Abstract
Background
Clinically meaningful change (CMC) for frailty index (FI) scores is little studied. We estimated the CMC by associating changes in FI scores with changes in the Clinical Frailty Scale (CFS) in hospitalized patients.
Methods
The Serious Outcomes Surveillance Network of the Canadian Immunization Research Network enrolled older adults (65+ years) admitted to hospital with acute respiratory illness (mean age = 79.6 ± 8.4 years; 52.7% female). Patients were assigned CFS and 39-item FI scores in-person at admission and via telephone at 1-month postdischarge. Baseline frailty state was assessed at admission using health status 2 weeks before admission. We classified those whose CFS scores remained unchanged (n = 1,534) or increased (n = 4,390) from baseline to hospital admission, and whose CFS scores remained unchanged (n = 1,565) or decreased (n = 2,546) from admission to postdischarge. For each group, the CMC was represented as the FI score change value that best predicted one level CFS change, having the largest Youden J value in comparison to no change.
Results
From baseline to admission, 74.1% increased CFS by ≥1 level. From admission to postdischarge, 61.9% decreased CFS by ≥1 levels. A change in FI score of 0.03 best predicted both one-level CFS increase (sensitivity = 70%; specificity = 69%) and decrease (sensitivity = 66%; specificity = 61%) in comparison to no change. Of those who changed CFS by ≥1 levels, 70.9% (baseline to admission) and 72.4% (admission to postdischarge) changed their FI score by at least 0.03.
Conclusions
A clinically meaningful change of 0.03 in the frailty index score holds promise as a benchmark for assessing the meaningfulness of frailty interventions.
Keywords: Aging, Clinical frailty scale, Frailty index, Clinically meaningful change
Frailty is the degree of vulnerability after experiencing a stressor, due to age-related decrements in multiple physiological systems (1). Severely frail people experience prolonged multisystem health complications and require more health care resources than their nonfrail counterparts. In many people, frailty can be prevented or successfully managed (2,3). Addressing frailty systematically is paramount for guiding patient care plans and informing public policy.
One approach for assessing this common age-associated state, the frailty index (FI), uses the proportion of deficits accrued in a set of health-related deficits to encapsulate an individual’s degree of vulnerability (4), providing a continuous score from 0 to 1. Any FI should encompass information about a variety of physiological systems, as well as manifestations of their single or combined deficiencies (eg, in cognition, function, mobility). There is no requirement that FIs always contain the same items or are measured in the same way; the FI pertains to a specific setting, while still being general enough to reflect an individual’s overall health. The FI must include a minimum of 30 items to robustly assess the multifactorial nature of frailty. It has shown strong utility for predicting risk of mortality (5–8), functional decline (7–9), and use of healthcare resources (10–12). The FI has been applied to research practice around the world, including an electronic FI which has been gaining traction in routine care in England (13) and the United States (14). In this context, defining the clinically meaningful change (CMC) for the FI can help patients better understand changes in their health and clinicians gauge the efficacy of frailty interventions.
The International Conference on Frailty and Sarcopenia Research Task Force recently commented that for frailty clinical studies, clinically meaningful outcome measures are needed to monitor disease progression and efficacy of interventions, and to design future clinical trials (15). A CMC refers to the difference in a continuous measure that best predicts the smallest clinically meaningful change in a strongly associated measure of the same outcome (16–18). The Clinical Frailty Scale (CFS) (19) is a useful benchmark for establishing CMC for frailty tools because it uses a clinician’s judgment of the patient’s frailty level and has distinct, graded categories. Change in a CFS score is meant to indicate that a health care professional has evaluated a patient’s health as being significantly different from their last evaluation (20). The convenience and utility of the CFS have led to its implementation internationally, and like the FI, the CFS strongly predicts adverse outcomes (21–23). Here, our objective was to explore the CMC in the FI by associating changes in the continuous FI scores with changes in the CFS.
Methods
Data Source
The Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN) collects longitudinal health data from older adults (aged 65+ years) admitted to hospital with acute respiratory illness. Each influenza season, CIRN SOS records real-time data on patient health status from 10 to 45 hospitals across Canada. Details regarding original data collection and ethics approval have been described previously (24,25). Secondary analysis for this study was exempt from research ethics board review in accordance with section 4.2.3 of the Nova Scotia Health Authority Standard Operating Procedure #NSHA-REB-SOP-4-001 (revised September 2017).
Sample
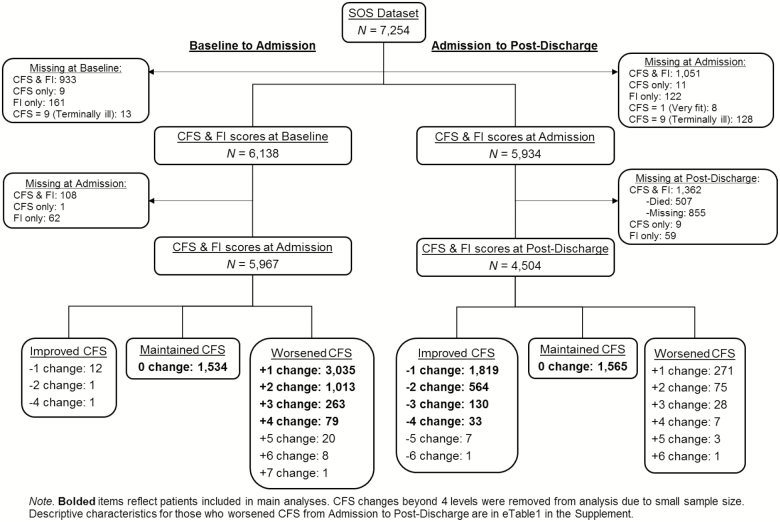
Here, we analyzed CIRN SOS health data collected during three influenza seasons (2011/2012, 2012/2013, and 2013/2014) for 7,254 patients aged 65 or older. Data were collected upon admission to hospital (also collected details on baseline functioning 2 weeks prior) and via telephone interview at 30 days postdischarge (“postdischarge”). Our two subgroups of interest were patients who were in the same or poorer health state upon admission compared to baseline and those who maintained or improved their health between admission and postdischarge (Figure 1).
Figure 1.
Flowchart
In total, 1,274 patients from baseline to admission analyses and 2,614 patients from admission to postdischarge analyses were excluded due to missing either CFS or FI scores; 507 patients died between admission and 30 days postdischarge. We also excluded 13 patients from the baseline to admission analyses, and 128 patients from admission to postdischarge analyses with CFS scores of 9 (“Terminally ill”) due to the unlikely possibility of change at follow-up. Similarly, eight patients with CFS scores of 1 (“Very fit”) at admission were excluded as these patients could not improve further between admission and postdischarge (Figure 1). We removed an additional 14 individuals who became less frail from baseline to admission, and 385 individuals who became frailer from admission to postdischarge. The 385 patients excluded from admission to postdischarge were older, less frail, and had a longer length of stay than those who maintained or improved CFS, but had similar proportions of females included (see Supplementary Table S1). These individuals were removed to better address the purpose of this paper, to look at the minimal frailty change expected for a typical hospitalization. It is generally expected that most people who develop acute illness are worse at admission compared to baseline and better at postdischarge compared to admission. Even so, some people (here 8.6%) are worse at postdischarge compared to admission due to a variety of reasons. These individuals, which were excluded here, should be included when examining recovery and changes in frailty during hospitalization. Final samples were 5,924 for baseline to admission analyses and 4,111 for admission to postdischarge analyses (Figure 1).
Clinical Frailty Scale
CFS scores initially ranged from 1 (“Very fit”) to 7 (“Severely frail”) (18); later, levels 8 (“Very severely frail”) and 9 (“Terminally ill”) were added. CFS scores were assigned by the SOS Network monitors who interviewed patients at admission, reviewed their medical records, and conducted telephone interviews 1 month after discharge from hospital.
Frailty Index
An FI was constructed using the deficit accumulation method described by Searle et al. (4), comprised of 39 health-related items (eg, cognition, I/ADLs, chronic conditions, medications) calculated for baseline, admission, and 1-month postdischarge (items and methodology described previously (24)). All FI items collected in-person at admission were also collected via telephone interview at postdischarge. Like the CFS, a higher FI score indicates more severe frailty. Theoretically, FI scores can range from 0 to 1; however, it is less common to see scores reflecting the presence of more than two thirds of the deficits being measured (ie, for this FI of 39 items, a submaximal limit of ~26 deficits) (4), and the 99% percentile is consistently less than 0.7. Beyond these observed limits, the rate of deficit accumulation is negligible (26).
Clinically Meaningful Change
To calculate the CMC, we utilized an anchor-based approach where the change in the outcome measure is compared to the smallest possible change in another clinical measure—the anchor—that is theoretically related and strongly correlated to the outcome measure (18,27). For this study, the CMC is represented by the degree of FI change that best predicts the smallest possible (one-level) increase or decrease in CFS compared to stability. It can also be calculated as the amount of frailty change needed to produce at least a small meaningful effect (a Cohen’s d ≥0.2) (28). Since the CMC value can be affected by the direction of patient health changes (18), it was calculated separately for prehospitalization (baseline to admission) and posthospitalization (admission to postdischarge). It is generally expected that a patient will worsen their condition until they have entered the hospital, and improve over the course of their admission.
Statistical Analyses
Descriptive characteristics of the sample are expressed as the mean scores ± standard deviation. Mean FI changes were calculated separately for baseline to admission and admission to postdischarge analyses. Worsened frailty was represented by an increase in CFS score (eg, from 2 to 6; a change of +4), and improved frailty by a decrease in CFS score (eg, from 3 to 1; a change of −2). Two receiver operating characteristic (ROC) curves mapped the sensitivity and 1-specificity values for each FI change value predicting a one-level CFS increase (baseline to admission) or a one-level CFS decrease (admission to postdischarge) in comparison to no change in the CFS. Inherent to the grading of CFS, moving up or down a category is considered the smallest significant change in state. Any CFS changes greater than this are informative but lie at least one level above the minimum. For the purpose of this study, defining the minimum change in FI that maps to the smallest significant change in CFS required excluding CFS changes larger than one level from the ROC analyses.
For each group, the CMC was reflected by the FI change value with the largest Youden Index (J) value, representing the point on the ROC curve with the largest vertical distance to the diagonal line of chance, the optimal combination of sensitivity and specificity. This value was compared to the change in FI needed to produce at least a small meaningful effect (a Cohen’s d ≥0.2), calculated as the product of d and the standard deviation of baseline (baseline to admission group) or admission (admission to postdischarge group) FI scores (28). Prevalence of the selected CMC and general mean changes in FI per degree of CFS change were also explored. All analyses were completed using IBM SPSS Statistics Version 25.0.
Results
Baseline to Admission
The first set of analyses comprises 5,924 individuals who increased (N = 4,390; MAge = 79.7 ± 8.3 years; 52.4% female) or maintained (N = 1,534; MAge = 79.4 ± 8.46 years; 54.4% female) their CFS scores from baseline to admission (Table 1). Spearman’s rank correlation coefficients between CFS and FI scores at baseline and admission were 0.68 and 0.74 (p < .001), respectively.
Table 1.
Descriptive Characteristics
| Baseline to Admission | Admission to Postdischarge | |||||||
|---|---|---|---|---|---|---|---|---|
| Maintained CFS (N = 1,534) | Worsened CFS (N = 4,390) | Maintained CFS (N = 1,565) | Improved CFS (N = 2,546) | |||||
| Age (Myears ± SD) | 79.4 ± 8.5 | 79.7 ± 8.3 | 79.4 ± 8.3 | 78.6 ± 8.1 | ||||
| Median | 80.0 | 80.0 | 80.0 | 79.0 | ||||
| % Female (N) | 54.4% (834) | 52.4% (2,300) | 54.2% (849) | 53.1% (1,353) | ||||
| LOS (Mdays ± SD) | 9.5 ± 10.4 | 11.5 ± 13.1 | 9.7 ± 9.3 | 10.0 ± 10.5 | ||||
| Median | 7.0 | 8.0 | 7.0 | 7.0 | ||||
| FI Change (M ± SD) | 0.02 ± 0.04 | 0.08 ± 0.07 | −0.03 ± 0.06 | −0.08 ± 0.07 | ||||
| Median | 0.00 | 0.06 | −0.02 | −0.06 | ||||
| CFS Change (M ± SD) | 0.0 ± 0.0 | 1.4 ± 0.7 | 0.0 ± 0.0 | −1.4 ± 0.6 | ||||
| Median | 0.0 | 1.0 | 0.0 | −1.0 | ||||
| Baseline | Admission | Baseline | Admission | Admission | Postdischarge | Admission | Postdischarge | |
| FI Score (M ± SD) | 0.23 ± 0.14 | 0.25 ± 0.14 | 0.22 ± 0.12 | 0.30 ± 0.13 | 0.27 ± 0.14 | 0.24 ± 0.14 | 0.27 ± 0.12 | 0.19 ± 0.11 |
| Median | 0.21 | 0.23 | 0.21 | 0.28 | 0.26 | 0.21 | 0.26 | 0.18 |
| CFS Scores (M ± SD) | 4.7 ± 1.6 | 4.7 ± 1.6 | 4.2 ± 1.4 | 5.6 ± 1.4 | 5.0 ± 1.5 | 5.0 ± 1.5 | 5.4 ± 1.2 | 4.0 ± 1.3 |
| Median | 4.0 | 4.0 | 4.0 | 6.0 | 5.0 | 5.0 | 5.0 | 4.0 |
| 1 (Very fit) | 7 | 7 | 37 | 0 | N/A | N/A | N/A | 22 |
| 2 (Well) | 48 | 48 | 244 | 13 | 31 | 31 | 7 | 147 |
| 3 (Managing well) | 407 | 407 | 1,257 | 150 | 296 | 296 | 85 | 875 |
| 4 (Vulnerable) | 350 | 350 | 1,150 | 896 | 338 | 338 | 612 | 657 |
| 5 (Mildly frail) | 183 | 183 | 850 | 978 | 228 | 228 | 650 | 444 |
| 6 (Moderately frail) | 269 | 269 | 587 | 1,154 | 329 | 329 | 659 | 318 |
| 7 (Severely frail) | 240 | 240 | 230 | 823 | 299 | 299 | 438 | 83 |
| 8 (Very severely frail) | 30 | 30 | 35 | 295 | 44 | 44 | 95 | 0 |
| 9 (Terminally ill) | N/A | N/A | N/A | 81 | N/A | N/A | N/A | N/A |
Note: Samples do not include CFS changes >±4 levels due to small sample size (see Figure 1). CFS = Clinical Frailty Scale. FI = Frailty Index; LOS = length of hospital stay; M = mean; SD = standard deviation.
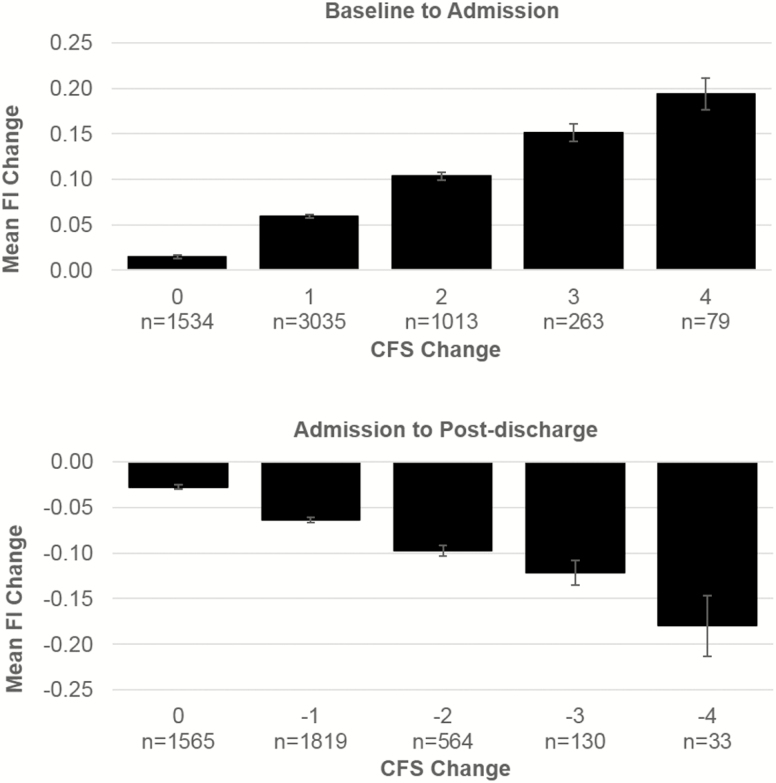
Overall, 25.9% of this sample had no change at CFS score at admission compared to baseline, 51.2% increased by one level, 17.1% by two levels, 4.4% by three levels, 1.3% by four levels, and 0.5% increased more than four levels (maximum seven levels change; Figure 1). On average, patients who worsened CFS by one level had a mean FI change of 0.06 ± 0.06, which increased steadily up to 0.19 ± 0.08 for those who worsened CFS by four levels (Figure 2).
Figure 2.
Mean FI change per level of CFS change. CFS = Clinical Frailty Scale; FI = Frailty Index.
The area under the ROC for predicting one-level worsening in CFS compared to no change was 0.76 (95% confidence interval [CI]: 0.74–0.77). An FI change of 0.03 was the most effective for predicting a one-level increase in CFS, having the largest Youden Index (J) of 0.40 (sensitivity = 70%, specificity = 69%; Table 2 and Supplementary Table S2). The optimal FI change was consistent when stratifying by sex (Table 2). The minimal FI change needed for a Cohen’s d ≥0.2 was 0.025 (0.2 × 0.1247). Approximately two thirds of the participants who worsened CFS by one level had an FI change of 0.03 or greater, and this proportion grew more substantial with each additional CFS level change (Table 3, Supplementary Table S3, and Supplementary Table S4).
Table 2.
Optimal FI Change Values for Predicting One-Level Change in CFS
| Baseline to Admission | N with no CFS Change | N with +1 CFS Change | AUC Predicting +1 CFS Change (95% CI) | Change in FI with Highest J | J (Sensitivity, Specificity) |
|---|---|---|---|---|---|
| All | 1,534 | 3,035 | 0.76 (0.74–0.77) | 0.026 | 0.40 (0.70, 0.69) |
| Males | 700 | 1,417 | 0.75 (0.73–0.77) | 0.026 | 0.40 (0.69, 0.71) |
| Females | 834 | 1,618 | 0.76 (0.75–0.78) | 0.029 | 0.40 (0.65, 0.76) |
| Admission to Postdischarge | N with no CFS Change | N with -1 CFS Change | AUC Predicting -1 CFS Change (95% CI) | Change in FI with Highest J | J (Sensitivity, Specificity) |
| All | 1,565 | 1,819 | 0.68 (0.66–0.70) | −0.032 | 0.27 (0.66, 0.61) |
| Males | 716 | 855 | 0.67 (0.64–0.69) | −0.025 | 0.25 (0.75, 0.50) |
| Females | 849 | 964 | 0.69 (0.66–0.71) | −0.031 | 0.30 (0.69, 0.61) |
Note: Only patients who maintained or changed CFS by 1 level were included in this analysis. AUC = area under the ROC curve; CFS = Clinical Frailty Scale; CI = confidence interval; FI = Frailty Index; J = Youden Index value for the change in FI that best predicts a one-level change in CFS.
Table 3.
Proportion of People Who Changed FI by more than 0.03 Based on Level of CFS Change
| Baseline to Admission | CFS Change | N | N (%) Who Changed FI ≥ 0.03 |
|---|---|---|---|
| 0 | 1,534 | 356 (23.2%) | |
| +1 | 3,035 | 1,899 (62.6%) | |
| +2 | 1,013 | 890 (87.9%) | |
| +3 | 263 | 246 (93.5%) | |
| +4 | 79 | 78 (98.7%) | |
| Admission to Postdischarge | CFS Change | N | N (%) Who Changed FI ≤ −0.03 |
| 0 | 1,565 | 623 (39.8%) | |
| −1 | 1,819 | 1,215 (66.8%) | |
| −2 | 564 | 481 (85.3%) | |
| −3 | 130 | 115 (88.5%) | |
| −4 | 33 | 32 (97.0%) |
Note: CFS = Clinical Frailty Scale; FI = Frailty Index. CFS change = 0 indicates no change. Percentages reflect the proportion of n within each degree of CFS change whose FI changed by at least +0.03 (baseline to admission) or −0.03 (admission to postdischarge). CFS changes beyond ±4 are not reported due to small sample size.
Admission to Postdischarge
Analyses were also done for 4,111 individuals who maintained (MAge = 79.4 ± 8.3 years; 54.2% female) or decreased (MAge = 78.6 ± 8.1 years; 53.1% female) their CFS scores from admission to postdischarge (Table 1). Spearman’s rank correlation coefficients between CFS and FI scores at admission and postdischarge were 0.72 and 0.68 (p < .001), respectively.
Overall, 38.1% of this group had no change of CFS score at postdischarge compared to admission, 44.3% decreased by one level, 13.7% by two levels, 3.2% by three levels, 0.8% by four levels, and 0.2% decreased by more than four levels (maximum six levels change; Figure 1). The mean FI change ranged from −0.06 ± 0.06 for patients who improved CFS by one level, up to −0.18 ± 0.09 for those who improved CFS by four levels (Figure 2).
An ROC where FI changes predicted a one-level decrease in CFS score compared to no change yielded an area under the curve of 0.68 (95% CI: 0.66, 0.70). The optimal FI change coordinate was −0.03, where J = 0.27 (sensitivity = 66%, specificity = 61%; Table 2 and Supplementary Table S2). This value was consistent when stratifying by sex, except that it was marginally smaller for males (Table 2). The minimal FI change needed for a Cohen’s d ≥0.2 was 0.026 (0.2 × 0.1292). Similar to the previous group, roughly two thirds of the participants who improved CFS by one level had an FI change of at least 0.03 with this proportion growing considerably with each additional CFS level change (Table 3, Supplementary Table S3, and Supplementary Table S4).
Discussion
This study showed that a CMC of 0.03 for the FI could identify changes in the level of frailty of hospitalized patients between baseline to admission and from admission to 1-month postdischarge. In an FI including 30 or more items, such as the one used in this study, this translates to at least a one-deficit change (ie, 1/30 or 1/40 = 0.03) indicating that at minimum, a one-deficit change is a significant improvement or deterioration in frailty state.
The FI is often used as a predictor in longitudinal observational studies but rarely as an outcome measure (29,30). The evidence is even more limited for using the FI as an outcome measure in interventional studies (29,31–33). When examined as a research outcome, the FI is usually tested as a continuous variable (29,30,34,35). Though the value of this is important for research purposes, the magnitude of any FI change may not always translate to clinical implications. Only one study (36) has identified what constitutes a clinically meaningful FI change. They identified a small CMC for the FI of 0.03, supporting our findings in this study. A small number of randomized control trials (RCT) have used change in FI as an outcome. The earliest considered any change in FI to be an improvement due to testosterone and nutritional supplementation (34). Others have used a one-deficit change as an appropriate benchmark (20), while others have used a specific change of 0.03 (35,37) to represent significant posttreatment change. An RCT examining the effects of multidomain lifestyle interventions on frailty in patients with diabetes found an approximately one deficit (~0.03 for their 38-item FI) drop upon 1-year follow-up (38). Another RCT targeting cognitive function found a reduction in FI of 0.02 (95% CI 0.02–0.03) after 6 months (39). A study using L-carnitine supplementation found that FI scores changed by 0.02 at 5-week follow-up from baseline, jumping to a difference of 0.04 by 10 weeks (40).
A limitation of this study is that, as is routine care for this clinical setting, baseline and admission medical health history were collected concurrently. It is generally expected that a patient’s condition will be worse in the lead up to hospital admission. While this expectation can lead to greater disparity between baseline and admission frailty scores, we expect that any bias that affects baseline frailty scores affected both CFS and FI in a similar manner. Therefore, while the magnitude of change within each tool can possibly become inflated, it is unlikely that the relationship between them was undermined. Further, the area under the curve for the admission to discharge ROC was 0.68—below the generally accepted level of 0.7. Lower AUCs are common in frailty studies and could be related to the heterogeneity of older adults within clinical settings and the multidomain nature of frailty. Also, a 0.03 change in the FI is not experienced equally at all levels of frailty. How this change manifests among these levels would be valuable to investigate.
Further, we excluded patients who died during hospitalization and a minority of patients who worsened between admission and postdischarge. These patients are important to include when examining the impact of hospitalization on frailty. The CFS increase was similar between the groups who worsened between baseline to admission and those who worsened between admission and postdischarge. Even so, the FI increase was much higher for the baseline to admission CFS worsening group with 70.9% of the patients experiencing an FI increase greater than 0.03; only 35.6% of the patients of the admission to postdischarge CFS worsening group experienced this CMC FI increase. It is even more surprising that 21.8% of this latter worsening group experienced an FI decrease greater than 0.03. CFS and FI measure the same construct but CFS relies on a clinician’s overall impression and FI on a series of examinations. It is possible that other factors not explained by medical tests could affect the subjective assessment of the clinician for this atypical group. Future studies should investigate this further.
Going forward, this CMC should be validated across different samples and FIs. This study followed patients over the course of hospitalization due to illness. More research should be done using the FI as an outcome in exercise and pharmaceutical interventions. Testing the CMC in such studies will further support its utility. Future work may also distinguish the CMC in different genres of FI, such as an FI constructed from objective markers (eg, FI based on laboratory tests).
This study used a cohort of acutely ill individuals who experienced short-term changes in frailty levels. In nonacutely ill older adults, health changes are likely to occur more gradually. Considering that our benchmark of 0.03 corresponds with the average annual rate of FI change for community-dwelling people (26,41), many individuals in our sample experienced changes much larger than that in a shorter period of time, though their net change from baseline to postdischarge may not have been drastic. Future research should replicate this study in nonacutely ill community-dwelling older adults.
The increasing number of frail people poses a great challenge on health care resources. We need to prioritize frailty management, as well as the continuous development and improvement of frailty assessment tools. Using the CMC identified for the FI, a widely used tool in clinical and research settings, to aid with the interpretation of frailty change can add to the effectiveness of frailty interventions and patient care plans and support the FI’s utility in future clinical trials.
Funding
This work was supported by funding from the Fountain Family Innovation Fund of the QEII Health Sciences Centre Foundation. The SOS Network was supported through an investigator-initiated Collaborative Research Agreement with GlaxoSmithKline Biologicals SA, and by the Public Health Agency of Canada and the Canadian Institutes of Health Research to the Canadian Immunization Research Network (CIRN).
Conflict of Interest
M.K.A. reports grant funding from GlaxoSmithKline (GSK), Pfizer, Sanofi, Canadian Institute of Health Research (CIHR), Public Health Agency of Canada (PHAC), and the Canadian Frailty Network, and honoraria from Pfizer, Sanofi, and the Canadian Frailty Network. S.A.M. has received research grants and consultancy fees from GlaxoSmithKline Biologicals SA and Pfizer and has participated in Clinical Trials funded by GSK, Merck, Novartis, Pfizer, and Sanofi Pasteur. K.R. is President and Chief Science Officer of DGI Clinical, which in the last 5 years has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, Otsuka) on individualized outcome measurement. In 2017, he attended an advisory board meeting with Lundbeck. Otherwise, any personal fees are for invited guest lectures and academic symposia, received directly from event organizers, chiefly for presentations on frailty. He is Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities, as well as, in its first phase (2013–2018), from Pfizer Canada and Sanofi Canada. The rest of the authors have no declarations of interest.
Supplementary Material
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults. JBI Database Syst. 2018;16(1):140–232. doi: 10.11124/JBISRIR-2017-003382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200. doi: 10.1093/ageing/afx162 [DOI] [PubMed] [Google Scholar]
- 6. Orkaby AR, Nussbaum L, Ho Y-L, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257–1264. doi: 10.1093/gerona/gly232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74:1271–1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 10. Han L, Clegg A, Doran T, Fraser L. The impact of frailty on healthcare resource use: a longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing. 2019;48:665–671. doi: 10.1093/ageing/afz088 [DOI] [PubMed] [Google Scholar]
- 11. Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. How do frail Medicare beneficiaries fare under bundled payments? J Am Geriatr Soc. 2019;67:2245–2253. doi: 10.1111/jgs.16147 [DOI] [PubMed] [Google Scholar]
- 12. Comans TA, Peel NM, Hubbard RE, Mulligan AD, Gray LC, Scuffham PA. The increase in healthcare costs associated with frailty in older people discharged to a post-acute transition care program. Age Ageing. 2016;45:317–320. doi: 10.1093/ageing/afv196 [DOI] [PubMed] [Google Scholar]
- 13. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a Medicare accountable care organization. J Gerontol A Biol Sci Med Sci. 2019;74:1771–1777. doi: 10.1093/gerona/glz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guralnik J, Bandeen-Roche K, Bhasin SAR, et al. Clinically meaningful change for physical performance: perspectives of the ICFSR Task Force. J Frailty Aging. 2020;9(1):9–13. doi: 10.14283/jfa.2019.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110:196–202. doi: 10.1002/cncr.22799 [DOI] [PubMed] [Google Scholar]
- 17. Houchen-Wolloff L, Evans RA. Unravelling the mystery of the ‘minimum important difference’ using practical outcome measures in chronic respiratory disease. Chron Respir Dis. 2019;16:1479973118816491. doi: 10.1177/1479973118816491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:171–84. doi: 10.1586/erp.11.9 [DOI] [PubMed] [Google Scholar]
- 19. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Theou O, Park GH, Garm A, Song X, Clarke B, Rockwood K. Reversing frailty levels in primary care using the CARES model. Can Geriatr J. 2017;20:105–111. doi: 10.5770/cgj.20.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregorevic KJ, Hubbard RE, Lim WK, Katz B. The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: a prospective cohort study. BMC Geriatr. 2016;16:117. doi: 10.1186/s12877-016-0292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 23. Kara I, Cicekci F, Nabi Undar H, Seven F, Sizer C. Can clinical frailty scale be used routinely in patients aged 50 years and older in intensive care units? Ann Med Res. 2019;26(2):185–189. doi: 10.5455/annalsmedres.2018.09.203 [DOI] [Google Scholar]
- 24. Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216(4):405–414. doi: 10.1093/infdis/jix282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nichols MK, Andrew MK, Hatchette TF, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: a pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network). Vaccine. 2018;36(16):2166–2175. doi: 10.1016/j.vaccine.2018.02.093 [DOI] [PubMed] [Google Scholar]
- 26. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–496. doi: 10.1016/j.mad.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 27. Lydick E, Epstein RS. Interpretation of quality of life changes. Qual Life Res. 1993;2:221–226. doi: 10.1007/bf00435226 [DOI] [PubMed] [Google Scholar]
- 28. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. PharmacoEcon. 1999;2:141–155. doi: 10.2165/00019053-199915020-00003 [DOI] [PubMed] [Google Scholar]
- 29. Trendelenburg AU, Scheuren AC, Potter P, Müller R, Bellantuono I. Geroprotectors: a role in the treatment of frailty. Mech Ageing Dev. 2019;180:11–20. doi: 10.1016/j.mad.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 30. Hoogendijk EO, Rockwood K, Theou O, et al. Tracking changes in frailty throughout later life: results from a 17-year longitudinal study in the Netherlands. Age Ageing. 2018;47:727–733. doi: 10.1093/ageing/afy081 [DOI] [PubMed] [Google Scholar]
- 31. Theou O, Squires E, Mallery K, et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018;18:139. doi: 10.1186/s12877-018-0823-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theou O, Chapman I, Wijeyaratne L, et al. Can an intervention with testosterone and nutritional supplement improve the frailty level of under-nourished older people? J Frailty Aging. 2016;5:247–252. doi: 10.14283/jfa.2016.108 [DOI] [PubMed] [Google Scholar]
- 35. Kehler DS, Giacomantonio N, Firth W, Blanchard CM, Rockwood K, Theou O. Association between cardiac rehabilitation and frailty. Can J Cardiol. 2020;36:482–489. doi: 10.1016/j.cjca.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 36. Jang IY, Jung HW, Lee HY, Park H, Lee E, Kim DH. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol A Biol Sci Med Sci. 2020;pii:glaa003. doi: 10.1093/gerona/glaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Godin J, Blodgett JM, Rockwood K, Theou O. Replacing sedentary time with light or moderate-vigorous physical activity across levels of frailty. J Aging Phys Act. 2019;28(1):18–23. doi: 10.1123/japa.2018-0361 [DOI] [PubMed] [Google Scholar]
- 38. Simpson FR, Pajewski NM, Nicklas B, et al. Impact of multidomain lifestyle intervention on frailty through the lens of deficit accumulation in adults with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. pii: glz197. In press. doi: 10.1093/gerona/glz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Souto Barreto P, Rolland Y, Maltais M, Vellas B; MAPT Study Group Associations of multidomain lifestyle intervention with frailty: secondary analysis of a randomized controlled trial. Am J Med. 2018;131:1382.e7–1382.e13. doi: 10.1016/j.amjmed.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 40. Badrasawi M, Shahar S, Zahara AM, Nor Fadilah R, Singh DK. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging. 2016;11:1675–1686. doi: 10.2147/CIA.S113287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.