Abstract
In this project, a recently synthesized DABCO-based catalyst is entrapped in agar to reduce its moisture sensitivity leading to enhancement of its stability and catalytic activity. After preparation and identification this new reagent is used as an efficient and environmentally safe catalyst for the preparation of 1, 2, 4-triazoloquinazolinone and some pyrimidine derivatives. This method is accompanied with some superiorities such as, simple operation, mild and green conditions, use of low cost and non-hazardous natural material, short reaction times, easy preparation methods and simple work-up procedures. The prepared catalyst can be re-used for several times in all of the studied reactions without any appreciable loss in its activity.
Keyword: DABCO, Agar-entrapped catalyst, Ionic liquids (ILs), Triazole, Uracile, Quinazolinone, Green chemistry, Gel-entrapped acidic catalyst (GEACs)
Graphical abstract
1. Introduction
Agar is a hydrophilic, colloidal substance consisting of the polysaccharides extracted from Gelidium cartilogineumn Gaillon, Gracilaria confervoides Greville, and related red algae [1], probably existing in the form of its calcium salt or a mixture of calcium and magnesium salts. It is a complex mixture of polysaccharides composed of two major fractions agarose, a neutral polymer, and agaropectin, a charged, sulfated polymer. Agarose, the gelling fraction, is a neutral linear molecule essentially free of sulfates, consisting of chains of repeating alternate units of β-1,3-linked D-galactose and α-1,4-linked 3,6-anhydro-L-galactose. Agaropectin, the non-gelling fraction, is a sulfated polysaccharide (3% to 10% sulfate), composed of agarose and varying percentages of ester sulfate, D-glucuronic acid, and small amounts of pyruvic acid. Agarose normally represents at least two-thirds of the natural agar (Fig. 1 ).
Fig. 1.
Agar/agarose structure and agar-entrapped sulfonated DABCO.
Because of their special structural characteristics, triazoles are considered as important class of heterocyclic compounds showing interesting biological, pharmaceutical and therapeutic activities including antifungal [2], antimicrobial [3], [4], [5], [6], [7], [8], anti-cancer [9], anticonvulsant [10], antihypertensive [11], and anti-viral [12]. In this regard many drugs containing triazole moiety are manufactured, which of them Ribavirin or Copegus (an antiviral), Alprazolam (an anxiolytic), Letrozole (an anticancer), and Flusilazole (an organosilicon fungicide) [13] are examples (Fig. 2 a).
Fig. 2.
The structures of some triazole (a) and uracil (b) containing drugs.
Uracil, as one of the four nucleobases in the nucleic acid, is a common and naturally occurring pyrimidine derivative. In RNA uracil binds to adenine via two hydrogen bonds, and in DNA the uracil nucleobase is replaced by thymine. This considerable interest is correlated with a huge range of biological activities such as, antitumor [14], antifolate [15], antihypertensive [16], and cardio tonic (may be prescribed when the heart is not pumping enough blood to supply other organs) [17]. A number of popular drugs including the uracil moiety are Sofosbuvir (a new antiviral for COVID-19) [18], Uramustine (a chemotropic drug that damage DNA) [19] and Uridine monophosphate (a nucleotide that is used as a monomer in RNA) [20] (Fig. 2b). Recently the remedial effect in COVID-19 (human coronavirus) patients was also observed that it has been proven by Ribavirin [21].
In recent years, a gel-entrapped-base catalysts (GEBCs), which in them the advantages of alkali and organic bases with those of heterogeneous supports are combined with each other is going to become an attractive concept for organic chemists. In this line, Salunkhe and co-workers reported the preparation of agar-agar entrapped-DABCO and its applicability in the promotion of the synthesis of 2-amino-4H-chromenes [22]. This strategy causes to reduce the amounts of the base used in the reactions and ease of the product isolation, along with making a cut in moisture absorption by the catalyst [23].
Recently we have reported the preparation of [DABCO] (SO3H)2(Cl)2 as an acidic IL and its use in the acceleration of some of the multi-component reactions [24], although the method is useful but its ability in the adsorption of moisture causes that its efficiency to be reduced. For this reason we motivated to use the above mentioned strategy to prepare, a gel entrapped acidic ionic liquid (GEAIL) by the entrapping of this reagent in agar for the first time. After identification the effect of this method on the moisture adsorption and catalytic ability of this reagent is studied in the synthesis of some triazole and uracil containing derivatives as an example.
2. Experimental
2.1. Materials and instruments
All solvents and materials, employed in this study, were purchased form Aldrich (Mumbai) and Merck Chemical Companies (Munich) and utilized without any further purification. Solvents were stored in airtight containers and had been distilled before being applied. To ensure about the purity of materials, they were checked with thin layer chromatography (TLC) on silica-gel poly-gram SILG/UV 254 plates and their melting points were compared with authenticated melting points in Merck and Aldrich indexes.
Melting points were determined by electro-thermal IA9100 melting point apparatus in capillary tubes. The melting point range was input manually through keyboard and the material changes were visually monitored. FT-IR spectra were recorded on a Perkin-Elmer spectrum BX series with KBr plates for solid samples. 1H NMR and 13C NMR spectra were recorded on Bruker AV-400 and -500 using TMS (0.00 ppm) as internal standard and DMSO‑d6 as the solvent.
2.2. Preparation of [DABCO](SO3H)2(Cl)2
The acidic ionic liquid was precisely prepared according to the reported procedure in the article [24].
2.3. Preparation of agar-entrapped IL
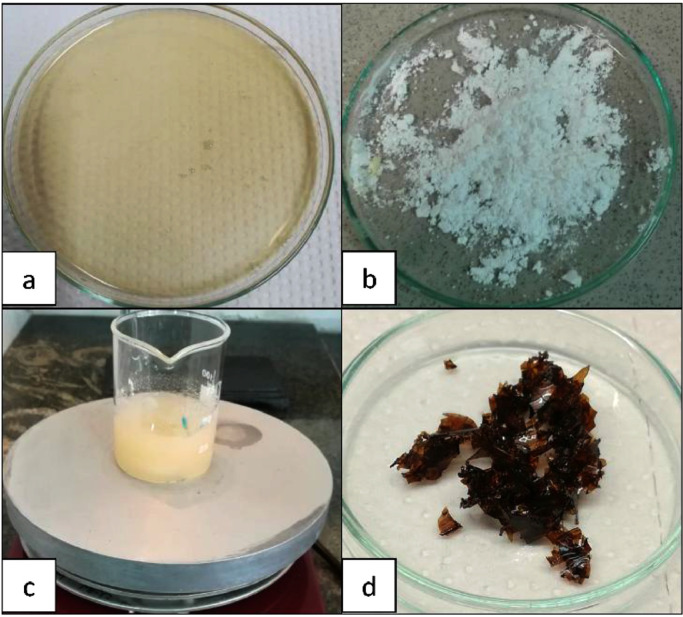
In a 150 mL flask, 5.0 g agar in 50 mL water was heated at boiling point to be completely dissolved (Fig. 3 -a). Then a solution of 2.5 g of [DABCO](SO3H)2(Cl)2 (7.2 mmol, Fig. 3-b), in warm water (50 mL) (Fig. 3-c), was added into preceding solution and stirred for 10 min. After that, the flask was poured into a petri dish and dried in vacuum oven for 12 h to obtain agar-entrapped IL as rough brown crystals (Fig. 3-d).
Fig. 3.
Agar in heated water (a), [DABCO](SO3H)2(Cl)2 (b), ionic liquid in warm water (c) and agar-entrapped ionic liquid (d).
2.4. General procedure for the preparation of 1,2,4-triazoloquinazolinones
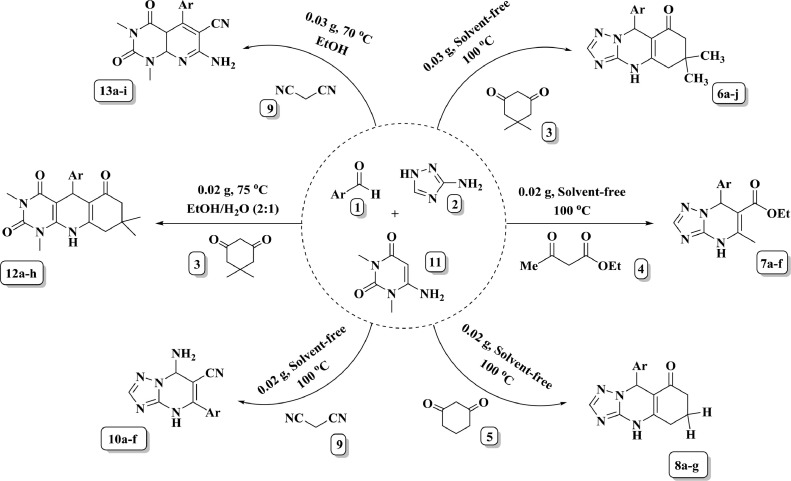
To a mixture of aromatic aldehyde 1 (1 mmol), 3-amino-1,2,4-triazole 2 (1 mmol), and β- diketone (dimedone 3, methyl acetoacetate 4 or 1,3-cyclohexadione 5) (1 mmol), agar-entrapped catalyst (0.03 g for 6, 0.02 g for 7 and 8) was added in a 25 mL round-bottom flask. Then the mixture was stirred magnetically in the absence of solvent in an oil bath (100 °C) for the appropriate time. The reaction process was carefully monitored by TLC (n-hexane: ethyl acetate; 8:3). After completion of the reaction, 5 mL water was poured into the reaction medium and filtered off to separate the catalyst. Finally, the obtained precipitate was recrystallized from ethanol to afford the required product (6a-j, 7a-f, and 8a-g). The spectral data of new compounds are as follow:
Diethyl-7,7′-(butane-1,4-diyl-bis(oxy)bis(4,1-phenylene))bis(5-methyl-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylate) (7e)
IR (KBr, ν/cm−1); 3422, 3135, 2934, 2871, 1692, 1642, 1581, 1510, 1473, 1427, 1383, 1248; 1H NMR (500 MHz, DMSO‑d 6), δ = 1.04 (t, J = 7.1 Hz, 3H, CH3), 1.80 (br, 2H, CH2), 2.41 (s, 3H, CH3), 3.98 – 3.91 (m, 4H, 2CH2), 6.21 (s, 1H, CH), 6.83 (d, J = 8.6 Hz, 2H, 2CH), 7.11 (d, J = 8.6 Hz, 2H, 2CH), 7.63 (s, 1H, CH), 10.76 (s, 1H, NH); 13C NMR (125 MHz, DMSO‑d6), δ =14.3, 18.8, 25.7, 59.3, 59.7, 67.5, 114.6, 115.3, 128.6, 132.2, 146.8, 147.3, 150.4, 158.6, 165.6.
Diethyl-7,7′-(hexane-1,6-diyl-bis(oxy)bis(4,1-phenylene))bis(5-methyl-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylate) (7f)
IR (KBr, ν/cm−1); 3381, 3097, 2987, 2867, 1695, 1653, 1591, 1510, 1489, 1415, 1370, 1266.; 1H NMR (500 MHz, DMSO‑d 6), δ = 1.03 (t, J = 7.5 Hz, 3H, CH3), 1.43 (br, 2H, CH2), 1.67 (br, 2H, CH2), 2.40 (s, 3H, CH3), 3.93 – 4.05 (m, 4H, 2CH2) 6.20 (s, 1H, CH), 6.82 (d, J = 8.0 Hz, 2H, 2CH), 7.10 (d, J = 7.8 Hz, 2H, 2CH), 7.63 (s, 1H, CH), 10.76 (s, 1H, NH); 13C NMR (125 MHz, DMSO‑d 6): δ =14.3, 18.8, 25.7, 29.0, 59.3, 59.7, 67.7, 114.5, 115.3, 128.6, 132.2, 146.8, 147.3, 150.4, 158.7, 165.6.
9-(2-Nitrophenyl)-5,6,7,9-tetrahydro-[1,2,4]triazolo[5,1-b]quinazolin-8(4H)-one (8d)
IR (KBr, υ, cm−1) 3444, 3213, 2910, 1643, 1569, 1357; 1H NMR (400 MHz, DMSO‑d 6): δ (ppm) 1.88–1.99 (2H, m, CH2), 2.17–2.27 (2H, m, CH2), 2.64–2.67 (2H, m, CH2), 6.98 (1H, s, CH), 7.30 (1H, d, J = 1.2 Hz, CH-Ph), 7.49 (1H, dt, J 1 = 7.6 Hz, J 2 = 0.8 Hz, CH-Ph), 7.61 (1H, dt, J 1 = 7.6 Hz, J 2 = 1.2 Hz, CH-Ph), 7.73 (1H, s, CH-triazole), 7.86 (1H, dd, J 1 = 8 Hz, J 2 = 1.2 Hz, CH-Ph), 11.32 (1H, s, NH); 13C NMR (100 MHz, DMSO‑d 6): δ (ppm) 21.1, 26.8, 36.4, 53.4, 106.19, 124.4, 129.4, 129.8, 133.8, 135.3, 147.2, 149.0, 150.8, 153.7, 193.9.
9,9′-(Butane-1,4-diylbis(oxy)bis(4,1-phenylene))bis(5,6,7,9-tetrahydro-[1,2,4]triazolo[5,1-b]quinazolin-8(4H)-one) (8f)
IR (KBr, ν/cm−1); 3422, 3230, 2926, 2833, 1644, 1584, 1511, 1476, 1416, 1362, 1250; 1H NMR (500 MHz, DMSO‑d6), δ = 1.79 (s, 2H, CH2), 2.05 – 1.83 (m, 2H, CH2), 2.30 – 2.20 (m, 2H, CH2), 2.70 – 2.58 (m, 2H, CH2), 3.94 (t, J = 5.2 Hz, 2H, CH2), 6.17 (s, 1H, CH), 6.81 (d, J = 8.4 Hz, 2H, 2CH), 7.10 (d, J = 8.3 Hz, 2H, 2CH), 7.67 (s, 1H, CH), 11.11 (s, 1H, NH); 13C NMR (125 MHz, DMSO‑d6), δ =21.1, 25.8, 26.8, 36.8, 57.5, 67.5, 114.5, 115.3, 128.6, 134.1, 147.1, 150.3, 152.7, 158.5, 193.7.
9,9′-(Hexane-1,6-diylbis(oxy)bis(4,1-phenylene))bis(5,6,7,9-tetrahydro-[1,2,4]triazolo[5,1-b]quinazolin-8(4H)-one) (8 g)
IR (KBr, ν/cm−1); 3423, 3229, 2931, 2868, 1648, 1581, 1511, 1474, 1414, 1360, 1246; 1H NMR (500 MHz, DMSO‑d6), δ = 1.41 (br, 2H, CH2), 1.67 (br, 2H, CH2), 1.98 – 1.86 (m, 2H, CH2), 2.29 – 2.20 (m, 2H, CH2), 2.69 – 2.58 (m, 2H, CH2), 3.89 (t, J = 6.7 Hz, 2H, CH2), 6.16 (s, 1H, CH), 6.80 (d, J = 8.2 Hz, 2H, 2CH), 7.09 (d, J = 8.1 Hz, 2H, 2CH), 7.66 (s, 1H, CH), 11.10 (s, 1H, NH); 13C NMR (125 MHz, DMSO‑d6), δ =21.1, 25.7, 26.8, 29.0, 36.8, 57.5, 67.7, 114.5, 115.3, 128.5, 134.0, 147.1, 150.3, 152.7, 158.6, 193.7.
2.5. General procedure for the preparation of 1, 2, 4-triazolo[4,3-a]pyrimidines
To a mixture of aromatic aldehyde 1 (1 mmol), 3-amino-1,2,4-triazole 2 (1 mmol), and malononitrile 9 (1 mmol), in a 25 mL round-bottom flask, agar-entrapped catalyst (0.02 g) was added. Then the mixture was stirred magnetically under solvent-free conditions in an oil bath (100 °C) for the appropriate time. The progress of the reaction was carefully monitored by TLC (n-hexane: ethyl acetate; 4:1). After completion of the reaction, 5 mL water was poured into the reaction medium and then filtered off to separate the catalyst. Finally, the obtained precipitate was recrystallized from ethanol to afford the required product (10a-f).
5-amino-7-(naphthalen-2-yl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carbonitrile (10f)
M.p. 281–282 °C; IR (KBr, υ, cm−1) 3360, 3256, 3183, 2189, 1657, 1629, 1526; 1H NMR (400 MHz, DMSO‑d 6): 5.56 (d, J = 2 Hz, 1H, CH), 7.31 (d, J = 2.8 Hz, 2H, NH2), 7.50–7.55 (m, 3H, CH-Ph), 7.77 (d, J = 10.8 Hz, 2H, CH-Ph), 7.90–7.97 (m, 3H, N CH—N and CH-Ph), 8.88 (d, J = 2.4 Hz, 1H, NH); 13C NMR (100 MHz, DMSO‑d 6): 54.3, 119.0, 124.4, 124.6, 126.2, 126.5, 127.5, 127.6, 127.9, 128.7, 132.5, 132.6, 140.3, 147.0, 151.9, 153.9.
2.6. General procedure for the preparation of pyrido[4,5-b]quinolone
To a mixture of aromatic aldehyde 1 (1 mmol), 6-amino-1,3-dimethyluracil 11 (1 mmol), dimedone 3 (1 mmol) in a 25 mL round-bottom flask, 0.02 g agar-entrapped catalyst was added and 5 mL of ethanol/water (2:1) was poured on it. The mixture was stirred magnetically to dissolve all components at reflux temperature. The reaction process was carefully checked by TLC (n-hexane: ethyl acetate; 4: 1). After completion, the mixture was filtered off to separate the catalyst, which is solvated in the solvent. Recrystallization of the product from absolute ethanol led to the pure product (12a-h).
2.7. General procedure for the preparation of pyrido[2,3-d]pyrimidines
Into a 25 mL round-bottom flask containing aldehyde 1 (1 mmol), 6-amino-1,3-dimethyluracil 11 (1 mmol), and malononitrile 9 (1 mmol), 5 mL ethanol and 0.03 g agar-entrapped catalyst were added. The mixture was stirred magnetically at 70 °C to dissolve all components. The precipitate of the product is appeared in the reaction medium after a short time. The reaction progress was traced to completion by TLC (n-hexane: ethyl acetate; 1: 4). After completion of the reaction, the mixture was filtered off and the obtained residue was washed with water to remove the catalyst. Finally, the crude product was recrystallized from ethanol if necessary (13a-i).
3. Results and discussion
3.1. Characterization of the catalyst
3.1.1. Fourier-Transform infrared spectroscopy (FT-IR)
When a molecule is entrapped in a linear polysaccharide through hydrogen bonding, it can be expected that the number and intensity of the peaks of its functional groups be decreases due to a lock in the structure (Fig. 4 ). This phenomena can be seen by the comparison of the FT-IR spectra of free IL and agar-entrapped IL (Fig. 5 ).
Fig. 4.
Hydrogen bond probability of agar-entrapped IL.
Fig. 5.
FT-IR spectra of DABCO (a), [DABCO] (SO3H)2Cl2 (b), agar (c) and agar-entrapped IL (d).
3.1.2. Scanning electron microscopy (SEM)
As shown in Fig. 6 , the morphology of DABCO changed going through the entrapping process. However, DABCO has porous and irregular shape with tiny holes, it change to an aggregated particles due to hydrogen bonding between hydroxyl groups of IL and agar.
Fig. 6.
SEM of DABCO (a and b) and agar-entrapped IL (c and d).
3.1.3. Thermal gravimetric analysis (TGA)
TGA diagrams of agar, IL, and agar-entrapped IL are represented at Fig. 7 , As shown, the major weight loss from agar-entrapped IL are happened between 369 and 681 °C (44.3%) which is related to decomposition of the catalyst. Before that, the weight loss (28.89%) between 219 and 312 °C is attributed to the thermal decomposition of IL which is entrapped in agar. The weight loss from the catalyst between 62 and 157 °C is owing to the decomposition of agar and the removal of physically adsorbed water and organic solvents, which were used in creating the catalyst.
Fig. 7.
TGA diagrams of agar (a) IL (b) and agar-entrapped IL (c).
3.2. Moisture-resistant property
In spite of great features of ionic liquids such as non-flammability, no miscibility with non-polar solvents, and negligible vapor pressure, these compounds are very sensitive on exposure to air and moisture. Moisture adsorption can be impacted on the properties of ILs; for example, can lead to a cut in the thermal stability or catalytic activity. For this reason, finding a protocol which increases the moisture resistance can be thoroughly vital.
In order to show the effect of agar-trapping on the moisture adsorption of the selected IL, we carried out loss on drying test for agar, IL, and agar-entrapped IL using Karl-Fischer method in 4 steps during 36 h at a standard temperature (23 °C) and 63% relative humidity in the laboratory.
The obtained results show an acceptable decrease in the amount of the moisture which can be observed by IL after its entrapping in agar (Fig. 8 ). As shown, the moisture adsorption decreased when the IL trapped in agar. It can be related to an increase in the H-bonding of catalyst with agar, as shown in Fig. 4, which cause to a cut in the positions which can H-bond with water [25].
Fig. 8.
A comparison between moisture resistant property of agar, IL, and agar-entrapped IL.
3.3. Catalytic activity
After ensuring about the preparation of the catalyst, the catalytic activity of the entrapped IL was investigated in the synthesis of 1,2,4-triazolo[4,3-a]pyrimidine, pyrido[2,3-d]pyrimidine, pyrido[4,5-b]pyrimidine and 1,2,4-triazoloquinazolinone derivatives.
At first, to gain the best conditions for the reactions, the synthesis of 4-cholorobenzaldehyde derivatives of these types of compounds (6b, 8b, 10b, 12b, 13b) was investigated as models for all reactions. In the case of compounds 7a-f the optimization studies were done on benzaldehyde as a selected model. The results are collected in Table 1 . As shown, different amounts of catalyst and various conditions were used to optimize the reaction conditions in accuracy. Which is distinguished in the table, agar-entrapped IL is more effective when thermal conditions and protic solvents were used (7a, 8b, 12b, and 13b). However, this catalyst was able to accelerate the synthesis of 6b and 10b in the absence of solvent. On the basis of the obtained results the best conditions are determined as shown in Scheme 1.
Table 1.
Optimization of the reaction conditions for the synthesis of 6b (entries 1–10), 7a (entries 11–18), 8b (entries 19–26), 10b (entries 27–35), 12b (entries 36–44), 13b (entries 45–52).
| Entry | Catalyst amount (g) | Solvent | Temp. ( °C) | Time (min.) | Conversion (Isolated yield%) |
|---|---|---|---|---|---|
| 1 | 0.01 | MeCN | r.t. | 120 | NRa |
| 2 | 0.03 | MeCN | Reflux | 120 | NRa |
| 3 | 0.01 | CH2Cl2 | r.t. | 120 | NRa |
| 4 | 0.03 | CH2Cl2 | Reflux | 120 | NRa |
| 5 | 0.03 | H2O | Reflux | 120 | NCb |
| 6 | 0.03 | EtOH/H2O | Reflux | 120 | NCb |
| 7 | 0.03 | EtOH | Reflux | 120 | NCb |
| 8 | 0.01 | – | 100 | 120 | 100 (70) |
| 9 | 0.02 | – | 100 | 90 | 100 (90) |
| 10 | 0.03 | – | 100 | 60 | 100 (91) |
| 11 | 0.20 | H2O | r.t. | 100 | Not NCb |
| 12 | 0.20 | MeCN | Reflux | 60 | NRa |
| 13 | 0.20 | MeCN | Reflux | 60 | NRa |
| 14 | 0.20 | CH2Cl2 | Reflux | 60 | NRa |
| 15 | 0.20 | CH2Cl2 | Reflux | 30 | NRa |
| 16 | 0.20 | H2O | 100 | 30 | 100 (90) |
| 17 | 0.10 | EtOH/H2O | 100 | 60 | 100 (70) |
| 18 | 0.20 | EtOH | 100 | 30 | 100 (95) |
| 19 | 0.20 | H2O | r.t. | 100 | NCb |
| 20 | 0.20 | MeCN | Reflux | 60 | NRa |
| 21 | 0.20 | MeCN | Reflux | 60 | NRa |
| 22 | 0.20 | CH2Cl2 | Reflux | 60 | NRa |
| 23 | 0.20 | CH2Cl2 | Reflux | 30 | NRa |
| 24 | 0.20 | H2O | 100 | 30 | 100 (90) |
| 25 | 0.10 | EtOH/H2O | 100 | 60 | 100 (70) |
| 26 | 0.20 | EtOH | 100 | 20 | 100 (95) |
| 27 | 0.01 | MeCN | r.t. | 75 | NRa |
| 28 | 0.01 | MeCN | Reflux | 90 | NRa |
| 29 | 0.01 | EtOH | Reflux | 90 | Trace |
| 30 | 0.01 | H2O | Reflux | 90 | Trace |
| 31 | 0.01 | EtOH/H2O | r.t. | 90 | NCb |
| 32 | 0.01 | EtOH/H2O | Reflux | 90 | NCb |
| 33 | 0.01 | – | 100 | 90 | 100 (90) |
| 34 | 0.01 | – | 100 | 40 | 100 (90) |
| 35 | 0.02 | – | 100 | 35 | 100 (98) |
| 36 | 0.02 | MeCN | r.t. | 120 | NRa |
| 37 | 0.03 | MeCN | Reflux | 120 | NRa |
| 38 | 0.02 | CH2Cl2 | r.t. | 120 | NCb |
| 39 | 0.03 | CH2Cl2 | Reflux | 120 | NCb |
| 40 | 0.03 | H2O | r.t. | 120 | NCb |
| 41 | 0.03 | EtOH | Reflux | 120 | 100 (70) |
| 42 | 0.02 | EtOH/H2O | Reflux | 100 | 100 (80) |
| 43 | 0.03 | EtOH/H2O (2:1) | Reflux | 75 | 100 (91) |
| 44 | 0.02 | EtOH/H2O (2:1) | Reflux | 75 | 100 (92) |
| 45 | 0.03 | MeCN | r.t. | 120 | NCb |
| 46 | 0.03 | MeCN | Reflux | 120 | NCb |
| 47 | 0.03 | H2O | Reflux | 120 | NCb |
| 48 | 0.03 | EtOH/H2O | Reflux | 120 | NCb |
| 49 | 0.03 | EtOH/H2O (2:1) | Reflux | 120 | NCb |
| 50 | 0.03 | EtOH | Reflux | 40 | 100 (80) |
| 51 | 0.04 | EtOH | Reflux | 40 | 100 (80) |
| 52 | 0.03 | EtOH | Reflux | 55 | 100 (97) |
No reaction.
Not completed.
Scheme 1.
Synthesis of 1,2,4-triazolo[4,3-a]pyrimidine, pyrido[2,3-d]pyrimidine and pyrido[4,5-b]pyrimidine, and 1,2,4-triazoloquinazolinone derivatives.
After optimization of the reaction conditions, we have developed our studies by utilization of miscellaneous aldehydes with withdrawal and/or donor functional groups. The obtained outcomes were collected in Table 2 . These results show that under the selected conditions the requested products can be obtained in high yields during acceptable reaction times with no considerable effect of the substituents on the aromatic ring. Because that, in spite of the aromatic aldehydes, a mixture of unidentified products were formed when aliphatic aldehydes were employed, the related results were not included in Table 2. In this study some new triazole derivatives are prepared from bis-aldehydes which their results are highlighted in Table 2.
Table 2.
Preparation of 1,2,4-triazolo quinazolinone (entries 1–23), 1,2,4-triazolo[4,3-a] pyrimidines (entries 24–29), pyrido[4,5-b]quinolone (entries 30–37) and pyrido[2,3-d]pyrimidines (entries 38–46) in the presence of agar-entrapped acidic IL as the catalyst.
| Entry | Ar | Subs. | Subs. | Pro. | Time (min.) | Yield (%)a | Melting point ( °C) | |
|---|---|---|---|---|---|---|---|---|
| Found | Rep. [Ref.] | |||||||
| 1 | C6H5- | triazole | DMb | 6a | 95 | 94 | 248–249 | 248–250 [26] |
| 2 | 4-ClC6H4- | triazole | DMb | 6b | 60 | 91 | 300–302 | 303–305 [27] |
| 3 | 2-MeOC6H4- | triazole | DMb | 6c | 85 | 97 | 299–300 | 298–300 [28] |
| 4 | 4-OHC6H4- | triazole | DMb | 6d | 90 | 95 | >300 | >300 [29] |
| 5 | 2-Naphtaldehyde | triazole | DMb | 6e | 80 | 95 | 289–292 | 287–290 [29] |
| 6 | 4-NO3C6H4- | triazole | DMb | 6f | 90 | 96 | 285–287 | 284–286 [26] |
| 7 | 3-NO3C6H4- | triazole | DMb | 6 g | 100 | 94 | 268–269 | 266–269 [29] |
| 8 | 2-NO3C6H4- | triazole | DMb | 6h | 110 | 94 | 292–294 | 290–292 [30] |
| 9 | 4-MeOC6H4- | triazole | DMb | 6i | 115 | 98 | 224–227 | 222–224 [26] |
| 10 | 3-MeOC6H4- | triazole | DMb | 6j | 110 | 95 | >300 | >300 [31] |
| 11 | C6H5- | triazole | MAAc | 7a | 30 | 95 | 193–194 | 193–194 [32] |
| 12 | 4-MeOC6H4- | triazole | MAAc | 7b | 60 | 90 | 233–234 | 233–235 [32] |
| 13 | 4-NO3C6H4- | triazole | MAAc | 7c | 45 | 90 | 257–258 | 257–258 [32] |
| 14 | 4-MeC6H4- | triazole | MAAc | 7d | 60 | – | Dec.d | — [32] |
| 15 | [(CH2)2OC6H4]2- | triazole | MAAc | 7e | 60 | 88 | >300 | New |
| 16 | [(CH2)3OC6H4]2- | triazole | MAAc | 7f | 80 | 85 | >300 | New |
| 17 | C6H5- | triazole | CHe | 8a | 20 | 91 | 299–300 | 300–301 [31] |
| 18 | 4-ClC6H4- | triazole | CHe | 8b | 20 | 95 | 294–295 | 294–295 [33] |
| 19 | 3-NO3C6H4- | triazole | CHe | 8c | 35 | 84 | 298–299 | 299–300 [31] |
| 20 | 2-NO3C6H4- | triazole | CHe | 8d | 45 | 86 | >300 | >300 [34] |
| 21 | 4-MeOC6H4- | triazole | CHe | 8e | 40 | 87 | >300 | >300 [31] |
| 22 | [(CH2)2OC6H4]2- | triazole | CHe | 8f | 60 | 89 | >300 | New |
| 23 | [(CH2)3OC6H4]2- | triazole | CHe | 8 g | 65 | 89 | >300 | New |
| 24 | C6H5- | triazole | MNf | 10a | 30 | 95 | >300 | >300 [35] |
| 25 | 4-ClC6H4- | triazole | MNf | 10b | 35 | 98 | 256–257 | 255–257 [35] |
| 26 | 2-ClC6H4- | triazole | MNf | 10c | 40 | 93 | 264–266 | 264–265 [35] |
| 27 | 4-MeOC6H4- | triazole | MNf | 10d | 40 | 94 | 220–223 | 220–221 [35] |
| 28 | 2-NO3C6H4- | triazole | MNf | 10e | 30 | 93 | 247–248 | 246–247 [35] |
| 29 | 2-Naphtaldehyde | triazole | MNf | 10f | 50 | 94 | 281–282 | 280–283 [36] |
| 30 | C6H5- | uracil | DMb | 12a | 55 | 92 | 270–271 | 269–270 [37] |
| 31 | 4-ClC6H4- | uracil | DMb | 12b | 75 | 92 | 288–289 | 287–288 [38] |
| 32 | 4-NO3C6H4- | uracil | DMb | 12c | 105 | 95 | 224–226 | 224–225 [37] |
| 33 | 3-NO3C6H4- | uracil | DMb | 12d | 110 | 93 | 223–224 | 221–223 [37] |
| 34 | 2-ClC6H4- | uracil | DMb | 12e | 80 | 92 | >300 | >300 [38] |
| 35 | 2-NO3C6H4- | uracil | DMb | 12f | 110 | 93 | 289–290 | 287–290 [39] |
| 36 | 4-MeOC6H4- | uracil | DMb | 12 g | 100 | 93 | >300 | >300 [38] |
| 37 | 2-MeOC6H4- | uracil | DMb | 12h | 80 | 94 | >300 | >300 [38] |
| 38 | C6H5- | uracil | MNf | 13a | 50 | 93.2 | >300 | >300 [40] |
| 39 | 4-ClC6H4- | uracil | MNf | 13b | 55 | 97 | >300 | >300 [41] |
| 40 | 2-ClC6H4- | uracil | MNf | 13c | 60 | 92 | >300 | >300 [41] |
| 41 | 4-MeOC6H4- | uracil | MNf | 13d | 50 | 94 | >300 | >300 [40] |
| 42 | 4-NO3C6H4- | uracil | MNf | 13e | 55 | 94 | >300 | >300 [42] |
| 43 | 2-Naphtaldehyde | uracil | MNf | 13f | 60 | 94 | >300 | >300 [43] |
| 44 | 3-BrC6H4- | uracil | MNf | 13 g | 65 | 95 | >300 | >300 [41] |
| 45 | 3-NO3C6H4- | uracil | MNf | 13h | 60 | 94 | >300 | >300 [41] |
| 46 | 2-NO3C6H4- | uracil | MNf | 13i | 60 | 95 | >300 | >300 [42] |
Isolated yields.
Dimedone.
Methyl acetoacetate.
Decomposition.
Cyclohexadione.
Malononitrile.
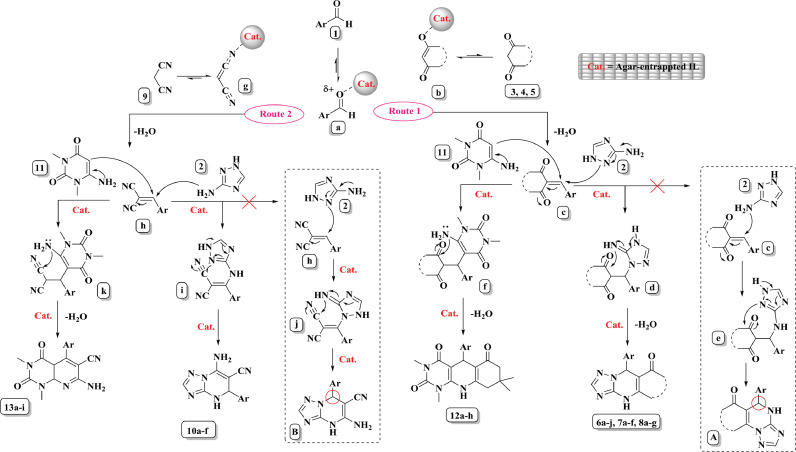
The mechanism starts from activation of aldehyde 1 via catalyst through H-bonding (intermadiate a). After that, the route divides to two pathways. In route 1, activated 1,3-diketone (enol b) makes for intermadiate a comes to c after loss water. If 3-amino-1,2,4-triazole 2 reacts with c through N2 or N3H2, the obtained products can be different. If N2attacks to c, the products 6a-j, 7a-f, and 8a-g produce after intra-molecular cyclization (intermediate d) and tautomerization. If intermediate c is attacked with N3H2, intermediate e produces which led to product A. But if it happened, aliphatic –CH in ring, assigned with red circle, should be split as a doublet with J = 2 Hz. This peak appeared as a singlet in the 1H NMR spectra of 6 h, 7e, 7f, 8f, and 8 g which approves 3-amino-1,2,4-triazole 2 participates in reaction through N2. On the other hand, owing to the replacement of 3-amino-1,2,4-triazole 2 with 6-amino-1,3-dimethyluracil 11, the products 12a-h were produced path through intermediate f.
In route 2, malononitrle 9 gets activated by catalyst to attack intermediate a and produce h as a key intermediate. Then whether 3-amino-1,2,4-triazole 2 or 6-amino-1,3-dimethyluracil 11, products 10a-f or 13a-i can be produced, going through intermediates i and k, respectively. But, by contrast, to produce product 10a-f, N3H2 from 3-amino-1,2,4-triazole 2 should attack h, if not, product B prepares. The doublet peak about 5.5 ppm with J = 2 Hz in the 1H NMR of 10f confirmed that the aliphatic –CH and –NH are in the neighborhood (Scheme 2)
Scheme 2.
The hypothesized mechanism for the synthesis of 1,2,4-triazolo[4,3-a]pyrimidine, pyrido[2,3-d]pyrimidine and pyrido[4,5-b]pyrimidine, and 1,2,4-triazoloquinazolinone derivatives in the presence of agar-entrapped IL.
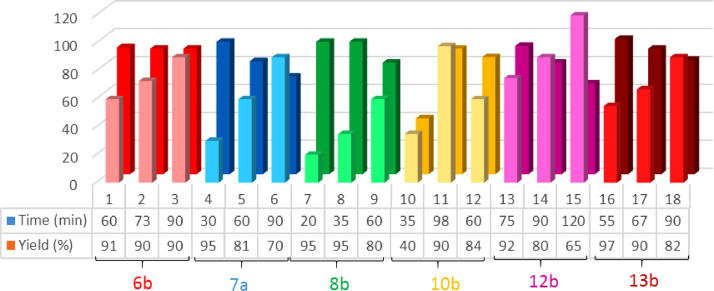
A brilliant feature of a catalyst which changes it as a convenient one is its recyclability. For investigation of this feature of our new catalyst, the synthesis of 4-chlorobenzaldehyde derivative in each reaction (6a, 8b, 10b, 12b, and 13b except 7a-f which benzaldehyde was used for the synthesis of 7a) is selected as model. After completion of the reaction and separation of the product the solvent is removed under vacuum at 50 °C. The obtained precipitate was eluted by diethyl ether and used for another reaction. This study showed that the catalyst can be reused at least for 3 consecutive runs without considerable decrease in its activity (Fig. 9 ).
Fig. 9.
Reusability of the catalyst in the synthesis of 1,2,4-triazoloquinazolinones (6b, 7a, 8b), 1,2,4-triazolo[4,3-a]pyrimidines (10b), pyrido[4,5-b]quinolone (12b) and pyrido[2,3-d]pyrimidines (13b).
In order to show the catalytic ability of the prepared reagent, the efficiency of agar-entrapped IL with some of the other catalysts in the synthesis of 5-amino-7-(4-chlorophenyl)-7,8-dihydro[1,2,4]triazolo[4,3-a]pyrimidine and 7-amino-5-(4-chlorophenyl)-1,3-dimethyl-2,4- dioxo-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile, is compered in Table 3 . This comparison implies that the selected reactions are carried out better using this new catalyst.
Table 3.
Comparison of the activity of agar-entrapped IL with those of other reported catalysts in the synthesis of 5-amino-7-(4-chlorophenyl)-7,8-dihydro[1,2,4]triazolo[4,3-a]pyrimidine (10b, entries1–6) and 7-amino-5-(4-chlorophenyl)-1,3-dimethyl-2,4- dioxo-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (13b, entries 7–11) compared performance.
| Entry | Catalyst/Conditions [Ref.] | Time (min.) | Yield (%) |
|---|---|---|---|
| 1 | NaOH/Reflux-EtOH [35] | 30 | 82 |
| 2 | NaOH/US-H2O [35] | 60 | 88 |
| 3 | 1,8-Diazabicyclo[5.4.0]undec‑7-ene (DBU) / Reflux-EtOH [44] | 15 | 90 |
| 4 | [H2-DABCO][H2PO4]2/Solvent-free, 100 °C [36] | 40 | 95 |
| 5 | [H2-DABCO][ClO4]2/Solvent-free, 100 °C [36] | 50 | 96 |
| 6 | Agar entrapped [DABCO](SO3H)2Cl2 Solvent-free -100 °C | 35 | 98 |
| 7 | Nano-MgO/H2O,80 oC [41] | 15 | 90 |
| 8 | Triethanolamine/H2O,80 °C [45] | 120 | 92 |
| 9 | Al-HMS-20/EtOH-r.t. [46] | 720 | 92 |
| 10 | [H2-DABCO][ClO4]2/EtOH, 70 °C [36] | 50 | 95 |
| 11 | Agar-entrapped [DABCO](SO3H)2Cl2/EtOH, 70°C | 55 | 97 |
4. Conclusions
We have used agar-entrapped [DABCO](SO3H)2(Cl)2 as a novel, highly efficient and green catalyst for the multi-component synthesis of 1,2,4-triazolo quinazolinone, 1,2,4-triazolo [4,3-a] pyrimidine, pyrido[4,5-b]quinolone and pyrido [2,3-d]pyrimidine derivatives. This new catalyst has the least absorption of humidity leading to more stability of the selected acidic ionic liquid. High yields, short reaction times, ease of separation, and stability and reusability of the catalyst are some of the important advantages of the method.
We hope that this newly reported idea can be a useful way for the stabilization of other moisture sensitive reagents leading to their broad range of applications in organic transformations.
Credit author statement
This article introduces agar-entrapped sulfonated DABCO as a gelly acidic catalyst for the acceleration of one-pot synthesis of 1,2,4-triazoloquinazolinone and some pyrimidine derivatives.
The main advantages of this method are: introduction of novel protocols for the synthesis of 1,2,4- triazoloquinazolinone and some pyrimidine derivatives in mild and green conditions. Moreover, using small amounts of non-metal catalysts, high yields of products, no by-product and simple work-up procedures are added advantages of these procedures. It should be emphasized that the submission is original, not under consideration for publication elsewhere, and that all authors are aware of the submission and agree to its publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to the Research Council of University of Guilan for the partial support of this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2020.129336.
Appendix. Supplementary materials
References
- 1.Tozkoparan B., Gokhan N., Aktay G., Yesilada E., Ertan M. 6-Benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6H)-onessubstituted with ibuprofen: synthesis, characterization and evaluation of anti-inflammatory activity. Eur. J. Med. Chem. 2000;34:743–750. doi: 10.1016/S0223-5234(00)00157-4. [DOI] [PubMed] [Google Scholar]
- 2.del Arco J., Galindo J., Clemente-Surez V.J., Corrales A., Fernndez-Lucas J. Sustainable synthesis of uridine-5′-monophosphate analogues by immobilized uracil phosphoribosyltransferase from Thermus thermophilus. BBA-Proteins Proteom. 2020;1868 doi: 10.1016/j.bbapap.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Holla B.S., Gonsalves R., Shenoy S. Studies on some N-bridged heterocycles derived from bis-[4-amino-5-mercapto-1,2,4-triazol-3-yl] alkanes. II Farmaco. 1998;53:574–578. doi: 10.1016/S0014-827X(98)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Ersan S., Nacak S., Berkem R. Synthesis and antimicrobial activity of N-[(α-methyl)benzylidene]-(3-substituted-1,2,4-triazol-5-yl-thio)acetohydrazides. II Farmaco. 1998;53:773–776. doi: 10.1016/S0014-827X(98)00095-0. [DOI] [PubMed] [Google Scholar]
- 5.Demirbas N., Karaoglu S.A., Demirbas A., Çelik E. Synthesis and antimicrobial activities of some new [1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles and [1,2,4]triazolo[3,4-b] [1,3,4]thiadiazines. ARKIVOC. 2005:75–91. doi: 10.3998/ark.5550190.0006.108. [DOI] [Google Scholar]
- 6.Ikizler A.A., Ucar F., Demirbas N., Yasa I., Ikizler A., Genzer T. Antimicrobial activities of some 4H-1,2,4-triazoles. Indian J. Pharm. Sci. 1999;61:271–274. https://www.ijpsonline.com/abstract/antimicrobial-activities-of-some-4h124triazoles-1442.html [Google Scholar]
- 7.Subhashini N.J.P., Kumara E.P., Gurrapu N., Yerragunta V. Design and synthesis of imidazolo-1,2,3-triazoles hybrid compounds by microwave-assisted method: evaluation as an antioxidant and antimicrobial agents and molecular docking studies. J. Mol. Struct. 2019;1180:618. doi: 10.1016/j.molstruc.2018.11.029. [DOI] [Google Scholar]
- 8.Thotla K., Noole V., Reddy K. Synthesis and antimicrobial activity of a novel hybrid benzo[b]thiophene-1,2,3-triazole analogues. Chem. Data Collect. 2020;27 doi: 10.1016/j.cdc.2020.100361. [DOI] [Google Scholar]
- 9.Demirbas N., Demirbas A., Karaoglu S.A. Synthesis and biological activities of new 1,2,4-triazol-3-one derivatives. Russ. J. Bioorg. Chem. 2005;31:387–397. doi: 10.1007/s11171-005-0054-0. [DOI] [PubMed] [Google Scholar]
- 10.Kritsanida M., Mouroutsou A., Marakos P., Pouli N., Papakonstantinou-Garoufalias S., Pannecouque C., Witvouw M., De. Clercq E. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. II Farmaco. 2002;57:253–257. doi: 10.1016/S0014-827X(01)01189-2. [DOI] [PubMed] [Google Scholar]
- 11.Alizadeh A. Synthesis of some new 1,2,4‐triazole derivatives by Mitsunobu chemistry. Helvetica. 2005;88:2777–2780. doi: 10.1002/hlca.200590218. [DOI] [Google Scholar]
- 12.Turan-Zitouni G., Kaplancikli Z.A., Erol K., Killic F.S. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. II Farmaco. 1999;54:218–223. doi: 10.1016/S0014-827X(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 13.Tozkoparan B., Gokhan N., Aktay G., Yesilada E., Ertan M. 6-Benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6H)-onessubstituted with ibuprofen: synthesis, characterizationand evaluation of anti-inflammatory activity. Eur. J. Med. Chem. 2000;34:743–750. doi: 10.1016/S0223-5234(00)00157-4. [DOI] [PubMed] [Google Scholar]
- 14.Grivaky E.M., Lee S., Siyal C.W., Duch D.S., Nichol C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem. 1980;23:327–329. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- 15.DeGraw J.I., Christie P.H., Clowell W.T., Sirotnak F.M. Synthesis and antifolate properties of 5,10-ethano-5,10-dideazaaminopterin. J. Med. Chem. 1992;35:320–324. doi: 10.1021/jm00080a017. [DOI] [PubMed] [Google Scholar]
- 16.Furuya S., Ohtaki T. Pyridopyrimidine derivatives, their production and use. Eur. Pat. Appl. EP. 1994 608565. [Google Scholar]
- 17.Heber D., Heers C., Ravens U. Positive inotropic activity of 5-amino-6-cyano-1,3-dimethyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. part 6: compounds with positive inotropic activity. Pharmazie. 1993;48:537–541. [PubMed] [Google Scholar]
- 18.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan G.Sh., Shah A., Rehman Zia-ur, Barker D. Chemistry of DNA minor groove binding agents. J. Photochem. Photobio. B. 2012;115:105–118. doi: 10.1016/j.jphotobiol.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Holguin S., Martinez J., Chow C., Wurtman R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008;22:3938–3946. doi: 10.1096/fj.08-112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezabakhsh A.A., Khodaei S.H. Novel coronavirus (COVID-19): a new emerging pandemic threat. J. Res. Clin. Med. 2020;8:5. doi: 10.34172/jrcm.2020.005. [DOI] [Google Scholar]
- 22.Shinde S.S., Rashinkar G., Salunkhe R. DABCO entrapped in agar-agar: a heterogeneous gelly catalyst for multi-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 2013;178:122–126. doi: 10.1016/j.molliq.2012.10.019. [DOI] [Google Scholar]
- 23.Bandgar B.P., Uppalla L.S. Gel entrapped base catalysed (GEBC) henry reaction: synthesis of conjugated nitroalkenes. Synth. Commun. 2000;30:2071–2075. doi: 10.1080/00397910008087384. [DOI] [Google Scholar]
- 24.Shirini F., Langarudi M.S.N., Seddighi M., Jolodar O.G. Bi-SO3H functionalized ionic liquid based on DABCO as a mild and efficient catalyst for the synthesis of 1,8-dioxo-octahydro-xanthene and 5-arylmethylene-pyrimidine-2,4,6-trione derivatives. Res. Chem. Intermed. 2015;41:8483–8497. doi: 10.1007/s11164-014-1905-1. [DOI] [Google Scholar]
- 25.Shirini F., Langarudi M.S.N., Daneshvar N., Jamasbi N., Irankhah-Khanghah M. Preparation and characterization of [H2-DABCO][ClO4]2 as a new member of DABCO-based ionic liquids for the synthesis of pyrimido[4,5-b]-quinoline and pyrimido[4,5-d]pyrimidine derivatives. J. Mol. Struct. 2018;1161:366–382. doi: 10.1016/j.molstruc.2018.02.069. [DOI] [Google Scholar]
- 26.Lipson V.V., Desenko S.M., Shirobokova M, G., Borodina V.V. Synthesis of 9-Aryl-6,6-dimethyl-5,6,7,9-tetrahydro-1,2,4-triazolo[5,1-b]quinazolin-8(4H)ones. Chem. Heterocycl. Compd. 2003;39:1213–1217. https://doi.org/10.1023%2FB%3ACOHC.0000008269.69460.ac. [Google Scholar]
- 27.Heravi M.M., Derikvand F., Ranjbar L. Sulfamic acid–catalyzed, three-component, one-pot synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives. Synth. Commun. 2010;40:677–685. doi: 10.1080/00397910903009489. [DOI] [Google Scholar]
- 28.Seyyedi N., Shirini F., Langarudi M.S.N., Jashnani S. A simple and convenient synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone and [1,2,4]triazolo[1,5-a]pyrimidine derivatives catalyzed by DABCO-based ionic liquids. J. Iran. Chem. Soc. 2017;14:1859–1867. https://doi.org/10.1007%2Fs13738-017-1125-x. [Google Scholar]
- 29.Heravi M.M., Ranjbar L., Derikvand F., Alimadadi B., Oskooie H.A., Bamoharram F.F. A three component one-pot procedure for the synthesis of [1,2,4]triazolo/benzimidazolo-quinazolinone derivatives in the presence of H6P2W18O62•18H2O as a green and reusable catalyst. Mol. Divers. 2008;12:181–185. doi: 10.1007/s11030-008-9086-8. https://doi.org/10.1007%2Fs11030-008-9086-8. [DOI] [PubMed] [Google Scholar]
- 30.Puligoundla R.G., Karnakanti Sh., Bantu R., Kommu N., Kondra S.B., Nagarapu L. A simple, convenient one-pot synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives by using molecular iodine. Tetrahedron Lett. 2013;54:2480–2483. doi: 10.1016/j.tetlet.2013.02.099. [DOI] [Google Scholar]
- 31.Shaabani A., Farhangi E, Rahmati A. Synthesis of tetrahydrobenzimidazo[1,2-b]quinazolin-1(2H)-one and tetrahydro-1,2,4-triazolo[5,1-b]quinazolin-8(4H)-one ring systems under solvent-free conditions. Comb. Chem. High Throughput Screen. 2006;9:771–776. doi: 10.2174/138620706779026060. [DOI] [PubMed] [Google Scholar]
- 32.Kumari K, Raghuvanshi D.S., Singh K.N. An expeditious synthesis of tetrahydro-1,2,4-triazolo[5,1-b]quinazolin-8(4H)-ones and dihydro-1,2,4-triazolo[1,5-a]pyrimidines. Org. Prep. Proced. Int. 2012;44:460–466. doi: 10.1080/00304948.2012.715062. [DOI] [Google Scholar]
- 33.A Shaabani, Farhangi E., Shaabani Sh. A rapid combinatorial library synthesis of benzazolo[2,1-b]quinazolinones and triazolo[2,1-b]quinazolinones. Iran. J. Chem. Chem. Eng. 2013;32:3–10. http://www.ijcce.ac.ir/article_5899.html [Google Scholar]
- 34.Haghighighat M, Shirini F., Golshekan M. Efficiency of NaHSO4 modified periodic mesoporous organosilica magnetic nanoparticles as a new magnetically separable nanocatalyst in the synthesis of [1,2,4]triazolo quinazolinone/pyrimidine derivatives. J. Mol. Struct. 2018;1171:168–178. doi: 10.1016/j.molstruc.2018.05.112. [DOI] [Google Scholar]
- 35.Ablajan K., Kamil W., Tuoheti A., Wan-Fu S. An efficient three component one-pot synthesis of 5-amino-7-aryl-7,8-dihydro-[1,2,4] triazolo[4,3-a]-pyrimidine-6-carbonitriles. Molecules. 2012;17:1860–1869. doi: 10.3390/molecules17021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamasbi N., Irankhah-Khanghah M., Shirini F., Tajik H., Langarudi M.S.N. DABCO-based ionic liquids: introduction of two metal-free catalysts for one-pot synthesis of 1,2,4-triazolo[4,3-a]pyrimidines and pyrido[2,3-d]pyrimidines. New J. Chem. 2018;42:9016–9027. doi: 10.1039/C8NJ01455H. [DOI] [Google Scholar]
- 37.Azimi S.C. Cellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of pyrimido and pyrazolo[4,5-b]quinolines under solvent-free conditions. Iran. J. Catal. 2014;2:113–120. http://ijc.iaush.ac.ir/article_552171.html [Google Scholar]
- 38.Shi D.Q., Ni S.N., Yang F., Shi J.W., Dou G.L., Li X.Y., Sh. Wang X., Ji S.J.J. An efficient synthesis of pyrimido[4,5‐b]quinoline and indeno[2′,1′:5,6]pyrido[2,3‐d]pyrimidine derivatives via multicomponent reactions in ionic liquid. Heterocycl. Chem. 2008;45:693–702. doi: 10.1002/jhet.5570450310. [DOI] [Google Scholar]
- 39.Mohammadi K., Shirini F., Yahyamdeh A. 1, 3-Disulfonic acid imidazolium hydrogen sulfate: a reusable and efficient ionic liquid for the one-pot multi-component synthesis of pyrimido [4, 5-b] quinoline derivatives. RSC Adv. 2015;5:23586–23590. doi: 10.1039/C5RA02198G. [DOI] [Google Scholar]
- 40.Abdolmohammadi S., Balalaie S. An efficient synthesis of pyrido[2,3-d]pyrimidine derivatives via one-pot three-component reaction in aqueous media. Int. J. Org. Chem. 2012;2:7–14. doi: 10.4236/ijoc.2012.21002. [DOI] [Google Scholar]
- 41.Mossafaii-Rad A., Mokhtary M. Efficient one-pot synthesis of pyrido[2,3-d]pyrimidines catalyzed by nanocrystalline MgO in water. Int. Nano. Lett. 2015;5:109–123. doi: 10.1007/s40089-015-0145-8. [DOI] [Google Scholar]
- 42.Shi D., Ji Sh., Niu L., Shi J., Wang X. One‐pot synthesis of pyrido[2,3‐d]pyrimidines via efficient three‐component reaction in aqueous media. J. Heterocycl. Chem. 2007;44:1083–1090. doi: 10.1002/jhet.5570440517. [DOI] [Google Scholar]
- 43.Jolodar O.G., Shirini F., Seddighi M. Efficient synthesis of pyrano[2,3-d]pyrimidinone and pyrido[2,3-d]pyrimidine derivatives in presence of novel basic ionic liquid catalyst. Chin. J. Cata. 2017;38:1245–1251. doi: 10.1016/S1872-2067(17)62827-4. [DOI] [Google Scholar]
- 44.Alluri H., Gonthina H., Ganta R.K., Ch M., Rao V. B. Efficient one-pot synthesis of multi-substituted triazolopyrimidines by using DBU as basic catalyst via MCR's. Der. Pharma Chem. 2015;7:515–520. [Google Scholar]
- 45.Bhattacharyya P., Paul S., Das A.R. Facile synthesis of pyridopyrimidine and coumarin fused pyridine libraries over a Lewis base-surfactantcombined catalyst TEOA in aqueous medium. RSC Adv. 2013;3:3203–3208. doi: 10.1039/c3ra23254a. [DOI] [Google Scholar]
- 46.Sabour B., Peyrovi M.H., Hajimohammadi M. Al-HMS-20 catalyzed synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines via three-component reaction. Res. Chem. Intermed. 2015;41:1343–1350. doi: 10.1007/s11164-013-1277-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.