Abstract
Background
Physical activity (PA) is important to maintaining functional independence. It is not clear how patterns of change in late-life PA are associated with contemporaneous changes in physical performance measures.
Methods
Self-reported PA, gait speed, grip strength, timed chair stand, and leg power were assessed in 3,865 men aged ≥ 65 years at baseline (2000–2002) and Year 7 (2007–2009). Group-based trajectory modeling, using up to four PA measures over this period, identified PA trajectories. Multivariate linear regression models (adjusted least square mean [95% confidence interval {CI}]) described associations between-PA trajectories and concurrent changes in performance.
Results
Three discrete PA patterns were identified, all with declining PA. Linear declines in each performance measure (baseline to Year 7) were observed across all three PA groups, but there was some variability in the rate of decline. Multivariate models assessing the graded response by PA trajectory showed a trend where the high-activity group had the smallest declines in performance while the low-activity group had the largest (p-for trend < .03). Changes in the high-activity group were the following: gait speed (−0.10 m/s [−0.12, −0.08]), grip strength (−3.79 kg [−4.35, −3.23]), and chair stands (−0.38 [−0.50, −0.25]), whereas changes in the low-activity group were the following: gait speed (−0.16 [−0.17, −0.14]), grip strength (−4.83 kg [−5.10, −4.55]), and chair stands (−0.53 [−0.59, −0.46]). Between-group differences in leg power trajectories across PA patterns were not significant.
Conclusions
Declines in functional performance were higher among those with lower PA trajectories, providing further evidence for the interrelationship between changes in PA and performance during old age.
Keywords: Physical activity, Patterns, Physical performance, Older men, Epidemiology
Maintaining function is central to both quality of life and longevity. More than half (52%) of the adults aged 65 years and older report limitations in walking, grasping, carrying, or pushing (1,2). Deficits in muscle strength, balance, and gait performance are common underlying traits among each of these limitations. Progression of such functional limitations is insidious and debilitating, and these limitations may increase vulnerability to a sedentary lifestyle and consequently exacerbate risk for future morbidity, dependency, hospitalization, and mortality (3–6).
Regular physical activity (PA) has been shown in numerous trials to help maintain physical functioning and slow down the physiological transitions of functional decline to overt disability, hypothetically, by preserving muscle strength, balance, and mobility (5,7,8). Previous findings indicate that baseline PA levels in older ages are not associated with greater risk of physical functioning decline later in life, and rather, cumulative activity versus activity measured at one time point may be a more important determinant of functioning over time in late life (9). Yet, there is a paucity of data that has prospectively demonstrated the joint association between changes in PA and changes in clinically measured physical performance measures (10), particularly among older men.
Using group-based trajectory models, we recently identified three discrete PA trajectory patterns, and their joint associations with changes in lean body mass (11) and subsequent mortality risk (12) in a large cohort of community-dwelling older men in the Osteoporotic Fracture in Men Study (MrOS). The purpose of the present study is to further examine the association between-PA trajectories measured in late life with contemporaneous changes in physical performance measures (ie, gait speed, grip strength, timed chair stands, leg power) over an average 7.1-year follow-up. We hypothesize that age-related losses in physical performance occurs at a slower rate among men who are engaged in- and maintain higher versus lower levels of PA over time.
Methods
Study Population
We studied participants enrolled in the Osteoporotic Fractures in Men (MrOS) Study, a prospective cohort study of community-dwelling older men. From 2000 to 2002, 5,994 community-dwelling men ≥ 65 years old were recruited from six geographic areas of the United States (13–15). Men with bilateral hip replacements and men who were unable to walk without the assistance of another person were not eligible for the study. All participants gave written informed consent, and the study was performed in accordance with the Declaration of Helsinki.
Physical Activity Measurements
PA was assessed by self-report using the Physical Activity Scale for the Elderly (PASE) questionnaire, which ascertains the intensity, frequency, and duration of a variety of activities over a period of 7 days. The PASE questionnaire includes walking; strenuous, moderate and light sports; muscle strength and endurance; occupational activities, including standing or walking; lawn work and gardening; caring for another person; home repairs; and heavy and light housework. The frequency and duration of each activity was multiplied by an empirically derived item weight and summed to compute the total PASE score. PASE scores have no units and provide a relative rather than absolute measure of PA levels. PASE has been previously validated with objective measures of PA and has high test–retest reliability (16). We used data from following study contacts: Baseline (Year 0) visit (2000–2002), Year 3.5 visit (sleep substudy in certain MrOS sites, 2003–2005), the Year 5 visit (all participants, 2005–2006), Year 7 visit (all participants, 2007–2009). Men who had returned to the Year 7 visit and had a PASE score and at least one clinical performance measures in Year 7, in addition to having at least one clinical performance measure and a PASE score for at least one of the prior study contacts were included in PA trajectory analysis (N = 3,891).
Analysis Sample
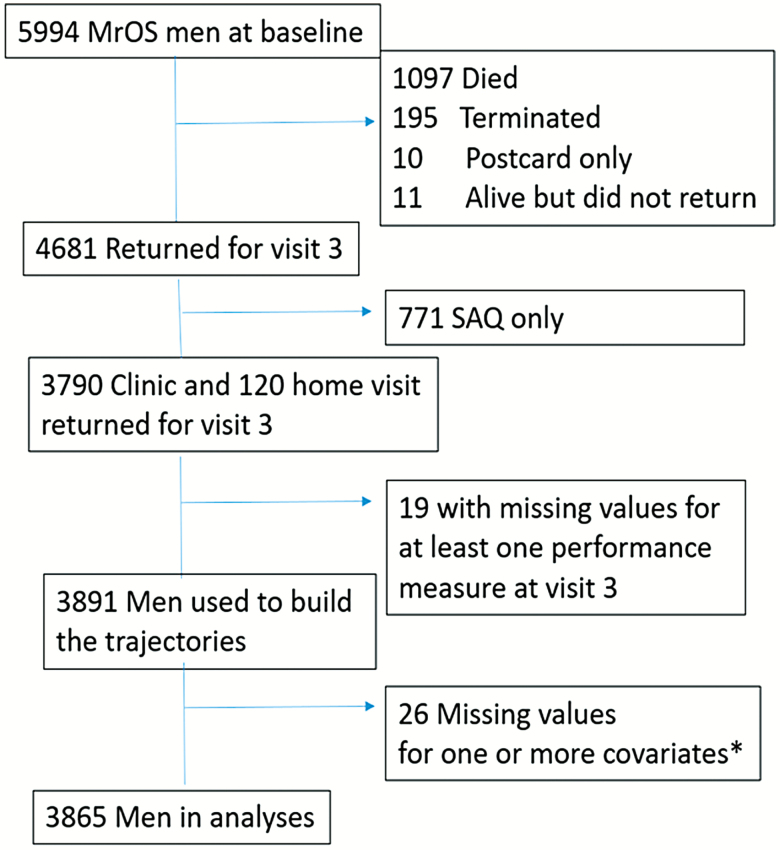
Current analyses included men in the trajectory analysis who did not have any missing covariates reported in the multivariate model (N = 3,865). Complete study flow diagram is shown in Figure 1.
Figure 1.
Flow chart describing MrOS sample subject characteristics using the 3,891 for physical activity trajectory building, and the final N = 3,865 for nonmissing values of adjusted covariates and Year 7 values of PASE score and at least one performance measure used in the final multivariate model for predicted change in performance. SAQ = short answer questionnaire. *Covariates included in multivariate model: age, race, clinic site, education, marital status, smoking, drinking, body mass index, self-rated health, Falls reported in the last year, SF12 mental health index, and comorbidity defined categorically (yes/no; hypertension, CVD, CHF, arthritis, diabetes).
Physical Performance Measurements
Physical performance was measured at baseline, Year 5, and Year 7 MrOS study visits with four tests: gait speed, grip strength, repeated chair stands, and leg extension power. Gait speed (meters per second) was assessed by measuring the time it took in seconds to complete a 6-m walking course performed at usual pace, using ambulatory aids as needed. The test was repeated, and the faster of the two measured times was included in the analysis. Grip strength, which measures voluntary muscle strength, was measured in kilograms (kg) using a Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL). Participants completed two trials for each hand, and the maximum effort across the trials was used for analyses. Chair stands were conducted to evaluate whether the participant was able to stand at least once, without using hands or arms, from a standard chair. Men who were able to stand once were asked to repeat the task five times, and the time to complete five stands was recorded to assess lower-extremity muscle strength and endurance and balance. Chair stand speed was calculated and used to create a variable equal to estimated number of chair stands per 10 seconds, with those who were unable to complete the test coded as zero. Leg extension power, which is highly correlated to other functional measures in older subjects (eg, chair-rising speed, stair-climbing speed and power, and walking speed) (17), was measured with the Nottingham power rig with nine trials for each leg as previously described (18). The maximum value (watts) from either leg was analyzed.
Other Covariates
At baseline, participants reported their age, race/ethnicity, education, marital status, smoking status, alcohol use, and self-rated health status. The SF-12 questionnaire was administered to assess self-rated mental and physical health status, with scores ranging from 0 to 100 points, with higher scores indicating better health status (19). Participants were asked to self-report a number of medical conditions, including hypertension, congestive heart failure, cardiovascular disease, diabetes, and arthritis and history of falls in the 12 months prior to the baseline visit. Weight was measured on a balance beam or digital scale, and height by wall-mounted stadiometers. Body mass index was calculated as weight (kg)/height2 (m2).
Statistical Methods
Group-based trajectory modeling was applied using PROC TRAJ in SAS to the repeated physical activity scores (PASE score) as previously described (11). Briefly, men who returned to the Year 7 visit, and who had completed at least one PASE questionnaire during the assessment period (baseline to Year 7 Visit) were included in the trajectory models, with age as the time scale. The maximum number of groups (trajectories) for each phenotype was decided a priori. A quadratic shape of the pattern of change per trajectory was specified for all models, as recommended by Jones et al. (2001) (20). Models with a different number of groups (minimum = 3) were compared using two times the change in the Bayesian Information Criterion (2DBIC). Models were assessed to ensure adequate sample size in each subgroup for assessing the risk of declining physical performance for each performance outcome. The final analytic model included three PA groups (Supplementary Table 1).
Distributions of posterior probabilities were evaluated to determine the internal reliability of trajectory groups. Plots of the mean estimated PA scores by age were used to visually confirm that trajectory analysis successfully grouped men with similar longitudinal patterns and that each PA patterns described in the trajectory classes were distinct from one another.
The simple linear change (from Baseline to Year 7) in gait speed (m/s) grip strength (kg), chair stands (n), and leg power were calculated. Least square means from multivariable linear regression models were calculated to demonstrate the association of PA trajectories with change in each performance measure and p values for the linear trend were reported. Characteristics of the participants were summarized by means and standard deviations (SD) for continuous variables and counts and percentages for categorical variables. Potential confounders at the baseline visit were selected a priori. Adjustment for covariates was considered for any measures or characteristics, or reported clinical or medical factor present at baseline that may potentially confound the relationship between PA and clinical performance, based on the previous literature (21,22). As such, all trajectory models were first minimally adjusted for baseline demographics (base model) including age, race, clinic site, and education. Multivariate models were adjusted for baseline demographics and smoking status, alcohol use, marital status, self-rated health, body mass index, presence of at least one comorbidity (yes/no), SF-12 mental health scores, and falls reported in the 12 months prior to visit 1. All p-values are two-tailed (α = 0.05). All analysis was performed using SAS 9.4.
Results
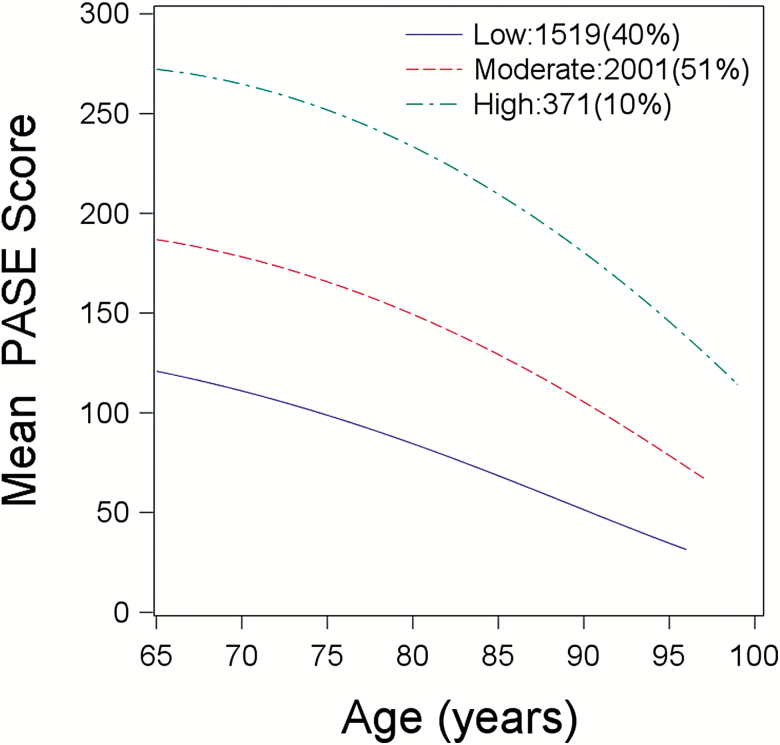
Three distinct patterns of PA change modeled over age were identified in this cohort, all characterized by declining activity, but distinguished by overall mean PA levels: “low-activity,” (N = 1,519), “moderate-activity,” (N = 2,001), and “high-activity,” (N = 371), representing 40%, 51%, and 10%, of the total sample, respectively. There was a decline over time in PA for each of the three patterns of PA change (pdifference between slopes = .003; Figure 2).
Figure 2.
Physical Activity Trajectories by Age; Total N = 3,891 men who returned to the Year 7 visit and had a PASE score and at least one clinical performance measures in Year 7, in addition to contributing at least one clinical performance measure and a PASE score during the physical activity assessment period (Baseline to Year 7 visit). Difference between slopes; p = .003.
Among men who were included in the analysis, those in the moderate and high-activity groups were shorter, weighed less, and were less likely to be current smokers; they reported having better physical, mental, and general health status and a lower prevalence of multiple comorbidity, compared to the low-activity group at baseline (Table 1; p < .01). Moreover, the men in high-activity PA trajectory group experienced smaller declines in grip strength (8.6%) and chair stands (10.0%) compared to those in the moderate-activity PA trajectory group (9.4% and 13.0%, respectively), whereas men in the lower-activity group experienced the greatest declines in grip strength (11.5%) and chair stand performance (20.0%). Similarly, linear declines in leg power were found across all three PA trajectories, with smaller declines reported among men in the high (15.0%) versus low (19.1%) PA trajectory.
Table 1.
Characteristics by Physical Activity Trajectory Over 7 Years in 3,865 MrOS Men at Baseline
| Cohort (N = 3,865) | Low Activity (N = 1,509) | Moderate Activity (N = 1,986) | High activity (N = 370) | p-Value* | |
|---|---|---|---|---|---|
| Age (years) | 72.44 ± 5.19 | 72.19 ± 5.20 | 72.53 ± 5.10 | 72.97 ± 5.6 | .02 |
| Weight (kg) | 83.59 ± 12.87 | 85.43 ±13.66 | 82.69 ± 12.18 | 80.92 ± 12.17 | <.0001 |
| Height (cm) | 174.62 ± 6.75 | 175.10 ± 6.93 | 174.41 ± 6.66 | 173.82 ± 6.31 | .0006 |
| Body Mass Index (kg/m2) | 27.38 ± 3.69 | 27.83 ± 3.95 | 27.15 ± 3.47 | 26.75 ± 3.56 | <.0001 |
| Caucasian | 3,489 (90.27) | 1,347 (89.26) | 1,808 (91.04) | 334 (90.27) | .22 |
| College Education | 2,192 (56.71) | 888 (58.85) | 1,099 (55.34) | 205 (55.41) | .101 |
| Married | 3,272 (84.66) | 1,246 (82.57) | 1,709 (86.05) | 317 (85.68) | .016 |
| Alcohol (none) | 1,235 (31.95) | 502 (33.27) | 607 (30.56) | 126 (34.05) | .22 |
| Alcohol (<7 drinks/wk) | 1,595 (41.27) | 620 (41.09) | 820 (41.29) | 155 (41.89) | |
| Alcohol (7+ drinks/wk) | 1,035 (26.78) | 387 (25.65) | 559 (28.19) | 89 (24.05) | |
| Current Smoking | 119 (3.08) | 59 (3.91) | 57 (2.87) | 3 (0.81) | .006 |
| SF-12 score | 55.99 ± 6.36 | 55.4 ± 6.82 | 56.44 ± 5.99 | 55.98 ± 6.17 | <.0001 |
| At least one comorbiditya | 2,738 (70.84) | 1,140 (75.55) | 1,374 (69.18) | 224 (60.54) | <.0001 |
| Self-reported health status (excellent/good vs fair/poor) | 3,449 (89.24) | 1,265 (83.83) | 1,828 (92.04) | 356 (96.22) | <.0001 |
| History of fall 12 mo prior to baseline | 742 (19.2) | 302 (20.01) | 354 (17.82) | 86 (23.24) | .031 |
| PASE score—baseline | 153.64 ± 67.12 | 105.33 ± 47.55 | 172.81 ± 50.05 | 247.76 ± 63.68 | <.0001 |
| PASE score—Year 7 | 130.14 ± 68.92 | 78.80 ± 43.78 | 149.56 ± 50.39 | 235.33 ± 65.99 | <.0001 |
| PASE score—change | −23.5 ± 69.19 | −26.53 ± 62.85 | −23.25 ±70.35 | −12.46 ± 84.88 | .0005 |
Note: LEP = leg extension power; PASE = Physical Activity Scale for the Elderly (PASE).
aComorbidities: Hypertension, CVD, Congestive Heart Failure, arthritis, diabetes.
*p-values indicate the overall difference among PA trajectories, calculated using chi-squared tests for categorical variables and ANOVA for continuous variables. Significant at p < .05.
Of note, PA levels, gait speed, grip strength, chair stand performance, and leg power were significantly higher at baseline for men included in the study compared to those who were not included (all p < .0001; data not shown).
At baseline, men in the high-activity PA trajectory group had higher PA levels and greater gait speed and grip strength, better chair stand performance (ie, ability to perform and faster time among those who were able to do the test), and leg power, while those in the low-activity trajectory group had lower levels of PA and lower scores in each performance measure (Supplementary Table 2). PA and each physical performance outcome declined over time across each of the three patterns of PA change. Overall, gait speed performance declined by 0.13 m/s (absolute percent change, 10.5%) over 7 years, but the decline was attenuated among those with higher versus lower PA trajectory, varying from 0.11 m/s (8.5%) in the high PA group to 0.15 m/s (12.3%) in the low PA group.
Patterns of PA change were generally associated with change in performance in unadjusted models with minimal attenuation seen between the base model (adjusted for age, race, and clinic site) and the multivariate models, and a linear reduction in risk by PA trajectory within unadjusted, base, and multivariate models (Table 2). Compared to men in the low-activity group, men in the moderate-activity and high-activity groups had a lower risk of declining in gait speed, and grip strength, in both base and multivariate models. Similarly, patterns of PA change were also associated with poor chair stand performance, with men in the high-activity trajectory group predicted to have better chair stands performance, despite a nominal increase in risk association after adjustment for covariates, while men in the low-activity group demonstrated worse performance. Furthermore, men in the moderate- and high-activity groups had a lower risk of declining leg power than those in the low-activity group; however, risk associations were attenuated and between-PA group differences in risk were not significant after multivariate adjustment.
Table 2.
The Association Between Physical Activity Trajectories and Change in Physical Performance Measures from Baseline to Year 7 Presented as Least Squares Mean
| Least Squares Mean Estimate (95% CI) | PA Trajectory Group | Unadjusted Model | Base Modela | Multivariateb Model |
|---|---|---|---|---|
| Gait speed (m/s) | Low activity | −0.15 (−0.17, −0.14) | −0.16 (−0.17, −0.15) | −0.16 (−0.17, −0.14) |
| Moderate activity | −0.12 (−0.13, −0.11) | −0.12 (−0.13, −0.11) | −0.12 (−0.13, −0.11) | |
| High activity | −0.10 (−0.13, −0.08) | −0.10 (−0.12, −0.08) | −0.10 (−0.12, −0.08) | |
| p-trend | <.0001 | <.0001 | <.0001 | |
| Grip strength (kg) | Low activity | −4.85 (−5.14, −4.57) | −4.89 (−5.17, −4.62) | −4.83 (−5.10, −4.55) |
| Moderate activity | −4.09 (−4.34, −3.84) | −4.08 (−4.32, −3.84) | −4.11 (−4.36, −3.87) | |
| High activity | −3.81 (−4.39, −3.23) | −3.68 (−4.24, −3.13) | −3.79 (−4.35, −3.23) | |
| p-trend | <.0001 | <.0001 | <.0001 | |
| Chair stands (n) | Low activity | −1.02 (−1.10, −0.94) | −0.99 (−1.07, −0.91) | −0.99 (−1.07, −0.91) |
| Moderate activity | −0.65 (−0.72, −0.59) | −0.67 (−0.74, −0.60) | −0.67 (−0.74 to −0.6) | |
| High activity | −0.5 (−0.65, −0.34) | −0.53 (−0.69, −0.37) | −0.53 (−0.69, −0.37) | |
| p-trend | <.0001 | <.0001 | <.0001 | |
| LEP (watts) | Low activity | −41.02 (−43.77, 38.26) | −40.76 (−43.47, −38.06) | −40.09 (−42.79, −37.38) |
| Moderate activity | −37.09 (−39.39, −34.78) | −36.98 (−39.24, −34.73) | −37.34 (−39.58, −35.09) | |
| High activity | −34.55 (−39.78, −29.33) | −36.01 (−41.14, −30.88) | −36.61 (−41.73, −31.49) | |
| p-trend | .01 | .03 | .12 |
Note: CI = confidence interval; LEP = leg extension power; PA = physical activity.
n missing: grip strength, n = 155; Gait speed, n = 42; Chair stands, n = 41; Leg power, n = 991.
aBase model adjusted for age, race, clinic site, education. bMultivariate model adjusted for age, race, clinic site, education, marital status, smoking, drinking, BMI, Self-rated health, Falls reported in the last year, SF12 mental health index, and comorbidity defined categorically (yes/no; hypertension, CVD, CHF, arthritis, diabetes).
*p for trend is the linear change from low-activity to moderate-activity to high-activity trajectory groups.
Discussion
Among this large cohort of older men, patterns of PA change, based primarily on baseline self-reported PA, were associated with concurrent declines in performance, including gait speed, grip strength, chair stands, and leg power over an average 7 years of follow-up. Declines in performance outcomes were consistent with the changes in PA across each PA trajectory group. Men in trajectory groups with higher levels of PA (high activity and moderate activity) had higher performance outcomes at Year 0 and Year 7, and experienced smaller declines in PA in nearly each performance outcome over the 7 year follow-up compared to men in the low-activity trajectory group. Importantly, the associations of higher PA maintained through older adulthood and performance decline were robust to adjustment for mental and physical health status and multiple morbidities that may contribute to declining physical functioning during old age.
Prior research on trajectories has assessed the impact of certain patterns of self-reported physical function over time as predictors of activity change in certain populations. Longitudinal models reported by Brach et al. who showed that women who were reportedly “always active” over the 14-year study period, had the highest functional status at the 14-year follow-up visit, compared to “never active” or “inconsistently active” women, and consistency of PA was a significant predictor of gait speed (23). In the present study, we identified three discrete PA trajectories in this cohort of older men that demonstrated roughly parallel, declining patterns over time, which were largely determined by the baseline level of PA levels (11). Moreover, we confirm and expand upon observations by Brach et al. (23) and other investigators (10,23–26) by showing that longitudinally, there were clear differences in performance decline observed across the three declining PA trajectories: Men who maintained higher levels of PA over time demonstrated higher initial performance and experienced smaller declines, whereas men with consistently low activity had lower baseline performance levels and experienced greater declines over the 7-year follow-up.
Gait speed has been considered to be a functional vital sign of overall health, prognosis and survival (27). In this cohort, men in each PA trajectory experienced clinically meaningful declines in 6-m gait speed (defined as >0.1 m/s) (28,29), with nominal, yet significant differences (0.02–0.05 m/s) observed among the PA groups over the 7-year follow-up. Furthermore, low-activity men on average presented with a mean gait speed of 1.06 m/s, and this group had a high proportion of men (26.47%) with gait speed below clinical thresholds (defined as <0.8 m/s (30,31) or <1.0 m/s (28,30) at the final follow-up year) compared to moderate-activity (16.3%) and high-activity men (11.8%). When the impact of PA declining patterns on gait speed were evaluated, a linear improvement (lower decline) was observed in higher versus lower trajectory groups, and there was minimal attenuation of risk after covariate adjustment. Men in the high-activity PA group experienced moderate declines in gait speed of 0.10 m/s, whereas more substantial declines (−0.12 to −0.16) in gait speed attributed to changes in PA patterns were found among men in the moderate—and low-activity groups (28,32).
Comparatively, on average, men in this cohort did not exhibit signs of clinical weakness based on established clinical cut points of grip strength (31), whereas cut points for 10-second chair stand performance have not been similarly validated. However, consistent with the hallmarks of aging, declines in grip strength, chair performance, and leg power over the 7-year follow-up were evident across all PA trajectory groups, suggesting that all men were becoming weaker as PA levels declined over time, while loss of strength (and hence weakness) may be attenuated among men who had higher initial PA levels and are able to maintain higher PA during old age. Accordingly, declines in chair stand performance attributed to decreasing PA patterns remained robust after covariate adjustment, suggesting that declining PA patterns may have a concomitant negative influence on overall lower-extremity strength as well as balance. In this context, these results may provide additional insights to understanding the increased vulnerability to adverse events such as falls, fracture, and disability that have shown to occur across the spectrum of activity and physical performance levels (33,34). Interestingly, leg power declined appreciably (15%–19%) over the 7-year follow-up, but the risk of decline attributed to declining PA become nominally attenuated after covariate adjustment, and differences in leg power decline among PA trajectories were statistically not significant. Given these findings and the fact that aging adults typically experience leg power declines earlier than declines in strength, suggesting that changes in leg power may be impacted by other factors such as self-rated health or the presence of comorbidities, which share common pathways with reduced working capacity and neuromuscular function (35), than by changing PA patterns per se. Other possible explanations for this finding include lower sample size and possible selection bias. Further, impairments in leg power are theorized to have a greater influence on muscle function than strength, with supporting evidence demonstrating its ability to predict gait speed, chair stand performance, and strength in older adults (6,17,36). Thus, the compounded effect of decreasing leg power may explain the significant and meaningful decrements in each performance measure seen across all PA trajectory groups, particularly among low-activity declining men. Indeed, it is plausible that the declining physical performance over time precludes the ability to initiate or maintain PA during aging, which is consistent with the concomitant declines in PA observed in our study, and corroborates findings from a prior tracking study (26).
Of course, low PA levels may be due to a greater preponderance of physical limitations and/or health problems that may hinder the ability to be physically active, triggering a vicious cycle that leads to increased sedentary behavior, reduced physical and functional capacity, exacerbated health (disease severity), and a greater performance decline over time. Some support comes from a cross-sectional analysis in 60–64-year olds showing that lower chair rise performance or lower grip strength was significantly associated with several chronic diseases (37). A recent study involving 17 countries showed that 1 SD decrease in grip strength was a significant prognostic indicator of cardiovascular mortality, even stronger than systolic blood pressure, in older adults over a 4-year follow-up (38). Health outcomes of this type may be related to greater performance declines like those observed among men who maintained lower versus higher PA levels over time. Men in the low-declining PA group reported having lower mental and physical health status (via SF-12), and had a higher prevalence of at least one comorbidity (ie, hypertension, CVD, CHF, arthritis, diabetes) at baseline compared to moderate- and high-activity declining PA groups, suggesting that residual confounding is at least moderately likely to explain our findings. Nonetheless, these findings support the need for evaluation of the effectiveness of targeted rehabilitation strategies that are designed to address management of health-related factors that may otherwise challenge PA engagement in populations at high risk for functional decline.
Strengths and Limitations
Noteworthy strengths include the large sample size and sufficient follow-up to detect group level differences in four clinically measured performance measures, repeated measures of PA using a common instrument over 7 years of follow-up, and inclusion of many potential confounders that may influence the relationship between PA and clinical performance (21,22). Study limitations include the observational design with potential residual confounding (eg, severity of comorbid conditions, clinic center), and reverse causality. It therefore remains unclear whether (and to what extent) PA has a causal impact on performance and overall health status versus merely reflecting underlying change in health status. Accordingly, analyses included healthier men who attended the Year 7 clinic visit, whereas selective drop-out or refusal of those who comparatively had lower performance outcomes and poor health status (data not shown) may have strengthened the association between baseline PASE score and changes in performance observed over the 7-year follow-up period. As such, the requirement to have repeat measures may have introduced selection bias for the study cohort. PA was measured using self-report and thus detailed measures such as intensity, frequency, duration of PA were not available. Thus, we are unsure whether smaller declines in physical performance among men represented in higher-PA declining groups were due to both the amount and intensity of PA, as demonstrated in prior studies (8,25). Last, the generalizability of the present study is limited since the source cohort is all male, mostly non-Hispanic white, and community-dwelling.
Conclusion
PA trajectories with decreasing PA were strong predictors of concurrent decreases in clinical performance measures of gait speed, grip strength, and chair stands. Those in the low-activity declining group had lower baseline performance measures and greater declines in performance compared with other groups.
Supplementary Material
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide the support: the National Institute on Aging (NIA); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS); the National Center for Advancing Translational Sciences (NCATS); and NIH Roadmap for Medical Research (grant numbers U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128). K.S. has institutional research grants from Merck. L.L. has institutional research grants from Abbott Nutrition.
Conflict of Interest
None reported.
References
- 1. Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421–426. [PubMed] [Google Scholar]
- 2. Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am. 2010;21:299–308. doi: 10.1016/j.pmr.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 3. Cawthon PM, Fox KM, Gandra SR, et al. ; Health, Aging and Body Composition Study Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc. 2011;59:781–787. doi: 10.1111/j.1532-5415.2011.03389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: the InCHIANTI study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE study investigators Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90:1711–1715. doi: 10.2522/ptj.20100270 [DOI] [PubMed] [Google Scholar]
- 8. Chalé-Rush A, Guralnik JM, Walkup MP, et al. Relationship between physical functioning and physical activity in the lifestyle interventions and independence for elders pilot. J Am Geriatr Soc. 2010;58:1918–1924. doi: 10.1111/j.1532-5415.2010.03008.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peeters G, Dobson AJ, Deeg DJ, Brown WJ. A life-course perspective on physical functioning in women. Bull World Health Organ. 2013;91:661–670. doi: 10.2471/BLT.13.123075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laddu DR, Wertheim BC, Garcia DO, et al. Associations between self-reported physical activity and physical performance measures over time in postmenopausal women: the women’s health initiative. J Am Geriatr Soc. 2017;65:2176–2181. doi: 10.1111/jgs.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laddu DR, Cawthon PM, Parimi N, et al. ; Osteoporotic Fractures in Men Study Research Group Trajectories of the relationships of physical activity with body composition changes in older men: the MrOS study. BMC Geriatr. 2017;17:119. doi: 10.1186/s12877-017-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laddu D, Parimi N, Cauley JA, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group The association between trajectories of physical activity and all-cause and cause-specific mortality. J Gerontol A Biol Sci Med Sci. 2018;73:1708–1713. doi: 10.1093/gerona/gly037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 14. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 15. MrOS Online. San Francisco Coordinating Center, California Pacific Medical Center Research institution & University of California, San Francisco, April 2017, mrosdata.sfcc-cpmc.net [Google Scholar]
- 16. Dinger MK, Oman RF, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the Physical Activity Scale for the Elderly (PASE). J Sports Med Phys Fitness. 2004;44:186–192. [PubMed] [Google Scholar]
- 17. Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82:321–327. doi: 10.1042/cs0820321 [DOI] [PubMed] [Google Scholar]
- 18. Blackwell T, Cawthon PM, Marshall LM, Brand R. Consistency of leg extension power assessments in older men: the Osteoporotic Fractures in Men (MrOS) Study. Am J Phys Med Rehabil. 2009;88:934–940. doi: 10.1097/PHM.0b013e3181bbddfb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ware JE, Keller SD, Kosinski M.. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA: Health Institute, New England Medical Center; 1995. [Google Scholar]
- 20. Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 21. Sims ST, Kubo J, Desai M, et al. Changes in physical activity and body composition in postmenopausal women over time. Med Sci Sports Exerc. 2013;45:1486–1492. doi: 10.1249/MSS.0b013e31828af8bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the women’s health initiative memory study. J Gerontol A Biol Sci Med Sci. 2010;65:300–306. doi: 10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brach JS, FitzGerald S, Newman AB, et al. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Arch Intern Med. 2003;163:2565–2571. doi: 10.1001/archinte.163.21.2565 [DOI] [PubMed] [Google Scholar]
- 24. Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Arch Intern Med. 2009;169:1476–1483. doi: 10.1001/archinternmed.2009.248 [DOI] [PubMed] [Google Scholar]
- 25. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x [DOI] [PubMed] [Google Scholar]
- 26. de Souto Barreto P, Ferrandez AM. Static or dynamic predictors of physical activity (PA)? A tracking study based on 12- and 38-month follow-ups in older adults. Arch Gerontol Geriatr. 2014;59:326–330. doi: 10.1016/j.archger.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 27. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 29. Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894 [DOI] [PubMed] [Google Scholar]
- 30. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 31. Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Studenski S. Current Geriatric Diagnosis and Treatment. In: Landefeld CS PR, Johnson MAet al. , ed. Exercise. New York, NY: McGraw-Hill; 2004:436–446. [Google Scholar]
- 33. Orwoll ES, Fino NF, Gill TM, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group The relationships between physical performance, activity levels, and falls in older men. J Gerontol A Biol Sci Med Sci. 2019;74:1475–1483. doi: 10.1093/gerona/gly248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin KR, Koster A, Murphy RA, et al. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003-04 and 2005-06. J Am Geriatr Soc. 2014;62:1263–1271. doi: 10.1111/jgs.12893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strollo SE, Caserotti P, Ward RE, Glynn NW, Goodpaster BH, Strotmeyer ES. A review of the relationship between leg power and selected chronic disease in older adults. J Nutr Health Aging. 2015;19:240–248. doi: 10.1007/s12603-014-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x [DOI] [PubMed] [Google Scholar]
- 37. Kuh D, Karunananthan S, Bergman H, Cooper R. A life-course approach to healthy ageing: maintaining physical capability. Proc Nutr Soc. 2014;73:237–248. doi: 10.1017/S0029665113003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leong DP, Teo KK, Rangarajan S, et al. ; Prospective Urban Rural Epidemiology (PURE) Study investigators Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.