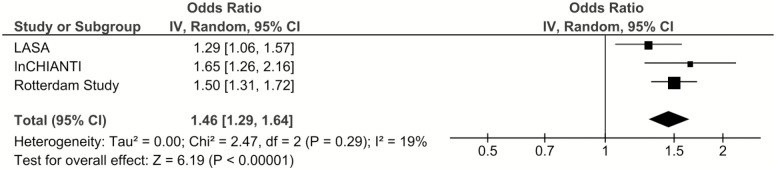Abstract
Background
Previous studies have suggested that the association between APOE ɛ 4 and dementia is moderated by physical activity (PA), but the results remain inconclusive and longitudinal data on cognitive decline are missing. In this study, we examine whether there is a gene–environment interaction between APOE and PA on cognitive decline in older adults using 9-year follow-up data of three cohort studies.
Methods
We followed 7,176 participants from three longitudinal cohort studies: Longitudinal Aging Study Amsterdam (LASA), InCHIANTI, and Rotterdam Study for 9 years. PA was assessed with self-reported questionnaires and was categorized in low, moderate, and high PA. Cognitive function was assessed with the Mini-Mental State Examination (MMSE) and cognitive decline was defined as a decrease of three points or more on the MMSE during 3 years follow-up. We fitted logistic regression models using generalized estimating equations adjusting for age, sex, education, depressive symptoms, and number of chronic disease. Interaction between APOE and PA was tested on multiplicative and additive scale.
Results
Cohorts were similar in most aspects but InCHIANTI participants were on average older and had lower education. APOE ɛ 4 carriers had higher odds of cognitive decline (odds ratio [OR] = 1.46, 95% confidence interval [CI]: 1.29–1.64) while PA was not significantly associated with cognitive decline overall (moderate PA: OR = 0.87, 0.67–1.13; high PA: OR = 0.71, 0.36–1.40). There was no evidence for an interaction effect between PA and APOE ɛ 4 in cognitive decline in older adults (APOE × moderate PA: p = .83; APOE × high PA: p = .90).
Conclusions
Previous claims of a gene–environment interaction between APOE ɛ 4 and PA in cognitive decline are not supported by our results.
Keywords: Gene–environment interaction, Longitudinal Aging Study Amsterdam, InCHIANTI, Rotterdam Study
Dementia is an increasing public health problem due to the increased life expectancy and the aging population (1,2). It has a complex genetic architecture and is largely influenced by lifestyle factors. In addition to dementia, there is an increasing burden of cognitive decline in the population.
Apolipoprotein E ε 4 (APOE ε 4) is the main genetic risk factor for dementia (3–5). Furthermore, the ε 4 isoform of the lipoprotein is directly involved in the biological pathway of dementia and cognitive decline. It reduces the clearance of soluble amyloid beta (Aβ) from the brain and increases its deposition in senile plaques (6,7). In addition, several studies have shown that APOE ε 4 carriers have more Aβ deposition in senile plaques in the brain compared to noncarriers (8,9).
Along with genetic factors, different lifestyle factors affect dementia and cognitive function in older individuals. Previous studies suggest that physical activity (PA) (10–12) has a positive effect on cognitive function. The exact mechanisms remain unknown but several theories are proposed such as enhancing cerebral perfusion, effects of PA on angiogenesis, neurogenesis, reduction of neuroinflammation, and reduction of Aβ deposition (13,14).
In the last years, a gene–environment interaction (GxE) between APOE and PA in Alzheimer’s disease (AD) has been suggested (15,16). A recent review by Bos and colleagues emphasizes the importance of identifying modifiable lifestyle factors for high-risk APOE ε 4 carriers (17). The underlying biological mechanisms of this interaction are not completely understood but it is hypothesized that APOE ɛ 4 carriers are more vulnerable to nonfavorable lifestyle factors compared to noncarriers. The study of Head and colleagues (14) showed that PA moderates the effect of APOE genotype on Aβ deposition with extra benefit in APOE ɛ 4 carriers.
However, current literature on APOE ε 4 by PA interaction is inconclusive and mainly focused on AD. Some studies suggest a stronger protective effect of PA on dementia in APOE ε 4 carriers compared to noncarriers (18,19). Conversely, findings from Cardiovascular Health Cognition Study and Canadian Study of Health and Aging analyzing the effect of PA on dementia suggest a stronger protective effect of PA in APOE ε 4 noncarriers compared to carriers (20,21).
Here, we aimed to explore whether PA might moderate the effect of APOE genotype on cognitive decline. Thus, we tested whether the GxE interaction between APOE genotype and PA was associated with cognitive decline in the general population of older individuals, using data from three prospective population-based cohort studies with longitudinal measurements.
Methods
Study Participants
This study includes data from three ongoing prospective cohort studies: the Longitudinal Aging Study Amsterdam (LASA), Invecchiare in Chianti (InCHIANTI), and Rotterdam Study (RS). A description of each study can be found in the Supplementary Material. The main analysis includes participants of Caucasian ethnicity, aged 55 years and older who underwent repeated cognitive assessment with the Mini-Mental State Examination (MMSE), and had a baseline MMSE score ≥18. Participants who were bedridden or in a wheelchair were excluded from the analysis. In total, 7,176 participants with 18,489 observations during 9-year follow-up were analyzed; respectively 1,736 participants with 4,255 observations from LASA, 843 participants with 2,132 observations from InCHIANTI study, and 4,597 participants with 12,102 observations from RS (Supplementary Figure 1). Mean follow-up period between two consecutive measurements was 3 years for LASA and InCHIANTI and 4 years for RS (Supplementary Table 1). Informed consent was obtained from all participants in each study and all studies have been approved by the respective Medical Ethics Committees.
Assessment of APOE
In LASA, both phenotyping and genotyping of APOE were available. Phenotyping was performed at the Immunochemistry Laboratory of VUmc, Amsterdam (22). Genotyping was performed using the Axiom-NL array from Affymetrix (Avera Institute for Human Genetics, South Dakota) or the Infinium Global Screening Array from Illumina (Genetic Laboratory, Erasmus MC, Rotterdam, the Netherlands). APOE could be inferred from two single-nucleotide polymorphisms (SNPs), rs429358 and rs7412. A comparison between phenotyping and genotyping status of APOE showed 96.9% agreement between the two methods. Because more participants had phenotyping data (N = 2,233) compared to genotyping data (N = 1,021), the status determined by phenotyping was included in the analysis.
In InCHIANTI, APOE status was inferred from rs429358 and rs7412 SNPs genotyped on Illumina Infinium HumanHap550 chip at the Laboratory of Neurogenetics of the U.S. National institute on Aging.
In RS, APOE status was assessed at study entry using polymerase chain reaction on coded DNA samples.
Assessment of PA
PA was assessed using different questionnaires per cohort which are described below. To make the PA measurements comparable between cohorts, PA was coded in three categories: low, moderate, and high PA (reference: low PA) following a similar procedure as described by Jonkman and colleagues (23).
In LASA, physical activity was assessed by using the validated LASA Physical Activity Questionnaire (LAPAQ) (24), based on the questionnaires by Vorrips and colleagues (25) and Caspersen and colleagues (26). The frequency and duration of the following activities in the previous 2 weeks was assessed: walking, cycling, light household activities, heavy household activities, and two most frequent sports. To take into account the intensity of each activity, metabolic equivalent of tasks (MET) was used. The MET-value is based on the ratio of work metabolic rate to resting metabolic rate and 1 MET is defined as 1 kcal/kg/h. The number of MET-hour for an individual spent on a specific activity was calculated by multiplying the MET-value by time spent on that specific activity (in hours) per week.
In InCHIANTI, PA was assessed using a modified standard interview-administered questionnaire (27) in which the participants provided data on current PA (28). Participants were asked about the frequency and duration of sports and recreational PA. By combining the responses and taking into account the intensity of each type of activity, participants were classified in one of the three categories:
(a) inactive, including participants who were completely inactive and those who performed low intensity PA.
(b) light PA, including participants who performed light intensity PA for 2–4 hours per week.
(c) moderate–high PA, including participants who performed at least moderate PA >3 hours per week or more and those who performed intense exercise many times per week.
In RS, PA was assessed by an adapted version of the validated Zutphen Physical Activity Questionnaire (26). The original questionnaire contains questions on walking, cycling, gardening, diverse sports, and hobbies. To obtain a more complete overview of physical activity, questions on housekeeping activities were added. Participants were asked how many hours they spent per week on these activities during the past 2 weeks. For some activities, like sports and gardening, participants were asked whether this activity was practiced only during summer or winter. When answered positive, the given period of time for that activity was divided by two (29). MET scores were assigned to each activity and subsequently the total MET hours/wk was calculated per each participant.
Study-specific tertiles were created based on the total MET hours/wk in LASA and RS.
Assessment of Cognitive Decline
All three studies assessed cognitive function at each visit using MMSE, a 20-item scale ranging from 0 to 30 with higher scores indicating better cognitive function (30). A decrease of three or more points in MMSE in the following visit was considered as cognitive decline. The same definition for cognitive decline has been used in other cohort studies (31,32). Furthermore, results from the LEILA study, using the reliable change indices method with repeated measurements every 1.5 years, found that a mean MMSE decline of three or more points in 3 years is considered a reliable change in population-based cohorts of older adults (33).
Assessment of Other Cognitive Tests in LASA
More specific aspects of cognition such as memory and information processing that are compromised in dementia were assessed in LASA. Episodic memory was assessed using the 15 Words Test, the Dutch version of the Auditory Verbal Learning Test (34). The test consists of 15 words that have to be learned during 3 trials. After every trial the respondent is asked to recall as many words as possible. After a distraction period of 20 minutes, the respondent is asked to name again the words he learned. The total number of words learned during three tests is the recall score (range 0–45). The number of words reproduced after 20 minutes is the delayed recall score (range 1–15) (22).
Information processing speed was assessed using an adjusted version of the Alphabet Coding Task-15 and has been described by Piccinin and Rabbit (35). The test was done in three cycles of one minute and the mean score of the three trials is used in the analysis (22).
These tests were either not available or different from the ones used in LASA in the other cohort studies; therefore, the analyses were only performed in LASA participants.
Assessment of Covariates
The following covariates were considered: age (years), sex, education (less than 9 years of education, 9–12 years of education, more than 12 years of education), clinically relevant depressive symptoms (Center for Epidemiological Studies Depression Scale ≥16), and number of chronic disease (0–1, 2, >2 diseases).
A detailed assessment of covariates is described in the Supplementary Material.
Supplementary Table 1 presents an overview of the number of participants per wave, methods, and instruments used to assess exposure, outcome, and covariates in each cohort.
Statistical Analysis
The main effect of APOE ɛ 4 and PA and then their interaction effect (APOE × PA) on cognitive decline were assessed using generalized estimating equations (GEE) with exchangeable correlation structure, taking into account repeated measurements. A binary logistic model was used to assess the unadjusted effect estimates. In Model 1, we adjusted for sex (dichotomous), age at baseline (continuous), and education (categorical, reference category: less than 9 years of education). Subsequently, we adjusted for clinically relevant depressive symptoms (dichotomous) and presence of chronic disease (categorical, reference category: 0–1 chronic disease) in Model 2.
After running GEE in each study site, the results were meta-analyzed using random effects to take into account possible heterogeneity between studies. The I2 statistic was calculated, which measures the percentage of variation between studies that is not due to chance: I2 < 25% is considered low heterogeneity. To determine whether the heterogeneity was statistically significant, the chi-square test of heterogeneity was used (36). For analyses indicating a high degree of heterogeneity, we performed three leave-one-out meta-analyses removing one cohort per time, in order to check the impact of potential outlier data on the results.
Sensitivity Analysis
Several sensitivity analyses were performed to test the robustness of the results against different aspects of cognitive function and scales of PA. First, since the reported levels of PA can be influenced by cognitive function, a sensitivity analysis was done including only individuals with good cognitive function at baseline (MMSE ≥24). Furthermore, to avoid loss of information due to the categorization of PA, a sensitivity analysis was conducted with PA assessed as continuous measure (total MET hours/wk divided by standard deviation) for studies in which continuous measures were available (ie, LASA and RS). Because interaction on the additive scale may reflect biological interaction better than interaction on the multiplicative scale (37) the relative excess risk due to interaction (RERI) was calculated according to the procedure proposed by Knol and colleagues (38), with 95% confidence intervals calculated by bootstrapping (N = 1,000).
Extra Analyses in LASA
Analyses assessing different aspects of cognition as memory (total recall score, delayed recall score) and information processing speed that might be more sensitive than MMSE were done in LASA participants. There are no clear cutoffs for what can be considered decline in the 15-word test and coding task; therefore, we used as outcome the difference in scores between two measurements. Linear regression models using GEE with exchangeable correlation structure was fitted.
Analyses in LASA and RS were done using IBM SPSS Statistics version 21.0 (IBM Corp, New York) and in InCHIANTI using STATA/SE 12.0. RERI was calculated using R (version 3.5.1). The meta-analysis of the results was done using the rmeta package and bootstrapping was done using boot package in R. The figures were made using Review Manager version 5.3.5 a tool from the Cochrane Collaboration (39).
Results
A total of 7,176 individuals were included in the main analysis. The sample characteristics per study are shown in Table 1 and the baseline characteristics of the participants without MMSE data at the first follow-up are shown in Supplementary Table 2.
Table 1.
Baseline Characteristics of the Participants Per Cohort
| LASA (N = 1,736) | InCHIANTI (N = 843) | Rotterdam Study (N = 4,597) | |
|---|---|---|---|
| Age at baseline (years), Mean (SD) | 69.8 (8.1) | 72.4 (7.2) | 67.8 (7.4) |
| Female, N (%) | 896 (51.6%) | 470 (55.7%) | 2,617 (56.9%) |
| APOE ɛ 4 carriers, N (%) | 467 (26.9%) | 128 (15.2%) | 1,268 (27.6%) |
| Physical activity | Range (MET h/wk) | N (%) | Range (MET h/wk) |
| Low | 0.2–41.4 | 122 (14.5%) | 2.8–63.4 |
| Moderate | 41.5–74.8 | 383 (45.4%) | 63.5–99.3 |
| High | 74.9–325.5 | 338 (40.1%) | 99.4–296.3 |
| MMSE at baseline, Mean (SD) | 27.5 (2.1) | 25.9 (2.7) | 27.9 (1.7) |
| Education, N (%) | |||
| Low (<9 y) | 685 (39.5%) | 742 (88.0%) | 505 (11.1%) |
| Intermediate (9–12 y) | 835 (48.2%) | 43 (5.1%) | 2,025 (44.4%) |
| High (>12 y) | 214 (12.3%) | 58 (6.9%) | 2,030 (44.5%) |
| Depression, N (%) | 213 (12.3%) | 245 (29.1%) | 428 (9.4%) |
| Number of chronic disease, N (%) | |||
| 0–1 | 1,343 (77.4%) | 545 (69.3%) | 4,080 (88.8%) |
| 2 | 279 (16.1%) | 147 (18.7%) | 428 (9.3%) |
| >2 | 113 (6.5%) | 95 (12.0%) | 89 (1.9%) |
Note: Low physical activity(PA) corresponds to the inactive category for InCHIANTI and first tertile for LASA and RS; Moderate PA corresponds to the light PA category for InCHIANTI and to the second tertile for LASA and RS; High PA corresponds to the moderate–high category for InCHIANTI and the highest tertile in LASA and RS.
Mean age at baseline was higher for InCHIANTI participants (72.4 [7.2] years) compared to LASA (69.8 [8.1] years) and RS (67.8 [7.4] years). MMSE score at baseline was lower (25.9 [2.7]) for InCHIANTI compared to LASA (27.5 [2.1]) and RS participants (27.9 [1.7]). Most InCHIANTI participants (88%) had completed up to 9 years of education while most LASA and RS participants had attained more than 9 years of education. LASA and RS participants had a similar percentage of APOE ɛ 4 carriers, respectively 26.9% and 27.6%, while only 15.2% of the respondents were carriers in InCHIANTI (Table 1). Participants who did not have MMSE data at the first follow-up were on average older, more often APOE ɛ 4 carriers, and had more often depression or chronic diseases (Supplementary Table 2).
The unadjusted effect estimates are presented in Supplementary Table 3. In the pooled adjusted analysis, APOE ɛ 4 carriers had higher odds of cognitive decline (odds ratio [OR] = 1.46, 95% confidence interval [CI]: 1.29, 1.64) compared to noncarriers and the results were similar across cohorts (I2 = 19%, p = .29; Figure 1).
Figure 1.
The association between APOE ɛ 4 and cognitive decline: Results from three longitudinal cohort studies. Model 2: Results adjusted for age, sex, education, depression, and chronic diseases.
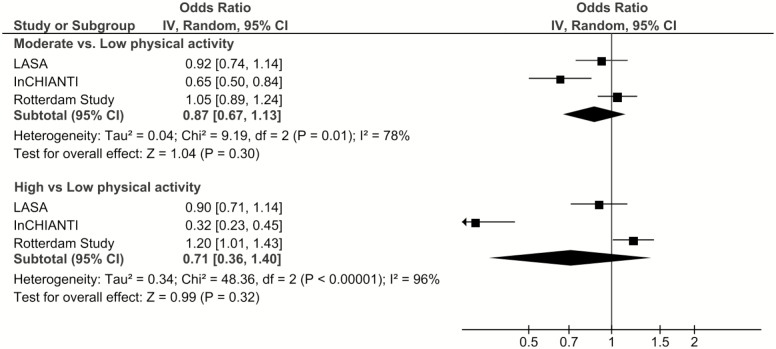
Overall, there was no association between PA and cognitive decline in the pooled analysis (moderate PA: OR = 0.87, 95% CI: 0.67–1.13; high PA: OR = 0.71, 95% CI: 0.36–1.40), but the heterogeneity between studies was substantial (moderate PA: I2 = 78%, p = .01; high PA: I2 = 96%, p < .0001; Figure 2). Similarly, there was no association between PA and cognitive decline when only LASA and InCHIANTI, LASA, and RS or InCHIANTI and RS were meta-analyzed (leave-one-out meta-analysis, Supplementary Table 4). In InCHIANTI, higher PA was associated with lower odds of cognitive decline (high PA compared to low PA, OR = 0.32, 95% CI: 0.23, 0.44), whereas no such associations were seen in LASA and RS.
Figure 2.
The association between physical activity and cognitive decline: Results from three longitudinal cohort studies. Model 2: Results adjusted for age, sex, education, depression, and chronic diseases.
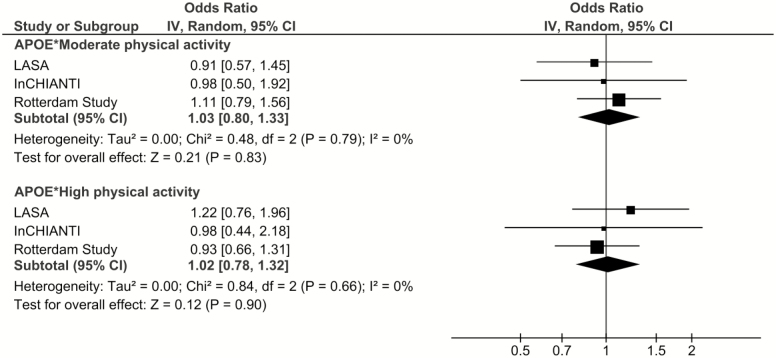
No gene-by-environment interaction between APOE and PA in cognitive decline was found in multiplicative scale (APOE × moderate PA: OR = 1.03, 95% CI: 0.80–1.33; APOE × high PA: OR = 1.02, 95% CI: 0.78–1.32) and there was no heterogeneity between studies (APOE × moderate PA: I2 = 0.0%, p = .79; APOE × high PA: I2 = 0.0%, p = .66; Figure 3).
Figure 3.
The association between the interaction effect of APOE and physical activity and cognitive decline: Results from three longitudinal studies. Model 2: Results adjusted for age, sex, education, depression, and chronic diseases.
Moreover, no evidence for interaction on additive scale between APOE and PA was found in LASA or InCHIANTI (LASA: moderate PA: RERI = −0.11, 95% CI: −0.75, 0.42; high PA: RERI = 0.20, 95% CI: −0.37, 0.59; InCHIANTI: moderate PA: RERI = −0.28, 95% CI: −1.70, 0.53; high PA: RERI = −0.50, 95% CI: −1.92, 0.28).
Sensitivity Analysis
Analyses including only cognitively healthy individuals at baseline (MMSE ≥24) showed similar results (Supplementary Table 5).
Furthermore, also the association of PA and APOE × PA assessed using PA in continuous scale in LASA and RS was consistent with the main findings (Supplementary Table 6).
Extra Analysis in LASA
In line with the main analyses, APOE ɛ 4 was associated with more decline in total recall score, delayed recall score, and information processing speed. There was no association of PA with specific aspects of cognition and no GxE interaction between APOE and PA in total recall score, delayed recall score or information processing speed was found (Supplementary Table 7).
Discussion
In this large study conducted among older adults in three prospective population-based cohort studies, we aimed to explore whether PA might moderate the effect of APOE genotype on cognitive decline. The present findings confirmed that APOE ɛ 4 allele was associated with higher risk of cognitive decline. Nevertheless, the GxE interaction between APOE genotype and PA was not associated with cognitive decline. Thus, the hypothesis that PA might modify the association between APOE and cognitive decline was not supported by our results.
Our results are in line with the findings from Whitehall II study (40), which reported no interaction between APOE ɛ 4 and PA in cognitive function in late midlife as well as with the findings from UK Biobank (41), which reported no interaction between APOE ɛ 4 and PA in cognitive abilities in persons aged 40–70 years. However, compared to our study, both studies had younger participants and did not have longitudinal data on cognitive function. Luck and colleagues analyzed data from the German study on Ageing, Cognition and Dementia in Primary care Patients (AgeCoDe) and reported no GxE interaction in multiplicative scale between APOE ɛ 4 and PA in dementia but they found a possible additive interaction in Alzheimer’s disease (15). In contrast to our findings, a cohort study of 347 older Dutch men found a stronger protective effect of PA in cognitive decline in APOE ɛ 4 carriers compared to noncarriers (31). The participants in this study were on average older, the follow-up period was 3 years compared to 9 years in our study and cognitive decline was assessed only once. The different outcome definitions used in the literature, the age of the participants, the follow-up time and physical activity assessment make it difficult to directly compare the results. To date, it can be postulated that the GxE interaction is present in AD but there is no evidence of this interaction in cognitive decline and cognitive functioning.
Overall, we found no association between PA and cognitive decline; however, the results were heterogeneous between the participating cohorts. PA was strongly associated with lower odds of cognitive decline in InCHIANTI while in LASA and RS this association was not statistically significant. The differences may be attributed to different questionnaires used per study or to specific population characteristics (ie, older age, lower educational level), suggesting that study characteristics should be taken into account when comparing results across studies.
Even though the majority of studies in the literature support a protective effect of PA in dementia and cognitive decline, the number of observational studies reporting no effect has increased in recent years. The evidence for a short-term effect (<5 years) is quite robust but there seems to be no effect in studies using a longer follow-up. In a recent publication from the Whitehall II study PA did not decrease the risk of dementia after 28 years follow-up (42). Likewise, when examining the association between PA and risk of dementia in RS, there was a short-term protective effect of PA using a follow-up up to 4 years but no association for longer follow-up (29). Furthermore, evidence from clinical trials does not support a long-term protective effect of PA on dementia or cognitive decline for follow-up 1 year or longer (43).
Strengths of our study include its prospective design, long follow-up period, large sample size, and large number of observations. To the best of our knowledge, this is the first study to analyze the GxE interaction between PA and APOE ε 4 in cognitive decline in three similar European, population-based, cohort studies. Variations in measurement methods were taken into account in the analysis plan by creating similar exposure, outcome, and covariate variables per study.
Nevertheless, the current study has some limitations. First, PA was assessed with self-reported questionnaires, which can be prone to information bias. Correlation of LAPAQ questionnaire with pedometer data in LASA was 0.56 (24), whereas the correlation of LAPAQ with wrist accelerometer data in RS was 0.3 (44). Second, each study assessed PA with different questionnaires, which may have contributed to heterogeneity in results. This was taken into account by using random effects meta-analysis to pool the data. Third, the MMSE is designed as a cognitive screening tool, and may not be very sensitive to assess decline in cognitive function. However, extra analysis using more specific tests such as 15 Word Test and Alphabet Coding Task-15 performed in LASA show similar results. Fourth, reverse causality might be present in the association between PA and cognitive decline. To account for possible reverse causation, a sensitivity analysis including only cognitively healthy participants (baseline MMSE ≥24) was performed and the results were similar with the main analysis. Fifth, our cohorts included only European ancestry participants and generalizability of the results in non-European populations should be taken with caution.
Assessment of PA with more objective measurements is needed in the future as these may better reflect the total amount of PA. Moreover, using a more sensitive test to assess cognitive decline can minimize the misclassification of the outcome and detect associations with more precision.
In spite of previous evidence suggesting a beneficial effect of PA in APOE ɛ 4 carriers, we found no evidence for a moderation effect by PA in the association between APOE genotype and cognitive decline. Based on our pooled results, PA is not associated with cognitive decline in older adults even though there was heterogeneity across the three cohorts. Further research is needed to identify subgroups where PA can protect against cognitive decline or type of activities that are more beneficial for preventing cognitive decline. Meanwhile PA in older adults should be encouraged for its protective effects in other conditions present at this age group.
In conclusion, the claims of a gene–environment interaction between APOE ɛ 4 and PA in cognitive decline are not supported by our results. There is a need for large sample replications before translating results from candidate GxE studies into clinical recommendations.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Eleonora Talluri, for her advice as biologist of InCHIANTI study, Dr. Hannie Comijs (GGZinGeest, Amsterdam) for her valuable input on cognitive tests used in the study and Prof. Jos Twisk (Department of Epidemiology and Biostatistics, Amsterdam UMC—location VUmc) for the advice on the statistical analyses. Furthermore, the authors would like to thank all the participants, researchers, interviewers, and supporting staff of LASA, InCHIANTI, and the Rotterdam Study.
Funding
This work is supported by the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care (LASA). The InCHIANTI study baseline was supported by the Italian Ministry of Health (ICS110.1/RF97.71) and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. R.S. is supported by the Erasmus Medical Centre and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health, Research and Development, the Research Institute for Disease in Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, and the Ministry of Health, Welfare and Sports. The funding agencies had no role in the study design, data collection and analysis, interpretation of results, writing, or publishing of the manuscript.
Authors’ Contribution
N.S., Nv.S., and Y.M. designed the study, prepared the statistical analysis plan, and drafted the manuscript. N.S. run the analysis for LASA, run the pooled analysis, and wrote the paper. F.J.W. and C.M.K. contributed to the data collection, data interpretation, methods description, and run the analysis for RS. Vd.P. contributed to the data interpretation, methods description, and run the analysis for InCHIANTI. T.V., S.B., M.A.I., and M.H. helped with the data acquisition and interpretation of the results. All the authors revised and approved the final version of manuscript.
Conflict of Interest
None reported.
References
- 1. Prince M, Adelina CH, Knapp M, Guerchet M, Karagiannidou M.. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. London, UK: Alzheimer’s Disease International; 2016. [Google Scholar]
- 2. He W, Luke LJ.. Older Americans with a Disability: 2008−2012 American Community Survey Reports, ACS-29. Washington, DC: U.S. Census Bureau and National Institute on Aging; 2014. [Google Scholar]
- 3. Henderson AS, Easteal S, Jorm AF, et al. Apolipoprotein E allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–1390. doi: 10.1016/s0140-6736(95)92405-1 [DOI] [PubMed] [Google Scholar]
- 4. Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55:964–968. doi: 10.1001/archneur.55.7.964 [DOI] [PubMed] [Google Scholar]
- 5. Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165:1231–1238. doi: 10.1093/aje/kwm085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verghese PB, Castellano JM, Garai K, et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci USA 2013;110:E1807–1816. doi: 10.1073/pnas.1220484110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polvikoski T, Sulkava R, Haltia M, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902 [DOI] [PubMed] [Google Scholar]
- 10. Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Curr Opin Psychiatry. 2006;19:190–193. doi: 10.1097/01.yco.0000214347.38787.37 [DOI] [PubMed] [Google Scholar]
- 11. Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denkinger MD, Nikolaus T, Denkinger C, Lukas A. Physical activity for the prevention of cognitive decline. Zeitschrift für Gerontologie und Geriatrie. 2012;45:11–16. doi: 10.1007/s00391-011-0262-6 [DOI] [PubMed] [Google Scholar]
- 13. Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luck T, Riedel-Heller SG, Luppa M, et al. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med. 2014;44:1319–1329. doi: 10.1017/S0033291713001918 [DOI] [PubMed] [Google Scholar]
- 16. Bates KA. Gene-environment interactions in considering physical activity for the prevention of dementia. AIMS Mol Sci. 2015;2:359–381. doi: 10.3934/molsci.2015.3.359 [DOI] [Google Scholar]
- 17. Bos MM, Noordam R, Blauw GJ, Slagboom PE, Rensen PCN, van Heemst D. The ApoE ε4 isoform: can the risk of diseases be reduced by environmental factors? J Gerontol A Biol Sci Med Sci. 2019;74:99–107. doi: 10.1093/gerona/gly226 [DOI] [PubMed] [Google Scholar]
- 18. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- 19. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B):2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092 [DOI] [PubMed] [Google Scholar]
- 21. Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ. Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: a population-based study. J Alzheimers Dis. 2017;56:297–303. doi: 10.3233/JAD-160424 [DOI] [PubMed] [Google Scholar]
- 22. Dik MG, Deeg DJ, Bouter LM, Corder EH, Kok A, Jonker C. Stroke and apolipoprotein E epsilon4 are independent risk factors for cognitive decline: a population-based study. Stroke. 2000;31:2431–2436. doi: 10.1161/01.str.31.10.2431 [DOI] [PubMed] [Google Scholar]
- 23. Jonkman NH, Colpo M, Klenk J, et al. Development of a clinical prediction model for the onset of functional decline in people aged 65-75 years: pooled analysis of four European cohort studies. BMC Geriatr. 2019;19:179. doi: 10.1186/s12877-019-1192-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 25. Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc. 1991;23:974–979. [PubMed] [Google Scholar]
- 26. Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol. 1991. 133:1078–1092. doi: 10.1093/oxfordjournals.aje.a115821 [DOI] [PubMed] [Google Scholar]
- 27. Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168 [DOI] [PubMed] [Google Scholar]
- 28. Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L; InCHIANTI Investigators Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60:760–767. doi: 10.1093/gerona/60.6.760 [DOI] [PubMed] [Google Scholar]
- 29. de Bruijn RF, Schrijvers EM, de Groot KA, et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol. 2013;28:277–283. doi: 10.1007/s10654-013-9773-3 [DOI] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 31. Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015 [DOI] [PubMed] [Google Scholar]
- 32. Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79 [DOI] [PubMed] [Google Scholar]
- 33. Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78:1298–1303. doi: 10.1136/jnnp.2006.109074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saan RJ, Deelman, BG. De 15-Woorden Tests A en B. (Een voorlopige handleiding). Groningen, Netherlands: afd Neuropsychologie, AZG (interne publicatie); 1986. [Google Scholar]
- 35. Piccinin AM, Rabbitt PM. Contribution of cognitive abilities to performance and improvement on a substitution coding task. Psychol Aging. 1999;14:539–551. doi: 10.1037//0882-7974.14.4.539 [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x [DOI] [PubMed] [Google Scholar]
- 38. Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–1118. doi: 10.1093/ije/dym157 [DOI] [PubMed] [Google Scholar]
- 39. Review Manager (RevMan). 5.3 ed.Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 40. Sabia S, Kivimaki M, Kumari M, Shipley MJ, Singh-Manoux A. Effect of Apolipoprotein E epsilon4 on the association between health behaviors and cognitive function in late midlife. Mol Neurodegener. 2010;5:23. doi: 10.1186/1750-1326-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyall DM, Celis-Morales C, Lyall LM, et al. Assessing for interaction between APOE ε4, sex, and lifestyle on cognitive abilities. Neurology. 2019;92:e2691–e2698. doi: 10.1212/WNL.0000000000007551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi: 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Souto Barreto P, Demougeot L, Vellas B, Rolland Y. Exercise training for preventing dementia, mild cognitive impairment, and clinically meaningful cognitive decline: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2017;73:1504–1511. doi: 10.1093/gerona/glx234 [DOI] [PubMed] [Google Scholar]
- 44. Koolhaas CM, van Rooij FJ, Cepeda M, Tiemeier H, Franco OH, Schoufour JD. Physical activity derived from questionnaires and wrist-worn accelerometers: comparability and the role of demographic, lifestyle, and health factors among a population-based sample of older adults. Clin Epidemiol. 2018;10:1–16. doi: 10.2147/CLEP.S147613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.