Abstract
Purpose:
Socket augmentation decrease the magnitude of alveolar ridge resorption, but the literature is limited in respect of quantifying soft tissue remodeling. The aim of this study was to determine the volumetric and linear dimensional changes at the buccal surface for both hard and soft tissues after socket augmentation treated with a xenogeneic collagen matrix in combination with bone grafting.
Materials and Methods:
Twenty-four individuals indicated for tooth extraction were enrolled in this investigation. Each participant was randomly assigned to one of two groups: 1) deproteinized bovine bone+ collagen plug, or 2) deproteinized bovine bone + xenogeneic collagen matrix. A cone beam computed tomography (CBCT) scan was taken prior to extraction and at month-6 post-extraction. Intra-oral scanning images were taken at baseline, month-3, and month-6 post extraction. Hard and soft tissue analyses were performed to compare linear ridge remodeling and volumetric changes by a non-contact reverse engineering software.
Results:
Both groups showed bone and soft tissue remodeling. For hard tissue remodeling there was no significant difference between collagen plug and collagen matrix groups. For soft tissue remodeling the collagen matrix group showed a reduced soft tissue loss compared to the collagen plug group. The volumetric analysis demonstrated that the average buccal soft tissue volume loss for the collagen matrix group was 68.6mm3 compared to 87.6mm3 found in the collagen plug group (p=0.009) over a 6-month period.
Conclusions:
This clinical investigation provides early evidence of using the total tissue volume to compare soft and hard tissue remodeling after socket augmentation. The results of this study demonstrated that the use of a xenogeneic collagen matrix reduced the buccal soft tissue loss after tooth extraction, but additional studies are necessary to evaluate the clinical significance of soft tissue augmentation after tooth extraction.
INTRODUCTION
Alveolar bone formation after tooth extraction is a naturally healing event that occurs based on the surrounding alveolar walls.(1, 2) However, the loss in hard and soft tissue volume may compromise esthetic rehabilitation and impair proper implant placement. Several techniques have been proposed aiming reduction of the magnitude of the alveolar crest resorption that occurs after tooth extraction.(3–6) Minimizing extraction trauma and limiting flap elevation are among these procedures. (7) The usage of bone fillers in socket augmentation also assists in preserving the remaining hard and soft tissues after tooth extractions and assists in decreasing additional bone grafting procedure for future implant placement.(3, 8, 9) However, the literature is limited in quantifying soft-tissue healing after socket augmentation protocols.
Resorbable and non-resorbable barrier membranes have been used for the preservation of the alveolar bone dimensions after tooth extraction and have demonstrated clinical advantages when combined with xenograft and allograft bone substitutes.(9) The use of a collagen plug as an adjunct to cover the bone grafting material during socket augmentation is commonly used in clinical practice. Unlike barrier membranes, the clinical implication of the collagen plug is to seal the site, prevent loss of the bone grafting material, and to aid in clot formation and platelet aggregation (10). However, due to material limitations, the use of collagen plug offers minimal advantage regarding the modulation of wound healing (11). Three-dimensional (3D) xenogeneic collagen matrix has been used over mineralized bone graft after minimally invasive tooth extraction in order to preserve hard and soft tissue volume for future implant placement (12). The application of such a xenogeneic collagen matrix appears to favor immediate blood clot stabilization leading to early vascularization (13, 14), facilitate soft tissue cell ingrowth (13), and enable excellent integration of the xenogeneic matrix within the surrounding tissues (13, 14). While it is straightforward to demonstrate these characteristics in vitro and in very controlled wound healing models, it is more difficult to measure the clinical effects in human subjects (15).
An accurate and reliable clinical quantification of hard and soft tissue volumetric changes has remained technically challenging. The use of cone-beam computed tomography (CBCT) has been used and developed into a reliable measurement tool (16, 17). However, this method also has several issues including the limitation for linear measurements analysis, the scattering effect that can affect the analysis, and the additional exposure to radiation of the patient for comparative analysis. The use of digital intra-oral optical scanning (IOS) and assessment methods were introduced to measure volume changes of oral tissues over time (18, 19) and provided a new perspective to measure and quantify soft tissue volume longitudinally after reconstruction or regenerative periodontal procedures.
The aim of the present investigation was to determine the volumetric and linear changes at the buccal surface for both hard and soft tissue after socket augmentation treated with a xenogeneic collagen matrix using CBCT and IOS generated models. To accomplish this, a novel 3D analysis using a non-contact reverse engineered software was developed and is described along with the outcomes.
MATERIALS & METHODS
This randomized controlled clinical trial enrolled 24 participants who were in need for tooth extraction and future implant placement. The study protocol was approved by the University of North Carolina at Chapel Hill human subjects internal review board. All recruited participants were previously treatment planned for extraction + implant placement by non-study personnel to avoid any potential conflict of interest. Participants were randomized by sealed envelopes containing one of the 2 assigned treatment groups: 1) Collagen plug group: Extraction and socket augmentation treated with deproteinized bovine bone (BioOss Collagen®, Geistlich Pharma AG) + collagen plug (HeliPlug®, Integra Miltex, Inc.), or 2) Collagen matrix group: Extraction treated with deproteinized bovine bone (BioOss Collagen®, Geistlich Pharma AG) + xenogeneic collagen matrix (Mucograft® Seal, Geistlich Pharma AG). Participants who required antibiotic prophylaxis prior to dental treatment or those with medical contraindication to dental treatment were excluded. To be eligible for the study, participants had to be adult males or females age 18 to 80 years old, having a maxillary premolar, canine, lateral incisor, or central incisor with a restorative or periodontal hopeless prognosis (20), in which a dental implant was indicated without any anticipated guided bone regeneration or sinus grafting required. In addition, all participants must be in a stable periodontal condition prior to the implant surgery. Participants with uncontrolled diabetes (HbA1c >7%) within 3 months prior to screening examination, history of intravenous bisphosphonates, current smokers, currently taking anticoagulant medications, high dose corticosteroids, radiation therapy or chemotherapy were excluded from this study. Women who were known to be pregnant, breastfeeding or planning to become pregnant within 6 months were also excluded from the study. Participants with dehisced, fenestrated, or discontinuous labial/buccal alveolar bone plate determined after baseline cone beam computed tomography (CBCT) prior extraction, or after tooth extraction where more than 50% of the buccal bone height was not present, were treated with guided bone regeneration and immediately excluded from the study. At the initial examination, all participants completed a full mouth clinical examination including probing pocket depth, clinical attachment level, bleeding on probing, and gingival index(21) on all teeth by a calibrated examiner. This randomized clinical trial was registered at the NIH Clinical Trials Registry (Clinical Trial Registration No. NCT02844569).
Surgical Procedure
Within two weeks of the initial examination, the pre-determined hopeless tooth was extracted, using a minimally traumatic approach. Facial and lingual intrasulcular incisions were made only at the tooth requiring extraction. A periotome was used in the interproximal spaces to sever subcrestal periodontal attachment fibers and expand the periodontal ligament space. If needed to facilitate periotome insertion, a fine long diamond bur (859–010; Brasseler, Inc.) was used to minimally remove interproximal bone alongside the tooth. An elevator was used to mobilize the tooth, and forceps were used to deliver the tooth. The socket was curetted to remove all granulomatous tissue, and the site was irrigated with sterile isotonic saline solution.
For the collagen plug group, deproteinized bovine bone was placed into the debrided socket in the necessary amount to successfully fill the extraction socket. The bone substitute material was rehydrated with the subject’s blood and/or sterile saline solution. Subsequently, a collagen plug was placed to cover the grafted extraction socket and sutured with a resorbable suture (5–0 Chromic Gut, Ethicon) to stabilize the wound (Figure 1). For the collagen matrix group, the same deproteinized bovine bone was placed into the debrided socket in the necessary amount to successfully fill the extraction socket to the level of bone. The bone substitute material was similarly rehydrated with the subject’s blood and/or sterile saline solution. A xenogeneic collagen matrix was used to cover the grafted extraction socket and sutured with a non-resorbable suture (6–0 Prolene, Ethicon) and resorbable suture to stabilize the collagen matrix over the extraction socket and maximize direct contact between the matrix and soft tissue of the socket opening (Figure 2).
Figure 1.
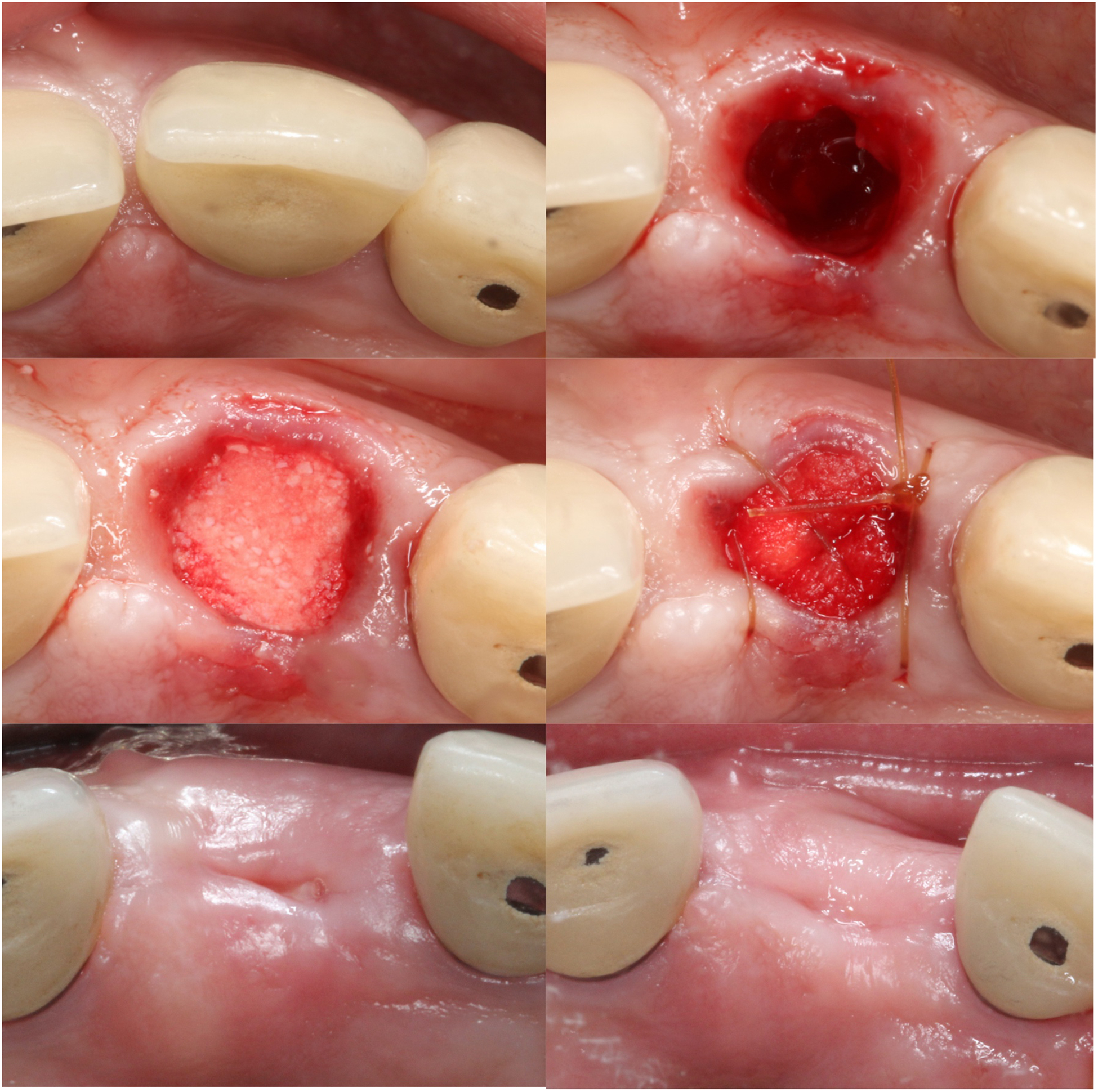
Clinical images of the procedures performed for the collagen plug group. A. Buccal view prior to the extraction of tooth #9; B. Occlusal view; C. Extraction socket after minimally invasive extraction; D. Extraction socket grafted with deproteinized bovine bone and covered with collagen plug; E. Occlusal view 6 months post-extraction; F. Buccal view 6 months post-extraction.
Figure 2.
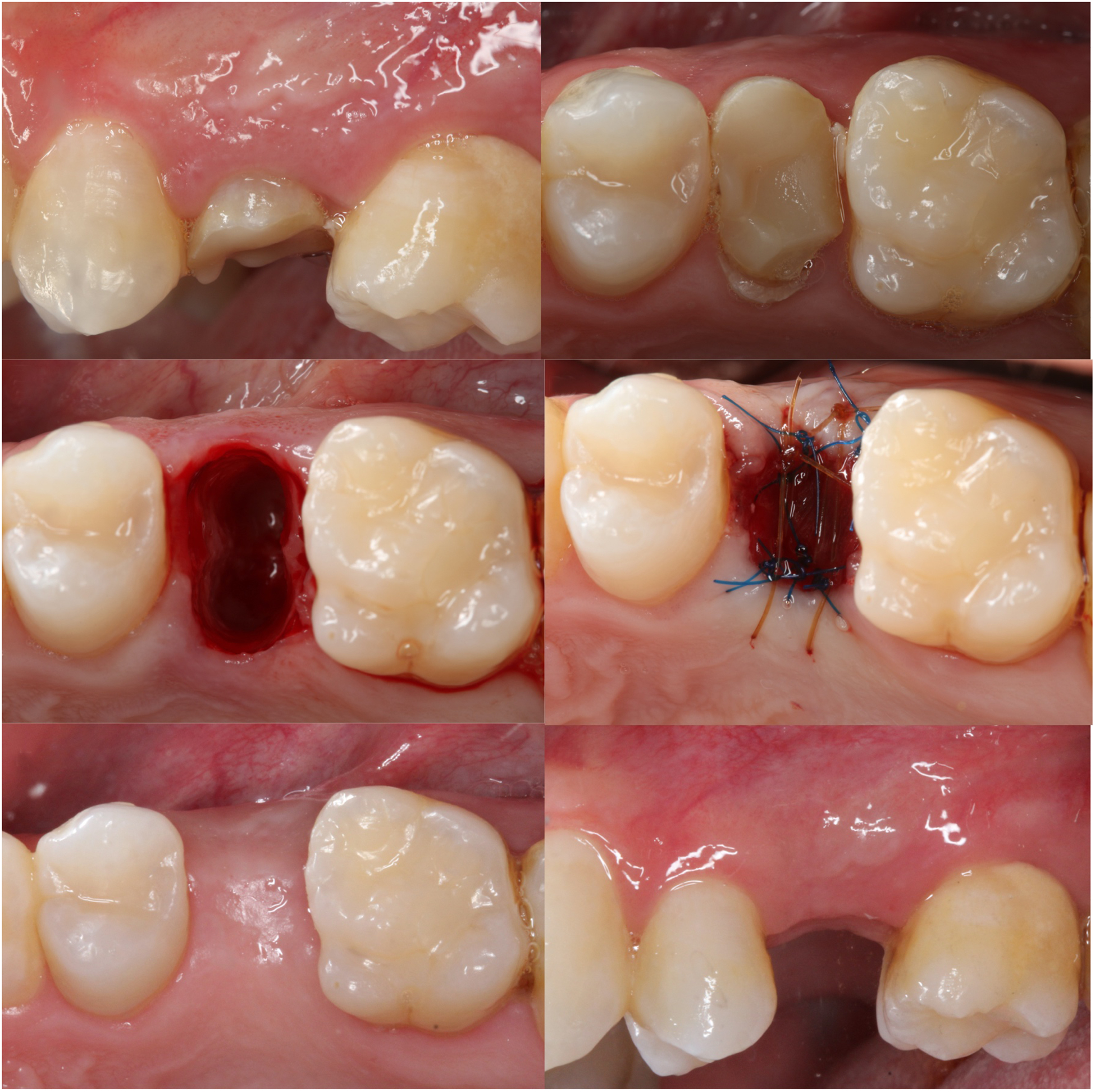
Clinical images of the procedures performed for the collagen matrix group. A. Buccal view prior to the extraction of tooth #13; B. Occlusal view; C. Extraction socket after minimally invasive extraction; D. Extraction socket grafted with deproteinized bovine bone and covered with collagen matrix; E. Occlusal view 6 months post-extraction; F. Buccal view 6 months post-extraction.
Medications prescribed to all participants included 500mg Amoxicillin (7 days) or 250mg Azithromycin (4 days) for participants who reported allergic to Amoxicillin, and 600mg Ibuprofen (7 days). All participants were instructed to rinse with 0.12% chlorhexidine gluconate for 30 seconds twice daily, and to avoid brushing or touching the surgical site for 2 weeks. Sutures were removed 2 weeks following the surgical appointment. Participants were recalled at 1, 2, 4, 12, and 24 weeks for monitoring of the healing process. Participants were permitted to wear a temporary removable prosthesis to replace the missing tooth. All removable prostheses were adjusted to remove any direct contact with the extraction site minimizing any direct effect of the prosthesis into the soft tissue healing.
Radiographic Analysis
Cone Beam Computed Tomography (CBCT) scans (New Tom 5G; 110kV; 2 mA) were taken following the screening visit and at 6 months post tooth extraction. To evaluate radiographic linear and volumetric changes from baseline to 6-months, data were converted to DICOM format and imported into InVesalius 3 software (22). Maxillary surface mesh models were generated using each CBCT data creating stereolithography (STL) files that were later analyzed using a non-contact reverse engineering software (Geomagic Control, 3D Systems). All radiographic data was analyzed by one calibrated examiner.
Soft Tissue Analysis
Soft tissue linear and volumetric analysis were performed to compare the soft tissue remodeling between the collagen matrix and collagen plug groups. IOS images captured with an intra-oral digital scanner (Trios 3, 3Shape) were collected at baseline, week-1, week-2, week-4, month-3, and month-6. To evaluate soft tissue linear and volumetric changes data were converted to STL files and analyzed by a non-contact reverse engineering software. Linear and volumetric changes were calculated based on the data measured at baseline, month-3, and month-6 visits.
Linear and Volumetric Assessments
For each participant, presurgical and the 6-month postsurgical radiographic models, and presurgical, 3- and 6-month postsurgical IOS models were superimposed. The superimposition technique included the selection of the same 3 teeth in each model. The software was then able to perform an automatic alignment and superimposition of the 2 models (e.g. presurgical and a follow-up model). The average error in alignment of the two data sets was kept below 0.1mm for all subjects. Prior to analysis, the presurgical model was selected as reference, while the postsurgical models were selected as the testing model against the reference. For each participant, to measure the volumetric remodeling, an area of interest at the buccal aspect of the extraction site was defined, and the volumetric remodeling at this area was measured. In addition, two-dimensional buccal-palatal cross sections were obtained in the center of the extraction site. Subsequently, the buccal linear remodeling between preoperative and post-surgical models were measured at 1-, 3-, and 5-mm below the crest for both radiographic and IOS models. Both linear and volumetric soft tissue analysis were performed by only one calibrated examiner,
Statistical Analyses
Descriptive statistics (mean and standard deviations) were calculated for the pooled data sets for each treatment group. A power analysis was performed using a statistical power calculator (SAS Power Procedure). The sample size of 24 subjects, 12 in each of two groups, allowed 90% power (α = 0.05) to detect a difference of 2.5mm in the horizontal ridge width measured at 3mm below the crest, assuming a standard deviation of 1.6mm as determined by a previous study (Jung et al. 2013). This power calculation accounted for a 10% subject drop-out rate. Differences were considered statistically significant at p<0.05.
For soft tissue analysis, the primary outcome was the within-participant difference between the linear and volume changes at the collagen matrix and collagen plug groups. These data were collected prior to surgery, as well as 3 and 6 months after surgery, leading to a series of three longitudinal differences for each participant. Linear mixed model (LMM) was used to analyze the data longitudinally, in which the actual linear and volume measures were modeled as a function of time, treatment group, and the interaction of time and treatment, while accounting for the repeated measures on each participant with a random participant effect. Due to the exploratory nature of the analysis, no Bonferroni correction for multiple testing was applied. Statistical significance was defined as a p-value < 0.05.
For hard tissue analysis a two-sample equal variance student t-test with a two-tailed distribution was performed comparing the two groups for each of the linear measurements as well as for the volumetric analysis. Statistical significance was defined as a p-value < 0.05.
RESULTS
A total of 28 individuals were screened for study eligibility; of these, 24 met study inclusion criteria and were randomized between the 2 study groups. Demographics baseline characteristics of this cohort have been described in Table 1. Each individual underwent a single tooth extraction. For the collagen plug group, 7 extractions were performed in the anterior area and 5 in the premolar area. For the collagen matrix group, 5 extractions were performed in the anterior area and 7 in the premolar region. All individuals completed the 6-month follow-up period, and the hard and soft tissue could be assessed in all preserved socket sites.
Table 1.
Study demographics
| Collagen Matrix | Collagen Plug | p-value | |
|---|---|---|---|
| Female | 7 | 7 | ND |
| Male | 5 | 5 | ND |
| Caucasian | 8 | 9 | ND |
| Non-Caucasian | 4 | 3 | ND |
| Mean Age (SD) | 45.2 (11.4) | 56.4 (12.2) | ND |
Data from horizontal linear soft tissue remodeling are demonstrated in Figures 3–4. Results showed that socket augmentation with the use of a deproteinized bovine bone + collagen plug showed a greater linear bucco-lingual loss of soft tissue at the 1 mm, 3 mm and 5 mm below the gingival margin at the 1, 3 and 6-month follow-up. Both groups showed similar horizontal linear soft tissue loss with most of the soft tissue loss concentrating 1mm below the gingival margin. The linear analysis between collagen matrix and collagen plug were not statistically significant at any of the evaluated time points.
Figure 3.
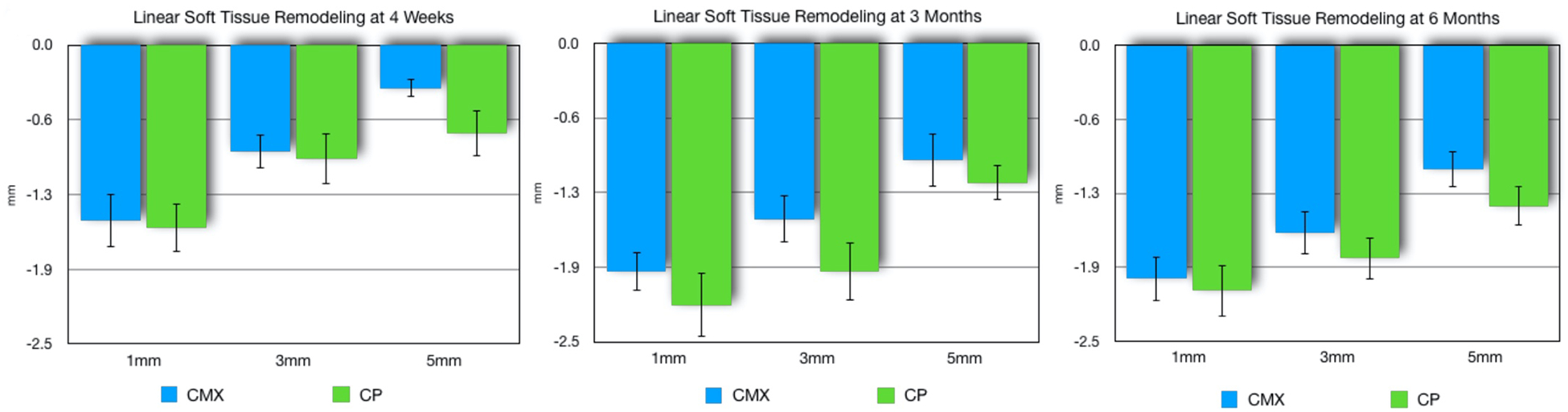
Linear (mean, SE) soft tissue loss for xenogeneic collagen matrix (CMX) and collagen plug (CP) groups at 1, 3, and 5 mm from the gingival margin at the mid-buccal of the extraction site at month 1 (A), 3 (B), and 6 (C). No statistically significant difference between groups (p>0.05).
Figure 4.
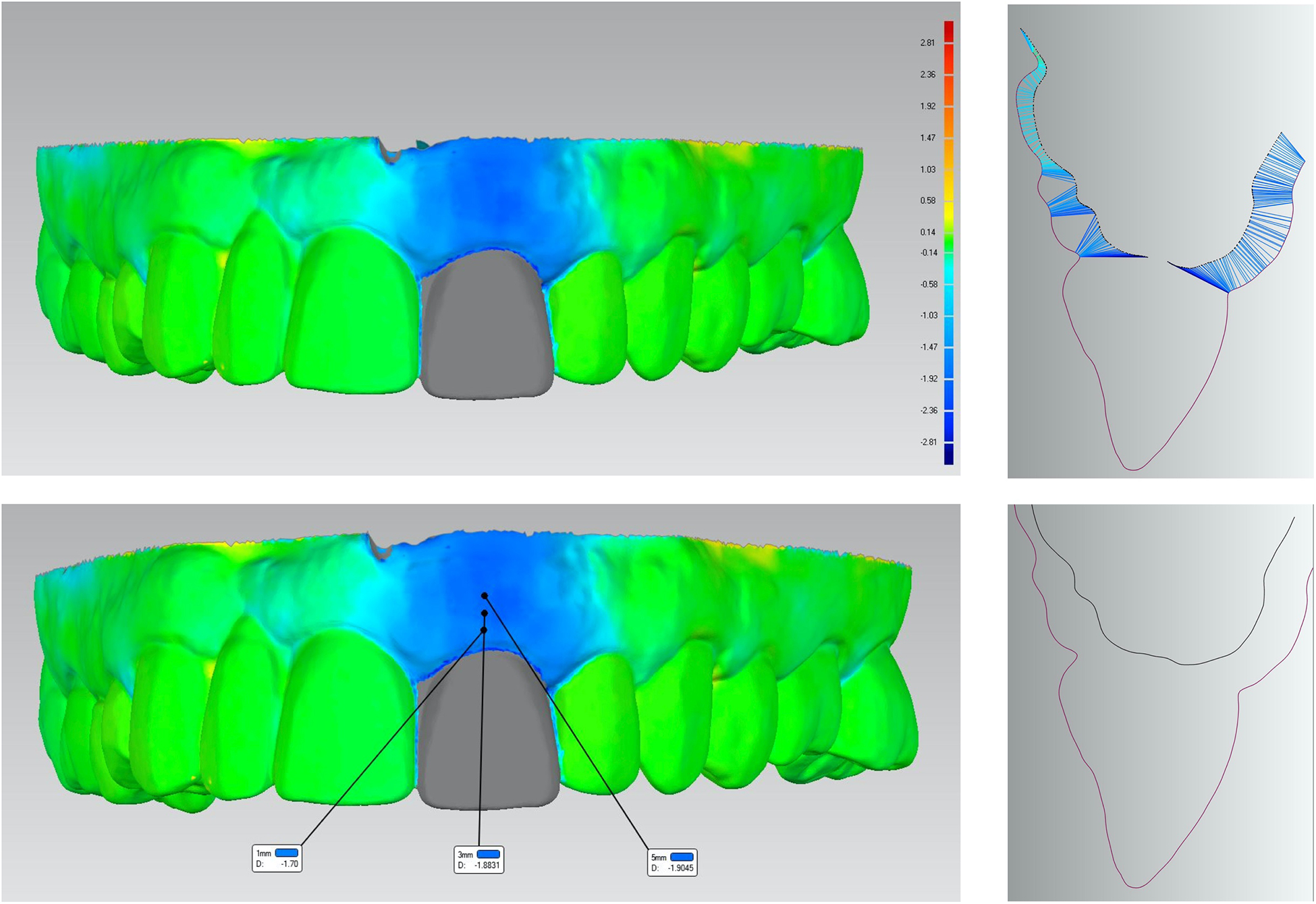
A. Example of a three-dimensional superimposition of scanned images at baseline and 6-month from the collagen plug group. Colored scale represents the linear soft tissue gain/loss (mm) at the buccal site. B. Cross-sectional image of the mid-buccal position at tooth #9 showing the linear soft tissue loss for both buccal and palatal sites. C. Three-dimensional superimposed scanned images showing the linear gain/loss in soft tissue at 1, 3, and 5mm below the gingival margin. D. Cross-sectional image at the mid-buccal site of tooth #9 showing baseline (red) and 6-month (black) soft tissue contour.
Data from horizontal linear hard tissue remodeling are demonstrated in Figures 5–6. Two collagen matrix group participants and four collagen plug group participants were excluded from the linear hard tissue analysis due to unacceptable discrepancies during the alignment of the baseline and 6-month CBCT data. Thus, a total of ten datasets were used for the collagen matrix group, and seven for the collagen plug. Results derived from the horizontal hard tissue linear analysis over six months showed similar amounts of linear buccal-lingual alveolar bone resorption from baseline at 1-, 3-, and 5mm below the alveolar crest. There was a significant difference in linear bone loss between groups at 5mm below the bone crest (p=0.029). The collagen plug group showed a mean linear bone loss of 0.92mm against 1.64mm for the collagen matrix group.
Figure 5.
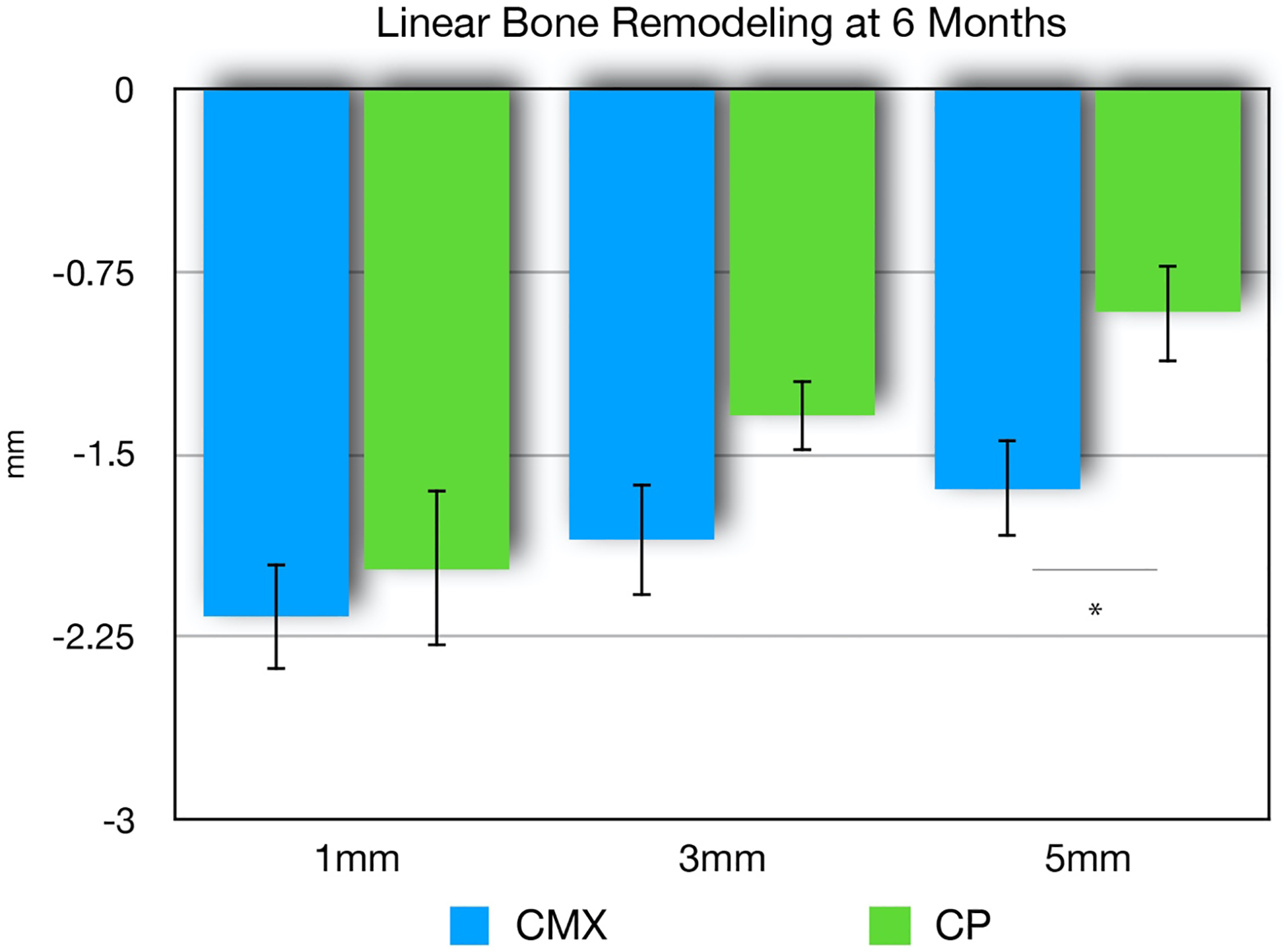
Linear (mean, SE) bone loss for xenogeneic collagen matrix (CMX) and collagen plug (CP) groups at six-months post-extraction and socket augmentation. Measures were obtained at 1mm, 3mm, and 5mm below the alveolar crest at the mid-buccal of the extraction site. No statistically significant differences at 1mm and 3mm (p>0.05). Statistically significant difference at 5mm (p<0.05) at 5mm in favor of the collagen plug group (*).
Figure 6.
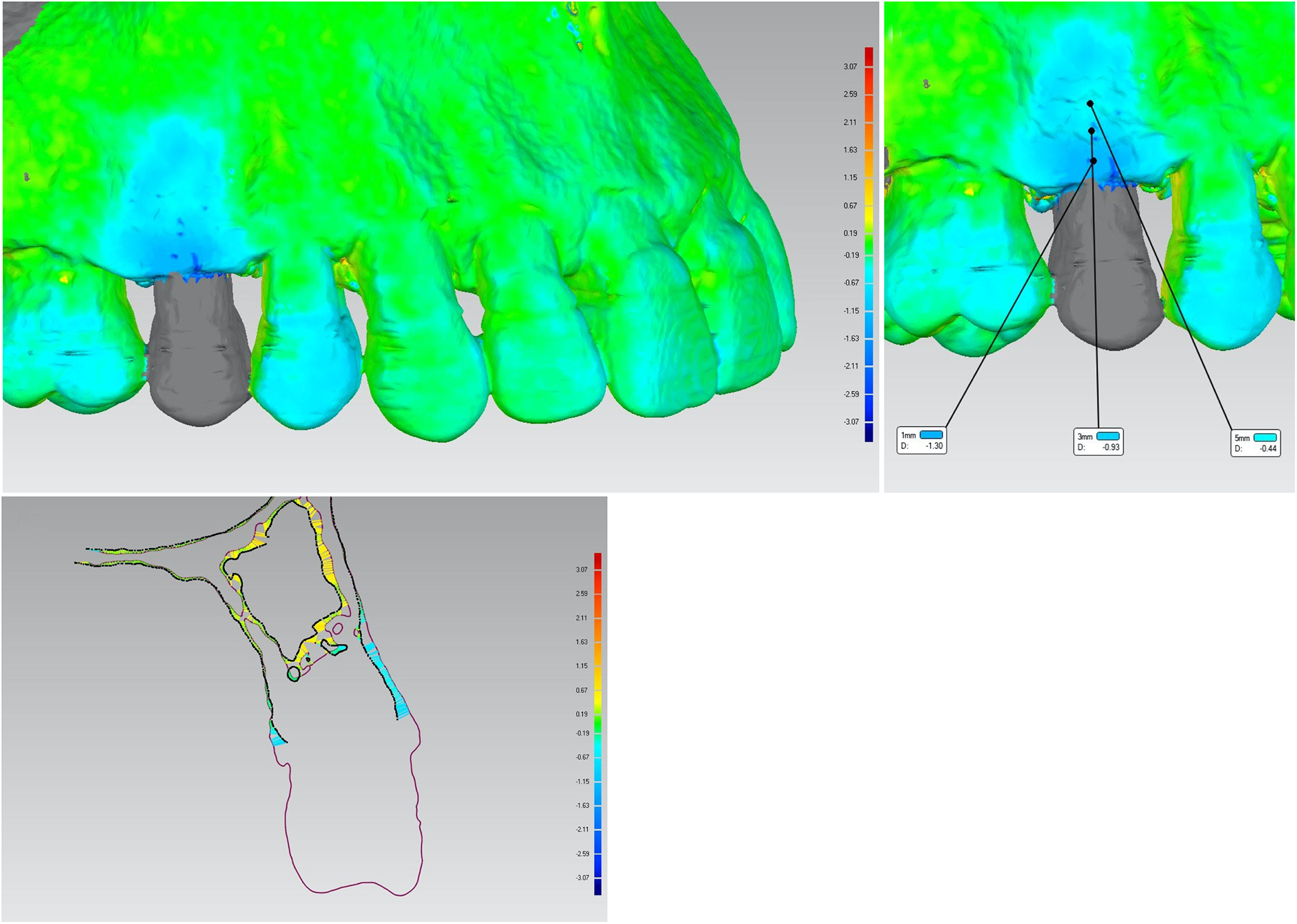
A. Three-dimensional superimposition of a baseline and a 6-month scanned image from a xenogeneic collagen matrix group. Colored scale represents the linear bone gain/loss (mm) at the buccal site. B. Three-dimensional superimposed scanned images showing the linear gain/loss in hard tissue at 1, 3, and 5mm below the bone crest. C. Cross-sectional image of the mid-buccal position at tooth #4 showing the linear bone loss for both buccal and palatal sites.
Data from soft tissue volumetric analysis demonstrated less facial soft tissue loss in favor of the collagen matrix group (Figures 7–8). At the 1-month follow-up, the collagen plug group demonstrated an average soft tissue loss of 50.8 mm3, while the collagen matrix group showed an average of 32.0 mm3 in volumetric soft tissue loss. At the 3-month assessment, the facial soft tissue volumetric analysis demonstrated that the collagen matrix group lost an average of 64.8 mm3 compared to 86.6 mm3 lost by the collagen plug group sites. At the 6-month time point, the collagen matrix group lost an average of 68.8 mm3 compared to 87.6 mm3 in the collagen plug sites. Comparing to the collagen plug group, the collagen matrix group exhibited an overall significant less soft tissue volumetric loss pattern over the 6-month healing period (p=0.009).
Figure 7.
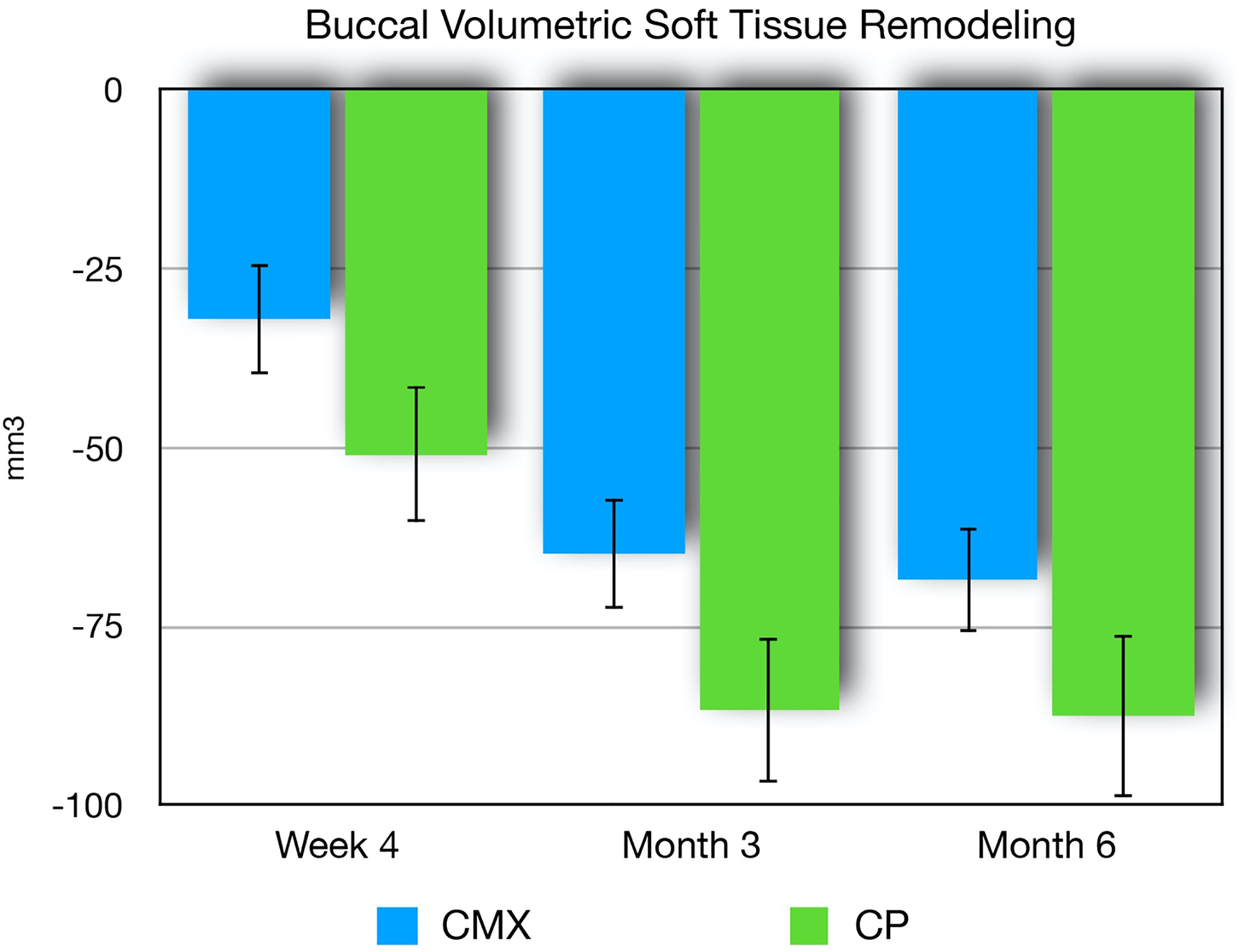
Soft tissue volume loss (mean, SE) for xenogeneic collagen matrix (CMX) and collagen plug (CP) groups at 1, 3, and 6 months post-extraction. A significantly statistical difference was found between groups at 6 months (p=0.009).
Figure 8.
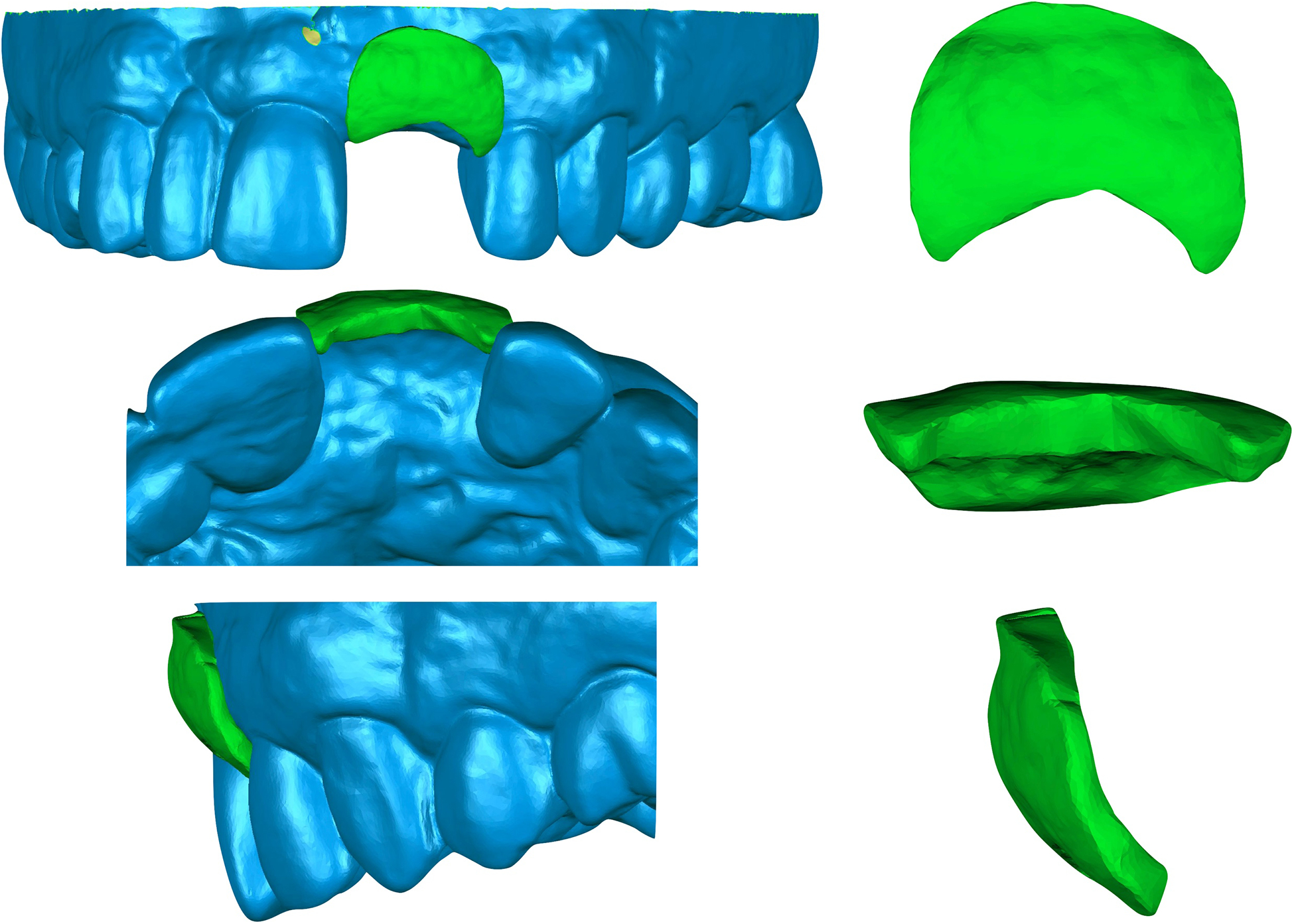
Visual representation of the soft tissue volumetric changes of superimposed images from a collagen plug treated site at site #9 from the A. Buccal, C. Occlusal and E. Lateral views. Isolated images of soft tissue changes from the B. Buccal, D. Occlusal and F. Lateral views.
Data from hard tissue volumetric analysis are demonstrated on Figures 9–10. Results revealed similar findings for both groups in terms of bone volume remodeling. The collagen plug group showed a mean volumetric hard tissue loss of 66.4mm3. The collagen matrix group showed a mean volumetric hard tissue loss of 72.6mm3. The difference between collagen matrix and collagen plug groups was not statistically significant (p=0.668).
Figure 9.
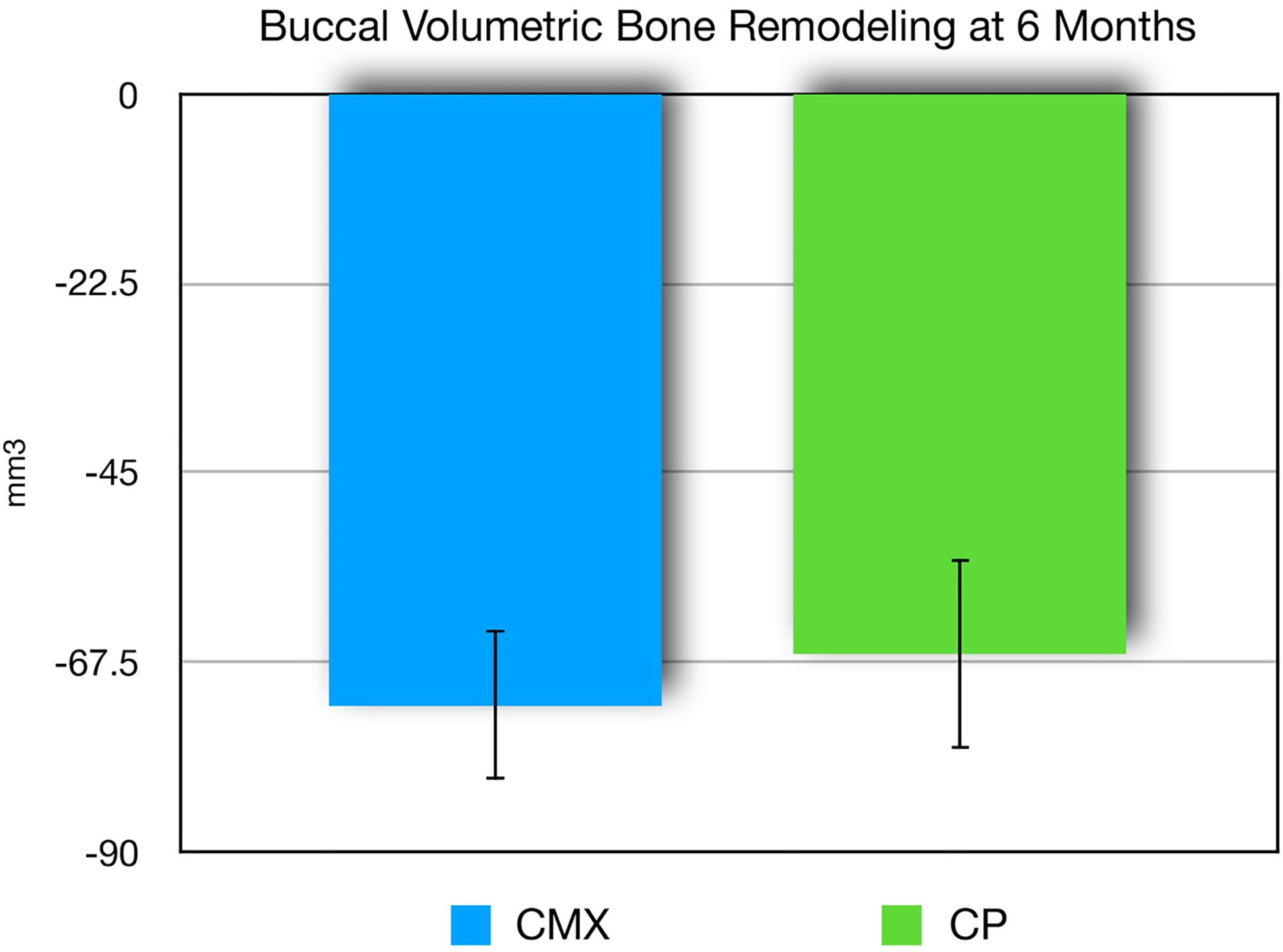
Bone volume loss (mean, SE) for xenogeneic collagen matrix (CMX) and collagen plug (CP) groups at six-months post-extraction and socket augmentation. No statistically significant difference between groups (p>0.05).
Figure 10.
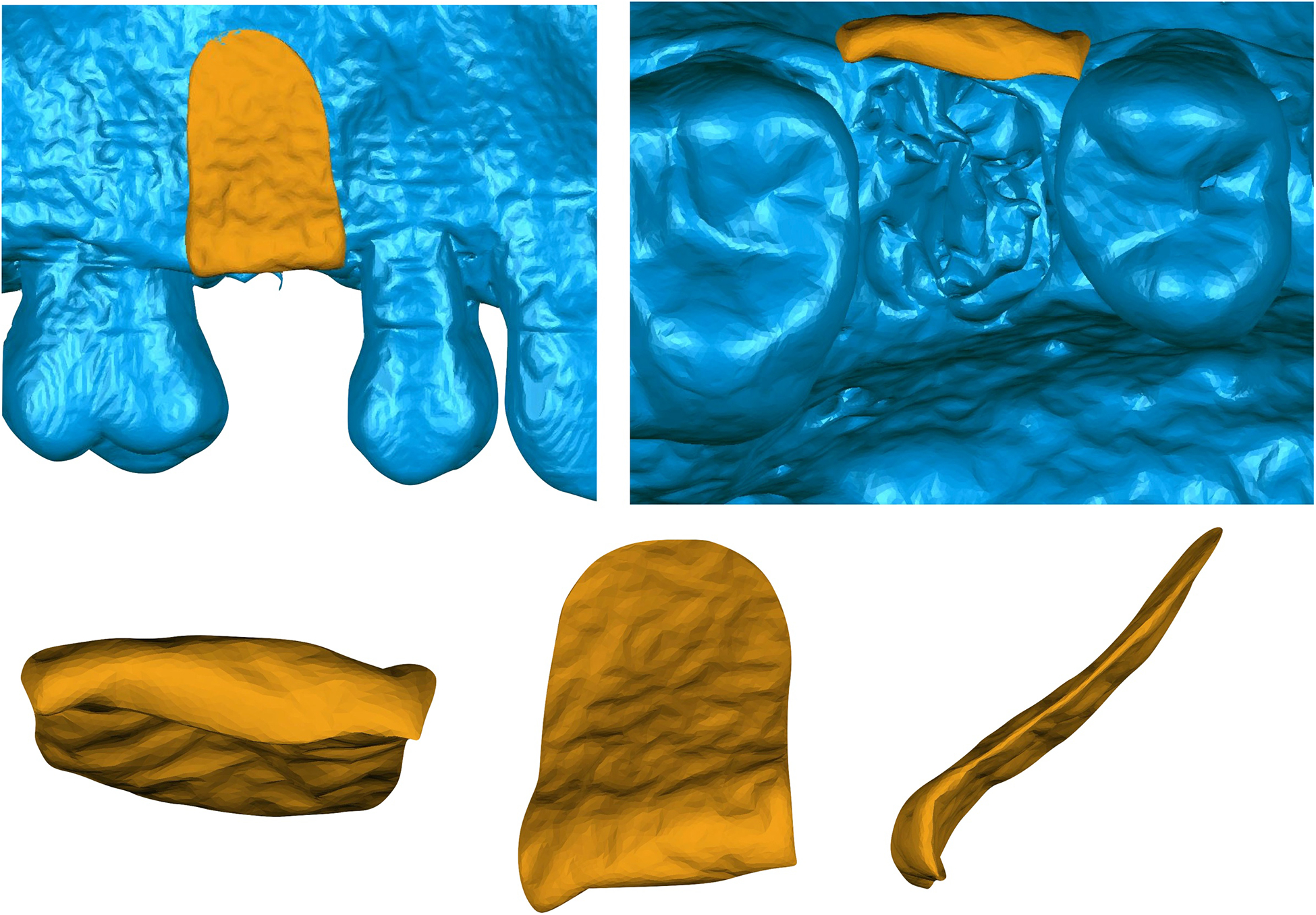
A. Buccal representation of the bone volumetric changes from a xenogeneic collagen matrix treated site. B. Occlusal representation of a 6-month scanned image superimposed with a baseline bone volume at site #4. Isolated representation of the bone volume loss at 6-months from the C. Occlusal, D. Buccal and E. Lateral views.
DISCUSSION
In this randomized clinical trial, we have demonstrated that the use of a xenogeneic collagen matrix seal over a bone graft material resulted in an increase of approximately 22% soft tissue volume at the facial site of extraction sites when compared to a collagen sponge plug over the same bone graft material. It has been widely demonstrated that socket augmentation techniques limit bone dimensional changes after tooth extraction, especially in esthetic zones (8, 23, 24). However, to our knowledge this is the first study to report the soft tissue 3D volumetric remodeling associated with socket augmentation. The esthetic zone is considered an area of high risk for ridge alteration consequent to tooth extraction (25). The maintenance of adequate soft tissue volume during socket augmentation procedures is crucial for the esthetics, diminishing the possibility of future additional soft tissue grafts.
In our study, linear measurement analysis demonstrated that sites treated with xenogeneic collagen matrix had a reduced soft tissue remodeling at 1mm (1.97mm collagen matrix; 2.07mm collagen plug), 3mm (1.58mm collagen matrix; 1.8mm collagen plug), and 5mm (1.05mm collagen matrix; 1.36mm collagen plug) below the gingival margin compared to collagen plug at the 6-month follow up visit. Our results are supported by other similar studies assessing the reduction of soft tissue loss by using a xenogeneic collagen matrix. A recent study by Natto and investigators compared the clinical and radiographic soft and hard tissue dimensional changes during socket augmentation using freeze-dried bone allograft (FDBA) with a collagen plug or a xenogeneic collagen matrix. Clinical soft tissue measurements were performed using radiographic stents. The linear measurements showed that both treatment modalities were effective in preserving the alveolar ridge. A slight increase in buccal gingival thickness at the coronal part was observed in both groups (0.9 mm collagen matrix and 0.5 mm collagen plug) (26). In a similar study using models that were scanned and analyzed with digital software, the linear soft tissue analysis showed that sites treated with a xenogeneic collagen matrix and free gingival graft had a reduced amount of soft tissue loss, 1.2 ± 0.5 mm and 1.2 ± 0.7 mm respectively, compared to sites treated with β-TCP not covered and non-grafted sites, 1.7 ± 0.7 mm and 1.8 ± 0.8mm respectively (27).
Our radiographic analysis demonstrated that the use of either collagen plug or xenogeneic collagen matrix over an extraction socket grafted with deproteinized bovine bone produces similar results in all volumetric measures, and in most linear measures. This finding is in agreement with previous studies, which have determined that there is no statistical difference in treatment outcomes based on material used for socket augmentation at the time of tooth extraction (8, 9). For example, Darby and collaborators evaluated thirty-seven human studies utilizing a variety of techniques and materials for post-extraction socket augmentation. It was determined that while socket augmentation procedures are indeed effective in minimizing horizontal and vertical ridge resorption, there is no evidence to support the efficacy of one technique or material as being superior to another (3). Another review and meta-analysis by Avila-Ortiz indicated that socket augmentation was effective in preserving vertical and horizontal dimensions compared to spontaneous healing, and that while a membrane and/or graft material did influence socket augmentation positively, the type of membrane or the type of grafting material (allograft vs xenograft) was not a determinant factor (9). Thus, as expected, the present study also did not show significant differences in the majority of radiographic bone measurements between the use of collagen plug compared with xenogeneic collagen matrix. The similarity in alveolar measurements could also be explained by the use of the same deproteinized bovine bone in the socket of both groups, with perhaps the material within the socket itself having a more dominant effect on the preservation of bone resorption more than the barrier material that was used as soft tissue protection over the graft.
It has been extensively reported that the amount of ridge resorption that occurs after tooth extraction is heavily influenced by the initial thickness of the buccal wall (28), with a thicker initial thickness generally leading to a smaller amount or resorption that occurs. In a separate study, the authors reported that the average ridge reduction in premolar sites was 18%, while in anterior sites, ridge reduction was significantly higher at 34%.(29) In this study, we did not find any significant correlation between initial buccal wall thickness and volumetric bone and soft tissue remodeling. In addition, we evaluated the data for premolar and anterior teeth separately and volumetric differences between the test and control groups were still not statistically significant. This can be explained by the low study sample size and that teeth with initial thin buccal wall (<0.5mm) were not considered for the study due to the higher potential of bucall wall fracture/fenestration during the extraction. In regard to the age range of the participants of the study (31 to 69 years), there was no significant correlation for age. A recent study in 547 patients evaluated the influence of age, sex, smoking status, and BMI in bone healing. Results reported that only smoking status significantly correlated with bone healing duration.(30)
In recent years, the advancement in 3D imaging technology has increased its use in maxillofacial surgery, dental implantology, and various other medical disciplines. The 3D model superimposition can facilitate treatment planning, predict and evaluate treatment outcomes. For the analysis of soft tissue independent of bone quantification, we used an intra-oral optical scanner to obtain 3D reconstructed images. In a validation study using the same IOS used in our study, Imburgia and collaborators showed a trueness value ranging from 50.2μm to 67.2μm and a precision value ranging from 24.5μm to 31.5μm (31), which does not significantly interfere in the results from our study. To evaluate the soft tissue dimensional variation we utilized a reverse engineered software in order to superimpose 3D models from different timepoints and subsequently calculate linear and volumetric changes. In a recent study, Gkantidis et al. evaluated 3D superimposition techniques on various skeletal structures using surface models and concluded that it can provide accurate, precise, and reproducible results (32). A precision analysis study using CAD files determined that surface reconstruction on Geomagic Qualify software provides a reliable analysis with a maximum deviation of 0.06 mm, standard deviation of 0.003 mm, and an average error of 0.002 mm (33). A validation study evaluating the reliability of 3D digital models obtained with a surface laser scanner and analyzed by using the Geomagic software demonstrated that linear measurements on digital models are accurate reproducible (34). In our study, using 3D models reconstructed from IOS and CBCT our average error was 0.04 mm and 0.07 mm respectively.
Soft tissue volume, color, and texture are key elements in achieving optimal esthetics in implant dentistry.(35) Thicker soft tissue not only appears to be important in implant esthetics, but also plays a pivotal role in maintaining a more favorable peri-implant health.(36) In our study, we demonstrated that the average buccal soft tissue loss for the collagen matrix group was 68.6mm3 compared to 87.6mm3 found in the collagen plug group (p=0.009) over a 6-month period. The use of volumetric unit (mm3) to measure soft and hard tissue changes have been published (37–39), but the clinical significance of the unit measured requires a different interpretation in relation to linear measurements reported in mm. The volume data in this study was mainly related to changes in soft tissue thickness located at the coronal portion of the extraction site. However, the clinical benefit is still questionable since the study did not analyze data related to peri-implant health and esthetics.
In summary, we used a novel method for evaluating soft and hard tissue changes using 3D superimposed images. We analyzed the images using a non-contact reverse engineering software that provides the potential to precisely measure tissue changes not only by numbers, but also by generating 3D images, giving an additional perspective for clinical research analysis. The results of this study demonstrated that the use of a xenogeneic collagen matrix reduced the buccal soft tissue loss after tooth extraction. However, additional studies are necessary to evaluate the clinical significance of soft tissue augmentation after tooth extraction.
ACKNOWLEDGMENTS
This study was funded by Geistlich, Pharma AG. Thiago Morelli was funded by NIH/NIDCR K23-DE025093. Shaoping Zhang was funded by NIH/NIDCR K99-DE027086. Julie Marchesan was funded by NIH/NIDCR K01-DE027087. The authors appreciate the assistance of Mrs. Caroline Butler in segmenting the radiographic dataset, Dr. John K. Spitznagel for his assistance in editing the manuscript.
REFERENCES
- 1.Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 2005;32:212–218. [DOI] [PubMed] [Google Scholar]
- 2.Tonetti MS, Jung RE, Avila-Ortiz G, Blanco J, Cosyn J, Fickl S, et al. Management of the extraction socket and timing of implant placement: Consensus report and clinical recommendations of group 3 of the XV European Workshop in Periodontology. J Clin Periodontol 2019;46 Suppl 21:183–194. [DOI] [PubMed] [Google Scholar]
- 3.Darby I, Chen ST, Buser D. Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants 2009;24 Suppl:260–271. [PubMed] [Google Scholar]
- 4.Cardaropoli D, Cardaropoli G. Preservation of the postextraction alveolar ridge: a clinical and histologic study. Int J Periodontics Restorative Dent 2008;28:469–477. [PubMed] [Google Scholar]
- 5.Rasperini G, Canullo L, Dellavia C, Pellegrini G, Simion M. Socket grafting in the posterior maxilla reduces the need for sinus augmentation. Int J Periodontics Restorative Dent 2010;30:265–273. [PubMed] [Google Scholar]
- 6.Bassir SH, Alhareky M, Wangsrimongkol B, Jia Y, Karimbux N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int J Oral Maxillofac Implants 2018;33:979–994. [DOI] [PubMed] [Google Scholar]
- 7.Fickl S, Zuhr O, Wachtel H, Bolz W, Huerzeler M. Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. J Clin Periodontol 2008;35:356–363. [DOI] [PubMed] [Google Scholar]
- 8.Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of Alveolar Ridge Preservation Interventions Following Tooth Extraction: A Systematic Review and Meta-Analysis. J Clin Periodontol 2019. [DOI] [PubMed] [Google Scholar]
- 9.Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res 2014;93:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotsakis G, Chrepa V, Marcou N, Prasad H, Hinrichs J. Flapless alveolar ridge preservation utilizing the “socket-plug” technique: clinical technique and review of the literature. J Oral Implantol 2012. [DOI] [PubMed] [Google Scholar]
- 11.Kim DM, Lim HC, Hong JY, Shin SI, Chung JH, Herr Y, et al. Validity of Collagen Plugs for Ridge Preservation in a Canine Model. Implant Dent 2017;26:892–898. [DOI] [PubMed] [Google Scholar]
- 12.Jung RE, Philipp A, Annen BM, Signorelli L, Thoma DS, Hammerle CH, et al. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: a randomized controlled clinical trial. J Clin Periodontol 2013;40:90–98. [DOI] [PubMed] [Google Scholar]
- 13.Ghanaati S, Schlee M, Webber MJ, Willershausen I, Barbeck M, Balic E, et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater 2011;6:015010. [DOI] [PubMed] [Google Scholar]
- 14.Rocchietta I, Schupbach P, Ghezzi C, Maschera E, Simion M. Soft tissue integration of a porcine collagen membrane: an experimental study in pigs. Int J Periodontics Restorative Dent 2012;32:e34–40. [PubMed] [Google Scholar]
- 15.Thoma DS, Sancho-Puchades M, Ettlin DA, Hammerle CH, Jung RE. Impact of a collagen matrix on early healing, aesthetics and patient morbidity in oral mucosal wounds - a randomized study in humans. J Clin Periodontol 2012;39:157–165. [DOI] [PubMed] [Google Scholar]
- 16.Januario AL, Barriviera M, Duarte WR. Soft tissue cone-beam computed tomography: a novel method for the measurement of gingival tissue and the dimensions of the dentogingival unit. J Esthet Restor Dent 2008;20:366–373; discussion 374. [DOI] [PubMed] [Google Scholar]
- 17.Amid R, Mirakhori M, Safi Y, Kadkhodazadeh M, Namdari M. Assessment of gingival biotype and facial hard/soft tissue dimensions in the maxillary anterior teeth region using cone beam computed tomography. Arch Oral Biol 2017;79:1–6. [DOI] [PubMed] [Google Scholar]
- 18.Thoma DS, Jung RE, Schneider D, Cochran DL, Ender A, Jones AA, et al. Soft tissue volume augmentation by the use of collagen-based matrices: a volumetric analysis. J Clin Periodontol 2010;37:659–666. [DOI] [PubMed] [Google Scholar]
- 19.Sanz-Martin I, Sailer I, Hammerle CH, Thoma DS. Soft tissue stability and volumetric changes after 5 years in pontic sites with or without soft tissue grafting: a retrospective cohort study. Clin Oral Implants Res 2016;27:969–974. [DOI] [PubMed] [Google Scholar]
- 20.Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol 2007;78:2063–2071. [DOI] [PubMed] [Google Scholar]
- 21.Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent 1986;8:3–6. [PubMed] [Google Scholar]
- 22.Amorim P M T, Silva J, Pedrini H . In Vesalius: An Interactive Rendering Framework for Health Care Support Advances in Visual Computing: Springer, Cham, 2015. [Google Scholar]
- 23.Cardaropoli D, Tamagnone L, Roffredo A, De Maria A, Gaveglio L. Alveolar Ridge Preservation Using Tridimensional Collagen Matrix and Deproteinized Bovine Bone Mineral in the Esthetic Area: A CBCT and Histologic Human Pilot Study. Int J Periodontics Restorative Dent 2018;38:s29–s35. [DOI] [PubMed] [Google Scholar]
- 24.Jung RE, Ioannidis A, Hammerle CHF, Thoma DS. Alveolar ridge preservation in the esthetic zone. Periodontol 2000 2018;77:165–175. [DOI] [PubMed] [Google Scholar]
- 25.Chappuis V, Engel O, Shahim K, Reyes M, Katsaros C, Buser D. Soft Tissue Alterations in Esthetic Postextraction Sites: A 3-Dimensional Analysis. J Dent Res 2015;94:187S–193S. [DOI] [PubMed] [Google Scholar]
- 26.Natto ZS, Parashis A, Steffensen B, Ganguly R, Finkelman MD, Jeong YN. Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: A randomized controlled clinical trial. J Clin Periodontol 2017;44:649–659. [DOI] [PubMed] [Google Scholar]
- 27.Schneider D, Schmidlin PR, Philipp A, Annen BM, Ronay V, Hammerle CH, et al. Labial soft tissue volume evaluation of different techniques for ridge preservation after tooth extraction: a randomized controlled clinical trial. J Clin Periodontol 2014;41:612–617. [DOI] [PubMed] [Google Scholar]
- 28.Chappuis V, Engel O, Reyes M, Shahim K, Nolte LP, Buser D. Ridge alterations post-extraction in the esthetic zone: a 3D analysis with CBCT. J Dent Res 2013;92:195S–201S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo MG, da Silva JCC, de Mendonca AF, Lindhe J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin Oral Implants Res 2015;26:407–412. [DOI] [PubMed] [Google Scholar]
- 30.Simon MH, Grunwald L, Schenke M, Dickschas J, Strecker W. Corrective osteotomies of femur and tibia: which factors influence bone healing? Arch Orthop Trauma Surg 2019. [DOI] [PubMed] [Google Scholar]
- 31.Imburgia M, Logozzo S, Hauschild U, Veronesi G, Mangano C, Mangano FG. Accuracy of four intraoral scanners in oral implantology: a comparative in vitro study. BMC Oral Health 2017;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gkantidis N, Schauseil M, Pazera P, Zorkun B, Katsaros C, Ludwig B. Evaluation of 3-dimensional superimposition techniques on various skeletal structures of the head using surface models. PLoS One 2015;10:e0118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang WG, Yi J . Precision Analysis of the Surface Reconstruction Model Based on Geomagic Qualify. Applied Mechanics & Materials 2014:443–447. [Google Scholar]
- 34.Sousa MV, Vasconcelos EC, Janson G, Garib D, Pinzan A. Accuracy and reproducibility of 3-dimensional digital model measurements. Am J Orthod Dentofacial Orthop 2012;142:269–273. [DOI] [PubMed] [Google Scholar]
- 35.Belser UC, Buser D, Hess D, Schmid B, Bernard JP, Lang NP. Aesthetic implant restorations in partially edentulous patients--a critical appraisal. Periodontol 2000 1998;17:132–150. [DOI] [PubMed] [Google Scholar]
- 36.Thoma DS, Naenni N, Figuero E, Hammerle CHF, Schwarz F, Jung RE, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin Oral Implants Res 2018;29 Suppl 15:32–49. [DOI] [PubMed] [Google Scholar]
- 37.Fickl S, Schneider D, Zuhr O, Hinze M, Ender A, Jung RE, et al. Dimensional changes of the ridge contour after socket preservation and buccal overbuilding: an animal study. J Clin Periodontol 2009;36:442–448. [DOI] [PubMed] [Google Scholar]
- 38.Baumer D, Zuhr O, Rebele S, Hurzeler M. Socket Shield Technique for immediate implant placement - clinical, radiographic and volumetric data after 5 years. Clin Oral Implants Res 2017;28:1450–1458. [DOI] [PubMed] [Google Scholar]
- 39.Cabanes-Gumbau G, Pascual-Moscardo A, Penarrocha-Oltra D, Garcia-Mira B, Aizcorbe-Vicente J, Penarrocha-Diago MA. Volumetric variation of peri-implant soft tissues in convergent collar implants and crowns using the biologically oriented preparation technique (BOPT). Med Oral Patol Oral Cir Bucal 2019;24:e643–e651. [DOI] [PMC free article] [PubMed] [Google Scholar]


