Abstract
By exploiting a uniquely reactive lysine residue (Lys99) for site-specific attachment of small molecules, the humanized catalytic antibody h38C2 has been used as bioconjugation module in the assembly of chemically programmed antibodies and antibody-drug conjugates. Treatment of h38C2 with β-lactam-functionalized small molecules has been previously shown to result in covalent conjugation by selective formation of a stable amide bond with the ε-amino group of the Lys99 residue. Here we report that heteroaryl methylsulfonyl (MS-PODA)-functionalized small molecules represent an alternative bioconjugation strategy through highly efficient, site-specific, and stable arylation of the Lys99 residue. A set of chemically programmed antibodies and antibody-drug conjugates assembled by Lys99 arylation provided proof-of-concept for the therapeutic utility of this alternative bioconjugation strategy. While being equally effective as β-lactam-functionalized ligands for bioconjugation with catalytic antibody h38C2, the MS-PODA moiety offers distinct synthetic advantages, making it highly attractive.
Keywords: Catalytic antibody, h38C2, bioconjugation chemistry, payload, cancer therapy
Graphical Abstract
INTRODUCTION
Antibody-small molecule conjugates are broadly used in basic research and for the diagnosis and therapy of diseases. For example, Food and Drug Administration (FDA)-approved and currently marketed therapeutic antibody-small molecule conjugates include five antibody-drug conjugates1 and one radioimmunoconjugate.2 Although none of these assemble antibody and small molecule by site-specific bioconjugation strategies, the recent utilization of natural or engineered uniquely reactive amino acids or carbohydrates affords highly homogeneous antibody-small molecule conjugates.3 By facilitating the manufacture and application of molecularly defined assemblies of antibody-small molecule conjugates, they have become state-of-the-art reagents backed by a rich preclinical and clinical pipeline.
While lysine (Lys) residues in antibodies have been predominantly utilized for random bioconjugation, a diverse set of strategies that facilitate site-specific Lys modification has been reported more recently.4–9 Based on a uniquely reactive Lys residue (Lys99) in its active site, the catalytic antibody 38C210,11 and its humanized version h38C212 can serve as bioconjugation modules for the assembly of highly homogeneous antibody-small molecule conjugates.4 Lys99 lies at the bottom of a deep hydrophobic pocket. Unlike surface Lys residues, it is deprotonated at physiological pH and highly nucleophilic. This has been harnessed for the site-specific covalent conjugation of small molecules that are derivatized with an electrophilic β-diketone or β-lactam group, which form enaminone or amide adducts, respectively, with the ε-amino group of the buried Lys99 residue. Chemically programmed antibodies that utilize h38C2 as a bioconjugation module to endow small molecules with the pharmacokinetic and pharmacodynamic properties of monoclonal antibodies (mAbs), have been investigated in phase I and II clinical trials.13 Furthermore, T-cell engaging bispecific antibodies have been equipped with h38C2 bioconjugation modules to link small molecules that target cell surface receptors to the power of immunotherapy.14,15 Finally, dual variable domain (DVD)-based antibody-drug conjugates (ADCs) have used h38C2 as a bioconjugation module for the rapid, precise, efficient and stable conjugation of highly cytotoxic payloads under mild conditions.16
Given the broadly demonstrated utility of the h38C2 bioconjugation module, we investigated alternative conjugation chemistry with the objective of providing additional options for payload derivatization. Specifically, we examined heteroaryl methylsulfones, which have been used for the conjugation of small molecules to mAbs having engineered cysteine (Cys) and selenocysteine (Sec) residues to afford higher serum stability compared to conventional maleimide conjugation.17,18 We hypothesized that the ε-amino group of the buried Lys99 residue of h38C2 and heteroaryl methylsulfone-functionalized small molecules present a compatible electron-pair donor/acceptor system. Accordingly, we analyzed the efficiency, site-specificity, and stability of such bioconjugates. Chemically programmed antibodies and ADCs generated by Lys arylation using methylsulfone oxadiazole derivatives and by Lys amidation using β-lactam derivatives were directly compared in functional assays. Collectively, we provide proof-of-concept for the practicality, versatility, and utility of this alternative bioconjugation strategy.
RESULTS AND DISCUSSION
In silico arylation of Lys99
The catalytic antibody 38C210,11 and its humanized version h38C212 covalently bind β-diketone and β-lactam haptens through a uniquely reactive Lys residue at position 99 (Lys99) in the variable heavy chain domain (VH) to afford highly homogeneous chemically programmed antibodies4 and ADCs.16 X-ray crystallography has revealed that Lys99 is positioned at the bottom of an 11-A deep hydrophobic pocket that forms the hapten binding site of 38C2 and h38C2. The ε-amino group of Lys99, which is essential for catalyzing aldol and retro-aldol reactions, has a perturbed pKa of ~6, rendering it largely unprotonated at physiological pH and highly nucleophilic.11 This feature has been utilized extensively for hapten-driven, site-specific, and irreversible covalent conjugation of β-lactam hapten derivatives of various payloads. In the concept of chemical programming, which has been translated to clinical trials, h38C2 IgG1 endows small or large molecules that bind to cell surface receptors with prolonged circulatory half-life and effector functions.13 In the concept of dual variable domain (DVD)-ADCs, an inner h38C2 Fv serves as attachment module for cytotoxic drugs.16
The nucleophilicity of the ε-amino group of Lys99 of h38C2 prompted us to investigate alternative irreversible covalent conjugation chemistries that could further increase the accessible payload space. Due to the hydrophobicity of the Lys99 microenvironment, we hypothesized that Lys arylation, which has not been reported for antibody conjugation,19 could provide a suitable route. Specifically, we were interested in testing heteroaryl methylsulfonyl compounds developed by Barbas and colleagues as serum-stable alternative to maleimide-based conjugation to antibodies with engineered free Cys residues.17,18 Our studies focused on the methylsulfone phenyloxadiazole (MS-PODA) (Figure 1A).
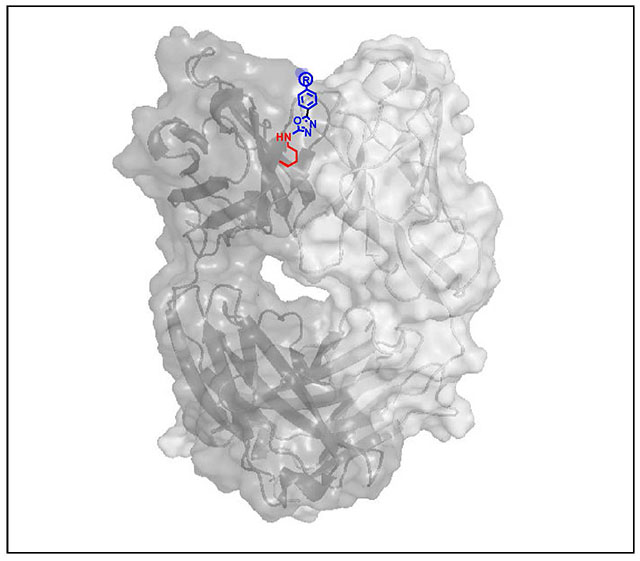
Figure 1. Heteroarylation of the reactive Lys residue of catalytic antibody h38C2.
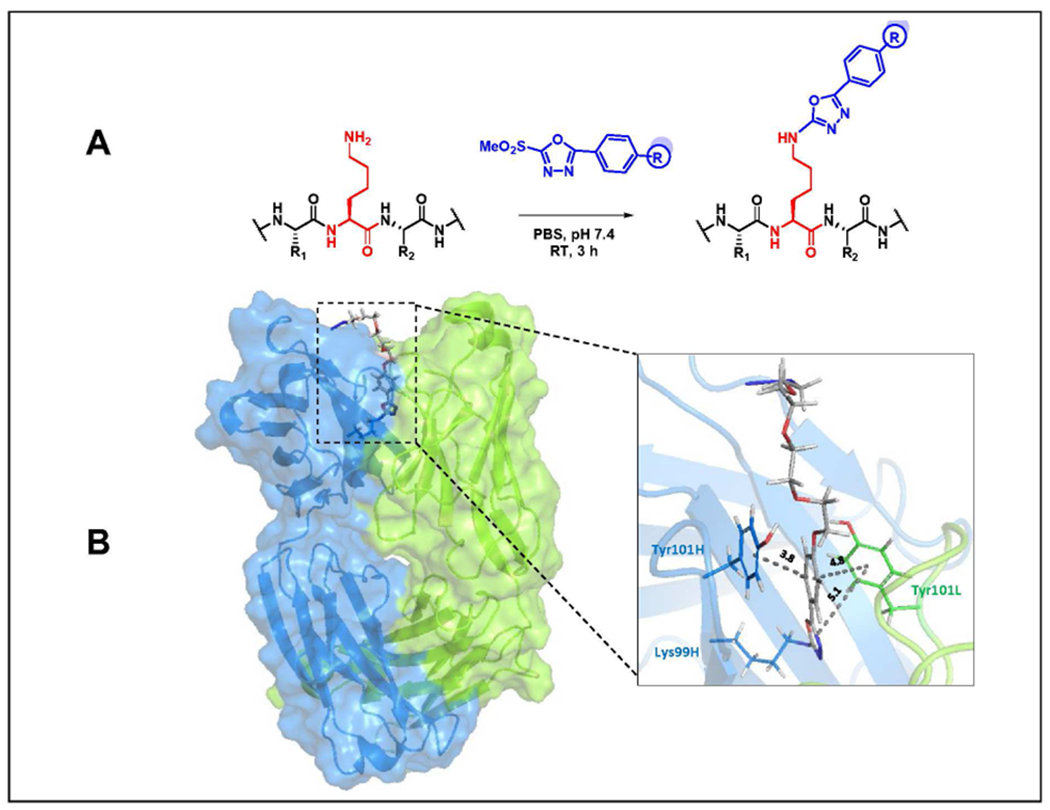
(A) Proposed reaction of MS-PODA with the ε-amino group of Lys99 under mild conditions. The side chains of the flanking Cys and threonine (Thr) residues are shown as R1 and R2, respectively. (B) In silico docking model of PODA-conjugated Lys99 in the hydrophobic pocket (box) of h38C2. The Fab’s heavy chain (VH-CH1) is shown in blue and the light chain (VL-CL) in green. Interatomic distances given in Å were calculated by PyMOL software.
Based on the proposed reaction of MS-PODA with the ε-amino group of Lys99 (Figure 1A), we used computational modeling to dock the compound into the hydrophobic pocket of h38C2. This was based on the recently solved crystal structure of h38C2 Fab with a Lys99Arg mutation (PDB ID 6U85).20 Arg99 was replaced with an azido-(PEG)4-PODA-derivatized Lys residue and subjected to energy minimization in silico. Residues in the hydrophobic pocket interacting with PODA were identified and their interatomic distances were calculated (Figure 1B). Two tyrosine (Tyr) residues, Tyr101 of VH and Tyr101 of the variable light chain domain (VL) dominated the interactions through π-π stacking with the phenyl ring of PODA (Suppl. Figure S1). Tyr101 of VL and a tryptophan (Trp) residue, Trp47 of VH, revealed a π-π stacking interaction with oxadiazole ring of PODA. Several hydrogen bonds bridged by water molecules also contributed to the interaction (Suppl. Figure S1). Collectively, computational modeling suggested that MS-PODA can serve as hapten-like compound for covalent conjugation to Lys99.
Efficient, selective, and stable arylation of Lys99 as probed with an MS-PODA derivative of fluorescein
To probe covalent conjugation to Lys99 in vitro, we used a previously described MS-PODA derivative of fluorescein (compound 1; Figure 2A).17 For comparison, we included a previously described β-lactam hapten derivative of tetramethylrhodamine (TAMRA) (compound 2; Figure 2A).20 To pinpoint conjugation at Lys99, we also cloned, expressed, and purified h38C2 IgG1 havina a Lvs99Ala mutation Followino incubation of h3802 and h38C2_Lys99Ala IgG1 with 5-fold molar excess (5 eq per each of the two Lys99 residues) of compounds 1 and 2 for 4 h at room temperature in PBS, unpurified antibody conjugates along with unconjugated antibody were separated by reducing and nonreducing SDS-PAGE and analyzed by Coomassie Blue staining and in-gel fluorescence (Figure 2B). This analysis revealed conjugation of both compounds to the 50-kDa heavy chain of h38C2 IgG1 but not to the 25-kDa light chain. No conjugation to h38C2_Lys99Ala was detectable, suggesting site-specific conjugation to the reactive Lys99 residue (Figure 2B). Mass spectrometry analysis of the PNGase F-treated (to remove N-glycosylation) and dithiothreitol (DTT)-treated (to reduce interchain disulfide bridges) unconjugated antibody revealed molecular weights of 49,384 Da (heavy chain; expected molecular weight without posttranslational modifications: 49,460 Da) and 23,953 Da (light chain; 23,955 Da) (Figure 2C). The correspondingly prepared antibody conjugate from the reaction of h38C2 IgG1 with compound 1 revealed an increase of the molecular weight of the heavy chain by 694 Da, indicating the covalent conjugation of one PODA-fluorescein molecule. The conjugation appeared to be highly efficient and selective as only ~5% unconjugated heavy chain, no conjugated light chain, and no multiple conjugated heavy chain were detectable (Figure 2C). Selective conjugation to the two hapten binding sites of h38C2 IgG1 was further shown by complete loss of catalytic activity mediated by Lys99 (Figure 2D).
Figure 2. Site-specific conjugation of MS-PODA to Lys99 of h38C2.
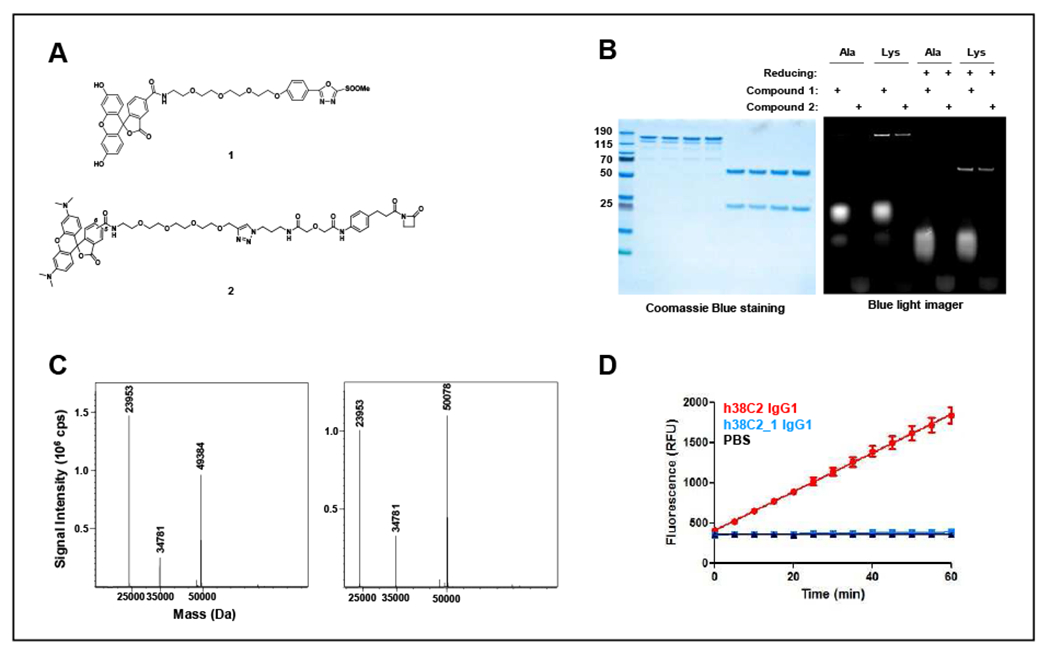
(A) Structures of the MS-PODA derivative of fluorescein (compound 1) and the β-lactam hapten derivative of TAMRA (compound 2) used in this study. (B) Unpurified fluorescein- and TAMRA-conjugated (5 eq) h38C2 (“Lys”) and h38C2_Lys99Ala (‘Ala”) IgG1 were separated by SDS-PAGE under nonreducing or reducing conditions and analyzed by Coomassie Blue staining and in-gel fluorescence. (C) MALDI-TOF analysis of the reduced and deglycosylated (PNGase F) unconjugated (left) and compound 1-conjugated (right) h38C2 IgG1. The expected masses for the unconjugated heavy and light chains were 49,460 Da and 23,955 Da, respectively. The expected mass for the heavy chain with one conjugated compound 1 was 50,055 Da. The peak at 34,781 Da corresponds to PNGase F. (D) Catalytic activity of h38C2 IgG1 before and after conjugation to compound 1. Unconjugated (red) and conjugated (blue) antibody (1 μM) was measured using the retro-aldol conversion of methodol to a detectable fluorescent aldehyde (RFU, relative fluorescent units) and acetone. Conjugated h38C2 IgG1 completely lost catalytic activity, revealing quantitative conjugation at the two reactive Lys residues. Mean ± SD values of triplicates were plotted.
Next, we examined the stability of the Lys99:PODA adduct by incubating the antibody conjugate with human plasma for up to 8 days at 37°C. Analysis by reducing SDS-PAGE followed by Coomassie Blue staining and in-gel fluorescence revealed high stability of the adduct without any detectable transfer of fluorescence to plasma proteins (Figure 3).
Figure 3. Human plasma stability of the conjugate of h38C2 IgG1 and the MS-PODA derivative of fluorescein.

h38C2 IgG1 was conjugated to compound 1, incubated with human plasma at 37°C, and analyzed after 0, 1, 2, 3, 4, 5, 6, 7, and 8 days by reducing SDS-PAGE followed by Coomassie Blue staining (top) and in-gel fluorescence (bottom). HSA, human serum albumin; HC, heavy chain; LC, light chain.
MS-PODA-mediated chemical programming
The efficient, selective, and stable conjugation of a fluorescein derivative of MS-PODA to Lys99 of h38C2 prompted us to investigate MS-PODA conjugation in the context of known therapeutic utilities of h38C2, including chemical programming.13 To endow h38C2 with high specificity and affinity for small molecule binding sites of two different cell surface receptors, we synthesized β-lactam hapten and MS-PODA derivatives of folate (compounds 3 and 5, respectively; Figure 4A) and LLP2A (compounds 4 and 6; respectively; Figure 4A). It is of note that compared to β-lactam-functionalized ligands, synthetic access to MS-PODA-containing constructs is more straight forward. Preparation of MS-PODA moieties can be achieved with readily available commercial reagents and incorporation into ligands can be performed directly on solid-phase resins without intermediate purification. In contrast, synthesis of β-lactam-functionalized ligands typically involves azide-alkyne click reactions that necessitate purification of the reaction products. Folate (vitamin B9), binds with nanomolar affinity to the folate receptor 1 (FOLR1 or folate receptor a), which is overexpressed in ovarian, lung, and other cancers.21 LLP2A is a picomolar-affinity ligand for the open conformation of integrin α4β1 that Lam and colleagues identified by screening a one-bead-one-compound combinatorial peptidomimetic library.22 The open conformation of integrin α4β1 (activated integrin α4β1) is found at elevated levels on malignant B cells and in other hematologic and solid malignancies, where it is involved in trafficking and metastasis.23 Thus, both FOLR1 and integrin α4β1 have emerged as attractive targets for cancer therapeutics.
Figure 4. Chemical programming of h38C2 IgG1.
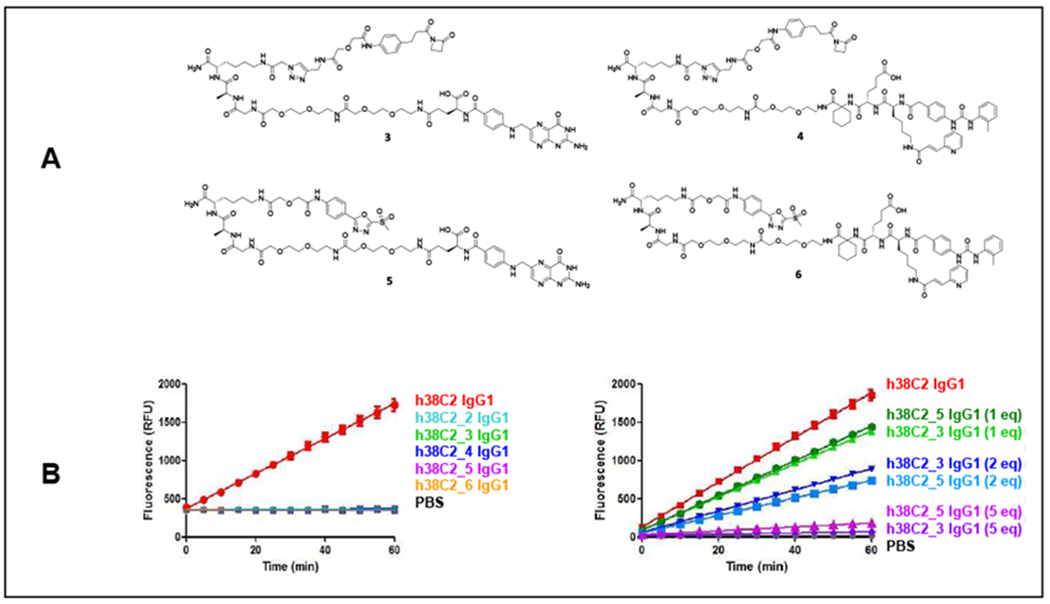
(A) Structures of the β-lactam hapten derivatives of folate (compound 3) and LLP2A (compound 4) and structures of the MS-PODA derivatives of folate (compound 5) and LLP2A (compound 6) used in this study. (B) (Left) Catalytic activity of h38C2 IgG1 (1 μM) before and after conjugation to 5 eq of compounds 2-6 (mean ± SD of triplicates). (Right) Catalytic activity of h38C2 IgG1 (1 μM) before and after conjugation to 1, 2, and 5 eq of compounds 3 and 5 (mean ± SD of triplicates).
Following incubation of h38C2 IgG1 with 5-fold molar excess of compounds 3-6 for 4 h at room temperature in PBS and removal of unconjugated compound, complete loss of catalytic activity for all incubation mixtures confirmed the equivalent efficiency of β-lactam hapten and MS-PODA-mediated conjugation (Figure 4B). At 1-fold and 2-fold molar excess (1 and 2 eq, respectively), no significant difference in the partial loss of catalytic activity was detectable between the two electrophiles (Figure 4B). With the loss of its catalytic activity, h38C2 gained the ability to bind FOLR1 and integrin α4βϊ when chemically programmed with the folate and LLP2A derivatives, respectively. This was first shown by ELISA using recombinant FOLR1 and Mn2+-activated24 integrin α4β1 for plate coating and horseradish peroxidase (HRP)-conjugated goat anti-human Fcy polyclonal antibodies (pAbs) for detection (Figure 5A and C). Subsequent flow cytometry analyses with the FOLR1-positive human ovarian cancer cell line IGROV-1 (Figure 5B) and the Mn2+-activated integrin α-displaying human T-cell line Jurkat (Figure 5D) confirmed the chemical programming. No difference between the established β-laetam hapten- and the new MS-PODA-mediated chemical programming was detectable. This demonstrated that arylation of its reactive Lys residue is suitable for chemical programming of h38C2.
Figure 5. Binding studies with chemically programmed h38C2 IgG1.
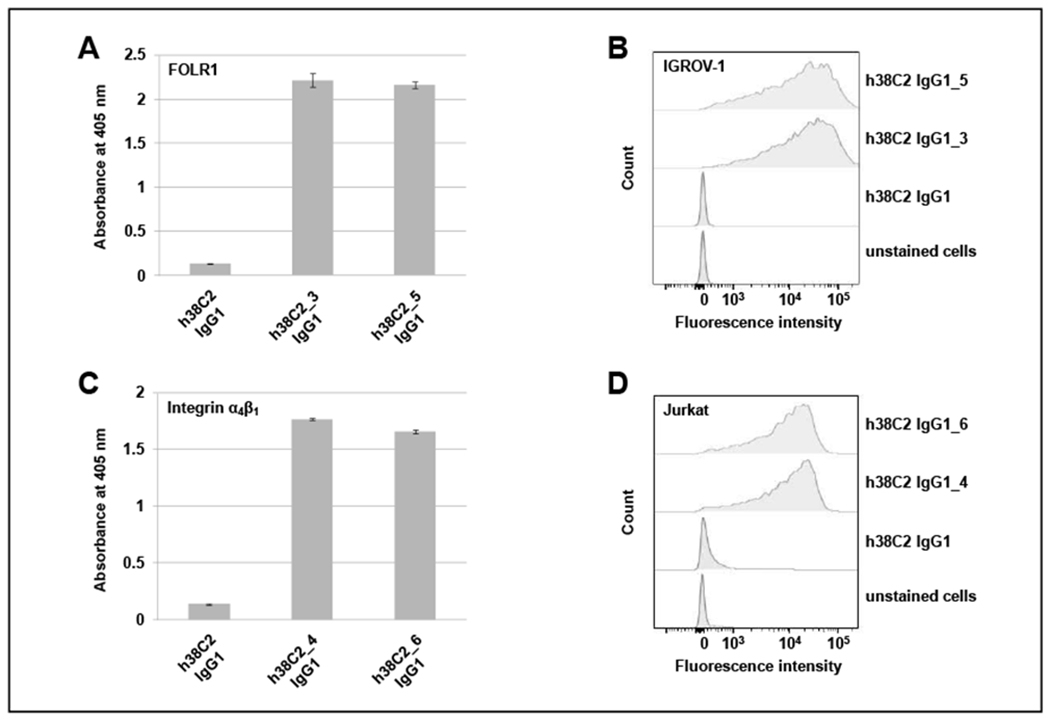
(A) ELISA of folate-conjugated h38C2 IgG1 via β-lactam hapten (compound 3) or MS-PODA (compound 5). Recombinant human FOLR1 in TBS was used for coating, 3% (v/v) skim milk in TBS for blocking, and HRP-conjugated goat anti-human Fcy-specific fragment pAbs for detection. Unconjugated h38C2 IgG1 served as negative control (mean ± SD of triplicates). (B) Flow cytometry analysis of the same antibody conjugates and negative control using FOLR1-positive human IGROV-1 cells and FITC-conjugated goat anti-human IgG-specific pAbs for staining. (C) ELISA of LLP2A-conjugated h38C2 IgG1 via β-lactam hapten (compound 4) or MS-PODA (compound 6). Recombinant human integrin α4β1 in TBS supplemented with 1 mM MnCl2 was used for coating (mean ± SD of triplicates) and the ELISA was carried out as described in (A). (D) Flow cytometry analysis of the same antibody conjugates and negative control using integrin α4β1-positive human Jurkat cells in the presence of 1 mM MnCl2. The cells were stained as in (B).
MS-PODA-mediated assembly of antibody-drug conjugates
Next, we investigated MS-PODA-mediated conjugation for assembling DVD-ADCs that consist of an outer trastuzumab-based Fv that targets HER2 on breast cancer cells and an inner h38C2-based Fv that facilitates site-specific conjugation of highly cytotoxic drugs. This concept has been established for a β-lactam hapten derivative of the tubulin polymerization inhibitor monomethyl auristatin F (MMAF), shown as compound 7 in Figure 6A.16 We previously reported a corresponding MS-PODA derivative of MMAF (compound 8; Figure 6A) for site-specific conjugation to antibodies with engineered Cys residues.25
Figure 6. Assembly and characterization of DVD-ADCs.
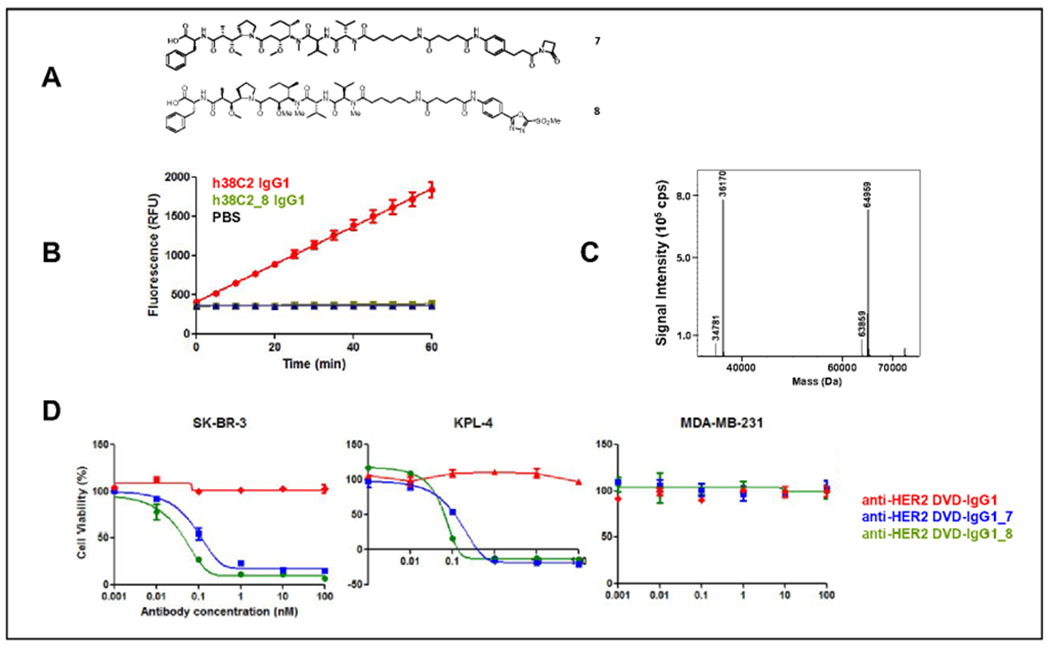
(A) Structures of the β-lactam hapten derivative of MMAF (compound 7) and the MS-PODA derivative of MMAF (compound 8) used in this study. (B) Catalytic activity of h38C2 IgG1 (1 μM) before and after conjugation to 5 eq of compound 8 (mean ± SD of triplicates). (C) MALDI-TOF analysis of the reduced and deglycosylated (PNGase F) compound 8-conjugated anti-FIER2 DVD-IgG1. The expected masses for unconjugated heavy and light chains were 63,878 Da and 36,175 Da, respectively. The expected mass for the heavy chain with one conjugated compound 8 was 64,980 Da. The peak at 34,781 Da corresponds to PNGase F. (D) Comparison of the cytotoxicity of compound 7- (blue) and compound 8-conjugated (green) anti-HER2 DVD-IgG1 following incubation with HER2-positive human SK-BR-3 and KPL-4 cells, and HER2-negative human MDA-MB-231 cells for 72 h at 37°C. Unconjugated anti-HER2 DVD-IgG1 (red) served as negative control. Mean ± SD values of triplicates were plotted.
The DVD IgG1 was incubated with compound 7 or 8 as before. Following removal of unconjugated compound, Lys99 conjugation was confirmed by loss of catalytic activity (Figure 6B). Next, the DVD-ADC assembled via MS-PODA conjugation was analyzed by mass spectrometry. The observed molecular weight of the unconjugated DVD IgG1 after PNGase and DTT treatment was 63,859 Da (heavy chain; expected molecular weight without posttranslational modifications: 63,878 Da) and 36,170 Da (light chain; 36,175 Da). The conjugated DVD IgG1 revealed an increase of the molecular weight of the heavy chain by 1,102 Da, indicating the covalent conjugation of one PODA-MMAF molecule. As noted for the MS-PODA derivative of fluorescein, the conjugation was highly efficient (~95%) and selective without detectable conjugated light chain or multiple conjugated heavy chain (Figure 6C). In the absence of DTT treatment, the observed molecular weight for the unconjugated and conjugated DVD IgG1 was 200,052 Da and 202,272 Da, respectively, indicating the conjugation of one PODA-MMAF molecule to each of the two reactive Lys99 residues (Suppl. Figure S2).
With the homogeneous assembly of the DVD-ADC confirmed, we next tested its ability to mediate potent and selective cytotoxicity. MS-PODA-and β-lactam hapten-assembled DVD-ADCs killed the HER2-positive human breast cancer cell lines SK-BR-3 and KPL-4 with IC50 values of 0.21 and 0.34 nM, and 0.1 and 0.09 nM, respectively (Figure 6D). Neither killed the HER2-negative human breast cancer cell line MDA-MB-231 at up to 100 nM, the highest concentration tested (Figure 6D). Collectively we conclude that MS-PODA-mediated conjugation of cytotoxic drugs to the two reactive Lys99 residues of DVD IgG1 having an inner h38C2-based Fv is equivalent in quality to DVD-ADCs, which we had previously reported employing β-lactam hapten-mediated conjugation.16 Overall, our study establishes a new conjugation chemistry for catalytic antibody h38C2 that is based on arylation of its reactive Lys99 residue and compatible with its therapeutic utilities. This new approach offers distinct synthetic advantages over β-lactam hapten-mediated conjugation strategies.
EXPERIMENTAL PROCEDURES
Cell lines
Human ovarian cancer cell line IGROV-1 was purchased from American Tissue Culture Collection (ATCC) and cultured in folate deficient RPMI-1640 medium supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS) and 1 × penicillin-streptomycin (containing 100 U/mL penicillin and 100 mg/mL streptomycin; all from Thermo Fisher Scientific). Human T-cell line Jurkat was cultured in RPMI-1640 medium supplemented with 10% (v/v) heat inactivated FBS and 1 × penicillin-streptomycin. Human breast cancer cell lines SK-BR-3 and MDA-MB-231 were purchased from ATCC. Human breast cancer cell line KPL-4 was kindly provided by Dr. Naoto T. Ueno based on an MTA with the University of Texas MD Anderson Cancer Center (Houston, TX) and with permission from Dr. Junichi Kurebayashi (Kawasaki Medical School; Kurashiki, Japan).26 All three cell lines were cultured in DMEM medium supplemented with 10% (v/v) heat inactivated FBS and 1 × penicillin-streptomycin. Expi293F cells were cultured in Expi293 expression medium supplemented with 1 × penicillin-streptomycin (all from Thermo Fisher Scientific).
Computational modeling
In the crystal structure20 of h38C2 Fab with a Lys99Arg mutation (PDB ID 6U85), Arg99 was replaced with an azido-(PEG)4-PODA-derivatized Lys residue in silico and subjected to energy minimization using Prime software (Schrodinger) and subjected to molecular dynamics simulations using Desmond software with the OPLS_2005 force field (Schrodinger). The coordinate was solvated in an orthorhombic box of 10 A each direction with TIP3 water, 150 mM NaCl, and counter ions. The system was pre-equilibrated using the NPT relaxation protocol, which consists of restrained/unrestrained minimizations and short simulations with isothermal and isobaric ensemble. A 10-ns molecular dynamics simulation was done subsequently at constant temperature (300 K) and pressure (1.01325 bar). Simulation quality analysis showed no significant fluctuation of system volume, pressure, temperature, and potential energy during the course of the simulation. Coordinates from the simulation between 1 and 10 ns were used to analyze and identify prominent interactions between the ligand (azido-(PEG)4-PODA) and the antibody (h38C2 Fab). Model figures and atom distances were created and calculated using PyMOL (Schrodinger). (Note: Crystal structure 6U85 is nearly identical to independent crystal structure 6DZR20 with a root-mean-square deviation (RMSD) of 0.446 A for 389 C a atoms; unlike 6DZR, 6U85 does not contain a sulfate ion that forms a salt bridge with Arg99 in the hydrophobic pocket).
Synthesis of MS-PODA and β-lactam hapten derivatives
The syntheses of compounds 1 (MS-PODA-fluorescein)17, 2 (β-lactam-hapten-TAMRA),20 7 (MS-PODA-MMAF),25 and 8 (β-lactam-hapten-MMAF)16 are published. The syntheses of compounds 3 (β-lactam-hapten-folate), 4 (β-lactam-hapten-LLP2A), 5 (MS-PODA-folate), and 6 (MS-PODA-LLP2A) and their characterization by 1H-NMR, 13C-NMR, HRMS, and LC-MS are provided in the Supplementary Information.
Antibodies
The amino acid sequences of VH and VL of h38C2 were published previously.12 Purified h38C2 IgG1 was a gift from the laboratory of Carlos F. Barbas III (The Scripps Research Institute; La Jolla, CA). To generate h38C2_Lys99Ala IgG1, light and mutated heavy chain encoding sequences of h38C2 IgG1 were cloned into mammalian expression vector pCEP4 via Nhel/Xhol (New England Biolabs). The two plasmids were co-transfected into a density of 3 – 106 cells/mL of Expi293F cell by using the ExpiFectamine 293 Transfection Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. After culturing transfected cells at 37°C, 5% CO2 for 5 days, the culture supernatant was collected and purified by affinity chromatography with a 1-mL HiTrap Protein A column in conjunction with an ÄKTA FPLC instrument (both from GE Healthcare). The sequences, cloning, expression, and purification of the anti-HER2 DVD-IgG1 were published previously.16,27
Antibody conjugation
10 μM of h38C2 IgG1 or h38C2_Lys99Ala IgG1 was incubated with 100 μM (5 eq per each of the two Lys99 residues) of compound 1 (MS-PODA-fluorescein) or compound 2 (β-lactam-hapten-TAMRA) in PBS for 3 h at RT. Of the reduced and nonreduced conjugation mixture, 2.5 vg was loaded onto a 10-well NuPAGE 4-12% Bis-Tris Protein Gel (Thermo Fisher Scientific). Fluorescent bands were visualized by blue light on an E-gel Imager and the gel was subsequently stained by PageBlue Protein Staining Solution (all form Thermo Fisher Scientific). Chemically programmed h38C2 IgG1_3, _4, _5, and _6 and the two ADCs (anti-HER2 DVD-IgG1_7 and _8) were assembled analogously, purified with illustra NAP-5 Columns (GE Healthcare), and concentrated with Amicon Ultra 0.5-mL Centrifugal Filters with 30-kDa MWCO.
Mass spectrometry
Unconjugated and conjugated antibodies at 10 μM were reduced with 50 mM DTT in PBS for 10 min at RT followed by enzymatic deglycosylation with PNGase F (New England Biolabs) overnight at 37°C. Following dilution into water, data were obtained on an Agilent Electrospray Ionization Time of Flight (ESI-TOF) mass spectrometer. Deconvoluted masses were obtained using Agilent BioConfirm Software.
Catalytic activity assay
Unconjugated and conjugated antibodies at 1 μM in 98 μL were dispensed into a 96-well plate (Corning) in triplicate. Subsequently, 2 μL of 10 mM methodol was added and the fluorescence (excitation/emission set to 330/452 nm) was measured in 5-min intervals for 1 h at RT using a Spectra Max M5 instrument (Molecular Devices).27
Human plasma stability assay
An equal volume of human plasma (Sigma-Aldrich) and 1 mg/mL h38C2 IgG1_1 in PBS were mixed and incubated at 37°C. After 0, 1, 2, 3, 4, 5, 6, 7, and 8 days, 2-μL aliquots were frozen and stored at −80°C. Under reducing condition, aliquots from all time points were analyzed using a 10-well NuPAGE 4-12% Bis-Tris Protein Gel. Fluorescent bands were visualized by blue light on an E-gel Imager and the gel was subsequently stained by PageBlue Protein Staining Solution.
ELISA
100 ng of Human recombinant folate receptor 1 (FOLR1) diluted at 100 ng/25 μL in Tris-buffered saline (TBS; Bio-Rad) and human recombinant integrin α4β1 (all from R&D systems) diluted at 100 ng/25 μL in TBS supplemented with 1 mM MnCl2 was placed in 96-well half-area microplates (Corning) and incubated at 4°C overnight. After 1-h blocking with 3% (v/v) skim milk in TBS, 5 μg/mL of unconjugated h38C2 IgG1 and h38C2 IgG1 chemically programmed with folate (compounds 3 and 5) or LLP2A (compounds 4 and 6) were added to the FOLR1-or integrin a4D1-coated wells, respectively, and incubated for 1 h. Subsequently, the wells were washed 3 times with 0.05% (v/v) Tween 20 (Sigma-Aldrich) in TBS. A 1:2,000 dilution of HRP-conjugated goat anti-human IgG Fcy-specific pAbs (Jackson ImmunoResearch) in 3% (v/v) skim milk in TBS was added and incubated for 1 h at RT. Following 3 washes as before, BioFX ABTS One Component HRP Microwell Substrate (Surmodics) was added to the wells following the manufacturer’s instructions. Absorbance at 405 nm was detected using a Spectra Max M5 instrument. The experiment was performed in triplicate.
Flow cytometry analysis
IGROV-1 cells (1 × 105) were incubated with h38C2 IgG1 chemically programmed with folate (compounds 3 and 5) diluted in 1% (v/v) BSA in TBS with 0.02% sodium azide (FACS buffer) for 1 h at RT. In parallel, an equal number of Jurkat cells was incubated with h38C2 IgG1 chemically programmed with LLP2A (compounds 4 and 6) in FACS buffer supplemented with 1 mM MnCl2. Following 3 washes with FACS buffer, a 1:1,000 dilution of FITC-conjugated goat anti-human IgG Fcy-specific pAbs (Jackson ImmunoResearch) in FACS buffer was added to the cells and incubated for 1 h. Following 3 washes as before, the cells were suspended in 4% (w/v) paraformaldehyde (Alfa Aesar) in PBS and flow cytometry was performed on a BD FACSCanto instrument. Data were analyzed with FlowJo software (Tree Star).
Cytotoxicity assay
Following a previously published procedure,20 SK-BR-3 (5 × 103 per well), MDA-MB-231 (3 × 103 per well), and KPL-4 (3×103 per well) were plated in 96-well tissue culture plates. Ten-fold serially diluted (0.001-100 nM) ADCs (anti-HER2 DVD-IgG1_7 and _8) along with anti-HER2 DVD-IgG1 as negative control were added to the wells and incubated at 37°C in an atmosphere of 5% CO2 for 72 h. Subsequently, cell viability was measured using CellTiter 96 Aqueous One Solution (Promega) following the manufacturer’s instructions and plotted as a percentage of untreated cells. IC50 values (mean ± SD) were calculated by GraphPad Prism software.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grants R01 CA174844, R01 CA181258, and R01 C204484 (to C.R.) and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (to T.R.B. Jr.). We thank Dr. Lee Pedzisa for synthesizing compounds 1 and 8, Dr. Napon Nilchan for synthesizing compound 2, and Dr. Alex R. Nanna for synthesizing compound 7, collectively under the supervision of Dr. William R. Roush (Department of Chemistry, The Scripps Research Institute, Jupiter, FL). This is manuscript # 29892 from The Scripps Research Institute.
ABBREVIATIONS
- Lys99
reactive lysine residue
- DVD
dual variable domain
- ADC
antibody-drug conjugate
- MS-PODA
methylsulfone phenyloxadiazole
- TAMRA
tetramethylrhodamine
- MMAF
monomethyl auristatin F
- FOLR1
folate receptor 1 or folate receptor α
Footnotes
SUPPORTING INFORMATION
Supplementary Experimental Procedures detailing the synthesis of MS-PODA and β-lactam hapten derivatives of folate and LLP2A. Supplementary Figures showing the small molecule-antibody interactions in the in silico docking model of PODA-conjugated Lys99 (Suppl. Figure S1) and Mass spectrometry analysis of nonreduced anti-HER2 DVD-ADC (Suppl. Figure S2). The Supporting Information is available free of charge on the ACS Publication website at DOI: 10.1021/acs.bioconjchem.
REFERENCES
- [1].Beck A, Goetsch L, Dumontet C, and Corvaia N (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nature Rev. Drug Discov 16, 315–337. [DOI] [PubMed] [Google Scholar]
- [2].Martins CD, Kramer-Marek G, and Oyen WJG (2018) Radioimmunotherapy for delivery of cytotoxic radioisotopes: current status and challenges. Expert Opin. Drug Deliv 15, 185–196. [DOI] [PubMed] [Google Scholar]
- [3].Agarwal P, and Bertozzi CR (2015) Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug. Chem 26, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rader C, Sinha SC, Popkov M, Lerner RA, and Barbas CF III (2003) Chemically programmed monoclonal antibodies for cancer therapy: adaptor immunotherapy based on a covalent antibody catalyst. Proc. Natl. Acad. Sci. U. S. A 100, 5396–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pham GH, Ou W, Bursulaya B, DiDonato M, Herath A, Jin Y, Hao X, Loren J, Spraggon G, Brock A, et al. (2018) Tuning a protein-labeling reaction to achieve highly site selective lysine conjugation. Chembiochem 19, 799–804. [DOI] [PubMed] [Google Scholar]
- [6].Matos MJ, Oliveira BL, Martinez-Saez N, Guerreiro A, Cal PMSD, Bertoldo J, Maneiro M, Perkins E, Howard J, Deery MJ, et al. (2018) Chemo- and regioselective lysine modification on native proteins. J. Am. Chem. Soc 140, 4004–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chilamari M, Kalra N, Shukla S, and Rai V (2018) Single-site labeling of lysine in proteins through a metal-free multicomponent approach. Chem. Commun 54, 7302–7305. [DOI] [PubMed] [Google Scholar]
- [8].Yu C, Tang J, Loredo A, Chen Y, Jung SY, Jain A, Gordon A, and Xiao H (2018) Proximity-induced site-specific antibody conjugation. Bioconjug. Chem 29, 3522–3526. [DOI] [PubMed] [Google Scholar]
- [9].Yamada K, Shikida N, Shimbo K, Ito Y, Khedri Z, Matsuda Y, and Mendelsohn BA (2019) AJICAP: affinity peptide mediated regiodivergent functionalization of native antibodies. Angew. Chem. Int. Ed. Engl 58, 5592–5597. [DOI] [PubMed] [Google Scholar]
- [10].Wagner J, Lerner RA, and Barbas CF III (1995) Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science 270, 1797–1800. [DOI] [PubMed] [Google Scholar]
- [11].Barbas CF III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjornestedt R, List B, Anderson J, Stura EA, Wilson IA, et al. (1997) Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science 278, 2085–2092. [DOI] [PubMed] [Google Scholar]
- [12].Rader C, Turner JM, Heine A, Shabat D, Sinha SC, Wilson IA, Lerner RA, and Barbas CF (2003) A humanized aldolase antibody for selective chemotherapy and adaptor immunotherapy. J. Mol. Biol 332, 889–899. [DOI] [PubMed] [Google Scholar]
- [13].Rader C (2014) Chemically programmed antibodies. Trends Biotechnol 32, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Walseng E, Nelson CG, Qi J, Nanna AR, Roush WR, Goswami RK, Sinha SC, Burke TR Jr., and Rader C (2016) Chemically programmed bispecific antibodies in diabody format. J. Biol. Chem 291, 19661–19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qi J, Hymel D, Nelson CG, Burke TR Jr, and Rader C (2019) Conventional and chemically programmed asymmetric bispecific antibodies targeting folate receptor 1. Front. Immunol 10, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nanna AR, Li X, Walseng E, Pedzisa L, Goydel RS, Hymel D, Burke TR Jr, Roush WR, and Rader C (2017) Harnessing a catalytic lysine residue for the one-step preparation of homogeneous antibody-drug conjugates. Nat. Commun 8, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Toda N, Asano S, and Barbas CF III (2013) Rapid, stable, chemoselective labeling of thiols with Julia-Kocienski-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew. Chem. Int. Ed. Engl 52, 12592–12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patterson JT, Asano S, Li X, Rader C, and Barbas CF III (2014) Improving the serum stability of site-specific antibody conjugates with sulfone linkers. Bioconjug. Chem 25, 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang C, Vinogradova EV, Spokoyny AM, Buchwald SL, and Pentelute BL (2019) Arylation chemistry for bioconjugation. Angew. Chem. Int. Ed. Engl 58, 4810–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hwang D, Nilchan N, Nanna AR, Li X, Cameron MD, Roush WR, Park H, and Rader C (2019) Site-selective antibody functionalization via orthogonally reactive arginine and lysine residues. Cell Chem. Biol 26, 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Low PS, and Kularatne SA (2009) Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol 13, 256–262. [DOI] [PubMed] [Google Scholar]
- [22].Peng L, Liu R, Marik J, Wang X, Takada Y, and Lam KS (2006) Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat. Chem. Biol 2, 381–389. [DOI] [PubMed] [Google Scholar]
- [23].Schlesinger M, and Bendas G (2015) Contribution of very late antigen-4 (VLA-4) integrin to cancer progression and metastasis. Cancer Metastasis Rev. 34, 575–591. [DOI] [PubMed] [Google Scholar]
- [24].Ye F, Kim C, and Ginsberg MH (2012) Reconstruction of integrin activation. Blood 119, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nilchan N, Li X, Pedzisa L, Nanna AR, Roush WR, and Rader C (2019) Dual-mechanistic antibody-drug conjugate via site-specific selenocysteine/cysteine conjugation. Antib. Ther in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, Mochizuki M, Nakamura H, and Sonoo H (1999) Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Brit. J Cancer 79, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nanna AR, and Rader C (2019) Engineering dual variable domains for the generation of site-specific antibody-drug conjugates. Methods Mol Biol 2033, 39–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


