Transplant biology and the immunological concepts underlying allograft rejection have progressed tremendously since the first successful kidney transplant was performed between identical twins in 19541. Current immunosuppressive regimens have dramatically improved immune tolerance and short-term graft acceptance2. However, the development of transplant arteriosclerosis (TA), a condition that narrows the graft vasculature and restricts blood flow, compromises long-term organ survival3. Although largely elusive, multifactorial loss of endothelial integrity, disruption of intimal homeostasis, smooth muscle cell (SMC) recruitment, matrix synthesis3 are considered important contributing factors. Immune responses have also been implicated in TA, with some similarities to native atherosclerosis3, 4. For example, Ly6Chi monocyte infiltration is aggravated by hyperlipidemia in both conditions5, 6 and statins reduce TA severity by inhibiting monocyte and T cell vascular recruitment7. Since hyperlipidemia is highly prevalent in transplanted patients, statins are routinely prescribed to delay TA and improve patient survival3.
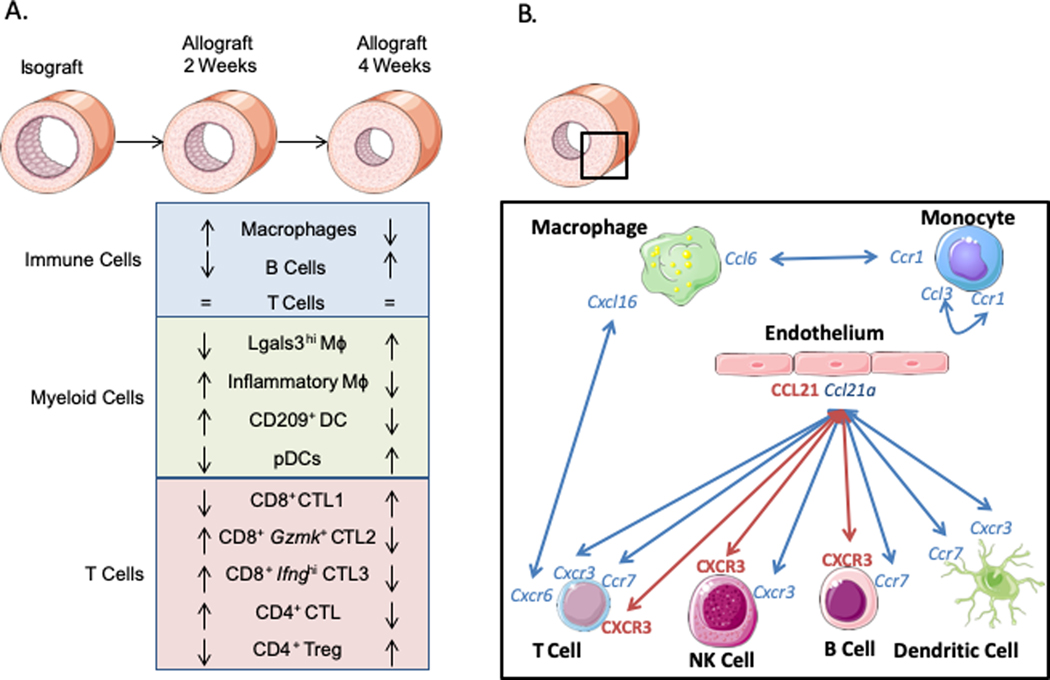
In this issue of Circulation Research, Cai et al.8 used single-cell RNA sequencing (scRNAseq) to provide a comprehensive cellular mapping of TA. In the allograft model used in this study, aortic segments from BALB/c mice were transplanted into the carotid of C57BL/6j mice and isografts served as controls. Mice achieved intimal hyperplasia at 2 weeks that worsened at 4 weeks. The cellular composition of the allografts was largely dominated by all major immune cell populations (i.e. monocytes, macrophages, dendritic cells (DC), T cells and B cells) which changed over time (Fig.1A). Macrophages decreased and B cells increased at 4 weeks, whereas T cells were equally represented at both time points. Overall, these data confirmed the key role of adaptive immunity at all stages of TA development and highlighted the critical contribution of innate immune responses at early stages.
Figure 1.
Immune cell contributions in allografts. A. Schematic of the compositional changes in major immune cell populations over time. B. Schematic of the main predicted (blue) and validated (red) interactions promoting chemotaxis.
The analysis of the myeloid compartment underscored specific macrophage alterations with three distinct clusters with inflammatory (Inf), interferon-related (Isg) and foam cell-like (Lgals3hi) functions. Surprisingly, the proinflammatory cytokine IL-1ß, was more robustly expressed by macrophages in the control isografts rather than in the allografts, suggesting that, unlike in atherosclerosis, IL-1ß signaling may not be critically involved in pathogenesis of TA. However, the possible contribution of post-transplant dyslipidemia3 on inflammasome-mediated IL-1ß activation9 was not measurable in the mouse model used in this study. The analysis of DCs revealed an enrichment of pDCs and monocyte-derived DCs in the allografts. Migratory-DCs and resident-like macrophages were predicted to act as antigen-presenting cells to CD8+ T cells, based on their high expression of MHCI, CD1, and co-stimulatory molecules. CD4+ T cells were also predicted to be antigen-presenting recipients of macrophages and DCs.
In the adaptive compartment, cytotoxic lymphocytes (CTL) accounted for the largest population in allografts. CTL profiles phenotypically shifted over time, with CD8+ (Gzmk+ and Ifnghi) and CD4+ CTLs prevalent at 2 weeks, and CD8+ CTL1 enriched at 4 weeks. While all CTLs expressed Ifng, the specific function of individual subsets remains to be determined. B cell immunity was also impacted over time. Transcriptional analysis of B cells suggested that early stage B cells have antigen-presentation functions, while humoral responses were evident at all stages of TA development. Of note, dividing B cells were predicted to differentiate into plasma B cells inside the allografts within tertiary lymphoid structures. These ectopic structures were detected in the adventitia at 4 weeks, presented a germinal center and correlated with the severity of intima media hyperplasia. Tertiary Lymphoid Organs (TLO) have recurrently been identified in chronically rejected allografts, are believed to escape tolerance regulation and contribute to cellular and humoral chronic rejection10. The identification of the allograft B cell diversity and differentiation trajectory suggests a strong local regulation of complex immune responses within TLO and an important involvement in TA development.
Fewer non-immune cells were detected in this study. The authors identified mesenchymal stromal cells, involved in tissue repair and regeneration, and endothelial cells (ECs) that were restored at 4 weeks following an initial loss of endothelial integrity at an early stage of TA development. They specifically identified an EC cluster that expressed a set of genes consistent with angiogenesis and vasculogenesis, as well as a cluster of lymphatic ECs. A major limitation of the study was that no vascular SMCs, a cell type that infiltrates the intima during TA development, were identified. Several possibilities could account for the lack of SMC detection, including a suboptimal digestion protocol or the loss of medial SMC as described in some vascular allograft models3.
To identify new cellular determinants of TA, cell-cell communications were inferred based on the expression of known ligand-receptor pairs (Fig1B.). Several putative chemotactic mechanisms were identified including monocyte self-recruitment (Ccl3-Ccr1), monocytes (Ccl6-Ccr1) and T cells (Cxcl16-Cxcr6) recruited by macrophages, and other immune cells (Ccl21a-Cxcr3, Ccl21a-Ccr7) recruited by ECs. The authors next prioritized interactions that involved chemokines (e.g. CCL21) with high systemic protein expression in the allograft recipient mice. This approach pinpointed a key role of lymphatic ECs expressing Ccl21a in recruiting Cxcr3 (DCs, NK cells, T cells) and Ccr7 (migratory-DCs, T cells and B cells) expressing cells. A causal role for CCL21-CXCR3 signaling in TA progression was confirmed experimentally by blocking either CCL21 or CXCR3 in vivo. These new data are consistent with previous observations implicating CCL21 signaling in mice11 and positive associations between CXCR3 systemic levels and TA progression in patients12.
Interestingly, lineage tracing experiments combined with scRNAseq analysis of the allografts identified c-kit+ immune cells that were primarily (>60%) composed of inflammatory and tissue-resident macrophages. C-kit+ tissue-resident macrophages were Folr2+, which expressed genes involved in endocytosis and chemotaxis, or RELMα+, which expressed genes involved in angiogenesis and lipid metabolic processes. The analysis of c-kit+ macrophages also revealed a cluster of foamy-like macrophages and TREM2hi macrophages which were described by others in atherosclerosis13, 14. Further, the authors identified a dividing macrophage cluster that resembled a stem-cell like macrophage cluster also identified in experimental atherosclerosis13. Overall, these data suggest that recipient c-kit+ stem cells may contribute to immune infiltration of the allograft and TA progression.
The authors also performed an integrated scRNAseq analysis of the allografts data with two published atherosclerosis datasets14, 15 and found a remarkable overlap of major immune cell frequencies. These similarities persisted despite the datasets being generated from mice with different genetic backgrounds and diet compositions. In contrast, TA and atherosclerosis diverged upon a more granular analysis, with the exception of B cells. Interestingly, B cells were detectable in APOE−/− mice, but not in the LDLR−/− model, with similar proportions to those found in the germinal center of the TLOs in TA. Overall, these data highlight similarities and differences in the immune response between TA and atherosclerosis, and revealed that B cells may have distinct roles in different mouse models of atherosclerosis. However, the translational potential of the immune alterations seen in this study needs to be confirmed to account for the influence that post-transplant dyslipidemia may have on specific immune responses.
Cai et al’s findings highlight that scRNAseq is a powerful approach to understand the cellular complexity of heterogenous populations at the tissue site. Intriguingly, the authors provide a first single-cell immune mapping of experimental TA that revealed distinct immune composition, origin, and specific functional states of infiltrating immune cells at different stages of TA development. A ligand-receptor interaction analysis predicted several chemokine and chemokine receptor involved in immune cell recruitment at the graft site including Ccl2/Cxcr3 signaling. As a proof-of-concept, they experimentally validated that blocking of either CCL21 or CXCR3 reduced TA progression in vivo, a finding that strongly supports the robustness of scRNAseq computational methods to identify key cell-specific mechanisms involved in complex human disease. The translational impact of this study may be intrinsically limited by the fact that the model used does not fully recapitulate the complexity of human TA. However, the findings of this study provide new conceptual advances in TA biology that could guide future studies and the future development of new preventive therapies for transplant patients.
Acknowledgments
Sources of funding
The authors acknowledge support by NIH grants R01HL153712 (CG) and T32HL007824 (DF), CZI INFL-0000000187 (CG), and AHA 20SFRN35210252 (CG).
Footnotes
Disclosures
None.
References
- 1.Merrill JP, Murray JE, Harrison JH and Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc. 1956;160:277–82. [DOI] [PubMed] [Google Scholar]
- 2.Enderby C and Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care. 2015;21:s12–23. [PubMed] [Google Scholar]
- 3.Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. [DOI] [PubMed] [Google Scholar]
- 4.Rahmani M, Cruz RP, Granville DJ and McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–15. [DOI] [PubMed] [Google Scholar]
- 5.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, et al. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiopu A, Nadig SN, Cotoi OS, Hester J, van Rooijen N, Wood KJ. Inflammatory Ly-6C(hi) monocytes play an important role in the development of severe transplant arteriosclerosis in hyperlipidemic recipients. Atherosclerosis. 2012;223:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin R, Zhu J, Shao H, Cheng X, Feng X, Li Z, Jing H. Inhibition of chemokine receptor CCR2 and CCR5 expression contributes to simvastatin-induced attenuation of cardiac allograft vasculopathy. J Heart Lung Transplant. 2007;26:485–93. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Deng J, Gu W, Ni Z, Liu Y, Kamra Y, Saxena A, Hu Y, Yuan H, Xiao Q,et al. Impact of Local Allo-Immunity and Recipient Cells in Transplant Arteriosclerosis. Circ Res. 2020; 127: xxx-xxx. [DOI] [PubMed] [Google Scholar]
- 9.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nykanen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, Wu Y, Pytowski B, Koskinen PK, Yla-Herttuala S, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–22. [DOI] [PubMed] [Google Scholar]
- 12.Kao J, Kobashigawa J, Fishbein MC, MacLellan WR, Burdick MD, Belperio JA, Strieter RM. Elevated serum levels of the CXCR3 chemokine ITAC are associated with the development of transplant coronary artery disease. Circulation. 2003;107:1958–61. [DOI] [PubMed] [Google Scholar]
- 13.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 15.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]