Abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic threatens global health thereby causing unprecedented social, economic, and political disruptions. One way to prevent such a pandemic is through interventions at the human-animal-environment interface by using an integrated One Health (OH) approach. This systematic literature review documented the three coronavirus outbreaks, i.e. SARS, MERS, COVID-19, to evaluate the evolution of the OH approach, including the identification of key OH actions taken for prevention, response, and control.
The OH understandings identified were categorized into three distinct patterns: institutional coordination and collaboration, OH in action/implementation, and extended OH (i.e. a clear involvement of the environmental domain). Across all studies, OH was most often framed as OH in action/implementation and least often in its extended meaning. Utilizing OH as institutional coordination and collaboration and the extended OH both increased over time. OH actions were classified into twelve sub-groups and further categorized as classical OH actions (i.e. at the human-animal interface), classical OH actions with outcomes to the environment, and extended OH actions.
The majority of studies focused on human-animal interaction, giving less attention to the natural and built environment. Different understandings of the OH approach in practice and several practical limitations might hinder current efforts to achieve the operationalization of OH by combining institutional coordination and collaboration with specific OH actions. The actions identified here are a valuable starting point for evaluating the stage of OH development in different settings. This study showed that by moving beyond the classical OH approach and its actions towards a more extended understanding, OH can unfold its entire capacity thereby improving preparedness and mitigating the impacts of the next outbreak.
Keywords: One Health, SARS, MERS, COVID-19, Collaboration, Disease ecology
Highlights
-
•
Different One Health understandings in practice complicate its operationalization.
-
•
Intersectoral collaboration under One Health can improve outbreak preparedness.
-
•
The role of the environment in OH lacks a clear definition and is still neglected.
-
•
Preventive actions at the human-animal-environment interface are needed.
1. Introduction
At the end of 2019, the novel Betacoronavirus, Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), crossed the animal-human barrier resulting in a public health emergency of international concern and a global pandemic [1]. With the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) in 2002/2003 and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012, this is the third time within the last 20 years that Betacoronaviruses have crossed the animal-human species barrier resulting in major zoonotic outbreaks [2].
The first SARS outbreak began in the Guangdong Province, China, in late 2002 with 300 cases of atypical pneumonia [3,4]. Despite the measures issued by the Chinese Government and the World Health Organization (WHO) to stop the transmission, SARS spread to “5 countries within 24 hours and to more than 30 countries on 6 continents within 6 months” ([5], p. 3), with over 8000 cases worldwide and a 10% fatality rate between 2002 and 2003 [6]. The primary source of infection was attributed to wild mammals traded in local markets for human consumption [5,7]. The epidemic was brought under control by a remarkable coordinated response [4], albeit impacting significantly 36 countries socially, politically, and economically [8].
In mid-2012, MERS emerged in Saudi Arabia due to human exposure to MERS-CoV-infected dromedary camels [9]. Further human-to-human transmission spread it to 27 countries across the globe [[10], [11], [12], [13], [14], [15], [16]] with a total of 2519 laboratory-confirmed cases globally and a case-fatality rate of 34.3% until January 2020 [17]. Measures to control and prevent MERS involved aggressive contact tracing, quarantine, and isolation of the cases [18,19].
On December 8, 2019, the first case of a new atypical pneumonia was confirmed in Wuhan City, China, and since then spread throughout the world [20]. Several million cases have been confirmed in approximately 188 countries as of September 2020 [21]. Based on countries' situations and capacities, various strategies have been implemented to control the pandemic. Those strategies include case detection followed by contact tracking and isolation, avoidance of close contacts between humans (e.g. physical distancing) through lockdown procedures [22], Water, Sanitation and Hygiene (WASH) interventions (particularly hand-hygiene) [23], and the use of personal protective equipment, in addition to increasing available capacities in the health care system.
Increasing human-animal interactions have amplified the likelihood of cross-species infections and spill-over events [24]. Epidemiological links of the three coronavirus outbreaks point to the human-animal interface, highlighting the importance of considering prevention strategies with an integrated approach such as “One Health” [25]. The One Health (OH) approach considers the human-animal-environmental interdependence through multi-sectoral collaborations [26] and recognizes that prevention and “zoonotic disease control programs are most effective when the broader socioeconomic and ecological determinants of health are included” ([27], p. 66).
The outbreaks of SARS, MERS, and COVID-19 represent ideal cases for analyzing how the OH approach has been used concerning those zoonotic diseases. Reviewing the experiences and lessons learned from the epidemics allows envisaging the future of public health and OH. Through a systematic literature review, this work contributes to the further development of the OH approach moving from surveillance to the prevention of public health emergencies. The study aims to understand the evolution of the OH approach in relation to SARS, MERS, and COVID-19, and to identify the key OH actions taken for prevention, response, and control of those.
2. Methods
2.1. Study selection
This study used a systematic literature review to analyse how the OH approach was framed in the context of several coronavirus outbreaks, thereby following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) selection method to identify relevant studies [28]. Eight databases were systematically searched (i.e. Google Scholar, JStor, PubMed, Science Direct, Springer Link, Taylor and Francis Online, Web of Science, and Wiley Online Library). The search terms were inspired by Elsevier's “Novel Coronavirus Information Center” [29], and further organized into coronaviruses and combined with OH: (i) coronavirus or “corona virus”, (ii) “COVID-19” or “2019-nCov” or “SARS-CoV-2”, (iii) “Severe Acute Respiratory Syndrome” or “SARS-CoV” or “SARS”, (iv) “Middle East Respiratory Syndrome” or “MERS-CoV” or “MERS”, and “One Health”. The literature search was repeated several times between April and September 2020 to identify newly published studies constantly.
Studies were considered suitable for inclusion if they specifically mentioned OH in their title or abstract in combination with either SARS, MERS, or COVID-19. Scientific publications in the form of case reports, correspondences, data articles, discussions, editorials, research and review articles, or short communications were included. However, books or book reviews, conference proceedings, and meeting abstracts, front matters, news, posters, practice guidelines, presentations, replication studies, or software and video publications were not considered. The year of the SARS outbreak (2002) was set as the starting point and only studies published in English were included. The full-texts of the studies that met the inclusion criteria were assessed for eligibility subsequently. Only studies that demonstrated an explicit and clear link between OH and coronaviruses were included in the final synthesis.
2.2. Synthesis of results
The studies were divided into two OH approaches observed in the general literature. The first denoted as “classical OH approach” addressed the “management of the disease threats to humans and animals” ([30], p. 372); the second, referred to as “extended OH approach” looked into the close interrelationship between humans and animals with ecosystems, environmental health, pathogens, and the broader social, cultural, and economic factors [[31], [32], [33]].
For classifying the studies, data were extracted into a purpose-built data extraction form in a Google Spreadsheet including details on study type, spatial scale, methodological approach, OH domains (animal, human, and environment), OH actions, and OH understanding. The varying nature of the studies did not allow for any internally consistent and comparable quality assessment.
The OH understandings were categorized into groups derived inductively from the wording used by the authors in their texts. Other classifications of OH proposed in the literature [e.g. [34], [35]] provided a basis for defining the groups. It was thereby possible for a study to be associated with more than one group. Three groups were identified following the patterns of how OH was utilized:
-
•
Institutional coordination and collaboration: OH was framed as a way to communicate, coordinate, and collaborate among stakeholders across sectors in a multi−/inter−/ and/or transdisciplinary manner to solve complex health challenges.
-
•
One Health in action/implementation: OH was used in a way to provide a frame for the actions introduced or proposed to prevent and control the respective disease outbreak, e.g. through surveillance and monitoring.
-
•
Extended One Health: OH was framed as a more comprehensive and extended understanding, thereby emphasising the role of the environment (either built or natural) and highlighting the complex interactions at the human-animal-environment interface as well as social, structural and ecological changes.
The documents were also scanned for specific actions that were used or proposed within the OH framework for prevention, control, or response, targeting either SARS, MERS, or COVID-19. The OH actions were classified into twelve sub-groups using terminology observed in the publications to determine the levels of application: (1) Animal movement and interaction between animals and with humans; (2) Awareness creation, operating protocols, and policies; (3) Control and understanding of pathogens; (4) Diagnosis, detection, and treatment capacities; (5) Environmental management and pollution control; (6) Food safety; (7) Human travel control and community control strategies; (8) Immunization; (9) Information management; (10) OH capacity development and research; (11) Preparedness and response plans; and (12) Surveillance and monitoring. The OH classical and extended approaches were used to segregate the OH actions further.
The results are presented quantitatively and are complemented by a qualitative synthesis in the discussion.
2.3. Limitations
Publication bias also applies to this review. However, the influence can be considered low because this study focused on how the respective authors framed OH in the context of coronaviruses, instead of specific (positive) outcomes. Moreover, selection bias can influence this study because this analysis only considered studies published in English. Due to the inclusion of reviews and editorials, it may be possible that some OH understandings and actions were counted more than once. This potential bias was addressed by grouping OH understandings and actions and not relying on individual studies for the analysis. Further consideration has to be given to the fact that the OH approach emerged in the early 2000s, was included in the global health discourse in 2004, and its adoption by international organizations occurred only in 2008 [36]. This may explain why the SARS outbreak in 2002/2003 is covered by far the least in the studies included.
The classification of OH understandings and actions was made based on the words used in the studies, which does not necessarily reflect on the general opinions of the authors concerning OH.
3. Results
3.1. Study selection
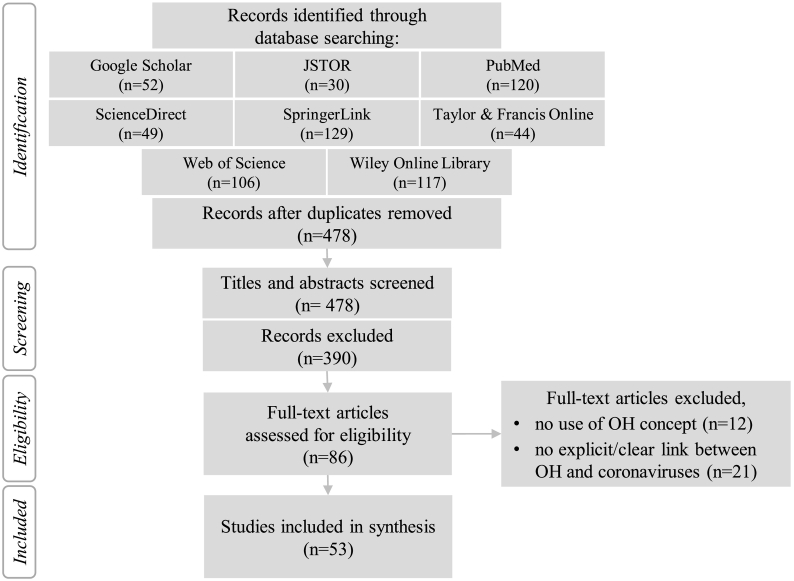
The initial online database search, after duplicates were removed, yielded 478 studies. The study selection process for final analysis is illustrated in the PRISMA flow chart (Fig. 1) [28]. Titles and abstracts of all studies were screened of which 86 were included for full-text assessment for eligibility. Out of those, 53 studies met the inclusion criteria and were included in the final synthesis (a list of all included studies can be found in supplementary material A).
Fig. 1.
PRISMA flow chart diagram of the systematic review showing the selection process of relevant studies [28].
3.2. Study characteristics
Table 1 displays the characteristics of the included studies. The majority of studies were conducted on a global scale, followed by studies at the national and regional scale, with the majority of countries in the Middle East and North Africa (MENA) that reported MERS outbreaks. Literature reviews and mini-reviews were the most numerous group. Opinions and perspectives, position statements, and commentaries, as well as editorials, guest editorials, and letters to the editor followed. Original research articles had the lowest count. There was a steep increase in publications in 2019 and 2020 explained by the outbreaks of MERS and COVID-19, after a period of relatively few studies between 2013 and 2017. Most studies focused on COVID-19, followed MERS and SARS.
Table 1.
Characteristics of all 53 studies included for the final synthesis.
| Group | Characteristic | No. | % |
|---|---|---|---|
| Spatial scale | Global | 34 | 64% |
| Regional | 10 | 19% | |
| National | 9 | 17% | |
| Study type | Editorial | 13 | 25% |
| Opinion and perspectives | 12 | 23% | |
| Original article | 10 | 19% | |
| Review | 18 | 34% | |
| Year | 2013 | 1 | 2% |
| 2015 | 2 | 4% | |
| 2016 | 2 | 4% | |
| 2017 | 2 | 4% | |
| 2019 | 7 | 13% | |
| 2020 | 39 | 74% | |
| Disease focus | SARS | 4 | 7% |
| MERS | 20 | 37% | |
| COVID19 | 37 | 69% |
Note: The sum of the disease focus sub-groups adds up to more than the total of studies because some focused equally on both SARS and MERS; the sum of percentage might not add up to 100% due to rounding.
3.3. Results of the studies: OH understandings
Three types of OH understandings were identified inductively, Table 2a, Table 2b display each study sorted by publication year with their respective study type, and OH understanding grouped into studies dealing with SARS or MERS (2013−2020) and COVID-19 (2020).
Table 2a.
One Health understandings identified in studies dealing with SARS or MERS (2013–2020).
| Study | Study type | One Health understanding |
|||
|---|---|---|---|---|---|
| Institutional coordination & collaboration | OH in action / implementation | Extended OH | |||
| SARS or MERS (2013–2020) | Heymann & Dixon (2013) | O | x | – | x |
| Crameri et al. (2015) | O | – | x | x | |
| Edelstein & Heymann (2015) | O&P | x | x | – | |
| Widagdo et al. (2016) | R | – | x | – | |
| Zumla et al. (2016) | E | x | x | x | |
| Alharbi (2017) | R | – | x | – | |
| Daly (2017) | E | x | x | – | |
| Dawson et al. (2019) | R | x | x | x | |
| Farag et al. (2019a) | O | x | x | – | |
| Farag et al. (2019b) | R | x | x | – | |
| Hemida (2019) | R | – | x | x | |
| Park et al. (2019) | O&P | x | x | – | |
| Ramadan & Shahib (2019) | R | x | x | x | |
| Werney et al. (2019) | R | x | x | – | |
| Al Awaidy et al. (2020) | R | x | x | – | |
| Hemida & Alnaeem (2020) | R | – | x | x | |
Note: E = Editorials; O&P = Opinion and perspective; O = Original research; R = Review.
Table 2b.
One Health understandings identified in studies dealing with COVID-19 (2020).
| Study | Study type | One Health understanding |
|||
|---|---|---|---|---|---|
| Institutional coordination & collaboration | OH in action / implementation | Extended OH | |||
| COVID-19 (2020) | Ahmad & Hui (2020) | E | x | x | x |
| Ahmad et al. (2020) | R | x | x | – | |
| Ayaji (2020) | E | x | x | x | |
| Amuasi et al. (2020) | O&P | x | – | x | |
| Bhatia (2020) | O&P | x | x | – | |
| Bonilla-Aldana et al. (2020a) | E | x | – | – | |
| Bonilla-Aldana et al. (2020b) | E | x | x | x | |
| Chauhan et al. (2020) | R | x | x | – | |
| Colunga-Salas & Hernandez-Canchola (2020) |
O&P | – | x | x | |
| Decaro et al. (2020) | O&P | x | x | x | |
| Dhama et al. (2020) | R | x | – | x | |
| Di Guardo & Vignoli (2020) | O&P | x | – | – | |
| Egeru et al. (2020) | O&P | x | – | x | |
| El Zowalaty & Järhult (2020) | O&P | x | x | x | |
| Enticott & Maye (2020) | O | x | – | – | |
| Espejo et al. (2020) | O | x | – | x | |
| Gollakner & Capua (2020) | O&P | x | x | – | |
| Helmy et al. (2020) | R | x | – | – | |
| Hemida & Abduallah (2020) | R | – | x | x | |
| Henley (2020) | E | x | – | x | |
| Kaphle (2020) | O | x | – | – | |
| Kasozi et al. (2020) | E | x | – | x | |
| KC et al. (2020) | R | x | – | – | |
| Konda et al. (2020) | R | x | – | – | |
| Lorusso et al. (2020) | O | x | x | – | |
| Majid et al. (2020) | O | – | x | – | |
| Marty & Jones (2020) | E | x | x | – | |
| Mobasheri (2020) | O&P | x | x | – | |
| Muraina (2020) | O&P | x | x | – | |
| Mushi (2020) | E | – | x | – | |
| Parry (2020) | E | x | – | – | |
| Pokharel et al. (2020) | E | x | – | – | |
| Poudel et al. (2020) | R | x | x | – | |
| Sun et al. (2020) | O | x | x | – | |
| Wang et al. (2020) | O | x | x | – | |
| Ward (2020) | E | x | x | – | |
| Yasobant et al. (2020) | R | – | x | – | |
Note: E = Editorials; O&P = Opinion and perspective; O = Original research; R = Review.
Across all studies, OH was most often framed as OH for institutional coordination and collaboration (81%), followed by OH in action/implementation, i.e. means for prevention and control (70%), and lastly in its extended meaning concerned with the (natural) environment and interactions at the human-animal-environment interface (38%).
Segregating the studies according to their study types revealed that OH was framed as institutional coordination and collaboration in most editorials (92%) and opinion and perspective studies (92%), while 80% of the original articles and only 67% of the reviews used it as such. Understanding OH as action/implementation was found in three-quarter of both opinion and perspective as well as reviews and in less than two-thirds of editorials and original articles. The extended understanding of the OH approach was mentioned more often in editorials (46%), followed by opinion and perspective (42%), reviews (33%), and original articles (30%).
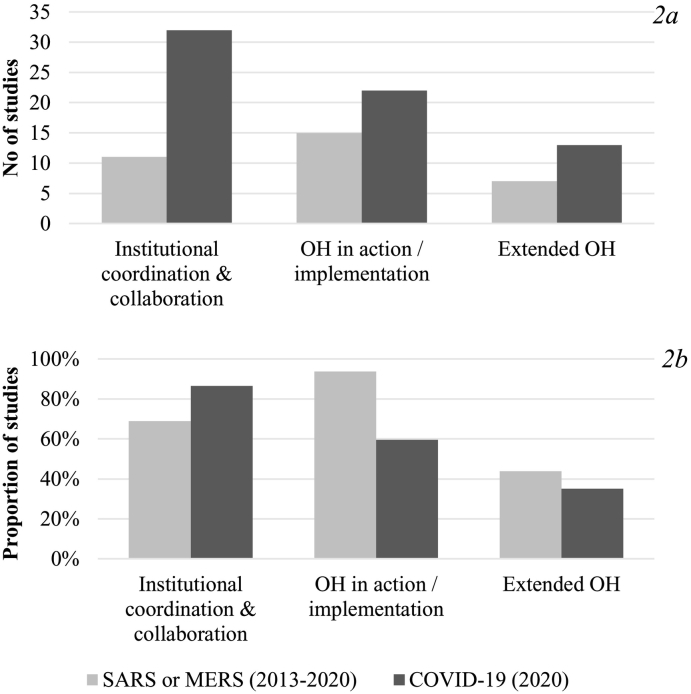
For further analysis, the studies were categorized by disease focus in two groups (i.e. SARS or MERS and COVID-19) (see Fig. 2). The number of studies on COVID-19 pursuing OH as institutional coordination and collaboration was much higher than for the SARS/MERS groups. The application of the extended OH approach has increased, however, it remains the least common understanding of OH. Examining the relative distribution of OH understandings by disease group (Fig. 2b) highlights that the dominant understanding of OH changed from OH in action/implementation, which decreased from 94% to 59%, to institutional coordination and collaboration. The application of an extended OH understanding decreased slightly from the SARS/MERS (2013–2020; 44%) group to the more recent studies on COVID-19 (2020; 35%).
Fig. 2.
a/b. Number (2a, top) and proportion (2b, bottom) of studies grouped by disease focus for each OH understanding.
3.4. Result of the individual studies: OH actions
The OH action types were split into actions under the classical and extended OH approaches, and grouped to determine the levels of application.
The actions at the human-animal interface displayed in Table 3 included measures to decrease the risk of viral shedding from human to human [37,38], from animal to animal [38,39], and from animal to human [40]. Further actions targeted the identification and understanding of pathogen reservoirs [38,[41], [42], [43], [44], [45], [46], [47], [48]], and awareness-raising on the diseases [45,[49], [50], [51], [52]]. The identified actions operated on various spatial levels: household level, i.e. to make dairy and meat safe for consumption [10,38] and the pasteurization of camel milk [40]; farm level, i.e. rapid identification of sick camels and their isolation [38,47]; abattoirs level, i.e. standardized protocols for the operation [38]; country and regional level, i.e. limitation of livestock mobility between neighbouring countries [38], improvement in the laboratories´ capacities [41,42,[53], [54], [55]], the collaboration between institutions for a coordinated response to outbreaks [56], and the improvement of surveillance and monitoring [[57], [58], [59], [60], [61], [62]].
Table 3.
One Health actions at the human-animal interface (i.e. “classical OH”) identified in each study.
| Group | References | |
|---|---|---|
| Classical | Animal movement and interaction between animals and with humans | Hemida & Alnaeem (2019); Hemida (2019); Konda et al. (2020); Zumla et al. (2016a); El Zowalaty & Järhult (2020); Colunga-Salas & Hernandez-Canchola (2020); Dhama et al. (2020); Ahmad et al. (2020) |
| Awareness creation, operating protocols, and policies | Farag et al. (2019a); Ajayi (2020); Helmy et al. (2020); Hemida & Alnaeem (2019); Ahmad et al. (2020); Dhama et al. (2020); Gollakner & Capua (2020); Ramadan & Shaib (2019); Hemida (2019); Konda et al. (2020); Lorusso et al. (2020); Mushi (2020) | |
| Control and understanding of pathogens | Al Awaidy et al. (2020); Di Guardo & Vignoli (2020); KC et al. (2020); Lorusso et al. (2020); Ahmad et al. (2020); Widagdo et al. (2016); Hemida & Alnaeem (2019); Konda et al. (2020); Gollakner & Capua (2020) | |
| Diagnosis, detection, and treatment capacities | Al Awaidy et al. (2020); Farag et al. (2019a); Sun et al. (2020); Ahmad et al. (2020); Bonilla-Aldana, D, Dhama, K, Rodriguez-Morales, A J (2020); Mushi (2020); Ahmad et al. (2020); Helmy et al. (2020); Lorusso et al. (2020); Bonilla-Aldana et al. (2020); Mobasheri (2020); Muraina (2020) | |
| Food safety | Hemida (2019); Hemida & Alnaeem (2019); Zumla et al. (2016a); Konda et al. (2020); Poudel et al. (2020); Ahmad et al. (2020) | |
| Human travel control and community control strategies | Ahmad et al. (2020), Mushi (2020); Ramadan & Shaib (2019); Zumla et al. (2016a) | |
| Immunization | Ramadan & Shaib (2019); Widagdo et al. (2016); Helmy et al. (2020); Bonilla-Aldana et al. (2020); Ahmad et al. (2020); Decaro et al. (2020); Lorusso et al. (2020) | |
| Information management | Farag et al. (2019a) | |
| OH capacity development and research | Bonilla-Aldana, D, Dhama, K, Rodriguez-Morales, A J (2020); Dhama et al. (2020); Mobasheri (2020); Konda et al. (2020); Helmy et al. (2020) | |
| Preparedness and response plans | Farag et al. (2019a); Hemida & Alnaeem (2019); Hemida & Abdullah (2020); Ramadan & Shaib (2019); Dhama et al. (2020) | |
| Surveillance, monitoring | Crameri et al. (2015); Hemida & Alnaeem (2019); Muraina (2020); Hemida & Abdullah (2020); Mobasheri (2020); Ahmad et al. (2020); Yasobant et al. (2020); Bhatia, R. (2020) |
Note: An extended version of this table with concrete actions can be found in Annex B.
A smaller set of control and response actions at the human-animal interface considered the environmental outcomes (see Table 4; a more detailed version of Table 3 and Table 4 can be found in supplementary material B). These include the banning and monitoring of animal transport and trade to avoid the zoonotic bridging in a food system related-environment [45,57,63], increased awareness of personal hygienic measures in occupational settings [50,52], vaccination and monitoring of domestic animals to reduce shedding the viruses to the environment [38,47,51,[64], [65], [66]], bio-surveillance in wet markets [37,61], and public education on zoonosis [37,46,54].
Table 4.
OH Actions with outcomes for the environment identified in each study.
| Group | References | |
|---|---|---|
| Classical towards care of environment | Animal movement and interaction between animals and with humans | Hemida & Abdullah (2020); Konda et al. (2020); Ahmad et al. (2020); Dhama et al. (2020) |
| Awareness, operating protocols, and policies | El Zowalaty & Järhult (2020); Bonilla-Aldana, D, Dhama, K, Rodriguez-Morales, A J (2020); Poudel et al. (2020); Hemida (2019); Helmy et al. (2020); Egeru et al. (2020) | |
| Control and understanding of pathogens | Poudel et al. (2020); Konda et al. (2020); Bonilla-Aldana, D, Dhama, K, Rodriguez-Morales, A J (2020); Ramadan & Shaib (2019); Al Awaidy et al. (2020); Di Guardo & Vignoli (2020); Parry (2020) | |
| Food safety | Henley (2020); Pokharel et al. (2020) | |
| Human travel control and community control strategies | Wang et al. (2020) | |
| Immunization | Alharbi (2017); Hemida & Alnaeem (2019); Daly (2017); Ramadan & Shaib (2019); Widagdo et al. (2016); Hemida (2019); Bonilla-Aldana et al. (2020) | |
| Information management | Al Awaidy et al. (2020); Ahmad et al. (2020); Yasobant et al. (2020) | |
| OH capacity development and research | Al Awaidy et al. (2020); Farag et al. (2019a); Edelstein and Heymann (2015); Ahmad et al. (2020); Henley (2020); KC et al. (2020); Muraina (2020); Park et al. (2019); Yasobant et al. (2020); Mobasheri (2020); Helmy et al. (2020); Egeru et al. (2020); Mushi (2020) | |
| Surveillance, monitoring | Dawson et al. (2019); Farag et al. (2019a); Hemida & Alnaeem (2019); Ramadan & Shaib (2019); Ahmad et al. (2020); Chauhan et al. (2020); Mushi (2020); Yasobant et al. (2020); Bonilla-Aldana et al. (2020), Hemida (2019); Heymann & Dixon (2013); Majid et al. (2020); Lorusso et al. (2020); Pokharel et al. (2020); El Zowalaty & Järhult (2020); Ajayi (2020) | |
| Extended | Awareness, operating protocols, and policies | Espejo et al. (2020) |
| Control and understanding of pathogens | Egeru et al. (2020); Hemida & Abdullah (2020) | |
| Environmental management and pollution control | Hemida & Abdullah (2020); Helmy et al. (2020); Dhama et al. (2020); Espejo et al. (2020); Bonilla-Aldana, D, Dhama, K, Rodriguez-Morales, A J (2020); Poudel et al. (2020); Hemida (2019); Hemida & Alnaeem (2019) | |
| Information management | Ramadan & Shaib (2019) | |
| OH capacity development and research | Kasozi et al. (2020); Helmy et al. (2020); Ramadan & Shaib (2019); Amuasi et al. (2020); Espejo et al. (2020); Ward (2020); Ajayi (2020); Enticott & Maye (2020); El Zowalaty & Järhult (2020) | |
| Surveillance, monitoring | Poudel et al. (2020); Helmy et al. (2020); Yasobant et al. (2020) |
Note: An extended version of this table with concrete actions can be found in Annex B.
The actions considered at the animal-human-environment interface addressed the environment as transmission and virus shedding route. These actions differentiated between measures applied in built environments such as environmental hygiene and cleanliness [50,57,63], biological waste disposal from abattoirs [38,52], measures applied in natural environments such as monitoring the concentration of the viruses in the air [57], urban land-use planning [67], chemical and pollutants management [38,52,67], and wastewater treatment plants for dealing with drugs and pathogens [54,67]. Further actions at the human-animal-environment interface were concern with the spatial identification of high-risk areas by utilizing Geographic Information Systems [40], and capacity development in a broad range of sectors and disciplines [37,46,49,50,61,68,69].
4. Discussion
4.1. Moving towards an extended OH approach that includes the environmental domain
The studies included in this systematic literature review have a strong focus on addressing zoonotic diseases at the animal-human interface. In contrast, only a few studies referred explicitly to interactions at the human-animal-environment interface [37,39,50,54,67,70], which may be explained by the apparent lack of a clear definition of “the environment”. More specifically, general references were made on disease transmission in healthcare settings [52,57], rather than the influence of the natural environment on disease emergence.
Crameri et al. [58] argued, “that when dealing with an emerging infectious disease with a complex epidemiology, conventional outbreak investigations may not resolve key questions, and thus there is a need for studies which might appear tangential” (p. 81). In line with this vision, other studies have indicated that environmental factors, e.g. temperature, rainfall, humidity, or vegetation, could play a role in the emergence of coronavirus diseases [39,52,54,57,70,71], calling for the equal inclusion of the environmental domain in the prevention of zoonosis.
Zoonotic disease emergence has been mostly mediated by the mobility of pathogens across species and ecological boundaries [72,73]. Human population growth has increased the demand for housing, food, trade, and tourism thereby directly or indirectly increasing human exposure to viral zoonoses through the expansion and modification of the built environment into natural habitats [[74], [75], [76], [77], [78]]. In addition, as a result of globalization and increased urbanization patterns, the demand for animal protein also increases, driving agricultural expansion and intensification, along with deforestation, as well as animal trade. This increases animal density and contact between animals and humans, leading to an increased risk of pathogen transfers [78]. This is evident in the emergence and re-emergence of viral zoonosis (e.g. Ebola or MERS-CoV) in Sub-Saharan Africa, the Middle East, and Asia [56,74,78]. Moreover, poor waste management leads to environmental degradation and increased exposure and susceptibility of humans to viral infections [79].
Fewer of the reviewed studies mentioned a broader socio-ecological perspective, by considering underlying drivers such as environmental and climate change, as well as anthropological and demographic changes [39,50,80]. In this context, some studies called for the inclusion of other specialists aside from the human and veterinary health disciplines, namely, ecologists, economists, and other natural and social scientists, to be involved in the effort to prevent and control disease outbreaks caused by coronaviruses [39,40,50,[81], [82], [83]].
The importance of the natural and built environments can be easily identified in the context of the COVID-19 outbreak. Recent studies on SARS-CoV-2 reported that the virus was isolated from toilet bowls, sinks [84,85], different kind of materialistic environmental surfaces, including plastic, stainless steel, paper [86], surgical masks, and personal protective equipment [87], indicating the role of the built environment. As for conditions of the natural environment, morbidity levels of COVID-19 have been related to temperature and humidity [87,88], and atmospheric pollution [89]. These findings suggest the potential importance of measures such as sewage surveillance, strict adherence to hygiene and sanitization measures [84,90], further research on airborne transmission through aerosols [85], and on the effects of weather parameters (e.g. solar radiation, temperature, and humidity) in different climatic zones [91].
4.2. Operationalization of OH through institutional collaboration and action
The different OH understandings make it challenging to operationalize OH, and thereby, highlighting the importance of institutional coordination and collaboration to create a common understanding of OH.
The majority of the studies highlighted the detection, prevention, and control of emerging zoonotic infections as the main motivation for institutional coordination and collaboration [10,37,44,54,62,92]. Those collaborations were implemented at various scales, such as international and national levels. Examples found in the review are the national OH strategies implemented in the countries of the Gulf Cooperation [56], and in Oman [41], based on human-animal health committees, enhancement of capacities to provide diagnostic for humans and animals, collaboration for epidemiological surveillance, joint research projects, multidisciplinary action, and inter-sectoral collaboration between ministries.
Along with the collaboration strategies, OH actions that are specific (e.g. spatial levels, i.e. household, food production units, food processing units, markets of live animals, transportation) and target-oriented (e.g. animal-human and animal-human-environment interfaces) are needed for the operationalization of OH [93]. At the animal-human interface, the understanding of zoonotic pathogen fatality and the spillover from wildlife to humans was the best action for effective prevention and control of zoonotic outbreaks [94]. Considering the environment, another group of actions proposed for the control and response to MERS and COVID-19 can be differentiated between those applied to the built environments and those applied in natural environments.
Combining institutional coordination with specific OH actions can drive the operationalization of OH, however, the initiatives taken until now have different understandings of the OH approach that might hinder effective coordination, collaboration, and action.
Challenges identified were related to different understandings of OH, sectoral power relations and priorities, and funding:
-
•
sectoral actions to reduce the risk of viral shedding, as well as therapeutic interventions, were framed within OH, causing potential biases on the understandings of OH;
-
•
the actions applied at the human-animal-environment interface reflected directly on the mortality and morbidity indicators of humans and animals, but not so on the improved environmental quality;
-
•
the distribution of financial funds was made in a sectoral manner, challenging the contribution of the parts involved in the funding of specific OH actions.
The classical OH approach is the natural choice to guide coordination between sectors at the early stages, moreover, to expand the OH scope to understand the mediating conditions that facilitate infection rates such as social, cultural, economic, or climatic, is a complex task that needs time to be addressed. Notwithstanding this, a more comprehensive understanding of infectious diseases is needed to address unanswered questions related to the complexity of diseases.
5. Conclusion
The evolution of the OH approach has created the opportunity to develop actions at the human-animal-environment interface more equitably. This has become apparent in the latest efforts to address MERS and COVID-19 epidemics, which focused not only on the human-animal interfaces but also started to include the environment. This extended OH approach provides an inclusive and comprehensive outlook on health issues, including the emergence of zoonotic diseases. However, there is still a long way to go for achieving institutional coordination and collaboration across disciplines and sectors, with the inclusion of social, cultural, and economic components.
The actions identified in this literature review are a good starting point for evaluating the stage of OH development in different settings, such as research or policy development. Moreover, the knowledge gaps identified were (1) for the general operationalization of OH: comparing the number of resources needed for OH coordination and actions, with the benefit obtained from those; and (2) for the inclusion of the environment in OH actions: the understanding of disease ecologies within the context of environmental factors (e.g. weather parameters, sewage surveillance, pathogens transmission through aerosols, environmental hygiene and sanitization measures).
The OH contribution to the current COVID-19 control can only be envisioned through therapeutic and preventive options. However, there is a great potential for promoting intersectoral action and active surveillance in natural reservoirs to be prepared and to mitigate the impacts of the next epidemics. Therefore, it is the right time to understand and extend the scope of OH, and appropriate actions need to be taken at the local, national, and global levels.
Funding
This study is funded by the Ministry of Culture and Science of North Rhine-Westphalia, Germany, through the Forschungskolleg “One Health and Urban Transformation”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All relevant data that supports the findings of this study are within the manuscript.
Authors' contributions
All authors contributed equally to the development of this study. As for the CRediT guidelines,
-
1.
Dennis Schmiege: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization, Project administration
-
2.
Ana Maria Perez Arredondo: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization, Project administration
-
3.
Joshua Ntajal: Conceptualization, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing
-
4.
Juliana Minetto Gellert Paris: Conceptualization, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing
-
5.
Merveille Koissi Savi: Conceptualization, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing
-
6.
Krupali Patel: Conceptualization, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing
-
7.
Sandul Yasobant: Conceptualization, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing
-
8.
Timo Falkenberg: Conceptualization, Writing – Review & Editing, Supervision
All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of Competing Interest
None.
Contributor Information
Dennis Schmiege, Email: d.schmiege@uni-bonn.de.
Ana Maria Perez Arredondo, Email: ana.perez@uni-bonn.de.
Joshua Ntajal, Email: joshuantajal@uni-bonn.de.
Juliana Minetto Gellert Paris, Email: jparismi@uni-bonn.de.
Merveille Koissi Savi, Email: s7mesavi@uni-bonn.de.
Krupali Patel, Email: Kpatel@uni-bonn.de.
Sandul Yasobant, Email: ysandul@uni-bonn.de.
Timo Falkenberg, Email: falkenberg@uni-bonn.de.
References
- 1.World Health Organization . Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Geneva. 2005. Statement on the second meeting of the International Health Regulations.https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov Available: [Google Scholar]
- 2.National Institute of Allergy and Infectious Diseases Coronaviruses. 2020. https://www.niaid.nih.gov/diseases-conditions/coronaviruses Available:
- 3.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey E.S., Fieldhouse J.K., Choi J.Y., Gray G.C. A mini review of the zoonotic threat potential of influenza viruses, coronaviruses, adenoviruses, and enteroviruses. Front. Public Health. 2018;6 doi: 10.3389/fpubh.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knobler S., Mahmoud A., Lemon S., Mack A., Sivitz L., Oberholtzer K., editors. Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. Washington (DC) 2004. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Cumulative Number of Reported Probable Cases of SARS: 1 Nov 2002 to 26 June 2003, 17:00 GMT+2. https://www.who.int/csr/sars/country/2003_06_26/en/ Available:
- 7.Demmler G.J., Ligon B.L. Severe acute respiratory syndrome (SARS): a review of the history, epidemiology, prevention, and concerns for the future. Semin. Pediatr. Infect. Dis. 2003;14:240–244. doi: 10.1016/S1045-1870(03)00056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arie S. Would today’s international agreements prevent another outbreak like SARS? BMJ Br. Med. J. 2014;348 doi: 10.1136/bmj.g4123. https://www.jstor.org/stable/26515393 Available: [DOI] [PubMed] [Google Scholar]
- 9.Farag E., Sikkema R.S., Mohamedani A.A., de Bruin E., Munnink B.B.O., Chandler F. MERS-CoV in camels but not camel handlers, Sudan, 2015 and 2017. Emerg. Infect. Dis. 2019;25:2333–2335. doi: 10.3201/eid2512.190882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zumla A., Alagaili A.N., Cotten M., Azhar E.I. Infectious diseases epidemic threats and mass gatherings: refocusing global attention on the continuing spread of the Middle East Respiratory syndrome coronavirus (MERS-CoV) BMC Med. 2016;14:132. doi: 10.1186/s12916-016-0686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10:93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H.-J., Yu H., Yu X.-J. Evidence for zoonotic origins of Middle East respiratory syndrome coronavirus. J. Gen. Virol. 2016;97:274–280. doi: 10.1099/jgv.0.000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasem S., Qasim I., Al-Hufofi A., Hashim O., Alkarar A., Abu-Obeida A. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J. Infect. Public Health. 2018;11:331–338. doi: 10.1016/j.jiph.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016;13:87. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog Glob. Health. 2015;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj V.S., Osterhaus A.D., Fouchier R.A., Haagmans B.L. MERS: emergence of a novel human coronavirus. Curr. Opin. Virol. 2014;5:58–62. doi: 10.1016/j.coviro.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization MERS Situation Update. Cairo. http://applications.emro.who.int/docs/EMCSR254E.pdf?ua=1 Available:
- 18.World Health Organization Middle East Respiratory Syndrome Coronavirus (MERS-CoV) https://www.who.int/emergencies/mers-cov/en/ Available:
- 19.Trilla A. One world, one health: the novel coronavirus COVID-19 epidemic. Med. Clin. 2020;154:175–177. doi: 10.1016/j.medcle.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Center for Systems Science and Engineering Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available:
- 22.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Water, Sanitation, Hygiene and Waste Management for COVID-19. 2020. https://www.who.int/publications-detail/water-sanitation-hygiene-and-waste-management-for-covid-19 Available:
- 24.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daszak P., Olival K.J., Li H. A strategy to prevent future epidemics similar to the 2019-nCoV outbreak. Biosaf. Heal. 2020;2:6–8. doi: 10.1016/j.bsheal.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinsstag J., Schelling E., Wyss K., Mahamat M.B. Potential of cooperation between human and animal health to strengthen health systems. Lancet. 2005;366:2142–2145. doi: 10.1016/S0140-6736(05)67731-8. [DOI] [PubMed] [Google Scholar]
- 27.Degeling C., Dawson A., Gilbert G. The ethics of One Health. In: Walton M., editor. One Planet, One Health. Sydney University Press; Sydney, Australia: 2019. pp. 65–84.https://ro.uow.edu.au/sspapers/4441/ Available: [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsevier Novel Coronavirus Information Center. Elsevier's Free Health and Medical Research on the Novel Coronavirus (SARS-CoV-2) and COVID-19. https://www.elsevier.com/connect/coronavirus-information-center Available:
- 30.Zinsstag J. Convergence of ecohealth and one health. Ecohealth. 2012;9:371–373. doi: 10.1007/s10393-013-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace R.G., Bergmann L., Kock R., Gilbert M., Hogerwerf L., Wallace R. The dawn of structural one health: a new science tracking disease emergence along circuits of capital. Soc. Sci. Med. 2015;129:68–77. doi: 10.1016/j.socscimed.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 32.Kock R. Structural one health - are we there yet? Vet. Rec. 2015;176:140–142. doi: 10.1136/vr.h193. [DOI] [PubMed] [Google Scholar]
- 33.Woldehanna S., Zimicki S. An expanded one health model: integrating social science and one health to inform study of the human-animal interface. Soc. Sci. Med. 2015;129:87–95. doi: 10.1016/j.socscimed.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett M.A., Osofsky S. one health: interdependencies of people, other species, and the planet. In: Katz D.L., Jekel J.F., Elmore J.G., DMG Wild, Lucan S.C., editors. Jekel’s Epidemiology, Biostatistics, Preventive Medicine, and Public Health. Philadelphia, PA. 2014. https://rmportal.net/groups/one-health-students-online-platform/one-health-interdependence-of-people-other-species-and-the-planet/view Available: [Google Scholar]
- 35.Galaz V., Leach M., Scoones I., Stein C. The Political Economy of One Health Research and Policy. Brighton, UK. 2015. https://steps-centre.org/publication/one-health-2/ Available:
- 36.Gibbs E.P.J. The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec. 2014;174:85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- 37.El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: a previously unknown SARS-CoV-2 virus of pandemic potential infecting humans–call for a one health approach. One Heal. 2020;100124 doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemida M.G., Alnaeem A. Some One Health based control strategies for the Middle East respiratory syndrome coronavirus. One Heal. 2019 doi: 10.1016/j.onehlt.2019.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zumla A., Dar O., Kock R., Muturi M., Ntoumi F., Kaleebu P. Taking forward a ‘One Health’ approach for turning the tide against the Middle East respiratory syndrome coronavirus and other zoonotic pathogens with epidemic potential. Int. J. Infect. Dis. 2016;47:5–9. doi: 10.1016/j.ijid.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Awaidy S., Al Hashami H. Zoonotic diseases in Oman: successes, challenges, and future directions. Vector-borne Zoonotic Dis. 2020;20:1–9. doi: 10.1089/vbz.2019.2458. [DOI] [PubMed] [Google Scholar]
- 42.Farag E., Nour M., Islam M.M., Mustafa A., Khalid M., Sikkema R.S. Qatar experience on One Health approach for middle-east respiratory syndrome coronavirus, 2012–2017: a viewpoint. One Heal. 2019;7 doi: 10.1016/j.onehlt.2019.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Guardo G., Vignoli M. CoViD-19, One Health, One Ocean, and Veterinarians. 2020. https://science.sciencemag.org/content/369/6506/956/tab-e-letters Available:
- 44.Lorusso A., Calistri P., Mercante M.T., Monaco F., Portanti O., Marcacci M. A “One-Health” approach for diagnosis and molecular characterization of SARS-CoV-2 in Italy. One Heal. 2020 doi: 10.1016/j.onehlt.2020.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konda M., Dodda B., Konala V.M., Naramala S., Adapa S. Potential zoonotic origins of SARS-CoV-2 and insights for preventing future pandemics through one health approach. CUREUS. 2020;12 doi: 10.7759/cureus.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poudel U., Subedi D., Pantha S., Dhakal S. Animal coronaviruses and coronavirus disease 2019: lesson for one health approach. Open Vet. J. 2020;10 doi: 10.4314/ovj.v10i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widagdo W., Okba N.M.A., Stalin Raj V., Haagmans B.L. MERS-coronavirus: from discovery to intervention. One Heal. 2017;3:11–16. doi: 10.1016/j.onehlt.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gollakner R., Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet. Ital. 2020;56:11–12. doi: 10.12834/VetIt.2246.12523.1. [DOI] [PubMed] [Google Scholar]
- 49.Ajayi A.O. The COVID-19 pandemic: critical issues and perspectives for infectious disease prevention in Africa. Infect. Ecol. Epidemiol. 2020;10 doi: 10.1080/20008686.2020.1798073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9 doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemida M.G. Middle East respiratory syndrome coronavirus and the one health concept. PeerJ. 2019;7:e7556. doi: 10.7717/peerj.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J., He W.-T., Wang L., Lai A., Ji X., Zhai X. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020 doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonilla-Aldana D.K., Dhama K., Rodriguez-Morales A.J. Revisiting the one health approach in the context of COVID-19: a look into the ecology of this emerging disease. Adv. Anim. Vet. Sci. 2020;8:234–237. doi: 10.17582/journal.aavs/2020/8.3.234.236. [DOI] [Google Scholar]
- 55.Mushi V. The holistic way of tackling the COVID-19 pandemic: the one health approach. Trop Med Health. 2020;48:69. doi: 10.1186/s41182-020-00257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farag ElmoubasherNour M., Islam M.M., Mustafa A., Khalid M., Sikkema R.S., Alhajri F. Vol. 7. 2019. Survey on Implementation of One Health Approach for MERS-CoV Preparedness and Control in Gulf Cooperation Council and Middle East Countries. One Heal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hemida M.G., Abduallah M.M.B. The SARS-CoV-2 outbreak from a one health perspective. One Heal. 2020 doi: 10.1016/j.onehlt.2020.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crameri G., Durr P.A., Barr J., Yu M., Graham K., Williams O.J. Absence of MERS-CoV antibodies in feral camels in Australia: implications for the pathogen’s origin and spread. One Heal. 2015;1:76–82. doi: 10.1016/j.onehlt.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muraina I.A. COVID-19 and zoonosis: control strategy through One Health approach. Asian Pac J Trop Med. 2020;13:381. [Google Scholar]
- 60.Mobasheri A. COVID-19, companion animals, comparative medicine and one health. Front. Vet. Sci. 2020;7:522. doi: 10.3389/fvets.2020.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasobant S., Patel K., Saxena D., Falkenberg T. COVID-19 in India: making a case for the one health surveillance system. Indian J. Public Health. 2020;64:S135–S138. doi: 10.4103/ijph.IJPH_488_20. [DOI] [PubMed] [Google Scholar]
- 62.Bhatia R. Need for integrated surveillance at human-animal interface for rapid detection & response to emerging coronavirus infections using One Health approach. Indian J. Med. Res. 2020 doi: 10.4103/ijmr.IJMR_623_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alharbi N.K. Vaccines against Middle East respiratory syndrome coronavirus for humans and camels. Rev. Med. Virol. 2017;27:e1917. doi: 10.1002/rmv.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daly J.M. Middle East respiratory syndrome (MERS) coronavirus: Putting one health principles into practice? Vet. J. 2017:52–53. doi: 10.1016/j.tvjl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonilla-Aldana D.K., Holguin-Rivera Y., Perez-Vargas S., Trejos-Mendoza A.E., Balbin-Ramon G.J., Dhama K. Importance of the One Health approach to study the SARS-CoV-2 in Latin America. One Heal. 2020;10 doi: 10.1016/j.onehlt.2020.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espejo W., Celis J.E., Chiang G., Bahamonde P. Environment and COVID-19: pollutants, impacts, dissemination, management and recommendations for facing future epidemic threats. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward M.P. SARS-CoV-2, where to now? Transbound. Emerg. Dis. 2020;67:1411–1413. doi: 10.1111/tbed.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enticott G., Maye D. Missed opportunities? Covid-19, biosecurity and one-health in the United Kingdom. Front. Vet. Sci. August 2020;7:1–6. doi: 10.3389/fvets.2020.00577. Article 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Decaro N., Martella V., Saif L.J., Buonavoglia C. COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us. Res. Vet. Sci. 2020;131:21–23. doi: 10.1016/j.rvsc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parry N.M.A. COVID-19 and pets: when pandemic meets panic. For. Sci. Int. Rep. 2020;2 doi: 10.1016/j.fsir.2020.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roosa K., Lee Y., Luo R., Kirpich A., Rothenberg R., Hyman J.M. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect. Dis. Model. 2020;5:256–263. doi: 10.1016/j.idm.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson P.K., Cunningham A.A., Patel N.G., Morales F.J., Epstein P.R., Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 74.Åsjö B., Kruse H. Zoonoses in the emergence of human viral diseases. Perspect Med. Virol. 2006;16:15–41. doi: 10.1016/S0168-7069(06)16003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood J.L.N., Leach M., Waldman L., MacGregor H., Fooks A.R., Jones K.E. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos. Trans. R Soc. B Biol. Sci. 2012;367:2881–2892. doi: 10.1098/rstb.2012.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Estrada-Peña A., Ostfeld R.S., Peterson A.T., Poulin R., de la Fuente J. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol. 2014;30:205–214. doi: 10.1016/j.pt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Nava A., Shimabukuro J.S., Chmura A.A., Luz S.L.B. The impact of global environmental changes on infectious disease emergence with a focus on risks for Brazil. ILAR J. 2017;58:393–400. doi: 10.1093/ilar/ilx034. [DOI] [PubMed] [Google Scholar]
- 78.Keusch G.T., Pappaioanou M., Gonzalez M.C., Scott K.A., Tsai P. National Research Council; 2009. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin.http://www.nap.edu/catalog/12625.html Available: [Google Scholar]
- 79.Karesh W.B., Dobson A., Lloyd-Smith J.O., Lubroth J., Dixon M.A., Bennett M. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El Zowalaty M.E., Jarhult J.D., Zowalaty] M.E. El, Järhult JD. From SARS to COVID-19: a previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans – call for a one health approach. One Heal. 2020;100124:9. doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henley P. COVID-19 and one health: shifting the paradigm in how we think about health. JBI Evid. Synth. 2020;18:1154–1155. doi: 10.11124/JBIES-20-00161. [DOI] [PubMed] [Google Scholar]
- 82.Kasozi K.I., Mujinya R., Bogere P., Ekou J., Zirintunda G., Ahimbisibwe S. Pandemic panic and anxiety in developing countries. Embracing one health offers practical strategies in management of COVID-19 for Africa. Pan Afr. Med. J. 2020:3. doi: 10.11604/pamj.2020.35.3.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang K., Gao J., Song X., Huang J., Wang H., Wu X. Fangcang shelter hospitals are a one health approach for responding to the COVID-19 outbreak in Wuhan, China. One Heal. 2020 doi: 10.1016/j.onehlt.2020.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv. 2020 doi: 10.1101/2020.03.23.20039446. [DOI] [Google Scholar]
- 86.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Hui-Ling Y., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30003-3. [cited 20 Apr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv. 2020;86 doi: 10.1101/2020.03.08.982637. : 2020.03.08.982637. [DOI] [Google Scholar]
- 91.Rapid Expert Consultation on SARS-CoV-2 . Rapid Expert Consultation on SARS-CoV-2 Survival and Incubation for the COVID-19 Pandemic. National Academies Press; 2020. Survival and incubation for the COVID-19 pandemic. [DOI] [Google Scholar]
- 92.Ahmad T., Hui J. One health approach and coronavirus disease 2019. Hum. Vacc. Immunother. 2020;00:1–2. doi: 10.1080/21645515.2020.1732168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yasobant S., Bruchhausen W., Saxena D., Falkenberg T. One health collaboration for a resilient health system in India: learnings from global initiatives. One Heal. 2019;8 doi: 10.1016/J.ONEHLT.2019.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cunningham A.A., Daszak P., Wood J.L.N. One health, emerging infectious diseases and wildlife: two decades of progress? Philos. Trans. R. Soc. B. 2017 doi: 10.1098/rstb.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data that supports the findings of this study are within the manuscript.