Abstract
Aims
The aim of this study was to evaluate the effect the lockdown imposed during COVID-19 outbreak on the glycemic control of people with Type 1 diabetes (T1D) using Continuous (CGM) or Flash Glucose Monitoring (FGM).
Materials and methods
We retrospectively analyzed glucose reading obtained by FGM or CGM in T1D subjects. Sensor data from 2 weeks before the lockdown (Period 0, P0), 2 weeks immediately after the lockdown (period 1, P1), in mid-lockdown (Period 2, P2) and immediately after end of lockdown (Period 3, P3) were analyzed.
Results
The study included 63 T1D patients, (FGM: 52, 82%; CGM:11, 18%). Sensor use (91%) were slightly reduced. Despite this reduction, Time in Range increased in P1 (62%), P2 (61%) and P3 (62%) as compared to P0 (58%, all p < 0.05 or less) with concomitant reduction in the Time Above Range (P0: 38%; P1: 34%, P2: 34%, P3: 32%, all p < 0.05 or less vs. P0). Average glucose and GMI improved achieving statistical difference in P3 (165 vs. 158 mg/dl, p = 0.040 and 7.2% (55 mmol/mol) vs. 7.0% (53 mmol/mol), p = 0.016) compared to P0. Time Below Range (TBR) and overall glucose variability remained unchanged. Bi-hourly analysis of glucose profile showed an improvement particularly in the early morning hours.
Conclusions
In T1D subjects with good glycemic control on CGM or FGM, the lockdown had no negative impact. Rather a modest but significant improvement in glycemic control has been recorded, most likely reflecting more regular daily life activities and reduces work-related distress.
Keywords: COVID-19; Type 1 diabetes; Flash glucose monitoring, continuous glucose monitoring; Lockdown; Time in range; Time above range; Time below range
1. Introduction
In the attempt to prevent the spreading of the COVID-19, the Italian Government, as other Governments, have imposed a national lockdown from March 9, 2020 to May 18, 2020. Such lockdown could be expected to have quite an impact on daily life of people with diabetes, including limitation of physical activity, change in diet habits, difficulties in contacting health care providers, concern about drug supplies. According to a survey carried out amongst 1678 adults with Type 1 diabetes, a less healthy diet was admitted by 36% of them, while 49% declared some degree of reduction of daily physical activity [1]. Moreover, many participants in this survey also disclosed concern and anxiety regarding the epidemic and its implications. This may translate into a stress condition and, in some case, depression which may affect compliance and adherence to diabetes management and treatment [2]. Brooks et al have recently emphasized how duration of quarantine, fears of infection, frustration, monotony, inadequate supplies and financial loss can represent powerful stressors and suggested potential long-lasting effects of the restrictions [3]. In spite of the above, the data so far reported [4], [5], [6], [7], [8] have shown, if any, an improvement of the glycemic control in T1D patients during lockdown, although they remain limited and may reflect local organization of health care delivery, degree of education of the persons with diabetes, severity of the epidemic. We now report further data also obtained in T1D patients using Flash Glucose Monitoring or Continuous Glucose Monitoring system that has allowed to monitor remotely, in real time, changes in glycemic control.
2. Materials and methods
2.1. Study design and participants
The study was conceived as a retrospective data collection and all the individuals had given written permission to access remotely their data and use them for research purposes when they started using the FGM/CGM devices.
2.2. Data collection
We have retrospectively analyzed the metabolic data of 63 T1D patients of whom 52 (82%) used Flash Glucose Monitoring (FGM) system (Freestyle Libre, Abbott Diabetes Care, Rome, Italy) and 11 (18%) Continuous Glucose Monitoring (CGM) system (Dexcom G6, Dexcom, Inc. San Diego, CA). Sensor’s data were uploaded from web-based software (Libreview or Diasend) to generate Ambulatory Glucose Profiles (AGP) and interpretive summary reports. To the purpose of this analysis, we analyzed Average Glucose, Glucose Management Indicator (GMI), Glucose Variability (calculated as the coefficient of variation, CV), Time In Range (TIR, 70–180 mg/dl), Time Above Range (TAR, >180 mg/dl) and Time Below Range (TBR, <70 mg/dl) [9], [10] across 4 lockdown periods: 14 days before lockdown (Period 0, P0: February 21 - March 6, 2020); early lockdown (Period 1, P1: March 11 - March 25, 2020); mid-lockdown (Period 2, P2: April 11 - April 25, 2020); after lockdown (Period 3, P3: May 22 - June 5, 2020). Finally, we assessed hourly glycemic variability and average hourly glucose. No proactive phone or e-mail contact were made throughout the lockdown period. All patients were contacted at the end of lockdown to record any change in their daily habits, physical activity and body weight.
2.3. Statistical analysis
Data analysis was performed using the SPSS statistics software. Continuous variables are given as mean ± SD values if normally distributed or as median ± IQR if not, and categorical variables as percentages. Normality was checked using the Shapiro–Wilk test. Comparisons across the 4 periods of interest were performed by ANOVA for repeated measures and post-hoc Bonferroni test. A p-value < 0.05 was considered statistically significant.
3. Results
The main clinical characteristics of the study cohort are shown in Table 1 . Out of 63 T1D patients 28 (44%) were male and 35 (56%) female; average age was 44 ± 12 years and median (IQR) diabetes duration 22 years (12–32); 35 (56%) was on MDI and 28 (44%) on CSII with an average Total Daily insulin Dose (TDD) of 43.3 ± 19.9 UI/die; HbA1c was 56 ± 10 mmol/mol (7.2 ± 0.9%), based on the latest available determination (all within 4-months before start of the lockdown). 56 (90%) patients were on home smart working, 22 (35%) claimed they exercise regularly at home (cyclette, treadmill…) several times a week. None of the subject was known to be infected by SARS-CoV-2 and/or hospitalized during the observational period.
Table 1.
Main anthropometric and clinical characteristic of the Type 1 diabetes study population.
| Number of Patients | 63 |
| Sex Male (n – %) | 28 (44%) |
| Age (years) | 44 ± 12 |
| Diabetes duration (years) | 22 (12–32) |
| Body Mass Index (kg/m2) | 25 (22.8–28.5) |
| FGM/CGM (n – %) | 52/11 (82/18%) |
| MDI/CSII (n – %) | 35/28 (56/44%) |
| Nephropathy (n – %) | 9 (14%) |
| Neuropathy (n – %) | 6 (10%) |
| Retinopathy (n – %) | 16 (25%) |
| Coronary artery disease (n – %) | 2 (3%) |
| Hypertension (n – %) | 13 (21%) |
| Total Daily Insulin Dose (UI/die) | 40 (29–56) |
| Metformin (n – %) | 4 (6%) |
| ACEi/ARB (n – %) | 18 (29%) |
| Statins (n – %) | 13 (21%) |
| Acetylsalicylic acid | 8 (13%) |
FGM: Flash Glucose Monitoring; CGM: Continuous Glucose Monitoring; MDI: Multiple dose insulin; CSII: Continuous SubCutaneous Insulin Infusion; ACE.: Angiotensin Converting Enzyme inhibitors; ARB: Angiotensin Receptor Blocker.
Table 2 summarizes the results of interest. During lockdown there was a slight reduction in percentage of the use of the glucose monitoring system. Nonetheless, average plasma glucose improved during P3 (165 vs. 158 mg/dl, p = 0.040) along with improvement in GMI (7.2% (55 mmol/mol) vs. 7.0% (53 mmol/mol), p = 0.016). The reduction of average glucose levels was associated with an increase in TIR and consensual reduction in TAR while no changed occurred in TBR in the whole population (Table 2). However, when the study population was stratified according to baseline TBR we found that those with a TBR > 10% (n = 10; 16 ± 4) improved during lockdown (P1: 10 ± 5%, p = 0.035; P2: 10 ± 3%, p = 0.012) with only a nominal improvement after lockdown (P3:12 ± 7%; p = NS). No changes occurred in those with a baseline TBR between 4 and 10% or below 4% /data not shown). No severe episode of hypoglycemia was reported throughout the entire observation period.
Table 2.
Results of interest.
| P0 | P1 | P2 | P3 | ||
|---|---|---|---|---|---|
| †‡System use (%) | 91 ± 12 | 88 ± 12* | 85 ± 15* | 86 ± 13* | |
| ‡Scan/day (n) | 8 ± 6 | 8 ± 4 | 8 ± 5 | 9 ± 7 | |
| Average Glucose (mg/dl) | 165 ± 29 | 161 ± 31 | 161 ± 29 | 158 ± 31* | |
| GMI (mmol/mol) (%) |
56 ± 8 7.2 ± 0.7 |
54 ± 8 7.1 ± 0.7 |
55 ± 7 7.2 ± 0.7 |
53 ± 8** 7.0 ± 0.7* |
|
| Coefficient of Variation (%) | 37.4 ± 6.0 | 37.6 ± 6.8 | 38.2 ± 5.9 | 38.3 ± 7.0 | |
| Time In Range % | 58 ± 15 | 62 ± 17* | 61 ± 15* | 62 ± 16* | |
| Time Above Range % | 38 ± 18 | 34 ± 18* | 34 ± 16* | 32 ± 17** | |
| Time Below Range % | 5 ± 6 | 5 ± 6 | 5 ± 5 | 6 ± 6 |
†Subjects using continuous glucose monitoring; ‡ Subjects using flash glucose monitoring; *p < 0.05; **p < 0.01 vs. P0.
Abbreviations: GMI: Glucose Management Indicator.
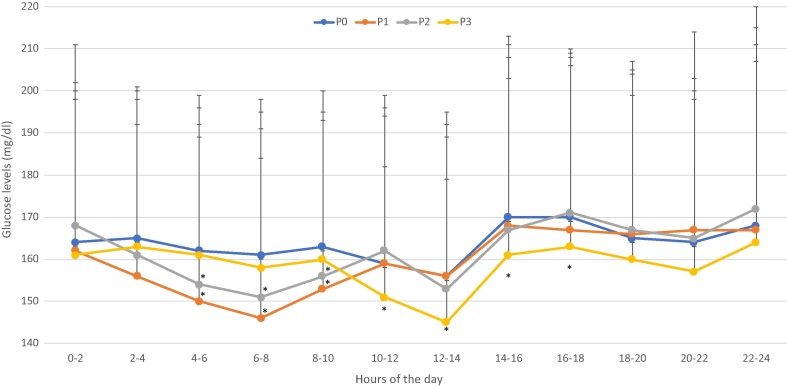
In the attempt to gain better insights in changes in daily glucose profile, we have calculated 2-hour average glucose over the 24 h (Fig. 1 ). This approach showed that the glucose levels were lower from 4am through 10am in P1 and P2 as compared to P0, whereas glucose levels from 10am though 6 pm were lower than in P0 in P3.
Fig. 1.
Bi-hourly daily glucose profile across the 4 periods of interest. *p < 0.05 or less vs. P0.
No significant chances in glucose metrics were found between subjects on MDI and those on CSII (data not shown).
There was a significant increase in body weight (P0: 75.0 ± 14.4; P2: 76.0 ± 14.8, p = 0.027) and in Total Daily insulin Dose (43.3 ± 19.9 vs 44.8 ± 20.5 UI/die, P0 and P2 respectively, p < 0.0001) during lockdown period.
4. Discussion
Recently, a simulation model was created using glycemic data from previous disaster, to estimate the effect of lockdown on glycemic control and on diabetes-related complications. According to this model a direct association was predicted between duration of the lockdown and worsening of glycemic control and risk of complications [11]. This model prediction is actually not supported by our results as well as those of other recent reports [4], [5], [6], [7], [8]. Thus, despite several potential interfering factors (physical activity, diet, psychological stress…) subjects with T1D during two-month lockdown, if any, showed an apparent improvement of their glycemic control.
Our data, as said, are in line with those so far reported but we also shed some light on the time courses of these changes as we found that glucose control improved immediately after lockdown (P1) to remained improved at mid-lockdown (P2) as well as after lockdown (P3). The improvement in average glucose levels was associated with GMI and, even more importantly, with an increase in the Time In Range and concomitant reduction of Time Above Range, while no significant change occurred in Time Below Range (Table 2). The lack of a significant change in TBR may be simply due to that fact that the rate of hypoglycemic events was already generally low in this population. Nonetheless, in patients with higher risk of hypoglycemia, i.e. those with a TBR P0 > 10% before lockdown, we observed a significant reduction of TBR in P1 and in P2.
We have analyzed in detail the changes in glucose profile throughout the 24-hrs by averaging glucose readings every 2 h. When daily glucose profiles were taken into consideration, it became apparent that most of overall improvement in glycemic control during lockdown (P1-P2) was mainly driven by a reduction in blood glucose levels in the early morning hours (from 4am through 10am). These changes seem to suggest a less pronounced ‘dawn phenomenon’. Of interest no significant changes occurred in basal insulin requirement (data not shown) while assessment of changes in prandial insulin is more difficult because the majority of our subjects were on CHO-counting. Conversely, it was glycemic control during these hours that worsened upon end of lockdown (P3). Though we cannot provide solid explanation for this observation, it is of interest that the “dawn phenomenon” is supported by the release of counterregulatory stress hormones [22]. Therefore, it is tempting to hypothesize that a more regular lifestyle and lighter smart working activities may have reduced the overall stress exposure and result in increased sleep quality and duration. In support to such an interpretation are data showing that work-related factors are associated with related distress intentional hyperglycemia at work [12].
Moreover, people with T1D have been shown to be at high risk for insufficient sleep duration and prior studies have shown that inadequate sleep may affect self-management behaviors and glycemic control [13]. Finally, it could be also speculated that because of the lockdown fewer dinner meals could have been eaten in restaurants where the carbohydrate and calorie content is often higher than what they may have had at home.
Altogether, these results suggest that T1D subjects well-educated on diabetes management who used Continuous (or Flash) Glucose Monitoring can effectively manage their glycemic control even under critical societal condition. With respect to that, the COVID-19 pandemic has provided incentives to the expansion of telemedicine for high-risk patients with diabetes, and especially for the management of type 1 diabetes.
Although the single-center nature of this study and the relatively small number of subjects can be seen as main limitations, yet our results corroborate those from other all of them concurring in claiming no worsening of glycemic control in type 1 diabetic subjects in spite of social distancing and limitations of their daily activities. Nonetheless, these results cannot be generalized to the whole category of type 1 diabetic patients since the subjects included in this study were well controlled to start and, more importantly, were well educated. If any, however, these results support the need for intensified educational programs for people with diabetes as this may indeed represent the basis for allowing them to deal with critical situations.
Funding
This work was supported by a grant from the University of Pisa (Punteggio Rating 2019).
Acknowledgments
Acknowledgments
The authors are grateful to the study participants and the nurses of the Diabetes Clinic of the Azienda Ospedaliero-Universitaria Pisana (AOUP) for the generous and continuous support.
Declaration of Competing Interest
The authors have no potential conflicts of interest relevant to this study.
Authors contributions
M.A., C.R., A.C. and R.G. contributed to the design of the study and the analysis and interpretation of data. F.C., A.B. C.B. and A.D. collected data and provided critical review of data interpretation: M.A. and C.R. wrote the first draft of the paper. S.D.P., A.C. provided relevant intellectual contribution to the development of the paper. All authors have provided substantial contribution to the acquisition of data, critically revised the final version of the paper and gave their final approval of the version submitted for publication., S.D.P. is the guarantor of this work, and as such, had full access to all the data of the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
References
- 1.dQ&A Impact of COVID-19 on the Diabetes Community Report April 2020.pdf | Con tecnologia Box. Accessed June 21, 2020. https://d-qa.app.box.com/s/8tysarjgoobbih7dj8m8kxhvm8n534sb.
- 2.Grenard J.L., Munjas B.A., Adams J.L., et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Int Med. 2011;26(10):1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks S.K., Webster R.K., Smith L.E., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonora B.M., Boscari F., Avogaro A., Bruttomesso D., Fadini G.P. Glycaemic control among people with type 1 diabetes during lockdown for the SARS-CoV-2 outbreak in Italy. Diabetes Ther. 2020 doi: 10.1007/s13300-020-00829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beato-Víbora P.I. No deleterious effect of lockdown due to COVID-19 pandemic on glycaemic control, measured by glucose monitoring, in adults with type 1 diabetes. Diabetes Technol Ther. 2020;1–10 doi: 10.1089/dia.2020.0184. [DOI] [PubMed] [Google Scholar]
- 6.Tornese G., Ceconi V., Monasta L., Carletti C., Faleschini E., Barbi E. Glycemic control in type 1 diabetes mellitus during COVID-19 quarantine and the role of in-home physical activity. Diabetes Technol Ther. 2020 doi: 10.1089/dia.2020.0169. [DOI] [PubMed] [Google Scholar]
- 7.Maddaloni E, Coraggio L, Pieralice S, Carlone A, Pozzilli P, Buzzetti R. Effects of COVID-19 Lockdown on Glucose Control: Continuous Glucose Monitoring Data From People With Diabetes on Intensive Insulin Therapy. Diabetes Care. Published online June 5, 2020. doi: 10.2337/dc20-0954 [DOI] [PubMed]
- 8.Capaldo B, Annuzzi G, Creanza A, et al. Blood Glucose Control During Lockdown for COVID-19: CGM Metrics in Italian Adults With Type 1 Diabetes. Diabetes Care. Published online June 15, 2020. 10.2337/dc20-1127. [DOI] [PMC free article] [PubMed]
- 9.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergenstal R.M., Beck R.W., Close K.L., et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosal S., Sinha B., Majumder M., Misra A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: a simulation model using multivariate regression analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):319–323. doi: 10.1016/j.dsx.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen U.M., Skinner T., Olesen K., Willaing I. Diabetes distress, intentional hyperglycemia at work, and glycemic control among workers with type 1 diabetes. Diabetes Care. 2019;42(5):797–803. doi: 10.2337/dc18-1426. [DOI] [PubMed] [Google Scholar]
- 13.Frye S.S., Perfect M.M., Silva G.E. Diabetes management mediates the association between sleep duration and glycemic control in youth with type 1 diabetes mellitus. Sleep Med. 2019;60:132–138. doi: 10.1016/j.sleep.2019.01.043. [DOI] [PubMed] [Google Scholar]