Abstract
Background
High-intensity statin is recommended in high-risk type 2 diabetes (T2D); however, statin dose dependently increases the risk of developing new-onset diabetes, can potentially worsen glycemic control in T2D, and may cause cognitive impairment. This study aimed to investigate the effect of statin intensification on glucose homeostasis and cognitive function in T2D.
Materials and Methods
T2D patients who were taking simvastatin ≤20 mg/day were randomized to continue taking the same dosage of simvastatin (low-dose simvastatin group; LS, n=63) for 12 weeks, or to change to atorvastatin 40 mg/day for 6 weeks, and if tolerated, atorvastatin was increased to 80 mg/day for 6 weeks (high-dose atorvastatin group; HS, n=62). Fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), plasma insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and of β-cell function (HOMA-B), cognitive functions using Montreal Cognitive Assessment (MoCA), and Trail Making Test (TMT) were assessed at baseline, 6 weeks, and 12 weeks.
Results
Mean age of patients was 58.8±8.9 years, and 72% were female. Mean baseline FPG and HbA1c were 124.0±27.5 mg/dl and 6.9±0.8%, respectively. No differences in baseline characteristics between groups were observed. Change in HbA1c from baseline in the LS and HS groups was −0.1% and +0.1% (p=0.03) at 6 weeks, and −0.1% and +0.1% (p=0.07) at 12 weeks. There were no significant differences in FPG, fasting plasma insulin, HOMA-B, HOMA-IR, MoCA score, or TMT between groups at 6 or 12 weeks.
Conclusion
Switching from low-dose simvastatin to high-dose atorvastatin in T2D resulted in a slight increase in HbA1c (0.1%) without causing cognitive decline.
Keywords: high-intensity statin, glucose homeostasis, cognitive function, type 2 diabetes, T2D
Introduction
Statin was shown to reduce cardiovascular events in patients with type 2 diabetes (T2D) when compared with placebo treatment.1 In addition, a meta-analysis of statin trials demonstrated the efficacy of statin therapy for primary and secondary prevention of cardiovascular diseases2 compared to placebo, including in patients with diabetes.3 Intensive plasma LDL-cholesterol (LDL-C) lowering with high-intensity statins resulted in a significantly greater reduction in cardiovascular events compared to low-dose statins.4,5 Therefore, high-intensity statins are recommended for high-risk T2D patients in most guidelines.6–8
Another meta-analysis of statin trials found statin use to be associated with a 9% increased risk of developing new-onset diabetes in non-diabetic patients.9 Worsened glycemic control in patients with T2D has also been reported.10,11 A recent meta-analysis of 9 randomized controlled trials that included 9696 type 2 diabetes patients demonstrated the mean glycated hemoglobin (HbA1c) level of patients on statin (pravastatin 10–20 mg/d, simvastatin 40 mg/d, or atorvastatin 10–80 mg/d) treatment to be 0.12% higher than those without statin treatment, which suggests that statin resulted in worsened glycemic control in patients with T2D as compared with no statin treatment.10 The mechanisms by which statins affect glucose homeostasis are still unclear. Increased insulin resistance or impaired β-cell function are the potential mechanisms of increased plasma glucose levels and the risk of developing new-onset diabetes.12 In February 2012, the US Food and Drug Administration (FDA) announced that statins can increase blood sugar and HbA1c levels.13 The risk of new-onset diabetes from statin was higher with intensive doses of statins than with low-dose statins.14,15 However, it is still unclear whether deterioration in glucose control would occur after statin intensification in T2D patients. Moreover, head-to-head comparison studies investigating the effects of different statins and different statin intensities on glucose homeostasis in patient with T2D are limited. In addition, most of the previous trials did not report the variations in the utilization of antidiabetic drugs, which might influence the impact of statins on glucose controls in T2D.
The relationship between lowered plasma LDL-C levels and coronary heart disease risk reduction is well established; however, previous study found too low of a plasma LDL-C level to be associated with hemorrhagic stroke,16,17 and may adversely affect cognitive function.18–21 Thus, the US FDA issued a warning that statin use may be associated with transient memory impairment in 2012.13,22 However, it remains unclear whether the adverse effect of statins on cognitive function can be attributed to low plasma LDL-C level or to other effects unique to this class of drugs. The results of systematic reviews and meta-analyses have been inconsistent in their findings relative to the adverse effects of statins on cognition, and the Statin Cognitive Safety Task Force in 2014 concluded that statins are not associated with these adverse effects.23
In an attempt to resolve some of the aforementioned disparities between and among studies, the present study was conducted to assess the effect of switching from low-dose simvastatin to high-dose atorvastatin compared to continuing with low-dose simvastatin on glucose homeostasis and neurocognitive function in patients with T2D.
Materials and Methods
Study Design
This study was a pre-specified analysis of a previously published randomized, open-label study.24 In brief, adult T2D patients who were receiving a stable dose of simvastatin up to 20 mg/day for at least 3 months prior to the start of the study, and who had a plasma LDL-C level less than 100 mg/dl at the time of randomization were enrolled.
Patients having any of the following conditions were excluded: history of dementia, decompensated liver cirrhosis or liver enzymes of ≥3 times above the upper normal range, stage 3 to 5 chronic kidney disease, unexplained elevation of plasma creatine kinase at enrollment, and/or concomitant use of other classes of lipid-lowering agents. Patients who took human insulin injection were excluded from the analysis of glucose homeostasis due to interference with plasma insulin measurement. One patient who had an extremely low plasma insulin level (0.2 µU/mL) was also excluded because a diagnosis of latent adult-onset type 1 diabetes was suspected in that case. The study drugs were discontinued if patients had elevation of liver enzymes or plasma creatine kinase greater than 3 and 5 times the upper normal limits, respectively. The study protocol was approved by the Siriraj Institutional Review Board (COA no. SI 680/2014). The trial was conducted in accordance with the principles described in the Declaration of Helsinki, and all included patients provided written informed consent. This study was registered with the Thai Clinical Trials Registry (trial registration no: TCTR20180812001).
Randomization and Study Treatment
Stratified randomization by age, gender, and baseline HbA1c was performed. Patients were randomized in a 1:1 ratio to continue using the same dosage of simvastatin for 12 weeks (low-dose simvastatin group, LS), or to switch to atorvastatin 40 mg/day for 6 weeks, and if tolerated, atorvastatin would be increased to 80 mg/day for another 6 weeks (high-dose atorvastatin group, HS). Simvastatin used in the LS group was classified as low- to moderate-intensity statins, and the statin used in HS group was classified as high-intensity statins according to the ACC/AHA guideline.
Hypoglycemic agents were unchanged throughout the study period. Fasting plasma glucose (FPG), HbA1c, fasting plasma insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and homeostasis model assessment of β-cell function (HOMA-B) were assessed at baseline, 6 weeks, and 12 weeks. Cognitive function was assessed using the Thai versions of the Montreal Cognitive Assessment (MoCA) test and Trail Making Test, part B (TMT).
Biochemical Analysis and Neurocognitive Function Assessment
A blood sample was collected after a 12-hour overnight fast. Plasma total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) were measured by enzymatic colorimetric method (Roche Diagnostics, Basel, Switzerland). Calculated LDL-C concentration was estimated by Friedewald formula. FPG and HbA1c were measured by enzymatic method and turbidimetric inhibition immunoassay, respectively. Fasting plasma insulin was measured by electrochemiluminescence immunoassay (ELISA). HOMA-IR and HOMA-B were calculated using the HOMA2 calculator, which was downloaded from https://www.dtu.ox.ac.uk/homacalculator/.
Neurocognitive function was assessed by the Thai versions of the Montreal Cognitive Assessment (MoCA) test and the Trail Making Test, part B (TMT) at baseline, 6 weeks, and 12 weeks. The MoCA test evaluates different types of cognitive abilities, including executive function,visuospatial ability, naming, memory, attention, language, abstraction, delayed recall, orientation, and clock-drawing test. MoCA scoring ranges from zero to 30 with a score of 26 or higher generally considered normal.25 The Thai version of the MoCA test was translated from English to Thai language, and was validated in Thai population.26,27 TMT was used to assess a variety of cognitive processes, including attention, visual search and scanning, sequencing and shifting, psychomotor speed, abstraction, flexibility, ability to execute and modify a plan of action, and ability to maintain two trains of thought simultaneously.28 Each participant was asked to draw lines to connect circled numbers and letters in an alternating numeric and alphabetic sequence (ie, 1-A-2-B, etc.) as rapidly as possible. A standard timer was used to record the time that each patient spent to complete the task. The performance of TMT was affected by age and education with a mean score of 80 seconds reported in those with education <12 years at a mean age of 54–59 years old.29
Sample Size Calculation
A previous study of statin trials in T2D including atorvastatin 10–80 mg/day found that HbA1C of statin users was 0.12% higher than those without treatment.10 Using a significance level of 0.05 and a power of 90% to detect the difference in HbA1c of 0.12% with an effect size of 0.6, a total of 60 patients per group were calculated. That sample size was increased by 5% to compensate for patient withdrawal for any reason, so 126 patients were recruited for this study.
Statistical Analysis
Data were analyzed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation for normally distributed variables, or median (interquartile range) for non-normally distributed variables. Change from baseline for each parameter was calculated using the values at 6 or 12 weeks minus the values at enrollment. Percent change from baseline was calculated using the following formula: (values at 6 or 12 weeks–baseline values)/baseline values x 100. Student’s t-test or Mann–Whitney U-test was used to compare differences in variables between two groups. For comparison of qualitative or categorical variables, chi-square test or Fisher’s exact test was used. A formal post hoc analysis was conducted as specified in each domain of cognitive function between 2 groups by repeated measures analysis of variance (ANOVA). Pearson correlation was used to assess correlation between percent changes in plasma LDL-C reduction and changes in neurocognitive function. A two-sided alpha level of 5% was used to indicate statistical significance (p-value <0.05).
Of the 150 patients eligible for enrollment, 127 patients were included in this study. Sixty-four patients were randomized to the LS group, and the other 63 patients were randomized to the HS group. One patient in each group was lost to follow up. One patient moved to the other province, and the other patient was no longer willing to participate in the study. Thus, the data from 63 and 62 patients in the LS and HS groups, respectively, were included in the final analysis. A flow diagram describing the enrollment and randomization protocol is shown in Figure 1. The baseline characteristics of the patients who were excluded were similar to those of patients included in the final analysis.
Figure 1.
Flow diagram of patient enrollment and group allocation.
Results
The mean age of patients was 58.8±8.9 years, and 72% were female. They had a long duration of diabetes, with a median duration of about 8 years. Our study patients had fairly good glycemic control at baseline with a mean FPG of 129.5±37.3 mg/dl, and a mean HbA1c of 7.0±0.9%. The mean baseline FPG and HbA1c were similar between groups (Table 1). The hypoglycemic agents used were also similar between groups. Ninety-seven percent of patients took metformin, and 55.2% of patients were on sulphonylurea. Insulin analogues were prescribed in 7.9% of LS group patients, and in 12.9% of HS group patients. The median dosage of insulin was 12 (12–35) units/day, and 21 (12–26) units/day in the LS and HS groups, respectively (p=0.85) (Table 2).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Patients
| Parameters | Low-Dose Simvastatin (n=63) | High-Dose Atorvastatin (n=62) | p-value |
|---|---|---|---|
| Age (years) | 58.2±9.5 | 58.4±8.9 | 0.91 |
| Female gender (n,%) | 48 (76.2%) | 42 (67.7%) | 0.29 |
| Body weight (kg) | 68.3±11.7 | 65.6±13.7 | 0.25 |
| Body mass index (kg/m2) | 28.0±4.3 | 26.9±4.2 | 0.15 |
| Waist circumference (cm) | 93.2±12.5 | 91.9±10.3 | 0.50 |
| Duration of diabetes (months) | 84 (42–144) | 96 (48–180) | 0.19 |
| Systolic blood pressure (mmHg) | 132.2±17.3 | 130.2±18.1 | 0.54 |
| Diastolic blood pressure (mmHg) | 72.7±10.2 | 72.9±9.3 | 0.88 |
| Hypertension | 46 (73.0%) | 47 (75.8%) | 0.72 |
| Chronic kidney disease | 5 (7.9%) | 2 (3.2%) | 0.44 |
| Microvascular complication | |||
|
11 (17.5%) 7 (11.1%) 4 (6.3%) |
11 (17.7%) 11 (17.7%) 5 (8.1%) |
0.51 0.44 0.74 |
| Education | |||
|
3 (4.8%) 20 (31.7%) 13 (20.6%) 9 (14.3%) 15 (23.8%) 3 (4.8%) |
3 (4.8%) 16 (25.8%) 6 (9.7%) 15 (24.2%) 18 (29.0%) 4 (6.5%) |
0.42 |
| MoCA, Thai | 20.8±4.0 | 21.0±3.8 | 0.80 |
| Trail Making Test, part B (second) | 122.0 (80.8–301.0) | 110.0 (72.5–301.0) | 0.84 |
| Simvastatin dosage (mg/day) | 15.1±5.1 | 14.8±5.2 | 0.73 |
| Cholesterol (mg/dl) | 147.8±19.5 | 147.3±18.6 | 0.86 |
| Triglyceride (mg/dl) | 113.0 (82.0–168.0) | 98.0 (71.0–133.0) | 0.06 |
| HDL-C (mg/dl) | 55.2±13.6 | 53.5±13.3 | 0.49 |
| Calculated LDL-C (mg/dl) | 67.4±14.1 | 72.7±13.2 | 0.03 |
| Fasting plasma glucose (mg/dl) | 121.5±21.6 | 126.5±32.3 | 0.30 |
| HbA1c (%) | 6.9±0.8 | 6.9±0.9 | 0.75 |
| Insulin (µU/mL) | 11.7 (7.9–17.9) | 10.7 (6.8–16.2) | 0.36 |
| HOMA-B (%) | 73.1 (54.8–100.2) | 62.7 (46.9–100.0) | 0.36 |
| HOMA-IR | 1.6 (1.1–2.5) | 1.5 (1.0–2.3) | 0.34 |
Note: Data presented as mean ± standard deviation (SD) or median (interquartile range), number and percentage.
Abbreviations: MoCA, Montreal Cognitive Assessment test; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; HOMA-B, homeostatic model assessment of beta call function; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 2.
Hypoglycemic Agents Used Compared Between the Two Study Groups
| Hypoglycemic Agent | Low-Dose Simvastatin (n=63), n (%) | High-Dose Atorvastatin (n=62), n (%) | p-value |
|---|---|---|---|
| Metformin | 61 (96.8%) | 60 (96.8%) | 1.00 |
| Sulfonylurea | 38 (60.3%) | 31 (50.0%) | 0.24 |
| Glinide | 0 (0.0%) | 1 (1.6%) | 0.50 |
| Pioglitazone | 14 (22.2%) | 14 (22.6%) | 0.96 |
| Alpha-glucosidase inhibitor | 3 (4.8%) | 5 (8.1%) | 0.49 |
| DPP-4 inhibitor (n, %) | 4 (6.3%) | 7 (11.3%) | 0.15 |
| GLP-1 receptor agonist | 1 (1.6%) | 0 (0.0%) | 1.00 |
| SGLT2-inhibitor | 0 (0.0%) | 1 (1.6%) | 0.50 |
| Insulin analogue | 5 (7.9%) | 8 (12.9%) | 0.53 |
Abbreviations: DPP4, dipeptidyl peptidase 4; GLP-1, glucagon like peptide 1; SGLT2, sodium glucose cotransporter 2.
The mean simvastatin dosage at study enrollment was 15.0±5.1 mg/day, and the mean baseline plasma LDL-C level of patients on simvastatin was 68.1±14.2 mg/dl. The HS group had a significantly higher baseline plasma LDL-C level than the LS group (72.7±13.2 vs 67.4±14.1 mg/dl, p=0.03). However, the HS group had a significantly (p<0.001) lower mean plasma LDL-C level with atorvastatin treatment of 40 mg/day at 6 weeks, and with atorvastatin treatment of 80 mg/day at 12 weeks compared to the LS group (both p<0.001). The mean difference in the percentage change in plasma LDL-C from baseline between HS and LS groups was 22.7% at 6 weeks (p<0.001), and 33.9% at 12 weeks (p<0.001).
Glucose Homeostasis
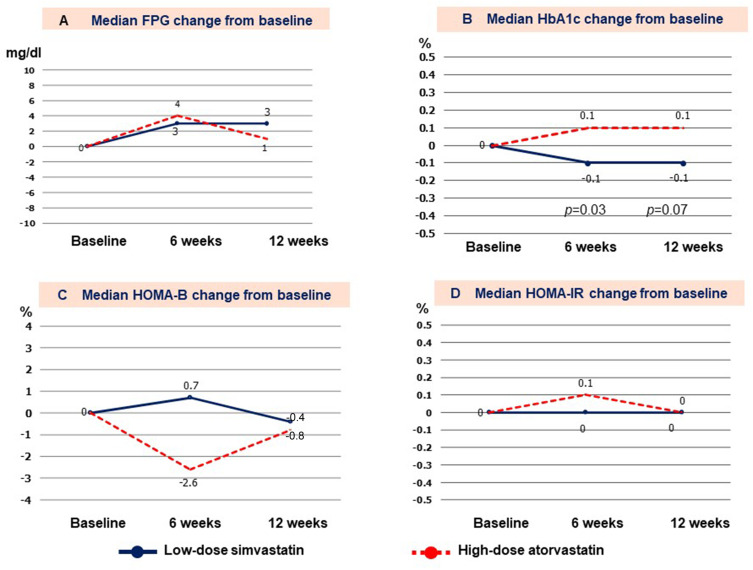
Median change in FPG from baseline in the LS and HS groups was +3 vs +4 mg/dl, (p=0.49) at 6 weeks, and +3 vs +1 mg/dl (p=0.93) at 12 weeks, as shown in Figure 2A. There was an increase in median HbA1C of 0.1% from baseline in the HS group, while HbA1c decreased by 0.1% in the LS group at both 6 and 12 weeks (Figure 2B). The difference in HbA1c between groups was significantly different only at 6 weeks (p=0.03); however, a trend towards statistical significance was observed at 12 weeks (p=0.07). Mean body weight was unchanged throughout the study period in both groups (Table 3).
Figure 2.
Changes from baseline to 6 and 12 weeks for (A) fasting plasma glucose (FPG); (B) glycated hemoglobin (HbA1c); (C) homeostatic model assessment of beta call function (HOMA-B); and, (D) HOMA of insulin resistance (HOMA-IR) compared between groups.
Table 3.
Anthropometric, Glycemic, and Lipid Parameters at 6 and 12 Weeks
| Parameters | 6 Weeks | 12 Weeks | ||||
|---|---|---|---|---|---|---|
| Low-Dose Simvastatin (n=63) | High-Dose Atorvastatin (n=62) | p | Low-Dose Simvastatin (n=63) | High-Dose Atorvastatin (n=62) | p | |
| Body weight (kg) | 68.3±11.9 | 65.7±13.8 | 0.28 | 67.8±11.7 | 65.5±13.9 | 0.33 |
| Waist circumference (cm) | 93.7±11.3 | 92.3±10.0 | 0.45 | 93.1±10.9 | 92.5±11.5 | 0.76 |
| Systolic blood pressure (mmHg) | 131.1±16.9 | 132.8±17.7 | 0.59 | 132.6±14.6 | 127.2±18.5 | 0.07 |
| Diastolic blood pressure (mmHg) | 74.1±11.3 | 73.2±10.5 | 0.67 | 73.9±10.2 | 72.1±10.7 | 0.34 |
| FPG (mg/dl) | 125.3±24.2 | 129.3±33.6 | 0.45 | 123.6±29.1 | 128.0±31.9 | 0.42 |
| HbA1c (%) | 6.8±0.8 | 6.9±0.9 | 0.60 | 6.9±0.8 | 7.0±0.8 | 0.69 |
| Insulin (µU/mL) | 11.7 (8.4–18.0) |
10.6 (7.4–16.1) |
0.42 | 11.8 (7.4–18.7) |
11.5 (6.9–16.5) |
0.68 |
| HOMA-B (%) |
65.6 (53.0–100.7) |
66.0 (45.7–99.6) |
0.54 | 64.6 (52.4–97.2) |
70.4 (47.0–97.2) |
0.87 |
| HOMA-IR | 1.7 (1.1–2.5) | 1.5 (1.1–2.4) | 0.42 | 1.7 (1.0–2.6) | 1.6 (1.0–2.3) | 0.60 |
| Cholesterol (mg/dl) | 148.0±24.5 | 128.4±24.6 | <0.01 | 152.1±23.7 | 125.8±31.7 | <0.01 |
| Triglyceride (mg/dl) | 115.0 (82.0–152.0) |
86.5 (63.0–111.0) |
<0.01 | 114.0 (80.0–147.0) |
84.0 (68.0–105.0) |
<0.01 |
| HDL-C (mg/dl) | 54.8±12.1 | 51.8±13.4 | 0.19 | 55.1±13.4 | 51.4±14.1 | 0.14 |
| Calculated LDL-C (mg/dl) | 67.7±19.0 | 56.8±15.4 | <0.01 | 73.1±20.1 | 55.4±26.4 | <0.01 |
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HOMA-B, homeostatic model assessment of beta call function; HOMA-IR, homeostatic model assessment of insulin resistance; HDL-C, high-density lipoprotein cholesterol; Calculated LDL-C, calculated low-density lipoprotein cholesterol.
There were no significant changes in median plasma insulin level from baseline to 6 and 12 weeks (+0.4 vs +1.0 µIU/mL [p=0.61] at 6 weeks, and +0.3 vs +0.2 [p=0.92] at 12 weeks), in median HOMA-B (+0.7% vs −2.6% [p=0.40] at 6 weeks, and −0.4% vs −0.8% [p=0.80] at 12 weeks), or in median HOMA-IR (0.0 vs +0.1 [p=0.89] at 6 weeks, and 0.0 vs 0.0 [p=0.65] at 12 weeks) between groups, as shown in Figure 2.
Switching to atorvastatin resulted in significant reductions in plasma LDL-C and triglyceride levels. Mean plasma cholesterol, triglyceride, and calculated LDL-C level were significantly lower in the HS group than in the LS group after atorvastatin therapy for 6 and 12 weeks, as shown in Table 3.
Neurocognitive Function
The mean MoCA score in the LS group was 20.8, 22.5, and 23.7 at baseline, 6 weeks, and 12 weeks, respectively; whereas, the mean MoCA score in the HS group was 21.0, 22.8, and 24.1, respectively. The median TMT results were 123.0 seconds, 116.5 seconds, and 105.5 seconds at baseline, 6 weeks, and 12 weeks, respectively, in the LS group; while those results were 110.0 seconds, 100.0 seconds, and 100.0 seconds, respectively, in the HS group. There were no significant differences in MoCA scores and TMT test scores at baseline, 6 weeks, or 12 weeks between groups (Tables 4 and 5). In addition, there was no significant correlation between percentage of LDL-C reduction and change in MoCA (r = −0.142, p=0.09), or percentage of LDL-C reduction and change in TMT test from baseline (r = 0.019, p=0.82). There were no significant differences between groups for any of the cognitive domains of the MoCA. Moreover, there was no self-report of memory loss or deterioration in neurocognition in patients with very low LDL-C levels (LDL-C <40 mg/dl) among 12 patients in the HS group, and 2 patients in the LS group as assessed by MoCA and TMT.
Table 4.
Neurocognition Testing Scores of Study Patients. Mean Montreal Cognitive Assessment (MoCA) Score and Median Trail Making Test, Part B (TMT) Score in Seconds Compared Between Groups at All Study Time Points
| Groups | Mean MoCA Score | Median TMT (Seconds) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Weeks | 12 Weeks | Baseline | 6 Weeks | 12 Weeks | |
| Low-dose simvastatin | 20.8±4.0 | 22.5±4.2 | 23.7±4.5 | 123.0 (70.8–301.0) |
116.5 (70.0–188.5) |
105.5 (69.5–222.0) |
| High-dose atorvastatin | 21.0±3.8 | 22.8±3.9 | 24.1±3.9 | 110.0 (72.5–301.0) |
100.0 (72.0–301.0) |
100.0 (65.0–301.0) |
| p-value* | 0.80 | 0.70 | 0.57 | 0.84 | 0.78 | 0.85 |
Note: *p-value reflects the difference between the LS and HS groups.
Table 5.
Neurocognition Testing Scores of Study Patients. Mean Change in MoCA Score and Median TMT Score in Seconds from Baseline to 6 and 12 Weeks Compared Between Groups
| Groups | Mean Change in MoCA Score from Baseline | Median Change in TMT from Baseline (Seconds) | ||
|---|---|---|---|---|
| 6 Weeks | 12 Weeks | 6 Weeks | 12 Weeks | |
| Low-dose simvastatin | +1.7±2.5 | +2.8±2.5 | −6.5 (−49.0 to +7.2) |
−14.5 (−45.8 to +0.3) |
| High-dose atorvastatin | +1.8±3.2 | +3.1±3.0 | 0.0 (−21.0 to 0.0) |
0.0 (−34.5 to +5.0) |
| p-value* | 0.83 | 0.62 | 0.57 | 0.29 |
Note: *p-value reflects the difference in change from baseline between the LS and HS groups.
Discussion
We found that switching from low-dose simvastatin to high-dose atorvastatin resulted in a slight deterioration in glucose homeostasis with an increase in HbA1c of 0.1% in patients with T2D compared to those who continued taking low-dose simvastatin. However, there was no deterioration in neurocognitive function, as assessed by MoCA and TMT.
Intensive statin treatment is recommended in high-risk T2D patients. All-cause mortality and risk of cardiovascular disease were decreased by approximately 10% and 20% per 1 mmol/L reduction in LDL-C, respectively.2,5 Although our study patients used statin for primary prevention, they had a long duration of T2D or had additional risk factors that qualified them for intensive plasma LDL-C lowering. Thus, statin intensification is recommended to reduce the risk of cardiovascular events based on the current guidelines.6–8 It is well documented that statins increase the risk of developing new-onset diabetes as compared to non-statin users. However, the effects of statins on glucose homeostasis in diabetic patients have been less studied. The WOSCOP (West of Scotland Coronary Prevention) study was the first study to show that pravastatin is associated with worsening glucose homeostasis compared to placebo.30 Later, rosuvastatin was reported to significantly increase the median glycated hemoglobin value compared to placebo.31 The increased risk of developing new-onset diabetes was subsequently confirmed in a meta-analysis of 13 randomized statin trials.9 Worsened glucose control in T2D was also reported in statin users compared to those that did not use statin.10 Another study reported a significant increase in HbA1c from baseline of 0.9% after one year of initiating statin treatment in well-controlled diabetes.32
While high-intensity statins are recommended in most guidelines in patients with high risk for cardiovascular diseases, reported data has suggested that the risk of developing new-onset diabetes from statins is greater with intensive-dose statins by 12% when compared with moderate-dose statins.15 This finding aroused our interest to see if this effect is also true for statin intensification by switching statin regimen in patients with T2D. A study comparing the effects of atorvastatin 10 mg/day and rosuvastatin 10 mg/day in non-diabetes found significant increase in HbA1c in both groups, but there was no significant difference between the two statin groups at 8 weeks.33 Another study comparing simvastatin 40 mg/day, rosuvastatin 10 mg/day, and simvastatin/ezetimibe 10/10 in non-diabetes found no significant changes of HbA1c from baseline, but there was a significant increase in HOMA-IR in all 3 groups at 12 weeks with no significant difference among groups.34 We found that statin intensification by switching from low-dose simvastatin to high-dose atorvastatin resulted in slight deterioration in glucose homeostasis with an increase in HbA1c of 0.1% in patients with T2D. Our study was different from previous studies because they compared glycemic control between similar statin intensity regimens using different drugs, whereas our study compared low-intensity statins with high-intensity statins. This explains why we observed slight deterioration in HbA1c, which is similar to the previous meta-analysis that compared low-dose and high-dose statins.15
The mechanisms by which statins affect glucose homeostasis are still unclear. Genetic studies suggested that the increased risk of new-onset T2D thought to be associated with statins is partly explained by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) inhibition.35 Increased insulin resistance or impaired β-cell were reported to be the potential mechanisms of increased plasma glucose and increased risk of new-onset diabetes.12 An open-label randomized controlled trial by Thongtang and colleagues demonstrated that atorvastatin 80 mg/day or rosuvastatin 40 mg/day significantly increased the median insulin level, which suggests that worsening glycemic control is caused by increased insulin resistance.36 However, the present study did not find changes in beta cell function or insulin resistance using HOMA-B and HOMA-IR in this study by comparing the two treatment regimens at the same timepoint. Longer follow-up may be required to detect such differences.
In contrast, a previous randomized single-blind crossover study reported that rosuvastatin and simvastatin significantly impaired glycemic control in well-controlled diabetes via impaired beta cell function at 12 months after statin initiation.32 However, the decline in beta cell function from baseline to 1 year could be due to disease progression during the clinical course of type 2 diabetes.
The brain contains 5–10 times more cholesterol than other organs. Previous studies reported too low of a plasma LDL-C level to be associated with hemorrhagic stroke,16,17 and associated with relatively poor performance on cognitive tests.18–20 However, post hoc analysis from the HOPE-3 study found no significant deterioration in neurocognitive function with rosuvastatin treatment, as assessed by MoCA and TMT.37 A meta-analysis by Ott et al38 did not find a significant difference in the incidence of dementia, confusion, or other adverse cognitive events between the statin and placebo groups among patients with normal cognition at baseline. Moreover, a cross-sectional study in coronary artery disease patients found high-dose statin to be associated with better cognition than low-dose statin, as assessed by MoCA and TMT.39 In addition, a meta-analysis of observational studies conducted by Macedo et al40 reported an odds ratio of 0.70 (95% CI: 0.59–0.83) for the development of all-type dementia and cognitive impairment without dementia in patients exposed to statins, suggesting a protective effect of statin use. With new lipid-lowering drugs, such as proprotein convertase subtilisin-kexin type 9 (PCSK9) monoclonal antibodies, a patient’s LDL-C level after treatment can be very low. The EBBINGHAS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) study also did not find significant worsening in neurocognition after PCSK9 monoclonal antibody treatment for 2 years using the Cambridge Neuropsychological Test Automated Battery.41 In agreement with these studies, we found that statin intensification by switching from low-dose simvastatin to high-dose atorvastatin did not significantly worsen neurocognition, as assessed by MoCA and TMT test in T2D patients, including the patients who had a plasma LDL-C level less than 40 mg/dl.
Limitations
Our study has some limitations. This study had a relatively small sample size and there was no parallel control group of patients on placebo. However, statins are recommended in most patients with T2D, thus using placebo could be considered to be unethical and medically negligent. Longer follow-up study is still needed to assess the long-term effect of statin on glucose homeostasis and neurocognition. β-cell function assessment with other methods, such as glycemic clamp study, are also required. The participants in our study had good glycemic control; therefore, our data may not be immediately generalizable to patients with poor glycemic control. In addition, this study was a randomized opened-label study; therefore, patients knew their plasma glucose levels during the study period. Even though the oral hypoglycemic agents were not allowed to be adjusted, patients might have modified their diet and lifestyle to improve their glucose controls if blood glucose levels increased at the 6-week follow-up. In cases where this might have happened, better glucose control would have been observed at the 12-week follow-up. This hypothesis could also explain the higher variability in changes in HbA1c at 12 weeks between groups compared to those observed at 6 weeks. This would also have the effect of reduced ability to detect statistically significant differences in HbA1c from baseline between groups. The results of MoCA and TMT testing could also have been affected by the education of the patient. However, although our study patients had a relatively low level of education, the baseline education between groups was similar. Slight improvement in MoCA score and TMT score from baseline was observed in both the LS and HS groups. It is possible, however, that these improvements were due to having taken the test previously.
Although we found slight impairment in glucose homeostasis, the established cardiovascular benefits of statin therapy far outweigh the risk of adverse effects;42 therefore, statin intensification is strongly recommended in high-risk type 2 diabetes patients.
Conclusion
Statin intensification by switching from low-dose simvastatin to high-dose atorvastatin resulted in slightly worsened glycemic control without causing cognitive decline, as assessed by MoCA and TMT tests in T2D patients without baseline dementia.
Funding Statement
This study was partially funded by a Siriraj Research Grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (grant no. R015936001), and partially funded by an investigator-initiated grant from Pfizer. Neither of the aforementioned funders influenced our interpretation of the data, the final conclusions drawn, the drafting of the report, or the decision to publish.
Abbreviations
ANOVA, analysis of variance; CI, confidence interval; COA, certificate of approval; DPP4, dipeptidyl peptidase 4; F/U, follow-up; FDA, Food and Drug Administration; FPG, fasting plasma glucose; GLP-1, glucagon-like peptide 1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; HOMA, homeostatic model assessment; HOMA-B, homeostatic model assessment of β-cell function; HOMA-IR, homeostatic model assessment of insulin resistance; HS, high-dose atorvastatin group; LDL-C, low-density lipoprotein cholesterol; LS, low-dose simvastatin group; MoCA, Montreal Cognitive Assessment; PCSK9, proprotein convertase subtilisin-kexin type 9; SGLT2, sodium glucose cotransporter 2; T2D, type 2 diabetes; TCTR, Thai Clinical Trials Registry; TMT, Trail Making Test, part B.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All study patients provided written informed consent to participate in this study. The study protocol was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (COA no. SI 680/2014).
Disclosure
All authors declare no personal or professional conflicts of interest relating to any aspect of this study. Nuntakorn Thongtang reports grants from Investigator-initiated research grant from Pfizer, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists' (CTT) Collaborators, Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–445. doi: 10.1016/j.jacc.2006.04.070 [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists' (CTT) Collaborators, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–S134. doi: 10.2337/dc20-S010 [DOI] [PubMed] [Google Scholar]
- 9.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 10.Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2014;57(12):2444–2452. doi: 10.1007/s00125-014-3374-x [DOI] [PubMed] [Google Scholar]
- 11.Hammad MA, Abdo MS, Mashaly AM, et al. The statins effects on HbA1c control among diabetic patients: an umbrella review of systematic reviews and meta-analyses of observational studies and clinical trials. Diabetes Metab Syndr. 2019;13(4):2557–2564. doi: 10.1016/j.dsx.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Betteridge DJ, Carmena R. The diabetogenic action of statins – mechanisms and clinical implications. Nat Rev Endocrinol. 2016;12(2):99–110. doi: 10.1038/nrendo.2015.194 [DOI] [PubMed] [Google Scholar]
- 13.FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. FDA [online] hwfgdduh.
- 14.Dormuth CR, Filion KB, Paterson JM, et al. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. doi: 10.1136/bmj.g3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi: 10.1001/jama.2011.860 [DOI] [PubMed] [Google Scholar]
- 16.Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the multiple risk factor intervention trial. Multiple risk factor intervention trial research group. Arch Intern Med. 1992;152(7):1490–1500. doi: 10.1001/archinte.1992.00400190110021 [DOI] [PubMed] [Google Scholar]
- 17.Stemmermann GN, Chyou PH, Kagan A, Nomura AM, Yano K. Serum cholesterol and mortality among Japanese-American men. The Honolulu (Hawaii) Heart Program. Arch Intern Med. 1991;151(5):969–972. doi: 10.1001/archinte.1991.00400050113021 [DOI] [PubMed] [Google Scholar]
- 18.Banach M, Rizzo M, Nikolic D, Howard G, Howard V, Mikhailidis D. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther. 2017;170:181–191. doi: 10.1016/j.pharmthera.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108(7):538–546. doi: 10.1016/S0002-9343(00)00353-3 [DOI] [PubMed] [Google Scholar]
- 20.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117(11):823–829. doi: 10.1016/j.amjmed.2004.07.041 [DOI] [PubMed] [Google Scholar]
- 21.Hammad MA, Syed Sulaiman SA, Aziz NA, Mohamed Noor DA. Evaluation of statins impacts on cognitive function among diabetic patients. Diabetes Metab Syndr. 2019;13(3):1797–1803. doi: 10.1016/j.dsx.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Marcum ZA, Vande Griend JP, Linnebur SA. FDA drug safety communications: a narrative review and clinical considerations for older adults. Am J Geriatr Pharmacother. 2012;10(4):264–271. doi: 10.1016/j.amjopharm.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas-Fernandez CH, Goldstein LB, Levey AI, Taylor BA, Bittner V; The National Lipid Association’s Safety Task F. An assessment by the statin cognitive safety task force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S5–S16. doi: 10.1016/j.jacl.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 24.Thongtang N, Piyapromdee J, Tangkittikasem N, Samaithongcharoen K, Srikanchanawat N, Sriussadaporn S. Efficacy and safety of switching from low-dose statin to high-intensity statin for primary prevention in Type 2 diabetes: a randomized controlled trial. Diabetes Metab Syndr Obes. 2020;13:423–431. doi: 10.2147/DMSO.S219496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 26.Tangwongchai SPM, Charernboon T, Akkayagorn L, Hemrungrojn S, Phanthumchinda K The validity of Thai version of The Montreal Cognitive Assessment (MoCA-T). International Psychogeriatric Association Conference; Montreal; September 2009, Abstract. [Google Scholar]
- 27.Tunvirachaisakul C, Supasitthumrong T, Tangwongchai S, et al. Characteristics of mild cognitive impairment using the Thai version of the consortium to establish a registry for alzheimer’s disease tests: a multivariate and machine learning study. Dement Geriatr Cogn Disord. 2018;45(1–2):38–48. doi: 10.1159/000487232 [DOI] [PubMed] [Google Scholar]
- 28.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39(4):222–232. doi: 10.1016/j.intell.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 30.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103(3):357–362. doi: 10.1161/01.CIR.103.3.357 [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 32.Bellia A, Rizza S, Lombardo MF, et al. Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis. 2012;223(1):197–203. doi: 10.1016/j.atherosclerosis.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 33.Her AY, Kim JY, Kang SM, et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J Cardiovasc Pharmacol Ther. 2010;15(2):167–174. doi: 10.1177/1074248409357922 [DOI] [PubMed] [Google Scholar]
- 34.Moutzouri E, Liberopoulos E, Mikhailidis DP, et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. Int J Clin Pract. 2011;65(11):1141–1148. doi: 10.1111/j.1742-1241.2011.02779.x [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–361. doi: 10.1016/S0140-6736(14)61183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thongtang N, Ai M, Otokozawa S, et al. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation. Am J Cardiol. 2011;107(3):387–392. doi: 10.1016/j.amjcard.2010.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch J, O’Donnell M, Swaminathan B, et al. Effects of blood pressure and lipid lowering on cognition. Results from the HOPE-3 study. Neurology. 2019;92(13):e1435–e1446. doi: 10.1212/WNL.0000000000007174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30(3):348–358. doi: 10.1007/s11606-014-3115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rej S, Saleem M, Herrmann N, Stefatos A, Rau A, Lanctot KL. Serum low-density lipoprotein levels, statin use, and cognition in patients with coronary artery disease. Neuropsychiatr Dis Treat. 2016;12:2913–2920. doi: 10.2147/NDT.S115505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. doi: 10.1186/1741-7015-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of Evolocumab. N Engl J Med. 2017;377(7):633–643. doi: 10.1056/NEJMoa1701131 [DOI] [PubMed] [Google Scholar]
- 42.Mach F, Ray KK, Wiklund O, et al. Adverse effects of statin therapy: perception vs. the evidence – focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526–2539. doi: 10.1093/eurheartj/ehy182 [DOI] [PMC free article] [PubMed] [Google Scholar]