Abstract
Background
Multiple major health organisations recommend the use of extracorporeal membrane oxygenation (ECMO) support for COVID-19-related acute hypoxaemic respiratory failure. However, initial reports of ECMO use in patients with COVID-19 described very high mortality and there have been no large, international cohort studies of ECMO for COVID-19 reported to date.
Methods
We used data from the Extracorporeal Life Support Organization (ELSO) Registry to characterise the epidemiology, hospital course, and outcomes of patients aged 16 years or older with confirmed COVID-19 who had ECMO support initiated between Jan 16 and May 1, 2020, at 213 hospitals in 36 countries. The primary outcome was in-hospital death in a time-to-event analysis assessed at 90 days after ECMO initiation. We applied a multivariable Cox model to examine whether patient and hospital factors were associated with in-hospital mortality.
Findings
Data for 1035 patients with COVID-19 who received ECMO support were included in this study. Of these, 67 (6%) remained hospitalised, 311 (30%) were discharged home or to an acute rehabilitation centre, 101 (10%) were discharged to a long-term acute care centre or unspecified location, 176 (17%) were discharged to another hospital, and 380 (37%) died. The estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 37·4% (95% CI 34·4–40·4). Mortality was 39% (380 of 968) in patients with a final disposition of death or hospital discharge. The use of ECMO for circulatory support was independently associated with higher in-hospital mortality (hazard ratio 1·89, 95% CI 1·20–2·97). In the subset of patients with COVID-19 receiving respiratory (venovenous) ECMO and characterised as having acute respiratory distress syndrome, the estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 38·0% (95% CI 34·6–41·5).
Interpretation
In patients with COVID-19 who received ECMO, both estimated mortality 90 days after ECMO and mortality in those with a final disposition of death or discharge were less than 40%. These data from 213 hospitals worldwide provide a generalisable estimate of ECMO mortality in the setting of COVID-19.
Funding
None.
Introduction
The severity of COVID-19-related acute hypoxaemic respiratory failure1, 2 and clinical evidence supporting extracorporeal membrane oxygenation (ECMO) in the acute respiratory distress syndrome (ARDS)3, 4, 5 prompted several international organisations including the World Health Organization (WHO),6 Surviving Sepsis Campaign,7 and Extracorporeal Life Support Organization (ELSO)8 to consider a role for ECMO support during the current pandemic.9 WHO recommended that expert centres with sufficient ECMO volume to maintain proficiency consider ECMO support in COVID-19-related ARDS with refractory hypoxaemia if lung protective mechanical ventilation10 was insufficient to support the patient.6 Despite such optimism for a possible role for ECMO in both acute respiratory and cardiac failure, early reports of patients with COVID-19 requiring ECMO suggested that mortality could be greater than 90%.11
ELSO is an international organisation that maintains a registry of ECMO cases among its member centres. In March, 2020, the ELSO Registry augmented its data capture with an addendum designed for ECMO-supported patients with COVID-19 to obtain additional information on these patients.12 In this study, we used data from the ELSO Registry to report the epidemiology, treatment, outcomes, and hospital characteristics of patients receiving ECMO with a confirmed diagnosis of COVID-19. Additionally, we examined whether patient factors and historical hospital ECMO case volume were associated with in-hospital mortality.
Research in context.
Evidence before this study
We searched PubMed for articles published in English or with English language abstracts up to Aug 7, 2020, with the Medical Subject Heading terms (“extracorporeal membrane oxygenation”) and either the supplementary concept (“COVID-19”) or (“severe acute respiratory syndrome coronavirus 2”). The search identified 71 manuscripts; review of these identified 13 additional manuscripts. Of the total 84 publications, 38 included clinical data from extracorporeal membrane oxygenation (ECMO)-supported patients with COVID-19. The 38 studies identified 202 ECMO-supported patients with COVID-19. These initial reports are small case series reporting outcomes across few centres.
Added value of this study
Novel findings in this study include determination of independent associations between mortality and risk factors for ECMO-supported patients with COVID-19. Identified risk factors were age, immunocompromised state, chronic respiratory disease, pre-ECMO cardiac arrest, degree of hypoxaemia, presence of acute kidney injury, and use of ECMO for temporary circulatory support (venoarterial ECMO support vs venovenous ECMO support). Strengths of this study include the breadth of international participation and its use of experienced and trained Extracorporeal Life Support Organization site data managers to collect data.
Implications of all the available evidence
This study of patients with COVID-19 who received ECMO in more than 200 hospitals in 36 countries provides a generalisable estimate of ECMO mortality, and supports existing recommendations to consider use of ECMO in refractory COVID-19-related respiratory failure when performed in experienced centres.
Methods
Data source and population
The ELSO Registry is an international ECMO registry with data for more than 125 000 patients. All ELSO site data managers receive detailed instructions and database definitions to guide data entry, and they all must pass the data entry exam in order to enter data into the ELSO Registry. Accuracy is augmented by a point-of-entry data assessment with error and validity checks. There is also a full record validation triggered on submission of the record that ensures all mandatory fields are completed.13
This analysis of anonymised data from the ELSO Registry was determined to be exempt from human participant review by the Institutional Review Board of the University of Michigan Medical School. The data collected consist of the standard elements reported for all ECMO runs and additional elements entered into the newly created COVID-19 addendum (appendix pp 10–11).
All patients diagnosed with COVID-19, aged 16 years or older, who had ECMO support initiated as recorded in the ELSO registry between Jan 16 and May 1, 2020, were included in the analysis. A case of COVID-19 was defined as the confirmed presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on laboratory testing. A priori, we chose to stop enrolling patients who were initiated on ECMO support after May 1, 2020. Follow-up data were updated until Aug 3, 2020.
We additionally report results for the subset of ECMO-supported patients with COVID-19 who met the following two criteria: (1) classified by the ELSO data manager as having ARDS, and (2) initial mode of ECMO support was venovenous ECMO. This subset of patients provided a more focused report on patients classified to have ARDS and receiving respiratory support alone via venovenous ECMO. We also report on the subgroup of patients without ARDS and the subgroup receiving ECMO with circulatory support (see appendix p 11 for description).
Outcomes
The primary outcome was in-hospital death in a time-to-event analysis assessed at 90 days after ECMO initiation. A time-to-event outcome was necessary because all patients might not have a final disposition at the time of database lock, and calculating in-hospital mortality without accounting for differential follow-up between patients would result in length-time bias. Records in which the last update indicated that the patient had not died, been discharged, or completed 90 days of follow-up after ECMO initiation were administratively censored at the time of their last update. A patient being discharged alive to home or to an acute rehabilitation centre, discharged to a long-term acute care centre or unspecified location, or discharged to another hospital were treated as distinct competing events for the primary outcome of in-hospital mortality. We also report proportion of in-hospital deaths in patients who reached a final disposition of death or discharge from the hospital.
Secondary outcomes were the proportion of patients remaining in the intensive care unit (ICU), discharged from the ICU but who remain hospitalised, discharged to home or an acute rehabilitation centre, discharged to a long-term acute care centre or unspecified location, and discharged to another hospital. We report ECMO duration, hospital length of stay, tracheostomy use, discharge location, acute kidney injury related to the current illness, use of renal replacement therapy during ECMO (regardless of indication), and the occurrence of complications while receiving ECMO. We also report the reason ECMO was discontinued, and we report the time to death after ECMO discontinuation in patients who died in hospital.
Statistical analysis
Descriptive statistics are provided as median (IQR) for continuous variables and as count and proportion for categorical variables. We calculated the Aalen–Johansen estimators of cumulative incidence of in-hospital mortality measured 90 days after ECMO initiation with discharged alive to home and discharged alive to another location treated as competing events.14 We estimated the cumulative incidence (rather than report the proportion) of in-hospital mortality 90 days after ECMO initiation because not all patients had died, been discharged from the hospital, or were followed up for 90 days. The validity of this estimator requires the standard independent censoring assumption15—namely, that the mechanism in which patients are censored is statistically independent of the mechanisms in which patients die or are discharged (appendix pp 11–12). To account for follow-up that is cut short by event incidence (death or discharge), we estimated potential follow-up using reverse Kaplan-Meier methodology.16 We estimated the distribution of ECMO duration and time to hospital discharge (for any reason) using Kaplan-Meier estimators.17
To estimate the relative risks between potential risk factors and mortality, we fit a Cox proportional hazards model for the primary outcome of death (appendix pp 12–14, 19). We censored patients who were still hospitalised at the time of the run's last registry update and those who were discharged alive at the time of their discharge date. The Cox model estimated the hazard for death as a function of a linear combination of the following prespecified set of patient-level variables: age, race, sex, chronic cardiac disease, chronic respiratory disease (excluding asthma), asthma, diabetes, cancer, immunocompromised state, duration of pre-ECMO intubation, the partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2:FiO2), the partial pressure of arterial carbon dioxide, a diagnosis of acute kidney injury, cardiac arrest before ECMO, and initial ECMO mode (venovenous vs venoarterial or venovenoarterial). Venovenous ECMO support drains and returns blood to the systemic venous system to support the lungs, whereas venoarterial ECMO support drains systemic venous blood and returns blood to the systemic arterial system and can provide heart and lung support. Venovenoarterial ECMO returns blood to both the venous and the arterial systems, and provides both heart and lung support.18
We generated a second Cox model to estimate the hazard for death that was identical to the previous one with addition of the centre-level covariate, 2019 adult ECMO case volume. This model excluded ECMO-supported patients with COVID-19 who received care at an ECMO centre that had no cases reported to the ELSO Registry in 2019 or earlier (a new ELSO centre). If a centre entered reported cases before 2019, but had no cases in 2019 (an existing ELSO centre), that centre's cases were included and the 2019 ECMO centre volume was measured as zero.
Multiple imputation was used to account for missing values in predictor variables. Briefly, we used fully specified chained equations in the R package.19 Ten imputed datasets were created and combined using between/within variance techniques to appropriately propagate uncertainty about the missing data (see appendix pp 13–14 for full details). All continuous variables apart from age were log2-transformed before model fitting. The parameter of interest from a Cox model is the hazard ratio (HR), which describes the relative risk of in-hospital mortality associated with a change in a covariate. Robust sandwich-type estimates of the standard errors were used to account for centre-level clustering. All analyses were done with R version 3.6.1.20, 21
Role of the funding source
There was no funding source for this study. RPB, PSB, and PTR had full access to all the data in the study. RPB, GM, and DB had final responsibility for the decision to submit for publication.
Results
In the ELSO Registry, 1093 patients aged 16 years or older with confirmed COVID-19 received ECMO support from Jan 16 to May 1, 2020. 57 patients were excluded because there was not a completed COVID-19 addendum (appendix pp 20, 32). One patient had a previous ECMO run before diagnosis of COVID-19, leaving 1035 patients for analysis.
The median age of eligible patients was 49 years (IQR 41–57) and median body-mass index was 31 kg/m2 (27–37; table 1 ). 764 (74%) of 1033 patients were men, and 724 (70%) of 1035 had at least one pre-ECMO comorbidity. 819 (79%) of 1035 patients were identified as having ARDS, 301 (29%) had acute kidney injury, 50 (5%) had acute heart failure, and 22 (2%) had myocarditis. 216 (21%) of 1035 patients were not identified as having ARDS (appendix pp 15, 21–22). Viral or bacterial co-infections were suspected or confirmed in 387 (37%) of 1033 patients (table 1 and appendix pp 23–27). Staphylococcus aureus was the most commonly cultured organism for bacterial pneumonia and bloodstream infections.
Table 1.
Patient characteristics before initiation of ECMO
| Full cohort (n=1035) | ARDS cohort*(n=779) | ||
|---|---|---|---|
| Age (years) | 49 (41–57) | 50 (42–57) | |
| BMI (kg/m2)† | 31 (27–37) | 32 (28–37) | |
| Sex‡ | |||
| Male | 764 (74%) | 572 (74%) | |
| Female | 269 (26%) | 206 (26%) | |
| Race and ethnicity | |||
| Black | 150 (14%) | 119 (15%) | |
| White (non-Hispanic) | 346 (33%) | 250 (32%) | |
| Asian | 152 (15%) | 86 (11%) | |
| Middle Eastern or North African | 35 (3%) | 26 (3%) | |
| Other | 27 (3%) | 21 (3%) | |
| Unknown | 54 (5%) | 38 (5%) | |
| Multiple | 53 (5%) | 51 (7%) | |
| Hispanic | 218 (21%) | 188 (24%) | |
| Pre-ECMO comorbidities | |||
| No comorbidity | 311 (30%) | 243 (31%) | |
| Cancer | 11 (1%) | 10 (1%) | |
| Immunocompromised | 24 (2%) | 21 (3%) | |
| Diabetes | 245 (24%) | 187 (24%) | |
| Pre-existing cardiac disease | 24 (2%) | 13 (2%) | |
| Pre-existing respiratory disease | 29 (3%) | 21 (3%) | |
| Pre-existing renal insufficiency | 21 (2%) | 14 (2%) | |
| Asthma | 110 (11%) | 91 (12%) | |
| Pregnancy | 22 (2%) | 13 (2%) | |
| Obesity (BMI >30 kg/m2) | 487 (47%) | 362 (47%) | |
| Acute illness | |||
| ARDS | 819 (79%) | 775 (100%) | |
| Acute heart failure | 50 (5%) | 25 (3%) | |
| Myocarditis | 22 (2%) | 7 (1%) | |
| Acute kidney injury | 301 (29%) | 247 (32%) | |
| Pre-ECMO cardiac arrest§ | 48 (5%) | 26 (3%) | |
| Pre-ECMO co-infection¶ | |||
| No co-infection | 646 (63%) | 479 (62%) | |
| Bacterial pneumonia | 337 (33) | 271 (35%) | |
| Viral co-infection | 90 (9%) | 60 (8%) | |
| Bloodstream infection | 123 (12%) | 106 (14%) | |
| Urinary tract infection | 38 (4%) | 31 (4%) | |
Data are median (IQR) or n (%). ARDS=acute respiratory distress syndrome. BMI=body-mass index. ECMO=extracorporeal membrane oxygenation.
The ARDS cohort were the subset of ECMO-supported patients with COVID-19 who met the following two criteria: (1) classified by the Extracorporeal Life Support Organization data manager as having ARDS, and (2) initial mode of ECMO support was venovenous ECMO.
Full cohort n=939; ARDS cohort n=698.
Full cohort n=1033; ARDS cohort n=778.
Full cohort n=1019; ARDS cohort n=766.
Full cohort n=1033; ARDS cohort n=777.
Most patients (704 [70%] of 1010) received care at another hospital before being transferred to an ELSO centre. Of these, 330 (47%) had ECMO initiated at an outside hospital and were transported while receiving ECMO support. ECMO support for patients with COVID-19 was provided at 213 centres in 36 countries. By comparison, non-COVID-19-related adult ECMO was provided in 395 centres in 48 countries during all of 2019. The median 2019 adult ECMO case volume was 23 (IQR 3–62; appendix pp 16, 28).
Before ECMO initiation, median PaO2:FiO2 within 6 h was 72 mm Hg (IQR 59–94) and most patients received neuromuscular blockade (729 [72%] of 1015), prone positioning (612 [60%] of 1019), and non-invasive ventilatory support before endotracheal intubation (606 [59%] of 1032; table 2 ). Patients were also commonly supported with vasoactive medications (606 [60%] of 1015) before ECMO initiation, such as norepinephrine in 561 (55%) of 1015 patients. 786 (76%) of 1035 patients received therapy targeting COVID-19; the most common therapy was hydroxychloroquine or chloroquine (538 [52%] of 1035 patients).
Table 2.
Supportive care and therapies delivered before ECMO
|
Full cohort (n=1035) |
ARDS cohort*(n=779) |
||||
|---|---|---|---|---|---|
| N | Median (IQR) or n (%) | N | Median (IQR) or n (%) | ||
| Non-invasive ventilation | |||||
| Non-invasive ventilation before intubation | 1032 | 606 (59%) | 776 | 434 (56%) | |
| BiPAP | 1032 | 185 (18%) | 776 | 119 (15%) | |
| CPAP | 1032 | 140 (14%) | 776 | 80 (10%) | |
| HFNC | 1032 | 357 (35%) | 776 | 285 (37%) | |
| Pre-ECMO intubation (days) | 914 | 4·0 (1·8–6·4) | 688 | 4·3 (2·0–6·5) | |
| Conventional ventilation† | 951 | 942 (99%) | 729 | 721 (99%) | |
| PEEP (cm H2O) | 868 | 14 (12–16) | 661 | 15 (12–18) | |
| PIP (cm H2O) | 699 | 33 (30–38) | 532 | 34 (30–38) | |
| FiO2 | 888 | 1·0 (0·90–1·0) | 672 | 1·0 (0·90–1·0) | |
| PaO2:FiO2 (mm Hg) | 868 | 72 (59–94) | 657 | 72 (60–93) | |
| PaCO2 (mm Hg) | 896 | 60 (50–74) | 678 | 60 (50–74) | |
| Pre-ECMO support | |||||
| Prone positioning | 1019 | 612 (60%) | 766 | 464 (61%) | |
| Neuromuscular blockade | 1015 | 729 (72%) | 762 | 567 (74%) | |
| Inhaled pulmonary vasodilators | 1019 | 293 (29%) | 766 | 242 (32%) | |
| Any vasoactive support | 1015 | 606 (60%) | 758 | 447 (59%) | |
| Norepinephrine | 1015 | 561 (55%) | 762 | 416 (55%) | |
| COVID-19 therapies and immunomodulators | |||||
| Any therapy | 1035 | 786 (76%) | 779 | 633 (81%) | |
| Glucocorticoids | 1035 | 420 (41%) | 779 | 331 (42%) | |
| Intravenous immunoglobin | 1035 | 29 (3%) | 779 | 22 (3%) | |
| Anticytokine | 1035 | 289 (28%) | 779 | 261 (34%) | |
| Lopinavir–ritonavir | 1035 | 116 (11%) | 779 | 103 (13%) | |
| JAK inhibition | 1035 | 7 (0·7%) | 779 | 5 (0·6%) | |
| Chloroquine or hydroxychloroquine | 1035 | 538 (52%) | 779 | 443 (57%) | |
| Remdesivir | 1035 | 84 (8%) | 779 | 69 (9%) | |
| Support type | |||||
| Respiratory | 1035 | 995 (96%) | 779 | 777 (99·7%) | |
| Cardiac | 1035 | 29 (3%) | 779 | 0 | |
| ECPR | 1035 | 11 (1%) | 779 | 2 (0·3%) | |
ARDS=acute respiratory distress syndrome. BiPAP=bilevel positive airway pressure. CPAP=continuous positive airway pressure. ECMO=extracorporeal membrane oxygenation. ECPR=extracorporeal cardiopulmonary resuscitation. FiO2=fraction of inspired oxygen. HFNC=high flow nasal cannula. JAK=Janus kinase. PaCO2=partial pressure of arterial carbon dioxide. PaO2:FiO2=ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen. PEEP=positive end-expiratory pressure. PIP=peak inspiratory pressure.
The ARDS cohort were the subset of ECMO-supported patients with COVID-19 who met the following two criteria: (1) classified by the Extracorporeal Life Support Organization data manager as having ARDS, and (2) initial mode of ECMO support was venovenous ECMO.
Mode of mechanical ventilation, the PEEP, PIP, FiO2, PaO2:FiO2, and PaCO2are measured within 6 h before ECMO initiation and are the measure nearest to ECMO initiation while still remaining pre-ECMO initiation.
The median duration from endotracheal intubation to ECMO initiation was 4·0 days (IQR 1·8–6·4). The overwhelming majority of patients received venovenous ECMO (978 [94%] of 1035); 44 (4%) of 1035 patients received venoarterial ECMO support, nine (0·9%) received venovenoarterial support, and four (0·4%) received other ECMO support. After ECMO initiation, the median peak inspiratory pressure was reduced by 9 cm H2O (IQR 4–13), and the median FiO2 was reduced by 0·40 (IQR 0·00–0·60; see appendix p 29 for ventilator settings and blood gas measures 24 h after ECMO initiation). The Kaplan-Meier median duration of ECMO support was 13·9 days (IQR 7·8–23·3). A tracheostomy was performed in nearly half of patients (444 [44%] of 1003; table 3 ).
Table 3.
Outcomes
| Full cohort (n=1035) | ARDS cohort*(n=779) | ||
|---|---|---|---|
| Patient status at study completion | |||
| Discharged alive to home or acute rehabilitation centre | 311 (30%) | 262 (34%) | |
| Discharged alive to long-term acute care centre or unspecified location | 101 (10%) | 79 (10%) | |
| Discharged to another hospital | 176 (17%) | 97 (12%) | |
| Remain in the hospital (discharged from ICU) | 11 (1%) | 10 (1%) | |
| Remain in the ICU | 56 (5%) | 40 (5%) | |
| In-hospital death | 380 (37%) | 291 (37%) | |
| Tracheostomy† | 444 (44%) | 353 (47%) | |
| Select complications‡ | |||
| Seizure | 6 (0·6%) | 5 (0·7%) | |
| CNS infarct | 7 (0·7%) | 5 (0·7%) | |
| CNS haemorrhage | 56 (6%) | 44 (6%) | |
| Haemolysis | 48 (5%) | 37 (5%) | |
| Membrane lung failure | 82 (8%) | 63 (9%) | |
| Pump failure | 8 (0·8%) | 6 (0·8%) | |
| Circuit change | 148 (15%) | 99 (13%) | |
Data are n (%). ARDS=acute respiratory distress syndrome. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit.
The ARDS cohort were the subset of ECMO-supported patients with COVID-19 who met the following two criteria: (1) classified by the Extracorporeal Life Support Organization data manager as having ARDS, and (2) initial mode of ECMO support was venovenous ECMO.
Full cohort n=1003; ARDS cohort n=756. Only 1003 patients in the full cohort and 756 patients in the ARDS cohort reported whether or not they had a tracheostomy; for the remainder it was missing.
Full cohort n=983; ARDS cohort n=738. Complications were only reported in 983 patients in the full cohort and 738 patients in the ARDS cohort; in the remainder it was missing.
Renal replacement therapy was used during ECMO support (regardless of indication) in 444 (44%) of 1006 patients (data not reported by 29 patients). Other than renal replacement therapy, 538 (55%) of 983 patients had one of many prespecified complications during or immediately following ECMO support (table 3 and appendix p 30). However, individual complications occurred relatively infrequently. Central nervous system haemorrhage occurred in 56 (6%) of 983 patients, central nervous system infarct occurred in seven (0·7%) patients, and seizures occurred in six (0·6%) patients. Collectively, any mechanical complications defined as a circuit change, membrane lung failure, cannula problems, or pump failure occurred in 277 (28%) of 983 patients supported with ECMO. Individually, circuit changes occurred in 148 (15%) of 983 patients, membrane lung failures occurred in 82 (8%) patients, cannula problems occurred in 57 (6%) patients, and pump failures occurred in eight (0·8%) patients.
At the time of analysis, 968 (94%) of 1035 patients were discharged from the hospital alive, died, or reached 90-day follow-up after ECMO initiation. 588 (57%) of 1035 patients were discharged alive from the hospital, of whom 311 (30%) of 1035 were discharged to home or an acute rehabilitation centre, 101 (10%) were discharged to a long-term acute care centre or unspecified location, and 176 (17%) were discharged to another hospital (figure 1 and table 3). No patients discharged alive to another facility were discharged to hospice. Of the 968 patients with a final disposition of death or hospital discharge, 380 (39%) died. 309 (81%) of 380 patients died within24 h of discontinuation of ECMO support (appendix p 33), and 322 (85%) were discontinued from ECMO support because of a poor prognosis (appendix p 31).
Figure 1.
Stacked bar plots of disposition over time for patients with COVID-19 who received ECMO
ECMO=extracorporeal membrane oxygenation. LTAC=long-term acute care. Discharged (home or rehab) refers to patients who were discharged to home or an acute rehabilitation centre. Discharged (LTAC or unspecified) refers to patients who were discharged to an LTAC centre or to an unspecified location. Discharge (hospital) refers to patients who were discharged to another hospital. Unknown status (censored) refers to patients who at the time of data analysis did not meet one of the following three criteria: (1) died, (2) discharged alive, or (3) survived at least 90 days after ECMO initiation. Hospitalised patients are those still in hospital at the Extracorporeal Life Support Organization Centre where ECMO support was delivered.
The estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 37·4% (95% CI 34·4–40·4; figure 2 and appendix p 34). In the subset of patients receiving venovenous ECMO and characterised as having ARDS, the estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 38·0% (95% CI 34·6–41·5). When considering in-hospital mortality 90 days after the initiation of ECMO stratified by ELSO geographical region, the results were not distinct (p=0·18) for an overall test of the simultaneous equality of these cumulative incidence curves for mortality (appendix pp 15–16, 35).
Figure 2.
Cumulative incidence of mortality from time of ECMO initiation
ECMO=extracorporeal membrane oxygenation. The solid line represents the estimated cumulative incidence of mortality and the shaded area represents the 95% CI.
The Kaplan-Meier median duration of hospital stay at the ELSO centre was 26·9 days (IQR 15·7–43·0). The observed median length of stay was 31·1 days (IQR 21·0–46·0) for survivors and 16·0 days (6·8–27·6) for non-survivors. In patients who survived to hospital day 40 or more and remained hospitalised, the estimated in-hospital mortality 90 days after the initiation of ECMO was 14·1% (95% CI 10·3–18·9; appendix p 36).
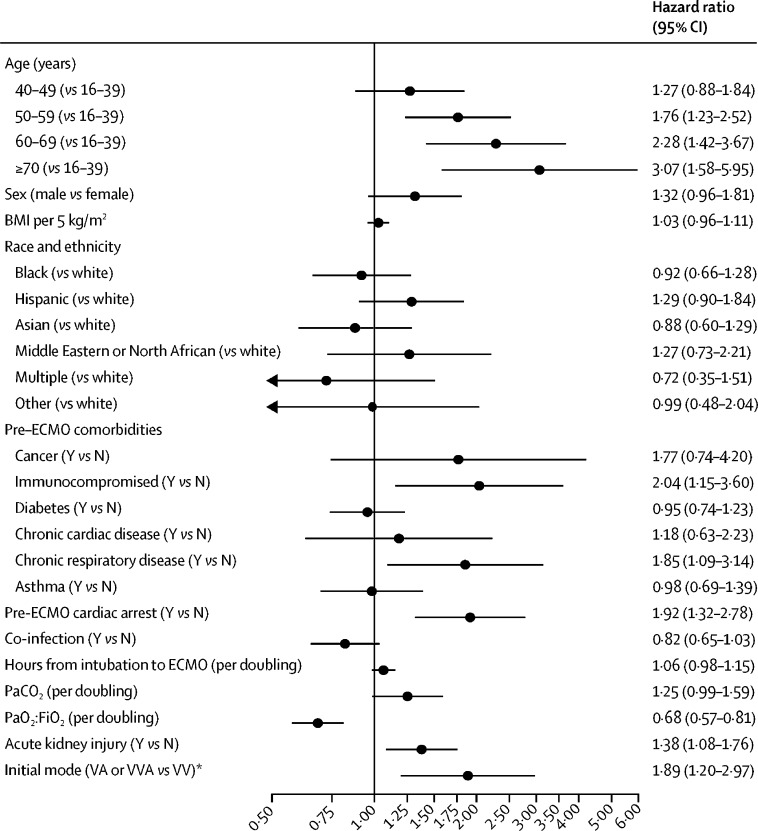
The Cox model showed that temporary circulatory support (venoarterial ECMO support) was significantly associated with in-hospital mortality (HR 1·89, 95% CI 1·20–2·97; figure 3 ). Increasing age was associated with a higher risk of in-hospital mortality for those 70 years or older relative to patients aged 16–39 years (HR 3·07, 95% CI 1·58–5·95); and higher PaO2:FiO2 was associated with lower mortality (HR 0·68 per doubling, 95% CI 0·57–0·81). Patients with acute kidney injury, chronic respiratory insufficiency, an immunocompromised state, or a pre-ECMO cardiac arrest had an associated higher risk of mortality. Sex, body-mass index, race, and hours from endotracheal intubation to ECMO initiation were not independently associated with mortality. The Cox model including centre volume suggests that higher adult 2019 hospital ECMO case volume was not substantively associated with lower mortality (HR 0·96 per doubling of 2019 hospital volume, 95% CI 0·90–1·03; appendix p 37).
Figure 3.
Cox model for factors associated with in-hospital mortality in patients with COVID-19 supported with ECMO
BMI=body-mass index. ECMO=extracorporeal membrane oxygenation. PaCO2=partial pressure of arterial carbon dioxide. PaO2:FiO2=ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen. VA=venoarterial. VV=venovenous. VVA=venovenoaterial. *Dataset of 1031 patients; four observations were excluded due to having an initial cannulation mode that was not venovenous, venoarterial, or venovenoarterial.
Discussion
This study from the international ELSO Registry provides data on 1035 ECMO-supported patients with COVID-19 who received care in 36 countries. Estimated in-hospital mortality 90 days after ECMO initiation was 37·4% (95% CI 34·4–40·4). This international report from the ELSO Registry is strengthened by its breadth, including 213 hospitals providing ECMO, and by employing experienced and trained ELSO site data managers to collect data.13 Additionally, our analytical plan (a time-to-event analysis) accounts for the potential bias that can be introduced when not all patients in the analysis have reached a final disposition.
High mortality in the initial published experience11 led some clinicians and investigators to recommend withholding ECMO support in patients with COVID-19.22 In ECMO-supported patients with COVID-19 and characterised as having ARDS, estimated in-hospital mortality 90 days after ECMO initiation was 38·0% (95% CI 34·6–41·5), consistent with previous mortality rates in non-COVID-19 ECMO-supported patients with ARDS and acute respiratory failure.3, 23 Our findings provide provisional support for the use of ECMO in COVID-19-related acute hypoxaemic respiratory failure.6, 7
In this study, higher 2019 hospital ECMO case volume was not associated with lower mortality. By contrast, a 2015 study from the ELSO Registry demonstrated that higher hospital ECMO case volume was associated with lower ECMO-supported patient mortality.24 The ELSO Registry data is a collection of self-selected and experienced centres dedicated to improving care for patients receiving ECMO support. Our findings cannot be extrapolated to inexperienced centres.
In the largest randomised controlled trial to date of ECMO for ARDS, the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial, 60-day mortality was 35% in the ECMO group versus 46% in the conventional management group (relative risk 0·76, 95% CI 0·55–1·04; p=0·09).3 A subsequent post-hoc Bayesian analysis of the EOLIA trial,4 a meta-analysis of trials of ECMO for ARDS in adults,5 and a network meta-analysis25 each provided credible support to the existence of a survival benefit of ECMO in refractory ARDS. The patients enrolled in the EOLIA study shared some similarities to the patients with ARDS in this ELSO Registry report with regards to pre-ECMO support, severity of ARDS, and mortality rates. The rate of pre-ECMO prone positioning in patients who received venovenous ECMO for COVID-19 and were characterised as having ARDS was 61% in this study and 59% in EOLIA before randomisation (appendix p 17). The median pre-ECMO PaO2:FiO2 ratio was 72 mm Hg in our study and the mean PaO2:FiO2 in EOLIA was 73 mm Hg.3 Nevertheless, it is unclear what the outcomes of patients with COVID-19 would have been had they not received ECMO.
Patients surviving critical illness,26 ARDS,27 and ECMO support23 often have disability that might require prolonged hospital stay or rehabilitation. In this study, few patients were discharged home; rather, the majority were transferred to either rehabilitation or long-term acute care facilities or another hospital to continue recovery. These data highlight the need for future studies to focus on the long-term outcomes of these patients.
In our study, nearly all patients had acute hypoxaemic respiratory failure, but only 79% were identified as meeting the definition for ARDS. In the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE) study, 78·5% (95% CI 74·8–81·8) of patients with severe ARDS were identified as having ARDS.28 In our study, it was unclear if patients not identified as having ARDS did not actually meet the Berlin Definition for ARDS or were simply not recognised as having the criteria for the diagnosis of ARDS (appendix pp 17–18).29
Our study has important limitations. First, the ELSO Registry did not externally validate the submitted data or validate that all consecutive cases initiated between Jan 16 and May 1, 2020, were submitted. The ELSO Registry does not contain data on all ECMO cases worldwide; data are collected from a subset of centres participating in the ELSO Registry. These centres were self-selected and needed to have the resources to submit patient data, particularly during a pandemic. Second, although the survival analyses for the primary outcome of mortality account for the incomplete follow-up, we might have underestimated the reported prevalence of complications during ECMO, given that not all patients had completed their ECMO course at the time of reporting. Third, our study was not a randomised controlled trial and thus we cannot draw any definitive conclusions as to whether ECMO should be used in patients with COVID-19 and severe respiratory failure. Fourth, the final outcome 90 days after ECMO initiation is unknown for patients who were discharged home or to an acute rehabilitation centre, discharged to a long-term acute centre or unspecified location, and discharged to another hospital. However, in patients who survived to hospital day 40 or more and remained hospitalised, the estimated in-hospital mortality 90 days after the initiation of ECMO was 14·1% (95% CI 10·3–18·9).
In patients with COVID-19 supported with ECMO, both estimated mortality 90 days after ECMO initiation and mortality in those who achieved a final disposition of death or discharge were less than 40%. The results were similar when the sample was limited to patients with COVID-19 who were characterised as having ARDS. Our results are also consistent with previously reported survival rates in acute hypoxaemic respiratory failure, supporting current recommendations that centres experienced in ECMO should consider its use in refractory COVID-19-related respiratory failure.
Acknowledgments
Acknowledgments
Additional contributors and collaborators are listed in the appendix pp 4–9.
Contributors
RPB, GM, and DB conceived the study. PSB conceived the analysis plan. RPB, GM, PSB, TJI, ASS, EF, RHB, RL, AC, and DB designed the study. RPB, GM, PSB, RL, AC, and DB drafted the initial manuscript. All authors analysed and interpreted the results, critically edited the manuscript, approved the final work, and agree to be accountable for the accuracy and integrity of the work.
Declaration of interests
RPB is the Extracorporeal Life Support Organization (ELSO) Registry Chair. GM, RHB, PTR, and DB are members of the ELSO Executive Committee. PSB has received funding from ELSO for statistical analysis unrelated to this study. SJH receives payment from ELSO to maintain the ELSO website and ELSO Data Entry System. PTR receives payment from ELSO in his role as executive director of ELSO. RPB, CLA, and KH are members of the ELSO Steering Committee. RL and AC are past members of the European ELSO Steering Committee. JET, RH, JJF, and MMA are members of the ELSO Registry committee. EF the chair of the ELSO Device Development Committee. RPB reports grants from the National Institutes of Health (NIH) to support research activities not specific to this study (K12 HL138039; R01 HL153519). TJI reports that he is a US government employee. ASS reports grants from the Canadian Institutes of Health Research (OV3-170344 and 137772) and personal fees from Baxter and Novalung/Xenios, which are unrelated to the submitted work. EF reports personal fees from ALung Technologies, Fresenius Medical Care, and MC3 Cardiopulmonary that are unrelated to the submitted work. JET reports grants from NIH as well as personal fees from LivaNova and Philips Healthcare, unrelated to the submitted work. RL reports personal fees from Medtronic, LivaNova, Eurosets, and PulseCath, unrelated to the submitted work. AC reports personal fees from Maquet, Baxter, and Xenios, unrelated to the submitted work. DB reports grants from ALung Technologies, reports a current relationship of medical advisory board with Hemovent, and reports personal fees from Baxter, Abiomed, and Xenios, all of which are unrelated to the submitted work. RD declares no competing interests.
Contributor Information
Extracorporeal Life Support Organization:
Peta Alexander, Nicholas Barrett, Jan Bělohlávek, Dale Fisher, John Fraser, Ali Ait Hssain, Jae Sung Jung, Michael McMullan, Yatin Mehta, Mark T. Ogino, Matthew L. Paden, Kiran Shekar, Christine Stead, Yasir Abu-Omar, Vanni Agnoletti, Anzila Akbar, Huda Alfoudri, Carlos Alviar, Vladimir Aronsky, Erin August, Georg Auzinger, Hilda Aveja, Rhonda Bakken, Joan Balcells, Sripal Bangalore, Bernard W. Barnes, Alaiza Bautista, Lorraine L. Bellows, Felipe Beltran, Peyman Benharash, Marco Benni, Jennifer Berg, Pietro Bertini, Pablo Blanco-Schweizer, Melissa Brunsvold, Jenny Budd, Debra Camp, Mark Caridi-Scheible, Edmund Carton, Elena Casanova-Ghosh, Anthony Castleberry, Christopher T. Chipongian, Chang Woo Choi, Alessandro Circelli, Elliott Cohen, Michael Collins, Scott Copus, Jill Coy, Brandon Crist, Leonora Cruz, Mirosław Czuczwar, Mani Daneshmand, Daniel Davis II, Kim De la Cruz, Cyndie Devers, Toni Duculan, Lucian Durham, Subbarao Elapavaluru, Carlos V. Elzo Kraemer, EDMÍLSON CARDOSO Filho, Jillian Fitzgerald, Giuseppe Foti, Matthew Fox, David Fritschen, David Fullerton, Elton Gelandt, Stacy Gerle, Marco Giani, Si Guim Goh, Sara Govener, Julie Grone, Miles Guber, Vadim Gudzenko, Daniel Gutteridge, Jennifer Guy, Jonathan Haft, Cameron Hall, Ibrahim Fawzy Hassan, Rubén Herrán, Hitoshi Hirose, Abdulsalam Saif Ibrahim, Don Igielski, Felicia A. Ivascu, Jaume Izquierdo Blasco, Julie Jackson, Harsh Jain, Bhavini Jaiswal, Andrea C. Johnson, Jenniver A. Jurynec, Norma M Kellter, Adam Kohl, Zachary Kon, Markus Kredel, Karen Kriska, Chandra Kunavarapu, Oude Lansink-Hartgring, Jeliene LaRocque, Sharon Beth Larson, Tracie Layne, Stephane Ledot, Napolitan Lena, Jonathan Lillie, Gösta Lotz, Mark Lucas, Lee Ludwigson, Jacinta J. Maas, Joanna Maertens, David Mast, Scott McCardle, Bernard McDonald, Allison McLarty, Chelsea McMahon, Patrick Meybohm, Bart Meyns, Casey Miller, Fernando Moraes Neto, Kelly Morris, Ralf Muellenbach, Meghan Nicholson, Serena O'Brien, Kathryn O'Keefe, Tawnya Ogston, Gary Oldenburg, Fabiana M. Oliveira, Emily Oppel, Diego Pardo, Diego Pardo, Sara J. Parker, Finn M. Pedersen, Crescens Pellecchia, Jose A.S. Pelligrini, Thao T.N. Pham, Ann R. Phillips, Tasneem Pirani, Paweł Piwowarczyk, Robert Plambeck, William Pruett, Brittany Quandt, Kollengode Ramanathan, Alejandro Rey, Christian Reyher, Jordi Riera del Brio, Rachel Roberts, David Roe, Peter P. Roeleveld, Janet Rudy, Luis F. Rueda, Emanuele Russo, Jesús Sánchez Ballesteros, Nancy Satou, Mauricio Guidi Saueressig, Paul C. Saunders, Margaret Schlotterbeck, Patricia Schwarz, Nicole Scriven, Alexis Serra, Mohammad Shamsah, Lucy Sim, Alexandra Smart, Adam Smith, Deane Smith, Maggie Smith, Neel Sodha, Michael Sonntagbauer, Marc Sorenson, Eric B Stallkamp, Allison Stewart, Kathy Swartz, Koji Takeda, Shaun Thompson, Bridget Toy, Divina Tuazon, Makoto Uchiyama, Obiora I. Udeozo, Scott van Poppel, Corey Ventetuolo, Leen Vercaemst, Nguyen V. Vinh Chau, I-Wen Wang, Carrie Williamson, Brock Wilson, and Helen Winkels
Supplementary Material
References
- 1.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 4.Goligher EC, Tomlinson G, Hajage D. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 5.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Clinical management of COVID-19: interim guidance. May 27, 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 7.Alhazzani W, Moller MH, Arabi YM. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett RH, Ogino MT, Brodie D. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 10.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Extracorporeal Life Support Organization SARS-CoV-2 registry addendum database definitions. 2020. https://www.elso.org/Registry/DataDefinitions,Forms,Instructions.aspx
- 13.Lorusso R, Alexander P, Rycus P, Barbaro R. The Extracorporeal Life Support Organization Registry: update and perspectives. Ann Cardiothorac Surg. 2019;8:93–98. doi: 10.21037/acs.2018.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scand Stat Theory Appl. 1978;5:141–150. [Google Scholar]
- 15.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990;46:813–826. [PubMed] [Google Scholar]
- 16.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Conrad SA, Broman LM, Taccone FS. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. 2018;198:447–451. doi: 10.1164/rccm.201710-2130CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buuren Sv, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 20.Therneau TM. A package for survival analysis in S; 2015. Version 2.38. https://CRAN.R-project.org/package=survival
- 21.Gray B. cmprsk: subdistribution analysis of competing risks; 2010. R package version 2.2-1. http://CRAN.R-project.org/package=cmprsk
- 22.Namendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–349. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peek GJ, Mugford M, Tiruvoipati R. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro RP, Odetola FO, Kidwell KM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoyama H, Uchida K, Aoyama K. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herridge MS, Chu LM, Matte A. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 27.National Heart Lung Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellani G, Laffey JG, Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 29.The ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.