Abstract
Background
Early recognition and diagnosis of allergic bronchopulmonary aspergillosis (ABPA) is critical to improve patient symptoms, and antifungal therapy may prevent or delay progression of bronchiectasis and development of chronic pulmonary aspergillosis.
Objective
A recently commercialized lateral flow assay (Aspergillus ICT) (LDBio Diagnostics, Lyons, France) detects Aspergillus-specific antibodies in <30 minutes, requiring minimal laboratory equipment. We evaluated this assay for diagnosis of ABPA compared to diseased (asthma and/or bronchiectasis) controls.
Methods
ABPA and control sera collected at the National Aspergillosis Centre (Manchester, UK) and/or from the Manchester Allergy, Respiratory and Thoracic Surgery research biobank were evaluated using the Aspergillus ICT assay. Results were read both visually and digitally (using a lateral flow reader). Serological Aspergillus-specific IgG and IgE, and total IgE titres were measured by ImmunoCAP.
Results
For 106 cases of ABPA versus all diseased controls, sensitivity and specificity for the Aspergillus ICT were 90.6% and 87.2%, respectively. Sensitivity for ‘proven’ ABPA alone (n = 96) was 89.8%, and 94.4% for ‘presumed’ ABPA (n = 18). ‘Asthma only’ controls (no bronchiectasis) and ‘bronchiectasis controls’ exhibited 91.4% and 81.7% specificity, respectively. Comparison of Aspergillus ICT result with Aspergillus-specific IgG and IgE titres showed no evident immunoglobulin isotype bias. Digital measurements displayed no correlation between ImmunoCAP Aspergillus-specific IgE level and ICT test line intensity.
Conclusions
The Aspergillus ICT assay exhibits good sensitivity for ABPA serological screening. It is easy to perform and interpret, using minimal equipment and resources; and provides a valuable simple screening resource to rapidly distinguish more serious respiratory conditions from Aspergillus sensitization alone.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an immunologically-mediated pulmonary disorder caused by hypersensitivity to the allergens of Aspergillus species (predominantly A. fumigatus) [1, 2]. The majority of cases complicate asthma—representing an estimated 1–4% of adult asthma cases worldwide [3]—but ABPA can also complicate cystic fibrosis (CF) [4] and in rarer instances, can occur in patients with neither condition [5, 6]. ABPA usually manifests clinically as poorly controlled asthma often with recurrent pulmonary infections, production of thick mucus plugs, and/or fatigue [7]. It remains an under-diagnosed disease in many settings [8, 9], and delays in diagnosis result in an increased likelihood of disease progression and permanent lung damage through the development of bronchiectasis and/or chronic pulmonary aspergillosis (CPA) if left untreated [1]. There are thought to be over 4.5 million adults with ABPA across the world, assuming about 2.5% of adults with asthma are affected [3]; ABPA is rare in children.
Recently revised diagnostic criteria for ABPA [10] relies primarily on serology, including the major requirements of raised total IgE and evidence of Aspergillus sensitivity by raised Aspergillus-specific (Asp) IgE or skin prick testing. In addition, two minor criteria must also be present and may include radiological features consistent with ABPA, eosinophilia, or raised levels of Asp IgG. A review comparing serological tests for identifying ABPA found that assays for total and Asp IgE demonstrated good sensitivity but poor to moderate specificity (40–80%), whereas the precipitins assay for the detection of Asp IgG (& IgA) [11, 12] had high specificity but poor sensitivity [13]. Other serological assays for the detection of Asp IgG are commercially available, such as enzyme-linked immunosorbent assay (ELISA)/enzyme immunoassay (EIA) [14], but their performance is dependent on cut-off values that vary between regional populations as well as for the specific condition being diagnosed [12].
LDBio Diagnostics (Lyons, France) has introduced a new point-of-care lateral flow assay (Aspergillus IgG-IgM ICT) for detection of Asp antibodies. The assay utilizes immunochromatographic technology (ICT) and has been validated against a spectrum of Aspergillus-related diseases [15], including a moderate number (n = 74) of ABPA cases. We recently evaluated patients with CPA and found this assay to have a sensitivity of 90.6% and a specificity of 98.0% [16]. Here we assess its performance in the serological diagnosis of ABPA.
Materials and methods
Serological samples
This study was performed using serum samples collected from 113 ABPA patients, using a combination of convenience sampling from patients identified at the National Aspergillosis Centre (NAC) (Manchester, UK) (n = 67) and samples archived in the Manchester Allergy, Respiratory and Thoracic Surgery (ManARTS) (Manchester, UK) research tissue bank (n = 46). The NAC is a nationally commissioned service providing long-term specialist care for patients with aspergillosis throughout the UK. For convenience sampling, residual sera from patient samples collected as part of routine care between 06/2018 and 01/2019 were identified. Control serum samples obtained from ManARTS were collected under ethical consent between 2011–2016 approved by the Local Research Ethics Committee (LREC, ethics approval 15/NW.0409). All sera were stored at -80°C until use. This study was assessed through the NHS Health Research Authority system (HRA) (http://www.hra-decisiontools.org.uk/research/) and was found to meet the UK NHS definition of a retrospective service evaluation for which formal ethical review was therefore not required.
Each ABPA diagnosis was confirmed by an experienced specialist clinician and classified as ‘proven’ (n = 95) or ‘presumed’ (n = 18). Using ISHAM guidelines [10, 11], the following three criteria were required for a ‘proven’ ABPA diagnosis: (1) total IgE >1000 IU/ml, (2) positive Aspergillus-specific IgE or skin prick test, and (3) any two of the following ‘other’ criteria: [a] positive Aspergillus-specific IgG or precipitins, [b] eosinophils >500 cells/μl, [c] radiological features consistent with ABPA. A ‘presumed’ ABPA diagnosis required (2) and (3) as above, with total IgE between 113–999 IU/ml. ImmunoCAP EIA (Thermo Scientific, Waltham, MA) was used for measurement of total IgE, Aspergillus-specific IgE (positive result >0.35 kUA/ml [kilo-units antigen-specific antibodies/milliliter] IgE), and Aspergillus-specific IgG (positive result >40mgA/L [milligrams antigen-specific antibodies/liter] IgG) levels. The Ouchterlony method [11] was used to detect Aspergillus precipitins (Microgen Bioproducts, Surrey, UK). Within the set of ‘proven’ ABPA cases, seven (7) had ABPA complicating cystic fibrosis and were analysed separately.
Diseased controls were obtained from the ManARTS biobank, a research tissue bank comprised of samples from consenting patients with respiratory and allergic diseases (and other related conditions) and patients undergoing thoracic surgery, at the Northwest Lung Research Centre at Wythenshawe Hospital (Manchester University NHS Trust Foundation). Serum samples from 164 patients with bronchiectasis and/or asthma were identified using two sets of inclusion criteria. ‘Asthma’ control cases (n = 93) were defined as asthmatic patients with a normal chest X-ray and total IgE <1000 IU/ml within one year of sample collection; and with no clinical or diagnostic evidence of CPA, ABPA, bronchiectasis or any other lung diseases that might predispose to CPA including COPD, sarcoidosis, prior tuberculosis, cancer, and rheumatoid arthritis. Patients with Aspergillus sensitivity, allergic rhinitis/nasal polyps, severe food or drug allergies, or anaphylaxis were also excluded from this control group. ‘Bronchiectasis’ control cases (n = 71) included patients with computed tomography evidence of bronchiectasis. Patients with a diagnosis of ABPA, CPA, or Aspergillus bronchitis, or signs of ABPA or CPA on radiology were excluded. Bronchiectasis cases with underlying asthma (n = 48) and/or COPD (n = 10) were included, as well as patients with microbiological (respiratory culture) evidence of Aspergillus colonization (n = 3) or raised Aspergillus-specific IgE (> 0.35 kUA/ml IgE) (n = 5).
Serological analysis
Each sample was tested using the Aspergillus ICT IgG-IgM (LDBio Diagnostics, Lyon, France) lateral flow assay. Test kits were shipped at ambient temperature and stored at 4°C upon receipt. Each batch (10/pack) of ICT cartridges were equilibrated to ambient laboratory temperature before use and were run according to the manufacturer’s instructions. Briefly, 15μl of sera was dispensed onto the ICT sample application pad, followed by four drops of eluting solution (provided with kit). The test was read at 30 minutes and results were interpreted both visually (by ‘eye’) and digitally using the Qiagen ESEQuant LR3 (Lake Constance, Germany) lateral flow reader. Both reads were conducted by the same user, with the visual reading conducted first to eliminate bias resulting from the digital reading. For visual reads, the test was determined positive by the appearance of two lines: a blue positive control (“C”) line, and a black positive test (“T”) line. The appearance of any black line at the “T” marker was considered positive, as recommended in the manufacturer’s guidelines. Using the LR3 lateral flow reader, a positive test was defined by detection of peaks (any height) between 46.0–48.0mm and between 53.5–55.5mm for control and test lines, respectively.
Routine diagnostics
In many cases, Aspergillus-specific IgG and IgE, and total IgE levels were measured on ABPA patient samples as part of routine clinical care. Testing was carried out by the Manchester University NHS Foundation Trust, Department of Immunology, using the automated ImmunoCAP Phadia 1000 System.
Statistical analyses
To assess diagnostic performance, we calculated Youden’s J statistic (sensitivity + specificity– 1), likelihood ratios and the diagnostic odds ratio (DOR) [17]. Binomial confidence intervals (95%) were calculated for sensitivity, specificity, and DOR. Pearson’s chi-square statistic was used for comparisons between subgroups. Spearman’s rank correlation coefficient (ρ) was used for correlation between ImmunoCAP titers and ICT results. For all results, a two-tailed P-value < 0.05 was considered statistically significant.
Results
Patients and sera
Patient characteristics for 106 ABPA patients and 141 diseased controls are shown in Table 1. Within the ABPA patient group, two subgroups were defined as ‘proven’ or ‘presumed’ according to total serum IgE level (‘proven’: >1000 IU/ml, ‘presumed’: 113–999 IU/ml). Diseased controls consisted of patients diagnosed with asthma and/or bronchiectasis. Subgroups for analysis were defined as (1) ABPA-S: serological ABPA (no bronchiectasis evident, ± asthma), and (2) ABPA-B: ABPA or control with bronchiectasis ± any other conditions (including asthma and COPD). Additionally, as part of routine testing, 64 of the 106 ABPA patients in this study had microbiological culture performed on respiratory samples (sputum), usually multiple specimens. High volume fungal culture technique was usually used, which has a higher yield than routine culture [18]. Seven (7) cases yielded no growth. Aspergillus fumigatus was the main pathogen isolated in the remaining 57 cases, and 21 of these also grew other Aspergillus species in culture. The following data was not available for ‘asthma only’ control subjects: (1) gender, (2) whether sputum culture was performed (and results).
Table 1. Patient and control characteristics.
| ABPA | Controls | |||||
|---|---|---|---|---|---|---|
| Characteristic | Alla (n = 106) | Proven (n = 88) | Presumed (n = 18) | Allb (n = 164) | Asthma (n = 93) | Bronchiectasis (n = 71) |
| Female gender, n (%) | 46 (43.4) | 36 (40.9) | 10 (55.6) | n/ac | n/a | 43 (60.6) |
| Mean age (years) | 63 | 63 | 67 | 52 | 45 | 60 |
| Age range (years) | 18–90 | 18–90 | 52–83 | 16–87 | 16–68 | 22–87 |
| Asthma, n (%) | 93 (87.7) | 78 (88.6) | 15 (83.3) | 141 (86.0) | 93 (100) | 48 (67.6) |
| Bronchiectasis, n (%) | 82 (76.4) | 66 (75.0) | 16 (88.9) | 71 (43.3) | 0 (0) | 71 (100) |
| Asthma + bronchiectasis, n (%) | 72 (67.9) | 59 (67.0) | 13 (72.2) | 48 (29.3) | 0 (0) | 48 (67.6) |
| COPDd, n (%) | 9 (8.5) | 8 (9.1) | 1 (5.6) | 7 (4.3) | 0 (0) | 10 (14.1) |
| Cystic fibrosis (CF), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Aspergillus fumigatus growth in sputum culture, n (%) | 57 (53.8) | 48 (54.5) | 9 (50.0) | 3 (1.9) | 0 (0) | 3 (4.2) |
| A. fumigatus only, n (%) | 36 (34.0) | 28 (31.8) | 8 (44.4) | n/a | 0 (0) | n/a |
| A. fumigatus + other Aspergillus spp., n (%) | 21e (19.8) | 20 (22.7) | 1 (5.6) | n/a | 0 (0) | n/a |
| Other Aspergillus spp. (only) growth in sputum culture, n (%) | 0 (0) | 0 (0) | 0 (0) | n/a | 0 (0) | n/a |
ICT results
For all 106 ABPA serum samples, 96 tested positive by ICT with 90.6% sensitivity (95% CI, 83.3% to 95.4%). Sensitivity for ‘proven’ and ‘presumed’ ABPA subgroups was 89.8% (95% CI, 81.5% to 95.2%) and 94.4% (95% CI, 72.7% to 99.9%), respectively, with no significant difference between these groups. No associations with any of multiple parameters were linked with false negative assay ICT assay. In the total diseased control group, 143 of the 164 sera tested negative by ICT with 87.2% specificity (95% CI, 81.1% to 91.9%) (Table 2).
Table 2. Summary of results for LDBio Aspergillus ICT IgG-IgM test and routine serological assay.
| Sensitivity | Specificity | Youden's | DOR | ||||
|---|---|---|---|---|---|---|---|
| Analysis group | % | (95% CI) | % | 95% CI (%) | Index | (95% CI) | |
| Overall | All ABPA (n = 106) | 90.6 | (83.3–95.4) | 0.816 | 65 (30–145) | ||
| 'Proven' ABPA (n = 88) | 89.8 | (81.5–95.2) | |||||
| 'Presumed' ABPA (n = 18) | 94.4 | (72.7–99.9) | |||||
| All diseased controls (n = 164) | 87.2 | (81.1–91.9) | |||||
| ABPA-S (Serological ABPA) | Serological ABPA (n = 24) | 79.2 | (57.9–92.9) | 0.706 | 62 (19–203) | ||
| Asthma only controls (n = 93) | 91.4 | (83.8–96.2) | |||||
| ABPA-B (ABPA + bronchiectasis) | ABPA + any bronchiectasis (n = 82) | 93.9 | (86.3–98.0) | 0.750 | 69 (23–204) | ||
| Bronchiectasis controls (n = 71) | 81.7 | (70.7–89.9) | |||||
Analysis of subgroups based on underlying conditions demonstrated a significant difference in sensitivity between the ABPA-B and ABPA-S subgroups (93.9% and 79.2% sensitivity, respectively; Pearson’s chi-square statistic = 4.719, P = 0.03). No significant differences in specificity were observed. For three (3) cases of bronchiectasis with evidence of Aspergillus colonization by positive sputum culture results, there were no ICT positive results (100% specificity) and for five (5) cases of bronchiectasis with raised Asp IgE, two (2) cases were ICT positive (60% specificity). We also evaluated serum from seven (7) cystic fibrosis patients with ‘proven’ ABPA and found 100% sensitivity using the ICT for this group. Youden’s J statistic calculated for overall ICT test results indicated a good balance between sensitivity and specificity. The test had an overall positive likelihood ratio of 7.07 (95% CI, 4.72 to 10.59) and negative likelihood ratio of 0.11 (95% CI, 0.06 to 0.20), with a high DOR of 65 (95% CI, 30 to 145). All tests were read both visually (i.e., by eye) and digitally (using the Qiagen ESEQuant LR3 lateral flow reader), with 100% agreement between methods.
In relation to Aspergillus species detected in sputum from ABPA patients (Table 3), the ICT performed well in detecting Asp antibodies for cases with A. fumigatus isolated alone (97.2% sensitivity) as well as for cases where non-A. fumigatus species were isolated in addition (95.2% sensitivity), with no significant difference (Pearson’s chi-square statistic = 0.154; P = 0.69). There was no significant difference in sensitivity between samples from patients with at least one Aspergillus species isolated by culture (‘culture positive’, 96.5% sensitivity) and patients whose sputum yielded no culture growth (‘culture negative’, 100% sensitivity). Of the 42 patients for whom no sputum culture was conducted, 34 were positive by ICT (81.0% sensitivity; 95% CI, 65.9% to 91.4%).
Table 3. LDBio Aspergillus ICT performance in ABPA cases with Aspergillus fumigatus and non-A. fumigatus species.
| Sputum culture result (n = 64) | n | ICT + (n) | % sensitivity (95% CI) |
|---|---|---|---|
| All Aspergillus growth (culture positive) | 57 | 55 | 96.5 (87.9, 99.6) |
| A. fumigatus only | 36 | 35 | 97.2 (85.5, 99.9) |
| A. fumigatus + other Aspergillus spp. | 21 | 20 | 95.2 (76.2, 99.9) |
| A. niger | 17 | 16 | 94.1 (71.3, 99.9) |
| A. nidulans | 3 | 3 | 100 (29.2, 100) |
| A. fischeri | 1 | 1 | 100 (2.5, 100) |
| A. montevidensis | 1 | 1 | 100 (2.5, 100) |
| A. pallidoflavus | 1 | 1 | 100 (2.5, 100) |
| A. terreus | 1 | 1 | 100 (2.5, 100) |
| A. versicolor | 1 | 1 | 100 (2.5, 100) |
| No Aspergillus growth (culture negative) | 7 | 7 | 100 (59, 100) |
Finally, we assessed three (3) different production lots of the LDBio Aspergillus ICT assay kits using 30 ABPA serum samples and observed 100% agreement of results across all lots tested (data not shown).
ICT detection of immunoglobulin isotypes
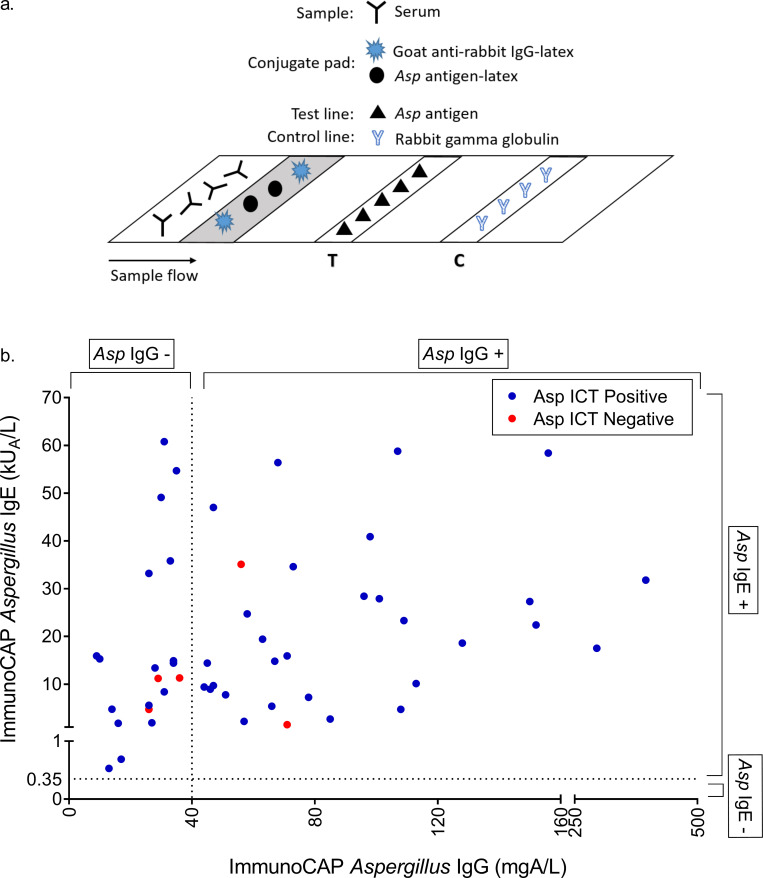
The Aspergillus ICT uses a homogeneous antigen sandwich format to detect Asp antibody in patient sera (Fig 1A), and may theoretically detect immunoglobulin isotypes other than (as claimed by the manufacturer) IgG and IgM. For 52 ABPA samples in this study, ImmunoCAP Asp IgE and IgG results were available and compared to the ICT result. We observed no evident immunoglobulin isotype bias for the ICT assay (Fig 1B), but did observe a significant correlation between Asp IgG and IgE titers (Spearman’s rank correlation coefficient ρ = 0.3524, P = 0.01).
Fig 1.
(a) LDBio Aspergillus ICT assay format and (b) Comparison of LDBio Aspergillus ICT result with ImmunoCAP Asp IgG and IgE titers (n = 52). Positive result cut-off for each assay denoted by dashed line (ImmunoCAP Aspergillus IgG = 40mg/L, ImmunoCAP Aspergillus IgE = 0.35 kUA/L).
Using data generated by digitally reading the ICT on the Qiagen LR3 reader, we found a weak but significant correlation between endpoint (30 minutes) test line peak height and ImmunoCAP Asp IgG titer (n = 46, Spearman’s rank correlation coefficient ρ = 0.2961, P = 0.046), supporting our previous findings[16]. However, as with the previous findings16, the correlation was not sufficient for quantification of Asp IgG. We found no significant correlation between peak height and ImmunoCAP total IgE (n = 48, Spearman’s rank correlation coefficient ρ = 0.2101, P = 0.15) or Asp IgE (n = 48, Spearman’s rank correlation coefficient ρ = 0.0295, P = 0.84) titers.
Discussion
Global prevalence of ABPA is estimated at 1–4% of adult asthma patients (approximately 4.8 million people) worldwide [3], but regional estimates may be higher. A study in Rio de Janiero found 20% prevalence among Brazilian asthmatic patients [19], and several studies in India found ABPA in approximately 5% [20] to 8% [5, 21], and up to 20% [22] of asthmatics, with up to half of patients being misdiagnosed as pulmonary tuberculosis [9]. The incidence of ABPA is also higher in patients with severe asthma and/or corticosteroid-dependent asthma (7–14%), and in patients with atopy [23]. Diagnosis of ABPA relies on a body of clinical, radiological, and immunological (and/or mycological) evidence; and the degree of difficulty in diagnosing ABPA largely depends on the staging and severity of disease. Diagnosis is relatively straightforward when a patient exhibits all clinical symptoms including bronchiectasis, and most patients are diagnosed at this stage [24]. Earlier diagnosis, however, is ideal to prevent development of bronchiectasis and irreversible tissue damage [25]. Serological tests are a useful tool for early detection of ABPA and comprise a significant portion of the recently suggested guidelines for ABPA diagnosis [10], with raised total IgE and Asp IgE (or positive skin prick test) being ‘major’ criteria and raised Asp IgG or positive precipitins test included under ‘minor’ criteria.
The LDBio Aspergillus ICT lateral flow assay is a new diagnostic test requiring minimal time and resources. Our recent evaluation in CPA patients (vs. healthy controls) determined the test to have good sensitivity (91.6%) and specificity (98.0%), and a significant improvement in performance over our current workhorse assay (ImmunoCAP EIA) to detect raised Asp IgG [16]. The current evaluation in ABPA patients and diseased controls from the United Kingdom has shown the Aspergillus ICT to have good overall sensitivity (90.6%) and specificity (87.2%) in distinguishing patients with ABPA from those with underlying respiratory disease. Not surprisingly, the test exhibited significantly better sensitivity for ABPA with bronchiectasis (ABPA-B) (93.9%) than for serological ABPA (ABPA-S) ± asthma (no evidence of bronchiectasis) (79.2%). Bronchiectasis is associated with progression of ABPA and a more severe form of disease [1]. Persistent Aspergillus infection may lead directly to bronchiectasis or drive recurrent exacerbations and progression of bronchiectasis already present due to ABPA or severe asthma [26, 27]. Once present, the pathophysiology of bronchiectasis can facilitate persistent infection through impairment in mucociliary clearance [28] and structural/tissue damage that creates a permissive environment for establishment of infection [26, 29, 30]. Patients with bronchiectasis (with or without ABPA) may develop Aspergillus bronchitis, and this entity is also associated with raised Asp IgG antibodies [31]. We did not explicitly assess a group of asthmatic patients with Aspergillus sensitisation for Aspergillus IgG with the ICT or ImmunoCap, and given the likely interaction with Aspergillus bronchitis, we would need to do so knowing if Aspergillus bronchitis was or was not present.
All cases in this study with Aspergillus species growth in high-volume sputum culture were positive for Aspergillus fumigatus and approximately one-third (37%) were also positive for other Aspergillus species. There was no significant difference in ICT performance between cases with A. fumigatus growth alone versus those with other species present. There were no cases in this study with non-A. fumigatus ABPA, however, a limited number of non-A. fumigatus CPA cases were evaluated in a previous study and we found no significant difference in ICT performance between A. fumigatus and non-A. fumigatus CPA cases [16]. The ability to detect antibody to non-A. fumigatus may be of particular importance in regions where ABPA due to non-A. fumigatus species is more prevalent [32]. For the diagnosis of ABPA, sputum culture is considered a supportive (not diagnostic) test [10]. In Indian patients thought to have ABPA, sputum tested by microscopy and culture was positive for Aspergillus species in only 32.5% of 203 cases [8]. Higher volume cultures yielded a positive in 56% of 75 cases (similar to this series), some of whom were on antifungal therapy compared with conventional culture (12.5%) [18]. Aspergillus ICT was more sensitive than this. Culture has the advantage of being able to check antifungal susceptibility, if positive, although direct detection of resistance is now possible [33–36].
Given the ‘major’ and ‘minor’ requirements of both raised Asp IgE and IgG for ABPA diagnosis, we sought to determine if the homogeneous antigen-sandwich format of the Aspergillus ICT rendered it capable of detecting IgE (in addition to IgG and IgM as claimed) and whether the test displayed an isotype bias. Although we found a significant correlation between levels of Asp IgG and Asp IgE, we did not observe any such isotype bias using the ICT. As expected based on our previous findings [16], we found the test to perform well at increasing levels of Asp IgG (ImmunoCAP ‘positive’, >40mgA/L) regardless of Asp IgE titer. The test also performed well at raised levels of Asp IgE paired with values of Asp IgG considered ‘negative’ by the recommended ImmunoCAP cut-off (≤ 40mgA/L). While this may possibly indicate the detection of Asp IgE by the ICT test format, we have previously found this assay to detect Asp antibody in patients with clinically confirmed CPA who have tested ‘negative’ by ImmunoCAP Asp IgG [16, 37]. Further studies would be necessary to evaluate the individual contributions of IgG and IgE from patient sera and the antibody class-specific performance of the Aspergillus ICT.
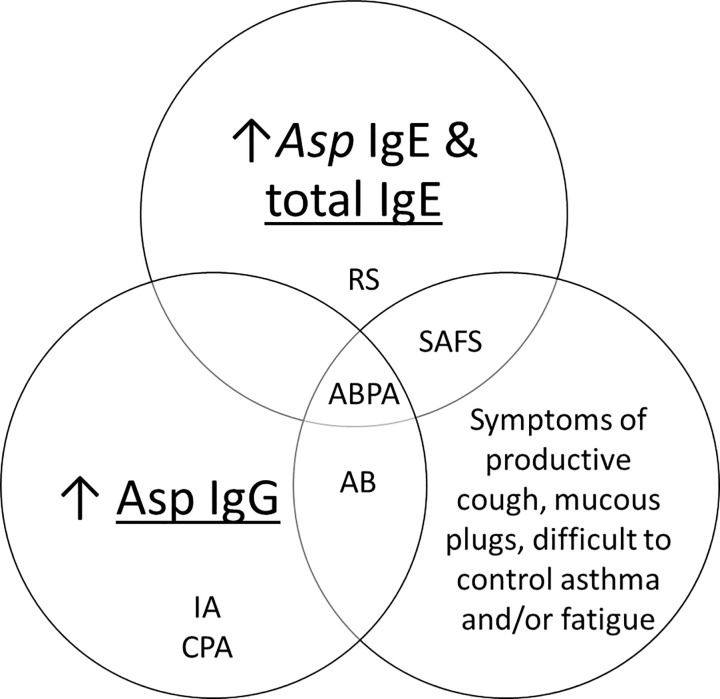
Individual serological tests alone for total IgE or Asp IgE or IgG are not specific for ABPA. It is important to note that routine tests used for ABPA diagnosis—raised Asp IgE, IgG and/or positive precipitins test—are also used in the diagnostic pathways for Aspergillus diseases other than ABPA including Aspergillus bronchitis (AB), acute and sub-acute invasive aspergillosis (IA), CPA, severe asthma with fungal sensitization (SAFS), and chronic or granulomatous Aspergillus rhinosinusitis (RS) [12]. The diagnostic interpretation of these tests, alone or in combination with each other and/or clinical presentation is summarized in Fig 2. Under generally accepted diagnostic criteria for Aspergillus disease, ABPA is the only condition using the combined requirements of raised Asp IgG and IgE. In practice however, it is not uncommon to see raised total and/or Asp IgE in CPA patients [38], especially those that have developed CPA as a result of untreated ABPA progression [39]. Additionally, although a raised level of Asp IgG is considered an exclusion criteria for SAFS, approximately 10% of all asthmatics present with raised Asp IgG [1] and there are likely to be cases of SAFS with increased levels of both Asp IgG and IgE, but lacking the clinical and radiological features of ABPA [12]. High titers of total serum IgE are encountered not only in ABPA, but also in cases of SAFS, Aspergillus rhinosinusitis, and in atopic asthmatics [40, 41]. Furthermore, cut-off values to distinguish these conditions are speculative and may even be different in ABPA complicating asthma versus ABPA complicating CF [4, 42].
Fig 2. Interpretation of Aspergillus serology and clinical presentation of ABPA for diagnosing Aspergillus disease: allergic bronchopulmonary aspergillosis (ABPA), Aspergillus rhinosinusitis (RS), severe asthma with fungal sensitization (SAFS), Aspergillus bronchitis (AB), invasive aspergillosis (IA), and chronic pulmonary aspergillosis (CPA).
In this study of clinically confirmed cases of ABPA compared to diseased controls, we found the LDBio Aspergillus ICT to have good sensitivity and specificity. The test effectively distinguished between Aspergillus-sensitization complicating asthma and/or bronchiectasis, and underlying conditions. It is rapid (result in <30 minutes) and easy to perform, with simple result interpretation by visible inspection. Overall, the LDBio Aspergillus ICT exhibits excellent performance as a screening tool in the ABPA diagnostic pathway.
Supporting information
(XLSX)
Acknowledgments
Diseased control sera were provided by the ManARTS research tissue bank, Wythenshawe Hospital, Manchester University NHS Foundation Trust (Manchester, UK).
Abbreviations used
- ABPA
allergic bronchopulmonary aspergillosis
- ABPA-B
allergic bronchopulmonary aspergillosis with bronchiectasis
- ABPA-S
serological allergic bronchopulmonary aspergillosis (no bronchiectasis evident
- CPA
chronic pulmonary aspergillosis
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- DOR
diagnostic odds ratio
- EIA
enzyme immunoassay
- ELISA
enzyme-linked immunosorbent assay
- ICT
immunochromatographic technology
- kUA/ml
kilo-units antigen-specific antibodies/millilitre (ImmunoCAP
- ManARTS
Manchester Allergy, Respiratory, and Thoracic Surgery
- mgA/L
milligrams antigen-specific antibodies/liter (ImmunoCAP)
- NAC
National Aspergillosis Centre
Data Availability
All relevant data are within the paper and supporting information files.
Funding Statement
This work was supported by the Medical Research Council (Newton grant MR/P017622/1 and the NIHR Manchester Biomedical Research Centre, both to DWD.
References
- 1.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest 2009;135(3):805–826. 10.1378/chest.08-2586 [DOI] [PubMed] [Google Scholar]
- 2.Hogan C, Denning DW. Allergic bronchopulmonary aspergillosis and related allergic syndromes. Semin Respir Crit Care Med 2011;32(6):682–692. 10.1055/s-0031-1295716 [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013;51(4):361–370. 10.3109/13693786.2012.738312 [DOI] [PubMed] [Google Scholar]
- 4.Baxter CG, Dunn G, Jones AM, Webb K, Gore R, Richardson MD, et al. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J Allergy Clin Immunol 2013;132(3):560–566 e510. 10.1016/j.jaci.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, Jindal SK. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest 2007;132(4):1183–1190. 10.1378/chest.07-0808 [DOI] [PubMed] [Google Scholar]
- 6.Muthu V, Sehgal IS, Prasad KT, Dhooria S, Aggarwal AN, Garg M, et al. Allergic bronchopulmonary aspergillosis (ABPA) sans asthma: A distinct subset of ABPA with a lesser risk of exacerbation. Med Mycol 2019;58(2):260–3. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Nath A, Aggarwal AN, Gupta D, Chakrabarti A. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses 2010;53(2):138–143. 10.1111/j.1439-0507.2008.01680.x [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti A, Sethi S, Raman DS, Behera D. Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses 2002;45(8):295–299. 10.1046/j.1439-0507.2002.00738.x [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest 2006;130(2):442–448. 10.1378/chest.130.2.442 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleira R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43(8):850–873. 10.1111/cea.12141 [DOI] [PubMed] [Google Scholar]
- 11.Longbottom JL, Austwick PK. Antigens and allergens of Aspergillus fumigatus. I. Characterization by quantitative immunoelectrophoretic techniques. J Allergy Clin Immunol 1986;78(1 Pt 1):9–17. [DOI] [PubMed] [Google Scholar]
- 12.Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis—quo vadis? Med Mycol 2015;53(5):417–439. 10.1093/mmy/myv020 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Maskey D, Aggarwal AN, Saikia B, Garg M, Gupta D, Chakrabarti A. Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: a latent class analysis. PLoS One 2013;8(4):e61105 10.1371/journal.pone.0061105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson MD, Stubbins JM, Warnock DW. Rapid enzyme-linked immunosorbent assay (ELISA) for Aspergillus fumigatus antibodies. J Clin Pathol 1982;35(10):1134–1137. 10.1136/jcp.35.10.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piarroux RP, Romain T, Martin A, Vainqueur D, Vitte J, Lachaud L, et al. Multicenter Evaluation of a Novel Immunochromatographic Test for Anti-aspergillus IgG Detection. Front Cell Infect Microbiol 2019;9:12 10.3389/fcimb.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stucky Hunter E, Richardson MD, Denning DW. Evaluation of LDBio Aspergillus ICT Lateral Flow Assay for IgG and IgM Antibody Detection in Chronic Pulmonary Aspergillosis. J Clin Microbiol 2019;57(9):e00538–19. 10.1128/JCM.00538-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56(11):1129–1135. 10.1016/s0895-4356(03)00177-x [DOI] [PubMed] [Google Scholar]
- 18.Vergidis P MC, Novak-Frazer L, Richardson R, Walker A, Denning DW, Richardson MD. High-volume culture and quantitative real-time PCR for the detection of Aspergillus in sputum. Clin Microbiol Infect 2020;In press. [DOI] [PubMed] [Google Scholar]
- 19.Serpa S. Aspergilose broncopulmonar alergica: prevalencia e criterios diagnosticos em pacientes asmaticos sensıveis ao Aspergillus fumigatus. [Dissertac-a˜o de mestrado] Rio de Janeiro. 1997. [Google Scholar]
- 20.Khan ZU, Sandhu RS, Randhawa HS, Menon MP, Dusaj IS. Allergic bronchopulmonary aspergillosis: a study of 46 cases with special reference to laboratory aspects. Scand J Respir Dis 1976;57(2):73–87. [PubMed] [Google Scholar]
- 21.Ghosh T, Dey A, Biswas D, Chatterjee S, Haldar N, Maiti PK. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis among asthma patients in eastern India. J Indian Med Assoc 2010;108(12):863–865. [PubMed] [Google Scholar]
- 22.Maurya V, Gugnani HC, Sarma PU, Madan T, Shah A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest 2005;127(4):1252–1259. 10.1378/chest.127.4.1252 [DOI] [PubMed] [Google Scholar]
- 23.Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc 2010;7(3):237–244. 10.1513/pats.200908-086AL [DOI] [PubMed] [Google Scholar]
- 24.Patterson R, Greenberger PA, Halwig JM, Liotta JL, Roberts M. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med 1986;146(5):916–918. 10.1001/archinte.146.5.916 [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Chopra D. Evaluation of allergic bronchopulmonary aspergillosis in patients with and without central bronchiectasis. J Asthma 2002;39(6):473–477. 10.1081/jas-120004905 [DOI] [PubMed] [Google Scholar]
- 26.De Soyza A, Aliberti S. Bronchiectasis and Aspergillus: How are they linked? Med Mycol 2017;55(1):69–81. 10.1093/mmy/myw109 [DOI] [PubMed] [Google Scholar]
- 27.Boyton RJ, Altmann DM. Bronchiectasis: Current Concepts in Pathogenesis, Immunology, and Microbiology. Annu Rev Pathol 2016;11:523–554. 10.1146/annurev-pathol-012615-044344 [DOI] [PubMed] [Google Scholar]
- 28.Oguma T, Asano K, Tomomatsu K, Kodama M, Fukunaga K, Shiomi T, et al. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J Immunol 2011;187(2):999–1005. 10.4049/jimmunol.1002257 [DOI] [PubMed] [Google Scholar]
- 29.Cole PJ. Inflammation: a two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl 1986;147:6–15. [PubMed] [Google Scholar]
- 30.King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis 2009;4:411–419. 10.2147/copd.s6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrdle A, Mustakim S, Bright-Thomas RJ, Baxter CG, Felton T, Denning DW. Aspergillus bronchitis without significant immunocompromise. Ann N Y Acad Sci 2012;1272:73–85. 10.1111/j.1749-6632.2012.06816.x [DOI] [PubMed] [Google Scholar]
- 32.Sehgal IS, Choudhary H, Dhooria S, Aggarwal AN, Bansal S, Garg M, et al. Prevalence of sensitization to Aspergillus flavus in patients with allergic bronchopulmonary aspergillosis. Med Mycol 2019;57(3):270–276. 10.1093/mmy/myy012 [DOI] [PubMed] [Google Scholar]
- 33.Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, et al. High-frequency triazole resistance found in non-culturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis 2011;52(9):1123–1129. 10.1093/cid/cir179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schauwvlieghe A, Vonk AG, Buddingh EP, Hoek RAS, Dalm VA, Klaassen CHW, Rijinders BJA. Detection of azole-susceptible and azole-resistant Aspergillus coinfection by cyp51A PCR amplicon melting curve analysis. J Antimicrob Chemother 2017;72(11):3047–3050. 10.1093/jac/dkx262 [DOI] [PubMed] [Google Scholar]
- 35.White PL, Posso RB, Barnes RA. Analytical and Clinical Evaluation of the PathoNostics AsperGenius Assay for Detection of Invasive Aspergillosis and Resistance to Azole Antifungal Drugs Directly from Plasma Samples. J Clin Microbiol 2017;55(8):2356–2366. 10.1128/JCM.00411-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anees-Hill SP HD, Masania R, Moore CB, Richardson MD, Rautemaa-Richardson R, Denning DW, et al. Deciphering Aspergillus fumigatus triazole resistance in situ in respiratory specimens via pyrosequencing of cyp51A. Manuscript submitted. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter E, Harris C, Wilopo B, Richardson M, Denning DW. Effect of patient immunodeficiencies on the diagnostic performance of serological assays to detect Aspergillus-specific antibodies in chronic pulmonary aspergillosis. Paper presented at: 9th Trends in Medical Mycology 2019; Nice, France. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003;37 Suppl 3:S265–280. [DOI] [PubMed] [Google Scholar]
- 39.Lowes D, Chishimba L, Greaves M, Denning DW. Development of chronic pulmonary aspergillosis in adult asthmatics with ABPA. Respir Med 2015;109(12):1509–1515. 10.1016/j.rmed.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Wang JL, Patterson R, Rosenberg M, Roberts M, Cooper BJ. Serum IgE and IgG antibody activity against Aspergillus fumigatus as a diagnostic aid in allergic bronchopulmonary aspergillosis. Am Rev Respir Dis 1978;117(5):917–927. 10.1164/arrd.1978.117.5.917 [DOI] [PubMed] [Google Scholar]
- 41.Patterson R, Fink JN, Pruzansky JJ, Reed C, Roberts M, Slavin R, et al. Serum immunoglobulin levels in pulmonary allergic aspergillosis and certain other lung diseases, with special reference to immunoglobulin E. Am J Med 1973;54(1):16–22. 10.1016/0002-9343(73)90078-8 [DOI] [PubMed] [Google Scholar]
- 42.Alghamdi NS, Barton R, Wilcox M, Peckham D. Serum IgE and IgG reactivity to Aspergillus recombinant antigens in patients with cystic fibrosis. J Med Microbiol 2019;68(6):924–929. 10.1099/jmm.0.000991 [DOI] [PubMed] [Google Scholar]