Abstract
The use of chloroquine in the treatment of COVID-19 has received considerable attention. The recent intense focus on this application of chloroquine stimulated an investigation into the effects of chloroquine at low doses on highly biologically-diverse models and whether it may induce hormetic-biphasic dose response effects. The assessment revealed that hormetic effects have been commonly induced by chloroquine, affecting numerous cell types, including tumor cell lines (e.g. human breast and colon) and non-tumor cell lines, enhancing viral replication, sperm motility, various behavioral endpoints as well as decreasing risks of convulsions, and enhancing a spectrum of neuroprotective responses within a preconditioning experimental framework. These diverse and complex findings indicate that hormetic dose responses commonly occur with chloroquine treatment with a range of biological models and endpoints. These findings have implications concerning study design features including the number and spacing of doses, and suggest a range of possible clinical concerns and opportunities depending on the endpoint considered.
Keywords: Chloroquine, Hydroxychloroquine, Hormesis, Dose-response, COVID-19, Biphasic dose response
Graphical abstract
1. Introduction
The use of chloroquine as a clinical treatment during the COVID-19 pandemic has generated vast public attention and hundreds of clinical trials (Ali and Alharbi, 2020; Cortegiani et al., 2020; Gendrot et al., 2020). This compound was first discovered in 1934 and gained recognition as an antimalarial agent in World War II, leading to further studies and trials on its potential treatment for various inflammatory, rheumatological, immunological and malignant disorders including malaria, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), prostate cancer etc. Further studies led to recognition of the structurally variant compound hydroxychloroquine, a less toxic metabolite of chloroquine (Pascolo, 2016; Pastick et al., 2020; Al-Bari, 2015). Despite the current heightened public attention given to chloroquine, it has long been a molecule of biological interest, attracting attention from broad interdisciplinary biological research perspectives, engaging highly diverse biological models and endpoints. Consequently, this diverse historical background of scientific interest in chloroquine allowed us to investigate distinct biological dose responses and the possibility that observations of chloroquine-induced hormetic dose responses may have been recognized only to a limited extent. Thus, we undertook an assessment of whether and to what extent chloroquine and hydroxychloroquine may have induced hormesis in the existing literature and its potential biological and biomedical implications.
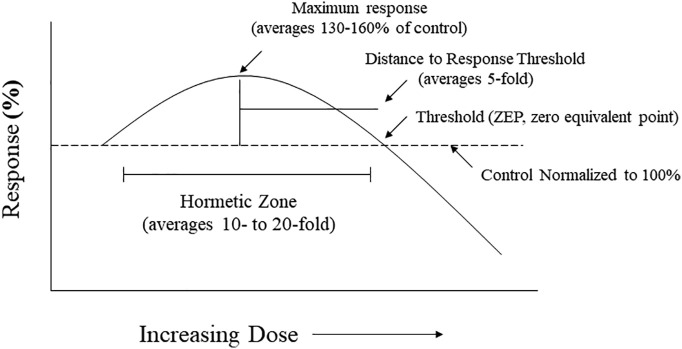
Hormesis is a biphasic dose response that is characterized by a low dose stimulation and a high dose inhibition. This biphasic dose response has specific quantitative features, with the maximum response being about 30–60% greater than the control group (Fig. 1 ) (Calabrese and Blain, 2005, Calabrese and Blain, 2011; Calabrese et al., 2019; Calabrese and Mattson, 2017). The dose/concentration width of the stimulation can be highly variable, depending on the biological model and endpoint measured. However, the dose/concentration width of the stimulation below the toxicological response is typically less than 50-fold (Calabrese and Blain, 2011). Hormesis may occur either as a result of a direct stimulation or as an overcompensation response following a disruption of homeostasis or a low to moderate induction of toxicity (Calabrese, 2008a, Calabrese, 2013). Regardless of the mechanisms of hormesis induction, biological model, endpoint measured, inducing agent, the potency of the inducing agent, and mechanism, the quantitative features of hormetic dose responses are similar. These observations suggest that the quantitative features of the hormetic dose response describe the limits of a type of biological plasticity (Calabrese and Mattson, 2011; Calabrese et al., 2016). As this article will demonstrate, these features of the hormetic dose response are manifested in the series of examples of hormesis assessed below for chloroquine, which we believe has consequences for its use in research and in the clinical practice.
Fig. 1.
Hormetic dose response, quantitative features (based on Calabrese and Baldwin, 1997).
2. Search strategy
PubMed, Web of Science and Google Scholar databases were searched for articles using the term hormesis or hormetic, biphasic dose response, U-shaped dose response, adaptive response, and preconditioning in combination with the term chloroquine or hydroxychloroquine. All relevant articles were iteratively evaluated for references cited and for all papers citing these articles. All research groups publishing these identified papers were assessed for possible relevant dose-response publications in the above databases.
3. Cell proliferation
3.1. Tumor cells
Chloroquine has been evaluated for its effect on cell proliferation in several tumor and non-tumor cell lines. In the case of tumor cells, research was initiated to assess the effects of anti-malarial agents on the survival of Ehrlich ascites tumor (EAT) infected mice. These studies indicated that the anti-malarial drugs mepacrine and primaquine enhanced mortality in the mice (Castelli et al., 1996). Follow up in vitro research indicated that oxygen consumption was significantly enhanced in tumor cells by these two anti-malaria drugs. This led to the suggestion that the higher oxygen consumption might affect the capacity to enhance tumor progression. Based on these findings, Rossi et al. (2007) assessed several anti-malarial agents, including chloroquine, on MCF-7 (human breast adenocarcinoma) and Vero cell (kidney epithelial cells from the African green monkey) lines. Using the MTT assay, the chloroquine treatment induced a biphasic dose response for each cell line. However, the responses were in the opposite directions. That is, the chloroquine treatment reduced the proliferation of the MCF-7 cells at low concentrations, while increasing it at higher concentrations, whereas the dose response pattern was reversed in the Vero cells (Fig. 2 ). Rossi et al. (2007) suggested that the high dose stimulation of the MCF-7 cells by chloroquine “must be considered a severe side-effect not to be underestimated when anti-malarial measures must be undertaken in an oncological patient…” This was particularly the case since malaria patients may require treatment for long durations. The chloroquine inhibited MCF-7 proliferation at a concentration < 5 mg/l, while enhancing cell growth at ≥25 mg/l. According to Pascolo (2016), the pleiotropic features of chloroquine may lead to an enhancement of tumor growth, a factor that needs consideration in future clinical studies.
Fig. 2.
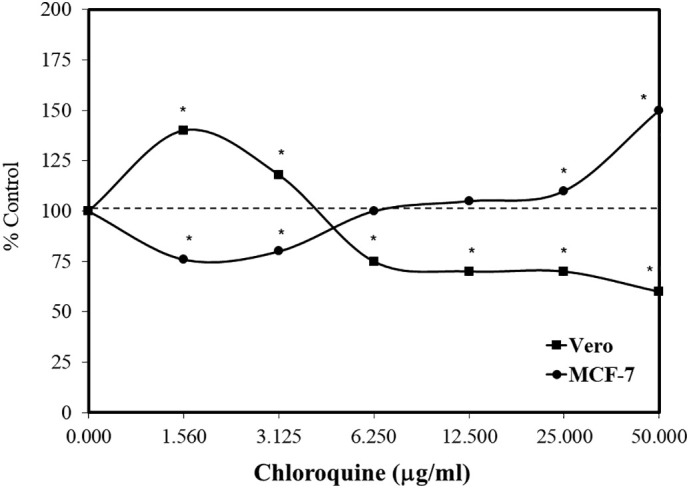
Effects of chloroquine on Vero and MCF-7 cell growth using the MTT assay (data: Rossi et al., 2007).
The research with the HCT-15 colorectal cell line was based on the goal of clarifying the role of autophagy in tumor development. Chloroquine is a lysosomotropic agent. As a weak base it can freely diffuse across lysosomal membranes in an unchanged state, becoming protonated and trapped within the acidic lysosome. Once protonated, the chloroquine may lead to the activation/release of hydrolytic lysosomal enzymes. Chloroquine can inhibit autophagy, preventing the formation of autophagolysosomes. Since tumor development may be enhanced by the upregulation of autophagy, chloroquine showed promise as a potential chemotherapeutic agent (Park and Lee, 2014). As cellular concentrations of chloroquine increase, they enhance lysosomal membrane permeabilization (LMP). The LMP process affects the release of cathepsins and other hydrolytic enzymes to the cytosol, activating processes that enhance occurrence of apoptosis. In a concentration response assessment using HCT-15 colorectal cells, chloroquine induced a biphasic response with cell survival being enhanced at low concentrations and inhibited at high concentrations (Fig. 3 ). Park and Lee (2014) hypothesized that chloroquine acted as an autophagy inhibitor at low concentrations, while mediating LMP at high concentrations. The application of antioxidants prevented the low dose stimulation and high dose inhibition. Such concentration response findings were also reported with the pancreatic cancer cell line, Panc-1 (Fig. 4 ) (Monma et al., 2018). However, the low concentration stimulation for the Panc-1 cells was optimized at a concentration that was 500-fold less than with the HCT-15 cells.
Fig. 3.
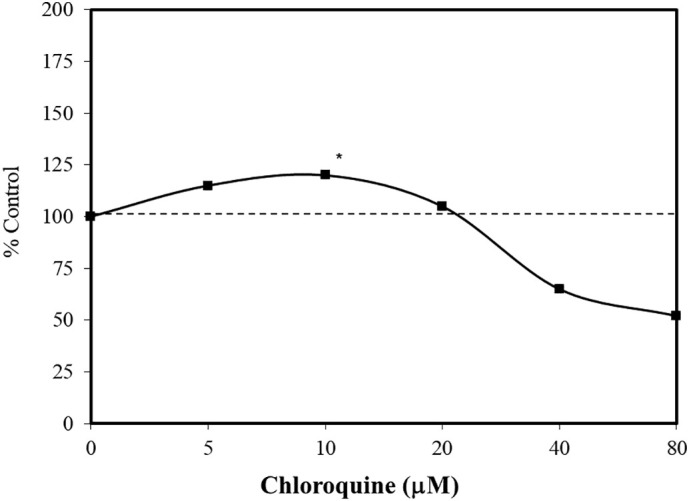
Effects of chloroquine on the growth of HCT-15 cells (MTT assay, 48 h) (data: Park and Lee, 2014).
Fig. 4.
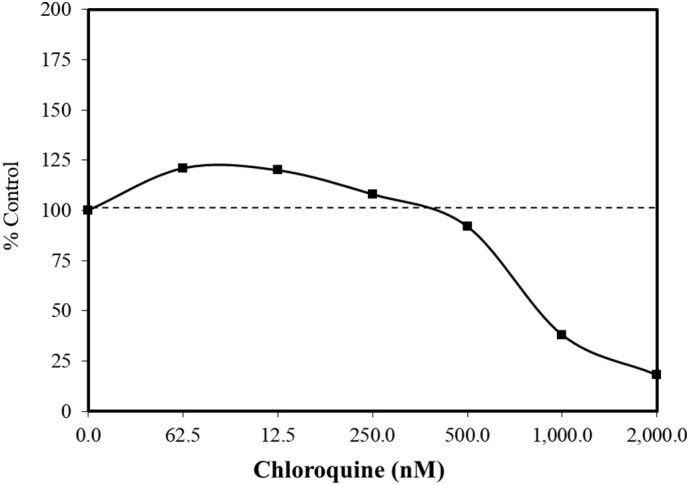
Effects of chloroquine on the pancreatic cell line, Panc-1, using the WST-8 assay (cell viability) (data: Monma et al., 2018).
3.2. Non-tumor cells
In similar studies with several non-tumor cell lines, biphasic concentration responses were also reported [e.g., Balb/c 3T3 cells, with a neutral red uptake assay (Cudazzo et al., 2019); HaCaT cells (immortalized human skin keratinocytes) with a crystal violet assay (Martins et al., 2013); and with human lymphocytes, using a tritiated thymidine uptake assay (Bygbjerg and Flachs, 1986)]. While the low concentration stimulation ranges were similar for the BALBc 3T3 (5–30 μM, Fig. 5 ; Cudazzo et al., 2019) and HaCaT cells (10–40 μM, Fig. 6 ; Martins et al., 2013), the stimulating concentrations for the human lymphocytes ranged from 100 to 1000 fold higher (Bygbjerg and Flachs, 1986). Chloroquine was selected by Cudazzo et al. (2019) due to its lysosomotropic features. These authors also reported that several other lysosomotropic agents such as nicotine and lapatinib also displayed similar biphasic dose responses using the neutral red uptake (NRU) assay. Of interest is that the increase in the uptake of the neutral red dye occurred in the absence of an increase in the number of cells. These findings challenged the utility of the NRU to establish cell proliferation for lysosomotrophic agents. However, they did not present the mechanism and biological significance of the uptake of the neutral red dye within the context of the observed biphasic dose response. In contrast, the studies of Cudazzo et al. (2019) and Martins et al. (2013) suggested that chloroquine enhanced the uptake of crystal violet dye in a biphasic concentration manner in HaCaT cells but that this biphasic response did not occur using the NRU assay. A similar divergent finding as seen in Cudazzo et al. (2019) was recently made by Kontar et al. (2020). They reported that sulforaphane induced biphasic dose responses in three types of L1210 leukemia cells with the MTS cell viability assay, while, at the same time, showing a dose-dependent decrease in cell number, in contrast to the expected increase in cell number. Possible reasons for this endpoint decoupling/divergence was suggested by Kontar et al. (2020). A similar type of focus for the divergent findings with the NRC assay with chloroquine remains to be made.
Fig. 5.
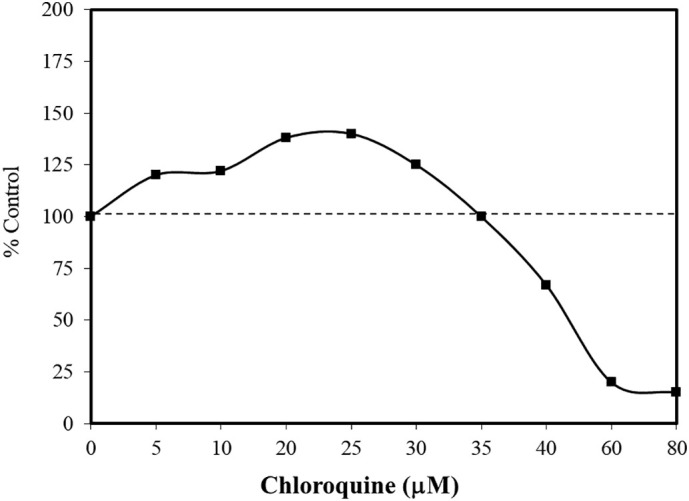
Effects of chloroquine on neutral red assay in BALB/c 3T3 cells (data: Cudazzo et al., 2019).
Fig. 6.
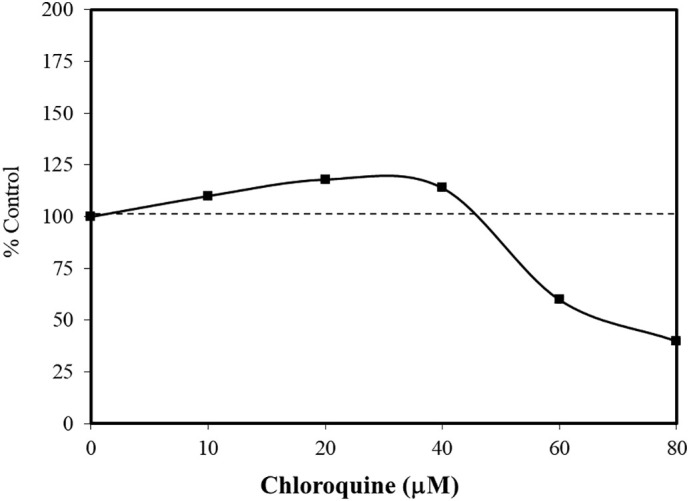
Effects of chloroquine on cell survival of HaCaT cells using the crystal violet assay (data: Martins et al., 2013).
4. Convulsions
Focal and generalized convulsions are often seen in patients with cerebral malaria. In fact, a sizeable literature suggests that high doses of chloroquine have a proconvulsant effect (N'Gouemo et al., 1994). In contrast, chloroquine has also been shown to enhance the effects of anti-epileptic drugs, although chloroquine itself is not an anti-seizure drug. The pro- and anti-convulsant effects of chloroquine led N'Gouemo et al. (1994) to hypothesize that chloroquine may induce dose-dependent biphasic responses for seizures (Fig. 7 ). This hypothesis was tested using the proconvulsant drug pentylenetetrazol (PTZ), which induces clonic or tonic-clonic-seizures in mice. Using Swiss mice as the model, six doses of chloroquine (1.0–50 mg/kg) were employed with 85 mg/kg PTZ, a dose producing tonic-clonic seizures. The authors were interested in assessing whether the chloroquine treatment could affect the seizure latency and mortality incidence. The findings indicated that low doses of chloroquine were highly protective by prolonging seizure latency and reducing mortality by 50%. The mechanisms of these protective effects suggested involvement of dopamine pathways. The biphasic nature of the chloroquine dose response is consistent with an extensive series of other pro-and anticonvulsant examples in the preclinical literature (Calabrese, 2008b).
Fig. 7.
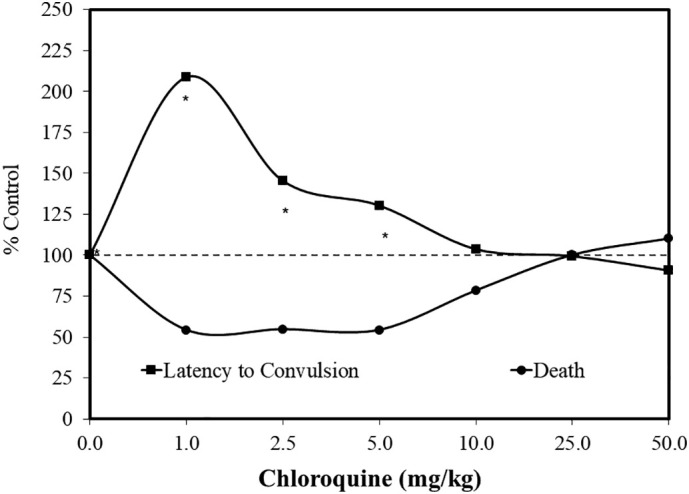
Effects of pretreatment of chloroquine on mortality induced by pentylenetetrazol (PTZ) in male Swiss mice (data: N'Gouemo et al., 1994).
5. Sperm function
Chloroquine has been assessed for its capacity to affect sperm function to a limited extent. This was initially reported by Norman and Gombe (1975), who suggested that the effects of chloroquine were both dose- and time- dependent. They found that a short-term or low dose treatment enhanced sperm motility and fertility in mammalian models. This low dose stimulating response was reversed at higher doses as repeated in later studies (Adeako and Dada, 1998; Okanlawon et al., 1993). Despite these suggestive findings, it was the report of Hargreaves et al. (1998) that assessed an extensive dose response continuum (2.5–2000 μg/ml). The effects of multiple antibiotics on sperm function were assessed since standard human therapeutic treatments may adversely affect human sperm function. Chloroquine is completely absorbed, being concentrated in tissues by 200–700-fold as compared to plasma (Hargreaves et al., 1998), thereby providing a basis for assessing a broad concentration range. In the latter in vitro human sperm study, chloroquine induced a biphasic concentration response of sperm motility (Fig. 8 ).
Fig. 8.
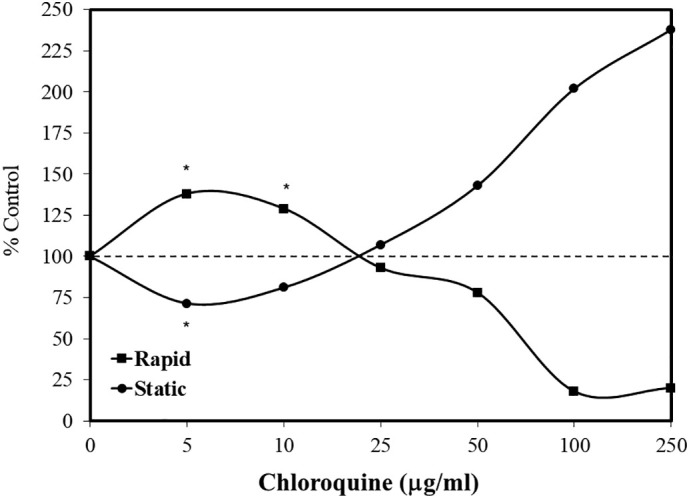
Effects of chloroquine on proportion of rapid and static human spermatozoa (data: Hargreaves et al., 1998).
These findings were significantly extended by Zhang et al. (2017) in an in vivo study on sperm motility in yellow catfish, with the chloroquine being injected behind the pectoral fin. The chloroquine treatment induced a biphasic dose response for multiple sperm motility parameters, including increased straight-line velocity, curvilinearity velocity, and average path velocity (Fig. 9 ). The authors suggested that the effects of chloroquine were mediated by its capacity to inhibit autophagy, but this could not explain the biphasic nature of the dose response as the autophagy dose response was linear.
Fig. 9.
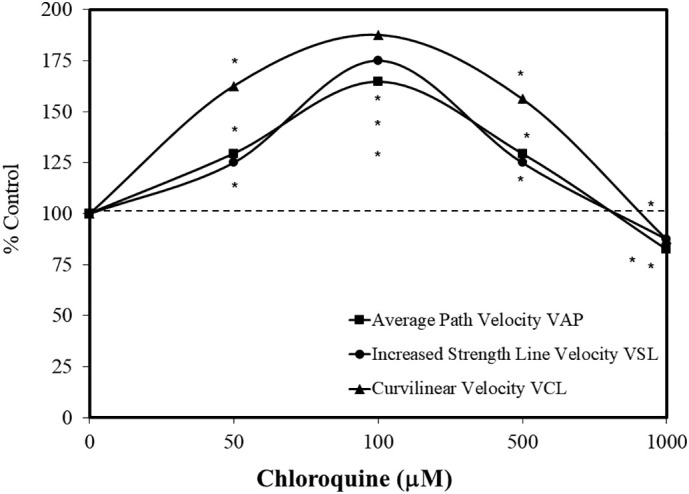
Effects of chloroquine on yellow catfish sperm kinematic parameters (data: Zhang et al., 2017).
6. Itching behaviors
Severe generalized pruritus is a well-known adverse effect of chloroquine when employed in the treatment of malaria and rheumatological disorders such as rheumatoid arthritis and systemic lupus erythematosus. Nearly 70% of adult Africans were affected with pruritus while receiving chloroquine for malaria treatment, significantly affecting compliance (Ajayi et al., 1989; Osifo, 1984; Onigbogi et al., 1999). A drug-induced aquagenic (postwetness) type pruritis induced by chloroquine was also observed in Caucasian populations treated for systemic lupus erythematosus (Jimenez-Alonso et al., 1998). Several studies have explored the capacity of chloroquine to induce scratching behavior within a dose response framework (Tarrason et al., 2017). These studies were extended to 11 mouse strains injected with a range of chloroquine doses, and a consistent U-shaped dose response pattern was observed. Based on these findings, the dosing strategy was such that the response to the highest dose employed did not differ from the response to the lowest dose (Green et al., 2006).
7. Virus growth
Severe acute respiratory syndrome (SARS) is a highly contagious human disease, with the causative agent identified as being part of the Coronaviridae family (i.e. enveloped, positive-sense, single-stranded RNA viruses). In response to the SARS epidemic, Saverino et al. (2003) suggested that chloroquine may be of clinical value in the management of SARS. This suggestion was based on its effectiveness as an antimalarial agent and its anti-viral activity against HIV type 1 (Tsai et al., 1990; Pardridge et al., 1998; Saverino et al., 2001) hepatitis virus (Kouroumalis and Koskinas, 1986), herpes simplex virus type 1 (Singh et al., 1996), and HCoV-229e (Blau and Holmes, 2001). Based on this literature, Keyaerts et al. (2004) provided a dose response inhibitory effect of chloroquine on the yield of SARS-COV when grown in Vero E6 cells. The viral replication was measured after one and three days, across a broad range of chloroquine concentrations (2–156 μM). The day one data revealed a dose-dependent reduction in virus yield, with a 99% reduction at 4 μM chloroquine. However, with the day three growth period, 16 μM of chloroquine were needed to achieve the same level of effectiveness. Not discussed in this paper was that the rate of virus synthesis from day one to day three for concentrations 2–8 μM far exceeded that of the control group (Keyaerts et al., 2004), suggesting an overcompensation hormetic response that has been commonly reported in dose/concentration-time relationships (Calabrese, 1999, Calabrese, 2001). These findings may have considerable clinical implications.
Other studies have shown that chloroquine directly enhanced viral replication for Esptein –Barr Virus in Raji cells (Karmali et al., 1978), recombinant vaccinia virus in HeLa cells (Chillakura et al., 1991), porcine circocirus 2 (PCV2) in procine kidney cells (PK-15) (Misinzo et al., 2008), human parvovirus B19 (B19V) in UT7/Epo cells (Bonsch et al., 2010; Quattrocchi et al., 2012), and Semliki Forest virus (SFV), encephalomyocarditis virus in mice (Maheshwari et al., 1991; Seth et al., 1999) and influenza A virus A/WSN/33 (H1N1) (Wu et al., 2015). Several viral studies employed dose response methods that revealed a hormetic-like biphasic dose response for chloroquine for Friend retrovirus (FV)-infected fibroblast of mice based on spleen weights (Sinet et al., 1996). The study of Bonsch et al. (2010) reported a striking biphasic dose response in a ten-concentration evaluation measuring B19 NS1 viral RNA. In that study, several chloroquine derivative agents (e.g. amodiaquine, mefloquine and piperaquine) also enhanced viral replication showing a hormetic dose response (Fig. 10 ), raising possible clinical concerns especially when chloroquine is utilized as an anti-viral agent.
Fig. 10.
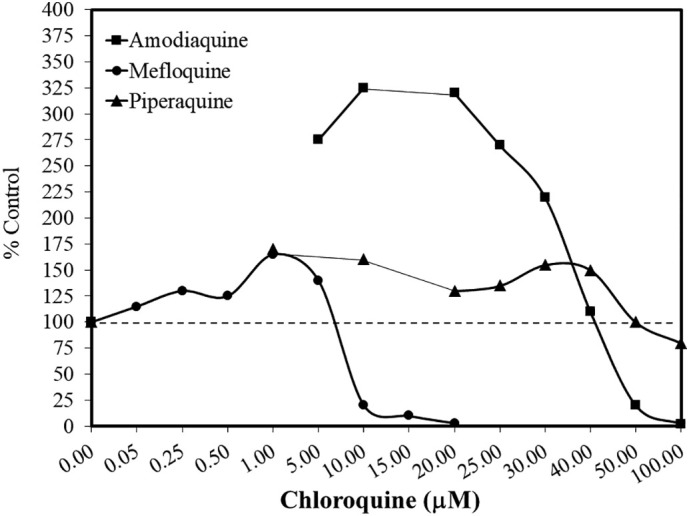
Effects of chloroquine derivatives on B19V viral replication (data: Bonsch et al., 2010).
8. Preconditioning
Preconditioning, as a means to induce acquired resilience, is an extensively documented adaptive phenomenon (Leak et al., 2018; Stone et al., 2018). When multiple separate conditioning doses are employed in preconditioning experiments, it has often been shown to follow a hormetic dose response (Calabrese, 2016a, Calabrese, 2016b). Chloroquine has been evaluated in a range of preconditioning studies concerning cardiac and renal ischemic/reperfusion injury (Todorovic et al., 2014; Tang et al., 2018; Shukla et al., 2015; Bourke et al., 2015).
Chloroquine has also been assessed with a preconditioning framework for its capacity to protect neuronal cells. That chloroquine might act in a neuroprotective manner was first suggested by Jarzyna et al. (1995) who also reported that chloroquine inhibited glutamate metabolism in the liver and kidney. Furthermore, chloroquine was suggested as a possible treatment for schizophrenia based on its potential to influence glutamate concentrations in neural tissue (Choi et al., 2007). Chloroquine was also shown to display a neuroprotective effect by preventing glutamate-induced neuronal cell death via the sigma-1 receptor in HT22 cells (Hirata et al., 2011).
At low μM concentrations (below the threshold to inhibit lysosomal activity), chloroquine enhances GM1 ganglioside accumulation in early endosomes and on the surface of PC12 cells (Yuyama et al., 2006). Since GM1 is a major brain ganglioside with a neuroprotective function, Hirata et al. (2011) hypothesized that its upregulation may be neuroprotective. In follow up studies using HT22 cells, an immortalized hippocampal cell line, chloroquine fully prevented cell death induced by co-treatment with glutamate while enhancing survival modestly beyond control group values at the optimized concentration. The concentration range suggested a U-shaped concentration response.
Based on the above investigations, Ma et al. (2019) assessed the capacity of chloroquine to affect glutamate-induced apoptosis in primary retinal ganglion cells of newborn Sprague-Dawley rats. In these studies, the effect on retinal ganglion cells (RGC) viability, measured by the MTT assay, showed a concentration-dependent progressively protective response (Fig. 10). In a follow up in vivo preconditioning study, Brown Norway male rats were exposed to chloroquine for seven days via gavage, and then exposed to glutamate. The glutamate reduced cell number by about 60%. The chloroquine blocked this cell toxicity, displaying a hormetic dose response pattern. At the optimal concentration, chloroquine treatment affected its protection via the induction of an anti-apoptosis response. The induction of BCL-2 was determined to reduce ROS, preventing apoptotic responses.
9. Discussion
The present assessment provides a substantial number of experimental models which demonstrated hormetic-like biphasic dose responses induced by chloroquine. Of considerable interest is the wide range of models/endpoints showing the hormetic dose/concentration response. These include the areas of epileptic seizures, itching behavior, virus growth, preconditioning with retinal cells, sperm cell motility and other functions, as well as cell proliferation for several tumor and non-tumor cells. An overall evaluation of the study designs employed indicates a median of six doses per experiment and a median of three doses below the threshold. The maximum hormetic stimulation was 155% (median)/177.6% (mean), with a stimulatory range of 4.5-fold (median), 33.2 fold (mean) (Table 1 ). These values are typical of a large body of hormetic dose responses (Calabrese and Blain, 2005, Calabrese and Blain, 2011; Calabrese et al., 2019).
Table 1.
Study characteristics and dose response features.
| Number of doses/concentrations per experiment | Number of doses/concentrations below threshold per experiment | Number of doses/concentrations with responses ≥110% per experiment | Max stimulation (%) | Stimulation width |
|---|---|---|---|---|
| 4.9 mean | 3.0 mean | 2.6 mean | 177.6 mean | 33.2 mean |
| 6.0 median | 3.0 median | 2.5 median | 155.0 median | 4.5 median |
Chloroquine displayed hormetic dose responses with both a direct stimulation framework or with a preconditioning experimental protocol. Numerous instances of chloroquine-induced preconditioning have been published, but only one study was designed to assess the possibility of a biphasic dose response using the HT22 immortalized mouse hippocampal cell line (Hirata et al., 2011), with the findings showing neuroprotective effects against glutamate induced oxidative stress.
Multiple studies incorporated a mechanistic component, providing an insight to the underlying basis of the reported biphasic dose response (Table 2 ). For example, chloroquine-induced cellular properties were generally related to the enhanced production of BCL-2 (Ma et al., 2019) and the upregulation of the P13/AKT signaling pathway. Likewise, its capacity to induce anti-inflammatory responses was related to its capacity to downregulate NLPP3 (Tang et al., 2018).
Table 2.
Hormetic mechanisms mediating chloroquine responses.
| Reference | Comments |
|---|---|
| Ma et al., 2019 | Page 287 – Low dose chloroquine prevented the capacity of clotamide to decrease intracellular GSH levels. Glutamate increases in Bax and caspase 3 were significantly decreased by chloroquine; BCL-2 and BCLxl levels were increased. |
| Hirata et al., 2011 | Chloroquine inhibits glutamate-induced death of a neural cell line by reducing ROS via sigma-1 receptor. |
| Bourke et al., 2015 | Preconditioning protection via hydroxychloroquine against cardiac ischemia/reperfusion injury was blocked by UO126 which inhibits ERK1/2 kinase. |
| Tang et al., 2018 | Preconditioning protection against renal ischemia/reperfusion injury by inhibitor of cathepsin mediated NLRP3 inflammasome activation by downregulating NF-KB signaling. |
| Xu et al., 2018 | Chloroquine protected against ischemic model injury by increasing HMGB1 (nuclear protein high mobility group tort). |
| Liu et al., 2018 | Low doses target P13K/AKT pathway increasing the P13K AKT ratio. |
| Zhang et al., 2017 | Acts via increasing the expression of LC3-II and inhibition of autophagy P13K-AKT signaling pathway upregulation. |
The present assessment focuses attention on chloroquine-induced dose response patterns, revealing the hormetic dose response to be a common occurrence, and widely distributed across models and endpoints. While these findings should be useful to researchers assessing biological effects of chloroquine at low doses, these findings also support the concept that hormesis is a highly generalized biological process with potentially important clinical and public health implications. The findings suggest the need for careful attention in the clinical application of this agent, especially within the context of possible considerable interindividual variability. Recognition of the possibility of hormetic dose responses has the potential to be an important new consideration for both the clinician and laboratory researcher.
CRediT authorship contribution statement
Edward J. Calabrese: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Funding acquisition. Jaap C. Hanekamp: Writing - review & editing. Yannic N. Hanekamp: Writing - review & editing. Rachna Kapoor: Writing - review & editing. Gaurav Dhawan: Writing - review & editing. Evgenios Agathokleous: Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
EJC acknowledges longtime support from the U.S. Air Force (AFOSR FA9550-19-1-0413) and ExxonMobil Foundation (S18200000000256). The U.S. Government is authorized to reproduce and distribute for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the author and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors had no involvement in study design, collection, analysis, interpretation, writing and decision to and where to submit for publication consideration.
Editor: Damia Barcelo
References
- Adeako A.O., Dada O.A. Chloroquine excretion in semen following antimalarial-drug administration. Andrologia. 1998;26:165–166. doi: 10.1111/j.1439-0272.1994.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Ajayi A.A., Oluokun O., Sofowora O., Akinleye A., Ajayi A.T. Epidemiology of antimalarial-induced pruritis in Africans. Eur. J. Clin. Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- Al-Bari A.A. Chloroquine analogues in drug discovery; new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I., Alharbi O.M.L. COVID-19: disease, management, treatment, and social impact. Sci. Total Environ. 2020;728:138861. doi: 10.1016/j.scitotenv.2020.138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau D., Holmes K. In: The Nidoviruses, Coronavirues and Arterivirues. Lavi E., editor. Kluwer; NY: 2001. Human coronavirus HcoV-229E enters susceptible ells via the endocyteic pathway; pp. 193–197. [DOI] [PubMed] [Google Scholar]
- Bonsch C., Kempf C., Mueller I., Manning L., Laman M., Davis T.M.E., Ros C. Chloroquine and its derivatives exacerbate B19V-assocaited anemia by promoting viral replication. Plos. 2010;4 doi: 10.1371/journal.pntd.0000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke L., McCormick J., Taylor V., Pericleous C., Blanchet B., Costedoat-Chalumeau N., Stuckey D., Lythgoe M.F., Stephanou A., Ioannou Y. Hydroxychloroquine protects against cardiac ischaemia/reperfusion injury in vivo via enhancement of ERK1/2 phosphorylation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygbjerg I.C., Flachs H. Effect of chloroquine on human lymphocyte proliferation. Trans. Royal Soc. Trop. Med. Hyg. 1986;80:231–235. doi: 10.1016/0035-9203(86)90021-0. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecototoxicology and Environmental Safety. 1999;42:135–137. doi: 10.1006/eesa.1998.1729. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Overcompensation stimulation: a mechanism for hormetic effects. Crit. Reviews of Toxicol. 2001;31:425–470. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Hormesis: why it is important to toxicology and toxicologists. Environ. Toxicol. Chem. 2008;27:1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Modulation of the epileptic seizure threshold: implications of biphasic dose responses. Crit. Rev. Toxicol. 2008;38:543–556. doi: 10.1080/10408440802014261. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013;43:580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Preconditioning is hormesis. Part 1: documentation, dose-response features and mechanistic foundations. Pharm. Res. 2016;110:242–264. doi: 10.1016/j.phrs.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Preconditioning is hormesis. Part II. How the conditioning dose mediates protection: dose optimization with temporal and mechanistic frameworks. Pharm. Res. 2016;110:265–275. doi: 10.1016/j.phrs.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Baldwin L.A. A quantitatively-based methodology for the evaluation of chemical hormesis. Hum. Ecol. Risk Asses. 1997;3:545–554. [Google Scholar]
- Calabrese E.J., Blain R.B. The occurrence of hormetic dose response in the toxicological literature, the hormesis data base: an overview. Toxicol. Appl. Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Blain R.B. The occurrence of hormetic dose responses in the toxicological literature. Reg. Toxicol. Pharm. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Mattson M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. Journ. Cell Comm. Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J., Mattson M.P. How does hormesis impact biology, toxicology and medicine. NPJ Aging Mech. Dis. 2017;3 doi: 10.1038/s41514-017-0013-z. (UNSP 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J., Dhawan G., Kapoor R., Iavicoli I., Calabrese V. Hormesis: a fundamental concept with widespread biological and biomedical applications. Gerontology. 2016;62:530–535. doi: 10.1159/000441520. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Agathokleous E., Kozumbo W.J., Stanek E.J., Leonard D.L. Estimating the range of the maximum hormetic stimulatory responses. Environ. Res. 2019;170:337–343. doi: 10.1016/j.envres.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Castelli M., Baggio G., Ruberto A.I., Tampieri A., Rossi T., Bossa M.R., an Galatulas, I. Influence of antimalarials chloroquine, quinine, primaquine, and mepacrine on the evolution of Ehrlich ascites tumour. Anticancer Res. 1996;16:1–3. [PubMed] [Google Scholar]
- Chillakura R.A., Ryu D.D., Yilma T. Propagation of recombinant vaccinia virus in HeLa cells: adsorption kinetics and replication in batch cultures. Biotechnol. Prog. 1991;7:85–92. doi: 10.1021/bp00008a002. [DOI] [PubMed] [Google Scholar]
- Choi M.M., Kim E.A., Choi S.Y., Kim T.U., Tae U., Yang S.J. Inhibitory properties of nerive-specific human glutamate dehydrogenase iszyme by chlroquine. J. Biochem. Molecul. Biol. 2007;40:1077–1082. doi: 10.5483/bmbrep.2007.40.6.1077. [DOI] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudazzo G., Smart D.J., McHugh D., Vanscheeuwijck P. Lysosomotropic-related limitations of the BALB/c 3T3 cell-based neutral red uptake assay and an alternative testing approach for assessing e-liquid cytotoxicity. Toxicol. in Vitro. 2019;61:104647. doi: 10.1016/j.tiv.2019.104647. [DOI] [PubMed] [Google Scholar]
- Gendrot M., Javelle E., Clerc A., Savini H., Pradines B. Chloroquine as a prophylactic agent against COVID-19? Int. J. Antimicrob. Agents. 2020;55:105980. doi: 10.1016/j.ijantimicag.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.D., Young K.K., Lehto S.G., Smith S.B., Mogil J.S. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Hargreaves C.A., Rogers S., Hills F., Rahman F., Howell R.J.S., Homa S.T. Effects of co-trimoxazole, erythromycin, amoxicillin, tetracycline and chloroquine on sperm function in vitro. Hum. Reprod. 1998;13:1878–1886. doi: 10.1093/humrep/13.7.1878. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Yamamoto H., Atta M.S.M., Mahmoud S., O-hashi K., Kiuchi K. Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. J. Neurochem. 2011;119:839–847. doi: 10.1111/j.1471-4159.2011.07464.x. [DOI] [PubMed] [Google Scholar]
- Jarzyna R., Zablocki K., Lietz T., Bryla J. Opposite effects of polyamines on glutamate deamination in isolated renal tubules and permeabilized kidney cortex mitochondria of rabbit. Biochem. Molecul. Biol. Intern. 1995;37:795–803. [PubMed] [Google Scholar]
- Jimenez-Alonso J., Tercedor J., Jaimez L., Garcia-Lora E. Antimalarial drug-induced aquagenic-type pruritis in patients with lupus. Arthritis Rheum. 1998;41:744–745. doi: 10.1002/1529-0131(199804)41:4<744::AID-ART26>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Karmali R.A., Horrobin D.F., Menezes J., Patel P., Musto J. Chloroquine enhance Epstein-Barr virus expression. Nature. 1978;275:444–445. doi: 10.1038/275444a0. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus b chloroquine. Biochem Biophys. Res. Comm. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontar S., Imrichova D., Bertova A., Mackova K., Poturnayova A., Sulova Z., Breier A. Ell death effects induced by sulforaphane and allyl isothiocyanate on P-glycoprotein positive and negative variants in L1210 cells. Molecules. 2020;25:2093. doi: 10.3390/molecules25092093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroumalis E.A., Koskinas J. Treatment of chronic active hepatitis B (CAH B) with chloroquine: a preliminary report. Ann. Acad. Med. Singap. 1986;15:149–152. [PubMed] [Google Scholar]
- Leak R.K., Calabrese E.J., Kozumbo W.J., Gidday J.M., Johnson T.E., Mitchell J.R., Ozaki C.K., Wetzker R., Bast A., Belz R.G., Botker H.E., Koch S., Mattson M.P., Simon R.P., Jirtle R.L., Andersen M.E. Enhancing and extending biological performance and resilience. Dose Response. 2018;16 doi: 10.1177/1559325818784501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Han C., Yu H., Zhu W., Cut H., Zheng L., Zhang C., Yue L. Chloroquine inhibits cell growth in human A549 lung cancer cells by blocking autophagy and inducing mitochondrial-mediated apoptosis. Oncol. Rep. 2018;39:2807–2816. doi: 10.3892/or.2018.6363. [DOI] [PubMed] [Google Scholar]
- Ma X., Zhang Y., Zhu D., Chen Z., Xu M., He L., Shi T., Huang I., Zou J. Low dosage chloroquine protects retinal ganglion cells against glutamate-induced cell death. Exper. Eye Res. 2019;181:285–293. doi: 10.1016/j.exer.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Maheshwari R.K., Srikantan V., Bhartiya D. Chloroquine enhances replication of Semliki Forest virus and encepalomycarditis virus in mice. J. Virol. 1991;6:992–995. doi: 10.1128/jvi.65.2.992-995.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins W.K., Severino D., Souza C., Stolf B.S., Baptista M.S. Rapid screening of potential autophagic inductor agents using mammalian cell lines. Biotechnol. J. 2013;8:730–737. doi: 10.1002/biot.201200306. [DOI] [PubMed] [Google Scholar]
- Misinzo G., Delputte P.L., Lefebvre D.J., Nauwynck H.J. Increased yield of porcine circovirus-2 b a combined treatment of PK-15 cells with interferon-gamma and inhibitors of endosomal-lysosomal system acidification. Arch. Virol. 2008;154:337–342. doi: 10.1007/s00705-007-1092-0. [DOI] [PubMed] [Google Scholar]
- Monma H., Iida Y., Moritani T., Okimoto T., Tanino R., Tajima Y., Harada M. Chloroquine augments TRAIL-induced apoptosis and induces G2/M phase arrest in human pancreatic cancer cells. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P., Attia M.B., Belaidi M. Effects of chloroquine on pentylenetetrazol-induced convulsions in mice. Pharm. Res. 1994;20(2):99–103. doi: 10.1016/1043-6618(94)80001-4. [DOI] [PubMed] [Google Scholar]
- Norman C., Gombe S. Stimulatory effect of the lysosomal stabilizer, chloroquine, on the respiration and motility of fresh and aged bovine spermatozoa. J. Reprod. Fert. 1975;44:481–485. doi: 10.1530/jrf.0.0440481. [DOI] [PubMed] [Google Scholar]
- Okanlawon A.O., Noronha C.C., Ashiru O.A. An investigation into the effects of chloroquine on fertility of male rats. E. African J. Med. 1993;12:118–121. [PubMed] [Google Scholar]
- Onigbogi O., Ajayi A.A., Ukponmwan O.E. Mechanisms of chloroquine-induced body-scratching behavior in rats: evidence of involvement of endogenous opioid peptides. Pharm Biochem Behav. 1999;65:333–337. doi: 10.1016/s0091-3057(99)00221-x. [DOI] [PubMed] [Google Scholar]
- Osifo N.F. Chloroquine-induced pruritis among patienws with malaria. Arch. Dermatol. 1984;120:80–82. doi: 10.1001/archderm.1984.01650370086015. [DOI] [PubMed] [Google Scholar]
- Pardridge W.M., Yang J., Diague A. Chloroquine inhibits HIV-1 rplicatio in human peripheral blood lymphyctes. Immunol. Lett. 1998;64:45–47. doi: 10.1016/s0165-2478(98)00096-0. [DOI] [PubMed] [Google Scholar]
- Park D., Lee Y. Biphasic activity of chloroquine in human colorectal cancer cells. Dev. Reprod. 2014;18:225–231. doi: 10.12717/DR.2014.18.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo S. Time to use a dose of chloroquine as an adjuvant to anti-cancer chemotherapies. Europ. J. Pharmacology. 2016;771:139–144. doi: 10.1016/j.ejphar.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Pastick K.A., Okafor E.C., Wang F., Lofgren S.M., Skipper C.P., Nicol M.R., Pullen M.F., Rajasingham R., McDonald E.G., Lee T.C., Schwartz I.S., Kelly L.E., Lother S.A., Mitjà O., Letang E., Abassi M., Boulware D.R. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open For. Inf. Dis. 2020;7 doi: 10.1093/ofid/ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi S., Ruprecht N., Bonsch C., Bieli S., Zurcher C., Boller K., Kempf C., Ros C. Characterizationof the early steps of human parvovirus B19 infection. J. Virol. 2012;86:9274–9284. doi: 10.1128/JVI.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi T., Coppi A., Bruni E., Ruberto A., Santachiara S., Baggio G. Effects of anti-malarial drugs on MCF-7 and vero cell replications. Anticancer Res. 2007;27:2555–2560. [PubMed] [Google Scholar]
- Saverino A., Gennero L., Sperber K., Boelaert J.R. The anit-HIV-1 activity of chlorquine. J. Clin. Virol. 2001;20:131–135. doi: 10.1016/s1386-6532(00)00139-6. [DOI] [PubMed] [Google Scholar]
- Saverino A., Boelaer J.R., Casson A., Majoori G., Caua R. Effects of chloroquine on vital infections: an old drug against today’s diseases? Lancet Infexct. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Mani H., Singh A.K., Banaudha K.K., Madhavan S., Sidhu G.S., Gaddipati J.P., Vogel S.N., Maheshwari R.K. Acceleration of viral replication and up-regulation of cytokine levels by antimalarials: implications in malaria-endemic areas. Amer. J. Trop Med Hyg. 1999;61:180–186. doi: 10.4269/ajtmh.1999.61.180. [DOI] [PubMed] [Google Scholar]
- Shukla A.M., Bose C., Karaduta O.K., Apostolov E.O., Kaushal G.P., Fahmi T., Segal M.S., Shah S.V. Impact of hydroxychloroquine on atherosclerosis and vascular stiffness in the presence of chronic kidney disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinet P.M., Verdier F., Charmot G., Desforges B., Gaudin C., Pocidalo J.J. Effets de la chloroquine sur la replication d’un retrovirus murin. Bull. Soc. Path. Ex. 1996;89:175–178. [PubMed] [Google Scholar]
- Singh A.K., Sidhu G.S., Friedman R.M., Maheshwari R.K. Mechanism of enhancement of the antiviral action of interferon against herpes simplex virus-1 by chloroquine. Journ. Interferon Cytokine Res. 1996;16:725–731. doi: 10.1089/jir.1996.16.725. [DOI] [PubMed] [Google Scholar]
- Stone J., Mitrofanis J., Johnstone D.M., Falsini B., Bisti S., Adam P., Nuevo A.B., George-Weinstein M., Mason R., Eells J. Acquired resilience: an evolved system of tissue protection in mammals. Dose Response. 2018;16 doi: 10.1177/1559325818803428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T.T., Lv L.L., Pan M.M., Wen Y., Wang B., Li Z.L., Wu M., Wang F.M., Crowley S.D., Liu B.C. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9:351. doi: 10.1038/s41419-018-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrason G., Carcasona C., Eichhorn P., Perez B., Gavaida A., Godessart N. Characterization of the chloroquine-induced mouse model of pruritus using an automated behavioural system. Exper. Derm. 2017;26:1105–1111. doi: 10.1111/exd.13392. [DOI] [PubMed] [Google Scholar]
- Todorovic Z., Medic B., Basta-Jovanovic G., Skodric S.R., Stojanovic R., Rovcanin B., Prosran M. Acute pretreatment with chloroquine attenuates renal I/R injury in rats. PLoS One. 2014;9:92673. doi: 10.1371/journal.pone.0092673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.P., Nara P.L., Kung S., Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retrovir. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- Wu L., Dai J., Zhao X., Chen Y., Wang G., Li K. Chloroquine enhances replication of influenza A virus A/WSN/33 (H1N1) in dose-, time-, and MOI-dependent manners in human lung epithelial cells Z549. Jour. Med. Virology. 2015;87:1096–1103. doi: 10.1002/jmv.24135. [DOI] [PubMed] [Google Scholar]
- Xu J., Cui X., Li J., Koutakis P., Pipinos I., Tzeng E., Chen A., Sachdev U. Chloroquine improves the response to ischemic muscle injury and increases HMCB1 after arterial ligation. J. Vasc. Surg. 2018;67:910–921. doi: 10.1016/j.jvs.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K., Yamamoto N., Yanagisawa K. Chloroquine-induced endocytic pathway abnormalities: cellular model of GM1 ganglioside-induced A beta fibrillogenesis in Alzheimer’s disease. FEB Letters. 2006;580:6972–6976. doi: 10.1016/j.febslet.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ma Wenge, Xie B., Gui J.F., Mei J. Beneficial effect and potential molecular mechanism of chloroquine on sperm motility and fertilizing ability in yellow catfish. Aquaculture. 2017;468:307–313. [Google Scholar]