ABSTRACT
Athletes exercising in heat stress experience increased perceived fatigue acutely, however it is unknown whether heat acclimation (HA) reduces the magnitude of this perceptual response and whether different HA protocols influence the response. This study investigated sensations of fatigue following; acute exercise-heat stress; short- (5-sessions) and medium-term (10-sessions) HA; and between once- (ODHA) and twice-daily HA (TDHA) protocols. Twenty male participants (peak oxygen uptake: 3.75 ± 0.47 L·min-1) completed 10 sessions (60-min cycling at ~2 W·kg-1, 45°C/20% relative humidity) of ODHA (n = 10) or non-consecutive TDHA (n = 10). Sensations of fatigue (General, Physical, Emotional, Mental, Vigor and Total Fatigue) were assessed using the multi-dimensional fatigue scale inventory-short form pre and post session 1, 5 and 10. Heat adaptation was induced following ODHA and TDHA, with reductions in resting rectal temperature and heart rate, and increased plasma volume and sweat rate (P < 0.05). General, Physical and Total Fatigue increased from pre-to-post for session 1 within both groups (P < 0.05). Increases in General, Physical and Total Fatigue were attenuated in session 5 and 10 vs. session 1 of ODHA (P < 0.05). This change only occurred at session 10 of TDHA (P < 0.05). Whilst comparative heat adaptations followed ODHA and TDHA, perceived fatigue is prolonged within TDHA.
Abbreviations
∆: Change; ANOVA: Analysis of variance; HA: Heat acclimation; HR: Heart rate; IL-6: Interleukin-6; MFS-SF: Multi-dimensional fatigue symptom inventory-short form (MFSI-SF); MTHA: Medium-term heat acclimation; Na+: Sodium; ODHA: Once daily heat acclimation; PV: Plasma volume; RH: Relative humidity; RPE: Rating of perceived exertion; SD: Standard deviation; SE: Standard error of the slope coefficient or intercept; SEE: Standard error of the estimate for the regression equation; STHA: Short-term heat acclimation; TDHA: Twice daily heat acclimation; TC: Thermal Comfort; Tre: Rectal temperature; TSS: Thermal sensation; V̇O2peak: Peak oxygen uptake; WBSL: whole-body sweat loss.
KEYWORDS: Heat stress, internal load, fatigue, heat acclimation, heat adaptation
Introduction
Exercise-heat stress, such as that forecasted for the Tokyo 2020 Olympic and Paralympic Games [1], induces physiological (e.g. hyperthermia, dehydration and cardiovascular load) and perceptual strain (e.g. elevated thermal sensation [TS], decreased thermal comfort [TC] and increased rating of perceived exertion [RPE]). These disruptions are associated with an increased risk of heat related illness [2] and/or compromised athletic performance [3], in comparison to equivalent exercise in temperate conditions. Sensations of fatigue are a complex emotion and can be self-assessed by single- (e.g. ratings of subjective or perceived fatigue [4]) or multi-dimensional Likert scales (e.g. via General, Physical, Emotional, Mental, Vigor and Total Fatigue scores [5]), to indicate an individual’s sense of tiredness and/or exhaustion before and after exercise [6]. These sensations of fatigue are typically experienced alongside changes in physiological responses (e.g. increased rectal temperature [Tre], heart rate [HR] and/or inflammatory/stress markers), which can further augment the magnitude of perceptual strain experienced [7,8]. For example, greater perceived fatigue has been found during exercise-heat stress (running at 60% of peak oxygen uptake [V̇O2peak] in 42°C, 18% relative humidity [RH]) compared to temperate conditions (22°C, 35% RH) [7,9]. Similarly, increased General and Physical Fatigue scores were reported whilst cycling at 2 W·kg−1 during the first of four sessions of exercise-heat stress (45°C, 30% RH), with reported symptoms of augmented lethargy and tiredness [10], which were correlated with an increased Tre (~39.0°C). These contributing factors and symptoms accompanying increased perceived fatigue, may manifest into unplanned cumulative fatigue, illness and/or potentially over-reaching if not monitored adequately during repeated and/or intensified training, especially within extreme environmental conditions [11–13]. As such, daily monitoring of perceptual wellbeing (e.g. perceived fatigue) and/or psychological status (e.g. mood, stress and anxiety) of high-performance athletes is common-place within elite sport [14,15] and has demonstrated positive relationships with physical performance in training [16].
One method to alleviate the aforementioned physiological and perceptual consequences of exercise-heat stress, is heat acclimation (HA) [17], which is a chronic heat alleviation strategy recommended for athletes [18] to be implemented in the preceding months before the Tokyo 2020 Olympic and Paralympic Games [1,19,20]. Physiological and perceptual adaptations following HA are well documented [17,21,22], however, an individual’s sensations of fatigue toward acute exercise-heat stress and subsequent adaptations following repeated exposures during HA of differing time-scales are less well understood [10]. This is a pertinent issue, given the required stimuli to optimize adaptations (e.g. elevated Tre and skin temperature, and profuse sweating) [17] within challenging environmental conditions (~40°C, 40% RH [21]) are also those which induce increased sensations of fatigue [10]. Additionally, a better understanding of the effects of acute heat stress on perceived fatigue is necessary, because HA interventions are commonly implemented alongside ongoing technical training and other physical preparation priorities. Previously, lower sensations of fatigue have been reported following four [10], seven [23], ten [9] and ten/eleven days of HA [22], alluding to a desirable negative relationship with the length of HA. However, in these experiments the HA method did not reflect the empirically recommended medium- to long-term isothermic model (e.g. 10–14 days of controlled hyperthermia [Tre ≥38.5°C]) [18], therefore, the perceived fatigue following this specific HA intervention remains unknown. Whilst single, once-daily HA (ODHA) sessions across a medium-term timescale are recommended [18], it has recently been observed that non-consecutive twice-daily HA (TDHA) presents similar heat adaptations, with no apparent differences in inflammatory/stress responses to ODHA [24]. The non-consecutive TDHA intervention presents individuals with a greater flexibility when prescribing HA, however it is unclear whether TDHA over short- and medium-term time-scales (e.g. 5 vs. 10-sessions) induces greater sensations of fatigue than ODHA.
Although the contributing factors to sensations of fatigue are multi-faceted, data suggest they may be influenced by inflammatory/stress markers. Following 4-weeks of repeated occupational specific-heat stress, fire service instructors reported increased General Fatigue, alongside chronic physiological strain and augmented inflammatory/stress responses, indicating an overtraining-type response [25]. Though inflammatory markers (e.g. interleukin-6 [IL-6]) and/or stress responses (e.g. cortisol) during HA [10,24,26,27] have been investigated, this data in conjunction with the sensations of fatigue has not been reported and may be an important element of an athlete-focused wellbeing monitoring strategy [28,29]. This requires attention given higher concentrations of IL-6 and cortisol appear to augment perceived fatigue and subsequently impair aerobic endurance [30] and cognitive performance [8], with evidence indicating correlations between perceived fatigue and cortisol concentrations, and body mass loss (e.g. dehydration) during exercise-heat stress [8].
Therefore, this study had the following aims; 1) describe the magnitude of sensations of fatigue during an acute exercise-heat stress exposure; 2) investigate whether STHA and MTHA reduce the sensations of fatigue; 3) understand whether training frequency elicited differences in the sensations of fatigue between ODHA and TDHA protocols; and 4) investigate factors which contribute to the changes in perceived fatigue. It was hypothesized that; 1) the sensations of fatigue will increase following an acute exercise-heat stress exposure; 2) MTHA would confer greater improvements in the sensations of fatigue compared to STHA, due to a greater dose of HA (e.g. 10- sessions [600-min] vs. 5-sessions [300-min]), thus enhancing heat acclimation state and alleviating any undesirable effects of repeated exercise-heat stress; 3) no differences would occur in the sensations of fatigue between ODHA and TDHA protocols, due to the same weekly dose of HA and similar physiological strain; and 4) increased physiological strain is associated with higher sensations of fatigue scores.
Methods
Participants and ethical approval
Twenty moderately-trained males volunteered to participate in this study having provided written informed consent. This study was approved by the institution’s Research Ethics and Governance Committee and conducted in accordance with the principles of the Declaration of Helsinki (World Medical Association 2013) [31]. Data presented within this study formed part of a larger study [24], however, the current study investigated different hypotheses and data focussing on the sensations of fatigue during HA over differing time-scales and with variances in HA protocols.
Experimental design and protocols
Following a graded cycling exercise test (SRM high performance model, Germany) within temperate conditions (22°C, 40% RH) to determine V̇O2peak [32] and a heat acclimation state test [33] [as described further in 24], participants were matched for biophysical characteristics and aerobic capacity, and assigned to consecutive ODHA (n = 10, age: 23 ± 6 years, body mass: 77.2 ± 10.0 kg, stature: 1.78 ± 0.08 m, V̇O2peak: 3.76 ± 0.46 L·min−1, body surface area: 1.95 ± 0.16 m2 and body fat: 14.9 ± 2.7%) or non-consecutive TDHA (n = 10, 25 ± 7 years, 75.3 ± 9.5 kg, 1.79 ± 0.04 m, 3.74 ± 0.50 L·min−1, 1.94 ± 0.13 m2 and 14.3 ± 3.7%). All participants completed ten, 60-min sessions in hot conditions (45°C, 20% RH) over a 12-day period. Isothermic HA was implemented to ensure equal absolute thermoregulatory strain was elicited throughout the intervention thus giving sufficient physiological strain for adaptation and providing equal strain to make comparisons across sessions [34]. HA started at a power output of 2.3 W·kg−1 [35] and a cadence of 80 rev·min−1, which was subsequently altered every 15-min corresponding with the participants’ ∆Tre and perceived effort [36,37] to target Tre of ≥38.5°C [34]. Participants avoided alcohol and caffeine 12-h before each visit and arrived euhydrated, as determined by urine; osmolality <700 mOsmol.kg−1 (Osmocheck, Vitech Scientific Ltd, Japan) specific gravity <1.020 (refractometer, Atago, Japan) and color <3 [38].
Perceptual measures
Thirty minutes pre and post session 1, 5 and 10, the sensations of fatigue via five subscales (General, Physical, Emotional, Mental, Vigor) and an overall Total Fatigue scale were measured using the multi-dimensional fatigue symptom inventory-short form (MFSI-SF) [5]. The MFSI-SF has been validated [5,39], implemented within previous heat stress research [10,25] and is assessed using 30 statements on a Likert scale from 0 (Not at all) to 4 (Extremely). Fatigue scores are added together as per Stein et al. [5], with high scores indicating larger levels of; General, Physical, Emotional, Mental and Total Fatigue, and low scores indicating lower levels of Vigor. Perceptions of RPE [40] from 6 (No exertion) to 20 (Maximal Exertion), thermal sensation (TSS [41]) from 0 (Very Very Cold), 4 (Neutral) to 8 (Very Very Hot), and TC [42] from 0 (Very Comfortable) to 5 (Very Uncomfortable), were collected during exercise at 5-min intervals during exercise heat stress. Familiarization to scales were provided and time was enabled for questions before each session.
Physiological measures
Participant’s Tre (Henley Medical Supplies rectal thermistor, UK and YSI 4600 Series Precision™ Thermometer, USA [accuracy: ± 0.115°C]) and HR (Polar, Electro Oy, Finland) were continuously monitored and recorded at 5-min intervals during exercise heat stress. Fluid intake was restricted for sessions 1, 5 and 10, to estimate whole-body sweat loss (WBSL) via pre-to-post session changes in nude body mass. Sweat samples were collected using an absorbent pad (Tegaderm+Pad 3MTM, USA) to assess sodium concentration ([Na+]) (Sweat-ChekTM Eli Tech Group, Wescor Inc., USA). To estimate ΔPV [43] between session 1, 5 and 10, a fingertip capillary blood sample was collected in triplicate and assessed for hemoglobin concentration (HemoCue, Ltd., Sweden) and hematocrit (Hawksley and Sons Ltd, England). A 10 mL venous blood sample was also analyzed for plasma IL-6 (Ready Set Go!®, eBioscience, Affymetrix Inc., USA) and cortisol (Sigma-Aldrich, USA) using commercially available ELISA kits. Data were corrected for ΔPV.
Data and statistical analyses
All data are reported as mean ± SD, with statistical significance set at P <0.05. Data were assessed and conformed to normality and sphericity prior to further statistical analysis. Analysis of data for HA (n = 20) combines data sets from both ODHA (n = 10) and TDHA (n = 10). To investigate intervention efficacy for HA, physiological data were analyzed using one-way repeated measures ANOVA, whereas perceptual data were analyzed using a Friedman test. To investigate changes following ODHA and TDHA, physiological and perceptual data were analyzed using two-way repeated measures ANOVA (Group*Time) for Group (ODHA and TDHA) and Time (session 1, 5 and 10, and, Δ between session 1–5 and 1–10). Following a significant F- (ANOVA) or X2-value (Friedman test), follow up Bonferroni-corrected post-hoc comparisons and Wilcoxon signed-rank tests were used, respectively. Relationships between perceptual and physiological measures, and the sensations of fatigue were examined using Spearman’s rank-order correlation coefficient (r), as per previous work [10,25,44]. Following the determination of significant linear relationships, statistically significant variables were entered into stepwise multiple regression analysis to better understand the correlations associated with the sensations of fatigue, as per previous work [45,46]. Relationships were interpreted as; <0.3 = weak, 0.3–0.5 = moderate, 0.5–0.7 = strong, 0.7–0.9 = very strong, 0.9–1.0 near perfect [47].
Results
Heat adaptations and exercise intensity data
Key markers of physiological (reductions in resting Tre and HR, conserved sweat [Na+], increased WBSL and PV expansion) and perceptual adaptations (reductions in RPE, TSS [e.g. “feeling cooler”] and TC [e.g. “feeling more comfortable”]) to heat stress were observed following 5 and 10-sessions of HA, ODHA and TDHA (all P <0.05) (Table 1). These physiological and perceptual adaptations were greater following 10-sessions compared to 5 for both ODHA and TDHA (P <0.05), with no between-group differences found (P >0.05) [24]). No main effect or interaction (all P >0.05) for exercise intensity (e.g. total work completed and mean power [W, % of V̇O2peak and W.kg−1]) were found between sessions 1, 5 and 10 for HA, ODHA and TDHA (Table 1). However, there was a main effect for ΔTre (P = 0.001), where a larger ΔTre was observed during session 5 and 10 compared to session 1 (P <0.05), but no interaction occurred (P = 0.597).
Table 1.
Mean ± SD changes (∆) in heat adaptations for session 1–5 and 1–10 and exercise intensity data for sessions 1, 5 and 10.
| ODHA and TDHA Combined (n = 20) |
ODHA (n = 10) |
TDHA (n = 10) |
||||
|---|---|---|---|---|---|---|
| Heat Adaptation | 1-5 | 1-10 | 1-5 | 1-10 | 1-5 | 1-10 |
| ∆Rest Tre (°C) | −0.20 ± 0.21* | −0.28 ± 0.16* | −0.18 ± 0.27* | −0.28 ± 0.22* | −0.22 ± 0.17* | −0.28 ± 0.19* |
| ∆Rest HR (b·min−1) | −5 ± 4* | −10 ± 4* | −5 ± 1* | −10 ± 3* | −5 ± 5* | −10 ± 4* |
| ∆PV (%) | +5.6 ± 3.9 | +9.1 ± 4.4* | +6.3 ± 4.0 | +10.1 ± 5.6* | +5.4 ± 4.0 | +8.5 ± 3.1* |
| ∆WBSL (mL) | +202 ± 176* | +463 ± 200* | +230 ± 207* | +533 ± 261* | +178 ± 142* | +398 ± 97* |
| ∆[Na+] (mmol·L−1) | −10 ± 10* | −20 ± 14* | −13 ± 13* | −27 ± 19* | −7 ± 6 | −14 ± 5* |
| ∆RPEpeak | −1 ± 1 | −2 ± 1* | −1 ± 1 | −2 ± 1* | −1 ± 1 | −2 ± 1* |
| ∆TSSpeak | −0.5 ± 0.5 | −0.9 ± 0.6* | −0.3 ± 0.4 | −0.7 ± 0.5* | −0.5 ± 0.5 | −0.9 ± 0.5* |
| ∆TCpeak | −1 ± 1 | −1 ± 1* | −1 ± 1 | −1 ± 1* | 0 ± 1 | −1 ± 1* |
| ∆[IL-6] (pg.mL·L−1) | +0.1 ± 0.8 | −0.1 ± 0.7 | +0.2 ± 0.8 | −0.1 ± 0.8 | 0.0 ± 0.8 | −0.1 ± 0.6 |
| ∆[Cortisol] (nmol·L−1) | +6 ± 25 | −17 ± 29 | +5 ± 20 | −26 ± 28 | +8 ± 31 | −8 ± 28 |
| Exercise Intensity | 1 | 5 | 10 | 1 | 5 | 10 | 1 | 5 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| Exercise time (min) | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 | 60 ± 0 |
| Total work (kJ) | 474 ± 51 | 482 ± 63 | 496 ± 52 | 476 ± 61 | 485 ± 56 | 490 ± 47 | 472 ± 41 | 479 ± 60 | 502 ± 58 |
| Mean power (W) | 137 ± 10 | 140 ± 10 | 143 ± 15 | 141 ± 10 | 141 ± 9 | 142 ± 16 | 134 ± 10 | 139 ± 11 | 144 ± 15 |
| Mean power (% V̇O2peak) | 48 ± 5 | 49 ± 6 | 50 ± 5 | 49 ± 5 | 49 ± 5 | 50 ± 3 | 47 ± 4 | 49 ± 8 | 50 ± 6 |
| Mean power (W·kg−1) | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.2 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.2 | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.2 |
| ∆Tre (°C) | 1.39 ± 0.23 | 1.54 ± 0.23 | 1.61 ± 0.27 | 1.42 ± 0.23 | 1.53 ± 0.23 | 1.58 ± 0.26 | 1.37 ± 0.24 | 1.56 ± 0.25 | 1.64 ± 0.28 |
| Mean HR (b·min−1) | 151 ± 12 | 150 ± 10 | 147 ± 11 | 151 ± 14 | 155 ± 9 | 150 ± 12 | 151 ± 9 | 145 ± 8 | 144 ± 9 |
| ∆body mass (%) | 1.4 ± 0.4 | 1.6 ± 0.4 | 2.0 ± 0.4 | 1.2 ± 0.3 | 1.4 ± 0.4 | 1.9 ± 0.4 | 1.5 ± 0.5 | 1.8 ± 0.3 | 2.1 ± 0.5 |
*represents a significant (P <0.05) pre- to post-intervention difference. Tabular data are adapted from Willmott et al. [24].
Sensations of fatigue
The sensations of fatigue data are presented in Table 2 and Figure 1 for HA, ODHA and TDHA.
Table 2.
Mean ± SD pre, post and changes in the sensations of fatigue for sessions 1, 5 and 10 during combined ODHA and TDHA.
| ODHA and TDHA Combined |
||||||
|---|---|---|---|---|---|---|
| Group |
1 |
5 |
10 |
|||
| Session | Pre | Post | Pre | Post | Pre | Post |
| General | 3.7 ± 3.0 | 10.6 ± 4.3* | 3.6 ± 2.4 | 8.2 ± 7.9* | 3.8 ± 3.6 | 6.0 ± 5.1*† |
| Physical | 1.9 ± 2.3 | 5.5 ± 4.0* | 1.8 ± 1.2 | 5.4 ± 5.2* | 2.1 ± 2.4 | 3.5 ± 3.1*† |
| Emotional | 1.7 ± 2.5 | 1.8 ± 2.7 | 0.5 ± 0.8 | 0.8 ± 1.0 | 1.6 ± 2.9 | 1.2 ± 2.7 |
| Mental | 1.8 ± 2.5 | 2.1 ± 2.7 | 1.1 ± 1.6 | 0.5 ± 1.5 | 1.2 ± 1.9 | 1.1 ± 2.6 |
| Vigor | 12.5 ± 4.9 | 7.6 ± 5.4* | 12.8 ± 5.2 | 10.1 ± 8.0* | 12.1 ± 5.8 | 12.5 ± 7.1† |
|
Total Fatigue |
−2.8 ± 9.0 |
12.4 ± 12.2* |
−4.1 ± 6.3 |
4.8 ± 20.7* |
−3.5 ± 10.0 |
2.7 ± 13.1*† |
| |
Within-session change |
|||||
| |
1 |
5 |
10 |
|||
| General | +6.9 ± 4.4* | +4.6 ± 7.4* | +2.2 ± 4.9* | |||
| Physical | +3.6 ± 4.3* | +3.7 ± 5.3* | +1.5 ± 2.7* | |||
| Emotional | +0.1 ± 1.7 | +0.4 ± 1.2 | −0.4 ± 2.1 | |||
| Mental | +0.3 ± 1.7 | −0.6 ± 2.0 | −0.1 ± 1.3 | |||
| Vigor | −4.9 ± 3.9* | −2.8 ± 5.9* | +0.4 ± 2.6 | |||
|
Total Fatigue |
+15.2 ± 12.2* |
+8.9 ± 20.3* |
+6.2 ± 7.4* |
|||
| |
Between-session change difference |
|||||
| |
01-May |
05-Oct |
01-Oct |
|||
| General | −2.3 ± 7.3 | −2.5 ± 7.0 | −4.8 ± 4.4† | |||
| Physical | +0.1 ± 6.3 | −2.2 ± 5.1 | −2.2 ± 3.9† | |||
| Emotional | +0.3 ± 1.3 | −0.7 ± 2.5 | −0.5 ± 2.3 | |||
| Mental | −0.9 ± 2.5 | +0.5 ± 2.5 | −0.4 ± 2.4 | |||
| Vigor | +2.2 ± 5.4 | +2.0 ± 3.9 | +5.3 ± 3.9† | |||
| Total Fatigue | −6.3 ± 18.1 | −2.7 ± 17.5 | −9.0 ± 9.4† | |||
Note: * difference (P <0.05) within session, † difference (P <0.05) between session 1 and 10.
Figure 1.
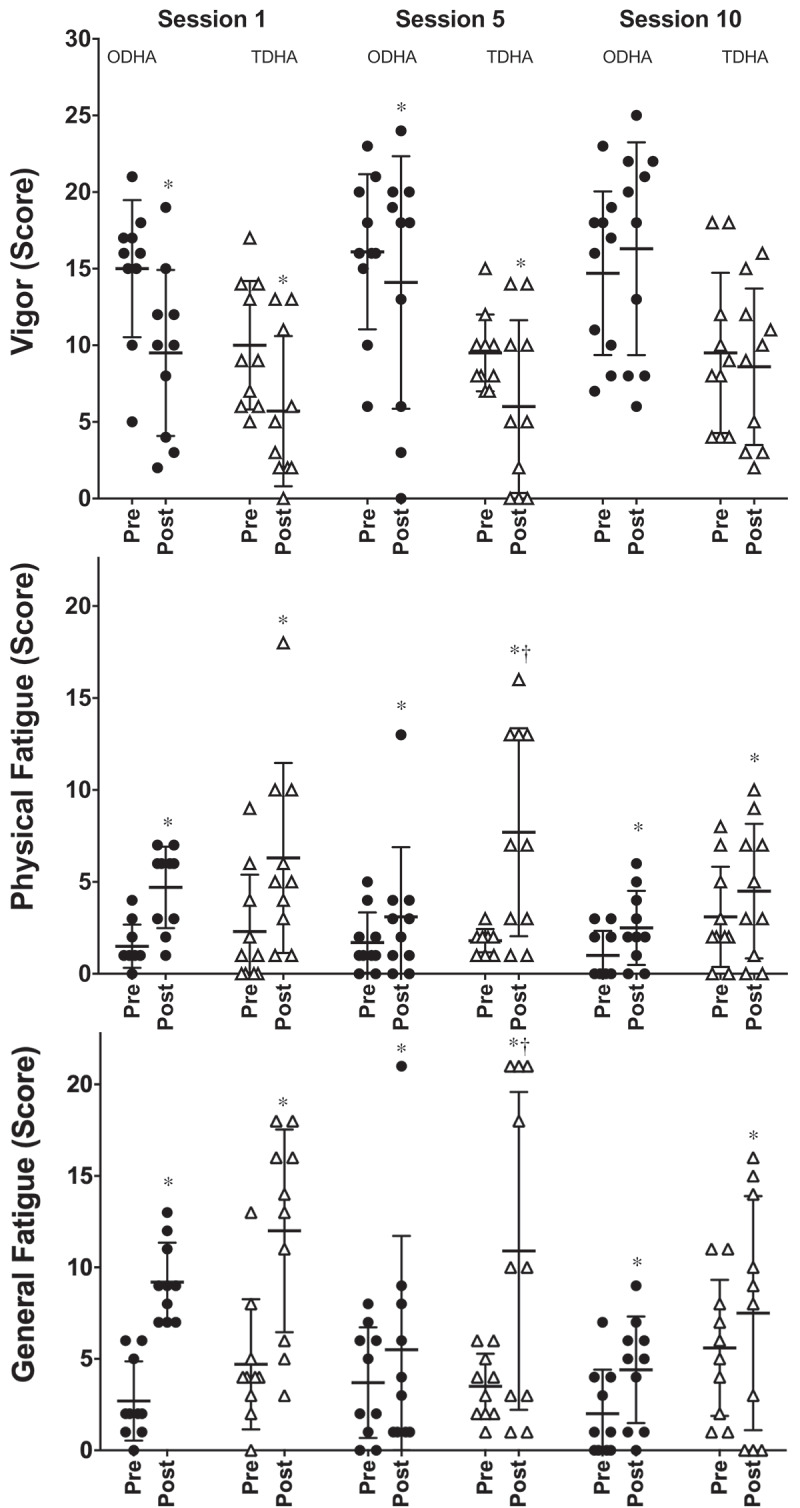
Mean ± SD pre and post session General Fatigue, Physical Fatigue and Vigor scores during ODHA and TDHA for sessions 1, 5 and 10 (* indicates a significant difference [P <0.05] between pre and post session scores, † indicates a significant difference [P <0.05] in the change of fatigue scores from pre to post between ODHA and TDHA).
Pre and post fatigue scores: No differences occurred for pre session fatigue scores (P >0.05) during HA however, there were lower General, Physical and Total Fatigue scores and higher Vigor scores (P <0.05) observed following session 10 compared to session 1 of HA. No differences (P >0.05) between ODHA and TDHA occurred for pre or post scores across each session.
Within-session: General, Physical and Total Fatigue increased from pre to post in session 1, 5 and 10 (P <0.05), whereas, Vigor reduced from pre to post in session 1 and 5 (P <0.05) for HA, ODHA and TDHA. No differences were observed in Emotional or Mental Fatigue (P >0.05). The changes in General, Physical and Total Fatigue scores from pre to post were larger (P <0.05) in session 5 for the TDHA group compared to ODHA, but no differences were found for session 1 or 10 (P >0.05).
Between-session: The pre to post change in General, Physical and Total Fatigue and Vigor were smaller in session 10 compared to session 1 for HA (P <0.05), but no changes were found for Emotional or Mental Fatigue (P >0.05). During ODHA, the pre to post change in General, Physical and Total Fatigue and Vigor were smaller (P <0.05) in session 5 and 10, compared to session 1. Whereas, during TDHA, the pre to post change in General, Physical and Total Fatigue were smaller (P <0.05) for session 10 only compared to session 1 and 5. Pre to post change in Vigor were also lower for session 10 compared to session 1 only for TDHA (P <0.05).
Inflammatory and stress markers
[IL-6] and [cortisol] increased from pre to post for session 1, 5 and 10 of ODHA and TDHA (all P <0.05) as per Willmott et al. [24], but no differences (P >0.05) were found within- or between-groups for the baseline levels or Δ[IL-6] and Δ[cortisol] across sessions 1, 5 or 10.
Relationships between parameters
The ΔGeneral and ΔPhysical Fatigue scores for session 1, 5 and 10 correlated with the Δbody mass, ΔTre, RPEpeak, Δ[IL-6] and Δ[cortisol], but as expected not with exercise intensity data (e.g. total work completed, mean power [W, W.kg−1 or % of V̇O2peak] or mean HR) (Table 4).
Table 4.
Correlation coefficients (r) between the within-session ΔGeneral and ΔPhysical Fatigue scores and, physiological and perceptual data during HA, ODHA and TDHA for sessions 1, 5 and 10.
| ΔGeneral Fatigue score |
ΔPhysical Fatigue score |
|||||
|---|---|---|---|---|---|---|
| |
1 |
5 |
10 |
1 |
5 |
10 |
| n = 20 | ODHA and TDHA Combined | |||||
| ∆body mass (%) | −0.64* | −0.71* | −0.75* | −0.67* | −0.75* | −0.80* |
| ∆Tre (°C) | 0.66* | 0.76* | 0.65* | 0.57* | 0.62* | 0.62* |
| RPEpeak | 0.67* | 0.62* | 0.48* | 0.41 | 0.66* | 0.52* |
| ∆[cortisol] (nmol·L−1) | 0.75* | 0.60* | 0.58* | 0.60* | 0.66* | 0.62* |
| ∆[IL-6] (pg·mL·L−1) | 0.45* | 0.68* | 0.34 | 0.63* | 0.70* | 0.64* |
| Total work (kJ) | 0.19 | 0.13 | 0.21 | 0.21 | 0.14 | 0.04 |
| Mean power (W) | 0.12 | 0.15 | 0.06 | 0.1 | 0.1 | 0.02 |
| Mean HR (b·min−1) |
0.17 |
0.13 |
0.13 |
0.13 |
0.01 |
0.22 |
| n = 10 |
ODHA |
|||||
| ∆body mass (%) | −0.37 | −0.76* | −0.61* | −0.05 | −0.81* | −0.73* |
| ∆Tre (°C) | 0.4 | 0.76* | 0.36 | −0.1 | 0.53 | 0.5 |
| RPEpeak | 0.33 | 0.74* | 0.32 | 0.11 | 0.73* | 0.11 |
| ∆[cortisol] (nmol·L−1) | 0.67* | 0.45 | 0.57* | 0.55* | 0.63* | 0.77* |
| ∆[IL-6] (pg·mL·L−1) | 0 | 0.64* | 0.1 | 0.29 | 0.63* | 0.12 |
| Total work (kJ) | 0.05 | 0.1 | 0.21 | 0.2 | 0.1 | 0.22 |
| Mean power (W) | 0.25 | 0.04 | 0.09 | 0.12 | 0.12 | 0.32 |
| Mean HR (b·min−1) |
0.06 |
0.19 |
0.14 |
0.17 |
0.14 |
0.35 |
| n = 10 |
TDHA |
|||||
| ∆body mass (%) | −0.75* | −0.54* | −0.89* | −0.84* | −0.62* | −0.86* |
| ∆Tre (°C) | 0.78* | 0.78* | 0.84* | 0.79* | 0.75* | 0.66* |
| RPEpeak | 0.92* | 0.63* | 0.58* | 0.57* | 0.69* | 0.71* |
| ∆[cortisol] (nmol·L−1) | 0.82* | 0.74* | 0.62* | 0.66* | 0.73* | 0.59* |
| ∆[IL-6] (pg·mL·L−1) | 0.57* | 0.65* | 0.55* | 0.71* | 0.71* | 0.84* |
| Total work (kJ) | 0.28 | 0.16 | 0.2 | 0.21 | 0.2 | 0.11 |
| Mean power (W) | 0.27 | 0.21 | 0.03 | 0.11 | 0.13 | 0.19 |
| Mean HR (b·min−1) | 0.2 | 0.14 | 0.12 | 0.19 | 0.17 | 0.28 |
Note: *P <0.05. Highlighted moderate-correlations (r = >0.5)
For combined HA data (n = 20), significant models (all P <0.001) from stepwise multiple regression analysis predicted ΔGeneral Fatigue scores for session 1 (r2 = 0.69: Δ[cortisol] and ΔTre) and 5 (r2 = 0.84: ΔTre, RPEpeak and Δ[cortisol]), and; ΔPhysical Fatigue scores for session 1 (r2 = 0.59: Δbody mass and Δ[IL-6]), 5 (r2 = 0.83: Δbody mass, Δ[cortisol] and RPEpeak) and 10 (r2 = 0.85: Δbody mass, Δ[IL-6] and RPEpeak). Significant models (all P <0.05) were also found for ODHA, which predicted; ΔGeneral Fatigue scores for session 1 (r2 = 0.75: Δ[cortisol] and ΔTre) and 5 (r2 = 0.83: ΔTre and Δbody mass), and; ΔPhysical Fatigue scores for session 5 (r2 = 0.97: Δbody mass, Δ[IL-6] and Δ[cortisol]). Likewise, a significant model (P <0.001) was found for TDHA, predicting; ΔGeneral Fatigue scores for session 1 (r2 = 0.94: RPEpeak and Δ[cortisol]) (full data is displayed in supplemental material).
Discussion
This study investigated the acute sensations of fatigue to an initial exercise heat stress session, and then investigated these responses following STHA and MTHA, as well as between ODHA and TDHA protocols. Our first aim was to describe changes in sensations of fatigue following acute exercise-heat stress. In line with our first hypothesis, General and Physical Fatigue scores increased, and Vigor scores decreased following session 1 of HA. Our second aim was to understand whether isothermic HA (irrespective of training frequency) would reduce sensations of fatigue. In agreement with our hypothesis, our data displays smaller within-session changes in General and Physical Fatigue scores following 10 sessions of HA, but not 5, thus supporting our hypothesis and reaffirming MTHA is both effective at inducing greater physiological adaptations and attenuates the increased sensations of fatigue reported during acute exercise-heat stress. Our third aim was to investigate whether training frequency influenced sensations of fatigue. Contrary to our third hypothesis, ODHA conferred smaller within-session changes in perceived fatigue following 5 and 10 sessions of HA, in comparison to non-consecutive TDHA, where lesser changes were only apparent after 10 sessions. Although lower scores in the sensation of fatigue occurred following STHA (ODHA only) and MTHA (both ODHA and TDHA), our results indicate an increased perceived fatigue is sustained during early stages of HA if completed twice-daily. Finally, our fourth aim was to explore the predictors of perceived fatigue, whereby, in agreement with our hypothesis, moderate-strong correlations are found between increased physiological strain (e.g. ∆Tre and ∆body mass) and ∆General and ∆Physical Fatigue scores. As ODHA and TDHA provide comparable heat adaptations, biomarker responses, and aerobic performance improvements [24], should practitioners wish to utilize the flexible non-consecutive TDHA approach, wellness monitoring (e.g. perceived fatigue) and recovery strategies (e.g. cooling) may be necessary. This may assist with the prevention of cumulative perceived fatigue and/or over-reaching responses, especially within the first 5 sessions of TDHA.
Overview of the sensations of fatigue
Acute
As expected during session 1 of HA, General, Physical and Total Fatigue scores increased, yet no between-group differences transpired. The increased sensations of fatigue within an acute exercise-heat stress exposure (General: +7 ± 4, Physical: +4 ± 4 and Total Fatigue: +15 ± 12) agree with previous findings from the first of four HA sessions (+6 ± 7, +3 ± 3 and +13 ± 15, respectively [10]) and are largely dependent upon the physiological strain experienced.
Chronic
Whilst STHA induced adaptation (Table 1), it was ineffective in reducing the degree of perceived fatigue experienced in this timescale when combing data from both HA groups (Table 2). However, when investigating HA protocols independently, ODHA exhibited smaller changes in perceived fatigue (i.e. General, Physical and Total Fatigue) following 5 sessions (i.e. STHA), thus confirming previous findings within ultra-marathon runners [10], and also, after 10 sessions (i.e. MTHA) compared to session 1 (Table 3). Interestingly, the within-session change in fatigue scores during ODHA were lower compared to TDHA, with reductions during TDHA only found following session 10 (Table 3). Nonetheless, the sensations of fatigue were lower following MTHA when implementing ODHA, in agreement with previous literature [9,48], and during non-consecutive TDHA, although between-group differences remain in the time-scale for perceptual improvements. Therefore, whilst ODHA and TDHA induce comparable physiological adaptations and exercise performance improvements [24], distinct differences arise in time-scales for improved sensations of fatigue. Interestingly, this is despite both HA groups completing the same weekly “dose” of HA (e.g. exposure time [300-min·week−1] and frequency [5-sessions·week−1]) and may be partly explained by recovery time during interventions and/or the inter-individual variability within the sensations of fatigue [24].
Table 3.
Mean ± SD pre, post and changes in the sensations of fatigue for sessions 1, 5 and 10 during ODHA and TDHA.
| ODHA | TDHA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group |
1 |
5 |
10 |
1 |
5 |
10 |
||||||
| Session |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
| General | 2.7 ± 2.2 | 9.2 ± 2.1* | 3.7 ± 3.0 | 5.5 ± 6.2* | 2.0 ± 2.4 | 4.4 ± 2.9* | 4.7 ± 3.6 | 12.0 ± ± 5.5* | 3.5 ± 1.8 | 10.9 ± 8.7* | 5.6 ± 3.7 | 7.5 ± 6.4* |
| Physical | 1.5 ± 1.2 | 4.7 ± 2.2* | 1.7 ± 1.6 | 3.1 ± 3.8* | 1.0 ± 1.3 | 2.5 ± 2.0* | 2.3 ± 3.1 | 6.3 ± 5.2* | 1.8 ± 0.6 | 7.7 ± 5.7* | 3.1 ± 2.7 | 4.5 ± 3.7* |
| Emotional | 1.7 ± 2.4 | 2.0 ± 2.4 | 0.4 ± 1.0 | 1.0 ± 1.3 | 0.5 ± 0.8 | 0.3 ± 0.5 | 1.6 ± 2.8 | 1.5 ± 3.1 | 0.5 ± 0.5 | 0.6 ± 0.5 | 2.6 ± 3.7 | 2.1 ± 3.7 |
| Mental | 2.2 ± 2.3 | 2.5 ± 2.6 | 1.1 ± 1.9 | 0.9 ± 2.0 | 0.3 ± 0.7 | 0.1 ± 0.3 | 1.4 ± 2.9 | 1.7 ± 2.8 | 1.0 ± 1.4 | 0.0 ± 0.0 | 2.0 ± 2.4 | 2.0 ± 3.6 |
| Vigor | 15.0 ± 4.5 | 9.5 ± 5.4* | 16.1 ± 5.1 | 14.1 ± 8.2* | 14.7 ± 5.3 | 16.3 ± 6.9 | 10.0 ± 4.2 | 5.7 ± 4.9* | 9.5 ± 2.5 | 6.0 ± 5.6* | 9.5 ± 5.2 | 8.6 ± 5.1 |
| Total Fatigue | −6.4 ± 7.0 | 8.9 ± 8.5* | −6.5 ± 7.6 | −3.6 ± 18.5* | −9.5 ± 5.5 | −3.7 ± 8.4* | 0.8 ± 9.7 | 15.8 ± 14.6* | −1.7 ± 2.5 | 13.2 ± 20.0* | 2.5 ± 10.1 | 9.1 ± 14.1* |
| |
Within-session change |
|||||
|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 1 | 5 | 10 | |
| General | +6.5 ± 3.3* | +1.8 ± 6.7*∂ | +2.4 ± 4.1* | +7.3 ± 5.4* | +7.4 ± 7.4* | +1.9 ± 5.9* |
| Physical | +3.2 ± 2.5* | +1.4 ± 4.1*∂ | +1.5 ± 1.2* | +4.0 ± 5.8* | +5.9 ± 5.6* | +1.4 ± 3.7* |
| Emotional | +0.3 ± 1.9 | +0.6 ± 1.5 | −0.2 ± 0.4 | −0.1 ± 1.4 | +0.1 ± 0.9 | −0.5 ± 3.0 |
| Mental | +0.3 ± 1.8 | −0.2 ± 2.5 | −0.2 ± 0.6 | +0.3 ± 1.6 | −1.0 ± 1.4 | 0.0 ± 1.7 |
| Vigor | −5.5 ± 4.3* | −2.0 ± 5.8* | +1.6 ± 2.4 | −4.3 ± 3.6* | −3.5 ± 6.3* | −0.9 ± 2.1 |
| Total Fatigue | +15.3 ± 10.5* | +2.9 ± 17.8*∂ | +5.8 ± 7.2* | +15.0 ± 14.3* | +14.9 ± 21.7* | +6.6 ± 7.9* |
| |
Between-session change difference |
|||||
|---|---|---|---|---|---|---|
| 01-May | 05-Oct | 01-Oct | 01-May | 05-Oct | 01-Oct | |
| General | −4.7 ± 6.6‡∂ | +0.6 ± 6.6∂ | −4.1 ± 3.3† | +0.1 ± 7.5 | −5.5 ± 6.3# | −5.4 ± 5.3† |
| Physical | −1.8 ± 5.8‡ | +0.1 ± 4.1∂ | −1.7 ± 2.7† | +1.9 ± 6.5 | −4.5 ± 5.1# | −2.6 ± 4.9† |
| Emotional | +0.3 ± 1.5 | −0.8 ± 1.7 | −0.5 ± 2.1 | +0.2 ± 1.1 | −0.6 ± 3.1 | −0.4 ± 2.5 |
| Mental | −0.5 ± 2.1 | 0.0 ± 3.1 | −0.5 ± 2.3 | −1.3 ± 1.8 | +1.0 ± 1.9 | −0.3 ± 2.6 |
| Vigor | +3.5 ± 5.5‡ | +1.8 ± 4.6 | +7.1 ± 2.7† | +0.8 ± 5.2 | +0.1 ± 2.9 | +3.4 ± 4.2† |
| Total Fatigue | −12.4 ± 17.1‡∂ | +2.9 ± 16.7∂ | −9.5 ± 7.1† | −0.1 ± 17.7 | −8.3 ± 17.3# | −8.4 ± 11.6† |
Note: * difference (P <0.05) within session, † difference (P <0.05) between session 1 and 10, and ‡ difference (P <0.05) between session 1 and 5, # difference (P <0.05) between session 5 and 10, and ∂ difference (P <0.05) between ODHA and TDHA.
The sensations of fatigue are complex and central in origin, yet likely influenced by thermal and non-thermal feedback from the periphery [49, 41, 50, 4, 51, 52]. This is in keeping with the contribution of skin temperature to TSS, reflecting the relative magnitude of perceived ambient temperature [53] and TC reflecting the perceptual indifference between Tre and the environmental conditions [54,51]. Therefore, improvements in the sensations of fatigue are in part, likely explained by the repeated experience of exercise-heat stress [9], and conceivably, the induced physiological (i.e. reductions in resting Tre and sweat setpoint, and augmented WBSL) and perceptual adaptations (i.e. lower TSS and RPE, and improved TC [Table 1]) [24]. The combination of these multi-factored reductions in perceived fatigue, exertion, thermal sensation and improved comfort are intriguing findings, particularly considering the physiological strain (e.g. ∆Tre), and total work completed and exercise intensity (e.g. mean power), were maintained throughout HA. Moreover, the specific subscales of the sensations of fatigue [5], indicate lower reported whole-body muscle aches and headache/syncope symptoms (i.e. Physical Fatigue), alongside lessened feelings of lethargy and tiredness (e.g. General Fatigue). As such, the consistent accumulation of these signs and symptoms of fatigue may lead to illnesses, maladaptation and/or over-reaching/training effects [11,13]. This is especially likely if individuals are not monitored frequently for health status [55]. Interestingly, no alterations appeared within Emotional nor Mental Fatigue scores throughout both protocols, suggesting a different mechanism to that which leads to impaired cognitive performance (e.g. attention tasks) in heat stress [56].
Predictors of the sensations of fatigue
Several potentially important contributors to changes in fatigue scores during HA were identified through Spearman’s rank-order correlations (Table 4) and stepwise multiple regression analysis (supplemental material) including; ∆body mass, ∆Tre, RPEpeak, ∆[cortisol] and ∆[IL-6]. However, it is acknowledged data should be interpreted with caution as some of the contributing variables are likely to be interlinked across physiological systems. Nonetheless, moderate-strong correlations were observed between ∆body mass and, ∆General and ∆Physical Fatigue scores (Table 4), potentially indicating that larger WBSL influences perceived fatigue [as per previously identified relationships by 8]. Consequently, dehydration, which has been shown to increase ∆[cortisol] during HA when fluid intake is restricted [27,57], may occur alongside feelings of stress [58] and impair cognitive performance [8,59]. As such our data indicates that heightened WBSL may induce perceived fatigue, especially during the initial stages of HA, which could be counterintuitive to preparation strategies. The relevance of ad libitum drinking vs. progressive dehydration on perceived fatigue during HA should therefore be examined.
Correlations were also observed between ∆[IL-6] and ∆Physical Fatigue scores during TDHA (Table 4), supporting indications that IL-6 may form one pathway that induces perceived fatigue [30] and may interfere with the central nervous system through the proposed neuro-inflammation model [60]. A likely reason for this only appearing during TDHA is the shorter-duration of recovery between sessions [61,62], as no between- or within-group differences in resting or ∆[IL-6] were observed. Nonetheless, TDHA provides ~6-h recovery during “HA specific days” (i.e. between sessions 1–2, 3–4, 6–7 and 8–9) followed by ~39-h between non-consecutive HA sessions (i.e. between session 2–3, 4–5, 7–8 and 9–10), whereas, ODHA offers ~23-h of consistent recovery. As such, varying recovery times are a likely contributor to larger sensations of fatigue [62], especially within STHA time-scales, as physiological data for each session did not differ between-groups [24].
Finally, relationships between ∆Tre and, ∆General and ∆Physical Fatigue scores were observed for TDHA (Table 4 and supplemental material), indicating within- and/or between-group variation, as no differences occurred in Tre responses between HA protocols (Table 1). With each group completing the same weekly “dose” of HA and perceived fatigue being assessed at the same time-of-day (i.e. session 1, 5 and 10 at 08:30 and 10:30-h), the TDHA group may have had a greater sensory association with their Tre (and plausibly TC, as no adaptation occurred following STHA [∆0 ± 1], although Tre reduced [∆-0.22 ± 0.17°C] [Table 1]). This may also explain the unaltered perceived fatigue scores in session 5 during TDHA. Nonetheless, whilst attenuated changes in perceived fatigue scores for session 10 were observed for both HA protocols, physiological signals from Tre continued to be an indicator of perceived fatigue during TDHA. Our findings agree with chronic heat exposure data within an occupational setting [25], but contrast data from STHA [10] and MTHA studies [9], which indicated heat acclimated individuals were less affected by temperature modulation, resulting in lower perceived fatigue. In agreement with the sensory association hypothesis for Tre [25] and disassociation of Tre signals following STHA [10], an intriguing interpretation of our data indicates a potential sensory associated learning and/or training effect during HA, where mean Tre was maintained yet larger sensations of fatigue were not observed. This is likely due to the repeated exercise-heat stress experience [9] and induced heat adaptations [24].
Application
An understanding of the perceptual responses and subsequent time-course for adaptations is important for those prescribing HA, allowing perceived fatigue to be somewhat predicted and potentially mitigated. As such, our research supports anecdotal evidence of increased tiredness and lethargy following exercise-heat stress [10], which is important to consider when prescribing HA, such as that for the Tokyo 2020 Olympic and Paralympic Games [1,20]. The cumulative effect of combined stressors and progressive physiological strain (e.g. controlled-hyperthermia, dehydration and/or biomarker responses) may induce negative and augmented sensations of fatigue within the initial HA session [10], thus affecting adherence and/or performance during subsequent HA sessions. However, chronic exposures of repeated exercise-heat stress can mitigate prevailing detriments [63], with perceptual adaptations that may in turn, aid endurance performance in the heat to a greater extent than in cool conditions [46]. Particular attention to the sensations of fatigue is necessary during STHA, which may be more preferable to athletes [64] preparing for Tokyo 2020, who must balance HA requirements and a need to maintain training quality. As such, whilst post-HA session recovery strategies (cooling interventions [e.g. cold-water immersion]) [65,66], seem counterintuitive (e.g. reducing the extended time spent with an augmented Tre), they may help athletes feel, sleep and/or perform better during the subsequent HA session and requires further investigation.
Limitations
It is acknowledged that the absence of a control group exercising in temperate conditions, the lack of female participants and recreationally active, rather than well-trained athletes as participants are limitations of this study. Follow up data should examine responses in these groups.
Conclusion
Acute exercise-heat stress increases the sensations of fatigue, which can be attenuated by implementing chronic HA strategies. Whilst comparative heat adaptations followed ODHA and non-consecutive TDHA, the increased sensation of fatigue during TDHA was only reduced after 10 sessions, whereas this response occurred by session 5 of ODHA. Monitoring wellness and/or undertaking recovery strategies may be considered when utilizing flexible TDHA interventions to optimize heat adaptations and exercise performance, especially within the initial stages.
Acknowledgments
The authors would like to thank all the participants who volunteered for this study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Gerrett N, Kingma BR, Sluijter R, et al. Ambient conditions prior to Tokyo 2020 olympic and paralympic games: considerations for acclimation or acclimatization strategies. Front Physiol. 2019;10:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Howe AS, Boden BP.. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384–1395. [DOI] [PubMed] [Google Scholar]
- [3].Guy JH, Deakin GB, Edwards AM, et al. Adaptation to hot environmental conditions: an exploration of the performance basis, procedures and future directions to optimise opportunities for elite athletes. Sports Med. 2015;45(3):303–311. [DOI] [PubMed] [Google Scholar]
- [4].Borg G. Borg’s perceived exertion and pain scales. Human Kinetics. 1998:1–104. [Google Scholar]
- [5].Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donovan KA, Stein KD, Lee M, et al. Systematic review of the multidimensional fatigue symptom inventory-short form. Support Care Cancer. 2015;23(1):191–212. [DOI] [PubMed] [Google Scholar]
- [7].Tamm M, Jakobson A, Havik M, et al. The compression of perceived time in a hot environment depends on physiological and psychological factors. Q J Exp Psychol. 2014;67(1):197–208. [DOI] [PubMed] [Google Scholar]
- [8].McMorris T, Swain J, Smith M, et al. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol. 2006;61(2):204–215. [DOI] [PubMed] [Google Scholar]
- [9].Tamm M, Jakobson A, Havik M, et al. Effects of heat acclimation on time perception. Int J Psychophysiol. 2015;95(3):261–269. [DOI] [PubMed] [Google Scholar]
- [10].Willmott AG, Hayes M, Waldock KA, et al. Short-term heat acclimation prior to a multi-day desert ultra-marathon improves physiological and psychological responses without compromising immune status. J Sports Sci. 2017;35(22):2249–2256. [DOI] [PubMed] [Google Scholar]
- [11].Buchheit M, Kuitunen S, Voss SC, et al. Physiological strain associated with high-intensity hypoxic intervals in highly trained young runners. J Strength Cond Res. 2012;26(1):94–105. [DOI] [PubMed] [Google Scholar]
- [12].Meeusen R, Duclos M, Foster C, et al. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European college of sport science and the American college of sports medicine. Med Sci Sports Exerc. 2013;45(1):186–205. [DOI] [PubMed] [Google Scholar]
- [13].Peiffer JJ, Abbiss CR. Influence of environmental temperature on 40 km cycling time-trial performance. Int J Sports Physiol Perform. 2011;6(2):208–220. [DOI] [PubMed] [Google Scholar]
- [14].Halson SL. Monitoring training load to understand fatigue in athletes. Sports Med. 2014;44(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saw AE, Main LC, Gastin PB. Monitoring athletes through self-report: factors influencing implementation. J Sports Sci Med. 2015;14(1):137. [PMC free article] [PubMed] [Google Scholar]
- [16].Gallo TF, Cormack SJ, Gabbett TJ, et al. Pre-training perceived wellness impacts training output in Australian football players. J Sports Sci. 2016;34(15):1445–1451. [DOI] [PubMed] [Google Scholar]
- [17].Sawka MN, Leon LR, Montain SJ, et al. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 2011;1:1883–1928. [DOI] [PubMed] [Google Scholar]
- [18].Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25:6–19. [DOI] [PubMed] [Google Scholar]
- [19].Pryor JL, Johnson EC, Roberts WO, et al. Application of evidence-based recommendations for heat acclimation: individual and team sport perspectives. Temperature. 2019a;6(1):37–49. doi: 10.1080/23328940.2018.1516537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Griggs KE, Stephenson BT, Price MJ, et al. Heat-related issues and practical applications for Paralympic athletes at Tokyo 2020. Temperature. 2019;1–21. doi: 10.1080/23328940.2019.1617030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tyler CJ, Reeve T, Hodges GJ, et al. The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 2016;46(11):1699–1724. [DOI] [PubMed] [Google Scholar]
- [22].Daanen HA, Racinais S, Periard JD. Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 2018;48(2):409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tian Z, Zhu N, Zheng G, et al. Experimental study on physiological and psychological effects of heat acclimatization in extreme hot environments. Build Environ. 2011;46(10):2033–2041. [Google Scholar]
- [24].Willmott AG, Hayes M, James CA, et al. Once‐and twice‐daily heat acclimation confer similar heat adaptations, inflammatory responses and exercise tolerance improvements. Physiol Rep. 2018a;6(24):e13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Watt PW, Willmott AG, Maxwell NS, et al. Physiological and psychological responses in fire instructors to heat exposures. J Therm Biol. 2016;58:106–114. [DOI] [PubMed] [Google Scholar]
- [26].Guy J, Pyne DB, Deakin G, et al. Acclimation training improves endurance cycling performance in the heat without inducing endotoxemia. Front Physiol. 2016;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Costello JT, Rendell RA, Furber M, et al. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine. 2018;110:277–283. [DOI] [PubMed] [Google Scholar]
- [28].Pyne DB, Guy JH, Edwards AM. Managing heat and immune stress in athletes with evidence-based strategies. Int J Sports Physiol Perform. 2014;9(5):744–750. [DOI] [PubMed] [Google Scholar]
- [29].Costa RJ, Gaskell SK, McCubbin A. Exertional-heat stress associated gastrointestinal perturbations during olympic sports: management strategies for athletes preparing and competing in the 2020 Tokyo Olympic Games. Temperature. 2019. doi: 10.1080/23328940.2019.1597676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Robson-Ansley PJ, Milander LD, Collins M, et al. Acute interleukin-6 administration impairs athletic performance in healthy, trained male runners. Can J Appl Physiol. 2004;29(4):411–418. [DOI] [PubMed] [Google Scholar]
- [31].World Medical Association . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- [32].Hayes M, Castle PC, Ross EZ, et al. The influence of hot humid and hot dry environments on intermittent-sprint exercise performance. Int J Sports Physiol Perform. 2014;9(3):387–396. [DOI] [PubMed] [Google Scholar]
- [33].Willmott AGB, Hayes M, Dekerle J, et al. The reliability of a heat acclimation state test prescribed from metabolic heat production intensities. J Therm Bio. 2015;53:38–45. [DOI] [PubMed] [Google Scholar]
- [34].Taylor NAS. Human Heat Adaptation. Compr Physiol. 2014;4:325–365. [DOI] [PubMed] [Google Scholar]
- [35].Gibson OR, Willmott AG, James C, et al. Power relative to body mass best predicts change in core temperature during exercise-heat stress. J Strength Cond Res. 2017;31(2):403–414. [DOI] [PubMed] [Google Scholar]
- [36].Gibson OR, Mee JA, Tuttle JA, et al. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol. 2015;49–50:55–65. [DOI] [PubMed] [Google Scholar]
- [37].Neal RA, Corbett J, Massey HC, et al. Effect of short‐term heat acclimation with permissive dehydration on thermoregulation and temperate exercise performance. Scand J Med Sci Sports. 2016a;26(8):875–884. [DOI] [PubMed] [Google Scholar]
- [38].Sawka MN, Burke LM, Eichner ER, et al. American college of sports medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–390. [DOI] [PubMed] [Google Scholar]
- [39].Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6(3):143–152. [DOI] [PubMed] [Google Scholar]
- [40].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- [41].Toner MM, Drolet LL, Pandolf KB. Perceptual and physiological responses during exercise in cool and cold water. Percept Mot Skills. 1986;62:211–220. [DOI] [PubMed] [Google Scholar]
- [42].Zhang H, Huizenga C, Arens E, et al. Thermal sensation and comfort in transient non-uniform thermal environments. Eur J Appl Physiol. 2004;92:728–733. [DOI] [PubMed] [Google Scholar]
- [43].Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–248. [DOI] [PubMed] [Google Scholar]
- [44].Willmott AG, Gibson OR, James CA, et al. Physiological and perceptual responses to exercising in restrictive heat loss attire with use of an upper-body sauna suit in temperate and hot conditions. Temperature. 2018b;5(2):162–174. doi: 10.1080/23328940.2018.1426949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gibson OR, Dennis A, Parfitt T, et al. Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones. 2014;19(3):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].James CA, Hayes M, Willmott AG, et al. Defining the determinants of endurance running performance in the heat. Temperature. 2017a;4(3):314–329. doi: 10.1080/23328940.2017.1333189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hopkins WG A scale of magnitudes for effect statistics. A new view of statistics. 2002. [Access 2019 Feb 1]. Available from: www.sportsci.org/resource/stats/effectmag.html..
- [48].Pryor JL, Pryor RR, Vandermark LW, et al. Intermittent exercise-heat exposures and intense physical activity sustain heat acclimation adaptations. J Sci Med Sport. 2019b;22(1):117–122. [DOI] [PubMed] [Google Scholar]
- [49].Gagge AP, Stolwijk JAJ, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res. 1969;2(3):209–229. [DOI] [PubMed] [Google Scholar]
- [50].Bainbridge FA. The physiology of muscular exercise. London: Longmans, Green and Co; 1919. p. 177. [Google Scholar]
- [51].Flouris AD, Schlader ZJ. Human behavioral thermoregulation during exercise in the heat. Scand J Med Sci Sports. 2015;25(1):52–64. [DOI] [PubMed] [Google Scholar]
- [52].St. Clair Gibson A, Baden DA, Lambert MI, et al. The conscious perception of the sensation of fatigue. Sports Med. 2003;33:167–176. [DOI] [PubMed] [Google Scholar]
- [53].Attia M. Thermal pleasantness and temperature regulation in man. Neurosci Biobehav Rev. 1984;8(3):335–342. [DOI] [PubMed] [Google Scholar]
- [54].Mercer J. Glossary of terms for thermal physiology. Third edition. Revised by the commission for thermal physiology of the international union of physiological sciences (IUPS thermal commission). Jpn J Physiol. 2001;51:245–280. [PubMed] [Google Scholar]
- [55].Borresen J, Lambert MI. The quantification of training load, the training response and the effect on performance. Sports Med. 2009;39(9):779–795. [DOI] [PubMed] [Google Scholar]
- [56].Qian S, Li M, Li G, et al. Environmental heat stress enhances mental fatigue during sustained attention task performing: evidence from an ASL perfusion study. Behav Brain Res. 2015;280:6–15. [DOI] [PubMed] [Google Scholar]
- [57].Neal RA, Massey HC, Tipton MJ, et al. Effect of permissive dehydration on induction and decay of heat acclimation, and temperate exercise performance. Front Physiol. 2016b;7:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vedhara K, Hyde J, Gilchrist ID, et al. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology. 2000;25:535–549. [DOI] [PubMed] [Google Scholar]
- [59].Hoffman JR, Maresh CM, Armstrong LE, et al. Effects of hydration state on plasma testosterone, cortisol and catecholamine concentrations before and during mild exercise at elevated temperature. Eur J Appl Physiol Occup Physiol. 1994;69(4):294–300. [DOI] [PubMed] [Google Scholar]
- [60].Vargas NT, Marino F. A neuroinflammatory model for acute fatigue during exercise. Sports Med. 2014;44(11):1479–1487. [DOI] [PubMed] [Google Scholar]
- [61].Ronsen O, Haugen O, Hallen J, et al. Residual effects of prior exercise and recovery on subsequent exercise-induced metabolic responses. E J Appl Physiol. 2004;92(4–5):498–507. [DOI] [PubMed] [Google Scholar]
- [62].Ronsen O, Lea T, Bahr R, et al. Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J Appl Physiol. 2002;92(6):2547–2553. [DOI] [PubMed] [Google Scholar]
- [63].James CA, Richardson AJ, Watt PW, et al. Short-term heat acclimation improves the determinants of endurance performance and 5-km running performance in the heat. Appl Physiol Nutr Metab. 2017b;42(3):285–294. [DOI] [PubMed] [Google Scholar]
- [64].Garrett AT, Creasy R, Rehrer NJ, et al. Effectiveness of short-term heat acclimation for highly trained athletes. E J Appl Physiol. 2012;112(5):1827–1837. [DOI] [PubMed] [Google Scholar]
- [65].Skein M, Wingfield G, Gale R, et al. Sleep quantity and quality during consecutive day heat training with the inclusion of cold-water immersion recovery. J Therm Bio. 2018;74:63–70. [DOI] [PubMed] [Google Scholar]
- [66].Vaile J, Halson S, Gill N, et al. Effect of hydrotherapy on recovery from fatigue. Int J Sports Med. 2008;29(07):539–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hopkins WG A scale of magnitudes for effect statistics. A new view of statistics. 2002. [Access 2019 Feb 1]. Available from: www.sportsci.org/resource/stats/effectmag.html..


