ABSTRACT
Tokyo 2020 will likely be the most heat stressful Olympics to date, so preparation to mitigate the effects of humid heat will be essential for performance in several of the 33 sports. One key consideration is heat acclimation (HA); the repeated exposure to heat to elicit physiological and psychophysical adaptations that improve tolerance and exercise performance in the heat. Heat can be imposed in various ways, including exercise in the heat, hot water immersion, or passive exposure to hot air (e.g., sauna). The physical requirements of each sport will determine the impact that the heat has on performance, and the adaptations required from HA to mitigate these effects. This review focuses on one key adaptation, plasma volume expansion (PVE), and how the mode of HA may affect the kinetics of adaptation. PVE constitutes a primary HA-mediated adaptation and contributes to functional adaptations (e.g., lower heart rate and increased heat loss capacity), which may be particularly important in athletes of “sub-elite” cardiorespiratory fitness (e.g., team sports), alongside athletes of prolonged endurance events. This review: i) highlights the ability of exercise in the heat, hot-water immersion, and passive hot air to expand PV, providing the first quantitative assessment of the efficacy of different heating modes; ii) discusses how this may apply to athletes at Tokyo 2020; and iii) provides recommendations regarding the protocol of HA and the prospect for achieving PVE (and the related outcomes).
KEYWORDS: Hypervolemia, Heat acclimation, heat stress, exercise performance, adaptation, plasma volume, athletic performance
Introduction
The Olympic and Paralympic games in Tokyo will likely be the most heat stressful to date, by virtue of the high humidity (~2.5–3 kPa) in conjunction with high dry bulb and associated radiant temperatures (dry bulb temperature ~27–31°C, [1]) [2,3]. The median wet bulb globe temperature (WBGT) in Tokyo during the Olympic period is expected to be higher than any of the previous Olympic games; ~4°C hotter than the Rio de Janeiro Olympics, ~12°C hotter than London, and ~3°C hotter than Beijing [2]. Compared to the previous Tokyo Olympics (1964), the games are being held in the hotter period of July/August (WBGT ~8°C higher than October) to satisfy overseas media interests, but subsequently compromising athlete performance and welfare.
High ambient heat stress impairs performance in a range of athletic events [4–9]. For instance, cycling in a warm environment (32–40°C dry bulb) can reduce power output by 6.5% [10] and repeat sprint ability by 9.5% [4], compared to temperate conditions. This is due, at least in part, to substantial cardiovascular strain during competitive exercise, which is exacerbated in the heat [11]. A humid environment further exacerbates this physiological challenge, as expected in Tokyo, and WBGT under-represents the impairment caused by higher humidity for well-trained male and female athletes alike [12,13], because of the lower maximal evaporative power [1]. Compared to 30°C and 24% relative humidity (RH), an increase to 60% or 80% RH leads to a 14 and 22 min reduction in time to exhaustion, respectively [14]. Even at the most modest of performance decrements, a 6.5% reduction in cycling time trial performance during the Rio De Janeiro Olympics – all else being equal – would have been the difference between gold and 21st position [15].
Spectators and athlete support staff may not be aware that these warm-humid environments have a disproportionately larger impact on the fittest athletes, but relatively less than people resting or working at low intensities. Specifically, (i) dry heat loss mechanisms (convection and radiation) can transfer little more than resting rates of heat production in warm environments, such that (ii) the additional transfer needed to sustain exercise requires evaporation, but the high ambient vapor pressure limits the gradient for evaporation and thus evaporative heat loss will become maximal at modest work rates, whilst (iii) any extra speed provides negligible additional convective or evaporative transfer (i.e., velocity-vs-heat loss is an exponential relation). So, whereas endurance performance is most impaired for less fit individuals in non-humid heat stress [16], the opposite may be true in humid heat because of the relatively larger importance of evaporation for elite endurance athletes. The heat stress elicited in the Tokyo Olympics (and Paralympics) will therefore concomitantly reduce the performance of sustained/repeated exercise and increase the risk of exertional heat illness [17]. Plans to move competition into the earlier hours have been proposed [18], but even at midnight the WBGT could be ~30°C [2], which would likely still impair performance and carry a risk of exertional heat illness [17].
The Tokyo 2020 Olympics will incorporate 33 sports, each requiring different physical characteristics for peak performance (e.g., a marathoner versus a fencer). The demand of the sport will influence i) the rate of heat production, ii) the potential to offload heat, and iii) the adaptations that may support performance. Endurance events such as road cycling, marathon, and race walking have a high sustained metabolic heat production, but they typically lose heat relatively effectively due to high sweat rates in conjunction with substantial airflow (not necessarily in Tokyo due to high absolute humidity). In this situation, several adaptations from heat acclimation (HA; Table 1), and further acute strategies (e.g., precooling, athlete rotation) could limit physiological strain [19] and aid performance. Sports such as fencing (i.e., skill/power/speed based) have an elevated metabolic heat production, but due to clothing or additional environmental limitations to evaporation, increased sweat rate could exacerbate dehydration and in doing so may reduce the psychomotor or cognitive function [20,21] that is crucial for success. This complexity and myriad contributing factors (to performance success) therefore make “one-size fits all” preparation programs complicated and often invalid by way of their lack of specificity.
Table 1.
Adaptations to heat acclimation and their importance for different sporting contexts.
| Adaptations | Resting/exercising Tc | Heat loss | Thermotolerance | Thermal comfort | Cardiac output | |
|---|---|---|---|---|---|---|
| Sport |
|
|
|
|
|
|
| Prolonged endurance outdoor (e.g., Marathon) | High Ḣ | High Ḣ | High Ḣ | Low cognitive demand | High CV demand | |
| Limited ability to manage | Limited ability to manage | Limited ability to manage | High skin temp | Already highly adapted CV system | ||
| |
|
High airflow |
High airflow |
Prolonged GI ischemia |
High airflow likely reduce discomfort |
Benefit late in exercise? E.g., defense of PV |
| |
|
|
|
|
|
|
| Intense endurance outdoor (e.g., 10,000 m) | Very high Ḣ | Very high Ḣ | Very high Ḣ | Low cognitive demand | High CV demand | |
| Limited ability to manage | Limited ability to manage | Limited ability to manage | High Skin Temp | Already highly adapted CV system | ||
| |
|
High airflow |
High airflow |
GI ischemia |
High thermal strain contributes to elevated perceived exertion |
Limited time to realize benefit of PVE |
| |
|
|
|
|
|
|
| Team sport (e.g., football/soccer) | Moderate – high Ḣ | Moderate – high Ḣ | Moderate – high Ḣ | Moderate – high cognitive demand | Moderate – high CV demand | |
| Breaks allow management | Breaks allow management | Breaks allow management | Breaks allow management | |||
| |
|
WU can elevate starting Tc |
Clothing could limit heat dissipation |
Prolonged GI ischemia |
|
Not “aerobically elite” |
| |
|
|
|
|
|
|
| Power, strength, skill (e.g., shooting) | Low Ḣ, short physical exertions | Low Ḣ, Limited exposure time | Low Ḣ, Limited exposure time | High cognitive demand | Low CV demand | |
| Opportunity to cool | Opportunity to cool | Opportunity to cool | Time gives opportunity to limit strain | |||
| Contribution to performance likely minimal | Contribution to performance likely minimal | Contribution to performance likely minimal | Attention, technique, and strategy critical | Not “aerobically elite” |
The traditionally recognised adaptations to heat acclimation (Columns) are broadly considered in the context of the different major sport categories. The first row (colour designation) corresponds to the importance of the respective adaptation for that category of sport. Black, grey, and white correspond to high, moderate, and low importance, respectively. This is a subjective assessment, based partly on information in other rows within that cell. The first row of text corresponds to the magnitude of strain expected in that sport category. The second row corresponds to the opportunity for athletes/support staff to offset this strain. The third row refers to pertinent sport/context specific factors that would alter the magnitude of this strain, or the specific relevance of the adaptive response. For instance, during intense endurance outdoor there will be a high metabolic heat production and thus a large thermal strain, meaning that core temperature, heat loss mechanisms, and thermotolerance will contribute substantially to performance success. Alternatively, cardiovascular load (or cardiac output) and metabolic heat production are not substantially stressed during power/strength/skill-based sports, and thus the adaptations to these systems/effectors would not meaningfully contribute to performance success. ‘Aerobically elite’ refers to an already highly adapted cardiovascular system. Abbreviations: CHO, carbohydrate; CV, cardiovascular; Ḣ, rate of heat production; PVE, plasma volume expansion; Tc, core temperature; WU, warm-up
Performance in the heat can be improved – or rather the performance decrement relative to temperate conditions and/or other athletes can be attenuated – following HA or acclimatization for well-trained endurance athletes [6,22–26] and elite team-sport athletes [25,27,28]. However, there is currently no published controlled-trial research on HA and performance in elite endurance athletes, despite the incorporation of control groups in HA trials with trained (not elite) participants becoming more common [29–31]. Further, studies that have used control groups have almost exclusively matched absolute rather than relative work, which inherently favors the more stressful condition, i.e., HA. The lack of control groups within the limited studies in elite athletes limits the interpretation and application of such findings and indicates the need for further research. While the complexity of these studies is acknowledged, only when these comparisons are forthcoming, can a valid and empirically driven assessment be made regarding the merits of HA for performance in elite athletes (discussed below).
HA leads to a range of physiological and psychophysical adaptations that improve one’s tolerance to heat stress. The general adaptations to HA are most demonstrably cardiovascular (e.g., increased plasma volume (PV) and reduced heart rate (HR)) and thermoregulatory (e.g., sweat rate and core temperature (Tc)). These collectively support improved thermal comfort and may contribute to potential metabolic [32–35], and possibly neuromuscular adaptations [36]. Increased cellular stress protection, and therefore thermotolerance, may also be induced and could be important in athletic performance contexts [37]. PV expansion (PVE) – a key outcome of HA – supports the maintenance of cardiac output via increased stroke volume and reduced HR [38–40], which increases cardiac reserve and could improve V̇O2 peak (most likely in untrained only) even in temperate conditions [41–44]. These adaptations are likely to contribute, collectively, to improved exercise performance in the heat (e.g., PVE, reduced Tc [45–47]). PVE therefore supports, and may indeed facilitate other functionally important markers of HA (see Figure 1).
Figure 1.
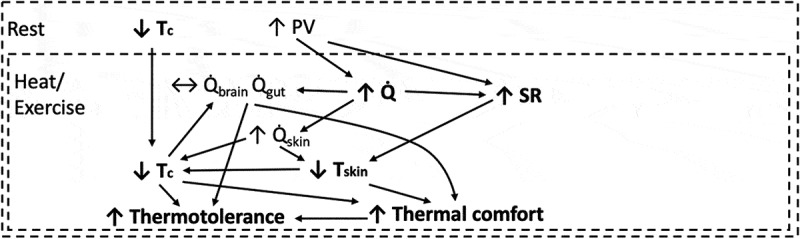
Heat acclimation initiates several adaptations that develop independently and inter-relatedly. Those bolded are mentioned within Table 1. Plasma volume (PV) expansion has several potential roles, albeit these are not definitively demonstrated, esp. for elite endurance athletes. Tc; Core Temperature, Q̇; Cardiac Output, Q̇brain; Cardiac Output to the Brain, Q̇gut; Cardiac Output to the Gut, Q̇skin; Cardiac Output to the Skin, SR; Sweat Rate, Tskin; Skin Temperature.
PVE is associated with improved exercise performance in hot environments following repeated heat exposure [26,45,47,48], and may help mediate other beneficial outcomes, as shown in Figure 1. The role of heat-induced PVE is difficult to determine, particularly for highly trained endurance athletes, because artificial PVE (via albumin/saline infusion or sodium loading) has shown both no improvement [39,40,49], and positive effects on endurance performance [42,50,51]. Untrained individuals gain cardiovascular and performance benefits from artificial or natural (e.g., HA) PVE in both temperate and hot environments. Athletes do not necessarily [40,52]. They already possess high PV in accordance with their training status and mode of exercise, but this is further expanded by short-term increases in training volume and with HA, so it presumably confers some benefit (e.g., for reasons indicated in Figure 1).
Table 1 highlights some principal limiting factors to exercise performance in the heat, and the associated adaptations observed following HA (as discussed above). The relevance (or not) of these adaptations to specific sporting contexts is seldom considered, despite different sports/events existing in thermally distinct contexts.
HA protocols typically vary from a few days, to more than 2 weeks, and may involve two fundamentally different phases in progressing from brief to prolonged phenotypes [37]. More prolonged exposures have been undertaken in rodents, but yet to be replicated in humans, thus major gaps in knowledge exist regarding the full spectrum of human heat adaptation; especially dose:response relations for multi-system physiological adaptation, and inter- and intra-individual factors contributing to the adaptive process. This is pertinent in the context of PVE, as PV contraction (relative to short-term adaptation) is a hallmark of the transition to a long-term HA phenotype [53]. The dosing of HA in time and mode may influence the pattern and outcome of all adaptation, but this has yet to be determined. Most HA studies also vary from 30 to 120 min per session and are completed either actively (exercise in the heat; ExH) or passively via hot air (e.g., sauna) or hot water immersion (HWI). Combinations of these modes have been reported [45,54–59], but mostly within rather than between sessions. Importantly, the mode of HA (ExH, passive air or HWI) may affect the type and volume of adaptations gained, due to the medium (air or water) or nature of the heat stress (active or passive), however, this notion remains almost unexamined despite its theoretical and practical value.
Beyond the HA protocol per se, the rate of adaptation also depends on the variable of interest. For example, it is purported that PVE can plateau within ~5 days (one session per day; volume per session not known) whilst sudomotor and vascular adaptations likely take more than 1 week to months [60–63]. These guidelines are often typically based on average responses, and therefore omit important information related to within-group differences [60,64,65]. Thus, the rate of heat adaptation is still poorly understood, but individual factors such as training status [66], previous heat exposure [67], sex [68], and the mode of HA likely have a role.
The protocol of HA employed, and the nature and extent of adaptation sought, must, therefore, consider the environment in which the athletes will likely compete, and the sport-specific determinants of success. Humid conditions, such as those at the Tokyo 2020 Olympics, could mean that increased PV and thus larger vascular volumes available to support high(er) sweat rates may have no benefit on heat loss due to skin wettedness from a low vapor pressure gradient. However, increased skin blood flow could still be important as it serves both dry and evaporative heat loss mechanisms, and PVE is involved a myriad of related and important adaptations (see Figure 1). Nevertheless, in this circumstance, it is unclear whether HA should be of primary importance, or greater focus placed on artificial strategies for reducing thermal strain (e.g., “warm up” and precooling) [69].
The intent for the remainder of the review is to i) highlight the capacity for typically available modes of HA (ExH, HWI, and passive hot air) to expand PV, ii) consider how this may apply to athletes attending Tokyo 2020, and iii) provide recommendations regarding PVE and the protocol of HA used. We feel that this approach (sport-, athlete-, and/or outcome-specific) is likely the most scientifically robust and practically appropriate method for assessing recommendations for HA (and wider training) in elite athletes. Comprehensive literature searches focused primarily on HA studies that included measures of blood or plasma volume, to provide a focussed and novel addition to the HA literature.
Plasma volume
PVE occurs across repetitive exposure to passive [70,71] and active heat exposure [72–74]. This section provides a brief description of the mechanisms that support PVE. Figure 2 illustrates how PVE can arise from several interrelated mechanisms. These mechanisms, which include fluid-regulating hormones and plasma proteins, are mentioned briefly because they may be important for the different modes of HA. Detailed reviews of PV control are also available elsewhere [75–78].
Figure 2.
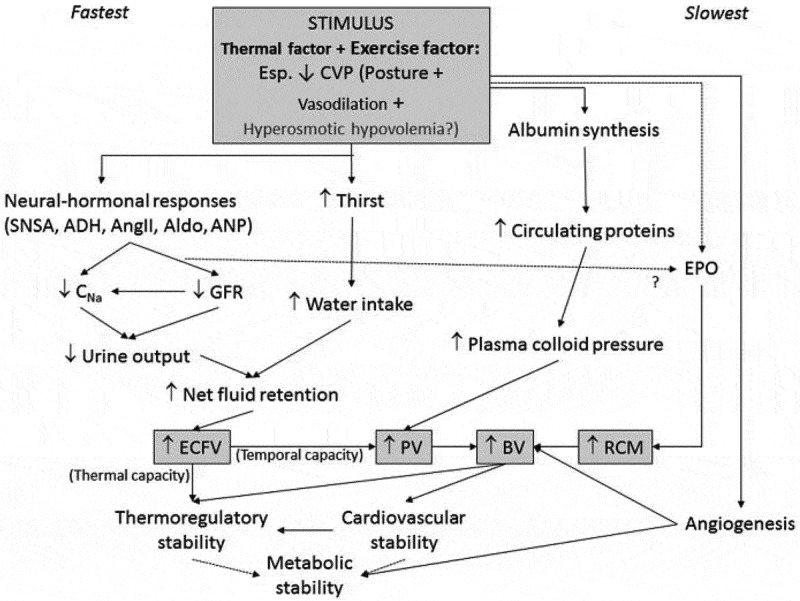
Illustration of the complex and interrelated mechanisms acting on fluid expansion. Used with permission from Akerman [34]. ADH, Anti-diuretic hormone; Aldo, aldosterone; AngII, Angiotensin II; ANP, Atrial natriuretic peptide; BV, Blood volume; CNa, sodium clearance; ECFV, Extracellular fluid volume; EPO, Erythropoietin; GFR, Glomerular filtration rate; PV, plasma volume; RCM, Red cell mass; SNSA, Sympathetic nervous system activity.
Fluid conservation can be increased directly through stimulation of the kidneys to retain fluid (via renin-angiotensin-aldosterone system (RAAS)), initiation of thirst (e.g., hypertonicity and anti-diuretic hormone (ADH)), increased fluid movement into the vascular space (e.g., protein translocation and production), and potentially through reductions in arterial blood pressure (ABP) and central venous pressures (that may modulate the above mechanisms).
PVE occurs predominantly through stimulation of fluid conserving hormones and accruing albumin in the intravascular space. Principal fluid conserving hormones are the RAAS and ADH. The RAAS initiates with the release of renin, which then stimulates the adrenal cortex to release angiotensinogen, which is converted to angiotensin I, and angiotensin II. Angiotensin II is a potent vasoconstrictor, that limits the renal loss of sodium, increases water absorption, promotes thirst [79,80], and stimulates aldosterone production [81]. Aldosterone limits the excretion of sodium and preserves the sodium/water balance, total sodium content, and retains water through changes in osmotic pressures [82]. ADH is released from the posterior pituitary gland into the bloodstream, inducing water retention through the kidneys, vasoconstriction of arterioles, and stimulating thirst [83,84].
Plasma proteins, in particular albumin, are also important in restoring and determining blood volume through changes in intravascular colloid osmotic pressures [38,85]. Albumin accounts for ~60–80% of the colloid osmotic pressure within the intravascular space and accrues in response to fluid regulatory stress, pulling water into the intravascular space and thus expanding blood volume [38,86]. The three main mechanisms responsible for increased intravascular albumin include increased lymphatic flow, reduced trans-capillary escape rate and increased albumin synthesis. Decreased albumin degradation after exercise may also play a role, but the slow rate of degradation (~3%/day) ensures that the net effect on intravascular albumin content is probably trivial compared with the other mechanisms (i.e., reduced degradation has likely a small effect on the total albumin lost) [87]. Given that the movement of 1 g of albumin into the intravascular space takes with it ~18 mL of water [85,88], PVE can, therefore, occur through albumin relocation or production. A reduction in ABP after exercise, referred to as post-exercise hypotension, has been shown to contribute to PVE through increasing albumin translocation into the intravascular space [89]. Post-exercise hypotension augments colloid osmotic pressure, and in turn increases fluid absorption [89]. However, post-exercise hypotension and PVE have some independence and do not necessarily occur together [90]; likely only a modest post-exercise hypotension is required to support fluid retention [91].
Given that disturbances to volume regulation stimulate the adaptations to these systems, dehydration may be an additional stressor to support adaptation. Specifically, dehydration could increase some fluid-regulating hormones (e.g., aldosterone [91]), which may support fluid retention and PVE. Garrett [92] demonstrated the potential positive effect of dehydrated versus euhydrated HA that has been further supported by Berland [93] (conference abstract), and Akerman [91] who demonstrated a positive effect of dehydration and heat versus heat alone on 24 h PVE. However, three other studies have shown no added effect of dehydration versus euhydration for adaptation [30,94,95], and we are unaware of any studies showing impaired adaptation. These inconclusive findings relating to the effect of dehydration on adaptation could be due to inter-individual responses to dehydration per se [34], HA, or the capacity for PVE. Alternatively, hydration state during HA bouts may have little impact on the net outcome at least for healthy young adults (discussed elsewhere; [34])
Methods of heat acclimation
This section summarizes how different modes of HA may differentially influence PVE via the mechanisms described above.
Passive air versus passive hot-water immersion
No study, to our knowledge, has investigated the adaptive response of passive hot air versus HWI. The difference in mediums may play a role on fluid-regulating hormones, albumin, and ABP. For example, fluid-regulating hormones are likely stimulated during both conditions due to a reduction in PV (see Figure 2) and an increase in Tc. However, ADH release may be inhibited during HWI due to the hydrostatic pressure of water increasing central venous pressure, atrial stretch, and atrial natriuretic peptide release, which inhibits ADH [96–98]. Hypotension during recovery may be important in PVE (see above), and occur in either condition but likely depends on the Tc, skin temperature, or vessel stress (i.e., shear stress), as they all affect local vascular conductance. Water immersion has also been shown to reduce sympathetic activation, which supports conductance and potentially hypotension, albumin translocation from the lymphatic systems, and PVE in recovery [99,100]. Albumin synthesis is stimulated partly by increased temperature, and given that HWI conducts thermal energy and inhibits evaporation to a greater extent than passive hot air, one could expect larger albumin-mediated PVE in hot water. Whether these adaptive responses are different when matched for thermal strain has yet to be determined. On the other hand, the lymphatic flow of albumin depends partly on decreased central venous pressure, which may not occur when immersed in water (i.e., with increased central venous pressure). Thus, the comparisons between methods of heat exposure for this specific variable (PVE), is complex given the multiple integrative pathways involved in fluid regulation. Direct comparisons between modes of heating, therefore, remain hypothetical and further investigation is warranted.
Passive versus active
The comparison between passive and active modes of heat exposure is complex, and only one study, to our knowledge, has presented the adaptive differences between exercise (in a temperate environment) and passive hot air [70]. The metabolic heat production associated with exercise increases heat production, which, when combined with a high ambient temperature (and/or humidity), increases Tc more so than temperate exercise alone. In comparison to passive heat exposure, the combination of hot air and metabolic heat production elicits a faster rise in Tc than hot air alone. However, the thermal impulse associated with hot water, rather than hot air, may be larger than exercise in a temperate environment [101], but the comparison to exercise in hot air is undetermined. Exercise could provide a greater stimulus for centrally- and peripherally mediated vasodilation, and thereby support lower ABP in recovery, but whether the magnitude of this stimulus is similar to passive exposure appears to be unknown. Further research is required to fully understand the differences between these methods of HA during and after the heat stress itself, especially when thermal strain is matched.
Figure 3 below highlights the different modes of heat stress that are regularly employed for HA, and the different dosing parameters that typically accompany them (e.g., intensity and duration) [61]. Some studies have utilized combined-mode HA, but none appear to have compared modes of HA on PVE, or any other adaptive response. To our knowledge, only Convertino [70] have compared exercise in temperate conditions to resting in hot air (between- rather than within-participants), and demonstrated significantly larger PVE following exercise.
Figure 3.
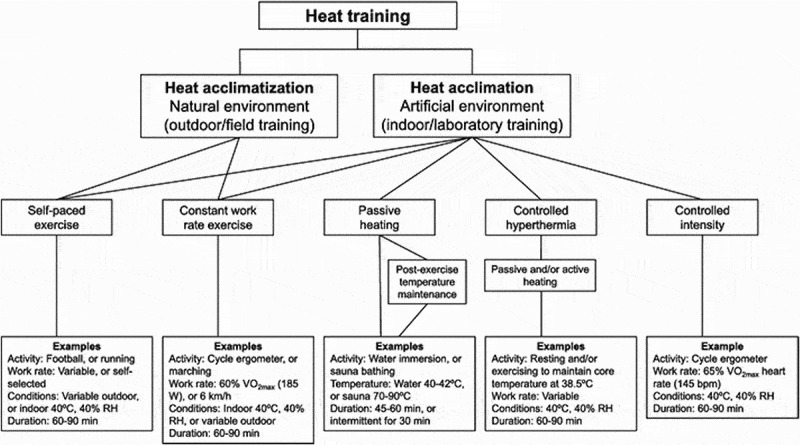
Schematic overview of methods for heat acclimation and heat acclimatization, with examples. RH; relative humidity, V̇O2 max; maximal oxygen utilization. Used with permission from Daanen [125].
In lieu of direct comparisons, between-study comparisons are possible. Considerable heterogeneity between studies makes this process difficult, however available data related to PVE and heat exposure methods are presented in Figure 4 below. While Figure 4A does not account for the considerable variability between studies (i.e., is not a meta-analysis), it provides the first quantitative assessment of PV responses to each of the four common modes of applying heat stress. Figure 4B extends these generalized data, by standardizing for duration and number of sessions to more appropriately compare modes of HA. Exercise is also presented in Figure 4 to highlight the role that endogenous factors alone have on PVE, and provide a valid comparison for HA-induced changes in PV. Included studies [22,25,28,30,31,57,70,72,73,85,88–92,94,102–124] were standardized for duration using a simple linear 60-min protocol (i.e., PV changes were divided by hours of exposure), notwithstanding that a linear correction may be, and is likely, inaccurate. This process is therefore warranted to provide some parity between protocols, but also theoretically appropriate given lack of sufficient information to inform the characteristics of a non-linear relation between exercise/heat dose and magnitude of PVE.
Figure 4.
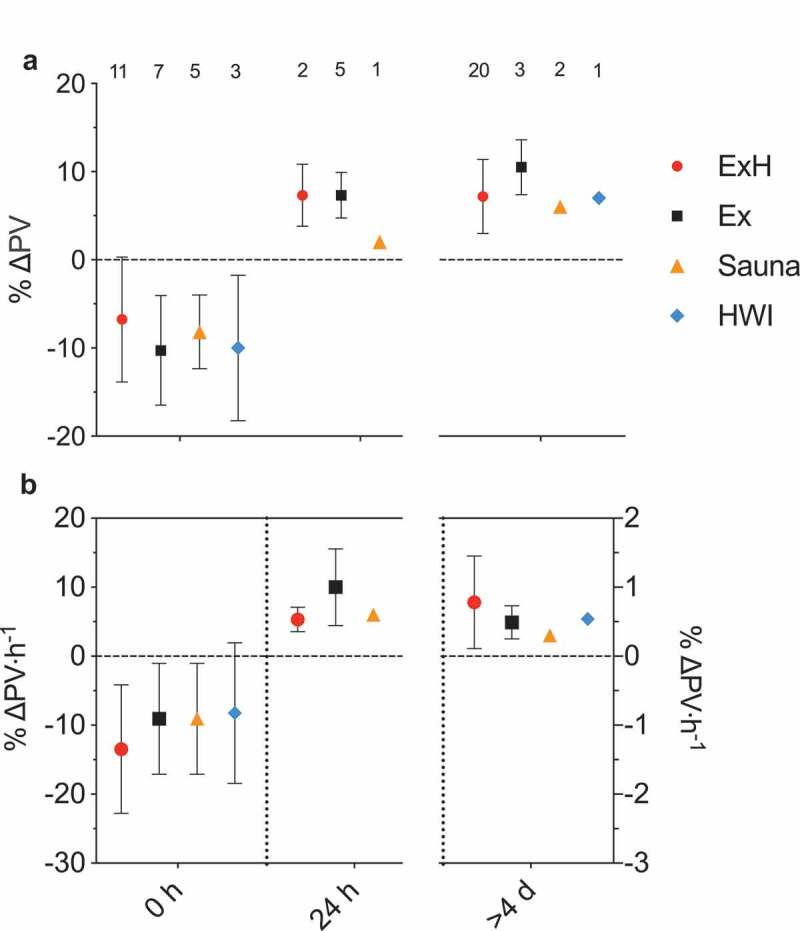
(A) Plasma volume (PV) changes from baseline, for exercise in heat (ExH), exercise in temperate air (Ex), passive hot-air exposure (Sauna), and hot-water immersion (HWI). PV changes are demonstrated for three periods of interest: within/immediately postexposure (0 h), at ~24 h post (24 h) and after a heat acclimation protocol of at least 4 sessions (≥4 d). (B) PV changes accounting for duration and number of sessions. The average increase in PV is compared when (linearly) controlled for total time under stress. It is likely that a non-linear relation exists, but in lieu of sufficient information to inform this relation, a linear response is appropriate. Numbers above the Figure indicate the quantity of studies represented at each datapoint. Conditions are missing when no data are available. Data presented are mean and SD.
Figure 4B therefore improves the validity of the comparisons between different modes, but there are still remaining sources of variability that could significantly impact PVE and its regulatory mechanisms. For instance, HA protocols likely vary with respect to temperature, humidity, exercise intensity (or Tc rise), participant training status, previous heat exposure, and participant ethnicity. The roles that some of these factors have on HA are highlighted elsewhere [60,125]. Therefore, Figure 4 comprises an initial assessment of responses to different HA modes, in the hope that future research will clarify factors associated with the variability in PVE – and its overall importance for elite performance. Figure 4 will be referred to below in its relation to PVE and each mode of HA.
Comparison of heat acclimation modes
PVE can occur through multiple mechanisms, and is likely stimulated within every mode of HA mentioned within this review. The focus below is to highlight, for each mode of HA; (i) their observed effect on PVE, (ii) the mechanisms that (may) support the fluid retention, and (iii) their relation to (elite) athletic performance.
Passive hot air
Context
Passive hot air, such as sauna, is a common HA protocol and accessible to many athletes. The high temperature (e.g., 50–90°C) promotes increased skin temperature and as a result, elevated thermal discomfort [126–128]. Due to the discomfort and thermal uncompensability of most saunas, studies often limit the exposure to ~30 min, or have intermittent breaks [129–132]; likely limiting the volume (~impulse) of thermal strain. These exposures to hot air can occur after exercise training [45,57], which can be time efficient and prolong the strain (both thermal and cardiovascular) from the preceding exercise session. HA via passive hot air seems unlikely to negatively affect subsequent training sessions, however, full body exposure to relatively hot environments (32°C) has been shown to impair glycogen resynthesis [133]. Thus, while the passive nature of this mode of heating will limit any excessive muscular fatigue associated with ExH (see section 4.3), the effect on recovery is still uncertain.
Mechanisms of PVE
Passive exposure to hot air is an effective means to increase the release of fluid conserving hormones. aldosterone rises with passive hot air exposure, peaking during recovery and remaining elevated for at least 2 h [134]. Concurrently, ADH and renin are both increased during exposure and remain elevated in recovery [135]. Changes in osmolality may directly affect ADH release, therefore a high sweat rate could support increased osmolality through water loss [135]. However, ADH concentration is significantly lower in passive hot air than in exercise, even when Tc is matched [70]. Therefore, exposure to passive hot air promotes a fluid retaining response, but it may be less effective than exercise per se.
Albumin content is an important determinant of PVE and likely increases after exposure to passive hot air. Convertino [70] demonstrated elevated albumin concentration within the vascular space after a single exposure, however the magnitude of this change was similar to the reduction in PV, meaning that the increased albumin could be due solely to increased concentration. However, as mentioned above, an increase in albumin concentration will increase colloid osmotic pressure and fluid movement into the vascular space. Further, Convertino [70] demonstrated a significant increase in albumin content after 4 and 8 days of exercise training, but no significant change with repeated exposure to passive hot air. Likely, the metabolic requirements of exercise, beyond thermal factors per se, may support increased albumin synthesis [70] and this may mediate the increased albumin synthesis associated with exercise alone [87,136].
The existing literature indicates that on average HA via passive hot air induces PVE (see Figure 4, and [137]). Convertino [70] demonstrated a 5% PVE in eight healthy males across 8 days (2 h/session) of passive hot humid air exposure (42°C; 93% RH); findings that are supported by others [138–140]. Further, post-exercise hot air exposure leads to an average PVE of between 7% Scoon [45] and 18% Stanley [57], and occurs even in endurance-trained participants (V̇O2 max: ~60 mL/min/kg [57]). Although the combination of exercise followed by hot-air exposure does not delineate the adaptive potential of hot-air exposure alone, these studies provide ecologically valid methods of HA and thus are important to highlight (similar reasoning to exercise before HWI; as discussed below).
Exercise performance
The only study to investigate exercise performance after repeated passive hot-air exposures (without prior exercise) demonstrated some benefit. Tyka [129] demonstrated that compared to a no heat control, 12 passive hot-air exposures of 3 × 15 min (separated by 4–6 min cool showers) increased total work by ~6% during an incremental test to exhaustion in 33°C, 50% RH in untrained males (V̇O2 max:~46 mL/min/kg). Other findings come from hot-air exposures following regular exercise training sessions [45,57]. For example, in a cross-over study, Scoon [45] demonstrated that running time to exhaustion improved by 32%, equating to a 1.9% improvement in 5-km time trial performance in temperate conditions after 12–13 sessions of post-exercise dry sauna bathing, relative to that after an equivalent period of control training. Conversely, Stanley [57] demonstrated no change in cycling peak power output when the test was performed 1 week after HA. The one-week delay before testing could contribute to this response. The positive association between PVE and performance benefit has been highlighted by Scoon [45] and others [26,47], and thus should be considered an important feature of the athletic heat acclimated phenotype.
Summary and recommendations
Passive hot-air is seemingly effective as a mode of HA in that it leads to a small but variable increase in PV (5–8% [70,139]; see Figure 4), which may be increased when completed immediately after exercise. HA with post-exercise hot-air exposure may also improve endurance performance, and this may be associated with the magnitude of PVE [26,45,47], however causation is not currently established. Passive hot-air exposure may be useful after exercise sessions where Tc is already elevated, when training quality is to be maintained, or in athletes such as skill/power-based (Table 1) who may benefit from being exposed to hot environments, e.g., to practice the skill in conditions in which they will compete. However, as mentioned above, exposure to hot environments could impair glycogen resynthesis and thus muscle recovery after exercise [133], which may be an issue to consider during HA if metabolic recovery is an important factor.
Passive hot-water immersion
Context
Spa baths/pools are commonly available in many westernized countries, but are seldom investigated as a mode of HA. Heat is transferred more readily (conduction and convection) in water, while evaporation is limited, which collectively supports a faster rate of heat gain versus passive hot air exposure (as discussed in section 3). HWI has been used before and after exercise training [54–56,58,59], and like post-exercise hot-air exposure, this allows for prolonged thermal and cardiovascular strain and is a time-efficient alternative to HWI alone. Post-exercise HWI may also support muscle recovery for subsequent training sessions [141], as the local application of heat (e.g., lower-limb immersion) has been shown to reduce delayed onset muscle soreness [142]. Thus, local application of heat stress may enhance the endurance phenotype through a range of pathways [35]. For instance, local heat exposure (applied with heat pads) can also support glycogen resynthesis [143] and this could occur with local HWI (e.g., lower-limb immersion), but not full body exposure (as discussed above) [133]. Further, HWI alone or after exercise likely avoids any impact on the quality of exercise in subsequent training sessions, and could, therefore, be considered a practically useful mode of heat exposure.
Mechanisms of PVE
As mentioned above, water immersion increases the release of atrial natriuretic peptide [144] which has an inhibitory effect on fluid retaining hormones [145–149], and it is also a diuretic and vasodilator. In HWI of 38°C, ADH was increased by 210 min of exposure but the rise in aldosterone did not occur at 30 or 210 min of immersion, but was evident 30 min into recovery [122]. Thermoneutral water immersion likely has an inhibitory effect on fluid conserving hormones (as mentioned above), however, this may be due to the often associated PVE during thermoneutral water immersion and its role on increasing central venous pressure, and atrial natriuretic peptide [150,151]. However, during HWI there may be a small increase in fluid conserving hormones [122], but further research is required in regard to this response and its relevance to PVE in recovery.
Limited research has investigated plasma albumin response to HWI. Hope [122] demonstrated a 7.8% or 3.3 g increase in albumin after 4 h immersed in 38.5°C saltwater. This increase has the potential to expand PV by ~60 mL [88], or ~2% [152]. However, the increase in albumin concentration is likely related to the loss of water via sweat, or immersion-associated diuresis [153]. Effects of HWI on the rate of albumin synthesis are currently unknown, so future studies might investigate its synthesis and content after HWI versus exercise to determine the roles of metabolic versus thermal factors.
As Figure 4 illustrates, repeated bouts of HWI cause PVE, but this has been examined in only one study as a singular mode of heating [71]. Bonner [71] demonstrated a 7% PVE after 13* 1-h sessions of HA in a 41°C bath located in a 40°C room. Albumin content was not reported, but a PVE of ~3% occurred following post-exercise HWI for 6 days (40 min cycling and ~40 min HWI) [54,55,59]. While this may indicate that the addition of exercise before HWI doesn’t facilitate further PVE, these combined-mode studies typically involve smaller volumes of HA and cooler air temperatures than other studies [71].
Exercise performance
Exercise performance has been investigated after repeated post-exercise HWI but not HWI alone. Post-exercise HWI has led to ~4% improvement in time trial performance in 33°C heat (but not in 18°C), with concurrent reductions in physiological and subjective strain (e.g., Tc, HR, rating of perceived exertion, skin temperature) during sub-maximal exercise [54]. PVE occurring in response to HWI alone likely facilitates the improvements in performance [26,45,47]. Further, a preliminary report demonstrated that 10 days of HWI (40°C) in conjunction with cycling for 70 min per session can increase intermittent walking time by 9 min during a heat stress test in impermeable clothing [56]. Further research should investigate (i) the effect of HWI as a standalone mode of HA for performance in the heat, (ii) how this compares to other modes of HA, (iii) the dependance (or not) of HA-mediated performance improvement on exercising and resting PVE [47], especially in elite athletes.
Summary and recommendations
The role of HWI, as a mode of HA, in facilitating PVE is still relatively unknown. The only study to our knowledge to demonstrate PVE with HWI alone elicited a 7% PVE, however further research is required to substantiate this relation. Practically, HWI provides a time-efficient and accessible mode relative to passive hot-air exposure, and a less muscle-dependent mode relative to ExH. Available research indicates the effectiveness of HWI (either alone or combined with exercise) in regard to PVE (see Figure 4 and [54,55,59,71]), however its role in elite athletes in relation to PVE or performance has not been reported. HWI may be useful in those needing to maintain subsequent training quality and those with limited equipment availability (e.g., heat chamber). Further, HWI may – in some instances – replace cold water immersion as a recovery tool and also provide physiological adaptations that could support improved exercise performance in the heat.
Exercise in heat
Context
ExH is the most common mode of HA and is a potent mediator of numerous physiological adaptations, but its comparative effectiveness to passive modes of HA are scarce (discussed above). ExH requires access to environments similar to that anticipated during the critical performance, or somewhat specialized equipment to simulate the environment (e.g., heat chamber). However, relatively crude equipment could be used (e.g., room with heater and vapor resistant clothing to increase humidity) to facilitate conditions more comparable to those in the heat [154]. Wearing additional clothing during normal training sessions may have no positive effect on heat adaptation [155], but it may provide an easy and cheap alternative should more specialized access not be possible.
ExH increases Tc and skin temperature, and therefore gradually augments cardiovascular, fluid regulatory, and metabolic strain. The heat stress is primarily metabolic, with the environment either impairing its offload or providing further gain. The most aerobically conditioned individuals may tolerate the workload required to rapidly raise Tc (+1.5°C in 20–30 min), however this may not be the case in those with lower aerobic power (e.g., team sport athletes). Whether ExH should be incorporated into elite training programs prior to competition has yet to be determined; this is likely dependent on the specific goals of the training period, the magnitude of deterioration in performance expected, and individual responses to training in the heat (see Table 2). The consideration of whether (or in what circumstances) HA should be implemented prior to elite performance, rather than merely whether it could be, is often neglected.
Table 2.
Factors to consider when determining the protocol of heat acclimation.
| Heat acclimation (HA) modes | Exercise in the heat | HWI | HWI after exercise | Passive hot air | Passive hot air after exercise | |
|---|---|---|---|---|---|---|
| Considerations | |
|
|
|
|
|
| Adaptive potential | Likely largest stimulus for adaptation (for complexity, systems, impulse) | Large thermal stimulus | Difficult to achieve air temperature sufficient to drive Tc strongly (unless sauna), but warm Tsk may aid cardiovascular effects | |||
| |
Validity in elite, i.e., already adapted? |
Little evidence |
Prolong stimulus or alter adaptive stimulus from preceding exercise? |
|
Prolong stimulus or alter adaptive stimulus from preceding exercise? |
|
| |
|
|
|
|
|
|
| Quality and quantity of regular training | Absolute intensity of training reduced beyond first ~15–20 minutes. Factor in to total training volume | Likely little effect due to passive nature | Maintain regular training | Likely little effect due to passive nature | Can maintain regular training | |
| |
Vs. regular training? When/how to periodise? |
Effect on training quality when engaging in HA and training on same day? |
Effect on quality of subsequent training? Impact previous adaptive stimulus? |
Effect on training quality when engaging in HA and training on same day? |
Effect on quality of subsequent training? Impact previous adaptive stimulus? |
|
| |
|
|
|
|
|
|
| Time efficiency | Large heat production | Strong conduction of heat so potent thermal stimulus | Can combine with regular training | Weaker thermal stimulus (unless sauna), so increased duration required | Can combine with regular training | |
| |
Vs. regular training? Especially considering likely financial and other burdens (e.g., environmental) |
|
Relative benefit vs. other recovery modes (e.g., sleep/nutrition/cold) |
|
Relative benefit vs. other recovery modes (e.g., sleep/nutrition/cold) |
|
| |
|
|
|
|
|
|
| Recovery | May cause muscular fatigue | Could promote muscle recovery | Could promote muscle recovery | Little evidence | Potential for impaired glycogen resynthesis | |
| Alter adaptive stimulus from preceding exercise? | Potential for impaired glycogen resynthesis | Alter adaptive stimulus from preceding exercise? | ||||
The considerations a practitioner must take into account when designing and implementing a heat acclimation program are highlighted with respect to the most common modes of heat acclimation. The first row (color designation) corresponds to the evidence available to suggest implementing the mode of heat acclimation, in the context of the given training/athletic consideration. Black, gray, and white correspond to highly useful, moderately useful, or not useful, respectively. The assessments are made based on the information in the rows below, and the evidence presented in Figure 4 and throughout the review. The first row highlights the positive and/or negative elements of the given heat acclimation mode for the given consideration, respectively. The second row provides notes for future research considerations. The table is intended as a brief practical guide and summary of evidence, as some caveats exist: i) The importance of the above adaptations are based on the physical requirements of the sport and the physiological adaptations that would enhance performance within hot and humid environments; ii) the training foci during this period may likely change and the individual situation must be considered; and iii) the effect each mode of heat acclimation has on the training foci (e.g., exercise in the heat impairing quality of the following training session) has not currently been investigated and the current perspectives are based on preliminary understandings of these modes; v) the mode of heat acclimation used will depend on factors additional to those mentioned above (e.g., time, equipment) [170], but mentioned throughout the review. Tc, core temperature.
Mechanisms of PVE
HA via ExH elicits PVE between ~4–18%, i.e., substantial variation has been reported (see Figure 4 and [73,92,94,103,106,107,113,114]). The combination of stressors likely facilitate these outcomes via large increases in fluid retaining hormones [70,106] that support PVE during recovery. Of note, aldosterone concentration increases during ExH [25,73,92,94,102,105,106,110,156,157], and typically returns to baseline within 12 h of exposure [102], compared with ~3 h after temperate exercise [85,117]. The magnitude of these responses is also mediated by external factors. For instance, in heat-acclimated males the ExH mediated release of ADH is potentiated by higher exercise intensity and hypohydration [158]. Thus, ExH substantially increases the stimulus for fluid-retention and likely to a greater extent than exercise or passive HA alone (see Figure 4), however further studies comparing passive and active HA to exercise alone are required to confirm this hypothesis.
Similar to HWI, atrial natriuretic peptide concentration increases with exercise, either in the heat [106,159] or in temperate environments [160,161]. The increase in atrial natriuretic peptide is likely due to increased HR (repetitive atrial stretch), catecholamines, and blood temperature [159,162]. However, the role of atrial natriuretic peptides on inhibition of PVE appears to be trivial in the context of a maximally stimulated fluid-retaining response. ExH also increases albumin content and concentration [70,72,106,113]. The initial increase may be due to a slower albumin transcapillary escape rate and higher lymphatic flow, but further increases after 24 h are more likely due to increased protein synthesis [70,163]. Plasma albumin content is increased across HA regimes of 8–19 days, and by an extent that explains nearly all of the PVE [106,107,113,164]. As mentioned previously, metabolic factors associated with exercise are important for the increased albumin content [70] through increased synthesis [87,136].
Exercise performance
ExH as a mode of HA, can improve endurance performance in the heat compared to a temperate control [22,23,25] but this is not always the case when compared to pre HA [28]. Similarly, intermittent sprint performance has been shown to be improved versus pre HA [24], but also have no effect when compared to a temperate control [25]. To effectively demonstrate the impact of HA on performance, control conditions must provide an ecologically valid comparison. Historically, the few studies including control conditions have matched for external workload [23,25,103] but cannot account for strain (e.g., HR, rating of perceived exertion, glycogen depletion) – although this is not always the case [29–31], these studies do not include elite endurance athletes. In meta-analytical approaches, longer HA protocols are associated with larger effects on performance in the heat [165], however the interpretation of these findings is problematic given high heterogeneity and high risk of bias associated with such studies. Understanding how this mode of HA affects elite athletes of different sports requires further research to properly inform an empirical basis for its use in this population.
Summary and recommendations
ExH is likely a potent mode of HA for PVE and other heat-related adaptations [70], however its superiority over other forms of HA requires further investigation. Collating available evidence indicates that it has the potential to elicit larger PVE than other modes (see Figure 4), but this is a preliminary analysis that must be substantiated. ExH is also likely associated with the most muscular fatigue, which could impair concurrent training sessions in a period where training quality may be vital (e.g., taper). Any benefits of ExH are likely due to the combined metabolic and external heat stress, which may be particularly important for those who are not aerobically trained (e.g., skill/power-based), and the familiarization to exercising in an environment they will compete in (e.g., runners, cyclists or triathletes). However, to limit the effect of fatigue within a large-volume HA protocol, ExH could be interspersed with passive heat to maintain training quality and adaptive potential. Alternatively, concurrent heat mitigation [166] and recovery strategies [69,167,168] may be employed alongside HA, however there is currently limited evidence to support these approaches for elite athletes.
Overall summary and conclusion
No study has compared the adaptive differences between ExH, HWI or passive-hot air (i.e., the most common modes of HA), thus the ability to directly compare conditions is difficult. Figure 4 summarizes the existing literature and allows for some insights into the difference, or lack of, in adaptive potential between these modes of HA. The above modes, as highlighted in Figure 4, have the potential to promote PVE, however the current literature indicates no clear advantage of either mode when the volume of HA is taken into account (Figure 4B). Thus, further research is required to compare these different modes of HA on PVE and other adaptations of HA.
When deciding on a HA protocol, other factors, aside from the adaptive potential, are likely to be important. Table 2 highlights our recommendations for HA, in the context of PVE, and illustrates some of the potential considerations for researchers, coaches, and athletes. These recommendations are based on i) the adaptations in PV likely required to support endurance performance, or any sport with a substantial cardiovascular load (also see Table 1), and ii) factors that are important to consider during this period of training. Table 2 provides an overview of several factors to consider when determining the mode of HA, but these are not exhaustive. Some clear caveats are highlighted, although many of these considerations are not novel with respect to the HA literature (see [165]). In particular, we highlight the limited research on HA in elite athletes, the lack of control groups/conditions, and expected rates (i.e., volume of HA) of adaptation being based on the average responses [60]; these will differ due to individual factors (e.g., aerobic fitness [66], sex [68], previous heat exposure [67]), and likely the mode of HA used. The recommendations of Table 2 (and throughout the manuscript), alongside other relevant reviews and perspectives [46,60,125,137,165,166,169,170], can help inform a practitioner, providing a framework for understanding how to design a preparation strategy that is specific to the requirements (and external demands) on the athlete.
Biographies
Lorenz S. Kissling is a recent Master of Physical Education graduate from the University of Otago. Lorenz is currently based at High-Performance Sport New Zealand as an Assistant Performance Physiologist. Lorenz’s main research interests are in thermoregulation and athletic performance.

Ashley P. Akerman is a Postdoctoral Research Fellow at the Human and Environmental Physiology Research Unit at the University of Ottawa. Ashley completed his PhD and previous postdoctoral positions at the University of Otago, and his research interests are the determinants of cardiovascular and autonomic responses to exercise, heat, and orthostatic stress.

James D. Cotter is a Professor of Exercise and Environmental Physiology at the University of Otago. Jim’s main research interests are in the separate and interactive effects of stressors within exercise (especially heat, dehydration, and orthostatic), acutely and chronically.

Funding Statement
This work was supported by the University of Otago Publishing Bursary; University of Otago [Postgraduate Publishing Bursary]
Abbreviations
- WBGT
Wet Bulb Globe Temperature
- RH
Relative Humidity
- °C
Degrees Celcius
- HA
Heat Acclimation
- PV
Plasma Volume
- PVE
Plasma Volume Expansion
- HR
Heart Rate
- Tc
Core Temperature
- V̇O2 peak
Peak Oxygen Consumption
- H
Rate of Heat Production.
- ExHAldo
Exercise in the Heat Aldosterone
- RAAS
Renin Angiotensin Aldosterone System
- HWI
Hot Water Immersion
- ADH
Anti-Diuretic Hormone
- AngII
Angiotensin II
- ANP
Atrial Natriuretic Peptide
- BV
Blood Volume
- CNa
Sodium Clearance
- ECFV
Extracellular Fluid Volume
- EPO
Erythropoietin
- GFR
Glomerular Filtration Rate
- RCM
Red Cell Mass
- SNSA
Sympathetic Nervous System Activity
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Gerrett N, Kingma BR, Sluijter R, et al. Ambient conditions prior to Tokyo 2020 olympic and paralympic games: considerations for acclimation or acclimatization strategies. Front Physiol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kakamu T, Wada K, Smith DR, et al. Preventing heat illness in the anticipated hot climate of the Tokyo 2020 Summer Olympic Games. Environ Health Prev Med. 2017;22(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kashimura O, Minami K, Hoshi A.. Prediction of WBGT for the Tokyo 2020 Olympic marathon. Jpn J Biometeorol. 2016;53(4):139–144. [Google Scholar]
- [4].Drust B, Rasmussen P, Mohr M, et al. Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol Scand. 2005;183(2):181–190. [DOI] [PubMed] [Google Scholar]
- [5].Périard JD, Cramer MN, Chapman PG, et al. Cardiovascular strain impairs prolonged self‐paced exercise in the heat. Exp Physiol. 2011;96(2):134–144. [DOI] [PubMed] [Google Scholar]
- [6].Racinais S, Périard JD, Karlsen A, et al. Effect of heat and heat acclimatization on cycling time trial performance and pacing. Med Sci Sports Exerc. 2015;47(3):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mohr M, Nybo L, Grantham J, et al. Physiological responses and physical performance during football in the heat. PLoS One. 2012;7(6):e39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tucker R, Rauch L, Harley YXR, et al. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflügers Archiv. 2004;448(4):422–430. [DOI] [PubMed] [Google Scholar]
- [9].Guy JH, Deakin GB, Edwards AM, et al. Adaptation to hot environmental conditions: an exploration of the performance basis, procedures and future directions to optimise opportunities for elite athletes. Sports Med. 2015;45(3):303–311. [DOI] [PubMed] [Google Scholar]
- [10].Tatterson AJ, Hahn AG, Martini DT, et al. Effects of heat stress on physiological responses and exercise performance in elite cyclists. J Sci Med Sport. 2000;3(2):186–193. [DOI] [PubMed] [Google Scholar]
- [11].Racinais S, Moussay S, Nichols D, et al. Core temperature up to 41.5 ºC during the UCI Road Cycling World Championships in the heat. Br J Sports Med. 2019;53(7):426–429. [DOI] [PubMed] [Google Scholar]
- [12].Lei TH, Stannard SR, Perry BG, et al. Influence of menstrual phase and arid vs. humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. J Physiol. 2017;595(9):2823–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lei TH, Cotter JD, Schlader ZJ, et al. On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol. 2019;597(1):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maughan RJ, Otani H, Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2012;112(6):2313–2321. [DOI] [PubMed] [Google Scholar]
- [15].IOC . Rio 2016 individual time trial men - Olympic Cycling Road: Rio 2016 individual time trial men - Olympic Cycling Road. 2016. [2019 Feb 15]. Available from: https://www.olympic.org/rio-2016/cycling-road/individual-time-trial-men.
- [16].Ely MR, Cheuvront SN, Roberts WO, et al. Impact of weather on marathon-running performance. Med Sci Sports Exerc. 2007;39(3):487–493. [DOI] [PubMed] [Google Scholar]
- [17].Armstrong LE, Casa DJ, Millard-Stafford M, et al. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. [DOI] [PubMed] [Google Scholar]
- [18].Kosaka E, Iida A, Vanos J, et al. Microclimate variation and estimated heat stress of runners in the 2020 Tokyo Olympic Marathon. Atmosphere. 2018;9(5):192. [Google Scholar]
- [19].Taylor L, Stevens CJ, Thornton HR, et al. Limiting the rise in core temperature during a rugby sevens warm-up with an ice vest. Int J Sports Physiol Perform. 2019;1–20. [DOI] [PubMed] [Google Scholar]
- [20].Adan A. Cognitive Performance and Dehydration. J Am Coll Nutr. 2012;31(2):71–78. [DOI] [PubMed] [Google Scholar]
- [21].Goodman SP, Moreland AT, Marino FE. The effect of active hypohydration on cognitive function: A systematic review and meta-analysis. Physiol Behav. 2019;204:297–308. [DOI] [PubMed] [Google Scholar]
- [22].Lorenzo S, Halliwill JR, Sawka MN, et al. Heat acclimation improves exercise performance. J Appl Physiol. 2010;109(4):1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nielsen B, Hales J, Strange S, et al. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460(1):467–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Castle P, Mackenzie RW, Maxwell N, et al. Heat acclimation improves intermittent sprinting in the heat but additional pre-cooling offers no further ergogenic effect. J Sports Sci. 2011;29(11):1125–1134. [DOI] [PubMed] [Google Scholar]
- [25].Sunderland C, Morris JG, Nevill ME. A heat acclimation protocol for team sports. Br J Sports Med. 2008;42(5):327–333. [DOI] [PubMed] [Google Scholar]
- [26].Karlsen A, Nybo L, Norgaard SJ, et al. Time course of natural heat acclimatization in well-trained cyclists during a 2-week training camp in the heat. Scand J Med Sci Sports. 2015;25(Suppl 1):240–249. [DOI] [PubMed] [Google Scholar]
- [27].Racinais S, Buchheit M, Bilsborough J, et al. Physiological and performance responses to a training camp in the heat in professional Australian football players. Int J Sports Physiol Perform. 2014;9(4):598–603. [DOI] [PubMed] [Google Scholar]
- [28].Pethick WA, Stellingwerff T, Lacroix MA, et al. The effect of a team sport-specific heat acclimation protocol on plasma volume in elite female soccer players. Sci Med Football. 2018;2(1):16–22. [Google Scholar]
- [29].Petersen CJ, Portus MR, Pyne DB, et al. Partial heat acclimation in cricketers using a 4-day high intensity cycling protocol. Int J Sports Physiol Perform. 2010;5(4):535–545. [DOI] [PubMed] [Google Scholar]
- [30].Pethick WA, Murray HJ, McFadyen P, et al. Effects of hydration status during heat acclimation on plasma volume and performance. Scand J Med Sci Sports. 2018:29:189–199. [DOI] [PubMed] [Google Scholar]
- [31].Keiser S, Fluck D, Huppin F, et al. Heat training increases exercise capacity in hot but not in temperate conditions: a mechanistic counter-balanced cross-over study. Am J Physiol Heart Circ Physiol. 2015;309(5):H750–61. [DOI] [PubMed] [Google Scholar]
- [32].Young AJ, Sawka MN, Levine L, et al. Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol. 1985;59(6):1929–1935. [DOI] [PubMed] [Google Scholar]
- [33].Febbraio MA, Snow RJ, Hargreaves M, et al. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol. 1994;76(2):589–597. [DOI] [PubMed] [Google Scholar]
- [34].Akerman AP, Tipton M, Minson CT, et al. Heat stress and dehydration in adapting for performance: Good, bad, both, or neither? Temperature. 2016;3(3):412–436. doi: 10.1080/23328940.2016.1216255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hawley JA, Lundby C, Cotter JD, et al. Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab. 2018;27(5):962–976. [DOI] [PubMed] [Google Scholar]
- [36].Racinais S, Wilson MG, Périard JD. Passive heat acclimation improves skeletal muscle contractility in humans. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R101–R7. [DOI] [PubMed] [Google Scholar]
- [37].Horowitz M. Heat acclimation: phenotypic plasticity and cues to the underlying molecular mechanisms. J Therm Biol. 2001;26(4):357–363. [Google Scholar]
- [38].Senay LC, Mitchell D, Wyndham CH. Acclimatization in a hot, humid environment: body fluid adjustments. J Appl Physiol. 1976;40(5):786–796. [DOI] [PubMed] [Google Scholar]
- [39].Sawka MN, Hubbard RW, Francesconi RP, et al. Effects of acute plasma volume expansion on altering exercise-heat performance. Eur J Appl Physiol Occup Physiol. 1983;51(3):303–312. [DOI] [PubMed] [Google Scholar]
- [40].Hopper MK, Coggan AR, Coyle EF. Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol. 1988;64(1):404–408. [DOI] [PubMed] [Google Scholar]
- [41].Coyle E, Hopper M, Coggan A. Maximal oxygen uptake relative to plasma volume expansion. Int J Sports Med. 1990;11(2):116–119. [DOI] [PubMed] [Google Scholar]
- [42].Berger NJA, Campbell IT, Wilkerson DP, et al. Influence of acute plasma volume expansion on V̇o2 kinetics, V̇o2peak, and performance during high-intensity cycle exercise. J Appl Physiol. 2006;101(3):707–714. [DOI] [PubMed] [Google Scholar]
- [43].Coyle EF, Hemmert M, Coggan AR. Effects of detraining on cardiovascular responses to exercise: role of blood volume. J Appl Physiol. 1986;60(1):95–99. [DOI] [PubMed] [Google Scholar]
- [44].Sawka MN, Young AJ, Cadarette BS, et al. Influence of heat stress and acclimation on maximal aerobic power. Eur J Appl Physiol Occup Physiol. 1985;53(4):294–298. [DOI] [PubMed] [Google Scholar]
- [45].Scoon GSM, Hopkins WG, Mayhew S, et al. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J Sci Med Sport. 2007;10(4):259–262. [DOI] [PubMed] [Google Scholar]
- [46].Chalmers S, Esterman A, Eston R, et al. Short-term heat acclimation training improves physical performance: a systematic review, and exploration of physiological adaptations and application for team sports. Sports Med. 2014;44(7):971–988. [DOI] [PubMed] [Google Scholar]
- [47].Racinais S, Mohr M, Buchheit M, et al. Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. Br J Sports Med. 2012;46(11):810–815. [DOI] [PubMed] [Google Scholar]
- [48].Convertino V, Brock P, Keil L, et al. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol. 1980;48(4):665–669. [DOI] [PubMed] [Google Scholar]
- [49].Watt MJ, Garnham AP, Febbraio MA, et al. Effect of acute plasma volume expansion on thermoregulation and exercise performance in the heat. Med Sci Sports Exerc. 2000;32(5):958–962. [DOI] [PubMed] [Google Scholar]
- [50].Sims ST, Rehrer NJ, Bell ML, et al. Preexercise sodium loading aids fluid balance and endurance for women exercising in the heat. J Appl Physiol. 2007;103(2):534–541. [DOI] [PubMed] [Google Scholar]
- [51].Luetkemeier MJ, Thomas EL. Hypervolemia and cycling time trial performance. Med Sci Sports Exerc. 1994;26(4):503–509. [PubMed] [Google Scholar]
- [52].Karlsen A, Racinais S, Jensen MV, et al. Heat acclimatization does not improve VO2max or cycling performance in a cool climate in trained cyclists. Scand J Med Sci Sports. 2015;25(Suppl 1):269–276. [DOI] [PubMed] [Google Scholar]
- [53].Horowitz M. Matching the heart to heat-induced circulatory load: heat-acclimatory responses. Physiology. 2003;18(6):215–221. [DOI] [PubMed] [Google Scholar]
- [54].Zurawlew MJ, Walsh NP, Fortes MB, et al. Post‐exercise hot water immersion induces heat acclimation and improves endurance exercise performance in the heat. Scand J Med Sci Sports. 2016;26(7):745–754. [DOI] [PubMed] [Google Scholar]
- [55].Zurawlew MJ, Mee JA, Walsh NP. Heat acclimation by post-exercise hot water immersion in the morning reduces thermal strain during morning and afternoon exercise-heat-stress. Int J Sports Physiol Perform. 2018;13(10):1–22. [DOI] [PubMed] [Google Scholar]
- [56].Weller A, Harrison M. Influence of heat acclimation on physiological strain during exercise-heat stress in men wearing clothing of limited water vapour permeability. J Physiol. 2001;531:51P.11179391 [Google Scholar]
- [57].Stanley J, Halliday A, D’Auria S, et al. Effect of sauna-based heat acclimation on plasma volume and heart rate variability. Eur J Appl Physiol. 2015;115(4):785–794. [DOI] [PubMed] [Google Scholar]
- [58].Weller A, Ellett C, Harrison M. The influence of a 4-day heat acclimation programme on indices of heat strain in hot dry and hot wet environments in clothed man. Proc J Physiol P. 1999;515:81P. [Google Scholar]
- [59].Zurawlew MJ, Mee JA, Walsh NP. Post-exercise hot water immersion elicits heat acclimation adaptations in endurance trained and recreationally active individuals. Front Physiol. 2018;9:1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Periard JD, Racinais S, Sawka MN. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand J Med Sci Sports. 2015;25(Suppl 1):20–38. [DOI] [PubMed] [Google Scholar]
- [61].Green DJ, Carter HH, Fitzsimons MG, et al. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588(9):1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Carter HH, Spence AL, Atkinson CL, et al. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol. 2014;114(4):859–865. [DOI] [PubMed] [Google Scholar]
- [63].Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol Part A. 2002;131(3):475–483. [DOI] [PubMed] [Google Scholar]
- [64].Pandolf K. Time course of heat acclimation and its decay. Int J Sports Med. 1998;19(S 2):S157–S60. [DOI] [PubMed] [Google Scholar]
- [65].Shapiro Y, Moran D, Epstein Y. Acclimatization strategies-preparing for exercise in the heat. Int J Sports Med. 1998;19(S 2):S161–S3. [DOI] [PubMed] [Google Scholar]
- [66].Pandolf K, Burse R, Goldman R. Role of physical fitness in heat acclimatisation, decay and reinduction. Ergonomics. 1977;20(4):399–408. [DOI] [PubMed] [Google Scholar]
- [67].Weller AS, Linnane DM, Jonkman AG, et al. Quantification of the decay and re-induction of heat acclimation in dry-heat following 12 and 26 days without exposure to heat stress. Eur J Appl Physiol. 2007;102(1):57–66. [DOI] [PubMed] [Google Scholar]
- [68].Mee J, Gibson O, Doust J, et al. A comparison of males and females’ temporal patterning to short‐and long‐term heat acclimation. Scand J Med Sci Sports. 2015;25:250–258. [DOI] [PubMed] [Google Scholar]
- [69].Adams EL, Vandermark LW, Pryor JL, et al. Effects of heat acclimation on hand cooling efficacy following exercise in the heat. J Sports Sci. 2017;35(9):828–834. [DOI] [PubMed] [Google Scholar]
- [70].Convertino VA, Greenleaf JE, Bernauer EM. Role of thermal and exercise factors in the mechanism of hypervolemia. J Appl Physiol. 1980;48(4):657–664. [DOI] [PubMed] [Google Scholar]
- [71].Bonner RM, Harrison MH, Hall CJ, et al. Effect of heat acclimatization on intravascular responses to acute heat stress in man. J Appl Physiol. 1976;41(5Pt. 1):708–713. [DOI] [PubMed] [Google Scholar]
- [72].Shapiro Y, Hubbard RW, Kimbrough CM, et al. Physiological and hematologic responses to summer and winter dry-heat acclimation. J Appl Physiol. 1981;50(4):792–798. [DOI] [PubMed] [Google Scholar]
- [73].Kirby CR, Convertino VA. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J Appl Physiol. 1986;61(3):967–970. [DOI] [PubMed] [Google Scholar]
- [74].Greenleaf J, Shevartz E, Keil L. Hemodilution, vasopressin suppression, and diuresis during water immersion in man. Aviat Space Environ Med. 1981;52(6):329–336. [PubMed] [Google Scholar]
- [75].Hormonal FN. Plasma volume alterations following endurance exercise. Sports Med. 1992;13(1):37–49. [DOI] [PubMed] [Google Scholar]
- [76].Sawka MN, Convertino VA, Eichner ER, et al. Blood volume: importance and adaptations to exercise training, environmental stresses and trauma sickness. Med Sci Sports Exerc. 2000;32(2):332–348. [DOI] [PubMed] [Google Scholar]
- [77].Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23(12):1338–1348. [PubMed] [Google Scholar]
- [78].Harrison MH. Heat and exercise. Effects on blood volume. Sports Med. 1986;3(3):214–223. [DOI] [PubMed] [Google Scholar]
- [79].Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144(6):2179–2183. [DOI] [PubMed] [Google Scholar]
- [80].Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–C97. [DOI] [PubMed] [Google Scholar]
- [81].Müller J. Aldosterone: The minority hormone of the adrenal cortex. Steroids. 1995;60(1):2–9. [DOI] [PubMed] [Google Scholar]
- [82].Morris DJ. The metabolism and mechanism of action of aldosterone. Endocr Rev. 1981;2(2):234–247. [DOI] [PubMed] [Google Scholar]
- [83].Hew-Butler T. Arginine vasopressin, fluid balance and exercise. Sports Med. 2010;40(6):459–479. [DOI] [PubMed] [Google Scholar]
- [84].Amabebe E, Idu F, Obika L. Relationship between thirst perception and plasma arginine vasopressin concentration in man. Niger J Physiol Sci. 2012;27(1):3–10. [PubMed] [Google Scholar]
- [85].Nagashima K, Mack GW, Haskell A, et al. Mechanism for the posture-specific plasma volume increase after a single intense exercise protocol. J Appl Physiol. 1999;86(3):867–873. [DOI] [PubMed] [Google Scholar]
- [86].Traylor RJ, Pearl RG. Crystalloid versus colloid versus colloid: all colloids are not created equal. Anesth Analg. 1996;83(2):209–212. [DOI] [PubMed] [Google Scholar]
- [87].Yang RC, Mack GW, Wolfe RR, et al. Albumin synthesis after intense intermittent exercise in human subjects circulations Albumin synthesis after intense intermittent exercise in human subjects. J Appl Physiol. 1998;84(14):584–592. [DOI] [PubMed] [Google Scholar]
- [88].Gillen CM, Lee R, Mack GW, et al. Plasma volume expansion in humans after a single intense exercise protocol. J Appl Physiol. 1991;71(5):1914–1920. [DOI] [PubMed] [Google Scholar]
- [89].Hayes P, Lucas J, Shi X. Importance of post-exercise hypotension in plasma volume restoration. Acta Physiol Scand. 2000;169(2):115–124. [DOI] [PubMed] [Google Scholar]
- [90].Graham MJ, Lucas SJ, Francois ME, et al. Low-volume intense exercise elicits post-exercise hypotension and subsequent hypervolemia, irrespective of which limbs are exercised. Front Physiol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Akerman AP, Lucas SJE, Katare R, et al. Heat and dehydration additively enhance cardiovascular outcomes following orthostatically-stressful calisthenics exercise. Front Physiol. 2017;8:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Garrett AT, Goosens NG, Rehrer NJ, et al. Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am J Hum Biol. 2014;26(3):311–320. [DOI] [PubMed] [Google Scholar]
- [93].Berland R, Briscoe C, Schleh M, editors. The effect of dehydration on heat acclimation. Int J Exercise Sci Conf Proc. 2016;8(4):46. [Google Scholar]
- [94].Neal RA, Massey HC, Tipton MJ, et al. Effect of permissive dehydration on induction and decay of heat acclimation, and temperate exercise performance. Front Physiol. 2016;7:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schleh MW, Ruby BC, Dumke CL. Short term heat acclimation reduces heat stress, but is not augmented by dehydration. J Therm Biol. 2018;78:227–234. [DOI] [PubMed] [Google Scholar]
- [96].Nielsen B, Rowell LB, Bonde-Petersen F. Cardiovascular responses to heat stress and blood volume displacements during exercise in man. Eur J Appl Physiol Occup Physiol. 1984;52(4):370–374. [DOI] [PubMed] [Google Scholar]
- [97].Nakamitsu S, Sagawa S, Miki K, et al. Effect of water temperature on diuresis-natriuresis: AVP, ANP, and urodilatin during immersion in men. J Appl Physiol. 1994;77(4):1919–1925. [DOI] [PubMed] [Google Scholar]
- [98].Norsk P, Epstein M. Effects of water immersion on arginine vasopressin release in humans. J Appl Physiol. 1988;64(1):1–10. [DOI] [PubMed] [Google Scholar]
- [99].Miwa C, Sugiyama Y, Mano T, et al. Sympatho-vagal responses in humans to thermoneutral head-out water immersion. Aviat Space Environ Med. 1997;68(12):1109–1114. [PubMed] [Google Scholar]
- [100].Weil JV, Chidsey CA. Plasma volume expansion resulting from interference with adrenergic function in normal man. Circulation. 1968;37(1):54–61. [DOI] [PubMed] [Google Scholar]
- [101].Thomas KN, van Rij AM, Lucas SJE, et al. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature. 2016;3(2):286–297. doi: 10.1080/23328940.2016.1156215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Costill DL, Branam G, Fink W, et al. Exercise induced sodium conservation: changes in plasma renin and aldosterone. Med Sci Sports. 1976;8(4):209–213. [PubMed] [Google Scholar]
- [103].Greenleaf JE, Sciaraffa D, Shvartz E, et al. Exercise training hypotension: implications for plasma volume, renin, and vasopressin. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(2):298–305. [DOI] [PubMed] [Google Scholar]
- [104].Armstrong LE, Francesconi RP, Kraemer WJ, et al. Plasma cortisol, renin, and aldosterone during an intense heat acclimation program. Int J Sports Med. 1989;10(1):38–42. [DOI] [PubMed] [Google Scholar]
- [105].Houmard JA, Costill DL, Davis JA, et al. The influence of exercise intensity on heat acclimation in trained subjects. Med Sci Sports Exerc. 1990;22(5):615–620. [DOI] [PubMed] [Google Scholar]
- [106].Nielsen B, Strange S, Christensen NJ, et al. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch. 1997;434(1):49–56. [DOI] [PubMed] [Google Scholar]
- [107].Patterson MJ, Stocks JM, Taylor NAS. Sustained and generalized extracellular fluid expansion following heat acclimation. J Physiol. 2004;559(1):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Garrett AT, Goosens NG, Rehrer NG, et al. Induction and decay of short-term heat acclimation. Eur J Appl Physiol. 2009;107(6):659. [DOI] [PubMed] [Google Scholar]
- [109].Hobson RM, Clapp EL, Watson P, et al. Exercise capacity in the heat is greater in the morning than in the evening in man. Med Sci Sports Exerc. 2009;41(1):174–180. [DOI] [PubMed] [Google Scholar]
- [110].Garrett AT, Creasy R, Rehrer NJ, et al. Effectiveness of short-term heat acclimation for highly trained athletes. Eur J Appl Physiol. 2012;112(5):1827–1837. [DOI] [PubMed] [Google Scholar]
- [111].Fujii N, Honda Y, Ogawa T, et al. Short-term exercise-heat acclimation enhances skin vasodilation but not hyperthermic hyperpnea in humans exercising in a hot environment. Eur J Appl Physiol. 2012;112(1):295–307. [DOI] [PubMed] [Google Scholar]
- [112].Costa RJ, Crockford MJ, Moore JP, et al. Heat acclimation responses of an ultra-endurance running group preparing for hot desert-based competition. Eur J Sport Sci. 2014;14(Suppl 1):S131–41. [DOI] [PubMed] [Google Scholar]
- [113].Patterson MJ, Stocks JM, Taylor NA. Whole-body fluid distribution in humans during dehydration and recovery, before and after humid-heat acclimation induced using controlled hyperthermia. Acta Physiol (Oxf). 2014;210(4):899–912. [DOI] [PubMed] [Google Scholar]
- [114].Fujii N, Tsuji B, Honda Y, et al. Effect of short-term exercise-heat acclimation on ventilatory and cerebral blood flow responses to passive heating at rest in humans. J Appl Physiol (1985). 2015;119(5):435–444. [DOI] [PubMed] [Google Scholar]
- [115].Mee JA, Peters S, Doust JH, et al. Sauna exposure immediately prior to short-term heat acclimation accelerates phenotypic adaptation in females. J Sci Med Sport. 2018;21(2):190–195. [DOI] [PubMed] [Google Scholar]
- [116].Pryor JL, Pryor RR, Vandermark LW, et al. Intermittent exercise-heat exposures and intense physical activity sustain heat acclimation adaptations. J Sci Med Sport. 2019;22(1):117–122. [DOI] [PubMed] [Google Scholar]
- [117].Altenkirch HU, Gerzer R, Kirsch KA, et al. Effect of prolonged physical exercise on fluid regulating hormones. Eur J Appl Physiol Occup Physiol. 1990;61(3–4):209–213. [DOI] [PubMed] [Google Scholar]
- [118].Ray CA, Delp MD, Hartle DK. Interactive effect of body posture on exercise-induced atrial natriuretic peptide release. Am J Physiol. 1990;258(5 Pt 1):E775–9. [DOI] [PubMed] [Google Scholar]
- [119].Gillen CM, Nishiyasu T, Langhans G, et al. Cardiovascular and renal function during exercise-induced blood volume expansion in men. J Appl Physiol. 1994;76(6):2602–2610. [DOI] [PubMed] [Google Scholar]
- [120].Haskell A, Nadel ER, Stachenfeld NS, et al. Transcapillary escape rate of albumin in humans during exercise-induced hypervolemia. J Appl Physiol. 1997;83(2):407–413. [DOI] [PubMed] [Google Scholar]
- [121].Mack GW, Yang R, Hargens AR, et al. Influence of hydrostatic pressure gradients on regulation of plasma volume after exercise. J Appl Physiol. 1998;85(2):667–675. [DOI] [PubMed] [Google Scholar]
- [122].Hope A, Aanderud L, Aakvaag A. Dehydration and body fluid-regulating hormones during sweating in warm (38 degrees C) fresh- and seawater immersion. J Appl Physiol (1985). 2001;91(4):1529–1534. [DOI] [PubMed] [Google Scholar]
- [123].Warburton D, Haykowsky MJ, Quinney HA, et al. Blood volume expansion and cardiorespiratory function: effects of training modality. Med Sci Sports Exerc. 2004;36(6):991–1000. [DOI] [PubMed] [Google Scholar]
- [124].Pilch W, Pokora I, Szygula Z, et al. Effect of a single finnish sauna session on white blood cell profile and cortisol levels in athletes and non-athletes. J Hum Kinet. 2013;39(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Daanen HAM, Racinais S, Périard JD. Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 2018;48(2):409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Gagge AP, Stolwijk J, Hardy J. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1(1):1–20. [DOI] [PubMed] [Google Scholar]
- [127].Cabanac M, Massonnet B, Belaiche R. Preferred skin temperature as a function of internal and mean skin temperature. J Appl Physiol. 1972;33(6):699–703. [DOI] [PubMed] [Google Scholar]
- [128].Gonzalez R, Nishi Y, Gagge A. Experimental evaluation of standard effective temperature a new biometeorological index of man’s thermal discomfort. Int J Biometeorol. 1974;18(1):1–15. [DOI] [PubMed] [Google Scholar]
- [129].Tyka A, Palka T, Tyka A, et al. Repeated sauna bathing effects on males’ capacity to prolonged exercise-heat performance. Med Sport. 2008;12(4):150–154. [Google Scholar]
- [130].Sohar E, Shoenfeld Y, Shapiro Y, et al. Effects of exposure to Finnish sauna. Isr J Med Sci. 1976;12(11):1275–1282. [PubMed] [Google Scholar]
- [131].Leppäluoto J, Tuominen M, Väänänen A, et al. Some cardiovascular and metabolic effects of repeated sauna bathing. Acta Physiol Scand. 1986;128(1):77–81. [DOI] [PubMed] [Google Scholar]
- [132].Kukkonen-Harjula K, Oja P, Laustiola K, et al. Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur J Appl Physiol Occup Physiol. 1989;58(5):543–550. [DOI] [PubMed] [Google Scholar]
- [133].Naperalsky M, Ruby B, Slivka D. Environmental temperature and glycogen resynthesis. Int J Sports Med. 2010;31(8):561–566. [DOI] [PubMed] [Google Scholar]
- [134].Kosunen KJ, Pakarinen AJ, Kuoppasalmi K, et al. Plasma renin activity, angiotensin II, and aldosterone during intense heat stress. J Appl Physiol. 1976;41(3):323–327. [DOI] [PubMed] [Google Scholar]
- [135].Leppaluoto J, Arjamaa O, Vuolteenaho O, et al. Passive heat exposure leads to delayed increase in plasma levels of atrial natriuretic peptide in humans. J Appl Physiol. 1991;71(2):716–720. [DOI] [PubMed] [Google Scholar]
- [136].Nagashima K, Cline GW, Mack GW, et al. Intense exercise stimulates albumin synthesis in the upright posture. J Appl Physiol. 2000;88(1):41–46. [DOI] [PubMed] [Google Scholar]
- [137].Heathcote SL, Hassmén P, Zhou S, et al. Passive heating: reviewing practical heat acclimation strategies for endurance athletes. Front Physiol. 2018;9:1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Saini J, Brandenberger G, Libert JP, et al. Nocturnal pituitary hormone and renin profiles during chronic heat exposure. J Appl Physiol (1985). 1993;75(1):294–300. [DOI] [PubMed] [Google Scholar]
- [139].Allsopp AJ, Sutherland R, Wood P, et al. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol Occup Physiol. 1998;78(6):516–521. [DOI] [PubMed] [Google Scholar]
- [140].Beaudin AE, Clegg ME, Walsh ML, et al. Adaptation of exercise ventilation during an actively-induced hyperthermia following passive heat acclimation. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R605–R14. [DOI] [PubMed] [Google Scholar]
- [141].Versey NG, Halson SL, Dawson BT. Water Immersion Recovery for Athletes: Effect on Exercise Performance and Practical Recommendations. Sports Med. 2013;43(11):1101–1130. [DOI] [PubMed] [Google Scholar]
- [142].Petrofsky J, Berk L, Bains G, et al. The efficacy of sustained heat treatment on delayed-onset muscle soreness. Clin J Sport Med. 2017;27(4):329–337. [DOI] [PubMed] [Google Scholar]
- [143].Slivka D, Tucker T, Cuddy J, et al. Local heat application enhances glycogenesis. Appl Physiol Nutr Metab. 2012;37(2):247–251. [DOI] [PubMed] [Google Scholar]
- [144].Epstein M, Norsk P, Loutzenhiser R. Effects of water immersion on atrial natriuretic peptide release in humans. Am J Nephrol. 1989;9(1):1–24. [DOI] [PubMed] [Google Scholar]
- [145].Epstein M, Pins DS, Sancho J, et al. Suppression of plasma renin and plasma aldosterone during water immersion in normal man. J Clin Endocrinol Metab. 1975;41(3):618–625. [DOI] [PubMed] [Google Scholar]
- [146].Epstein M, Saruta T. Effect of water immersion on renin-aldosterone and renal sodium handling in normal man. J Appl Physiol. 1971;31(3):368–374. [DOI] [PubMed] [Google Scholar]
- [147].Crane MG, Harris JJ. Suppression of plasma aldosterone by partial immersion. Metabolism. 1974;23(4):359–368. [DOI] [PubMed] [Google Scholar]
- [148].Claybaugh JR, Pendergast DR, Davis JE, et al. Fluid conservation in athletes: responses to water intake, supine posture, and immersion. J Appl Physiol. 1986;61(1):7–15. [DOI] [PubMed] [Google Scholar]
- [149].Epstein M, Pins DS, Miller M. Suppression of ADH during water immersion in normal man. J Appl Physiol. 1975;38(6):1038–1044. [DOI] [PubMed] [Google Scholar]
- [150].Gordon CJ, Fogarty AL, Greenleaf JE, et al. Direct and indirect methods for determining plasma volume during thermoneutral and cold-water immersion. Eur J Appl Physiol. 2003;89(5):471–474. [DOI] [PubMed] [Google Scholar]
- [151].Johansen LB, Foldager N, Stadeager C, et al. Plasma volume, fluid shifts, and renal responses in humans during 12 h of head-out water immersion. J Appl Physiol. 1992;73(2):539–544. [DOI] [PubMed] [Google Scholar]
- [152].Davy KP, Seals DR. Total blood volume in healthy young and older men. J Appl Physiol. 1994;76(5):2059–2062. [DOI] [PubMed] [Google Scholar]
- [153].Graveline DE, Jackson MM. Diuresis associated with prolonged water immersion. J Appl Physiol. 1962;17(3):519–524. [DOI] [PubMed] [Google Scholar]
- [154].Willmott AG, Gibson OR, James CA, et al. Physiological and perceptual responses to exercising in restrictive heat loss attire with use of an upper-body sauna suit in temperate and hot conditions. Temperature. 2018;5(2):162–174.doi: 10.1080/23328940.2018.1426949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Stevens CJ, Heathcote SL, Plews DJ, et al. Effect of two-weeks endurance training wearing additional clothing in a temperate outdoor environment on performance and physiology in the heat. Temperature. 2018;5(3):267–275. doi: 10.1080/23328940.2018.1474672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Francesconi RP, Sawka MN, Pandolf KB. Hypohydration and heat acclimation: plasma renin and aldosterone during exercise. J Appl Physiol. 1983;55(6):1790–1794. [DOI] [PubMed] [Google Scholar]
- [157].Armstrong LE, Francesconi RP, Kraemer WJ, et al. Plasma cortisol, renin, and aldosterone during an intense heat acclimation program. Int J Sports Med. 1989;10(1):38–42. [DOI] [PubMed] [Google Scholar]
- [158].Montain SJ, Laird JE, Latzka WA, et al. Aldosterone and vasopressin responses in the heat: hydration level and exercise intensity effects. Med Sci Sports Exerc. 1997;29(5):661–668. [DOI] [PubMed] [Google Scholar]
- [159].Nose H, Takamata A, Mack GW, et al. Right atrial pressure and forearm blood flow during prolonged exercise in a hot environment. Pflugers Archiv Eur J Physiol. 1994;426(3–4):177–182. [DOI] [PubMed] [Google Scholar]
- [160].Ray CA, Cureton KJ, Ouzts HG. Postural specificity of cardiovascular adaptations to exercise training. J Appl Physiol. 1990;69(6):2202–2208. [DOI] [PubMed] [Google Scholar]
- [161].Nagashima K, Nose H, Yoshida T, et al. Relationship between atrial natriuretic peptide and plasma volume during graded exercise with water immersion. J Appl Physiol. 1995;78(1):217–224. [DOI] [PubMed] [Google Scholar]
- [162].Bilder GE, Schofield TL, Blaine EH. Release of atrial natriuretic factor. Am J Physiol Renal Physiol. 1986;251(5):F817–F21. [DOI] [PubMed] [Google Scholar]
- [163].Nagashima K, Wu J, Kavouras SA, et al. Increased renal tubular sodium reabsorption during exercise-induced hypervolemia in humans. J Appl Physiol. 2001;91(3):1229–1236. [DOI] [PubMed] [Google Scholar]
- [164].Goto M, Okazaki K, Kamijo Y, et al. Protein and carbohydrate supplementation during 5-day aerobic training enhanced plasma volume expansion and thermoregulatory adaptation in young men. J Appl Physiol. 2010;109(4):1247–1255. [DOI] [PubMed] [Google Scholar]
- [165].Tyler CJ, Reeve T, Hodges GJ, et al. The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 2016;46(11):1699–1724. [DOI] [PubMed] [Google Scholar]
- [166].Alhadad SB, Tan PM, Lee JK. Efficacy of heat mitigation strategies on core temperature and endurance exercise: a meta-analysis. Front Physiol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].James CA, Richardson AJ, Watt PW, et al. Short-term heat acclimation and precooling, independently and combined, improve 5-km time trial performance in the heat. J Strength Cond Res. 2018;32(5):1366–1375. [DOI] [PubMed] [Google Scholar]
- [168].Skein M, Wingfield G, Gale R, et al. Sleep quantity and quality during consecutive day heat training with the inclusion of cold-water immersion recovery. J Therm Biol. 2018;74:63–70. [DOI] [PubMed] [Google Scholar]
- [169].Taylor NA. Human heat adaptation. Compr Physiol. 2014;4(1):325–365. [DOI] [PubMed] [Google Scholar]
- [170].Periard JD, Travers GJS, Racinais S, et al. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton Neurosci. 2016;196:52–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- IOC . Rio 2016 individual time trial men - Olympic Cycling Road: Rio 2016 individual time trial men - Olympic Cycling Road. 2016. [2019 Feb 15]. Available from: https://www.olympic.org/rio-2016/cycling-road/individual-time-trial-men.


