Abstract
Makorin RING finger protein 3 (MKRN3) is a key inhibitor of the hypothalamic–pituitary–gonadal axis. Loss-of-function mutations in MKRN3 cause familial and sporadic central precocious puberty (CPP), while polymorphisms are associated with age at menarche. To date, 115 patients with CPP carrying MKRN3 mutations have been described, harboring 48 different genetic variants. The prevalence of MKRN3 mutations in genetically screened populations with CPP is estimated at 9.0%. Girls are more commonly and more seriously affected than boys. MKRN3 is expressed in humans and rodents in the central nervous system. Circulating levels in humans and hypothalamic expression in rodents decrease during pubertal progression. Although some MKRN3 regulators have been identified, the precise mechanism by which MKRN3 inhibits the hypothalamic–pituitary–gonadal axis remains elusive. The role of makorins in developmental physiology and organ differentiation and the role of maternal imprinting are discussed herein.
Keywords: Puberty, Makorin, Estradiol, GnRH, Kisspeptin, Development, Imprinting
Central precocious puberty
Puberty is a complex developmental process allowing organisms to acquire full sexual maturation and reproductive capacity. It is the result of a coordinated sequence of events controlled by genetic, neurochemical, metabolic, and environmental cues. Neural elements are activated within an intricate hypothalamic network, which culminate in GnRH neuron stimulation and hypothalamic–pituitary–gonadal (HPG) axis activation [1,2]. The HPG axis is active during various stages of human life: there is strong evidence for HPG axis activity during the final months of fetal development, with sustained and detectable activation for several months after birth (commonly referred to as minipuberty). Subsequently, the HPG axis remains physiologically quiescent during childhood.
Central precocious puberty (CPP) is the result of early reactivation of the HPG axis, revealed by the occurrence of the first signs of puberty more than 2–2.5 standard deviations earlier than the general population with similar genetic backgrounds. In Western countries, this definition roughly corresponds to the onset of pubertal changes before the age of 8 years in girls and 9 years in boys, although thresholds are likely changing in recent decades. Precocious activation of the HPG axis leads to sex steroid production and secretion by the gonads, with a remarkable impact on adult height, body proportions, and psychological health, as well as associations with adverse health outcomes in later life [3].
Table 1 summarizes the major causes of CPP and their relative frequency. Diencephalic lesions and environmental factors, historically evoked as the most common etiologies of CPP, have more recently given way to a wider landscape of etiologies, most notably the recent identification of causative genetic mutations in the imprinted MKRN3 and delta-like noncanonical Notch ligand 1 (DLK1) genes in familial and sporadic forms of CPP [4,5].
Table 1.
Etiologies of central precocious puberty and relative frequency of causes.
| Etiology | Frequency |
|---|---|
| Genetic | |
| MKRN3 loss-of-function mutations (MIM #603856) | >>> |
| DLK1 loss-of-function mutations (MIM #176290) | > |
| KISS1 gain-of-function mutations | > |
| KISS1R (former GPR54) gain-of-function mutations (MIM #604161) | > |
| Syndromic | |
| Type 1 neurofibromatosis (MIM #162200) | > |
| Tuberous sclerosis (Bourneville disease, MIM #191100) | > |
| Temple syndrome (MIM #616222) | > |
| Silver-Russell syndrome (MIM #180860) | > |
| Prader–Willi syndrome (MIM #176270) | > |
| Williams-Beuren syndrome (MIM #194050) | > |
| Diencephalic tumors | |
| Hamartoma (hypothalamus) | > |
| Gliomas/oligodendrogliomas | > |
| Hydrocephaly | > |
| Other intracranial hypothalamic tumors (rare) | > |
| Environmental factors | |
| Adoption/early life social stressors | >> |
| Nutritional excess | > |
| Traumatic brain injury | > |
| Cranial irradiation | > |
| Prepubertal exposure to sex steroids | > |
| Prepubertal exposure to endocrine disruptors | > |
| Idiopathic | >>> |
Frequency of etiology: >>> (>5%), >> (2–5%), and > (<2%).
Makorin RING finger protein family function and molecular properties
Makorin RING finger proteins contain a characteristic array of zinc finger motifs conserved in many eukaryotes. They typically contain several C3H zinc fingers, which suggest RNA binding properties, a Cys- and His-rich makorin motif, and a specific RING pairwise zinc finger domain [6] (Figure 1). The presence of a RING finger domain is characteristic of RING-class E3 ubiquitin protein ligases, which transfer ubiquitin from an E2 enzyme to a substrate protein, supporting the hypothesis that makorin proteins act in cell processes modulated by protein ubiquitination, such as protein degradation (by addition of polyubiquitin chains), protein–protein interactions, and other changes in protein stability (monoubiquitination or oligoubiquitination). A recent study showed that makorin RING finger protein 1 (MKRN1) participates in the ribosome-associated quality control complex, mediating the recognition of messenger RNA with prematurely polyadenylated tails to prevent the production of aberrant proteins, thereby maintaining proteome integrity [7]. Nevertheless, despite extensive predictions through the study of functional domains and some evidence from experimental studies, the exact function and the networking ecosystem of the makorins remain largely elusive.
Figure 1. Schematic depiction of the makorin RING finger protein 3 (MKRN3) structure and location of mutations reported in patients with central precocious puberty.
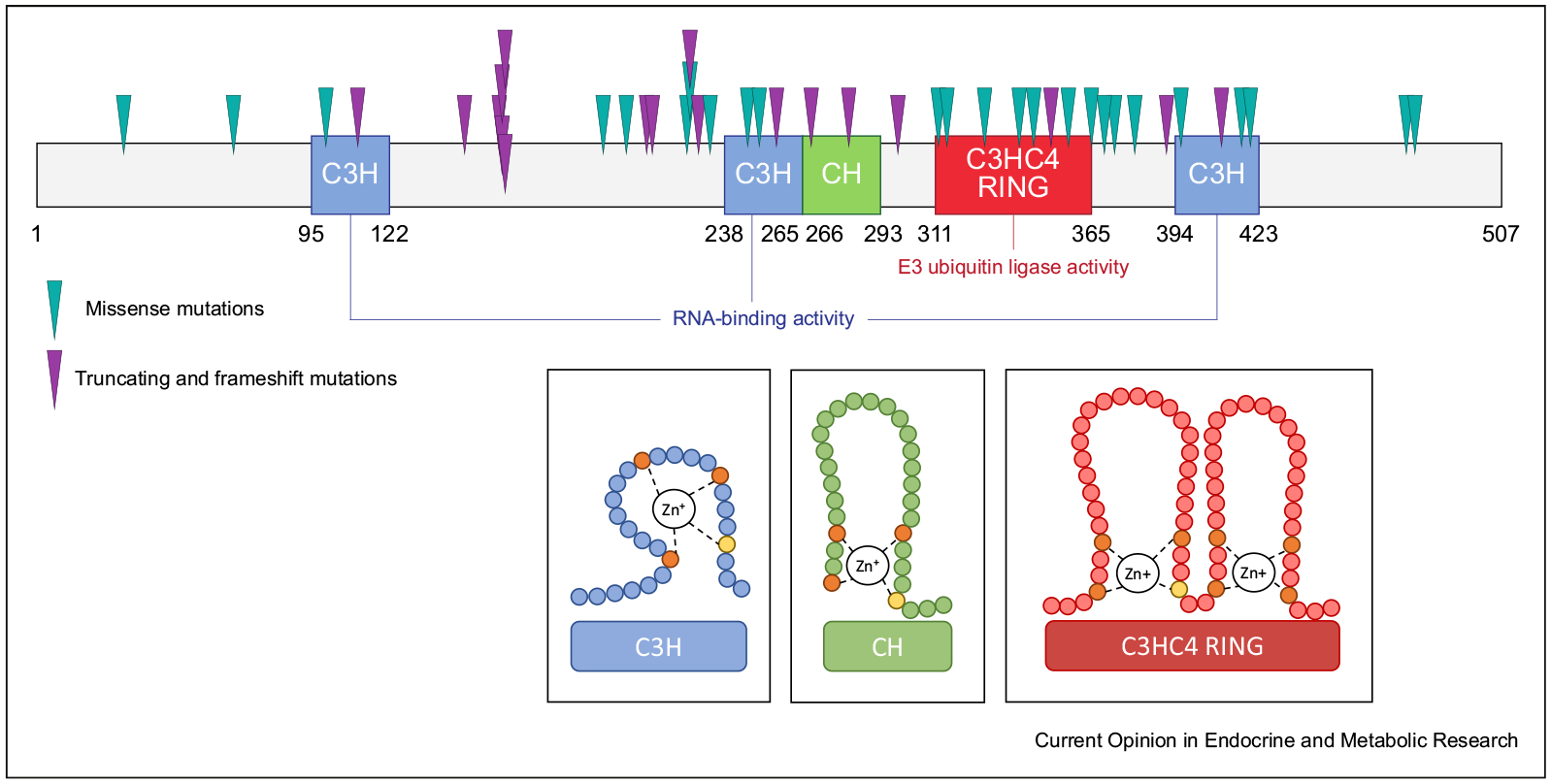
Protein structure: numbers indicate amino acids from 1 to 507. The canonical zinc fingers (C3H) typical of RNA binding activity are depicted in blue, the makorin-type Cys–His (CH)–rich hinge region is depicted in green, and the RING finger domain (C3HC4) characteristic of E3 ubiquitin ligase activity is depicted in red. The locations of mutations in MKRN3 identified in patients with central precocious puberty that result in amino acid substitutions are indicated by green arrows (missense variants) and those resulting in protein truncation or frameshift mutations are indicated by purple arrows (nonsense variants). The insets show two-dimensional details of the different MKRN3 domains.
MKRN1 is ubiquitously expressed in humans but appears to be more restricted to the gonads and central nervous system (CNS) in mice. Mkrn1-null mice are viable and fertile but are leaner and resistant to obesity under high-fat diet [8]. Makorin RING finger protein 2 (MKRN2) expression is prevalent in the gonads. In both human males and male rodents, MKRN2 alterations are associated with infertility and sperm abnormalities. A detailed analysis of testicular tissues revealed that, among the different stages of spermatogenesis, spermiation is the most disrupted stage in Mkrn2-null mice [9,10]. Makorin RING finger protein 3 (MKRN3) has been widely implicated in pubertal onset (see the following section). Makorin RING finger protein 4 exists in the human genome, but it has been annotated as a pseudogene, and its function is currently undetermined.
In addition to mammals, makorins are present in a broad range of species, including invertebrates and plants, suggesting a highly conserved role during evolution. In Drosophila melanogaster, the makorin ortholog Mkrn1 regulates the timing of larval developmental and body size [11]. Embryonic patterning and gonadal commitment in Drosophila are tightly regulated by the interaction of the Mkrn1 ortholog with poly(A)-binding proteins [12]. In Caenorhabditiselegans, the makorin ortholog Lep-2 regulates neuronal and sexual maturation by promoting Lin28 degradation [13]. In rice and in peas, the makorin ortholog is expressed in a temporal and spatial manner, and its levels rise during imbibition and progressively decline after germination and after maximal somatic elongation [14].
Taken together, data regarding the different makorins suggest that a highly regulated temporal expression is a common characteristic in this class of proteins. A progressive decline in makorin expression occurs after maximal cell body elongation, organ differentiation, or neural development. All of these findings support a role for the makorin family in development.
Makorin RING finger protein 3
In humans, MKRN3 is an intronless gene located in the Prader–Willi syndrome region (chromosomal location 15q11.2-q13). This genomic region is maternally imprinted and is expressed solely from the paternal allele. As with other makorins, Mkrn3 transcript levels in several species are maximal during early developmental stages and progressively decrease over time [4,15]. Although ubiquitous in the CNS in rodents in early postnatal life, Mkrn3 transcripts begin to be restricted more discretely in the hypothalamus in key areas important for the control of puberty, such as the arcuate nucleus and the anteroventral periventricular nucleus [16]. Moreover, MKRN3 was recently shown to selectively repress the gene promoter activity of key GnRH secretagogues, such as KISS1 and TAC3 (Figure 2) [17].
Figure 2. Schematic depiction of hypothalamic regions involved in MKRN3 action.
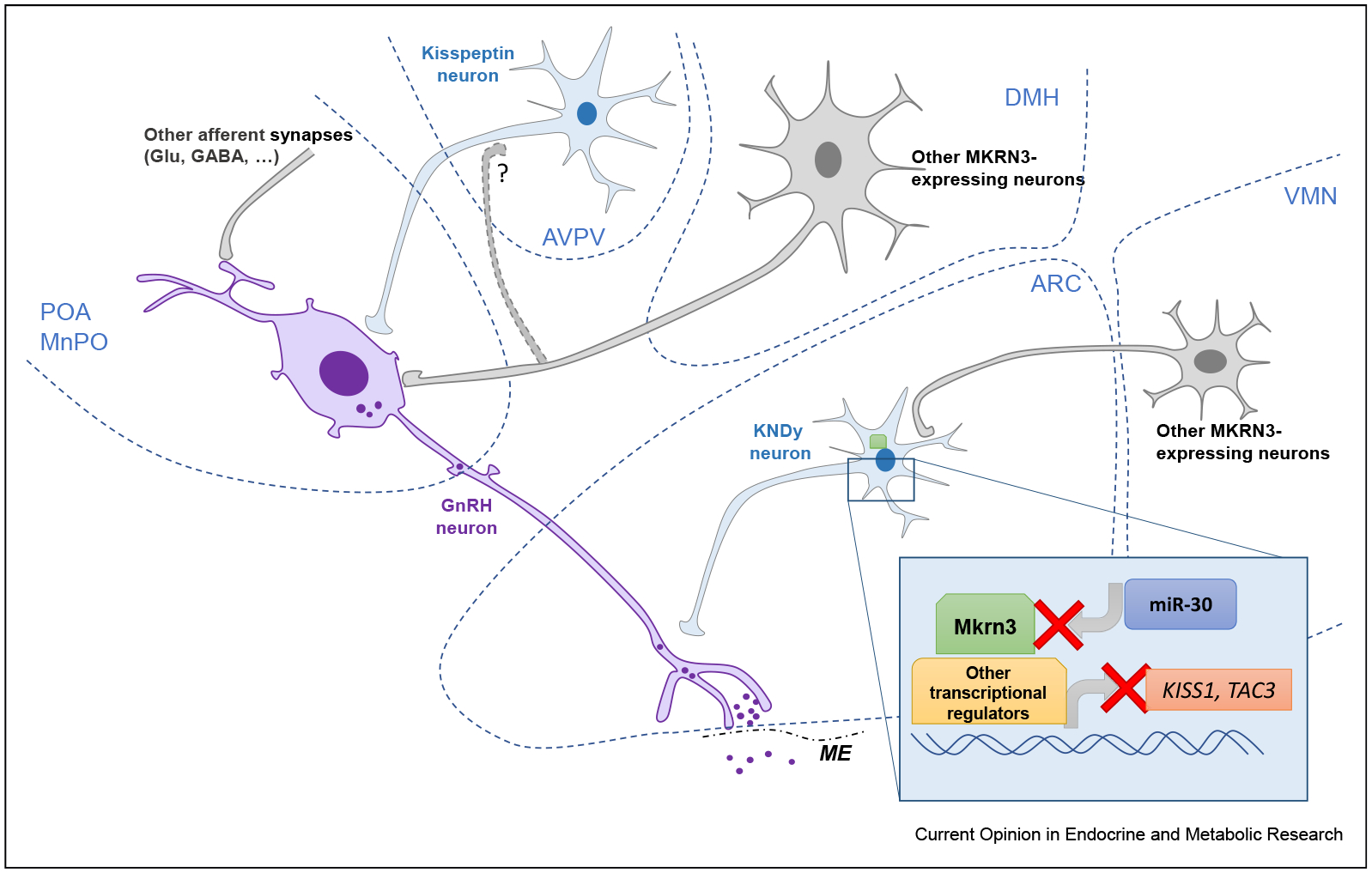
This figure summarizes the predominant sites of expression of MKRN3 within the hypothalamus. Putative regions and nuclei are encircled by dotted blue lines. The GnRH neuron is depicted in purple. Kisspeptin and KNDy neurons are shown in blue. Other putative MKRN3-expressing neurons are shown in gray. The inset shows the intracellular site of action of MKRN3 and the currently proposed mechanisms of action and regulation. The red X indicates inhibitory action. ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; DMH, dorsomedial hypothalamic nucleus; ME, median eminence; MnPO, median preoptic nucleus; POA, preoptic area; VMN, ventromedial nucleus.
Rat Mkrn3 has recently been shown to be specifically inhibited by the micro-RNA miR-30 [15]. In the rat mediobasal hypothalamus, miR-30 levels increase as Mkrn3 transcripts decline, and transfection studies showed that miR-30 directly targets a regulatory region in the Mkrn3 3’-untranslated region. Furthermore, central administration of a miR-30 blocker during the juvenile-to-puberty transition delayed puberty in rats and maintained hypothalamic Mkrn3 expression [15]. The regulatory mechanism upstream of miR-30 controlling the temporal expression of this noncoding RNA molecule to drive Mkrn3 suppression and consequently HPG onset remains to be elucidated.
In mice, Mkrn3 is also abundantly expressed in the testis, especially in gametes in late postmeiotic spermatogenic stages, including in mature spermatozoa. As Mkrn3 must remain active in the paternal lineage, it has been proposed that this expression could contribute to the setting of the paternal-specific gametic imprint at this locus [17].
In vitro protein–protein interaction analyses after mass spectrometry purification showed a wide range of proteins interacting with MKRN3, including those involved in RNA transport and metabolism and cell–cell adhesion, cell cycle regulators, and other zinc finger proteins [18]. Empirical computational approaches evaluating MKRN3 codon changes within human databases and interspecies alignments confirm positive selection, especially within regions involved in ubiquitin ligase activity and in RNA binding [19]. A recent protein–protein interaction study showed that MKRN3 may interact with targets involved in neural lineage specification, such as the neural pentraxin-1, a CNS-restricted extracellular matrix protein involved in synapse remodeling and synaptic macromolecule uptake [20].
MKRN3 mutations in patients with CPP
The first families with MKRN3 mutations in association with CPP were described in 2013 [4]. Pedigrees belonging to five different families reported herein clearly showed a pattern of maternal imprinting. An example of a pedigree illustrating the paternal transmission of MKRN3 and CPP is shown in Figure 3 [21]. From 2013 to 2019, a total of 115 patients carrying MKRN3 mutations have been described in familial and sporadic cases of CPP (Table 2). A clear prevalence of affected females (approximately 6:1) is found in almost all published series.
Figure 3. Pedigree showing a family with the M268Vfs*23 MKRN3 mutation associated with central precocious puberty (CPP).
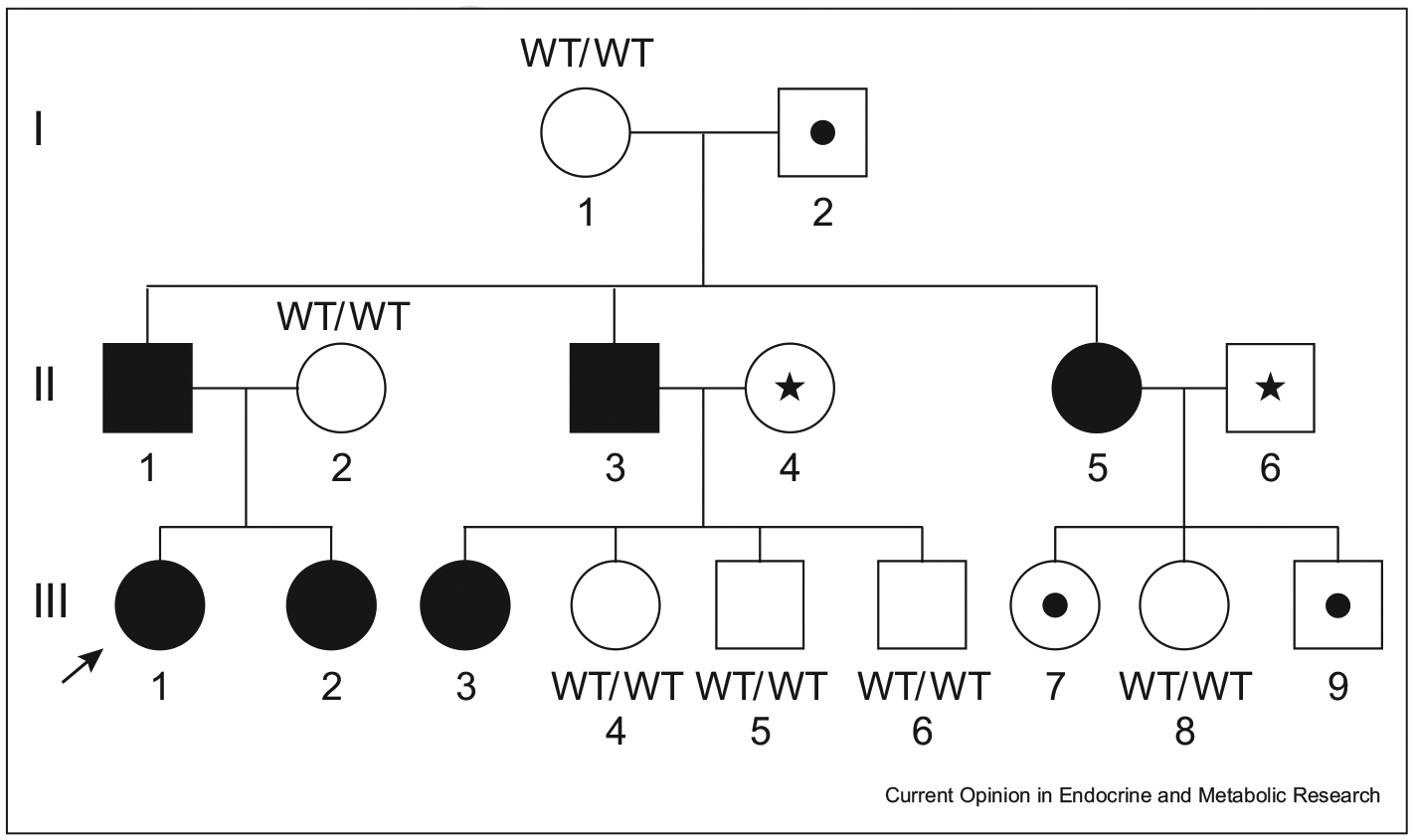
Squares indicate males, circles indicate females, black symbols indicate CPP-affected members, and symbols with a black dot inside indicate asymptomatic carriers. Symbols with a star inside indicate DNA sample unavailability. The arrow indicates the proband. Reprinted from the study by Simsek et al. [21] with permission. Please note that only individuals inheriting the mutation from the father are affected. By contrast, those inheriting the mutation from their mother are unaffected carriers.
Table 2.
MKRN3 mutations identified in patients with CPP (updated through April 2020).
| Nucleotide change | Protein change | No. of reported cases | Inheritance pattern | GnomAD | Reference |
|---|---|---|---|---|---|
| g.−865G>A | – | 1 | Sporadic | – | [34] |
| g.−166G>A | – | 1 | Sporadic | – | [34] |
| g.−886C>T | – | 1 | Sporadic | – | [34] |
| g.+13C>T | – | 1 | Sporadic | – | [34] |
| c.−150_−147delTCAG | – | 1 | Sporadic | – | [35] |
| c.−81C>T | – | 1 | Sporadic | – | [36] |
| c.89C>T | P30L | 1 | Sporadic | 1.20 e–5 | [37] |
| c.203G>A | R68H | 1 | Sporadic | 1.20 e–5 | [38] |
| c.298A>T | I100F | 1 | Sporadic | None | [39] |
| c.331G>T | E111* | 2 | Familial | None | [40] |
| c.441_441delG | H148Tfs*23 | 3 | Familial | 4.00 e–6 | [21] |
| c.477_485del | P160Cfs*14 | 3 | Familial | None | [41] |
| c.482delC | P161Rfs*10 | 3 | Sporadic | None | [42] |
| c.482_483insC | P161Rfs*16 | 4 | Familial/sporadic | None | [43,44] |
| c.475_476insC | A162Gfs*14 | 9 | Familial/sporadic | None | [4,45] |
| c.482insC | A162Gfs*15 | 13 | Familial | None | [37] |
| c. 587G>T | G196V | 2 | Sporadic | 9.21 e–5 | [39,46] |
| c.611T>C | I204T | 1 | Sporadic | 3.99 e–6 | [39] |
| c.630_650delins GCTGGGC | P211Lfs*16 | 2 | Familial | None | [47] |
| c.637delC | R213Gfs*73 | 4 | Familial/sporadic | None | [4,45] |
| c.673C>G | L225V | 1 | Sporadic | None | [48] |
| c.675_676insA | Q226Tfs*6 | 1 | Sporadic | None | [43] |
| c.677A>C | Q226P | 1 | Sporadic | None | [39] |
| c.683_684insA | E229Rfs*3 | 2 | Familial | None | [49] |
| c.699G>C | K233N | 1 | Sporadic | None | [39] |
| c.737A>G | Y246C | 1 | Sporadic | None | [37] |
| c.749G>A | G250E | 1 | Sporadic | None | [46] |
| c.766_767delA | E256Gfs*36 | 1 | Sporadic | None | [43] |
| c.802–803del | M268Vfs*23 | 7 | Familial | 3.98 e–6 | [21] |
| c.841C>T | Q281* | 3 | Familial | None | [39] |
| c.891A>T | E298* | 3 | Familial | None | [50] |
| c.934G>A | G312D | 2 | Familial | None | [51] |
| c.943A>G | M315V | 2 | Familial | None | [37] |
| c.982C>T | R328C | 6 | Familial/sporadic | 3.98 e–6 | [45,52] |
| c.1018T>G | C340G | 2 | Familial | None | [53] |
| c.1034G>A | R345H | 1 | Sporadic | 2.12 e–5 | [54] |
| c.1053_1056delACAG | R351Sfs*44 | 1 | Sporadic | None | [42] |
| c.1071C>G | I357M | 2 | Familial | None | [48] |
| c.1095G>T | R365S | 4 | Familial | 3.98e–6 | [4,45] |
| c.1212C>G | S368C | 1 | Sporadic | 3.98e–6 | [55] |
| c.1118C>T | P373L | 2 | Familial | None | [37] |
| c.1138G>A | E380K | 3 | Familial/sporadic | None | [48] |
| c.1171_1172insA | Y391* | 2 | Familial | None | [4] |
| c.1188C>A | S396R | 1 | Sporadic | 2.78 e–5 | [39] |
| c.1229G>A | C410* | 1 | Sporadic | None | [52] |
| c.1249T>A | F417I | 1 | Familial | None | [37,43] |
| c.1259T>G | H420Q | 5 | Familial | None | [56] |
| c.1420T>A | L474M | 1 | ? | None | [48] |
| c.1430G>A | R477Q | 1 | Sporadic | 5.66 e–5 | [46] |
CPP, central precocious puberty; GnomAD, Genome Aggregation Database.
GnomAD: allele frequency from the GnomAD, a public database, https://gnomad.broadinstitute.org.
Girls with MKRN3 mutations had signs of pubertal onset at a median age of 6.0 years (range: 3.0–7.8), whereas boys manifest signs of pubertal onset at a significantly older age (8.5 years, range: 5.9–9.2). Girls with MKRN3 mutations exhibited a more marked advance in first signs of pubertal development (i.e. at lower standard deviations with respect to female reference populations) than boys, indicating a sexually dimorphic effect of MRKN3 on pubertal advance. Girls typically present with premature thelarche, whereas boys present with testicular enlargement. In addition, boys are more commonly diagnosed because of the presence of CPP in a first-degree relative, typically a sister, and the borderline age of onset of pubertal signs may in part explain the gender imbalance reported in observational studies. Girls with MKRN3 mutations also had higher FSH levels at the time of diagnosis, and a greater difference was observed between chronological and bone age [22]. Patients with MKRN3 mutations have not been reported to manifest clinical characteristics other than pubertal advance. Similar to CPP caused by other etiologies, MKRN3-related CPP responds to pharmacological inhibition of the HPG axis by GnRH analogs [23].
The age at the onset of first pubertal signs appears to vary based on the type of mutation; patients with more deleterious mutations (truncating or frameshift) develop signs of pubertal onset at a younger age than those with missense variants. At the mildest end of this spectrum are MKRN3 polymorphisms, which were shown to predict age at menarche in a parent-of-origin–specific manner in large genome-wide association studies [24]. To date, a total of 48 mutations in the MKRN3 gene (coding sequence or regulatory regions) have been described in association with CPP. Table 2 reports the individual MKRN3 mutations, as well as the number of reported cases of each mutation and the relative frequency of variants in a reference population.
These include 4 nonsense, 13 frameshift, and 27 missense variants as well as 5 variants in the upstream promoter or regulatory regions. These genetic events do not appear to be scattered throughout the sequence, but clustered in specific protein domains, suggesting the importance of critical functional domains and the need to disrupt them to produce a phenotype (Figure 1). Indeed, an enrichment of missense mutations is found within the second zinc finger domain, the makorin-type cysteine-rich hinge region, and the RING finger domain (Figure 1). Methylation defects and whole-gene deletions have not yet been reported in patients with CPP [25]. Overall, the prevalence of MKRN3 mutations in patients with CPP has been estimated at 9.0% (95% confidence interval, 0.04 to 0.15) in a recent meta-analysis, with variations based on sex, family history, and geographical distribution [22].
It is worth noting that, among the aforementioned mutations, 11 of them have also been found in the Genome Aggregation Database, a public database. However, the finding of these variants in the general population does not necessarily rule out a causal role in CPP. First, the maternally imprinted nature of this gene implies that mutations will not lead to any clinical manifestations if inherited from the mother. The parent-of-origin allelic status is not reported in the Exome Aggregation Consortium database or Genome Aggregation Database. Second, it is possible that some presumed healthy controls included in these databases may have manifested some signs of early puberty during their growth and development that were undiagnosed or unreported.
The MKRN3 level has been measured in peripheral blood. Similar to the decline in Mkrn3 expression in the CNS in rodents, circulating MKRN3 levels decrease progressively in association with pubertal onset [26]. Serum MKRN3 levels were reported to be lower in patients with CPP than in matched controls [27]. These findings suggest that, regardless of the cause of CPP, a decline in serum MKRN3 levels appears to be a common pattern associated with HPG reactivation and pubertal onset. However, the source and physiological significance of peripheral MKRN3 in humans is not yet known [26].
In vitro studies conducted on mutant MKRN3 variants show that truncated and some missense variants impair the repressive action on human KISS1 and TAC3 promoters and/or the MKRN3-dependent ubiquitination [16].
Evolutionary significance of MKRN3 imprinting on pubertal timing
The MKRN3 gene is located in a maternally imprinted genomic region. This feature is not shared by other members of the makorin family. Interestingly, loss-of-function mutations in DLK1, another maternally imprinted gene, were recently identified as a cause of genetic CPP [5]. Genome-wide association studies showed an enrichment of menarche signals in several imprinted regions, including DLK1-WDR25, MKRN3-MAGEL2, and KCNK9 [24]. Recent theories on imprinting suggest that maternally expressed genes might promote early sexual maturation that is beneficial for the mother at the expense of the child’s survival [28]. By contrast, paternally expressed genes confer a greater likelihood for a child to grow and survive. Indeed, MKRN3 and DLK1 genes are paternally expressed and act as inhibitors of pubertal development.
In addition, the timing of menarche has been associated with changes in birth size, body size, and body composition, with earlier age at menarche associated with low weight at birth but higher fat mass in childhood [29]. It is of interest that, taken as a whole, several maternally imprinted genes, such as DLK1, IGF2, PEG1, PEG3, and RASGRF1, have been found to promote growth, especially during embryo development [30]. MKRN3 has not been demonstrated to promote growth directly as its function only seems to prevent the onset of puberty. However, in various species, including humans, a relationship between puberty and growth exists, with the onset of puberty being a major determinant to influence adult height [31]. Hence, by preventing progression through puberty, MKRN3 might indirectly have a permissive effect on growth.
Conclusion
Mutations in MKRN3 have been identified in 115 patients with CPP and represent the most common genetic cause of CPP. Forty-eight variants have been described, either within the coding sequence or in regulatory regions. The genomic location of MKRN3 and the more recently identified DLK1 in maternally imprinted regions underscore the important role of genomic imprinting in the regulation of puberty initiation. The E3 ubiquitin ligase activity of MKRN3 suggests an inhibitory action on post-translational regulation of proteins. Although GnRH and its known activators, kisspeptin and neurokinin B, have been proposed as the most likely targets of Mkrn3 action [16], recent studies report additional potential targets, including neural pentraxin-1 and Lin28b, involved in neural differentiation and in regulation of age at menarche, respectively [20,32]. Moreover, Mkrn3 has recently been shown to be specifically inhibited by miR-30, unveiling for the first time the role of hypothalamic miRNAs in regulating Mkrn3 expression [15]. Makorin proteins have been identified in a broad range of species, including invertebrates and plants. They have been demonstrated to decline progressively after body elongation, organ differentiation, and/or neural development, supporting the hypothesis of an important and conserved role in embryonic and postnatal development. Understanding the hypothalamic network surrounding the action of Mkrn3 will be a major challenge for future research to fully understand the regulation of puberty initiation.
Acknowledgements
This work was supported by grant NIH / NICHD R01 HD082314 (to UBK) and in part by the French Society of Endocrinology Research Award (to LM).
Footnotes
Note added in proof
While this work was under revision, Li et al. [33] described a mouse model lacking Mkrn3. These mice manifested an early onset of puberty, as indicated by timing of vaginal opening and first estrus in females and preputial separation in males, mirroring the central precocious phenotype seen in humans with loss-of-function MKRN3 mutations.
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Avendano MS, Vazquez MJ, Tena-Sempere M: Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update 2017, 23:737–763. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE: Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 2016, 12:452–466. [DOI] [PubMed] [Google Scholar]
- 3.Latronico AC, Brito VN, Carel JC: Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol 2016, 4:265–274. [DOI] [PubMed] [Google Scholar]
- 4.**.Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, et al. : Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med 2013, 368:2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper first identified MKRN3 mutations in patients with familial central precocious puberty. It also demonstrated for the first time that Mkrn3 transcripts progressively decrease in mice hypothalami during the first two postnatal weeks.
- 5.Dauber A, Cunha-Silva M, Macedo DB, Brito VN, Abreu AP, Roberts SA, et al. : Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab 2017, 102:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naule L, Kaiser UB: Evolutionary conservation of MKRN3 and other makorins and their roles in puberty initiation and endocrine functions. Semin Reprod Med 2019, 37:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrandt A, Bruggemann M, Ruckle C, Boerner S, Heidelberger JB, Busch A, et al. : The RNA-binding ubiquitin ligase MKRN1 functions in ribosome-associated quality control of poly(A) translation. Genome Biol 2019, 20:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MS, Han HJ, Han SY, Kim IY, Chae S, Lee CS, et al. : Loss of the E3 ubiquitin ligase MKRN1 represses diet-induced metabolic syndrome through AMPK activation. Nat Commun 2018, 9:3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian X, Wang L, Zheng B, Shi ZM, Ge X, Jiang CF, et al. : Deficiency of Mkrn2 causes abnormal spermiogenesis and spermiation, and impairs male fertility. Sci Rep 2016, 6:39318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian YC, Xie YX, Wang CS, Shi ZM, Jiang CF, Tang YY, et al. : Mkrn2 deficiency induces teratozoospermia and male infertility through p53/PERP-mediated apoptosis in testis. Asian J Androl 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran HT, Cho E, Jeong S, Jeong EB, Lee HS, Jeong SY, et al. : Makorin 1 regulates developmental timing in Drosophila. Mol Cell 2018, 41:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dold A, Han H, Liu N, Hildebrandt A, Bruggemann M, Ruckle C, et al. : Makorin 1 controls embryonic patterning by alleviating Bruno1-mediated repression of oskar translation. PLoS Genet 2020, 16, e1008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson H, Vuong E, Miller RM, Kiontke K, Fitch DH, Portman DS: The Makorin lep-2 and the lncRNA lep-5 regulate lin-28 to schedule sexual maturation of the C. elegans nervous system. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arumugam TU, Davies E, Morita EH, Abe S: Sequence, expression and tissue localization of a gene encoding a makorin RING zinc-finger protein in germinating rice (Oryza sativa L. ssp. Japonica) seeds. Plant Physiol Biochem 2007, 45: 767–780. [DOI] [PubMed] [Google Scholar]
- 15.*.Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, Sanchez-Tapia MJ, Ruiz-Pino F, Roa J, et al. : Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol 2019, 17, e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated that rat Mkrn3 expression is under upstream inhibitory regulation by miR-30. The identification of this micro-RNA opens new perspectives for the hierarchical regulation of the hypothalamic network controlling the gonadotrope axis.
- 16.Abreu Apt CA, Song YB, Navarro VM, Bosch MA, Eren A, Liang JN, Carroll RS, Latronico AC, Rønnekleiv OK, Aylwin CF, Lomniczi A, Ojeda S, Kaiser UB: MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest 2020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jong MT, Gray TA, Ji Y, Glenn CC, Saitoh S, Driscoll DJ, et al. : A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet 1999, 8:783–793. [DOI] [PubMed] [Google Scholar]
- 18.Yellapragada V, Liu X, Lund C, Kansakoski J, Pulli K, Vuoristo S, et al. : MKRN3 interacts with several proteins implicated in puberty timing but does not influence GNRH1 expression. Front Endocrinol 2019, 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad MJ, Ahmad HI, Adeel MM, Liang A, Hua G, Murtaza S, et al. : Evolutionary analysis of makorin ring finger protein 3 reveals positive selection in mammals. Evol Bioinform Online 2019, 15, 1176934319834612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Kong X, Chen F: Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget 2017, 8:85102–85109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simsek E, Demiral M, Ceylaner S, Kirel B: Two frameshift mutations in MKRN3 in Turkish patients with familial central precocious puberty. Horm Res Paediatr 2017, 87:405–411. [DOI] [PubMed] [Google Scholar]
- 22.*.Valadares LP, Meireles CG, De Toledo IP, Santarem de Oliveira R, Goncalves de Castro LC, Abreu AP, et al. : MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc 2019, 3:979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work provided a systematic review and meta-analysis of the clinical features of all patients with central precocious puberty carrying MKRN3 mutations.
- 23.Ramos CO, Macedo DB, Canton A, Cunha-Silva M,Antonini SRR, Stecchini MF, et al. : Outcomes of patients with central precocious puberty due to loss-of-function mutations in MKRN3 gene after treatment with gonadotropin-releasing hormone analog. Neuroendocrinology 2019. [DOI] [PubMed] [Google Scholar]
- 24.Perry JR, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, et al. : Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki E, Shima H, Kagami M, Soneda S, Tanaka T, Yatsuga S, et al. : (Epi)genetic defects of MKRN3 are rare in Asian patients with central precocious puberty. Hum Genome Var 2019, 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagen CP, Sorensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A: Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab 2015, 100:1920–1926. [DOI] [PubMed] [Google Scholar]
- 27.Ge W, Wang HL, Shao HJ, Liu HW, Xu RY: Evaluation of serum makorin ring finger protein 3 (MKRN3) levels in girls with idiopathic central precocious puberty and premature thelarche. Physiol Res 2020, 69:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotler J, Haig D: The tempo of human childhood: a maternal foot on the accelerator, a paternal foot on the brake. Evol Anthropol 2018, 27:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT: Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab 2006, 91: 4369–4373. [DOI] [PubMed] [Google Scholar]
- 30.Barlow DP, Bartolomei MS: Genomic imprinting in mammals. Cold Spring Harb Perspect Biol 2014, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plant TM: Neuroendocrine control of the onset of puberty. Front Neuroendocrinol 2015, 38:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, et al. : Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 2009, 41:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Lu W, Yang L, Li Z, Zhou X, Guo R, Wang J, Wu Z, Dong Z, Ning G, Shi Y, Gu Y, Chen P, Hao Z, Han T, Yang M, Wang W, Huang X, Li Y, Gao S, Hu R: MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. National Science Review 2020, 7:671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanis P, Skordis N, Toumba M, Papaioannou N, Makris A, Kyriakou A, et al. : Central precocious puberty caused by novel mutations in the promoter and 5’-UTR region of the imprinted MKRN3 gene. Front Endocrinol 2019, 10:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macedo DB, Franca MM, Montenegro LR, Cunha-Silva M,Best DS, Abreu AP, et al. : Central precocious puberty caused by a heterozygous deletion in the MKRN3 promoter region. Neuroendocrinology 2018, 107:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu W, Wang J, Li C, Sun M, Hu R, Wang W: A novel mutation in 5’-UTR of Makorin ring finger 3 gene associated with the familial precocious puberty. Acta Biochim Biophys Sin 2018, 50: 1291–1293. [DOI] [PubMed] [Google Scholar]
- 37.Simon D, Ba I, Mekhail N, Ecosse E, Paulsen A, Zenaty D, et al. : Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol 2016, 174:1–8. [DOI] [PubMed] [Google Scholar]
- 38.Ortiz-Cabrera NV, Riveiro-Alvarez R, Lopez-Martinez MA, Perez-Segura P, Aragon-Gomez I, Trujillo-Tiebas MJ, et al. : Clinical Exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Horm Res Paediatr 2017, 87:88–94. [DOI] [PubMed] [Google Scholar]
- 39.Lee HS, Jin HS, Shim YS, Jeong HR, Kwon E, Choi V, et al. : Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res 2016, 48:118–122. [DOI] [PubMed] [Google Scholar]
- 40.Schreiner F, Gohlke B, Hamm M, Korsch E, Woelfle J: MKRN3 mutations in familial central precocious puberty. Horm Res Paediatr 2014, 82:122–126. [DOI] [PubMed] [Google Scholar]
- 41.Grandone A, Cantelmi G, Cirillo G, Marzuillo P, Luongo C, Miraglia del Giudice E, et al. : A case of familial central precocious puberty caused by a novel mutation in the makorin RING finger protein 3 gene. BMC Endocr Disord 2015, 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrova-Mladenova MS, Stefanova EM, Glushkova M, Todorova AP, Todorov T, Konstantinova MM, et al. : Males with paternally inherited MKRN3 mutations may Be asymptomatic. J Pediatr 2016, 179:263–265. [DOI] [PubMed] [Google Scholar]
- 43.Macedo DB, Abreu AP, Reis AC, Montenegro LR, Dauber A, Beneduzzi D, et al. : Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab 2014, 99:E1097–E1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stecchini MF, Macedo DB, Reis AC, Abreu AP, Moreira AC, Castro M, et al. : Time course of central precocious puberty development caused by an MKRN3 gene mutation: a prismatic case. Horm Res Paediatr 2016, 86:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bessa DS, Macedo DB, Brito VN, Franca MM, Montenegro LR, Cunha-Silva M, et al. : High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology 2017, 105:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin WD, Wang CH, Tsai FJ: Genetic screening of the makorin ring finger 3 gene in girls with idiopathic central precocious puberty. Clin Chem Lab Med 2016, 54:e93–e96. [DOI] [PubMed] [Google Scholar]
- 47.Aycan Z, Savas-Erdeve S, Cetinkaya S, Kurnaz E, Keskin M, Muratoglu Sahin N, et al. : Investigation of MKRN3 mutation in patients with familial central precocious puberty. J Clin Res Pediatr Endocrinol 2018, 10:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T, Chen L, Wu H, Xie R, Wang F, Chen X, et al. : Low frequency of MKRN3 and DLK1 variants in Chinese children with central precocious puberty. Internet J Endocrinol 2019, 2019:9879367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishioka J, Shima H, Fukami M, Yatsuga S, Matsumoto T, Ushijima K, et al. : The first Japanese case of central precocious puberty with a novel MKRN3 mutation. Hum Genome Var 2017, 4:17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christoforidis A, Skordis N, Fanis P, Dimitriadou M, Sevastidou M, Phelan MM, et al. : A novel MKRN3 nonsense mutation causing familial central precocious puberty. Endocrine 2017, 56: 446–449. [DOI] [PubMed] [Google Scholar]
- 51.Neocleous V, Shammas C, Phelan MM, Nicolaou S, Phylactou LA, Skordis N: In silico analysis of a novel MKRN3 missense mutation in familial central precocious puberty. Clin Endocrinol 2016, 84:80–84. [DOI] [PubMed] [Google Scholar]
- 52.Grandone A, Capristo C, Cirillo G, Sasso M, Umano GR, Mariani M, et al. : Molecular screening of MKRN3, DLK1, and KCNK9 genes in girls with idiopathic central precocious puberty. Horm Res Paediatr 2017, 88:194–200. [DOI] [PubMed] [Google Scholar]
- 53.Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A: Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab 2014, 99:E647–E651. [DOI] [PubMed] [Google Scholar]
- 54.Kansakoski J, Raivio T, Juul A, Tommiska J: A missense mutation in MKRN3 in a Danish girl with central precocious puberty and her brother with early puberty. Pediatr Res 2015, 78:709–711. [DOI] [PubMed] [Google Scholar]
- 55.Pagani S, Calcaterra V, Acquafredda G, Montalbano C, Bozzola E, Ferrara P, et al. : MKRN3 and KISS1R mutations in precocious and early puberty. Ital J Pediatr 2020, 46:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vries L, Gat-Yablonski G, Dror N, Singer A, Phillip M: A novel MKRN3 missense mutation causing familial precocious puberty. Hum Reprod 2014, 29:2838–2843. [DOI] [PubMed] [Google Scholar]


