Abstract
The burden of Alzheimer’s disease and related dementias (ADRD) has increased substantially in the United States, particularly in health disparity populations. Little is known about the epidemiology of ADRD in American Indian (AI) adults, although they have a high prevalence of ADRD risk factors including hypertension, diabetes, obesity, and smoking. Using electronic health records from a large health care organization during 2016-18, we describe characteristics of AI patients aged ≥55 years with and without an ADRD diagnosis, assess ADRD risk factors and contrast findings with results from age- and sex-matched non-Hispanic White (NHW) patients. To identify factors associated with ADRD diagnoses, we estimated population-averaged prevalence rate ratios to approximate relative risk (RR) using generalized estimating equations models adjusted for age, sex, and marital and rural residency status. The age-adjusted prevalence of ADRD diagnosis was 6.6% of AI patients, compared with 4.4% in NHW patients. Patient age and diagnosis of hypertension, depression, hyperlipidemia, or diabetes were significantly associated with higher risk of ADRD diagnosis in AIs (RR range: 1.1-2.8) whereas female sex or being married/having a partner were associated with lower risk of ADRD diagnosis (each RR=.7). ADRD risk factors were generally similar between AI and NHW patients, except for sex and marital status. However, the adjusted risk of ADRD was approximately 49% higher in AI patients. To our knowledge, our study is the first to examine ADRD diagnoses and comorbidities in AIs across a large geographical region in southwest United States. Future efforts to confirm our findings in diverse AI communities are warranted.
Keywords: American Indians, Alzheimer’s Disease, Dementia, Electronic Health Records, Dementia Risk Factors
Introduction
As the US population ages, degenerative diseases such as Alzheimer’s disease and related dementias (ADRD) are a growing public health concern, particularly in racial/ethnic minority populations. By 2060, the CDC estimates the prevalence of ADRD will increase 178% among all Americans aged ≥65 years, and minority groups will experience the fastest growing rates.1
In 2012, the US National Plan for Alzheimer’s Disease prioritized inclusion of health disparities groups in ADRD research,2 yet few studies of those populations exist.3 Little is known about ADRD epidemiology and risk factors in racial and ethnic minorities, particularly American Indian (AI) and Alaska Native (AN) adults,3 who are often aggregated together despite substantial heterogeneity in health status and risk factors. One small community survey in 192 Cree Indians aged ≥65 years estimated a dementia prevalence of 4.2%.4 Two larger population-based studies using medical records and/or claims data have identified disparities in ADRD among population subgroups defined by race and ethnicity. A study using 2014 Medicare and census data for the US population aged ≥65 years noted significant disparities in dementia between racial and ethnic groups, though the dementia prevalence of 9.1% in AIs and Alaska Natives (AN) combined was slightly lower than that in non-Hispanic Whites (NHW) (10.3%).1 Another study in the Kaiser Permanente Northern California Health system found age-adjusted incidence rates of dementia to be highest in African American and AI/AN racial groups.5
Large administrative datasets, such as electronic health record (EHR) data from managed care encounters maintained by health care systems, hold promise as efficient and cost-effective resources for population-representative ADRD research, including studies of AI/AN populations. Compared with studies relying on primary data collection, EHR-based studies are considerably less expensive, require less time to complete, and have the potential to include substantially larger, diverse and more generalizable populations.6 However, EHR data are prone to specific biases such as selection bias that may limit their use for public health and epidemiologic research.7 Advanced statistical methods and linkages with external data have been used to address some limitations of using EHR data, but researchers must carefully consider whether EHR data quality are sufficient to achieve specific research aims.8 We conducted an analysis using health network EHR data to characterize ADRD and its risk factors in AI patients, and contrast associations with those from a sample of NHW patients. We also describe some of the challenges of using the EHR data to address these aims.
Methods
Setting
Headquartered in Arizona, Banner Health is one of the largest nonprofit health care systems in the country. The system owns and operates 28 acute-care hospitals, Banner Health Network, Banner – University Medicine, academic and employed physician groups, long-term care centers, and outpatient surgery centers. It delivers an array of other services through Banner Urgent Care, family clinics, home care and hospice services, pharmacies and a nursing registry. Banner Health is located in six states: Arizona, California, Colorado, Nebraska, Nevada and Wyoming.
Data
We obtained anonymous data for all inpatient and outpatient encounters for AI patients aged ≥55 years in the Banner Health system from 2016-2018. We initially requested data for AN patients also, but there were too few (<10 patients) for stratified analysis. For comparison purposes, we also requested age and sex-matched data for NHW patients (matched 1:1). Race and ethnicity were self-reported in the medical record. We requested patient characteristics (sex, marital status, zip code, ethnicity, and primary language) and measurements recorded during encounters (age, systolic blood pressure [SBP], diastolic blood pressure [DBP], height, and weight), as well as details about encounters (date and type—inpatient, outpatient, acute care or emergency department). Zip codes were grouped according to rural-urban commuting area (RUCA) codes approximated for zip codes (version 3.1). RUCA codes delineate areas into 33 urban and rural categories based on data from the 2010 Census work-commuting data, the 2012 Census Bureau revised urban area definition, and 2013 zip codes.9 We classified categories using codes as described in a report by Danaher et al.10 Due to small numbers in the rural categories, we further classified codes into urban or rural (grouping large rural/town, small rural/town and isolated small/rural town together).
We requested medical codes, if any, for the main outcome of ADRD. In addition, we requested codes for the following clinical conditions known to be risk factors for ADRD in other populations11: diabetes, hypertension, stroke, dyslipidemia, depression, and tobacco use disorders. While tobacco use disorder specifically reflects nicotine abuse and dependence, others have found it to be a reasonable proxy for smoking.12 To prepare lists of medical codes to define each condition, we reviewed published literature and ultimately used codes as recommended by the Centers for Medicare Chronic Conditions Warehouse.13 The specific codes that we used to identify each condition are available in supplemental table 1, available from the corresponding author. The Warehouse also recommends algorithms for use with claims data. Because we only had access to inpatient or outpatient encounter data (and not nursing facility or home health care data), we defined each condition as the presence of one of the applicable ICD-10 codes in the EHR during the study time period.
The Washington State University institutional review board reviewed and approved all study procedures. This study met regulatory guidelines for expedited review, and was granted a waiver of informed consent, per the code of federal regulations for records research, 45CFR46.116(d)(3).
Analysis
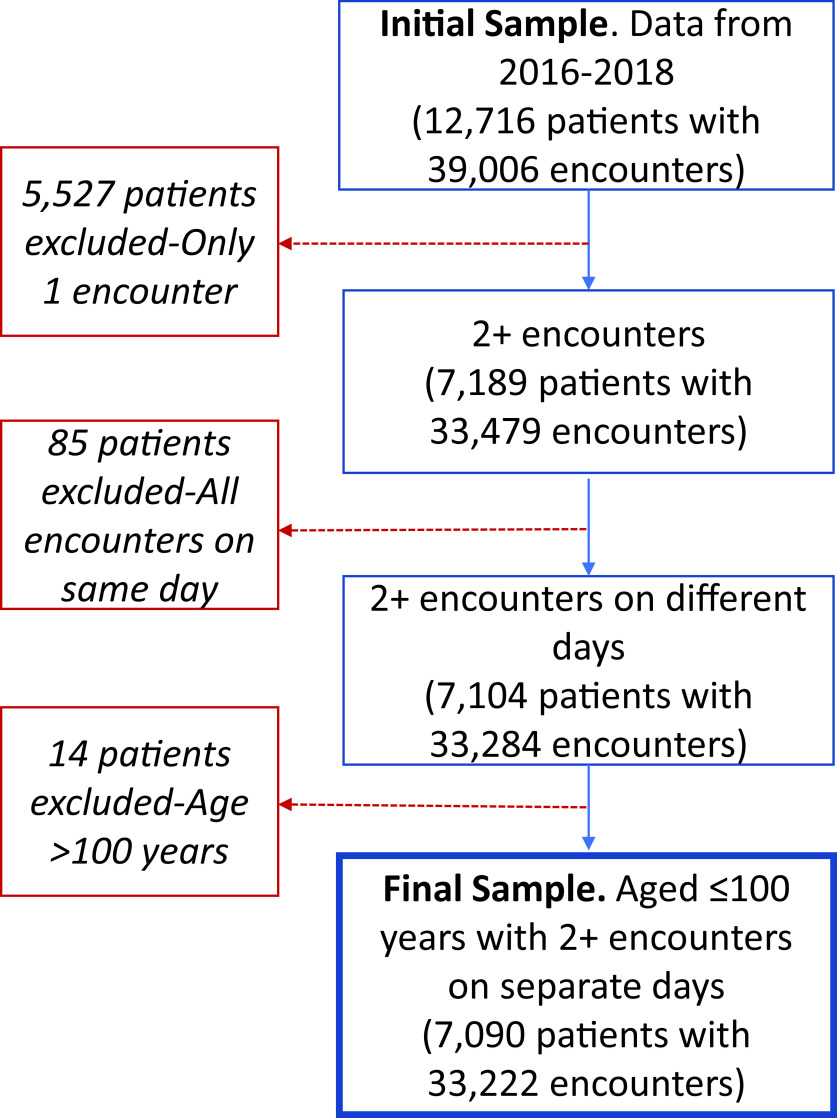
Data obtained from Banner Health were reviewed for consistency and completeness. As a result of these quality control processes, several exclusion criteria were applied (Figure 1). We limited the dataset to patients with at least two encounters on separate days to minimize inclusion of referral patients who did not receive regular care at Banner Health. In addition, we excluded 14 individuals who were aged >100 years, due to concerns about generalizability, and accuracy of age in the EHR. Height and weight were used to estimate body mass index (BMI) and biologically implausible height and weight values were excluded: height <100cm or >250cm and weight <34kg or >318 kg.
Figure 1. Patient Data Flow Chart for American Indians and non-Hispanic Whites.
We prepared descriptive statistics of variables and then contrasted diagnoses in AIs vs NHWs. For these comparisons, we used generalized estimating equations with log link, Poisson distribution, time in days, and clustering on patient to estimate population-averaged prevalence rate ratios as risk ratios (RR) with 95% CI. We included AI and NHW individuals in the same model, using the diagnosis of interest as the outcome and included an indicator of AI race as an independent variable in the model to formally compare the two racial groups. Matching variables (age and sex) were included in all models as recommended by Pearce et al.14 Models were also adjusted for marital status (married or life partner vs none), and rural residency status (yes/no).
To measure associations between ADRD diagnosis and ADRD risk factors, we used models as described above to estimate RRs and stratified by race. All models were adjusted for age, sex, marital status, rural residency status and clinical covariates. In sensitivity analyses, we included a variable indicating the number of medical encounters in the models to reduce potential bias due to informed presence.15 Informed presence bias occurs because the existence of EHR data is usually a result of an individual seeking medical services for some condition or illness and thus individuals in EHR are systematically different from those not in EHR (eg, more chronic conditions in the former).15
In exploratory models, we tested whether associations between ADRD and other factors varied by race by including a multiplicative interaction term in each model. To account for multiple testing in these exploratory analyses, we applied a conservative Bonferroni correction to the p-value threshold for significance, P<.007 (or P=.05/7).
In sensitivity analyses, we addressed high proportions of missing data for the measures expected at every visit: BMI, SBP and DBP. We imputed values for these variables for outpatient visits only due to concerns about potential biases between measurement in outpatient vs inpatient settings. Due to missing height and weight values, 60% of patients (65% of AIs and 54% of NHWs) were missing BMI at all outpatient encounters. SBP and DBP were missing at all outpatient visits in 62% of AI patients and 52% of NHW patients. Using multiple imputation chained equations,16 we imputed SBP, DBP, height, and weight for all patients and calculated BMI based on the imputed values of height and weight. For the imputations, we used available demographic and diagnostic code data, the number of medical encounters, and 100 imputations. In analyses including the imputed data, we estimated parameter and standard error estimates that accounted for the variability in SBP, DBP and BMI across imputed datasets. Due to small cell sizes in the outpatient only models, some key variables had to be removed from AI models with imputed data so they would converge. Stata/SE 14.2 (StataCorp LLC, College Station, TX) was used for all analyses.
Results
After the data exclusion steps (Figure 1), we had data for 3464 AI and 3626 NHW patients. Characteristics of the patients at their first medical encounter are shown in Table 1. The majority (58.7%) of AI patients were female. Higher proportions of AI than NHW patients were rural dwelling (31.5 vs. 11.0%) and single (33.5 vs. 10.2%). The median (intra-quartile range) age of AI patients at their first encounter was younger than NHWs, 64 (59-71) years vs 68 (61-75) years, in spite of the initial matching. ADRD was a rare diagnosis in both groups (Table 2): 5.7% of AI patients aged ≥55 years had an ADRD diagnosis, compared with 5.0% of NHW patients. Given differences in the age distribution between the racial groups, we also estimated the age-adjusted prevalence estimates for ADRD diagnosis, which were 6.6% in AIs and 4.4% in NHWs. AIs had 60% higher risk of ADRD diagnosis compared with NHWs in models adjusted for demographic factors. Further adjustment for clinical factors (diagnosis of diabetes, stroke, hyperlipidemia, hypertension, and tobacco use disorders), attenuated the RR slightly, but it remained significantly elevated (RR=1.5; 95%CI: 1.2-1.9, P=.001). Other diagnoses with higher risk in AIs included hypertension (20% higher risk) and diabetes (220% higher risk), whereas AIs had significantly lower risk of diagnoses of depression, and tobacco use disorders than NHWs. Both AI and NHW patients had a median of 3 encounters during the study period; other encounter characteristics are summarized in supplemental table 2 available from the corresponding author.
Table 1. Characteristics of unique patients, based on first encounter during 2016-2018.
| Characteristic | AI (n=3464) | NHWa (n=3626) | ||
| Age in years, median (IQR) | 64 | (59-71) | 68 | (61-75) |
| Female, n (%) | 2032 | (58.7) | 2192 | (60.5) |
| Hispanic, n (%) | 152 | (4.4) | 0 | (0.0) |
| Language, n (%) | ||||
| English | 3337 | (96.3) | 3614 | (99.7) |
| Other | 119 | (3.4) | 12 | (0.3) |
| Unknown | 8 | (0.2) | 0 | (0.0) |
| Married/life partner, n (%) | 1360 | (39.3) | 2273 | (62.7) |
| Zipcode, n (%) | ||||
| Urban | 2370 | (68.4) | 3220 | (88.8) |
| Rural | 1092 | (31.5) | 400 | (11.0) |
| Unknown | 2 | (0.1) | 6 | (0.2) |
| BMI in kg/m2, mean±SD b | 30.6 | ± 8.5 | 29.9 | ± 7.7 |
1. NHW were selected at random and matched by age and sex to AI sample at EHR data extraction
2. BMI data are missing for 32.6% of patients. BMI mean (95%CI) including the imputed data are 30.3 (29.5-31.1) and 29.2 (28.5-29.8) for AI and NHW, respectively.
Table 2. Frequency of diagnoses for selected conditions in the patient populations during 2016-18.
| AI, n=3464 | NHW, n=3626 | Risk of condition in AI vs. NHWb | ||
| CMS condition | N (%)a | N (%)a | RR (95% CI) | P |
| Dementia | 196 (5.7) | 180 (5.0) | 1.60 (1.27-2.01) | <.001 |
| Stroke | 174 (5.0) | 205 (5.7) | .99 (.79-1.24) | .918 |
| Tobacco use disorders | 916 (26.4) | 1298 (35.8) | .69 (.63-.75) | <.001 |
| Hypertension | 2033 (58.7) | 1993 (55.0) | 1.20 (1.13-1.26) | <.001 |
| Depression | 354 (10.2) | 554 (15.3) | .68 (.58-.78) | <.001 |
| Hyperlipidemia | 1138 (32.9) | 1387 (38.3) | .98 (.90-1.06) | .541 |
| Diabetes | 1666 (48.1) | 853 (23.5) | 2.18 (2.00-2.36) | <.001 |
a. Number and race-specific percentage of unique patients with at least one code.
b. RR reflects the risk of AI having the condition compared with the risk of NHW having the condition in models adjusted for age, sex, marital status, rural status and considering each condition separately.
Using race-specific, covariate-adjusted models, we estimated risk factor associations with ADRD. Age and diagnosis of hypertension, depression, hyperlipidemia, or diabetes were significantly associated with increased risk of an ADRD diagnosis in AIs (RRs ranging from 1.1- 2.8 and all P<.02), whereas being female or being married/having a life partner were associated with ~30% lower risk of an ADRD diagnosis (P<.03) (Table 3). The most robust associations were observed for diagnoses of hypertension or depression, for which the risk of an ADRD diagnosis increased approximately 3-fold (RR = 2.8; 95% CI: 1.8-4.3 and RR = 2.5; 95% CI:1.8-3.5, respectively). Rural residence or tobacco diagnosis was not associated with ADRD diagnosis in AIs. Findings for the NHW sample were generally similar, with the exception of the female sex and marital status. Neither was significantly associated with ADRD diagnosis in NHWs and point estimates were close to the null. Although not significant in either racial group, rural residence trended to a lower risk of ADRD in NHWs (RR=.6; 95% CI: .3-1.1).
Table 3. Risk factors for ADRD diagnosis in AI and NHW.
| AI, n=3440 | NHW, n=3615 | |||
| Characteristic | RRa (95% CI) | P | RRa (95% CI) | P |
| Age | 1.12 (1.11-1.14) | <.001 | 1.09 (1.08-1.12) | <.001 |
| Female sex | .70 (.52-.94) | .018 | .95 (.68-1.31) | .735 |
| Married/life partner | .67 (.48-.94) | .020 | 1.07 (0.77-1.49) | .699 |
| Rural residence | 1.06 (0.79-1.43) | .704 | .59 (.31-1.12) | .108 |
| Tobacco diagnosis | 1.16 (0.86-1.55) | .333 | 1.17 (0.89-1.53) | .265 |
| Hypertension diagnosis | 2.75 (1.75-4.31) | <.001 | 3.43 (2.44-4.83) | <.001 |
| Depression diagnosis | 2.49 (1.79-3.46) | <.001 | 2.05 (1.53-2.75) | <.001 |
| Hyperlipidemia diagnosis | 1.41 (1.08-1.84) | .013 | 1.49 (1.15-1.91) | .002 |
| Diabetes diagnosis | 2.07 (1.41-3.03) | <.001 | 1.50 (1.13-1.99) | .005 |
a. RR reflects the risk of having an ADRD diagnosis in race-stratified models adjusted for age, sex, marital status, rurality, and having a diagnosis of tobacco use, hypertension, depression, hyperlipidemia, or diabetes.
In models combining the racial groups, we also assessed effect modification of the risk factor-ADRD association by race for variables significantly associated with ADRD diagnosis in either race in the main models. However, no race interactions met the threshold for statistical significance after accounting for multiple testing. P-values unadjusted for multiple testing for the variables tested include: sex (P=.59); age (P=.02); marital status (P=.04), and diagnosis of hypertension (P=.73), depression (P=.70), hyperlipidemia (P=.78) or diabetes (P=.49).
In sensitivity models adjusting for the number of medical encounters during the time period to account for potential informed presence bias, no substantial differences from the main model results were observed. Along with outpatient visit models with imputed SBP, DBP and BMI (and existing data only, for comparison purposes), these data can be found in supplemental tables 3, 4a and 4b, available from the corresponding author.
Sample sizes were considerably smaller due to the focus on outpatient visits only, which limited power to detect modest associations. Few differences in associations were observed between models with imputed data and models with existing data only. No significant associations between ADRD diagnosis with BMI, SBP or DBP were observed in adjusted models in either AIs or NHWs. Many point estimates for other variables in these models were of similar magnitude to the main models, though much less precise and no longer significant. Marital status, hypertension, and diabetes were no longer significantly associated with ADRD diagnoses in AIs in models including BMI, SBP and DBP whereas ADRD associations with depression were more robust. In NHW models including BMI, SBP and DBP, only age was significantly associated with ADRD diagnosis.
Discussion
Although ADRD in racial/ethnic minority populations is a topic of interest in recent policy and research discussions, few studies have focused on AI populations.17 As a result, little is known about the epidemiology of ADRD in AI adults.18 Here, we report characteristics associated with an ADRD diagnosis in AI patients who used Banner Health from 2016-18. We found that 5.7% of AI patients aged ≥55 years had an ADRD diagnosis, compared with 5.0% of NHW patients. The corresponding age-adjusted ADRD estimates are 6.6% and 4.4% for AIs and NHWs, respectively. Our estimates for both racial groups are lower than the ADRD prevalence of 8.4% -10.9% reported in the overall US population, which were based on health claims data or population surveys of older populations aged ≥65 years.1,19,20 We focused on a younger-aged sample because studies indicate that AIs are at greater risk of death at earlier ages and have lower life expectancies than the overall US population.21 Thus, we might expect incidence of ADRD (and other conditions) in AIs at younger ages than in NHWs. The few ADRD estimates specifically for AIs range from 4.2% in a small community survey of Cree Indians4 to 9.1% in AI/AN combined from the Medicare population.1
We observed that AI patients had higher risk of diagnoses of ADRD, hypertension and diabetes, but lower risk of having diagnoses of depression, and tobacco use disorders than NHW patients. Further, we found that age and diagnosis of hypertension, depression, hyperlipidemia, or diabetes were significantly associated with higher risk of an ADRD diagnosis in AI patients, whereas being female or being married/having a life partner were associated with lower risk of having an ADRD diagnosis.
Other literature supports the importance of older age, cardiovascular disease and depression in the development of ADRD.22-25 In contrast to our findings, the prevalence of ADRD is generally higher in females than males,26 though some studies report no association between ADRD and sex,24,27 and many researchers assert that the higher ADRD prevalence in women is due to their longer life-expectancy.22,28 Recent evidence also suggests a more complex relationship between sex and ADRD subtype in which women may be at higher risk of AD, and men at higher risk for vascular dementia.28,29 In addition, differences in the clinical presentation, diagnosis or duration of ADRD may contribute to observed sex differences in prevalence and rates.27,30 We also observed no association between urban/rural status and ADRD diagnosis in AIs. Some evidence suggests higher risk of dementia in rural vs urban populations.31 In contrast, a study in Medicare beneficiaries residing in Kentucky and West Virginia found an 11% lower prevalence of ADRD in rural counties compared with urban ones, perhaps due to underdiagnosis in the rural areas.32 Interestingly, our findings in NHWs, but not AIs trended toward a decreased risk of ADRD in patients with rural residence, but the associations were not significant.
Study Limitations
This analysis of EHR data is subject to potential biases that deserve mention. EHR data present unique issues related to the representativeness of the patient sample, and complete data capture.6,7,33 Others have addressed these challenges by linking EHR data with community survey data, or focusing on regional populations that receive most or all of their care within a particular system, or using health claims databases that reflect all care, such as Medicare.1,5,33 Unfortunately, these options were not available in our study. Though Banner has a health plan, only about .3% of enrollees are AI. Many AIs receive health care through the Indian Health Service, an agency that provides comprehensive health service to members of federally recognized tribes. Some AI patients may come to Banner Health for referral appointments, tests or specialty care not available in Indian Health Service facilities, and thus may lack a comprehensive medical history in the EHR. We limited patients to those with two or more visits on separate days to minimize the chances of including referral-only patients.
It is also possible that we have incomplete ADRD ascertainment in our sample because a diagnosis was not recorded in the Banner EHR, or the condition was not observed or treated by a specialist. Indeed, ADRD is often underdiagnosed, and among those with a diagnosis, many may not be aware of their diagnosis.1 Moreover, some argue that ascertainment bias in the clinical diagnosis of AD, in particular, is likely unless clinicians consider a patient’s longitudinal course,34 which requires observation of a patient during visits over time. Ironically, attending more medical encounters also introduces a potential bias because the likelihood of a prevalent condition being diagnosed increases with each successive clinic visit. EHR data are only collected when a person seeks medical care. This bias, termed informed presence bias, affects EHR data used for research, and adjustment for the number of encounters may help to reduce this bias.15
In sensitivity analyses, we adjusted for the number of encounters, but did not observe meaningful differences in point estimates, perhaps partly because we already limited our sample to include only patients with two or more encounters. Additional biases may be induced by interacting with a health system. For example, selection bias could be induced by a patient receiving care in one part of a health facility instead of another, or a patient’s choice of a particular health system could induce information bias.15 We included all types of encounters in our analyses, so we do not expect that results are unduly influenced by the selection bias described. However, information bias (or differential data capture by group) is a potential limitation of our study. This bias might occur, for example, if AI patients sought most of their care through a different health system. Further, missing data may not be missing at random, a requirement of some statistical models. For example, missing data limited our assessment of BMI, SBP and DBP in our main models; and we found that younger patients, females, AIs and rural residents were more likely to have missing data. Accordingly, we observed some differences in associations when limiting the data to outpatient visits only and using the imputed BMI and blood pressure estimates compared with excluding these variables. These differences should be interpreted cautiously due to model and sample differences, and the high proportion of missing data for the imputation. Finally, we lacked education level and APOE genotype data, both important ADRD risk factors. Overall, these biases may affect generalization of our findings to the underlying population of AI adults, but we expect they have less of an effect on our comparisons between AI and NHW patients within the same health system.
Conclusion
As the population at risk of ADRD grows in size and diversity, the need for ADRD research in subpopulations is critical for future planning, prevention and treatment efforts. This EHR study in a population greatly underrepresented in ADRD research avoids some of the challenges of participant recruitment and retention common in population research. Even after applying stringent exclusion criteria, we had large numbers of AIs with longitudinal data, few missing demographic variables and we were able to measure important risk factor associations with ADRD in AIs. Little is known about ADRD in racial/ethnic minorities, particularly AI adults. The National Plan for Alzheimer’s Disease prioritized including health disparities groups such as AIs in ADRD research, yet several years later, few studies have been published.3 Our study is the first to examine ADRD in AIs across a large geographical region in southwest United States and report relationships between risk factors and ADRD in AI.
With the exception of being female and marital status, we found that ADRD risk factors were generally similar between AI and NHW patients, though the adjusted risk of ADRD was approximately 49% higher in AI patients in this regional health network. As one of the largest EHR analyses of ADRD in AI to date, our study is an important step in determining the ADRD burden in the AI population and regional community. Future efforts include confirmation of our findings in another sample, and linkage of EHR data in AI cohorts with behavioral data. This linkage would allow investigation of modifiable lifestyle factors, in addition to clinical factors, in ADRD risk determination.
Acknowledgments / Ethical Standards
This research is supported by the National Institute on Aging under award numbers P30AG059295, P30AG15297 and P30AG019610; by the National Center for Minority Health and Health Disparities under award number P60MD000507; and the state of Arizona (Reiman, PI).
Research involving Human Participants: The Washington State University institutional review board reviewed and approved all study procedures. This study met regulatory guidelines for expedited review, and was granted a waiver of informed consent, per the code of federal regulations for records research, 45CFR46.116(d)(3). All study procedures were in accordance with the ethical standards of the IRB and the Helsinki Declaration of 1975, as revised in 2000.
Informed consent: not applicable
References
- 1.Matthews KA, Xu W, Gaglioti AH, et al. . Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17-24. 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services National Plan to Address Alzheimer’s Disease.(2012). Last accessed July 7, 2020 from https://aspe.hhs.gov/national-plan-address-alzheimers-disease.
- 3.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72-83. 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- 4.Hendrie HC, Hall KS, Pillay N, et al. . Alzheimer’s disease is rare in Cree. Int Psychogeriatr. 1993;5(1):5-14. 10.1017/S1041610293001358 [DOI] [PubMed] [Google Scholar]
- 5.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37(1):61-81. 10.1146/annurev-publhealth-032315-021353 10.1146/annurev-publhealth-032315-021353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortreed SM, Cook AJ, Coley RY, Bobb JF, Nelson JC. Challenges and opportunities for using big health care data to advance medical science and public health. Am J Epidemiol. 2019;188(5):851-861. 10.1093/aje/kwy292 [DOI] [PubMed] [Google Scholar]
- 8.Haneuse S, Daniels M. A General framework for considering selection bias in EHR-based studies: what data are observed and why? EGEMS (Wash DC). 2016;4(1):1203. 10.13063/2327-9214.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rural Health Research Center RUCA Data: Zip Code RUCA Approximation. Last accessed July 7, 2020 from https://depts.washington.edu/uwruca/ruca-approx.php.
- 10.Danaher BG, Hart LG, McKay HG, Severson HH. Measuring participant rurality in Web-based interventions. BMC Public Health. 2007;7(1):228. 10.1186/1471-2458-7-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. 10.1016/S0140-6736(17)31363-6 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 12.Wiley LK, Shah A, Xu H, Bush WS. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20(4):652-658. 10.1136/amiajnl-2012-001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Center for Medicaid and Medicare Services Chronic Conditions Data Warehouse: Your Source for National CMS Medicare and Medicaid Research Data. (2018). Last accessed July 7, 2020 from https://www2.ccwdata.org/web/guest/home/
- 14.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. 10.1136/bmj.i969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184(11):847-855. 10.1093/aje/kww112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681-694. 10.1002/(SICI)1097-0258(19990330)18:63.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services, Centers for Medicare and Medicaid Services Emerging LTSS Issues in Indian Country: Alzheimer’s and Dementia. 2016. Last accessed July 7, 2020 from https://bit.ly/3ec6fLk
- 18.Garrett MD, Baldridge D, Benson W, Crowder J, Aldrich N. Mental health disorders among an invisible minority: depression and dementia among american Indian and alaska native elders. Gerontologist. 2015;55(2):227-236. 10.1093/geront/gnu181 [DOI] [PubMed] [Google Scholar]
- 19.Koller D, Bynum JP. Dementia in the USA: state variation in prevalence. J Public Health (Oxf). 2015;37(4):597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langa KM, Larson EB, Crimmins EM, et al. . A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51-58. 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Disparities in premature deaths from heart disease—50 States and the District of Columbia, 2001. MMWR Morb Mortal Wkly Rep. 2004;53(6):121-125. [PubMed] [Google Scholar]
- 22.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30(3):421-442. 10.1016/j.cger.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55(9):809-815. 10.1001/archpsyc.55.9.809 [DOI] [PubMed] [Google Scholar]
- 24.Kukull WA, Higdon R, Bowen JD, et al. . Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737-1746. 10.1001/archneur.59.11.1737 [DOI] [PubMed] [Google Scholar]
- 25.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538. 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plassman BL, Langa KM, Fisher GG, et al. . Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125-132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018;136(6):873-885. 10.1007/s00401-018-1908-x 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley RF, Waller M, Masters CL, Dobson A. To what extent does age at death account for sex differences in rates of mortality from alzheimer disease? Am J Epidemiol. 2019;188(7):1213-1223. 10.1093/aje/kwz048 [DOI] [PubMed] [Google Scholar]
- 29.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685-691. 10.1001/archpsyc.62.6.685 [DOI] [PubMed] [Google Scholar]
- 30.Mayeda ER. Examining sex/gender differences in risk of alzheimer disease and related dementias-challenges and future directions. Am J Epidemiol. 2019;188(7):1224-1227. 10.1093/aje/kwz047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weden MM, Shih RA, Kabeto MU, Langa KM Secular trends in dementia and cognitive impairment of u.s. rural and urban older adults. Am J Prev Med. 2018;54(2):164-172. https://doi.org/ 10.1016/j. amepre.2017.10.021 PMID:29246677 [DOI] [PMC free article] [PubMed]
- 32.Abner EL, Jicha GA, Christian WJ, Schreurs BG. Rural-urban differences in Alzheimer’s disease and related disorders diagnostic prevalence in Kentucky and West Virginia. J Rural Health. 2016;32(3):314-320. 10.1111/jrh.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowie MR, Blomster JI, Curtis LH, et al. . Electronic health records to facilitate clinical research. Clin Res Cardiol. 2017;106(1):1-9. 10.1007/s00392-016-1025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storandt M, Morris JC. Ascertainment bias in the clinical diagnosis of Alzheimer disease. Arch Neurol. 2010;67(11):1364-1369. 10.1001/archneurol.2010.272 [DOI] [PMC free article] [PubMed] [Google Scholar]