Abstract
Objective
To assess the predicted performance of the American College of Obstetrics and Gynecology (ACOG)’s recommended endometrial thickness (ET) of ≥4mm via transvaginal ultrasound (TVUS) for a simulated cohort of US Black women with postmenopausal bleeding (PMB).
Main Outcome Measure
Performance characteristics of 3+, 4+, and 5+mm ET thresholds were assessed including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Receiver Operator Characteristic (ROC) curves, and the area under the curve (AUC).
Methods
We used endometrial cancer parameters from ET studies upon which guidelines are based, as well as documented population characteristics of US Black women, to simulate a cohort of US Black women with PMB. Annual endometrial cancer (EC) prevalence overall and by histology type (I and II), history and current diagnosis of uterine fibroids, and visibility of endometria were estimated. Sensitivity analyses were performed to assess performance changes with quality of baseline parameters and impact of fibroids on ET visibility.
Results
In the main model with the 4+mm recommended threshold, TVUS ET showed a sensitivity of 47.5% (95% CI: 46.0-49.0%); specificity of 64.9% (95% CI: 64.4-65.3%); PPV of 13.1% (95% CI: 12.5-13.6%); NPV of 91.7% (95% CI: 91.4-92.1%), and AUC of .57 (95% CI: .56-.57).
Conclusions
Among a simulated cohort of US Black women, the recommended 4+mm ET threshold to trigger diagnostic biopsy for EC diagnosis performed poorly, with more than 50% of cases missed and an 8-fold higher frequency of false negative results than reported for the general population.
Keywords: Endometrial Cancer, Health Care Disparities, African American, Quality of Health Care, Cancer Early Detection, Ultrasonography
Introduction
Endometrial cancer (EC) is the 4th most common cancer in the United States with an estimated 61,880 newly diagnosed cases and 12,160 cancer deaths in 2019.1 After adjustment for the underlying high prevalence of hysterectomy among US women, the incidence rate of EC increases by 60%2 and is higher among Black women aged >50 years (99.2/100,000) than among White women (88.4/100,000).3 The racial disparity in mortality is marked, with a more than 90% higher rate of mortality among Black women compared to White women.2,4 There are several components to this disparity, including both higher likelihood of advanced stage at diagnosis among Black women and a larger proportion of high-risk EC (non-endometrioid histology, or “Type II”) among Black women compared with White women.5,6
Among women with endometrial cancer diagnoses, around 90% experienced postmenopausal bleeding (PMB) before their diagnosis, making this a good indicator for further diagnostic testing.7-11 Currently, the American College of Obstetrics and Gynecology (ACOG) recommends one of three strategies in the event a woman presents with PMB – an endometrial biopsy, a uterine dilation and curettage (D&C), or the use of transvaginal ultrasound (TVUS) to measure endometrial thickness (ET) in order to determine if either biopsy or D&C are necessary. Emphasis in the guidelines, clinical care, and prior studies is made for the use of TVUS to avoid unnecessary invasive procedures among women with PMB, with reported 99-100% negative predictive value of this strategy.7,12 Only if the ET meets a specific threshold, recommended as >4mm by ACOG, would these women be further tested by endometrial biopsy for diagnostic confirmation of endometrial cancer.7 If not, no such biopsy is performed.
Although these guidelines and ET threshold are based on several large population-based studies from Scandinavia,13,14 Italy,15 and Hong Kong,16 these data may be suboptimal sources from which to base guidelines for Black women for two reasons. Women with fibroids are underrepresented via exclusions,16 underrepresentation,13,15 or going unreported.14 The rationale to exclude women with uterine leiomyoma (fibroids) is that the presence of these lesions can distort the ability to measure ET, yet fibroids are up to 80% prevalent among Black women.17 In addition, the ET threshold has differential performance by EC histology type, with less accuracy for Type II cancers.18-20 In the United States, among Black women with EC, approximately 30% are diagnosed with non-endometroid Type II EC, a frequency 3-fold higher than all other groups of women.2,6 These TVUS data upon which guidelines are established may not accurately represent the performance of TVUS screening for PMB among a cohort of US Black women.
In light of the consistent pattern of Black women diagnosed at advanced stage EC, despite insurance and health care access,6 we sought to investigate the current performance of ACOG recommended TVUS ET thresholds for biopsy among a simulated cohort of symptomatic women.
Methods
The goal of this analysis was to assess the hypothetical performance of different ET thresholds in detecting EC among US postmenopausal Black women aged ≥45 years. First, we created a dataset representative of Black women aged ≥45 years with PMB in the United States. Case counts were identified from the Surveillance, Epidemiology, and End Results (SEER) national cancer registry SEER-18) dataset from 2012-2016 using SEER*Stat and were averaged to estimate the mean number of EC cases in any given year with the assumption that case counts were relatively stable during this time period.21
An average incidence rate was calculated from this data using the SEER base population averaged over those same years.21 This mean incidence rate was then multiplied by the average US Black female population aged ≥45 years (using the same 5-year range of 2012-2016)22 to estimate the number of incident cases in the United States in a year. Based on reported literature, we used the estimation that 10% of women with PMB have EC and used this ratio to calculate the total number of symptomatic Black women in a given year.7-10,23 Women were grouped into 5-year age groups. This process was conducted for overall EC counts and by histology type. Histology type was classified using traditional Type I and II EC given that these are the categories also used to report variation in ET by histology in the literature. Type I included endometrioid type and Type II included serous, carcinosarcoma, clear cell, and mixed types using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes. (Table 1)24
Table 1. List of ICD-O-3 codes included to calculate EC case counts3,25.
| EC Type | ICD-O-3 Code |
| Type I | |
| Endometroid | 8260, 8262, 8384, 8140, 8210, 8380, 8381, 8382, 8383, 8440, 8480, 8481, 8482, 8560, 8570, 8050 |
| Type II | |
| Serous | 8450, 8441, 8460, 8461 |
| Carcinosarcoma | 8950, 8951, 8980, 8981 |
| Clear cell | 8313, 8310 |
EC, endometrial cancer.
We used data from four previous studies upon which the guidelines were developed as well as an additional article from our comprehensive literature search to determine the value parameters for ET measurements. These studies included women who were recruited in Scandinavia,13,14 Italy,15 Hong Kong,16 and Jamaica.20 Using results from TVUS, ET was grouped into categories of <3, 3, 4, 5, and >5mm for each study based on histology type (I or II). We added the fifth study as it specifically focused on Black (Jamaican) women who were not represented by the four primary studies. Average proportions were calculated for each ET category from the five reference studies and we calculated the proportional distribution of women with EC falling into each of these categories for our simulated cohort. This process was repeated for the proportion of women not assigned to have EC.
The estimated proportion of women with a history of fibroids and those who would have a new diagnosis of fibroids was calculated from the National Institute of Environmental Health Sciences uterine fibroid study to determine the overall fibroid prevalence for this simulated population.17 As population data indicated that a history of fibroids increases the risk of EC overall by 42% and the risk of Type II EC three-fold over Type I,25 we incorporated these associations into assignment of EC in the simulated population to account for this correlation between fibroids and EC.
Visibility of ET on TVUS was assigned as a binary yes/no based on the estimated proportion of visible endometrium of women with and without fibroids (50.6% vs 63.7%, respectively).26 Lists of parameters used to generate the simulated dataset are shown (Table 2, Table 3).
Table 2. Parameters for cohort simulation of US Black women with postmenopausal bleeding.
| Parameter | Value/Range | Reference(s) |
| Average annual population of Black women in US (2012-2016) | 8,213,830 | 23 |
| Average annual # women with EC overall (2012-2016), main model | 4,462 | 21 |
| Type I | 2,848 | |
| Type II | 1,614 | |
| % women with EC with PMB | 90 | 7-10, 24 |
| ET distribution for EC Type I (average %) – main model | 7, 13-16, 20 | |
| <3mm | 0.6 | |
| 3mm | 5.4 | |
| 4mm | 5.8 | |
| 5mm | 5.63 | |
| >5mm | 82.63 | |
| ET distribution for EC Type II (average %) | 18, 19 | |
| <3mm | 9.2 | |
| 3mm | 9.2 | |
| 4mm | 9.2 | |
| 5mm | 19.8 | |
| 5+mm | 52.6 | |
| ET distribution for women without EC (average %) – main model | 7, 13-16, 20 | |
| <3mm | 15.5 | |
| 3mm | 18.5 | |
| 4mm | 17.4 | |
| 5mm | 10.5 | |
| >5mm | 38.1 | |
| % Postmenopausal women with history of fibroids | 74 | 17 |
| Relative risk of EC given history of fibroids | 1.42 | 26 |
| Relative risk of Type II vs Type I EC given history of fibroids | 3.00 | 26 |
| % women with current fibroids with fibroid history | 87% | 17 |
| % women with current fibroids without fibroid history | 60% | 17 |
| % of endometria visible without fibroids present | 63.7 | 27 |
| % of endometria visible with fibroids present | 50.6 | 27 |
PMB, postmenopausal bleeding; ET, endometrial thickness; EC, endometrial cancer
Table 3. Parameters for cohort simulation of US Black women with postmenopausal bleeding - Sensitivity Analysis.
| Parameter | Value/Range | Reference(s) |
| ET Distribution for EC Type I (Average %) | 7, 13-16, 20 | |
| <3mm | .7 | |
| 3mm | .5 | |
| 4mm | 1.0 | |
| 5mm | 2.4 | |
| >5mm | 95.4 | |
| ET Distribution for EC Type II (Average %) | 18, 19 | |
| <3mm | 9.2 | |
| 3mm | 9.2 | |
| 4mm | 9.2 | |
| 5mm | 19.8 | |
| 5+mm | 52.6 | |
| ET Distribution for Women without EC (Average %) | 7, 13-16 | |
| <3mm | 16.8 | |
| 3mm | 19.0 | |
| 4mm | 17.7 | |
| 5mm | 8.5 | |
| >5mm | 38.1 |
ET, endometrial thickness; EC, endometrial cancer.
We completed two sensitivity analyses: first, given the overall lower quality of data (based on sample size) from the lone study that included Black women, we repeated the modeling using only the larger population-level estimates. Additionally, given the strong influence in the models of ET visibility in postmenopausal women with fibroids and the fact that this threshold is based on a published abstract but not full report, we varied this parameter of ET visibility with fibroids from the reported 50%26 to more generous estimates of 70% and 80%, and without fibroids from 63.7% to 83.1% and 93.1% (maintaining the relative difference in those with and without fibroids found by Rotenberg, et al).26
Statistics
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), receiver operating characteristic (ROC) curves, and area under the curve (AUC) were calculated using Stata 15.127 to assess the theoretical performance of ET measurement cut points of ≥3 (“3+”), ≥4 (“4+”), and ≥5 (“5+”) mm in identifying EC overall among Black women.
Results
Values used to parameterize the simulated cohort of US postmenopausal Black women with vaginal bleeding are described in Table 2. The estimated case count of endometrial cancer in any given year was 4,462 with a corresponding estimated prevalence of PMB among Black women in any given year of 44,611. Of these EC cases, 2,848 were EC Type I and 1,614 were Type II. A larger proportion of women with Type II EC had thinner endometria than those with Type I (Table 2). A total of 33,012 women were assigned a history of uterine fibroids; 2,086 were EC Type I and 1,491 were Type II. Of those women with a history of fibroids, 3,112 women with EC (1,815 Type I and 1,297 Type II) and 25,608 women without EC were assigned to currently have fibroids. ET visibility among women with fibroids was distributed as follows: 1,841 with EC (1,148 Type I, 694 Type II) and 16,210 without EC had visible endometria. ET visibility among women without fibroids was distributed as follows: 522 with EC (367 Type I, 155 Type II) and 5,168 without EC had visible endometria.
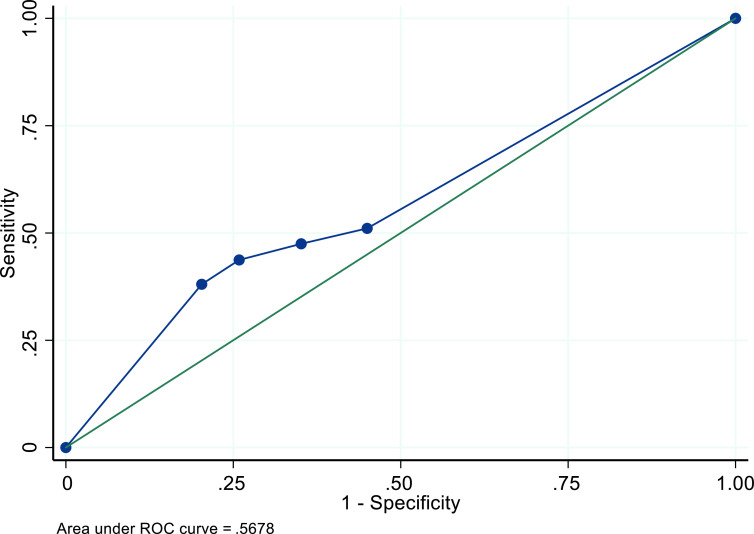
In the main model, among all women with all cancer types, with and without the presence of fibroids, and with and without visible endometria, the recommended TVUS ET threshold of 4+mm resulted in a sensitivity of 47.5% (95%CI: 46.0-49.0%); specificity of 64.9% (95% CI: 64.4-65.3%); PPV of 13.1% (95% CI: 12.5-13.6%); NPV of 91.7% (95% CI: 91.4-92.1%); and AUC of .57 (Table 4). The ROC curve is displayed in Figure 1. A threshold of 3+mm resulted in slightly increased sensitivity and a decrease in specificity, PPV, and NPV. The 5+mm threshold increased specificity, PPV, and NPV at the cost of decreased sensitivity (Table 4).
Table 4. Performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding – main model.
| Threshold | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) |
| Main Model | |||||
| 3+ mm | 51.1% (49.6-52.6%) | 55.0% (54.5-55.5%) | 11.2% (10.8-11.6%) | 91.0% (90.6-91.4%) | .57 (.56-.57) |
| 4+ mm | 47.5% (46.0-49.0%) | 64.9% (64.4-65.3%) | 13.1% (12.5-13.6%) | 91.7% (91.4-92.1%) | - |
| 5+ mm | 43.7% (42.3-45.2%) | 74.1% (73.7-74.5%) | 15.8% (15.2-16.5%) | 92.2% (91.9-92.5%) | - |
TVUS, transvaginal ultrasound; ET, endometrial thickness; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval
Figure 1. ROC curve for performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding - Main Model.
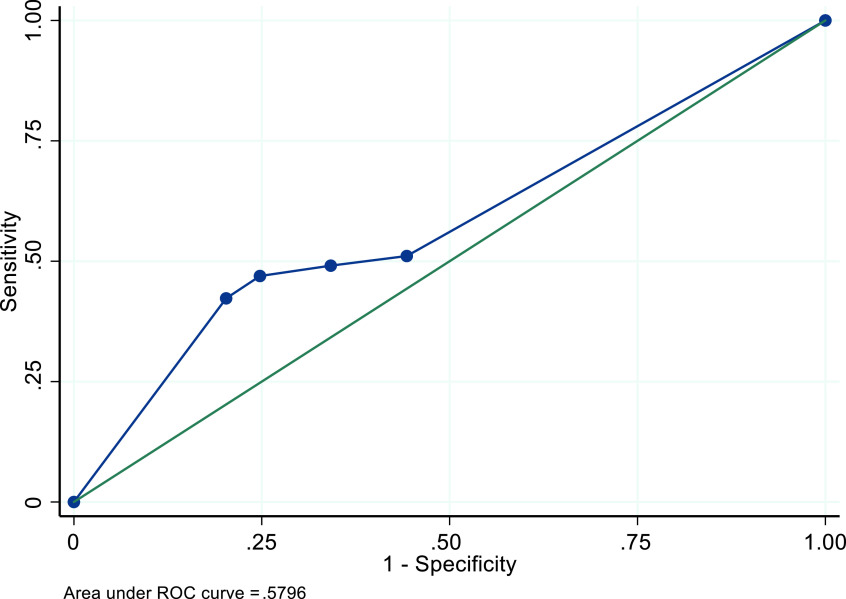
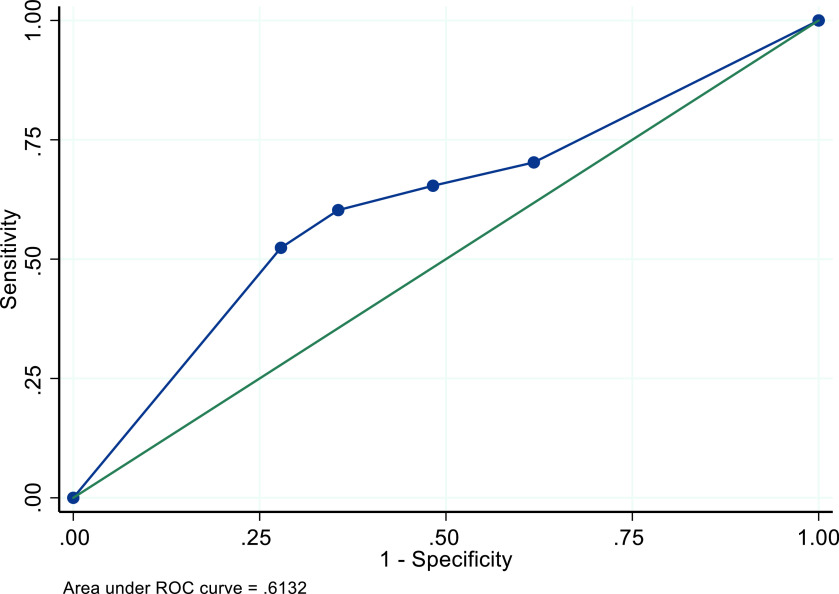
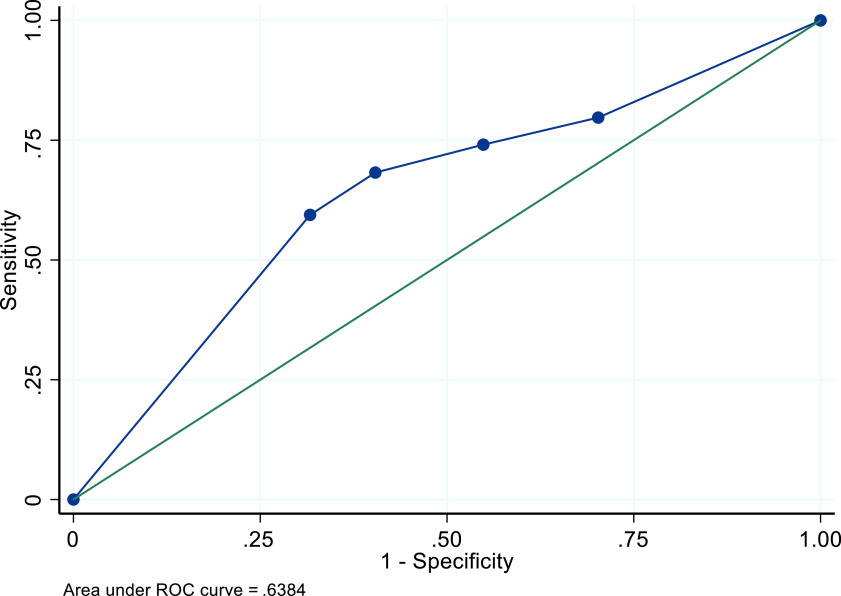
In the sensitivity analysis for which we excluded parameters from the study of Black Jamaican women, the recommended 4+mm threshold performed slightly better across all four performance characteristics, with the most increase in sensitivity performance (49.1%, 95% CI: 47.6-50.6%). The AUC remained approximately the same at .58 (Table 5, Figure 2). The sensitivity analysis for which we varied the ET visibility to 70.6% among women with fibroids and 83.1% among women without fibroids resulted in an increase of all performance characteristics, particularly for sensitivity (65.4%, 95% CI: 51.7-52.2%) (Figure 3). Increasing ET visibility to 80.6% and 93.1% among women with and without fibroids, respectively, resulted in even better performance (sensitivity = 74.1%, 95% CI: 72.8-75.4%) and an NPV of 94.0% (95 CI: 93.7-94.3%, Table 5) (Figure 4).
Table 5. Performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding – sensitivity analyses.
| Threshold | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) | |
| Excluding parameters from Jamaican women study | ||||||
| 3+ mm | 51.1% (49.6-52.6%) | 55.7% (55.2-56.2%) | 11.4% (10.9-11.8%) | 91.1% (90.7-91.5%) | .58 (.57-.58) | |
| 4+ mm | 49.1% (47.6-50.6%) | 65.8% (65.4-66.3%) | 13.8% (13.2-14.3%) | 92.1% (91.8-92.4%) | - | |
| 5+ mm | 46.9% (45.5-48.4%) | 75.2% (74.8-75.7%) | 17.4% (16.7-18.1%) | 92.7% (92.4-93.0%) | - | |
| Using 70.6% and 83.1% visibility with and without fibroids | ||||||
| 3+ mm | 70.3% (68.9-71.6%) | 38.2% (37.7-38.7%) | 11.2% (10.9-11.6%) | 92.0% (91.6-92.4%) | .61 (.61-.62) | |
| 4+ mm | 65.4% (64.0-66.8%) | 51.7% (51.2-52.2%) | 13.1% (12.6-13.5%) | 93.1% (92.7-93.4%) | - | |
| 5+ mm | 60.3% (58.8-61.7%) | 64.5% (64.0-64.9%) | 15.9% (15.3-16.4%) | 93.6% (93.3-93.9%) | - | |
| Using 80.6% and 93.1% visibility with and without fibroids | ||||||
| 3+ mm | 79.7% (78.5-80.9%) | 29.8% (29.3-30.2%) | 11.2% (10.9-11.6%) | 93.0% (92.5-93.4%) | .64 (.63-.64) | |
| 4+ mm | 74.1% (72.8-75.4%) | 45.1% (44.6-45.6%) | 13.0% (12.6-13.5%) | 94.0% (93.7-94.3%) | - | |
| 5+ mm | 68.2% (66.9-69.6%) | 59.6% (59.1-60.1%) | 15.8% (15.3-16.3%) | 94.4% (94.1-94.7%) | - | |
TVUS, transvaginal ultrasound; ET, endometrial thickness; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval
Figure 2. ROC curve for performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding - excluding model parameters of Jamaican women.
Figure 3. ROC curve for performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding – increased visibility of 70.6% and 83.1% among those with and without fibroids.
Figure 4. ROC curve for performance of TVUS ET thresholds for identifying endometrial cancer among a simulated cohort of US Black women with postmenopausal bleeding – increased visibility of 80.6% and 93.1% among those with and without fibroids.
Discussion
In an effort to assess if the ACOG-recommended threshold is useful for identifying Black women who should be further evaluated for endometrial cancer, we created a simulated cohort of the estimated population of US Black women with PMB, a much larger cohort than was included in any of the studies upon which guidelines were developed. When we applied parameters derived from these four studies (and included parameters from the single study of ET measurements among Black Jamaican women)13-16,20 to our Black cohort, we found that the 4mm recommended threshold performed very poorly – more than 50% of Black women with endometrial cancer were categorized as false negatives by either an inability to visualize the endometrium or incorrect assignment into the non-cancer categorization with a measured endometrium. The interpretation of AUC ranges from 1.00, a test with perfect performance, to .50, a test that has no discriminating power. The calculated AUC for the ROC curve for the 4mm threshold was .57, indicating poor performance. Our results suggest that ACOG’s recommendation for TVUS as a primary evaluation for endometrial cancer likely disproportionately underperforms for Black women.
Sensitivity analyses with increasing proportions of visible, and therefore measurable, endometria among women with and without fibroids resulted in increases in performance for the 4+mm ET threshold. If visibility among Black women is higher than hypothesized in our main model (based on a single study among Black women),26 it is possible that the 4+mm threshold does perform better than we would expect. However, even if the visibility of ET is approximately 80% among Black women with fibroids and 93% among Black women without fibroids, quite high proportions, the diagnostic test would still miss more than a quarter of endometrial cancer cases among Black women.
TVUS as a strategy to screen and avoid unnecessary endometrial biopsy is based on a high likelihood of ET visibility and a low penalty if the test is wrong – delay in diagnosis of a relatively low-risk cancer. When considering the overall US population, low-risk Type I EC predominates and is known to have an indolent course. For Black women, however, nearly a third have aggressive Type II EC and more than 50% have high-risk EC (including high-grade Type I) at the time of diagnosis.2,6 In addition, the extraordinarily high prevalence of fibroids among Black women compromises the accuracy of the screening measure itself – endometrial thickness.17 Small differences in the performance of this clinical strategy have a large impact on Black women at risk for EC. On an individual level, more than 50% of Black women with EC would either screen negative – not requiring a biopsy – or not be served (inability to visualize ET) by a TVUS first strategy. Although guidelines recommended endometrial sampling (biopsy or D&C) if ET is not visualized,7 it is unknown with what fidelity this occurs, especially in the setting of a TVUS finding of fibroids, another common cause of abnormal vaginal bleeding. A TVUS first strategy may introduce further opportunity for care delay and care gaps for this at-risk group, given that prior work suggests Black women themselves are at risk of misattribution of abnormal bleeding to fibroids28 and Black women are less likely to receive guideline concordant diagnostic procedures prior to an EC diagnosis.8
In this analysis, 8.3% of Black women would screen negative (ET visualized, no biopsy required) when they in fact have EC. Although a modest false negative rate may seem appropriate, this test functions as a gateway to definitive diagnosis through an office-based biopsy. This false-negative rate is not acceptable when Black women are at the highest risk for aggressive, fast-growing EC in the population.
Study Limitations
Our study has important limitations to consider. Our model was parameterized by necessity with several distributions that were drawn from studies of women who were not Black, perhaps most importantly the distribution of ET measurements across our age groups. We had to assume that these distributions were reflective of the actual distributions of Black women, which means the accuracy of the performance of ET threshold in our models may be an overestimation if Black women actually have thinner endometria and an underestimation if Black women actually have thicker endometria. Our endometrial cancer case counts were drawn from the SEER-18 database and extrapolated to the entire United States, which is the standard method by which cancer statistics are reported by the US government29 and relies on the deliberate sampling strategy of SEER to represent all racial/ethnic groups, rural/urban variation, and socioeconomic strata.30 Finally, several parameters, including history of fibroids, concurrent presence of fibroids, and visibility of ET among Black women were based, by necessity, on a few studies17,25,26 and might not be generalizable to a larger population. Despite these limitations, we strove to create as accurate a representation of postmenopausal symptomatic Black women in the United States as was possible, given the data available, by including the only study of TVUS ET performance among Black women and using sensitivity analyses to vary the most impactful parameters to the model.
Conclusion
Racial inequity in a health outcome is often complex and multi-layered, as it represents one symptom of the root problem of differential value assigned to life by racial categorization in the United States. To achieve equity, we must disentangle these multiple influences that overlap to create vulnerability and poor outcomes. In this case, our analysis suggests that the guideline-recommended strategy of TVUS screening to determine appropriateness for endometrial biopsy among women with postmenopausal bleeding is not optimized for Black women. These findings support the need for a prospective cohort study to evaluate both current guidelines and alternative innovative strategies to screen symptomatic postmenopausal Black women at risk of EC.
Acknowledgments
This work was supported by funding from the Robert Wood Johnson Foundation. SR would like to acknowledge her positionality and potential bias as a White woman.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometroid cancers. J Clin Oncol. 2019;37(22):1895-1908. 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll KM, Winn AN. Assessing endometrial cancer risk among US women: long-term trends using hysterectomy-adjusted analysis. Am J Obstet Gynecol. 2019;221(4):318. e1-318.e9. https://doi.org/ 10.1016/j ajog.2019.05.024 PMID:31125544 [DOI] [PubMed]
- 4.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. 10.3322/caac.21555 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
- 5.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1407-1415. 10.1158/1055-9965.EPI-15-0316 [DOI] [PubMed] [Google Scholar]
- 6.Doll KM, Snyder CR, Ford CL. Endometrial cancer disparities: a race-conscious critique of the literature. Am J Obstet Gynecol. 2018;218(5):474-482.e2. 10.1016/j.ajog.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists ACOG committee opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131(5):e124-e129. 10.1097/AOG.0000000000002631 [DOI] [PubMed] [Google Scholar]
- 8.Doll KM, Khor S, Odem-Davis K, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol. 2018;219(6):593. e1-593.e14. https://doi.org/ 10.1016/j ajog.2018.09.040 PMID:30291839 [DOI] [PubMed]
- 9.Coates RJ, Click LA, Harlan LC, et al. Differences between black and white patients with cancer of the uterine corpus in interval from symptom recognition to initial medical consultation (United States). Cancer Causes Control. 1996;7(3):328-336. 10.1007/BF00052938 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20(10):1025-1036. 10.7863/jum.2001.20.10.1025 [DOI] [PubMed] [Google Scholar]
- 11.Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(9):1210-1222. 10.1001/jamainternmed.2018.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long B, Clarke MA, Morillo ADM, Wentzensen N, Bakkum-Gamez JN. Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: systematic review and meta-analysis. [published online January 30, 2020]. Gynecol Oncol. 2020;157(3):624-633. 10.1016/j.ygyno.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 13.Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;188(2):401-408. 10.1067/mob.2003.154 [DOI] [PubMed] [Google Scholar]
- 14.Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172(5):1488-1494. 10.1016/0002-9378(95)90483-2 [DOI] [PubMed] [Google Scholar]
- 15.Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S, Dordoni D. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7(5):315-321. 10.1046/j.1469-0705.1996.07050315.x [DOI] [PubMed] [Google Scholar]
- 16.Wong AS, Lao TT, Cheung CW, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG. 2016;123(3):439-446. 10.1111/1471-0528.13342 [DOI] [PubMed] [Google Scholar]
- 17.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107. 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101(1):120-125. 10.1016/j.ygyno.2005.09.042 [DOI] [PubMed] [Google Scholar]
- 19.Billingsley CC, Kenne KA, Cansino CD, et al. The use of transvaginal ultrasound in type II endometrial cancer. Int J Gynecol Cancer. 2015;25(5):858-862. 10.1097/IGC.0000000000000423 10.1097/IGC.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 20.Phillip H, Dacosta V, Fletcher H, Kulkarni S, Reid M. Correlation between transvaginal ultrasound measured endometrial thickness and histopathological findings in Afro-Caribbean Jamaican women with postmenopausal bleeding. J Obstet Gynaecol. 2004;24(5):568-572. 10.1080/01443610410001722671 10.1080/01443610410001722671 [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence – SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, November 2018. (1975-2016 varying) Sub-Linked to County Attributes – Total US, 1969-2017 Counties. Released April 2019; based on November submission, and available from www.seer.cancer.gov.
- 22.United States Census Bureau , Population Division. Annual Estimates of the Resident Population by Sex, Age, Race, and Hispanic Origin for the United States and States: April 1, 2010 to July 1, 2017. Washington, D.C.: U.S. Census Bureau; 2018. Last accessed February 24, 2020 from https://factfinder.census.gov/faces/tableservices/jsf/pages/productview. xhtml?src=bkmk.
- 23.Musonda P, Burbos N, Duncan TJ, Crocker SG, Morris EP, Nieto JJ. Comparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):433-438. 10.1016/j.ejogrb.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Fritz A, Percy C, Jack A, et al., eds. International Classification of Diseases for Oncology (ICD-O). 1st rev 3rd ed. Geneva, Switzerland: WHO Press; 2013, https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf?sequence=1&isAllowed=y. Accessed February 24, 2020. [Google Scholar]
- 25.Wise LA, Sponholtz TR, Rosenberg L, et al. History of uterine leiomyoma and risk of endometrial cancer in black women. Cancer Causes Control. 2016;27(4):545-552. 10.1007/s10552-016-0728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotenberg O, Escobar P, Fridman D, Dar P. Factors associated with inconsistency in visibility of the endometrial echo on transvaginal ultrasound of postmenopausal women [ISUOG Education abstract P14.10]. Ultrasound Obstet Gynecol. 2017;50(suppl 1):199-200. 10.1002/uog.18140 [DOI] [Google Scholar]
- 27.Stata Statistical Software [computer program]. Version 15.1. College Station, TX: StataCorp; 2017.
- 28.Doll KM, Hempstead B, Alson J, Sage L, Lavallee D Assessment of Prediagnostic Experiences of Black Women With Endometrial Cancer in the United States. JAMA Netw Open. 2020;3(5):e204954. https://doi. org/ 10.1001/jamanetworkopen.2020.4954 PMID:32412636Accepted March 12th, 2020. MS#J NO19-4447R [DOI] [PMC free article] [PubMed]
- 29.Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 years. J Natl Cancer Inst. 2019;111(12):1279-1297. 10.1093/jnci/djz106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, Epidemiology, and End Results (SEER) database. JAMA Surg. 2018;153(6):588-589. 10.1001/jamasurg.2018.0501 [DOI] [PubMed] [Google Scholar]