Abstract
Background
Type 2 diabetes and obesity increase the accumulation of health deficits and may accelerate biological aging. Multidomain lifestyle interventions may mitigate against this.
Methods
Within a large, randomized clinical trial of intensive lifestyle intervention including caloric restriction, increased physical activity, dietary counseling, and risk factor monitoring compared with diabetes support and education, we examined the accumulation of health deficits across 8 years. We used two complementary frailty indices (FIs) based on deficit accumulation, one modeled on work in the Systolic Blood Pressure Intervention Trial and the other including additional deficits related to obesity and type 2 diabetes mellitus. Differences between intervention groups and their consistency among subgroups were assessed with re-randomization tests.
Results
Data from 4,859 adults (45–76 years at baseline, 59% female) were analyzed. Random assignment to intensive lifestyle intervention was associated with lower FI scores throughout follow-up as captured by areas under curves traced by longitudinal means (p ≤ .001), over which time mean (SE) differences between intervention groups averaged 5.8% (0.9%) and 5.4% (0.9%) for the two indices. At year 8, the percentage of participants classified as frail (FI > 0.21) was lower among intensive lifestyle intervention (39.8% and 54.5%) compared with diabetes support and education (42.7% and 60.9%) for both FIs (both p < .001). Intervention benefits were relatively greater for participants who were older, not obese, and without history of cardiovascular disease at baseline.
Conclusions
Eight years of multidomain lifestyle intervention create a buffer against the accumulation of age-related health deficits in overweight or obese adults with type 2 diabetes.
ClinicalTrials.gov Identifier: NCT00017953
Keywords: Multidomain lifestyle intervention, Aging, Diabetes mellitus, Obesity
Diabetes and obesity are often described as accelerators of biological aging due to associations with decreased life span, increased risk of disability, and reductions in health-related quality of life with increasing age (1–3). The accumulation of age-related deficits in health and functional outcomes, that is, the deficit accumulation model of frailty, serves as a marker of an individual’s “aging-related health state” (4) and is recognized as a major risk factor for poorer function, disability, and death, in general and specifically within the context of diabetes (5–7). This has spurred interest in developing interventions that might slow or even reverse the progression of frailty, including multidomain lifestyle interventions that simultaneously target multiple behaviors, including diet, physical activity, and risk factor monitoring.
The strongest evidence that such lifestyle interventions can reduce frailty, quantified via deficit accumulation, is from the Multidomain Alzheimer’s Preventive Trial (MAPT), which reported that lifestyle intervention targeting nutrition, physical activity, and cognitive training slowed the increase in a deficit accumulation frailty index (FI) over 3 years in a cohort of older individuals at enhanced risk for cognitive decline (8). The Action for Health in Diabetes (Look AHEAD) trial provides the opportunity to assess whether a multidomain intervention targeting weight loss and increased physical activity slows the accumulation of health deficits over 8 years in a cohort of individuals with diabetes and overweight/obesity aged 45–76 years (9).
Methods
The Look AHEAD design, methods, and CONSORT diagram have been published previously (9,10). It was a multisite, single-blind RCT that recruited 5,145 individuals (during 2001–2004) who were overweight or obese and had type 2 diabetes. At enrollment, participants were 45–76 years of age with body mass index (BMI) > 25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) < 11%, systolic/diastolic blood pressure < 160/<100 mm Hg, and triglycerides <600 mg/dL. Protocols and consent forms were approved by local Institutional Review Boards.
Interventions
Participants were randomly assigned with equal probability to intensive lifestyle intervention (ILI) or the control condition of diabetes support and education (DSE). The multidomain ILI included diet modification and increased physical activity designed to induce weight loss to an average >7% at 1 year and maintain this over time (11). ILI participants were assigned a daily calorie goal (1,200–1,800 based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and a minimum of 15% of total calories from protein. The physical activity goal was >175 min/wk through activities similar in intensity to brisk walking. Measurements of blood pressure, lipids, glycosolated hemoglobin (HbA1c) were obtained: participants were provided results of these tests, and when levels did not conform to clinical guidelines, data were shared with their clinicians. During the first 6 months of ILI, participants attended three group meetings and one individual session per month. For the remainder of the first year, participants were provided two groups and one individual meeting per month. In months 13–48, participants attended monthly individual meetings that were followed approximately 14 days later with phone calls or e-mails from interventionists. Optional monthly group meetings were also offered. After this, ILI participants were encouraged to continue individual monthly sessions and annual campaigns were used to promote maintenance of weight loss.
DSE participants were invited to attend group sessions focused on diet, physical activity, and social support (12). Four meetings were offered in year 1, three per year in years 2–4, and one meeting per year thereafter. Attendance at these meetings was optional. Participants did not receive specific diet, activity, or weight goals or information on behavioral strategies; however, risk factor monitoring was identical in both interventions.
Relative to the DSE, the ILI produced sustained weight losses and increases in physical function (10). Interventions were terminated September 2012, after an average of 10 years (range 8–11). This manuscript is limited to the first 8 years of Look AHEAD to compare ILI and DSE during delivery.
Frailty Indices Based on Deficit Accumulation
The deficit composition of FIs has varied widely among studies. It is recommended that they include 30 or more components, each related to aging, with the deficits not being overly redundant or exceedingly rare (13). We constructed an FI modeled after the Systolic Blood Pressure Intervention Trial (SPRINT; Supplementary Table S1) (14). Its FI included 37 health factors, 8 of which were not directly available in Look AHEAD (self-reported atrial fibrillation, potassium, sodium, blood urea nitrogen, orthostatic hypotension, Montreal Cognitive Assessment Orientation Score, and the Logical Memory Delayed Recall task). It also included deficits related to being underweight (BMI < 18.5 kg/m2) and diabetes, both of which are not applicable as all Look AHEAD participants had diabetes and none reached the cut point for underweight. Look AHEAD did not have an objective measure of global cognitive function: instead, we used self-reported abilities on thinking, memory, and decision making (15). It also had no objective measure of physical function (eg, gait speed): instead, we used a self-report assessment of walking ability. We refer to this index with the above modification as FISPR.
SPRINT excluded individuals with type 2 diabetes. Thus, it may not be as sensitive to diabetes-related aging as an index including additional components related to diabetes and obesity in older individuals. Conversely, if such additional components do not materially affect the performance of the index in Look AHEAD, this reinforces the generalizability of indices across diverse cohorts. We augmented the FISPR with nine additional deficits related to diabetes and obesity to create a 38-item FI, which we label FIAUG (Supplementary Table S2): self-reports of sleep apnea, body stiffness after sleep or rest, urinary incontinence, worsening eyesight or hearing, poorly healing wounds, diabetic nephropathy, and use of insulin determined by audits of prescription medications. We further categorized each FI as fit (FI ≤ 0.10), pre-frail (0.10 < FI ≤ 0.21), or frail (FI > 0.21) (14,16): we use these classifications as a convenient ordering rather than diagnostic criteria. At baseline, the distribution among these groupings in the SPRINT cohort was 19% fit, 54% pre-frail, and 28% frail (14).
Collection of FI Components During Follow-up
At enrollment and annual follow-up visits, self-reported lifestyle characteristics, health conditions, and clinical histories were assessed using standardized questionnaires (9). Prescription medications were verified, and weight and blood pressure were measured. Following 12-hour fasting, metabolic risk factors (lipid/lipoproteins, glucose, and creatinine) were measured annually through year 4 and every other year thereafter. History of cardiovascular disease at baseline was based on self-report of prior myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, and class I/II heart failure. Hypertension was defined by current treatment or measured blood pressure ≥ 140/90 mm Hg. Depressive symptoms were assessed with the Beck Depression Index.
Statistical Analysis
Our analyses used de-identified data developed for investigators outside of the core Look AHEAD study group. Of the 5,145 Look AHEAD participants, 4,901 (95.3%) provided consent for this data sharing. Forty-two did not provide sufficient follow-up data to compute the FIs, resulting in our analytical cohort of 4,859. Baseline characteristics between intervention groups were compared using t-test and chi-square test. We calculated FIs at each annual visit when at least 80% of their deficits were evaluable. Rates of individuals lost to follow-up or for whom we were otherwise unable to compute FIs increased over time. At year 1, these were 5% (DSE) and 3% (ILI); at year 8, these were 15% (DSE) and 13% (ILI).
The covariance structure of the longitudinal assessments was complex, depending on both historical self-reported events (eg, history of stroke) and current measures (eg, obesity status) and the nature of any missing data. Because of this, we used re-randomization tests for inference, which while potentially yielding less statistical power than other approaches, required few assumptions about the distribution of data (17). To capture differences between groups, we computed the mean FI value at each year of follow-up and the area under the curve traced by these means across 8 years. This measure can be thought of as a cumulative summary of the average FI values over time. To generate a sampling distribution for this statistic under the null hypothesis of no differences between groups, we randomly assigned participants to intervention groups 1,000 times, preserving the observed sample sizes, and recorded the proportion that yielded more extreme (positive or negative) summary measures than observed as the two-sided p value for the inference. We report SE as the SD of this sampling distribution. We repeated this approach for predefined subgroups based on baseline characteristics: age, sex, BMI, duration of diabetes, and history of cardiovascular disease. We also compared the distribution of frailty status (fit, pre-frail, or frail) between the intervention groups at baseline and year 8 using chi-square tests.
Results
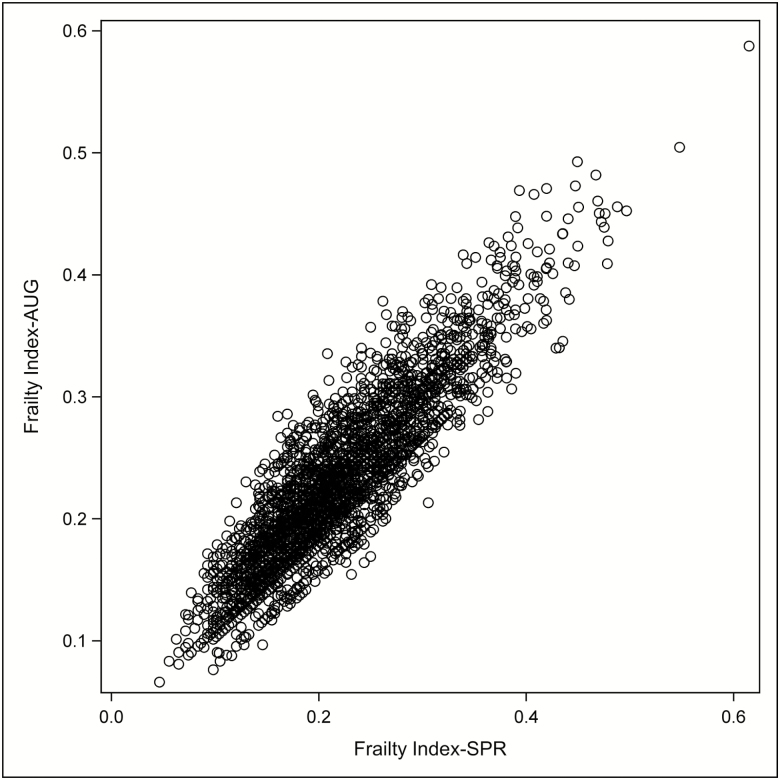
Table 1 describes baseline characteristics of the cohort. The balance afforded by the randomization was preserved, and both FISPR and FIAUG were similar between intervention groups (p > .35). Figure 1 portrays the distribution of the two FIs, which were highly correlated (r = .90).
Table 1.
Characteristics at Look AHEAD Enrollment Grouped by Intervention Assignment: N (%) or Mean (SE)
| Diabetes Support and Education | Intensive Lifestyle Intervention | ||
|---|---|---|---|
| N = 2,432 | N = 2,427 | p Valuea | |
| Age, y | |||
| 45–59 | 1,341 (55.2) | 1,375 (56.6) | .30 |
| 60–76 | 1,090 (44.8) | 1,052 (43.4) | |
| Sex | |||
| Female | 1,427 (58.7) | 1,420 (58.5) | .90 |
| Male | 1,005 (41.3) | 1,007 (41.5) | |
| Race/ethnicity | |||
| African American | 399 (16.4) | 396 (16.3) | .86 |
| Hispanic | 337 (13.9) | 336 (13.8) | |
| Non-Hispanic White | 1,615 (66.4) | 1,603 (66.0) | |
| Other, multiple | 81 (3.3) | 92 (3.8) | |
| BMI, kg/m2 | |||
| Overweight: 25–29 | 342 (14.1) | 374 (15.4) | .18 |
| Obese: ≥30 | 2,089 (85.9) | 2,051 (84.6) | |
| HbA1c, % | |||
| <7.0 | 1,104 (45.4) | 1,136 (46.8) | .47 |
| 7.0–8.9 | 1,240 (51.0) | 1,196 (49.3) | |
| 9.0–11.0 | 88 (3.6) | 95 (3.9) | |
| Insulin use, missing = 178 | |||
| No | 1,962 (83.7) | 1,989 (84.6) | .43 |
| Yes | 381 (16.3) | 362 (15.4) | |
| Diabetes duration, y | |||
| 0–4 | 1,092 (45.1) | 1,125 (46.7) | .28 |
| ≥5 | 1,327 (54.9) | 1,285 (53.3) | |
| Hypertension | |||
| No | 405 (16.6) | 393 (16.2) | .66 |
| Yes | 2,027 (83.4) | 2,034 (83.8) | |
| Smoking missing = 11 | |||
| Never | 1,218 (50.2) | 1,188 (49.0) | .64 |
| Former | 1,107 (45.6) | 1,127 (46.5) | |
| Current | 100 (4.1) | 108 (4.5) | |
| History of cardiovascular disease | |||
| No | 2,102 (86.4) | 2,077 (85.6) | .40 |
| Yes | 330 (13.6) | 350 (14.4) | |
| SPRINT Frailty Index (FISPR), mean | 0.200 (0.064) | 0.201 (0.067) | .43 |
| ≤0.10 | 44 (1.8) | 58 (2.4) | |
| >0.10 to ≤0.20 | 1,465 (60.2) | 1,457 (60.0) | .37 |
| >0.21 | 923 (38.0) | 912 (37.6) | |
| Augmented Frailty Index (FIAUG), mean | 0.202 (0.062) | 0.211 (0.065) | .37 |
| ≤0.10 | 20 (0.8) | 16 (0.7) | |
| >0.10 to ≤0.20 | 1,333 (54.8) | 1,296 (53.4) | .46 |
| >0.21 | 1,079 (44.4) | 1,115 (45.9) |
Notes: BMI = body mass index. aChi-square or t-test.
Figure 1.
Scatterplot of the FISPR and FIAUG deficit accumulation frailty indices at baseline (r = .90).
Table 2 provides mean baseline FISPR and FIAUG scores for subgroups based on traditional risk factors for aging. Although some differences include contributions of factors included as deficits in the indices (eg, obesity, hypertension, and smoking), the overall patterns are not unexpected. Racial/ethnic minorities, obese individuals, smokers, and those with poorer diabetes control, longer durations of diabetes, and hypertension had significantly higher mean baseline FI than those without these characteristics.
Table 2.
Differences in Baseline Deficit Accumulation Frailty Indices Among Subgroups
| Baseline Characteristic | FISPR | FIAUG |
|---|---|---|
| Mean (SE)a | Mean (SE)a | |
| Age, y | ||
| 45–59 | 0.202 (0.002) | 0.211 (0.002) |
| 60–76 | 0.199 (0.002) | 0.210 (0.002) |
| p = .392 | p = .785 | |
| Sex | ||
| Female | 0.200 (0.002) | 0.206 (0.002) |
| Male | 0.202 (0.002) | 0.216 (0.002) |
| p = .430 | p < .001 | |
| Race/ethnicity | ||
| African American | 0.205 (0.002) | 0.216 (0.002) |
| Hispanic | 0.206 (0.003) | 0.210 (0.002) |
| Non-Hispanic White | 0.198 (0.001) | 0.210 (0.001) |
| Other, Multiple | 0.193 (0.005) | 0.208 (0.005) |
| p < .001 | p < .001 | |
| BMI, kg/m2 | ||
| Overweight: 25–29 | 0.171 (0.003) | 0.181 (0.003) |
| Obese: ≥30 | 0.207 (0.002) | 0.217 (0.002) |
| p < .001 | p < .001 | |
| HbA1c, % | ||
| <7.0 | 0.190 (0.002) | 0.200 (0.002) |
| 7.0–8.9 | 0.210 (0.002) | 0.220 (0.002) |
| 9.0–11.0 | 0.198 (0.005) | 0.208 (0.005) |
| p < .001 | p < .001 | |
| Insulin use | ||
| No | 0.196 (0.002) | 0.200 (0.002) |
| Yes | 0.226 (0.003) | 0.259 (0.002) |
| p < .001 | p < .001 | |
| Diabetes duration, y | ||
| 0–4 | 0.194 (0.002) | 0.200 (0.002) |
| ≥5 | 0.206 (0.002) | 0.219 (0.002) |
| p < .001 | p < .001 | |
| Hypertension | ||
| No | 0.178 (0.003) | 0.190 (0.003) |
| Yes | 0.205 (0.002) | 0.215 (0.002) |
| p < .001 | p < .001 | |
| Smoking | ||
| Never | 0.188 (0.002) | 0.200 (0.002) |
| Former | 0.210 (0.002) | 0.218 (0.002) |
| Current | 0.241 (0.005) | 0.244 (0.005) |
| p < .001 | p < .001 |
Note: BMI = body mass index. aMeans and inference are from analyses of covariance with adjustment for age, sex, and race/ethnicity.
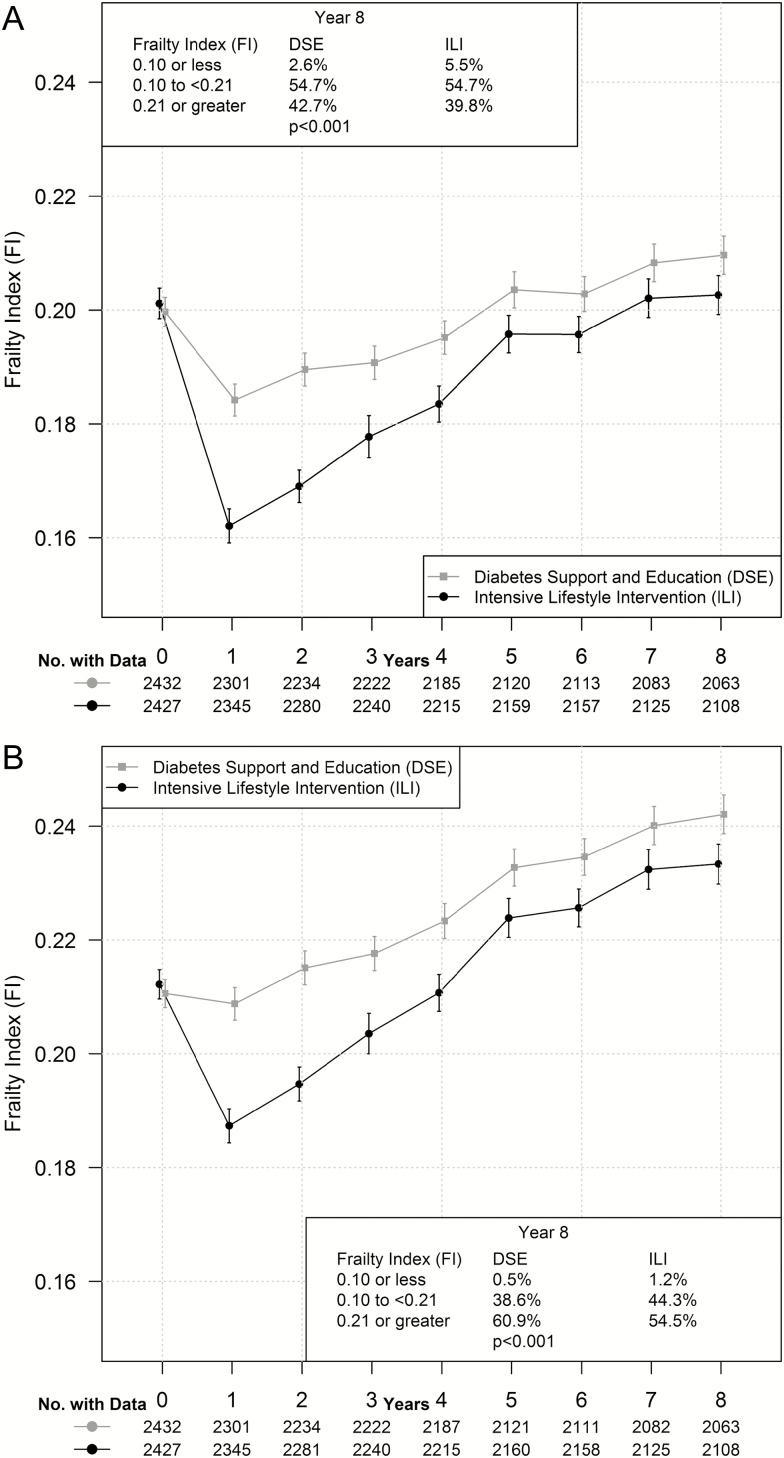
Figure 2A portrays mean 8-year trajectories of FISPR by intervention assignment. Within the DSE cohort, mean scores dipped slightly from baseline to year 1, but then rose gradually through the remainder of follow-up. Mean scores for the ILI cohort dropped more markedly between baseline and year 1, and then rose steadily, narrowing the gap between intervention groups. Overall, mean trajectories varied markedly between groups (p ≤ .001). Averaged over time, the mean (SE) FISPR scores for the ILI cohort were 5.8% (0.9%) lower than those for the DSE cohort. Relative to DSE, the year 8 distributions of scores among ILI participants were shifted towards lower levels of frailty (p < .001).
Figure 2.
Trajectory of mean FISPR (A) and FIAUG (B) with bars from 95% confidence intervals over follow-up by intervention assignment.
Figure 2B is a parallel presentation for scores from the FIAUG. With inclusion of additional obesity- and diabetes-related deficits, there was less of an initial buffer induced at year 1 between the two groups than for FISPR, but also slightly less attenuation of differences between groups across follow-up. Differences between intervention groups were highly statistically significant (p ≤ .001) and, averaged over time, were 5.4% (0.9%) lower among ILI participants and shifted toward less frailty at year 8.
Table 3 examines whether differences between interventions varied among important subgroups. Listed are mean differences in areas under the trajectories for the DSE minus ILI intervention groups, with positive values indicating a relative benefit for ILI compared with DSE. Results are fairly consistent for FISPR and FIAUG. ILI benefits were comparable for women and men and independent of diabetes duration. However, overall relative benefits of ILI were greater for older participants, with interaction p = .023 (FISPR) and p = .031 (FIAUG). Similarly, ILI appeared to provide relatively greater benefit for FISPR among overweight compared with obese individuals (interaction p = .023), and for those without baseline history of cardiovascular disease: interaction p = .020 (FISPR) and p = .016 (FIAUG). Supplementary Exhibit 3 portrays the mean trajectories of FISPR for participants grouped by intervention assignment for subgroups based on age, baseline BMI, and history of cardiovascular disease.
Table 3.
Estimated Mean Differences Between Intervention Groups in the Area Under the Curve (SE) Traced by Mean Values of Frailty Indices Over Time: Over Time and Among Subgroups
| Subgroup | N | AUC (SE) Difference Between Intervention Groups Over Time | |
|---|---|---|---|
| FISPR | FIAUG | ||
| Overall | 4,859 | 0.0912 (0.002) | 0.0976 (0.002) |
| p < .001 | p < .001 | ||
| Sex | |||
| Female | 2,847 | 0.0999 (0.002) | 0.0949 (0.003) |
| Male | 2,012 | 0.0789 (0.003) | 0.1019 (0.003) |
| p = .263 | p = .409 | ||
| Age group | |||
| 44–59 | 2,716 | 0.0621 (0.002) | 0.0688 (0.003) |
| 60–76 | 2,142 | 0.1265 (0.003) | 0.1324 (0.003) |
| p = .023 | p = .031 | ||
| Diabetes duration, y | |||
| 0–4 | 2,217 | 0.0896 (0.003) | 0.0879 (0.003) |
| ≥5 | 2,612 | 0.0924 (0.003) | 0.1048 (0.003) |
| p = .453 | p = .297 | ||
| Body mass index, kg/m2 | |||
| 25–29 | 716 | 0.1377 (0.003) | 0.1326 (0.003) |
| ≥30 | 4,140 | 0.0786 (0.002) | 0.0869 (0.003) |
| p = .023 | p = .080 | ||
| CVD history | |||
| No | 2,179 | 0.1045 (0.003) | 0.1123 (0.003) |
| Yes | 680 | 0.0420 (0.003) | 0.0387 (0.003) |
| p = .020 | p = .016 | ||
Notes: AUC = area under curve; CVD = cardiovascular disease. Inference is based on re-randomization tests. Parenthesis represents SE. Positive values reflect relative benefit for intensive lifestyle intervention.
Among all deficits included in the FISPR and FIAUG, those that increased most across 8 years are reflective of diabetes and obesity: neuropathy, insulin usage, and sleep apnea. The prevalence of participants reporting stopped breathing during sleep increased from 12% to 33% in DSE participants and from 13% to 30% among ILI participants. The prevalence of insulin use increased from 16% to 37% in DSE individuals and from 15% to 31% in ILI individuals. The prevalence of participants reporting a diagnosis of diabetic neuropathy increased from 12% to 25% in DSE participants and from 13% to 26% in ILI participants.
Discussion
We showed that a multidomain lifestyle intervention administered to overweight and obese adults with type 2 diabetes in midlife and early late-life appears to buffer against the accumulation of age-related deficits when compared with diabetes support and education. This benefit was apparent irrespective of the precise composition of deficits used in the two FIs we explored. Adding components sensitive to diabetes and obesity did not materially improve the performance of the FI: this provides support that the precise composition of FIs may not be as important as having sufficient numbers of nonoverlapping components, whose incremental increases over time collectively contribute to changes in the FI. The overall magnitude of the benefit was not large, at least compared with the range of FI scores at baseline. Across follow-up, FI scores for ILI participants were 5%–6% lower, and at year 8, the prevalence of frailty was 3%–6% lower. The significant intervention effects documented in Look AHEAD for disability-free life years (6%–8%, depending on age) and all-cause hospitalizations (11%) were also modest but important (18,19).
After the initial year of intervention, FIs in the DSE group increased by about 0.03 units over the subsequent 7 years, that is, about 0.0043 units per year. For comparison, the FI used in the Longitudinal Aging Study Amsterdam representative sample of adults aged 65 (mean 76) years and older at baseline, increased at a rate of 0.013 units per year across 17 years of follow-up (20), that is, about three times the rate of DSE participants who were 20 years younger.
Diabetes and obesity have been described as accelerating biological aging, resulting in lost muscle mass and strength (21), vascular diseases such as atherosclerosis and microvascular dysfunction (22–24), telomere shortening (25,26), accrual of age-related chronic diseases (27), changes in brain structure and function (28), cognitive decline (29), cell senescence (30), and age-related changes in immunological function (31). To capture broadly these interdependent processes, an index cutting across many potential underlying health domains may be more informative than measures focused on individual pathways. An advantage of the deficit accumulation approach is that indices can be assembled from data collected in many contexts, as long as there is a sufficiently rich panel of age-related deficits (32). Multidomain lifestyle interventions have the potential to slow many of the processes listed above. Complex mediation analyses would be required to identify the most influential mechanisms toward benefit, and the relative importance of individual components for predicting outcomes may vary across clinical subgroups, for example, by sex (33).
It is unclear the extent that FIs reflect biological aging. Further work is necessary for validation by examining the associations of FIs with other indices of aging, including health span. Importantly, the ability of any index to serve as a surrogate for an outcome such as biological aging in a clinical trial can only be established in the context of an effective intervention (34,35). Demonstrating that the Look AHEAD multidomain lifestyle intervention conveyed benefits on the FIs, and also on the other accepted measures of disability-free life years and multimorbidity (18,19), indicates the potential value of the trial as a resource to validate surrogate makers of health span and aging-related health status.
Relative benefits from ILI on FI appeared to accrue within the first few years. It is during this time that ILI was most intense and the greatest weight loss was achieved (10). Of some potential concern however, following the initial decline in FI scores from baseline to year 1, scores in the ILI group thereafter appeared to increase at a slightly greater rate in the ILI compared with DSE cohort, although never closing the gap between cohorts completely. This convergence may correspond to a mean regain of weight (primarily adipose tissue) in the ILI group following the initial year (36). Increased adipose tissue is associated with greater cellular senescence and inflammation, biological mechanisms thought to lead to functional and metabolic decline (3), which may account for these findings. In the ILI cohort, weight cycling, compared with maintained weight loss, was associated with poorer physical function (37).
ILI appeared to be equally beneficial toward buffering against increases in the FIs over time for women and men and for individuals with diabetes durations of less than 5 years versus longer durations. Importantly, there is evidence that the ILI effect on the FIs was stronger for older individuals, nonobese individuals, and those without history of cardiovascular disease. In Look AHEAD, older individuals achieved greater weight loss and comparable increases in physical activity than younger individuals (38). Although obese participants in ILI were successful in losing weight (39), they predominantly remained obese, which may have counteracted any potential benefits from ILI. Prevalent cardiovascular disease may identify individuals who have passed a window of opportunity for ILI benefits. In Look AHEAD, ILI appeared to benefit a number of individual age-related conditions more strongly among these subgroups: physical function among older participants (39); several metabolic risk factors (10,41), cognitive function (42), nephropathy (43), and cognitive impairment (44) among those with lower BMI; and health care costs (19), physical function (45), and cognitive function (46) for those without cardiovascular disease history.
The model of deficit accumulation is very different from the frailty phenotype of Fried (47) and should not be conflated. The phenotypic conception of frailty reflects clinicians’ impressions of highly vulnerable patients and focuses on measures in domains identified by geriatricians. Conversely, the model of deficit accumulation derives from engineering, where frailty refers to the likelihood of a material or system failure. These approaches are related: the five factors included in the phenotype could be included within an FI. However, the phenotype is problematic in trials of caloric restriction as weight loss is one of its criteria: unless intent is somehow considered, a caloric restriction intervention may appear to exacerbate frailty simply by inducing weight loss. Changes in the phenotype also depend on an individual crossing measurement thresholds for gait speed and grip strength. Given the number of deficits typically included (≥30), FIs tend to have a more dynamic range, likely improving sensitivity to change and providing greater statistical power.
Limitations
The Look AHEAD cohort consists of eligible volunteers for a randomized weight-loss trial and may not reflect more general clinical populations. The components we have included in the two FIs are based on data collected by Look AHEAD; other sets of components may yield different results. We have relied on self-reported clinical events rather than adjudication, consistent with other FIs. We did not address mortality in our analyses, although the mortality rate was similar between the groups (11). FIAUG includes nine additional deficits that have not been previously used in other studies: future studies should validate this index. The additional deficits included in FIAUG mostly reflect disease-specific severity: it is possible that improvements in diabetes-specific complications or glucose levels contribute to observed improvements in FIAUG.
Summary
Based on a deficit accumulation approach, our results provide further evidence that multidomain lifestyle interventions may buffer against declines in individuals’ age-related health status.
Funding
This research was funded by two diversity supplements to the Action for Health in Diabetes Extension Study Biostatistics Research Center (3U01DK057136-19S1 and 3U01DK057136-19S2). The funding for the parent award is from U01DK057136. The Action for Health in Diabetes is supported through the following cooperative agreements from the National Institutes of Health (DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992). The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. Additional support was received from the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical and Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); Frederic C. Bartter General Clinical Research Center (M01RR01346); and the Wake Forest Alzheimer’s Disease Core Center (P30AG049638-01A1).
Supplementary Material
Acknowledgments
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson and Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. This manuscript is based on a subset of the Look AHEAD cohort: participants from the Southwest Native American sites are not included. The complete cohort has been described (The Look AHEAD Research Group. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) research study. Diabetes Vasc Dis Res. 2006;3:202–215. NIH Registration: NIHMS81811). The analyses performed herein were not conducted at the Look AHEAD Data Coordinating Center. This does not represent the work of the Look AHEAD study group. All authors contributed to the conceptualization and preparation of this manuscript. F.S., D.O., and M.A.E. collaborated on the analysis. F.S. and M.A.E. wrote the initial draft. N.M.P., B.N., S.K,. A.B., and F.I. reviewed and edited drafts, provided critical expertise and insights, and have approved the final submission.
Conflict of Interest
None reported.
References
- 1. Morley JE. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 2008;24:395–405. doi:10.1016/j.cger.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 2. Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15:996–997. doi:10.1038/nm0909-996 [DOI] [PubMed] [Google Scholar]
- 3. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi:10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc. 2019;67:1559–1564. doi:10.1111/jgs.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kane AE, Gregson E, Theou O, Rockwood K, Howlett SE. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience. 2017;39:221–229. doi:10.1007/s11357-017-9967-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 2018;35:838–845. doi:10.1111/dme.13644 [DOI] [PubMed] [Google Scholar]
- 8. de Souto Barreto P, Rolland Y, Maltais M, Vellas B; MAPT Study Group Associations of multidomain lifestyle intervention with frailty: secondary analysis of a randomized controlled trial. Am J Med. 2018;131:1382.e7–1382.e13. doi:10.1016/j.amjmed.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 9. The Look AHEAD Research Group. Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi:10.1016/S0197-2456(03)00064-3 [DOI] [PubMed] [Google Scholar]
- 10. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med. 2013;369:145–154. doi:10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi:10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Look AHEAD Research Group. The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials. 2011;8:320–329. doi:10.1177/1740774511405858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pajewski NM, Williamson JD, Applegate WB, et al. ; SPRINT Study Research Group Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi:10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espeland MA, Dutton GR, Neiberg RH, et al. ; Action for Health in Diabetes (Look AHEAD) Research Group Impact of a multidomain intensive lifestyle intervention on complaints about memory, problem-solving, and decision-making abilities: the action for health in diabetes randomized controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2018;73:1560–1567. doi:10.1093/gerona/gly124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi:10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 17. Proschan MA, Dodd LE. Re-randomization tests in clinical trials. Stat Med. 2019;38:2292–2302. doi:10.1002/sim.8093 [DOI] [PubMed] [Google Scholar]
- 18. Gregg EW, Lin J, Bardenheier B, et al. ; Look AHEAD Study Group Impact of intensive lifestyle intervention on disability-free life expectancy: the Look AHEAD Study. Diabetes Care. 2018;41:1040–1048. doi:10.2337/dc17-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Espeland MA, Glick HA, Bertoni A, et al. ; Look AHEAD Research Group Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37:2548–2556. doi:10.2337/dc14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoogendijk EO, Rockwood K, Theou O, et al. Tracking changes in frailty throughout later life: results from a 17-year longitudinal study in the Netherlands. Age Ageing. 2018;47:727–733. doi:10.1093/ageing/afy081 [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Kim D, Kim C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: a meta-analysis. Diabetes Ther. 2017;8:459–473. doi:10.1007/s13300-017-0258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scuteri A, Cunha PG, Rosei EA, et al. ; MARE Consortium Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis. 2014;233:654–660. doi:10.1016/j.atherosclerosis.2014.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assar ME, Angulo J, Rodríguez-Mañas L. Diabetes and ageing-induced vascular inflammation. J Physiol. 2016;594:2125–2146. doi:10.1113/JP270841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzik TJ, Cosentino F. Epigenetics and immunometabolism in diabetes and aging. Antioxid Redox Signal. 2018;29:257–274. doi:10.1089/ars.2017.7299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monickaraj F, Aravind S, Gokulakrishnan K, et al. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem. 2012;365:343–350. doi:10.1007/s11010-012-1276-0 [DOI] [PubMed] [Google Scholar]
- 26. Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev. 2012;11:220–229. doi:10.1016/j.arr.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 27. Smith AE, Molton IR, Jensen MP. Self-reported incidence and age of onset of chronic comorbid medical conditions in adults aging with long-term physical disability. Disabil Health J. 2016;9:533–538. doi:10.1016/j.dhjo.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 28. Kullmann S, Callaghan MF, Heni M, et al. Specific white matter tissue microstructure changes associated with obesity. Neuroimage. 2016;125:36–44. doi:10.1016/j.neuroimage.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi:10.1001/archinte.163.13.1524 [DOI] [PubMed] [Google Scholar]
- 30. Yuan Q, Hu CP, Gong ZC, et al. Accelerated onset of senescence of endothelial progenitor cells in patients with type 2 diabetes mellitus: role of dimethylarginine dimethylaminohydrolase 2 and asymmetric dimethylarginine. Biochem Biophys Res Commun. 2015;458:869–876. doi:10.1016/j.bbrc.2015.02.050 [DOI] [PubMed] [Google Scholar]
- 31. Yang H, Youm YH, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi:10.1182/blood-2009-03-213595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi:10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 33. Dallmeier D, Braisch U, Rapp K, et al. Frailty index and sex-specific 6-year mortality in community-dwelling older people: the ActiFE Study. J Gerontol A Biol Sci Med Sci. 2019. doi:10.1093/Gerona/glz075 [DOI] [PubMed] [Google Scholar]
- 34. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi:10.1093/jnci/djn515 [DOI] [PubMed] [Google Scholar]
- 35. Espeland MA, O’Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi:10.1186/1468-6708-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pownall HJ, Schwartz AV, Bray GA, et al. ; Look AHEAD Research Group Changes in regional body composition over 8 years in a randomized lifestyle trial: the Look AHEAD study. Obesity (Silver Spring). 2016;24:1899–1905. doi:10.1002/oby.21577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beavers KM, Neiberg RH, Houston DK, et al. Body weight dynamics following intentional weight loss and physical performance: the Look AHEAD Movement and Memory Study. Obes Sci Pract. 2015;1:12–22. doi:10.1002/osp4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Espeland MA, Rejeski WJ, West DS, et al. Intensive weight loss intervention in individuals aged 65 years or older. J Am Geriatr Assoc. 2013;61:912–922. doi:10.1111/jgs.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unick JL, Beavers D, Bond DS, et al. ; Look AHEAD Research Group The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126:236–242, 242.e1. doi:10.1016/j.amjmed.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Houston DK, Neiberg RH, Miller ME, et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: the Look AHEAD Study. J Gerontol A Biol Sci Med Sci. 2018;73:1552–1559. doi:10.1093/gerona/glx204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four year results of the Look AHEAD Trial. Arch Intern Med. 2010;170:1566–1575. doi:10.1001/archinternmed.2010.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Espeland MA, Rapp SR, Bray GA, et al. ; Action for Health In Diabetes (Look AHEAD) Movement and Memory Subgroup; Look AHEAD Research Group Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69:1101–1108. doi:10.1093/gerona/glu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:801–809. doi:10.1016/S2213-8587(14)70156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Espeland MA, Luchsinger JA, Baker LD, et al. ; Look AHEAD Study Group Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88:2026–2035. doi:10.1212/WNL.0000000000003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rejeski WJ, Bray GA, Chen SH, et al. Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci. 2015;70:345–353. doi:10.1093/gerona/glu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Espeland MA, Carmichael O, Hayden K, et al. Long-term impact of weight loss intervention on changes in cognitive function: exploratory analyses from the Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2018;73:484–491. doi:10.1093/gerona/glx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.