Abstract
STUDY QUESTION
Is maternal polycystic ovary syndrome (PCOS) associated with increased risks for a broad spectrum of psychiatric and mild neurodevelopmental disorders in offspring?
SUMMARY ANSWER
Maternal PCOS and/or anovulatory infertility is independently, and jointly with maternal obesity, perinatal problems, cesarean delivery and gestational diabetes, associated with increased risks in offspring for almost all groups of psychiatric and mild neurodevelopmental disorders with onset in childhood or adolescence.
WHAT IS KNOWN ALREADY
Maternal PCOS was previously associated with autism spectrum disorder, attention-deficit/hyperactivity disorders and possibly developmental delay in offspring. Few studies have investigated the association between maternal PCOS and other psychiatric and neurodevelopmental disorders in offspring.
STUDY DESIGN, SIZE, DURATION
This was a population-based cohort study in Finland including all live births between 1996 and 2014 (n = 1 105 997). After excluding births to mothers with symptoms similar to PCOS, a total of 1 097 753 births by 590 939 mothers remained. Children were followed up until 31 December 2018, i.e. up to the age of 22 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
National registries were used to link data of the included births and their mothers. Data from 24 682 (2.2%) children born to mothers with PCOS were compared with 1 073 071 (97.8%) children born to mothers without PCOS. Cox proportional hazards modeling was used to evaluate the hazard ratio (HR) and 95% CI for the risk of neuropsychiatric disorders in relation to maternal PCOS. Stratified analyses were performed to test the independent role of PCOS and the joint effects of PCOS with maternal obesity, perinatal problems, cesarean delivery, gestational diabetes and use of fertility treatment. The analysis was adjusted for maternal age, country of birth, marriage status at birth, smoking, parity, psychiatric disorders, prescription of psychotropic N05/N06 during pregnancy and systemic inflammatory diseases when applicable.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 105 409 (9.8%) children were diagnosed with a neurodevelopmental or psychiatric disorder. Firstly, maternal PCOS was associated with any psychiatric diagnosis (HR 1.32; 95% CI 1.27–1.38) in offspring. Particularly, the risk was increased for sleeping disorders (HR 1.46; 95% CI 1.27–1.67), attention-deficit/hyperactivity disorders and conduct disorders (HR 1.42; 95% CI 1.33–1.52), tic disorders (HR 1.42; 95% CI 1.21–1.68), intellectual disabilities (HR 1.41; 95% CI 1.24–1.60), autism spectrum disorder (HR 1.40; 95% CI 1.26–1.57), specific developmental disorders (HR 1.37; 95% CI 1.30–1.43), eating disorders (HR 1.36; 95% CI 1.15–1.61), anxiety disorders (HR 1.33; 95% CI 1.26–1.41), mood disorders (HR 1.27; 95% CI 1.18–1.35) and other behavioral and emotional disorders (ICD-10 F98, HR 1.49; 95% CI 1.39–1.59). In short, there was no significant difference between sexes. The results were robust when restricting the analyses to the first-born children or births to mothers without psychiatric diagnosis or purchase of psychotropic medication. Secondly, stratified analysis according to maternal BMI showed that the risk of any neuropsychiatric disorder was increased in offspring to normal-weight mothers with PCOS (HR 1.20; 95% CI 1.09–1.32), and markedly higher in those to severely obese mothers with PCOS (HR 2.11; 95% CI 1.76–2.53) compared to offspring to normal-weight mothers without PCOS. When excluding perinatal problems, mothers with PCOS were still associated with increased risks of any neuropsychiatric disorders in offspring (HR 1.28; 95% CI 1.22–1.34) compared to mothers without PCOS. However, an additional increase was observed for PCOS in combination with perinatal problems (HR 1.99; 95% CI 1.84–2.16). Likewise, excluding cases with maternal gestational diabetes (HR 1.30; 95% CI 1.25–1.36), cesarean delivery (HR 1.29; 95% CI 1.23–1.35) or fertility treatment (HR 1.31; 95% CI 1.25–1.36) did not eliminate the associations.
LIMITATIONS, REASONS FOR CAUTION
The register-based prevalence of PCOS was lower than previously reported, suggesting that this study may capture the most severe cases. To combine anovulatory infertility with PCOS diagnosis as PCOS exposure might introduce diagnostic bias. It was not feasible to distinguish between subtypes of PCOS. Furthermore, familial factors might confound the association between maternal PCOS and neuropsychiatric disorders in offspring. Maternal BMI was available for birth cohort 2004–2014 only and there was no information on gestational weight gain.
WIDER IMPLICATIONS OF THE FINDINGS
This study provides further evidence that maternal PCOS and/or anovulatory infertility, independently and jointly with maternal obesity, perinatal problems, gestational diabetes and cesarean delivery, implies a broad range of adverse effects on offspring neurodevelopment. These findings may potentially help in counseling and managing pregnancies.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the joint research funding of Shandong University and Karolinska Institute (SDU-KI-2019-08 to X.C and C.L.), THL Finnish Institute for Health and Welfare: Drug and pregnancy project [M.G.], the Swedish Research Council [2014-10171 to C.L.], the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institute Stockholm County Council [SLL20170292 to C.L.], the Swedish Brain Foundation [FO2018-0141 and FO2019-0201 to C.L.]. X.C. was supported by the China Scholarship Council during her training in Karolinska Institute. L.K. was supported by the China Scholarship Council for his PhD study in Karolinska Institute. The authors have no competing interests to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: polycystic ovary syndrome, offspring, psychiatric disorder, neurodevelopmental disorder, transgenerational, obesity, gestational diabetes, anovulatory infertility
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women at reproductive age, with an estimated prevalence of 6–20% (Escobar-Morreale, 2018). The syndrome is characterized by a combination of androgen excess and ovarian dysfunction after excluding other specific diagnoses. The etiology of PCOS is unknown; but there is growing evidence for an interaction between genetic susceptibility and prenatal androgen exposure (Risal et al., 2019).
PCOS status, together with associated obesity, insulin resistance, and pregnancy complications, is potentially to provide an altered intrauterine environment, which may negatively influence the offspring (Vanky et al., 2019). Maternal PCOS has been proposed as a model of exposure to excessive androgens in uterus, reporting that the affected children had increased risks of neuropsychiatric disorders including autism spectrum disorder (ASD), attention deficit/hyperactivity disorders (ADHDs) and possibly Tourette syndrome/chronic tic disorders (Palomba et al., 2012; Kosidou et al., 2016; Kosidou et al., 2017; Cherskov et al., 2018; Cesta et al., 2019). In addition, a cohort study found that offspring with maternal PCOS were more likely to have developmental delay before age 3 (Bell et al., 2018). Also, a PCOS-like phenotype in mice was associated with anxiety-like behaviors in offspring (Hu et al., 2015). These findings suggest that maternal PCOS might have a broad adverse effect on neurodevelopment. However, to our knowledge, a comprehensive picture of the association between maternal PCOS and offspring neurodevelopmental and psychiatric disorders is less known.
Moreover, overweight and obesity are more prevalent in women with PCOS (Escobar-Morreale, 2018). Studies on offspring ASD and ADHD revealed a tendency of increasing risks in mothers with PCOS with worsening metabolic profiles (Kosidou et al., 2016; Kosidou et al., 2017); however, the risk for neuropsychiatric disorders at full BMI strata in mothers with PCOS is not determined.
In addition, subfertility is a common trait in women with PCOS and many of them seek help from ART due to anovulation (Escobar-Morreale, 2018; Teede et al., 2018). Further, mothers with PCOS are more likely to develop pregnancy complications including gestational diabetes mellitus (GDM) and perinatal problems such as preterm birth and small for gestational age (Teede et al., 2018). Studies in the general population have revealed that perinatal problems, GDM, preeclampsia, cesarean delivery and possibly fertility treatment are risk factors for neuropsychiatric disorders in offspring (Bay et al., 2013; Sucksdorff et al., 2015; Walker et al., 2015; Kong et al., 2018; Zhang et al., 2019). But it is unknown whether, and to what extent the association between maternal PCOS and offspring neuropsychiatric disorders could be explained by them.
Thus, this study aimed to explore whether maternal PCOS is associated with a wide spectrum of offspring neuropsychiatric disorders using a nationwide register cohort in Finland. The unique effects of PCOS in normal weight and its joint effects with obesity were examined. The potential roles of perinatal problems, GDM, cesarean delivery and fertility treatment in mothers with PCOS were tested by stratified analyses.
Materials and methods
Data source and study population
The index cases in this study were all live births during 1996–2014 in Finland, registered in the Drugs and Pregnancy Database (Artama et al., 2011) and originally identified from the Medical Birth Register (MBR). Clinical diagnoses for offspring and mothers were identified from the Finnish Care Register for Health Care (HILMO). Medication purchases (prescription-only) were retrieved from the Finnish Register on Reimbursement Drugs (Supplementary Methods).
Births to mothers with symptoms similar to PCOS were excluded from this study (n = 8244): pituitary adenoma (ICD-9: 227.3; ICD-10: D35.2), disorders of the pituitary glands including hypo/hyper function (ICD-9: 253; ICD-10: E22, E23), disorders of the adrenal glands including congenital adrenal hyperplasia and Cushing’s syndrome (ICD-9: 255; ICD-10: E24/E25/E27), galactorrhea (ICD-9: 611.6; ICD-10: N64.3), suprarenal tumor (ICD-9: 194; ICD-10: C74) and Turner syndrome (ICD-9: 758.6; ICD-10: Q96).
Ethical consideration
This study was approved by the data providing authorities and the data protection authority in Finland. According to Finnish regulations, analysis of anonymous register data required no informed consent. Data from different datasets and registries were linked and combined using the personal identification number issued to each Finnish citizen and permanent resident. The data analysis was performed between 1 June 2019 and 10 July 2020.
Exposure ascertainment
Maternal PCOS was identified from the HILMO based on the diagnosis of PCOS (ICD-9: 256.4; ICD-10: E28.2) or anovulatory infertility (ICD-9: 628.0; ICD-10: N97.0), given that PCOS is the most common cause for anovulatory infertility. Such a PCOS diagnosis was defined as exposure irrespective of year because for this disorder the hormonal and metabolic manifestations persisted through the women’s life span. A total of 1 097 753 births by 590 939 mothers was yielded. Of them, 24 682 children (11 949 females, 12 733 males) were born to mothers with PCOS and 1 073 071 (524 538 females, 548 533 males) to non-PCOS mothers.
Information on maternal BMI, available from 2004, was calculated as pre-pregnancy weight (recorded at their first prenatal visits, round gestation weeks 7–10) divided by the height squared, obtained from the MBR.
GDM and preeclampsia were identified based on ICD-10 O24.4 and O14, respectively. Fertility treatment refers to IVF/ICSI only (check-box in the MBR). Perinatal problems are defined as birth before gestational week 37 or a birth weight <2500 g, or small for gestational age, which is a birth weight and/or length >2 SD below the sex- and gestational age-specific reference mean (Sankilampi et al., 2013), based on the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society (Clayton et al., 2007). Information on cesarean delivery (yes/no) was obtained from the Drugs and Pregnancy database.
Definition of offspring neurodevelopmental and psychiatric disorders
The cases with neurodevelopmental or psychiatric disorders were identified from the HILMO, defined as a primary or secondary diagnosis: F30–39 and F92 (mood disorders), F40–43 and F93 (anxiety disorders), F50 (eating disorders), F51 (sleeping disorders), F60–69 (personality disorders), F70–79 (intellectual disabilities), F80–83 (specific developmental disorders), F84 (ASD), F90–91 (ADHD and conduct disorders), F95 (tic disorders) and F98 (other behavioral and emotional disorders) (Supplementary Table SI). The diagnoses were grouped on the basis of symptom similarities. The coverage and accuracy of HILMO on adult mental and behavioral disorders range from satisfactory to very good, with a completeness above 95% during recent years and a positive predictive value between 75% and 99% for common diagnoses (Sund, 2012). Already in 1991, another study reported that 99% of registrations on hospitalizations relating to mental disorders were under the correct ICD chapter and 98% of the main diagnoses were correct at the three-digit ICD-code level (Keskimäki and Aro, 1991). One study has reported good validity (96% predictive value) for pediatric ASD in HILMO (Lampi et al., 2010).
Information on offspring purchases of psychotropic medications was retrieved from the Finnish Register on Reimbursement Drugs with the following ATC codes: N05 (antipsychotics, anxiolytics, hypnotics, and sedatives), N06A (antidepressants) and N06B (stimulants).
Covariates
Information on maternal age at delivery, parity (0 or ≥1), country of birth (Finland or other), mother married at birth (yes/no), smoking during pregnancy (yes/no), diagnoses of systemic inflammatory disorders (ICD-10: M30–M36), psychiatric disorders (in-patient care due to mental health disorders before pregnancy according to ICD-8: 290–317, ICD-9: 290–319 and ICD-10: F00–F99) and purchase of N05 and N06 during pregnancy (yes/no) were obtained from the Drugs and Pregnancy Database.
Statistical analysis
All statistical analyses were performed using SAS versions 9.3 and 9.4 (SAS Institute, Inc, Cary, NC, USA). Cox proportional hazards modeling was used to evaluate the hazard ratio (HR) and 95% CI for the risk of offspring neuropsychiatric disorders. First, births with maternal PCOS and anovulatory infertility were examined separately, to confirm that it was plausible to combine PCOS and anovulatory infertility as PCOS exposure. Births to mothers with neither PCOS nor anovulatory infertility were used as the reference. Covariates were adjusted for as indicated in tables and figures.
Next, births to mothers with PCOS or anovulatory infertility were combined into the PCOS-exposed group. Using PCOS-unexposed births as the reference, results were examined for all study population first, and then in sex-stratified models.
To test the effects of PCOS with and without obesity, mothers were categorized according to pre-pregnancy BMI into five groups based on the World Health Organization classification: (i) underweight: <18.5, (ii) normal weight: 18.5 ≤ BMI <25, (iii) overweight: 25≤ BMI <30, (iv) moderately obese: 30 ≤ BMI <35 and (v) severely obese: ≥35 kg/m2. Using births to normal-weight mothers without PCOS as the reference, the HR and its 95% CI for offspring neuropsychiatric disorders, as well as purchases of psychotropic medications, was examined. Because maternal BMI was available since 2004, the analyses were performed using birth cohort 2004–2014. The underweight category was not included in the analysis due to restricted sample size.
Lastly, we tested the perinatal problems, GDM, cesarean delivery and fertility treatment for effect modification. PCOS-exposed births were classified into groups of with and without the putative modifier. Using births to mothers with no PCOS and no such modifier as the reference, we explored whether, and to what extent the associations between maternal PCOS and neuropsychiatric disorders in offspring could be attributed to these factors. When testing the effects of perinatal problems, births by cesarean delivery and those born to mothers with GDM and fertility treatment were included in the reference, and vice versa, to avoid creating a control group that was not representative.
Information on mothers’ preeclampsia was also available. However, the sample size for births to mothers with both PCOS and preeclampsia was too few for stratified analysis.
Results
Description of the study population
Of the total 1 097 753 offspring, 9.8% (n = 105 409) were diagnosed with a neurodevelopmental or psychiatric disorder (ICD-10: F30–39 and F92, F40–43 and F93, F50, F51, F60–69, F70–79, F80–84, F90–91, F95 and F98) between 1996 and 2018, that is, 4–22 years for the children. We identified a total of 6789 (0.6%) children with maternal PCOS, and 18 625 (1.7%) with maternal anovulatory infertility, with 4712 (0.4%) overlapped between them. There were elevated risks for most neuropsychiatric disorders studied for both the offspring with maternal PCOS and the offspring with maternal anovulatory infertility (Table I). There were only minor differences in the effect sizes of these increased risks for offspring between those exposed to maternal PCOS and those exposed to anovulatory infertility (Table I), with a pointwise HR being 0.2 higher in PCOS for mood disorders, anxiety disorders, specific developmental disorders, ASD and other behavioral and emotional disorders and 0.4 higher in PCOS for ADHD and conduct disorders. Therefore, we combined PCOS and anovulatory infertility as PCOS exposure (n = 24 682).
Table I.
Hazard ratios (95% CI) for offspring psychiatric and mild neurodevelopmental disorders in relation to maternal PCOS (birth cohort 1996–2014 in Finland).
| Diagnosis | Any F diagnosis | Mood disorders | Anxiety disorders | Eating disorders | Sleeping disorders | Personality disorders | Intellectual disabilities | Specific developmental disorders | Autism spectrum disorders | ADHD and conduct disorders | Tic disorders | Other behavioral and emotional disorders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 24 682)a | n = 2532 | n = 876 | n = 1248 | n = 138 | n = 202 | n = 65 | n = 245 | n = 1651 | n = 323 | n = 881 | n = 143 | n = 859 |
| 1.32 (1.27–1.38) | 1.27 (1.18–1.35) | 1.33 (1.26–1.41) | 1.36 (1.15–1.61) | 1.46 (1.27–1.67) | 1.19 (0.93–1.52) | 1.41 (1.24–1.60) | 1.37 (1.30–1.43) | 1.40 (1.26–1.57) | 1.42 (1.33–1.52) | 1.42 (1.21–1.68) | 1.49 (1.39–1.59) | |
| Maternal PCOS (n = 6789) | n = 560 | n = 140 | n = 248 | n = 23 | n = 52 | n = 10 | n = 69 | n = 443 | n = 91 | n = 236 | n = 32 | n = 233 |
| 1.41 (1.30–1.53) | 1.34 (1.14–1.59) | 1.61 (1.42–1.82) | 1.45 (0.96–2.19) | 1.60 (1.22–2.10) | 1.28 (0.69–2.39) | 1.72 (1.36–2.18) | 1.62 (1.48–1.78) | 1.81 (1.47–2.23) | 1.95 (1.72–2.22) | 1.50 (1.06–2.13) | 1.82 (1.60–2.07) | |
| Maternal anovulatory infertility (n = 18 625) | n = 1658 | n = 527 | n = 781 | n = 89 | n = 134 | n = 37 | n = 170 | n = 1 168 | n = 240 | n = 577 | n = 106 | n = 614 |
| 1.33 (1.27–1.40) | 1.34 (1.23–1.47) | 1.41 (1.32–1.51) | 1.43 (1.16–1.77) | 1.40 (1.18–1.67) | 1.21 (0.88–1.67) | 1.43 (1.23–1.67) | 1.44 (1.36–1.52) | 1.55 (1.36–1.76) | 1.52 (1.40–1.65) | 1.54 (1.27–1.87) | 1.59 (1.47–1.72) | |
| Male (n = 12 733) | n = 1545 | n = 385 | n = 542 | n = 25 | n = 103 | n = 18 | n = 156 | n = 1151 | n = 240 | n = 666 | n = 108 | n = 508 |
| 1.30 (1.24–1.37) | 1.22 (1.11–1.35) | 1.30 (1.19–1.41) | 1.43 (0.96–2.12) | 1.33 (1.09–1.61) | 1.00 (0.63–1.59) | 1.41 (1.20–1.66) | 1.36 (1.28–1.44) | 1.35 (1.19–1.53) | 1.40 (1.29–1.51) | 1.40 (1.15–1.69) | 1.48 (1.36–1.62) | |
| Female (n = 11 949) | n = 987 | n = 491 | n = 706 | n = 113 | n = 99 | n = 47 | n = 89 | n = 500 | n = 83 | n = 215 | n = 35 | n = 351 |
| 1.36 (1.28–1.45) | 1.31 (1.20–1.43) | 1.36 (1.26–1.46) | 1.35 (1.12–1.63) | 1.61 (1.32–1.97) | 1.27 (0.95–1.70) | 1.40 (1.13–1.73) | 1.38 (1.26–1.51) | 1.57 (1.27–1.96) | 1.51 (1.32–1.73) | 1.51 (1.08–2.11) | 1.49 (1.34–1.66) |
PCOS was identified by ICD-9 256.4 and ICD-10 E28.2. Anovulatory infertility was identified by ICD-9 628.0 and ICD-10 N97.0. The analyses were adjusted for maternal age, mother’s country of birth (Finland or not), mother married at birth (yes/no), maternal smoking (yes/no), parity (0 or ≥1), maternal psychiatric disorder (yes/no), maternal purchase of N05 and N06 during pregnancy (yes/no) and maternal systemic inflammatory disease (yes/no). For each outcome, birth cohort 1996–2014 was used and followed up until 2018.
ADHD, attention-deficit/hyperactivity disorders; ICD, international classification of disease; PCOS, polycystic ovary syndrome.
A total of 4712 births were diagnosed with both maternal PCOS and anovulatory infertility. Reference group was total, male or female births to mothers with no PCOS no anovulatory infertility.
PCOS-exposed children, compared with PCOS-unexposed, more often had mothers being ≥30 years, overweight or obese, first-time pregnant, married, non-smokers, and developing GDM, preeclampsia and perinatal problems, and undergoing fertility treatment and cesarean section (Table II).
Table II.
Demographic characteristic of birth cohort between 1996 and 2014 in Finland stratified by maternal PCOS.
| Maternal PCOS (n = 24 682) | No maternal PCOS (n = 1 073 071) | |
|---|---|---|
| Maternal age at delivery (years) | ||
| <25 | 3335 (13.5) | 199 630 (18.6) |
| 25–29 | 7622 (30.9) | 340 611 (31.7) |
| 30–34 | 8541 (34.6) | 334 348 (31.2) |
| ≥35 | 5184 (21.0) | 198 484 (18.5) |
| Pre-pregnancy BMI (kg/m2) | ||
| <18.5 | 419 (1.7) | 22 471 (2.1) |
| 18.5–24 | 8656 (35.1) | 373 050 (34.8) |
| 25–29 | 3897 (15.8) | 129 421 (12.1) |
| 30–34 | 2216 (9.0) | 47 180 (4.4) |
| ≥35 | 1301 (5.3) | 22 210 (2.1) |
| Missing | 8193 (33.2) | 478 739 (44.6) |
| Parity ≥1 | 12 147 (49.2) | 441 121 (41.1) |
| Mother born in Finland | 22 670 (91.8) | 987 139 (92.0) |
| Mother married at delivery | 16 554 (67.1) | 636 044 (59.3) |
| Non-smoking during pregnancy | 20 865 (84.5) | 885 129 (82.5) |
| Maternal systemic inflammatory disorders | 384 (1.6) | 11 124 (1.0) |
| Maternal psychiatric disorders | 619 (2.5) | 19 867 (1.9) |
| Maternal prescription of psychotropic N05/N06 during pregnancy | 1596 (6.5) | 47 650 (4.4) |
| Cesarean delivery | 5698 (23.1) | 154 953 (14.4) |
| Fertility treatment | 3466 (14.0) | 22 706 (2.1) |
| Preeclampsia | 1037 (4.2) | 28 962 (2.7) |
| GDM | 3764 (15.2) | 89 384 (8.3) |
| Perinatal problems | 4287 (17.4) | 137 163 (12.8) |
| Female offspring | 11 949 (48.4) | 524 38 (48.9) |
Values are expressed as n (%). PCOS refers to polycystic ovary syndrome defined by ICD-9: 256.4 and 628.0; ICD-10: E28.2 and N97.0. Perinatal problem is defined as birth before gestational week 37, or birth weight <2500 g, or small for gestational age according to Finnish sex-specific standards (Sankilampi et al., 2013), based on the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society (Clayton et al., 2007). GDM defined by ICD-10 O24.4. Preeclampsia was identified by ICD-10 O14. Fertility treatment refers to in vitro fertilization/intracytoplasmic sperm injection (check-box in the MBR).
GDM, gestational diabetes mellitus.
Increased risk for neuropsychiatric disorders for both sexes of PCOS-exposed offspring
Compared with PCOS-unexposed offspring, children to mothers with PCOS were at higher risks of any neuropsychiatric disorder (HR 1.32; 95% CI 1.27–1.38). For most examined categories of pediatric neurodevelopmental and psychiatric disorders, an increased risk of 30–50% was observed in the presence of maternal PCOS exposure. Sex-stratified analyses showed similar risk estimates for male (HR 1.30; 95% CI 1.24–1.37) and female offspring (HR 1.36; 95% CI 1.28–1.45) (Table I). The results were robust when restricting to first-born births (Supplementary Table SII) or excluding mothers with psychiatric disorders or purchase of psychotropic N05/06 medications (Supplementary Table SIII).
Offspring neuropsychiatric disorders in relation to maternal PCOS and BMI
Among PCOS-exposed births, maternal pre-pregnancy BMI was overweight in 15.8%, moderately obese in 9.0%, and severely obese in 5.3% of the cases. Corresponding proportions in PCOS-unexposed births were 12.1%, 4.4% and 2.1% (Table II). For PCOS-unexposed births, maternal BMI above normal were associated with increased risks of neuropsychiatric disorders in offspring. Moreover, mothers with PCOS, being normal-weight, had increased risks of having an offspring with any neuropsychiatric disorder (HR 1.20; 95% CI 1.09–1.32), intellectual disabilities (HR 1.44; 95% CI 1.12–1.85), ASD (HR 1.39; 95% CI 1.12–1.73), other behavioral and emotional disorders (HR 1.38; 95% CI 1.21–1.57), anxiety disorders (HR 1.25; 95% CI 1.08–1.45), and specific developmental disorders (HR 1.19; 95% CI 1.08–1.32), compared to normal-weight mothers without PCOS, indicating an association between maternal PCOS and adverse neuropsychiatric outcomes in offspring independent of BMI (Table III and Fig. 1).
Table III.
Hazard ratios (95% CI) for offspring psychiatric and mild neurodevelopmental disorders in relation to maternal PCOS and BMI (birth cohort 2004–2014 in Finland).
| Any F diagnosis | Mood disorders | Anxiety disorders | Eating disorders | Sleeping disorders | Personality disorders | Intellectual disabilities | Specific developmental disorders | Autism spectrum disorders | ADHD and conduct disorders | Tic disorders | Other behavioral and emotional disorders | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No maternal PCOS | ||||||||||||
| Normal weight | n = 17661 | n = 3710 | n = 7516 | n = 508 | n = 1663 | n = 167 | n = 2115 | n = 16 289 | n = 2770 | n = 8120 | n = 1494 | n = 8145 |
| 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Overweight | n = 6752 | n = 1464 | n = 2828 | n = 149 | n = 606 | n = 48 | n = 945 | n = 6883 | n = 1166 | n = 3405 | n = 497 | n = 3005 |
| 1.12 (1.09–1.15) | 1.18 (1.11–1.25) | 1.09 (1.05–1.14) | 0.85 (0.71–1.03) | 1.06 (0.97–1.17) | 0.87 (0.63–1.19) | 1.27 (1.18–1.37) | 1.22 (1.19–1.26) | 1.25 (1.17–1.34) | 1.25 (1.20–1.30) | 1.01 (0.91–1.11) | 1.10 (1.06–1.15) | |
| Moderately obese | n = 2924 | n = 630 | n = 1266 | n = 56 | n = 199 | n = 37 | n = 438 | n = 3221 | n = 546 | n = 1582 | n = 195 | n = 1241 |
| 1.35 (1.30–1.40) | 1.41 (1.30–1.53) | 1.36 (1.28–1.44) | 0.93 (0.70–1.22) | 0.96 (0.83–1.12) | 1.84 (1.29–2.63) | 1.65 (1.49–1.83) | 1.61 (1.55–1.67) | 1.68 (1.53–1.84) | 1.60 (1.52–1.69) | 1.13 (0.98–1.32) | 1.28 (1.20–1.36) | |
| Severely obese | n = 1587 | n = 381 | n = 655 | n = 22 | n = 94 | n = 9 | n = 261 | n = 1906 | n = 273 | n = 962 | n = 91 | n = 664 |
| 1.61 (1.53–1.69) | 1.93 (1.74–2.15) | 1.57 (1.45–1.70) | 0.83 (0.54–1.27) | 0.97 (0.79–1.19) | 1.00 (0.51–1.95) | 2.15 (1.89–2.45) | 2.09 (1.99–2.19) | 1.86 (1.65–2.11) | 2.16 (2.02–2.31) | 1.18 (0.95–1.46) | 1.50 (1.38–1.62) | |
| Maternal PCOS | ||||||||||||
| Normal weight | n = 427 | n = 69 | n = 179 | n = 16 | n = 50 | n = 5 | n = 63 | n = 392 | n = 84 | n = 169 | n = 33 | n = 228 |
| 1.20 (1.09–1.32) | 1.02 (0.81–1.30) | 1.25 (1.08–1.45) | 1.63 (0.99–2.69) | 1.30 (0.98–1.72) | 1.58 (0.65–3.86) | 1.44 (1.12–1.85) | 1.19 (1.08–1.32) | 1.39 (1.12–1.73) | 1.13 (0.97–1.32) | 1.07 (0.76–1.51) | 1.38 (1.21–1.57) | |
| Overweight | n = 200 | n = 28 | n = 73 | n = 5 | n = 24 | n = 3 | n = 27 | n = 215 | n = 26 | n = 119 | n = 20 | n = 109 |
| 1.25 (1.08–1.43) | 0.95 (0.65–1.37) | 1.14 (0.91–1.44) | 1.14 (0.47–2.74) | 1.40 (0.93–2.09) | 2.17 (0.69–6.79) | 1.35 (0.93–1.98) | 1.44 (1.25–1.64) | 0.97 (0.66–1.43) | 1.76 (1.47–2.11) | 1.46 (0.94–2.28) | 1.47 (1.21–1.77) | |
| Moderately obese | n = 172 | n = 34 | n = 57 | n = 2 | n = 15 | n = 0 | n = 27 | n = 176 | n = 33 | n = 83 | n = 12 | n = 78 |
| 1.92 (1.65–2.23) | 2.08 (1.49–2.92) | 1.60 (1.23–2.07) | 0.90 (0.23–3.61) | 1.51 (0.91–2.51) | NA | 2.46 (1.68–3.59) | 2.13 (1.84–2.47) | 2.26 (1.61–3.19) | 2.16 (1.74–2.68) | 1.62 (0.92–2.85) | 1.89 (1.51–2.36) | |
| Severely obese | n = 115 | n = 26 | n = 52 | n = 2 | n = 10 | n = 0 | n = 12 | n = 105 | n = 17 | n = 57 | n = 6 | n = 57 |
| 2.11 (1.76–2.53) | 2.46 (1.67–3.62) | 2.28 (1.73–2.99) | 1.43 (0.36–5.73) | 1.64 (0.88–3.06) | NA | 1.77 (1.01–3.13) | 2.08 (1.72–2.52) | 1.89 (1.17–3.04) | 2.42 (1.87–3.14) | 1.31 (0.59–2.91) | 2.26 (1.74–2.94) | |
PCOS was identified by ICD-9 256.4 and ICD-10 E28.2. Anovulatory infertility was identified by ICD-9 628.0 and ICD-10 N97.0. The analyses were adjusted for maternal age, mother’s country of birth (Finland or not), mother married at birth (yes/no), maternal smoking (yes/no), parity (0 or ≥1), maternal psychiatric disorder (yes/no), maternal purchase of N05 and N06 during pregnancy (yes/no) and maternal systemic inflammatory disease (yes/no). For each outcome, the birth cohort was followed up until 2018. Reference group was normal-weight mothers with no PCOS. Births to underweight mothers with PCOS were small in number (n = 336) and hence not reported. Birth cohort 2004–2014 was used due to availability of maternal pre-pregnancy BMI. ADHDs, attention deficit/hyperactivity disorders.
Figure 1.
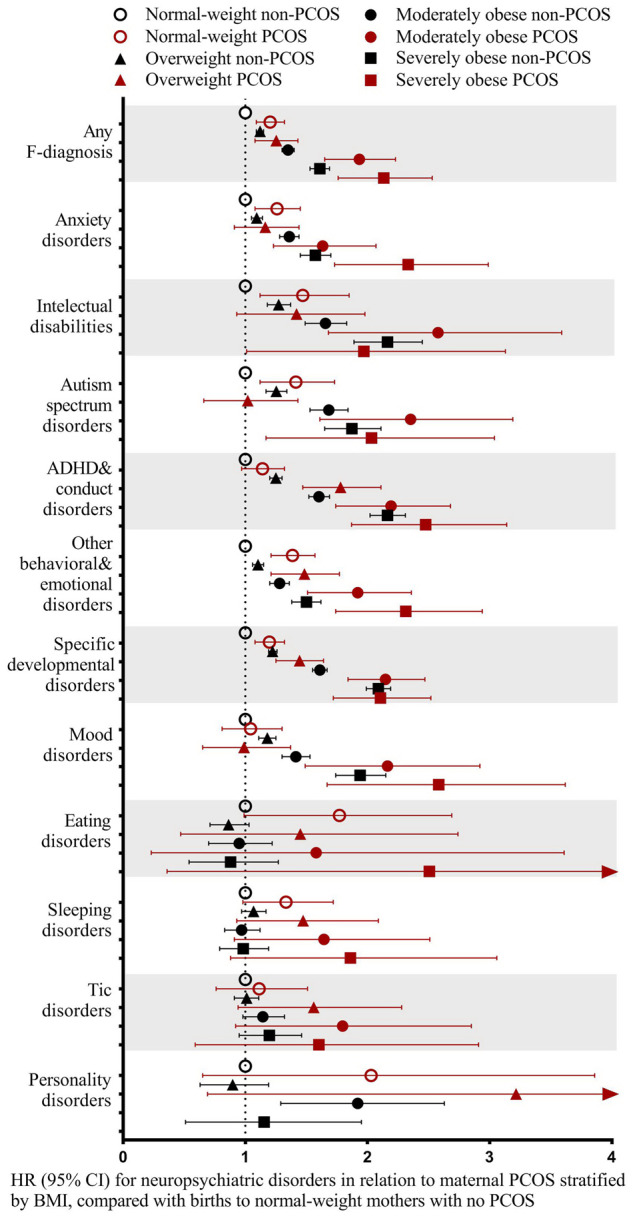
Normal-weight (18.5 ≤ BMI < 25 kg/m2) mothers with PCOS were associated with increased risks of neuropsychiatric disorders in offspring, which tended to increase in obese mothers with PCOS. PCOS refers to ICD-9: 256.4 and 628.0; ICD-10: E28.2 and N97.0. The analyses were adjusted for maternal age, mother’s country of birth (Finland or not), mother married at birth (yes/no), maternal smoking (yes/no), parity (0 or ≥1), maternal psychiatric disorder (yes/no), maternal purchase of N05 and N06 during pregnancy (yes/no) and maternal systemic inflammatory disease (yes/no). All children were followed up until 2018. The reference was births to normal-weight mothers with no PCOS. Births to underweight mothers with PCOS were small in number (n = 336) and hence not reported. Birth cohort 2004–2014 was used due to availability of maternal pre-pregnancy BMI. ADHDs, attention deficit/hyperactivity disorders; ICD, international classification of disease; PCOS, polycystic ovary syndrome.
Among births to mothers with PCOS with BMI above normal, the risk of neuropsychiatric disorders tended to increase with maternal BMI. The risk of any neuropsychiatric disorder in offspring to mothers with PCOS and obesity was significantly higher than that of maternal obesity alone: for moderate obesity (HR 1.92; 95% CI 1.65–2.23 vs HR 1.35; 95% CI 1.30–1.40), for severe obesity (HR 2.11; 95% CI 1.76–2.53 vs HR 1.61; 95% CI 1.53–1.69) or that of maternal normal-weight PCOS (HR 1.20; 95% CI 1.09–1.32). The risks for most categories of neuropsychiatric disorders examined were increased among births to mothers with PCOS and obesity compared with those to normal-weight mothers without PCOS. In particular, there were significantly higher risks among births to moderately obese mothers with PCOS compared to moderately obese mothers without PCOS for ADHD and conduct disorders (HR 2.16; 95% CI 1.74–2.68 vs HR 1.60; 95% CI 1.52–1.69), specific developmental disorders (HR 2.13; 95% CI 1.84–2.47 vs HR 1.61; 95% CI 1.55–1.67) and other behavioral and emotional disorders (HR 1.89; 95% CI 1.51–2.36 vs HR 1.28; 95% CI 1.20–1.36). Among births to severely obese mothers with PCOS compared to severely obese mothers without PCOS, there were increased risks for anxiety disorders (HR 2.28; 95% CI 1.73–2.99 vs HR 1.57; 95% CI 1.45–1.70), and other behavioral and emotional disorders (HR 2.26; 95% CI 1.74–2.94 vs HR 1.50; 95% CI 1.38–1.62). This suggests synergistic effects of PCOS and obesity on neurodevelopment (Table III and Fig. 1).
Association of maternal PCOS and BMI with psychotropic medication purchase in offspring
Additionally, we also analyzed psychotropic drug purchases until 2014, including antipsychotics, hypnotics, and anxiolytics (N05), antidepressants (N06A), and stimulants (N06B) as a complementary measure for offspring neuropsychiatric disorders. The sample size of purchases was limited, however. Overweight mothers with PCOS were associated with increased risk for any psychotropic medication purchase in offspring (HR 1.28; 95% CI 1.02–1.61). The highest signal was observed in offspring to severely obese mothers with PCOS (HR 1.64; 95% CI 1.16–2.32). Moreover, overweight (HR 1.49; 95% CI 1.01–2.19) and moderately obese (HR 1. 75; 95% CI 1.09–2.82) mothers with PCOS were also associated with increased risks of stimulant purchases in offspring (Supplementary Table SIV).
The influence of perinatal problems, gestational diabetes, cesarean section and fertility treatment on the association between maternal PCOS and offspring neuropsychiatric disorders
We explored if the increased neuropsychiatric risks in relation to maternal PCOS were modified by perinatal problems, GDM, cesarean delivery or fertility treatment. When excluding perinatal problems, PCOS was still associated with increased risks of any neuropsychiatric disorders in offspring (HR 1.28; 95% CI 1.22–1.34) compared to mothers without PCOS. However, an additional increase was observed for PCOS in combination with perinatal problems (HR 1.99; 95% CI 1.84–2.16), with pronounced risks for sleeping disorders (HR 2.22; 95% CI 1.69–2.92 vs HR 1.37; 95% CI 1.17–1.62), intellectual disabilities (HR 2.91; 95% CI 2.31–3.65 vs HR 1.41; 95% CI 1.21–1.64), specific developmental disorders (HR 2.26; 95% CI 2.06–2.49 vs HR 1.32; 95% CI 1.25–1.40), ADHD and conduct disorders (HR 1.97; 95% CI 1.72–2.26 vs HR 1.39; 95% CI 1.29–1.50) and other behavioral and emotional disorders (HR 2.29; 95% CI 2.00–2.61 vs HR 1.43; 95% CI 1.32–1.55) (Fig. 2). Likewise, while GDM could not explain the increased risk of offspring neuropsychiatric disorders by maternal PCOS exposure, higher risks were found for PCOS joint with GDM than for mothers with PCOS with no GDM (HR 1.70; 95% CI 1.53–1.88 vs HR 1.30; 95% CI 1.25–1.36) (Supplementary Table SV). Similarly, using births to mothers with no PCOS and no cesarean delivery as the reference, the HR for births to mothers with PCOS without cesarean delivery was significantly increased (HR 1.29; 95% CI 1.23–1.35), and was even larger for births to mothers with PCOS and cesarean delivery (HR 1.71; 95% CI 1.58–1.84) (Supplementary Table SVI). However, fertility treatment implied little further risks (HR 1.46; 95% CI 1.31–1.63 vs HR 1.31; 95% CI 1.25–1.36; Supplementary Table SVII).
Figure 2.
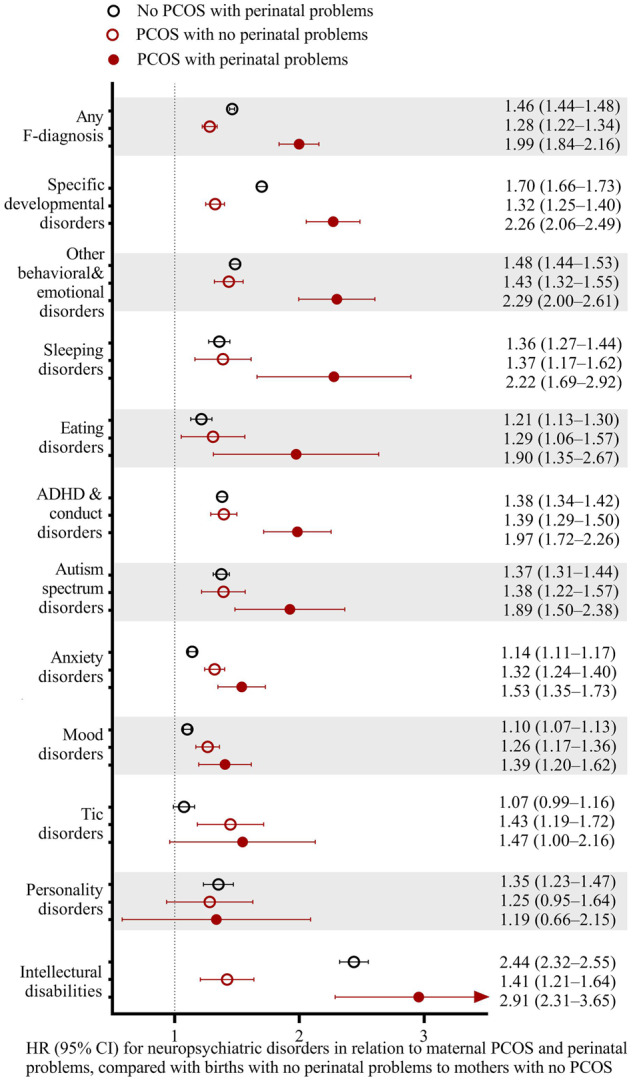
PCOS was associated with increased risks of neuropsychiatric disorders in offspring irrespective of, but higher with perinatal problems. PCOS refers to ICD-9: 256.4 and 628.0; ICD-10: E28.2 and N97.0. Perinatal problems were defined as birth before gestational week 37 or a birth weight <2500 g, or small for gestational age. Births to mothers with no PCOS and no perinatal problems were used as the reference. The analyses were adjusted for maternal age, mother’s country of birth (Finland or not), mother married at birth (yes/no), maternal smoking (yes/no), parity (0 or ≥1), maternal psychiatric disorder (yes/no), maternal purchase of N05 and N06 during pregnancy (yes/no) and maternal systemic inflammatory disease (yes/no). All children were followed up until 2018. Reference group was births to mothers with no PCOS and no perinatal problems (n = 935 908).
Discussion
In this nationwide prospective cohort study, we identified associations of maternal PCOS and/or anovulatory infertility with a wide spectrum of pediatric neurodevelopmental and psychiatric disorders in offspring: mood disorders, anxiety disorders, eating disorders, sleeping disorders, intellectual disabilities, specific developmental disorders, ASD, ADHD and conduct disorders, tic disorders and other behavioral and emotional disorders, with similarly increased risks in male and female offspring. Normal-weight mothers with PCOS were associated with increased risks for neuropsychiatric disorders in offspring, which tended to be broader in categories and larger in effect sizes when joint with obesity. Perinatal problems, cesarean delivery and GDM, but not fertility treatment, could explain a part of the effect size in these associations.
Previous studies have reported increased risks of ASD, ADHD and Tourette’s syndrome/tic disorders among children with maternal PCOS (Kosidou et al., 2016; Kosidou et al., 2017; Cherskov et al., 2018; Cesta et al., 2019; Palomba et al., 2012). This study confirmed findings on ASD, ADHD and tic disorders, and extended that maternal PCOS was also associated with many other neuropsychiatric disorders with onset during childhood and adolescents, including mood disorders, anxiety disorders, eating disorders, sleeping disorders, intellectual disabilities, specific developmental disorders and other behavioral and emotional disorders. In consistence, a recent large cohort study reported that children to mothers with PCOS demonstrated signs of developmental delay even before the age of 3 years (Bell et al., 2018). These results indicated a broad negative influence of maternal PCOS on neurodevelopment.
PCOS, also in normal-weight mothers, was associated with increased risk of neuropsychiatric disorders in offspring, with pronounced increases particularly for anxiety disorders, specific developmental disorders, ASD, intellectual disabilities and other behavioral and emotional disorders, supporting an association between PCOS and offspring neuropsychiatric disorders independent of maternal BMI (Kosidou et al., 2016; Kosidou et al., 2017). When PCOS was combined with obesity, the risks were further increased. For anxiety disorders, and other behavioral and emotional disorders, the risk was significantly higher for maternal PCOS joint with severe obesity than either alone, indicating multiplicative effects of PCOS and obesity. In consistence, Kosidou and colleagues also revealed increasing odds of ASD and ADHD in offspring with maternal PCOS with worsening cardiometabolic conditions (Kosidou et al., 2016; Kosidou et al., 2017). Evidence from mice with PCOS-phenotype also showed joint effects of androgen excess and obesity on anxiety in offspring (Manti et al., 2018). Taken together, our findings suggest that PCOS status alone is a risk factor for offspring neurodevelopment, with more detrimental influences if joint with obesity. Given that mothers with PCOS are often obese before pregnancy and are likely to gain excess weight during gestation (Bahri Khomami et al., 2019), health professionals should be aware of their offspring as targets for mental health care.
Elevated circulating androgens are present in many women with PCOS before and during pregnancy (Tata et al., 2018). There are also signs of intrauterine androgen exposure for their offspring (Anderson et al., 2010; Barrett et al., 2018). Prenatal exposure to sex hormones influences neurodevelopment through pathways including brain lateralization, neurological processes, neuronal spine, synaptic plasticity, and brain dopaminergic activity (McCarthy and Arnold, 2011; Lombardo et al., 2012; Hatanaka et al., 2015; Hu et al., 2015). By revealing increased risks of neuropsychiatric disorders in a wide range besides ASD and ADHD, which remained when obesity was excluded, this study supported the hypothesis of broad detrimental effects on neurodevelopment by hormonal disturbances in PCOS.
In addition, altered gut-microbiota in women with PCOS has been recently reported (Qi et al., 2019), which might provide an additional link between maternal PCOS and offspring neurodevelopment through a putative maternal gut-fetal brain axis (Kim et al., 2017). Moreover, chronic low-grade inflammation is present in PCOS and aggravated by obesity. The broader spectrum of neuropsychiatric disorders in mothers with PCOS and obesity than PCOS alone may indicate worsened intrauterine environment by hyperandrogenism, hyperinsulinemia, lipotoxicity and long-term inflammation (Sanchez et al., 2018; Gumusoglu and Stevens, 2019).
Furthermore, familial factors including genetic predispositions of psychiatric conditions could not be excluded, although maternal psychiatric disorders were adjusted for in this study. Cohort studies reported increased risks of ASD and ADHD both in mothers with PCOS and their children (Berni et al., 2018; Cherskov et al., 2018; Katsigianni et al., 2019). A large cohort study found increased risks of a range of psychiatric disorders in women with PCOS (Cesta et al., 2016). In consistence, this study revealed a similar pattern of increased neuropsychiatric disorders in PCOS-exposed offspring, even at childhood and adolescents. A Swedish cohort study accounting for familial confounding by a cousin-comparison supported the role of prenatal androgen exposure in offspring ASD and ADHD (Cesta et al., 2019). Moreover, it has been recently reported that daughters with maternal PCOS were more likely to be diagnosed with PCOS, probably due to altered gene expression by prenatal androgen exposure (Risal et al., 2019). It is recommended that offspring to mothers with PCOS should be screened for both mental and reproductive health.
In addition, this study revealed that the increased risks of neuropsychiatric disorders were comparable between daughters and sons with maternal PCOS, in line with the studies by Kosidou et al. (Kosidou et al., 2016; Kosidou et al., 2017). Conversely, some studies found stronger associations of ASD and ADHD in PCOS-exposed girls than boys (Palomba et al., 2012; Cesta et al., 2019). There was also evidence from rodent models that female offspring were affected to a larger extent than the male (Manti et al., 2018). In contrast, Cherskov et al. (2018) found a higher risk of ASD in sons but not daughters of mothers with PCOS. From the perspective of prenatal androgen exposure, higher risks for female offspring than male might be expected because of the opposite sex steroids. However, PCOS is associated with disturbances of a series of hormones, including anti-Müllerian hormone and LH besides androgens (Tata et al., 2018). Moreover, maternal inflammation and gut-fetal brain pathways might influence neurodevelopment irrespective of sex (Kim et al., 2017; Gumusoglu and Stevens, 2019).
This study revealed that the increased risks of neuropsychiatric disorders by maternal PCOS could be explained, in part, by perinatal problems, cesarean delivery and GDM. We found that the risks remained increased among PCOS when perinatal problems were excluded, but it was much higher when PCOS was in conjunction with perinatal problems, particularly for sleeping disorders, specific developmental disorders, ADHD and conduct disorders, intellectual disabilities, and other behavioral and emotional disorder. Likewise, more pronounced risks were observed for PCOS with GDM. Due to small sample size for PCOS with preeclampsia, stratified analysis was not performed in this study. In Swedish register-based cohort studies, Kosidou et al. examined the influence of preterm birth, Apgar score, small for gestational age and preeclampsia on ASD and ADHD in PCOS-exposed offspring by further adjusted models (Kosidou et al., 2016; Kosidou et al., 2017). They reported little attenuation in the odds ratio after adjusting for these factors, though this may relate to the low percentage (1.3–7.0%) of affected births relative to the unaffected (Kosidou et al., 2016; Kosidou et al., 2017). Our findings were in line with studies in the general population which revealed perinatal problems and GDM as risk factors for neurodevelopment (Sucksdorff et al., 2015; Kong et al., 2018; Money et al., 2018). In addition, this study revealed increased risk of neuropsychiatric disorders in offspring to mothers with PCOS after excluding those with cesarean delivery, indicating other factors than delivery effects for the altered neurodevelopment. Thus, prophylactic measures to reduce pregnancy and obstetrical complications in mothers with PCOS might help offspring neurodevelopment. However, births to maternal PCOS without such reproductive complications are also at-risk populations.
Our study did not reveal contributions of fertility treatment for neuropsychiatric disorders in PCOS-exposed offspring, in line with recent evidence that the increased incidence of neuropsychiatric disorders in offspring born to women with fertility problems might be attributed to the underlying causes of infertility, rather than the treatment (Bay et al., 2013). However, the present study included IVF/ICSI treatment only, restricting us to explore potential effects of other assisted reproduction technologies such as ovulation induction, in vitro maturation of the oocytes and isolated role of embryo freezing.
Strengths of our study lie in that we include a large, population-based sample with prospectively collected data from inpatients and out-patient settings. This study design reduces the potential for reporting and recall bias and loss to follow-up, thus increasing the generalizability of our findings. The large sample size enables us to examine a wide spectrum of neurodevelopmental and psychiatric disorders in relation to maternal PCOS, taking into account many relevant factors.
Our study has some limitations. First, the observed prevalence of PCOS was 2.2%, lower than previously reported. Similarly underestimated prevalence of PCOS has been identified in the Swedish and UK national registries (Kosidou et al., 2017; Cherskov et al., 2018; Cesta et al., 2019). Therefore, the register-based studies may capture only the seriously affected PCOS that seek medical treatment. In addition, anovulatory infertility was combined with PCOS to represent maternal PCOS exposure. A potential for diagnostic bias could not be completely ruled out though PCOS is the leading cause of anovulatory infertility and extensive exclusions have been made. However, subgroup analysis between PCOS and anovulatory infertility showed similar patterns of increased risks for neuropsychiatric disorders. In addition, recently published studies suggested a separate and perhaps stronger effect of the excess androgen subtypes of PCOS (Lee et al., 2017; Robinson et al., 2020); it’s valuable to distinguish between subtypes of PCOS however not feasible in this study as ICD codes could not give information on the subtypes.
Second, unmeasured familial factors might confound the association between PCOS and neuropsychiatric disorders in offspring. As mentioned above, a wide range of psychiatric disorders has been reported in mothers with PCOS (Cesta et al., 2016; Berni et al., 2018). This study adjusted for maternal inpatient psychiatric diagnoses before pregnancy and psychotropic drug purchases, including N05 and N06 (antipsychotics, anxiolytics, hypnotics, sedatives, antidepressants, psychostimulants and nootropics) during pregnancy. Maternal out-patient psychiatric diagnoses were unavailable for this study. Moreover, maternal psychiatric diagnoses in this study were grouped because of the overlap in genetic susceptibility variants. Information on fathers, including age, medical disorders or psychiatric disorders was not available either. Discordant-exposed sibling comparison was impossible due to early onset and persisting hormonal disturbances in PCOS. To further disentangle familial confounding with PCOS exposure, cousin-comparison design might be informative. However, the study by Cesta and colleagues reported that accounting for familial factors by cousin-comparison did not change the estimates significantly (Cesta et al., 2019), supporting a role of maternal PCOS in the development of offspring neuropsychiatric disorders.
Third, offspring neuropsychiatric disorders were grouped to have enough sample size, which restricted estimates for single disorder. Further, neuropsychiatric disorders and psychotropic drug purchases were analyzed separately, as the permission for data was until 2018 for diagnoses related to mental and behavioural disorders and until 2014 for psychotropic medications.
Fourth, only pre-pregnancy BMI data were available and only from 2004 onwards. Thus, estimates for the joint association of obesity and PCOS were conducted in a sub-cohort of 2004–2014, with no information on gestational weight gain.
Conclusions
PCOS exposure increased the risks of a wide spectrum of neurodevelopmental and psychiatric disorders in the offspring. The negative influence of PCOS on neuropsychiatric disorders was independent of but increased with obesity. Perinatal problems, cesarean delivery and GDM explained part of the effect sizes of the increased risks. These findings are relevant to women with PCOS with respect to pregnancy counseling and offspring developmental monitoring. Further studies are warranted to confirm our results and investigate underlying pathways and mechanisms linking PCOS exposure to long-term neurodevelopmental consequences.
Authors’ roles
X.C. conceptualized the study, interpreted the data, drafted and revised the manuscript. L.K. interpreted the data and revised the manuscript. T.T.P. provided critical inputs on the study design and revised the manuscript. M.G. provided critical inputs on the study design, performed data cleaning and statistical analyses and revised the manuscript. C.L. conceptualized the study, interpreted the data and revised the manuscript.
Funding
This study was supported by the joint research funding of Shandong University and Karolinska Institute (SDU-KI-2019-08), THL Finnish Institute for Health and Welfare: Drug and pregnancy project [M.G.], the Swedish Research Council [2014-10171 to C.L.], the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet Stockholm County Council [SLL20170292 to C.L.], the Swedish Brain Foundation [FO2018-0141 and FO2019-0201 to C.L.]. X.C. was supported by the China Scholarship Council during her training in Karolinska Institute. L.K. was supported by the China Scholarship Council for his PhD study in Karolinska Institute. The sponsor had no role in the study design, data analysis or interpretation, manuscript preparation or the decision for submission.
Conflict of interest
Authors have no competing interests to disclose.
Supplementary Material
References
- Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A.. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab 2010;95:2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artama M, Gissler M, Malm H, Ritvanen A; Drugs, Pregnancy Study Group. Nationwide register-based surveillance system on drugs and pregnancy in Finland 1996-2006. Pharmacoepidemiol Drug Saf 2011;20:729–738. [DOI] [PubMed] [Google Scholar]
- Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Arora C, Silagy M, Misso ML, Teede HJ, Moran LJ.. The role of maternal obesity in infant outcomes in polycystic ovary syndrome—a systematic review, meta-analysis, and meta-regression. Obes Rev 2019;20:842–858. [DOI] [PubMed] [Google Scholar]
- Barrett ES,, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN, Swan SH.. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis 2018;9:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay B, Mortensen EL, Hvidtjorn D, Kesmodel US.. Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. BMJ 2013;347: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GA, Sundaram R, Mumford SL, Park H, Mills J, Bell EM, Broadney M, Yeung EH.. Maternal polycystic ovarian syndrome and early offspring development. Hum Reprod 2018;33:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni TR, Morgan CL, Berni ER, Rees DA.. Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J Clin Endocrinol Metab 2018;103:2116–2125. [DOI] [PubMed] [Google Scholar]
- Cesta CE, Mansson M, Palm C, Lichtenstein P, Iliadou AN, Landen M.. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 2016;73:196–203. [DOI] [PubMed] [Google Scholar]
- Cesta CE, Oberg AS, Ibrahimson A, Yusuf I, Larsson H, Almqvist C, D'Onofrio BM, Bulik CM, Fernandez de la Cruz L, Mataix-Cols D. et al. Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychol Med 2019;50:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S.. Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry 2018;8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A.. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 2007;92:804–810. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]
- Gumusoglu SB, Stevens HE.. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol Psychiatry 2019;85:107–121. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Wada K, Kabuta T.. Abnormal instability, excess density, and aberrant morphology of dendritic spines in prenatally testosterone-exposed mice. Neurochem Int 2015;85–86:53–58. [DOI] [PubMed] [Google Scholar]
- Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP. et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci U S A 2015;112:14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsigianni M, Karageorgiou V, Lambrinoudaki I, Siristatidis C.. Maternal polycystic ovarian syndrome in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry 2019;24:1787–1797. [DOI] [PubMed] [Google Scholar]
- Keskimäki I, Aro S. Accuracy of data on diagnoses, procedures and accidents in the Finnish Hospital Discharge Register. Int J Health Sci 1991;2:7. [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017;549:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Norstedt G, Schalling M, Gissler M, Lavebratt C.. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics 2018;142:1–11. [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM.. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry 2016;21:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM.. Maternal polycystic ovary syndrome and risk for attention-deficit/hyperactivity disorder in the offspring. Biol Psychiatry 2017;82:651–659. [DOI] [PubMed] [Google Scholar]
- Lampi KM, Sourander A, Gissler M, Niemela S, Rehnstrom K, Pulkkinen E, Peltonen L, Von Wendt L.. Brief report: validity of Finnish registry-based diagnoses of autism with the ADI-R. Acta Paediatr 2010;99:1425–1428. [DOI] [PubMed] [Google Scholar]
- Lee BK, Arver S, Widman L, Gardner RM, Magnusson C, Dalman C, Kosidou K.. Maternal hirsutism and autism spectrum disorders in offspring. Autism Res 2017;10:1544–1546. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Lai MC, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S.. Fetal programming effects of testosterone on the reward system and behavioral approach tendencies in humans. Biol Psychiatry 2012;72:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manti M, Fornes R, Qi X, Folmerz E, Linden Hirschberg A, de Castro Barbosa T, Maliqueo M, Benrick A, Stener-Victorin E.. Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. FASEB J 2018;32:4158–4171. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP.. Reframing sexual differentiation of the brain. Nat Neurosci 2011;14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KM, Barke TL, Serezani A, Gannon M, Garbett KA, Aronoff DM, Mirnics K.. Gestational diabetes exacerbates maternal immune activation effects in the developing brain. Mol Psychiatry 2018;23:1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB.. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf )2012;77:898–904. [DOI] [PubMed] [Google Scholar]
- Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L. et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019;25:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019;25:1894–1904. [DOI] [PubMed] [Google Scholar]
- Robinson SL, Ghassabian A, Sundaram R, Trinh MH, Bell EM, Mendola P, Yeung EH.. The associations of maternal polycystic ovary syndrome and hirsutism with behavioral problems in offspring. Fertil Steril 2020;113:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH, Fuemmeler BF.. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev 2018;19:464–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L.. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med 2013;45:446–454. [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, Sourander A.. Preterm birth and poor fetal growth as risk factors of attention-deficit/hyperactivity disorder. Pediatrics 2015;136:e599–e608. [DOI] [PubMed] [Google Scholar]
- Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012;40:505–515. [DOI] [PubMed] [Google Scholar]
- Tata B, Mimouni NEH, Barbotin AL, Malone SA,, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundstrom-Poromaa I, Piltonen TT. et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018;24:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanky E, Engen Hanem LG, Abbott DH. Children born to women with polycystic ovary syndrome-short- and long-term impacts on health and development. Fertil Steril2019;111:1065–1075. [DOI] [PubMed] [Google Scholar]
- Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr 2015;169:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Sidorchuk A, Sevilla-Cermeno L, Vilaplana-Perez A, Chang Z, Larsson H, Mataix-Cols D, Fernandez de la Cruz L.. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e1910236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


