-4000 Optical Topography System; Hitachi Medical Co., Japan) with a temporal resolution of 10 Hz. The same probeset placement was used as in previous studies of our group on the CCN in rumination27,62,69. It consisted of two frontal and one parietal probeset positioned on an Easycap with sponge rings for additional fixation.
After the assessment, data was further analyzed using MATLAB R2019a (MathWorks Inc, Natick, USA). We used the relatively new motion correction method of Fishburn et al.70: Temporal Derivative Distribution Repair (TDDR). Briefly, the method uses an adaptive algorithm to identify and correct sharp high amplitude movement artefacts based on the signal’s amplitude distribution70. Afterwards, we performed the correlation based signal improvement method of Cui et al.71,72 to reduce low amplitude artefacts that would not be captured by the TDDR. Further, interpolation of single artifact-loaded channels was performed, before data were bandpass filtered (0.1–0.01 Hz). As hemodynamic data might be confounded by global physiological artefacts through respiration, we performed a global signal reduction with a spatial Gaussian kernel filter73 with a standard deviation of σ = 40. Finally, the signal was z-standardized before event-related averages were computed.
In the MT and IT instruction paradigm, a 10 s baseline correction in a 70 s window was used. Mean activity was extracted between 10 and 40 s, as auditory instructions were given in the first 10 s of each trial. For data in the emotion regulation task, a 10 s baseline correction in a 32 s window was used for linear detrending. Data were averaged for the anticipation of negative and neutral videos (0–7 s) and the watching of negative and neutral videos (7–27 s).
Data was averaged in 5 regions of interest (ROI) comprising the superior parietal lobule (SPL), bilateral dlPFC and inferior frontal gyrus (IFG). ROIs were selected according to previous studies on the CCN27,62,69.
Data analysis
Statistical analysis has been conducted with IBM SPSS State Statistics 24. Figures have been created with the ggplot2 package in R. Brain maps have been created with self-written MATLAB routines. Multiple comparisons of post-hoc tests were corrected by the Benjamini–Hochberg procedure. As this study was a pilot study to investigate the usefulness of a blocked mindfulness short intervention and the potential effects on emotion regulation, we conducted a sensitivity analysis given an alpha level of α = 0.05 and a power of β = 0.80 with G-power. Given the sample size of N = 67, we were able to detect effect sizes up to f2(V) = 0.19 for comparisons between two groups (main effect instruction group/rumination) with 4 dependent variables and f2(V) = 0.21 for 5 dependent variables, which are both moderate effect sizes. A within-between interaction needed to have an effect of f(V) = 0.34 for two groups (e.g. instruction group *condition) and f(V) = 0.42 for 4 groups (instruction group *rumination), which are moderate to large effect sizes.
We separately analyzed the first (instruction group) and second (emotion regulation) paradigm, as well as changes in emotion, decentering and state-rumination. In all analyses we used mixed MANOVAs with the between subject factors rumination (high vs. low) and instruction group (MT vs. IT) and the within-subject factor condition (MT: focus on breath vs. emotion; IT: focus on past vs. present; ER task: negative vs. neutral videos) of the related instruction. Dependent variables (DVs) were the behavioral scores (ratings) during the paradigm and NIRS data of the five ROI. Prior to the analysis, the assumptions of MANOVA were checked. Data for the behavioral ratings were transformed to achieve normal distribution. Post-hoc analysis was conducted on the DVs that further showed significant results in the univariate statistics. Univariate analyses following the MANOVAs were corrected by the Benjamini–Hochberg procedure. According to our hypothesis, we were mainly interested in main effects and interactions of the between subject factors. In each instruction group, we analyzed subjective ratings first as part of the manipulation check and subsequently fNIRS data.
Statistical analyses of paradigm 1: instruction groups
Behavioral data in the instruction groups was analyzed using a 2 (rumination: high vs. low) by 2 (instruction: MT vs. IT) by 2 (condition: focus/past vs. equanimity/future) MANOVA with 4 DVs (rating: attentional shifts vs. effort vs. emotional distress vs. success). As post hoc tests, we performed planned comparisons between (1) the groups and (2) the conditions within the groups (in MT: focus vs. equanimity; in IT: past vs. future). Interactions with the factor rumination were explored by comparing the subgroups (MT-rumination low, MT-rumination high, IT-rumination low, IT-rumination high) and the higher order interactions in the same manner (see Supplementary Table S1).
fNIRS data in the first paradigm was analyzed using a 2 (rumination: high vs. low) by 2 (instruction group: MT vs. IT) by 2 (condition: focus/past vs. equanimity/future) MANOVA with 5 DVs (ROI: left DLPFC, left IFG, right DLPFC, right IFG, SPL). Planned comparisons were equivalent to those of the behavioral data. Additionally, for descriptive purposes we performed tests of O2Hb values against baseline (t-test against zero) to assess the general activation pattern during the intervention paradigm (see Supplementary Analysis Table S4).
Statistical analyses of paradigm 2: emotion regulation task
In the emotion regulation task we directly analyzed difference scores of the experimental contrast (negative videos vs. neutral videos) for reasons of simplicity. Behavioral data in the emotion regulation task was analyzed using a 2 (rumination: high vs. low) by 2 (instruction group: MT vs. IT) MANOVA with 2 DVs (rating: emotional distress vs. avoidance). Post-hoc tests were performed in DVs with significant univariate effects and included comparisons between the instruction groups and subgroups of rumination (high ruminators MT vs. IT; low ruminators MT vs. IT) in the ratings.
fNIRS data were analyzed with a 2 (rumination: high vs. low) by 2 (instruction group: MT vs. IT) by 2 (phase: anticipation vs. watching) MANOVA for the contrast of the conditions (negative—neutral) on 5 DVs (ROI: left DLPFC vs. left IFG vs. right DLPFC vs. right IFG vs. SPL). Post-hoc tests included comparisons between the instruction groups and rumination groups separately for the different ROI (see Supplementary Table S1).
Statistical analysis of state variables over the course of the experiment
To analyze changes in negative emotion ratings, state-rumination and decentering, mixed MANOVAs with the factors instruction, rumination and time (measurement point) were conducted. We used time-related contrasts (linear, quadratic and cubic) to track changes over the course of the experiment. Planned comparisons included interactions of the time factor with instruction and rumination (see Supplementary Table S1).
Ethics approval
The ethics committee at the University Hospital and University of Tuebingen approved this project and all subjects gave written informed consent. All used methods and procedures in this study were in accordance to the current guidelines of the World Medical Association's Declaration of Helsinki.
Results
First paradigm: MT vs. IT
Behavioral data
During the instruction, we observed a main effect of instruction group (F(4,60) = 5.618, p < 0.001, Wilk’s Λ = 0.728, partial η2 = 0.27), a main effect of condition (F(4,60) = 5.849, p < 0.001, Wilk’s Λ = 0.719, partial η2 = 0.28) and an interaction of condition by instruction group (F(4,60) = 6.652, p < 0.001, Wilk’s Λ = 0.693, partial η2 = 0.31). No main effect of or interaction with rumination was found (all p > 0.1).
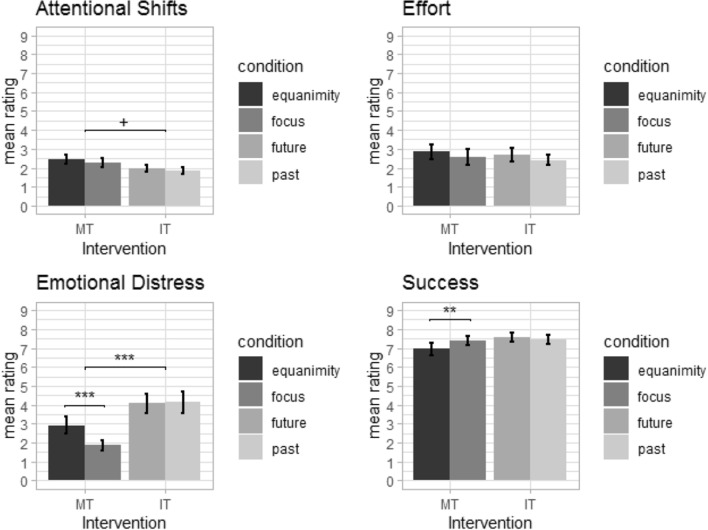
Univariate analysis of the main effect of instruction group was significant for the DVs emotional distress (F(1,63) = 18.765, p < 0.001, partial η2 = 0.23) and attention shifts (F(1,63) = 5.048, p < 0.05, partial η2 = 0.07); however, the latter finding was not significant after correction for multiple comparisons in the univariate ANOVAs. The main effects indicated lower emotional distress and marginally more attention shifts in the MT group in comparison to the IT group (see Fig. 2).
Figure 2.
Ratings (Likert scale from 1 to 9) during the instruction paradigm in the MT group and IT group. ***p < 0.001, **p < 0.01, *p < 0.05, +p < 0.05 without correction for multiple comparisons.
Further, the interaction of condition by group showed significant results for the DVs emotional distress (F(1,63) = 16.001, p < 0.001, partial η2 = 0.20) and perceived success (F(1,63) = 9.669, p < 0.01, partial η2 = 0.13). Post-hoc tests were only performed between the conditions of each instruction group, as the main effect already showed significant differences between the groups. Post-hoc tests revealed that the MT group showed reduced emotional distress (t(33) = 3.940, p < 0.001, d = 0.73) and higher perceived success of task completion (t(33) = 3.422, p < 0.01, d = 0.39) during the focus condition in comparison to the equanimity condition, while no differences in these variables were observable between the conditions of the IT group (see Fig. 2).
Cortical activation
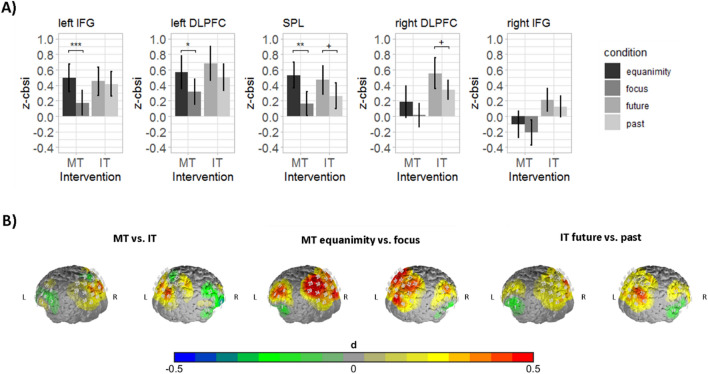
The analysis of fNIRS data during the instruction paradigm showed a marginally significant effect of instruction group (F(5,58) = 2.025, p < 0.1, Wilk’s Λ = 0.851, partial η2 = 0.15), a significant main effect of condition (F(5,58) = 3.631, p < 0.01, Wilk’s Λ = 0.762, partial η2 = 0.24) and a marginally significant interaction of condition by instruction group (F(5,58) = 2.354, p < 0.1, Wilk’s Λ = 0.831, partial η2 = 0.17). The marginal main effect of instruction group was characterized by a tendency towards reduced CCN activity in the MT group in comparison to the IT group.
In summary, only the condition effect was significant, implying a similar pattern of activity in the equanimity/future condition and focus/past condition in the instruction groups. The univariate comparison of the main effect of condition showed that both groups combined had significantly higher activity in the equanimity/future condition than in the focus/past condition in the left IFG (F(1,62) = 8.295, p < 0.01, partial η2 = 0.12), left DLPFC (F(1,62) = 9.825, p < 0.01, partial η2 = 0.14), right DLPFC (F(1,62) = 8.121, p < 0.01, partial η2 = 0.12), and SPL (F(1,62) = 18.160, p < 0.001, partial η2 = 0.22). As combined comparisons of the instruction groups on the condition factor during the short intervention are somehow misleading, e.g. as the equanimity condition of the MT group is not directly comparable/equivalent to the future condition of the IT group, we reanalyzed the condition effect for both groups separately. Here again, we observed a main effect for condition in the MANOVA in both groups (MT: F(5,28) = 3.667, p < 0.05, Wilk’s Λ = 0.604, partial η2 = 0.40); IT: F(5,26) = 2.622, p < 0.05, Wilk’s Λ = 0.665, partial η2 = 0.33). Univariate analysis for the MT group indicated significantly higher activity in the equanimity than in the focus condition in left IFG (F(1,32) = 15.027, p < 0.001, partial η2 = 0.32), left DLPFC (F(1,32) = 7.154, p < 0.05, partial η2 = 0.18) and SPL (F(1,32) = 11.522, p < 0.01, partial η2 = 0.27). In contrast, no univariate comparison in the IT group survived the correction for multiple comparisons. Tendencies towards higher activity in the future in comparison to the past condition were observed in the left DLPFC (F(1,30) = 3.204, p < 0.1, partial η2 = 0.10), right DLPFC (F(1,30) = 5.013, p < 0.05, partial η2 = 0.14) and SPL (F(1,30) = 7.064, p < 0.05, partial η2 = 0.19). The different impact of the condition factor in both groups might further explain the marginally significant interaction of condition by instruction group in the main analysis (see Fig. 3).
Figure 3.
Activation during the short intervention. (A) Bar plots depicting the average z-score of the correlation based signal improvement (CBSI) O2Hb value for each region of interest (ROI) in the condition for the MT- and IT group. The focus condition in the MT group and the past condition in the IT group is colored in dark grey, the equanimity condition in the MT group and the future condition in the IT group is colored in light grey. IFG inferior frontal gyrus, DLPFC dorsolateral prefrontal cortex, SPL superior parietal lobule, ***p < 0.001, **p < 0.01, *p < 0.05, +p < 0.05 without correction for multiple comparisons. (B) Contrast head maps for the observed effects. MT mindfulness instruction, IT instructed thinking, L left, R right. Brain maps were created using self-written MATLAB routines.
Second paradigm: emotion regulation task
Behavioral data
After the short intervention, all subjects participated in the emotion regulation task. With respect to behavioral ratings during the emotion regulation task, we found a significant main effect of condition (negative vs. neutral) as indicated by a significant constant of the experimental contrast (F(2,60) = 135.483, p < 0.001, Wilk’s Λ = 0.181, partial η2 = 0.82). Univariate analysis indicated higher emotional distress (F(1,61) = 275.084, p < 0.001, partial η2 = 0.82) and higher avoidance (F(1,61) = 89.545, p < 0.001, partial η2 = 0.60) during watching of the negative movies in comparison to neutral movies (see Fig. 4). Further, we found a main effect of instruction group (F(1,62) = 3.160, p < 0.05, Wilk’s Λ = 0.905, partial η2 = 0.10). Univariate analysis showed significant results for emotional distress (F(1,61) = 5.099, p < 0.05, partial η2 = 0.08) and avoidance (F(1,61) = 4.781, p < 0.05, partial η2 = 0.07), but both effects did marginally not survive the correction for multiple comparisons (pkrit = 0.025, pemp = 0.028).
Figure 4.
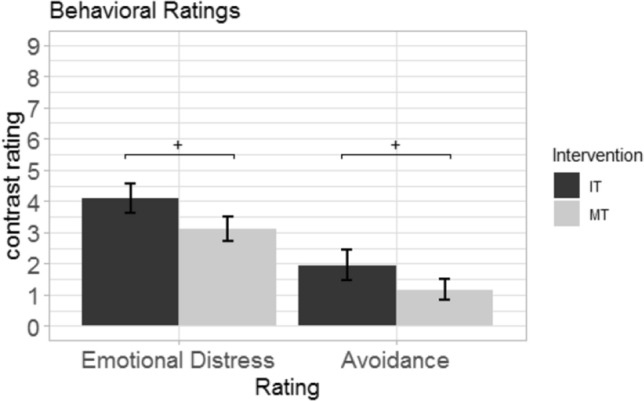
Mean ratings of emotional distress and avoidance during the emotion regulation task. +p < 0.05 without correction for multiple comparisons.
No main effect of or interaction with rumination was found (all p > 0.1).
Cortical activation
With respect to cortical O2Hb levels during the emotion regulation task, we observed a main effect of the constant of the contrast values (negative-neutral movies) (F(5,57) = 12.855, p < 0.001, Wilk’s Λ = 0.470, partial η2 = 0.53), a main effect of instruction group (F(5,57) = 2.986, p < 0.05, Wilk’s Λ = 0.792, partial η2 = 0.21) and a main effect of phase (F(5,57) = 3.318, p < 0.05, Wilk’s Λ = 0.775, partial η2 = 0.23).
Univariate analysis of the constant term showed significantly increased O2Hb levels in the left IFG (F(1,61) = 19.705, p < 0.001, partial η2 = 0.24), left DLPFC (F(1,61) = 16.125, p < 0.001, partial η2 = 0.21), right DLPFC (F(1,61) = 7.599, p < 0.01, partial η2 = 0.11) and SPL (F(1,61) = 43.790, p < 0.001, partial η2 = 0.42), indicating increased cortical blood oxygenation in these brain areas during negative in comparison to neutral movie clips.
The main effect of instruction group showed significantly increased O2Hb levels in the MT in comparison to the IT group in the left DLPFC (F(1,61) = 4.589, p < 0.05, partial η2 = 0.07), although the effect did not survive the correction for multiple comparisons for the univariate analysis, and a marginally significant effect in the right IFG (F(1,61) = 3.394, p < 0.1, partial η2 = 0.53) (see Fig. 5).
Figure 5.
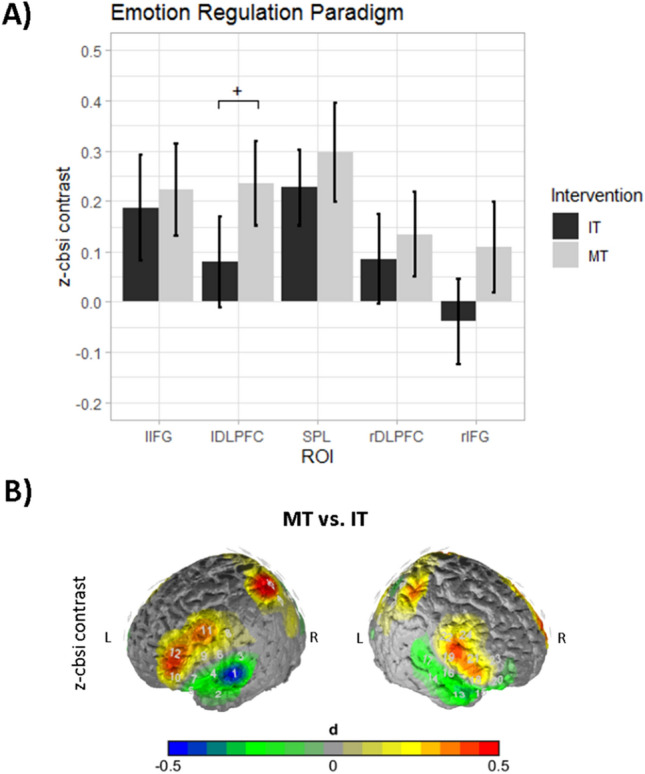
Activation during the emotion regulation task. (A) Bar plots depicting the main effect of instruction group. Dependent variable is the z-score of the correlation based signal improvement (CBSI) O2Hb value. +p < 0.05 without correction for multiple comparison. (B) Contrast head maps for the observed effects. The contrast of the MT vs. IT group is depicted with values in effect size d. Warm colors indicate higher activity in the mindfulness group. Brain maps were created using self-written MATLAB routines.
Finally, the univariate analysis of the main effect of phase showed significantly increased O2Hb levels during anticipation in comparison to watching in the left IFG (F(1,61) = 12.793, p < 0.001, partial η2 = 0.17) and SPL (F(1,61) = 4.586, p < 0.05, partial η2 = 0.07).
No main effect of or interaction with rumination was found (all p > 0.1).
Changes in decentering, subjective emotion, and state-rumination over the course of the experiment
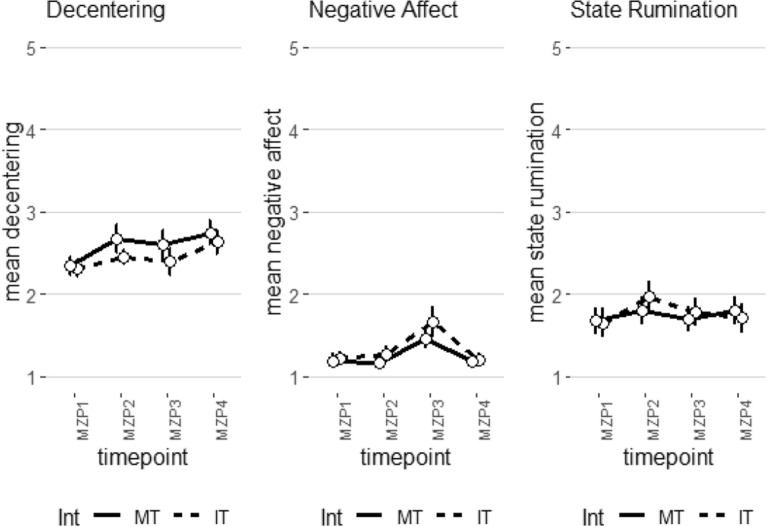
Lastly, we analyzed changes in decentering, negative emotion and state rumination over the course of the experiment. Our results of the multivariate analysis of variance showed a significant main effect for rumination (F(3,57) = 6.454, p < 0.001, Wilk’s Λ = 0.746, partial η2 = 0.24) and time (F(9,51) = 13.182, p < 0.001, Wilk’s Λ = 0.301, partial η2 = 0.70). The univariate analysis of the main effect of rumination showed significantly increased state rumination (F(1,59) = 19.157, p < 0.001, partial η2 = 0.25), and negative affect (F(1,59) = 4.187, p < 0.05, partial η2 = 0.07) in the high ruminators. However, the latter effect was not significant after correction for multiple comparisons. The main effect of time showed significant effects in all DVs: negative affect (F(3,177) = 25.939, p < 0.001, partial η2 = 0.31), state rumination (F(3,177) = 5.633, p < 0.001, partial η2 = 0.09) and decentering (F(3,177) = 8.911, p < 0.001, partial η2 = 0.13). The change in negative affect was characterized by a quadratic (F(1,59) = 29.686, p < 0.001, partial η2 = 0.34) and cubic (F(1,59) = 42.293, p < 0.001, partial η2 = 0.42) change with a relatively steady level until the beginning of the emotion regulation task, an increase in negative affect after this task and a decrease 10 min later (see Fig. 6). For decentering the linear cubic (F(1,59) = 17.157, p < 0.001, partial η2 = 0.22) and cubic (F(1,59) = 6.727, p < 0.05, partial η2 = 0.10) term reached significance. In this case, the effects were driven by a linear increase over the course of the experiment with a first peak after the short intervention, a subsequent decrease after the emotion regulation task and a maximal peak 10 min following the end of the emotion regulation task (see Fig. 6). Finally, with respect to state rumination, post-hoc testing indicated a quadratic (F(1,59) = 6.569, p < 0.05, partial η2 = 0.10) and cubic (F(1,59) = 11.071, p < 0.01, partial η2 = 0.16) change. The effect was driven by an increase in state rumination following the short intervention, a decrease after the emotion regulation task and a steady level 10 min later (see Fig. 6).
Figure 6.
Changes in decentering, negative affect and state rumination over the course of the experiment in the instruction groups. MZP1 = measurement before the short intervention, MZP2 = measurement after the short intervention/before the emotion regulation task, MZP3 = measurement directly after the emotion regulation task, MZP4 = measurement 10 min after the emotion regulation task.
Reanalysis controlling for age, sex and experience with relaxation or meditation
Although we randomized subjects to the instruction groups, there were significant differences in the average age between the treatment conditions. Further, sex was not equally distributed within the sample and prior experience to any kind of relaxation or meditation showed tendencies to an unequal distribution as well. Therefore, we repeated the analysis with these variables as covariates with MANCOVA. In Supplementary Table S5, we summarize the effects of the prior analysis and the controlled analysis. In the reanalysis, all previously reported results were still significant, but the main effect of instruction group on the behavioral ratings during the emotion regulation task showed only marginally significant results. When inspecting the univariate analysis of the effect, the effect of the intervention on avoidance was no longer significant, but the effect on emotional distress showed significance. Therefore, the effect of the instruction group on behavioral ratings during the emotion regulation task has to be interpreted with caution. However, the change in significance level might also be attributed to the limited power of this pilot sample.
Discussion
The present study aimed to investigate an adapted mindfulness short intervention and corresponding effects on behavioral and neural parameters of emotion regulation in low and high habitual ruminators. To this end, 67 habitual high and low ruminators either underwent a mindfulness or instructed thinking short intervention while functional near-infrared spectroscopy (fNIRS) was assessed. Afterwards, all subjects watched either affectively neutral or negative movie clips during an emotion regulation task.
As expected, we observed reduced emotional distress in the MT in comparison to the IT group on a behavioral level. Further, emotional distress within the MT group was lower in the focus when compared to the equanimity condition. Likewise, perceived success was reduced in the equanimity in comparison to the focus condition. No differences in these variables were observed between the conditions of the IT group. On a neural level, contrary to our hypothesis, we observed a marginally significant effect of the instruction group towards reduced activity in the CCN in the MT group. Further, we found a significant condition effect in the whole sample, reflecting higher activity in the equanimity/future condition than the focus/past condition in the instruction groups. As both groups did perform different tasks, we subsequently performed the post-hoc tests for the condition effect separately for both groups, without comparing the instruction groups directly (within-subject comparison vs. between-subject comparison). Post-hoc analysis showed an expected higher activity during the equanimity than the focus condition in the MT group in the left DLPFC, left IFG and SPL. The supplementary analysis of the activation pattern in the two conditions further indicated that this effect was due to no CCN activation during the focus condition but during the equanimity condition. In the IT group differences between conditions were not significant after correction for multiple comparisons, pointing only to tendencies towards higher activation in the future than in the past condition in the bilateral DLPFC and SPL. However, the supplementary analysis of the activation during the two conditions showed that the past as well as the future focus induced activity in the CCN different from zero. In the design of the study and the formulation of the hypotheses, we did not expect that the IT conditions would induce such high CCN activity. The differences between the MT conditions, however, point towards two different neural processes that are characterized by different levels of emotional distress and perceived success in the task completion. One explanation for this might be that the mindful focus on the emotional state provoked effortful control for emotion regulation to maintain a distanced mindful focus on the emotional state. This interpretation is in line with the model of model-based and model-free emotion regulation of Etkin et al.74. In their framework, the authors suggest that—during model-based emotion regulation—plans and internal models are used to guide behavior and that areas of the CCN are involved in this process74. The mindful focus on the emotional state versus the breath might just be more related to follow internal plans, which would be associated with higher model-based control. Interestingly, this interpretation might also be related to findings from meditation stages. For example, Hasenkamp et al.75 postulate that during meditation four stages are involved: mindwandering, awareness of mindwandering, attention shifting and sustained attention. In their study, Hasenkamp and colleagues observed increased activity within areas of the default mode network during mindwandering, increased activity within the salience network during awareness of mindwandering and increased activity in the cognitive control network during attention shifting and sustained attention75. Associations of CCN activation and attentional control/shifting have also been shown in other fNIRS and fMRI studies52,69,76–79. Correspondingly, we propose that our results might reflect a naturally occurring effect that is present during mindfulness meditation when reflecting on emotions in a mindful state. The non-judgmental reflection of these emotional processes may be accompanied by increased activity in areas of the cognitive control network, before attention is reallocated, similar to the observed increased O2Hb levels during the equanimity condition.
Following the short intervention, all subjects underwent an emotion regulation task during which emotional video clips were first cued and then watched by all participants. Interestingly, on a behavioral level we again observed a main effect of the instruction group on behavioral ratings of emotional distress and avoidance, although both effects were significant only without correction for multiple comparisons in the univariate analysis. Therefore, this effect needs to be interpreted with caution, but hints towards a marginally significant improvement when looking at both variables separately. However, emotional distress and emotional avoidance were moderately positively correlated in this study (r = 0.60, p > 0.001) and in light of the significant multivariate test might be interpreted in terms of improved overall affective well-being. This effect is well in line with the work of Arch and Craske57 who found that a short mindfulness induction reduces negative affect and increases willingness to be confronted with negative material in comparison to an unfocussed attention and worry intervention57. In line with this, Creswell et al.58 observed reduced perceived stress and increased cortisol responses during the Trier Social Stress Test in subjects after 3 days of a mindfulness training in comparison to a control training. Additionally, the training was most effective in subjects that showed low pre-existing levels in dispositional mindfulness58. From this point of view, we suggest that the training of a mindful state within the utilized block design is comparable to the effects of mindfulness on mood found in the literature56,58, 59.
On a neural level we observed a main effect of the constant indicating higher CCN activity during negative vs. neutral movie clips and a main effect of phase that was characterized by increased cortical oxygenation already in the anticipation of the negative movie clips. Similar effects have been reported with other emotionally relevant material80,81, which underlines the assumption that the regulation of the emotional process already starts with the anticipation of emotional events. Further, in the multivariate analysis we observed a main effect of instruction group implying increased CCN activity within the MT group in the experimental contrast negative versus neutral videos. However, the univariate analysis showed that this effect was mainly driven by the left DLPFC and was only significant without correction for multiple comparisons. Therefore, this effect has to be interpreted with caution and can only be related to the overall CCN activation. However, the finding of improved affective well-being and increased CCN activity after a mindfulness instruction is in line with the existing literature on the topic. A prior study on the effects of trait mindfulness on CCN activity during emotion regulation implicated a positive association of mindfulness and activity in the CCN during reappraisal82. Similarly, trait mindfulness was positively associated with prefrontal activity during affect labeling in an fMRI study83. Further evidence comes from an fMRI study of Lutz et al. (2014), who used a short mindfulness intervention before an emotion regulation task was assessed. They observed increased prefrontal activation during expectation of negative material in the mindfulness group relative to controls84. In another study, Hölzel et al.54 observed increased prefrontal activity in an affect labeling task following an MBSR intervention in GAD54. Nonetheless, our results must be interpreted with caution, as the univariate analysis for the behavioral and neural effects was only significant without correction for multiple comparisons. However, as this study was designed as a pilot study, we might suspect that increases in the dose of intervention might increase the observed tendencies found in this study. Further, we hypothesize that the overall effect of the blocked instruction paradigm, which differs substantially from traditional meditation settings in terms of timing and interruptions, might have the potential to be effective in a higher dosage.
Importantly, the factor of habitual rumination showed no significant effect, neither in the instruction paradigm nor in the emotion regulation task. In previous investigations using clinical analogue samples, we found encouraging effects in high habitual ruminators, despite the non-clinical groupings27,62. In general, it must be noted that reactivity in negative emotion and state rumination were rather low. As expected, high trait ruminators showed elevated general levels of state rumination and negative emotion. Surprisingly, interactions of instruction group, rumination and time were not significant, indicating that time-related changes in state rumination, negative affect and decentering were not dependent on these factors. The first explanation for this might be that the implemented factors to influence these dependent variables—e.g. the intervention and the instructed thinking task—were not strong enough to have an impact. Interestingly, the significant main effect of time indicates that state rumination increased in all subjects regardless of the instruction group, implying also increases in state rumination in the MT group following the instruction paradigm. It might be the case that the MT instruction had indeed some kind of negative side effects. Other studies have found that mindfulness based interventions may have “side effects” in terms of actualization and realization of emotionally loaded problems during meditation85. This effect may be in accordance with higher emotional distress in the MT group while focusing on one’s emotional state with a mindful focus. Another explanation may be that the instruction was too short for influencing ruminative tendencies.
Furthermore, state rumination did not increase during the emotion regulation task in high ruminators, which emphasizes the use of subjectively relevant emotional situations for the induction of state rumination27,62. Indeed, in subjectively relevant stress paradigms like those used by our group, the reactive negative emotion and state rumination were much higher than in the current study. It may be the case that group differences were not significant in this study, as the absolute effect of the paradigms utilized on subjective emotion and state rumination was rather small. In future studies on the effects of mindful emotion regulation, it might be of advantage to use paradigms such as the Trier Social Stress Test86,87.
Despite these promising findings, some limitations have to be noted. First, this study was conceptualized as a pilot study to investigate the implementation of a block-designed mindfulness instruction and the estimation of potential effect. Although the results are encouraging, the power of the used sample size is limited, resulting in the conflicting results following the correction for multiple comparisons. In future studies the estimated effect sizes of the short intervention in this study might be used to a priori estimate a sufficient sample size for the correction of multiple comparisons. Further, fNIRS only allows measurement of the upper layers of the cortex, because the penetration depth of the NIR light is limited88,89. However, in terms of ecological validity, fNIRS is the best available option for measuring cortical hemodynamic changes90. Further, it is important to bear in mind that the current study used a non-clinical population and future studies are needed to support the usage of the implemented mindfulness training in clinical samples. However, because one aim of the current study was to investigate the effects of the paradigm, our sample used also had some advantages. For example, high ruminators were medication-free, meaning our study was free from confounding of the observed effects with medication status of the high ruminating group. Also, although the sample was non-clinical, high ruminators showed significantly elevated levels of subclinical depression scores, indicating that this subgroup is potentially at-risk90–93. Another limitation concerns the instruction during the emotion regulation paradigm. During the emotion regulation paradigm, we did not give direct instructions how to regulate in the IT group, as we wanted to contrast a naturally occurring spontaneous emotion regulation in the IT group versus mindful emotion regulation in the MT group. However, without a direct instruction how to regulate, the IT group might have used a variety of different emotion regulation strategies. On the other hand, control instructions—such as not to regulate—themselves might induce neural activity, which is why we decided to design the paradigm in the current way. In future investigations it might also be interesting to investigate the spontaneous emotion regulation strategies in the non-intervention group in detail. Lastly, although most probably due to the limited sample size of this pilot study, the investigated subgroups of this study showed significant differences with respect to their age. However, controlling for the age of participants did not affect the results of our analysis. This is most probably due to the relatively low differences between groups in age, as all subject groups were on average between 22 and 29 years old. Most age-related changes in brain functioning occur in certain developmental windows. Following adolescence, brain functioning may be comparable across different age groups (e.g. 22 and 29 year old subjects) within adults until age-related decline starts. A last limitation concerns the study design. The development of control conditions for laboratory mindfulness inductions is rather complicated. We did not choose to use a non-intervention group—e.g. just resting for 15 min—as subjects in such a group could be mindful while performing the control condition. Instead, we used a non-mindful instructed thinking instruction that might have contributed to the differences between the groups. In future studies, it may be preferable to investigate a third non-intervention group. However, such study designs are cost and time expensive. In future studies it should be assessed if subjects practice Yoga more explicitly, as such a practice could improve mindfulness skills. Furthermore, in future studies additional physiological measures (e.g. heart rate) might be assessed, and larger sample sizes should be collected to allow the analysis of brain-behavior-correlations.
In conclusion, we showed that an fNIRS mindfulness block design showed promising results on brain activation and behavioral indices of emotion regulation. Moreover, different mindfulness states were accompanied by different involvement of areas of the cognitive control network demonstrated in a within-subject comparison. The results of this study show that the intervention paradigm might be useful in future studies to investigate the neural correlates of mindfulness-based interventions in situ using fNIRS. In future studies, the current training paradigm should be further investigated in clinical populations and longitudinal investigations to track changes in CCN activity through longer training periods.
Supplementary information
Acknowledgements
The authors would like to thank Ramona Taeglich, Betti Schopp, and Isabell Int-Veen for their excellent work and their valuable support with the measurements. We acknowledge support by Open Access Publishing Fund of University of Tübingen.
Authors contributions
D. R., A.K., and A.-C.E. contributed in the analysis and interpretation of the data for the work and did the primary drafting. J.H., J.R., M.M., T.S., L.W., L.G., D.M., D.F. and A.J.F. contributed in the design and acquisition of the work and revised it critically for important intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Funding
Ann-Christine Ehlis was partly supported by IZKF Tübingen (Junior Research Group 2115-0-0). David Rosenbaum and Florian Metzger were partly supported by STORZ MEDICAL AG.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71952-5.
References
- 1.The global burden of disease: 2004 update. (World Health Organization, 2008).
- 2.Ormel J, Kessler RC, Schoevers R. Depression: more treatment but no drop in prevalence. Curr. Opin. Psychiatry. 2019;1:1. doi: 10.1097/YCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 3.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J. Consult. Clin. Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinert C, Hofmann M, Kruse J, Leichsenring F. Relapse rates after psychotherapy for depression—stable long-term effects? A meta-analysis. J. Affect. Disord. 2014;168:107–118. doi: 10.1016/j.jad.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Evans MD, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch. Gen. Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- 6.Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006;11:12–21. doi: 10.1017/S1092852900015212. [DOI] [PubMed] [Google Scholar]
- 7.Segal ZV, et al. Antidepressant monotherapy vs. sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch. Gen. Psychiatry. 2010;67:1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JM, Alloy LB. A roadmap to rumination: a review of the definition, assessment, and conceptualization of this multifaceted construct. Clin. Psychol. Rev. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalak J, Hölz A, Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression: rumination as a predictor of relapse. Psychol. Psychother. Theory Res. Pract. 2011;84:230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- 10.Teismann T. Kognitive Verhaltenstherapie Depressiven Grübelns. Berlin: Springer; 2012. [Google Scholar]
- 11.Eshun S. Role of gender and rumination in suicide ideation: a comparison of college samples from Ghana and the United States. Cross Cult. Res. 2000;34:250–263. doi: 10.1177/106939710003400303. [DOI] [Google Scholar]
- 12.Ito T, Takenaka K, Tomita T, Agari I. Comparison of ruminative responses with negative rumination as a vulnerability factor for depression. Psychol. Rep. 2006;99:763–772. doi: 10.2466/PR0.99.7.763-772. [DOI] [PubMed] [Google Scholar]
- 13.Koval P, Kuppens P, Allen NB, Sheeber L. Getting stuck in depression: the roles of rumination and emotional inertia. Cogn. Emot. 2012;26:1412–1427. doi: 10.1080/02699931.2012.667392. [DOI] [PubMed] [Google Scholar]
- 14.Depressive rumination: nature, theory, and treatment. (Wiley, Berlin, 2004).
- 15.Spasojevic J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Lyubomirsky S, Kasri F, Zehm K. Dysphoric rumination impairs concentration on academic tasks. Cogn. Ther. Res. 2003;27:309–330. doi: 10.1023/A:1023918517378. [DOI] [Google Scholar]
- 17.Hertel PT, Benbow AA, Geraerts E. Brooding deficits in memory: focusing attention improves subsequent recall. Cogn. Emot. 2012;26:1516–1525. doi: 10.1080/02699931.2012.668852. [DOI] [PubMed] [Google Scholar]
- 18.Slavish DC, Graham-Engeland JE. Rumination mediates the relationships between depressed mood and both sleep quality and self-reported health in young adults. J. Behav. Med. 2015;38:204–213. doi: 10.1007/s10865-014-9595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 20.Ottaviani C, et al. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- 21.Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkhouse KL, et al. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cogn. Affect. Behav. Neurosci. 2016;10:470–478. doi: 10.3758/s13415-016-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones NP, Fournier JC, Stone LB. Neural correlates of autobiographical problem-solving deficits associated with rumination in depression. J. Affect. Disord. 2017;218:210–216. doi: 10.1016/j.jad.2017.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longe O, et al. Having a word with yourself: Neural correlates of self-criticism and self-reassurance. NeuroImage. 2010;49:1849–1856. doi: 10.1016/j.neuroimage.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum D, et al. Aberrant functional connectivity in depression as an index of state and trait rumination. Sci. Rep. 2017;7:2174. doi: 10.1038/s41598-017-02277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum D, et al. Stress-related dysfunction of the right inferior frontal cortex in high ruminators: an fNIRS study. NeuroImage Clin. 2018;18:510–517. doi: 10.1016/j.nicl.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lydon-Staley DM, et al. Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Transl. Psychiatry. 2019;9:234. doi: 10.1038/s41398-019-0560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldbeck F, et al. The positive brain—resting state functional connectivity in highly vital and flourishing individuals. Front. Hum. Neurosci. 2019;12:540. doi: 10.3389/fnhum.2018.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum D, et al. State-dependent altered connectivity in late-life depression: a functional near-infrared spectroscopy study. Neurobiol. Aging. 2016;39:57–68. doi: 10.1016/j.neurobiolaging.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Buckner RL. The serendipitous discovery of the brain’s default network. NeuroImage. 2012;62:1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 34.Ray RD, et al. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 2005;5:156–168. doi: 10.3758/CABN.5.2.156. [DOI] [PubMed] [Google Scholar]
- 35.Vanderhasselt M-A, et al. How brooding minds inhibit negative material: an event-related fMRI study. Brain Cogn. 2013;81:352–359. doi: 10.1016/j.bandc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Mennin DS, Fresco DM. What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clin. Psychol. Publ. Div. Clin. Psychol. Am. Psychol. Assoc. 2013;20:258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuyken W, et al. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry. 2016;73:565–574. doi: 10.1001/jamapsychiatry.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins E, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br. J. Psychiatry. 2011;199:317–322. doi: 10.1192/bjp.bp.110.090282. [DOI] [PubMed] [Google Scholar]
- 39.van der Velden AM, et al. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin. Psychol. Rev. 2015;37:26–39. doi: 10.1016/j.cpr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Fresco DM, Mennin DS, Heimberg RG, Ritter M. Emotion regulation therapy for generalized anxiety disorder. Cogn. Behav. Pract. 2013;20:282–300. doi: 10.1016/j.cbpra.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mennin DS. Emotion regulation therapy: an integrative approach to treatment-resistant anxiety disorders. J. Contemp. Psychother. 2006;36:95–105. doi: 10.1007/s10879-006-9012-2. [DOI] [Google Scholar]
- 42.Jacobs RH, et al. Targeting ruminative thinking in adolescents at risk for depressive relapse: rumination-focused cognitive behavior therapy in a pilot randomized controlled trial with resting state fMRI. PLoS ONE. 2016;11:e0163952. doi: 10.1371/journal.pone.0163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mennin DS, Fresco DM, Ritter M, Heimberg RG. An open trial of emotion regulation therapy for generalized anxiety disorder and cooccurring depression. Depress. Anxiety. 2015;32:614–623. doi: 10.1002/da.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mennin DS, Fresco DM, O’Toole MS, Heimberg RG. A randomized controlled trial of emotion regulation therapy for generalized anxiety disorder with and without co-occurring depression. J. Consult. Clin. Psychol. 2018;86:268–281. doi: 10.1037/ccp0000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renna ME, et al. A pilot study of emotion regulation therapy for generalized anxiety and depression: findings from a diverse sample of young adults. Behav. Ther. 2018;49:403–418. doi: 10.1016/j.beth.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Toole MS, Renna ME, Mennin DS, Fresco DM. Changes in decentering and reappraisal temporally precede symptom reduction during emotion regulation therapy for generalized anxiety disorder with and without co-occurring depression. Behav. Ther. 2019;50:1042–1052. doi: 10.1016/j.beth.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 2007;33:11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 48.Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? Explore. 2009;5:265–271. doi: 10.1016/j.explore.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol. 2010;78:169. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin. Psychol. Sci. Pract. 2003;10:125–143. doi: 10.1093/clipsy.bpg015. [DOI] [Google Scholar]
- 51.Tang Y-Y, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn. Sci. 2012;16:330–337. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gundel F, et al. Meditation and the brain—neuronal correlates of mindfulness as assessed with near-infrared spectroscopy. Psychiatry Res. Neuroimaging. 2018;271:24–33. doi: 10.1016/j.pscychresns.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Sperduti M, Martinelli P, Piolino P. A neurocognitive model of meditation based on activation likelihood estimation (ALE) meta-analysis. Conscious. Cogn. 2012;21:269–276. doi: 10.1016/j.concog.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Hölzel BK, et al. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage Clin. 2013;2:448–458. doi: 10.1016/j.nicl.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doll A, et al. Mindful attention to breath regulates emotions via increased amygdala–prefrontal cortex connectivity. NeuroImage. 2016;134:305–313. doi: 10.1016/j.neuroimage.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 56.Broderick PC. Mindfulness and coping with dysphoric mood: contrasts with rumination and distraction. Cogn. Ther. Res. 2005;29:501–510. doi: 10.1007/s10608-005-3888-0. [DOI] [Google Scholar]
- 57.Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behav. Res. Ther. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Tang Y-Y, et al. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. 2007;104:17152. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J. Pers. Soc. Psychol. 1999;77:1061–1072. doi: 10.1037/0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- 61.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 1991;100:569–582. doi: 10.1037/0021-843X.100.4.569. [DOI] [PubMed] [Google Scholar]
- 62.Rosenbaum D, et al. Disrupted prefrontal functional connectivity during post-stress adaption in high ruminators. Sci. Rep. 2018;8:15588. doi: 10.1038/s41598-018-33777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of beck depression inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 64.Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self-report: the kentucky inventory of mindfulness skills. Assessment. 2004;11:191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- 65.Fresco DM, et al. Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering. Behav. Ther. 2007;38:234–246. doi: 10.1016/j.beth.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Gecht J, et al. Measuring decentering in self-reports: psychometric properties of the experiences questionnaire in a German sample. Psychother. Res. 2014;24:67–79. doi: 10.1080/10503307.2013.821635. [DOI] [PubMed] [Google Scholar]
- 67.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 68.Desbordes G, et al. Moving beyond mindfulness: defining equanimity as an outcome measure in meditation and contemplative research. Mindfulness. 2015;6:356–372. doi: 10.1007/s12671-013-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenbaum D, et al. Neurophysiological correlates of the attention training technique: a component study. NeuroImage Clin. 2018;19:1018–1024. doi: 10.1016/j.nicl.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fishburn FA, Ludlum RS, Vaidya CJ, Medvedev AV. Temporal derivative distribution repair (TDDR): a motion correction method for fNIRS. NeuroImage. 2019;184:171–179. doi: 10.1016/j.neuroimage.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brigadoi S, et al. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage. 2010;49:3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Noah JA, Hirsch J. Separation of the global and local components in functional near-infrared spectroscopy signals using principal component spatial filtering. Neurophotonics. 2016;3:015004. doi: 10.1117/1.NPh.3.1.015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 75.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59:750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Rosenbaum D, et al. Comparison of speed versus complexity effects on the hemodynamic response of the trail making test in block designs. Neurophotonics. 2018;5:045007. doi: 10.1117/1.NPh.5.4.045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cogn. Ther. Res. 2007;31:235–262. doi: 10.1007/s10608-006-9118-6. [DOI] [Google Scholar]
- 78.Maier MJ, et al. Forgiveness and cognitive control—provoking revenge via theta-burst-stimulation of the DLPFC. NeuroImage. 2018;183:769–775. doi: 10.1016/j.neuroimage.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 79.Maier MJ, et al. Disinhibited revenge—an fNIRS study on forgiveness and cognitive control. Front. Behav. Neurosci. 2019;13:223. doi: 10.3389/fnbeh.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenbaum D, et al. Cortical oxygenation during exposure therapy—in situ fNIRS measurements in arachnophobia. NeuroImage Clin. 2020;26:102219. doi: 10.1016/j.nicl.2020.102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenbaum, D., Leehr, E. J., Kroczek, A., Rubel, J. A., Int-Veen, I., Deutsch, K., Maier, M. J., Hudak, J., Fallgatter, A. J. & Ehlis, A-C. Neuronal correlates of spider phobia in a combined fNIRS-EEG study. Scientific Reports10(1) (2020). [DOI] [PMC free article] [PubMed]
- 82.Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc. Cogn. Affect. Neurosci. 2010;5:369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom. Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 84.Lutz J, et al. Mindfulness and emotion regulation—an fMRI study. Soc. Cogn. Affect. Neurosci. 2013;9:776–785. doi: 10.1093/scan/nst043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shapiro DH., Jr Adverse effects of meditation: a preliminary investigation of long-Tenn meditators. Int. J. Psychosom. 1992;39:63. [PubMed] [Google Scholar]
- 86.Rosenbaum D, et al. Cortical hemodynamic changes during the trier social stress test: an fNIRS study. NeuroImage. 2018;171:107–115. doi: 10.1016/j.neuroimage.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 87.De Witte S, et al. The effect of neurostimulation applied to the left dorsolateral prefrontal cortex on post-stress adaptation as a function of depressive brooding. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;96:109687. doi: 10.1016/j.pnpbp.2019.109687. [DOI] [PubMed] [Google Scholar]
- 88.Haeussinger FB, et al. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS ONE. 2011;6:e26377. doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haeussinger F, et al. Reconstructing functional near-infrared spectroscopy (fNIRS) signals impaired by extra-cranial confounds: an easy-to-use filter method. NeuroImage. 2014;95:69–79. doi: 10.1016/j.neuroimage.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 90.Ehlis A-C, Schneider S, Dresler T, Fallgatter AJ. Application of functional near-infrared spectroscopy in psychiatry. NeuroImage. 2014;85:478–488. doi: 10.1016/j.neuroimage.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 91.Horwath E, Johnson J, Klerman GL, Weissman MM. What are the public health implications of subclinical depressive symptoms? Psychiatr. Q. 1994;65:323–337. doi: 10.1007/BF02354307. [DOI] [PubMed] [Google Scholar]
- 92.Fava GA. Subclinical symptoms in mood disorders: pathophysiological and therapeutic implications. Psychol. Med. 1999;29:47–61. doi: 10.1017/S0033291798007429. [DOI] [PubMed] [Google Scholar]
- 93.Warner V, Weissman M, Fendrich M, Wickramaratne P, Moreau D. The course of major depression in the offspring of depressed parents: incidence, recurrence, and recovery. Arch. Gen. Psychiatry. 1992;49:795–801. doi: 10.1001/archpsyc.1992.01820100039008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.