Abstract
Background
Lifestyle change interventions (LCI) for prevention of type 2 diabetes are covered by Medicare, but rarely by US Medicaid programs that constitute the largest public payer system in the USA. We estimate the long-term health and economic implications of implementing LCIs in state Medicaid programs.
Methods
We compared LCIs modeled after the intervention of the Diabetes Prevention Program versus routine care advice using a decision analytic simulation model and best available data from representative surveys, cohort studies, Medicaid claims data, and the published literature. Target population were non-disability-based adult Medicaid beneficiaries aged 19–64 years at high risk for type 2 diabetes (BMI ≥25 kg/m2 and HbA1c ≥ 5.7% or fasting plasma glucose ≥ 110 mg/dl) from eight study states (Alabama, California, Connecticut, Florida, Iowa, Illinois, New York, Oklahoma) that represent around 50% of the US Medicaid population. Incremental cost-effectiveness ratios (ICERs) measured in cost per quality-adjusted life years (QALYs) gained, and population cost and health impact were modeled from a healthcare system perspective and a narrow Medicaid perspective.
Results
In the eight selected study states, 1.9 million or 18% of non-disability-based adult Medicaid beneficiaries would belong to the eligible high-risk target population – 66% of them Hispanics or non-Hispanic black. In the base-case analysis, the aggregated 5- and 10-year ICERs are US$226 k/QALY and US$34 k/QALY; over 25 years, the intervention dominates routine care. The 5-, 10-, and 25-year probabilities that the ICERs are below US$50 k (US$100 k)/QALY are 6% (15%), 59% (82%) and 96% (100%). From a healthcare system perspective, initial program investments of US$800 per person would be offset after 13 years and translate to US$548 of savings after 25 years. With a 20% LCI uptake in eligible beneficiaries, this would translate to upfront costs of US$300 million, prevent 260 thousand years of diabetes and save US$205 million over a 25-year time horizon. Cost savings from a narrow Medicaid perspective would be much smaller. Minorities and low-income groups would over-proportionally benefit from LCIs in Medicaid, but the impact on population health and health equity would be marginal.
Conclusions
In the long-term, investments in LCIs for Medicaid beneficiaries are likely to improve health and to decrease healthcare expenditures. However, population health and health equity impact would be low and healthcare expenditure savings from a narrow Medicaid perspective would be much smaller than from a healthcare system perspective.
Electronic supplementary material
The online version of this article (10.1007/s40258-020-00565-w) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Eighteen percent of the non-disability-based adult Medicaid population is at high risk for developing type 2 diabetes. |
| Life-style change intervention programs to prevent type 2 diabetes in Medicaid beneficiaries at high risk for type 2 diabetes are likely to be cost effective in the long-term from a healthcare system perspective. |
| The cost effectiveness is lower from a narrow Medicaid perspective and the population-level health impact of intervening in beneficiaries at high risk for type 2 diabetes is small. |
Introduction
Diabetes mellitus is a burdensome and costly disease that disproportionally affects minorities and low-income populations [1–3]. Pursuant to the Affordable Care Act, as of November 2018, 37 states had expanded Medicaid to individuals with a family income < 138% of the federal poverty level (FPL) [4]. With that change, an even larger proportion of people with or at risk for type 2 diabetes are being covered by Medicaid programs, putting substantial financial pressure on the state’s Medicaid budgets [5]. The U.S. Diabetes Prevention Program study (DPP), the Da Qing Diabetes Prevention study, and subsequent translation studies have shown that structured lifestyle interventions lead to sustainable reductions in diabetes incidence in people at high risk of diabetes and reduced cardiovascular and all-cause mortality decades after the intervention stopped [6–11]. The National DDP (NDDP), a national public–private partnership connecting health departments, employers, insurers, healthcare professionals, and community-based organizations, provides the infrastructure for implementing DPP-like lifestyle change interventions (LCI) and has motivated many private payers and Medicare to pay for this program [12–15]. But despite encouraging studies on the feasibility of DPP-like LCI in the Medicaid population [13, 16, 17] and the intriguing opportunity to diminish health disparities through Medicaid benefits, to date, few Medicaid programs pay for LCIs [18–20]. Information about the budget impact and the economic implications is important for policy makers. However, data on the number of eligible beneficiaries at high risk of type 2 diabetes are scarce and, owing to differences in socio-demographic and contextual factors, the generally favorable evidence on the cost effectiveness of DPP-like LCI in the general population at high risk of type 2 diabetes [21–24] might be not applicable to Medicaid beneficiaries. The current study addresses this evidence gap and aims to analyze the size of the eligible Medicaid population at high risk of type 2 diabetes, as well as the cost-effectiveness, economic, and health equity impact of implementing DDP-like LCI in state Medicaid programs.
Methods
Study Design and Data Sources
To answer these questions, we combined nationally representative data sources, population-based cohort studies, Medicaid claims data, and published data on the effect of DPP-like LCI and ran simulations using the CDC-RTI diabetes model [25]. Owing to great heterogeneity in demographic, epidemiological, and economic characteristics between state Medicaid programs, we present state-specific analyses for eight states (Alabama, California, Connecticut, Florida, Iowa, Illinois, New York, and Oklahoma) that capture the country’s regional and demographic heterogeneity and represent approximately 50% of the country’s adult Medicaid population. We present population-size-weighted average and/or cumulative estimates for the combined data of the eight states as main results and report additionally state-specific estimates. Details on the selection criteria for the states are presented in Online Appendix A-M1.
The study was conducted in compliance with ethical standards and in all studies from which data were used participants gave informed consent.
Characteristics and Size of the Eligible Population
Eligibility
We used clinical eligibility criteria close to those defined by the Medicare DPP, i.e. a BMI ≥ 25 kg/m2 and a laboratory result of either Hba1c ≥ 5.7% or a fasting plasma glucose (FPG) level ≥ 110 mg/dL [26]. As there is no compelling evidence on the program’s feasibility and effectiveness in the disabled population, and as most dually eligible beneficiaries will be eligible for DPP-like LCI through the Medicare DPP [26, 27], we restricted our analyses to non-disability-based Medicaid beneficiaries aged 19–64 years with full benefits.
Population Size and Characteristics
We sampled participants without diabetes and insured under Medicaid or with a family income below 138% FPL from the nationally representative National Health and Nutrition Examinations Surveys (NHANES, waves 2006–2016) who matched the age, sex, and race/ethnicity characteristics of Medicaid beneficiaries without diabetes in Medicaid claims files (2008–2012) for the eight selected states. The prevalence of people at high risk of type 2 diabetes and their demographic and clinical characteristics were then taken from this merged NHANES–Medicaid claims data set. We then combined data on the total number of non-disability-based adult beneficiaries with full benefit with the estimated prevalence of people with high risk of type 2 diabetes to calculate the number of non-disability-based adult beneficiaries with full benefit that are at high risk of type 2 diabetes [28, 29]. Details of these steps are described in Online Appendix A-M2 and A-M4.
Design and Input Parameters of the Simulation Scenarios
Intervention and Comparators
We compared in-person DPP-like LCIs delivered by trained and certified clinic staff, community health workers, peers in the workplace and church and community settings, as well as virtual programs, as delivered in several studies in the Medicaid population, with a counterfactual of routine care advice for people who are identified as having increased risk for type 2 diabetes in their usual care setting [17, 30]. DPP-like LCI programs focus on healthy eating, physical activity, and coping skills and generally consist of 16 weekly core sessions over 4 months plus 8 monthly follow-up sessions. Programs have been adapted for various ethnic and racial groups [9, 31–34], and evidence from various studies has shown that the delivery of LCI versions tailored to the needs of the Medicaid population is feasible and results in clinically relevant weight loss [17, 18, 35]. Recent demonstration projects further indicated that the tools and infrastructure built by the Centers for Disease Control and Prevention (CDC) and its partners [16, 36–38] might be successfully used to facilitate implementation of DPP-like LCI in state Medicaid programs [16, 30].
Simulation Model
Cost and health effects of the LCI were projected using the decision analytic CDC-RTI diabetes computer simulation model. The CDC-RTI diabetes cost-effectiveness model is a Markov model that uses annual transition probabilities to simulate cohorts through different health states including ‘pre-diabetes’ (i.e. people at high risk for type 2 diabetes), type 2 diabetes, and death. Each health state is associated with a distinct set of costs for treatment and quality of life (QoL) decrements and the model accumulates incremental costs and health benefits, measured in quality-adjusted life years (QALYs) in each intervention arm [25].
The disease pathways and complications that are modelled in the diabetes module include nephrology, neuropathy, retinopathy, coronary heart disease, and stroke. The respective key transition probabilities are mainly based on data from the United Kingdom Prospective Diabetes Study (UKPDS) [39] and the risk equations of the American College of Cardiology/American Heart Association (ACC/AHA) [40].
The ‘prediabetes’ module follows individuals from the time of diagnosis of ‘prediabetes’ to diagnosis of type 2 diabetes or death, whichever comes first. People with ‘prediabetes’ may already have some complications at diagnosis of ‘prediabetes’ and may also experience coronary heart disease, stroke, early stages of nephropathy and neuropathy, or death while in the ‘prediabetes’ phase. Most of the model’s disease progression parameters are based on data of [41] the DPP study, the UKPDS and the ACC/AHA risk equations [39–41].
In both disease modules, intervention effects can be modelled through changes in the annual probability of transitioning from ‘pre-diabetes’ to type 2 diabetes, as well as changes in BMI, systolic and diastolic blood pressure, and total cholesterol and high-density lipoproteins.
The model has been validated against the results of large longitudinal studies/trials [25] and has been used successfully for economic evaluations of various prevention and treatment strategies in clinical and non-clinical settings [21, 23, 42]. Details of the model and simulation structure are provided in Online Appendix A-M3.
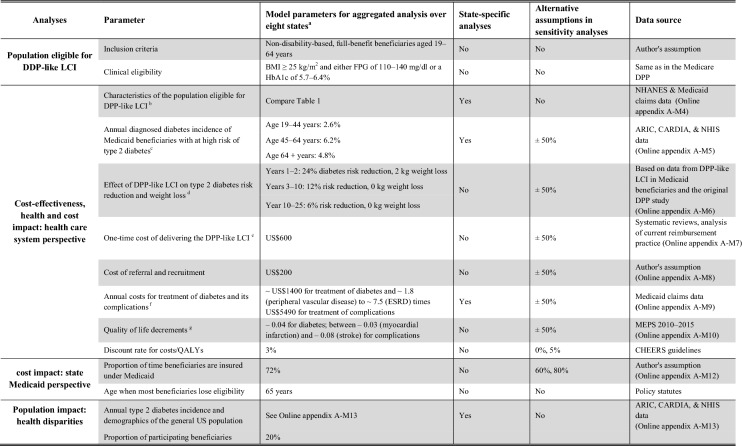
Model Parameters
Details on the data sources and methods for estimating Medicaid-specific input parameters are described in Online Appendix A-M4–A-M10. The most important model parameter is the effect of the LCI on type 2 diabetes incidence and modifiable risk factors. To obtain valid and reliable estimates on these effectiveness parameters we used systematic reviews that tested the efficacy of LCIs versus routine care in RCTs [10, 43, 44], reviews on randomized and non-randomized studies that tested interventions modelled after the DPP in more real-world settings [9, 45], observational data from the NDPP registry [20] as well as observational data from studies that implemented DPP-like interventions in the Medicaid population [46]. Following this combined evidence, we assumed that the LCI induces a type 2 diabetes risk reduction of 24% in years 1 and 2, of 12% in years 3–10 and of 6% in years 11–25. Conservatively, we also assumed that the intervention induces a weight loss of 2 kg in the years 1–2 and no effect on other risk factors. We assumed that these effectiveness parameter did not differ between LCI delivery modes (for details on these assumptions see Online Appendix A-M6).
Other crucial input parameters comprise characteristics of the Medicaid population at high risk for diabetes (directly estimated from Medicaid claims and NHANES data, for details see Online Appendix A-M4), their annual background probability for developing type 2 diabetes [estimated from the National Health Interview Surveys (NHIS), the Atherosclerosis Risk in Communities (ARIC) Study, and the Coronary Artery Risk Development in Young Adult (CARDIA) Study, for details see Online Appendix A-M5], the cost for recruitment, referral and delivery of the DPP-like LCI (based on previous studies and current practice, for details see Online Appendix A-M7 and A-M8), as well as the costs (directly estimated from Medicaid Analytic eXtract files of the eight states, for details see Online Appendix A-M9) and QoL decrements [estimated from the Medical Expenditure Panel Survey (MEPS), for details see Online Appendix A-M10] associated with diabetes and its complications.
An overview of the resulting parameters is described in Table 1. For example, the annual probability of developing type 2 diabetes of a Medicaid enrollee eligible for LCI are between 4% and 8%, the combined costs of recruitment, referral and delivering of the DPP-like LCI are around US$800, annual excess costs of treating diabetes versus remaining in the pre-diabetes state are around US$1400, the QoL decrement for diabetes is −0.04 and the QoL decrements for complications lies between –0.03 (myocardial infarction) and –0.08 (stroke).
Table 1.
Summary of relevant model assumptions
DPP Diabetes Prevention Program, LCI lifestyle change intervention, ESRD end-stage renal disease, FPG fasting plasma glucose, HbA1c hemoglobin A1c, BMI body mass index, NHANES National Health and Nutrition Examination Survey, NHIS National Health Interview Survey ARIC Atherosclerosis Risk in Communities Study; CARDIA Coronary Artery Risk Development in Young Adults Study, MEPS Medical Expenditure Panel Survey, QALYs quality adjusted life years
aAlabama, California, Connecticut, Florida, Iowa, Illinois, New York, and Oklahoma; bPopulation characteristics of the Medicaid population at high risk for type 2 diabetes were derived by matching individual-level Medicaid claims with data from NHANES; cIncidence of type 2 diabetes in this population was directly estimated from the NHIS, the ARIC Study, and the CARDIA Study; dPre–after DPP-like LCI weight-loss data from studies in the Medicaid population [18, 35] were combined with data on weight loss and type 2 diabetes incidence reduction from the original DPP and other RCTs [7, 41] to infer the expected diabetes incidence reduction that results from the intervention; dCosts of delivering DPP-like LCI are based on cost estimates from previous studies and on current reimbursement practices [22, 54]; eCosts for treatment of diabetes and diabetic complications were directly estimated from Medicaid claims data from the states of Alabama, California, Connecticut, Florida, Iowa, Illinois, New York, and Oklahoma; fQuality of life decrements were directly estimated from data from the Medical Panel Expenditure Surveys (MEPS)
State-Specific Parameters and Assumptions
For the clinical and demographic characteristics of the population at high risk of type 2 diabetes, the annual background incidence of type 2 diabetes, and the costs of treating diabetes and its complications we could derive state-specific input parameters and used them in the state-specific model scenarios. For the effectiveness and the costs of the DPP-like LCI and the impact of diabetes and diabetes-related complications on health-related QoL we had no state-specific data and assumed that they are the same in each of the 8 states (for details see Table 1).
Cost-Effectiveness Analyses
A healthcare system perspective was chosen because the societal perspective includes indirect costs that are not directly relevant to the Medicaid program or other payers in the healthcare system [47]. We simulated individuals at high risk for type 2 diabetes over 5, 10, and 25 years from the start of a DPP-like LCI. Twenty-five years was chosen as maximum time horizon as this approximately coincides with the longest follow-up of current LCI studies and as every effect beyond this time horizon was considered to be quite hypothetical. Both costs, consisting of costs for referral, intervention, and treatment of diabetes and complications, and health effects, described in QALYs, a measure that combines length and QoL, were discounted at 3% annually. Costs are indexed to the year 2018. Incremental costs and QALYs were used to calculate incremental cost-effectiveness ratios (ICERs). To capture structural and stochastic uncertainties, we conducted univariate and probabilistic sensitivity analyses. In the univariate sensitivity analyses we varied crucial model parameters by ± 50%. In the probabilistic sensitivity analyses we permuted parameters simultaneously (for details, see Online Appendix A-M11). We also estimated the maximal intervention cost at which the ICERs are below US$50,000/QALY and US$100,000/QALY in the base case analysis [48]. Analysis and reporting are based on the recommendations of the Consolidated Health Economic Evaluation Reporting Standards [33].
Return on Investment (ROI) from a Health Care System and Medicaid Perspective
Monetary return on investment (ROI) from a health care system perspective equals the cost outcome from the cost-effectiveness analyses. Given the specific Medicaid policy context, we conducted additional analysis in which we considered factors relevant to the ROI for state Medicaid programs. First, non-disability-based Medicaid enrollees are generally not eligible for Medicaid beyond the age of 64 years. We therefore assumed that savings that occur from preventing type 2 diabetes and its complications beyond age 64 years won’t be captured by the Medicaid system [27]. Second, Medicaid enrollees typically move in and out of Medicaid eligibility, a phenomenon often referred to as ‘churning’. Data show that average non-disability-based Medicaid beneficiaries are enrolled 8.6 months or 72% of the fiscal year in Medicaid [49]. In our adjusted ROI model scenario, we therefore pragmatically assumed that until Medicaid beneficiaries turn 65 only 72% of savings that occur from preventing type 2 diabetes and its complications will be captured by Medicaid (for details, see Online Appendix A-M12).
Population Health, Health Equity and Cost Impact
To estimate the expected upfront investments and the long-term cost and health impact on a population level, in a next step, we combined data on the number of expected participants with the per-participant ROI estimates. Furthermore, using the CDC-RTI model and the background type 2 incidence of race/ethnicity and income strata in the Medicaid and non-Medicaid populations, we calculated the cumulative type 2 incidence in the general US adult population with and without implementing LCI for eligible Medicaid beneficiaries at high risk of type 2 diabetes. We then calculated the absolute and relative narrowing in the difference of the cumulative diabetes incidence between white and non-Hispanic black, and Hispanics, and between people below and above 138% FPL in the general US adult population. For all those analyses, we assumed that 20% of eligible beneficiaries participated in DPP-like LCI (for details, see Online Appendix A-M13).
Analyses and simulations were run in 2018.
Results
Eligible Population Size and Population Characteristics
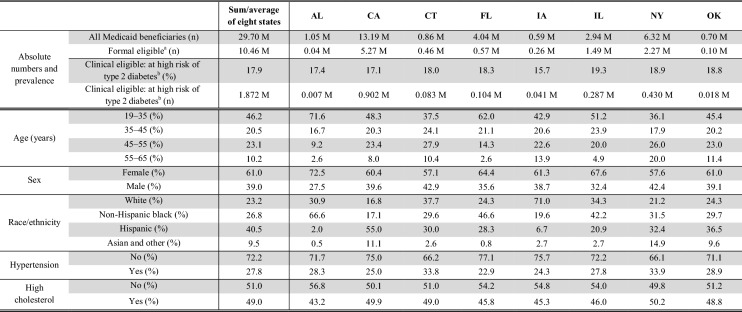
In the eight study states, 30 million people are insured under Medicaid, and approximately 18% or 1.9 million of the 10.5 million non-disability-based, adult, full-benefit Medicaid beneficiaries fall in our category of having increased risk of type 2 diabetes. The number of those high-risk beneficiaries ranges from 7000 in Alabama to 902,000 in California (Table 2). On average, this at high-risk population is young, diverse (two-thirds are non-Hispanic blacks or Hispanics), and at high risk for cardiovascular diseases (28% have hypertension, and 49% have high cholesterol). Considerable differences in demographic and clinical characteristics exist between states.
Table 2.
Numbers and characteristics of medicaid beneficiaries eligible for DPP-like lifestyle change interventions
AL Alabama, CA California, CT Connecticut, FL Florida, IA Iowa, IL Illinois, NY New York, OK Oklahoma, M million
aNon-disability-based, full-benefit beneficiaries aged 19–64 years
bBody mass index ≥ 25 kg/m2 and either fasting plasma glucose of 110–140 mg/dL or hemoglobin A1c of 5.7–6.4%
Hypertension: blood pressure ≥ 140/90 mmHg; high cholesterol: total cholesterol ≥ 200 mg/dL
Cost-effectiveness
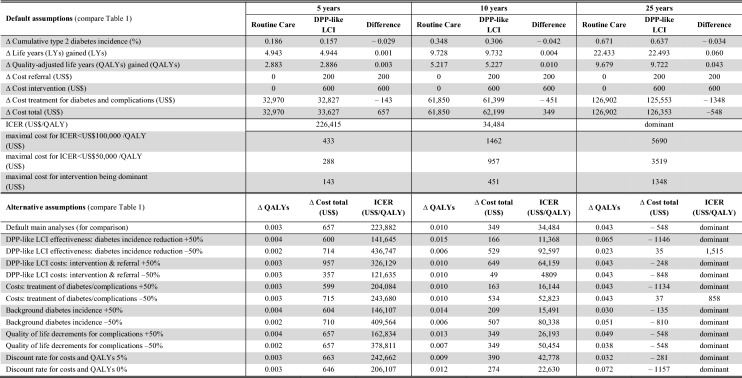
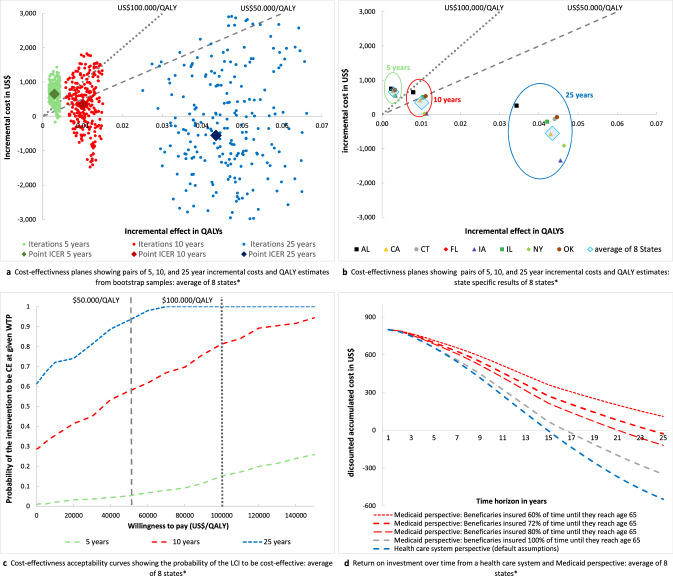
Given our default assumptions on weight change and relative type 2 diabetes incidence reduction, the LCI translates to absolute risk reductions of 3.4% for type 2 diabetes and 0.05% (end-stage renal disease) to 0.68% (microalbuminuria) for complications over a 25-year time horizon (Online Appendix A-R-Table 1 + 2). For the combined data of the eight states, over a 5-year and 10-year time horizon, this leads to a gain of 0.003 and 0.010 QALYs at costs of US$657 and US$349, resulting in ICERs of US$226 k/QALY and US$34 k/QALY. Over 25 years, the intervention leads to a per-person QALY gain of 0.043 at savings of US$548 meaning that the LCI intervention dominates routine care (Table 3 and Fig. 1a). The probability that the intervention is cost effective at willingness to pay (WTP) thresholds of US$50 k and US$100 k per QALY is 6% and 15% over 5 years, 59% and 82% over 10 years, and 96% and 100% over 25 years, respectively (Fig. 1c). Given a WTP threshold of US$50 k (US$100 k) per QALY, the maximal upfront intervention costs need to be below US$288 (US$433), US$957 (US$1462), and US$3519 (US$5690) to make the intervention cost effective over a time horizon of 5, 10, and 25 years. There is substantial variation in the ICERs between states; however, over a 10- or 25-year time horizon, the intervention is dominant or cost effective in all eight analyzed Medicaid programs (Fig. 1b, Online Appendix A-R-Table 2). The main driver of the variance in ICERs is the difference in costs of treatment for diabetes and complications. The univariate sensitivity analyses show that the results are most sensitive to the effectiveness of the LCI and the costs of treating diabetes, but that even under most conservative assumptions the intervention is likely to be cost effective over 10 and 25 years (compare lower part of Table 3).
Table 3.
Total and incremental healthcare costs and health effects of DPP-like LCI per participant from a healthcare system perspective: average of eight states
Results are based on simulation scenarios from a Markov-style decision analytic simulation model with default and alternative assumptions on crucial model parameters (compare Table 1)
Dominant: less costly and more beneficial
DPP Diabetes Prevention Program, ICER incremental cost-effectiveness-ratio, LCI lifestyle change intervention, LYs life years, QALYs quality-adjusted life years
Fig. 1.
a Cost-effectiveness plane for the combined data of the eight study states with pairs of QALYs and cost estimates from n = 250 bootstrap samples. Green dots show bootstrap samples of pairs of QALYs and cost estimates over a 5-year time horizon, red dots show bootstrap samples over a 10-year time horizon, and blue dots show bootstrap samples over a 25-year time horizon. The large diamonds represent the mean of cost QALY and cost estimates. The gray dotted lines show the willingness to pay thresholds of US$50,000/QALY and US$100,000/QALY; realizations below these lines are considered to be cost-effective under the given willingness to pay threshold. b Cost-effectiveness plane with pairs of incremental QALYs and cost estimates for each of the eight study states. The large blue diamonds represent the pairs of population size-weighted incremental QALYs and cost estimates for the combined data of the eight study states (identical to 1a). The green circle frames the state-specific results of the analyses over a 5-year time horizon, the red circle frames the state-specific results of the analyses over a 10-year time horizon, and the blue circle frames the state-specific results of the analyses over a 25-year time horizon. The gray dotted lines show the willingness to pay thresholds of US$50,000/QALY and US$100,000/QALY; realizations below these lines are considered to be cost-effective under the given willingness to pay threshold. c Cost-effectiveness acceptability curves for the combined data of the eight study states with the WTP on the vertical axis and the probability of the intervention being cost-effective at a given WTP on the horizontal axis. Curves are derived on the basis of net benefit values from n = 250 bootstrap samples of incremental cost and QALY estimates. The green curve shows the 5-year time horizon, the red curve shows the 10-year time horizon, and the blue curve shows the 25-year time horizon. The gray dotted lines show the willingness to pay thresholds of US$50,000/QALY and US$100,000/QALY. d Curves of the per participant return on investment for the combined data of the eight study states with the time horizon on the horizontal axis and the accumulated costs on the vertical axis. The accumulated costs represent the value of upfront costs of US$800 for the intervention minus cost savings associated with prevention of diabetes and complications. The blue line represents the base-case scenario from a healthcare system perspective, the gray and red curves show the ROI from a Medicaid perspective assuming that beneficiaries are insured for 60–100% of their lifetime under Medicaid before they lose eligibility at age 65 years. Negative accumulated costs indicate a positive return on investment. * AL Alabama, CA California, CT Connecticut, FL Florida, IA Iowa, IL Illinois, NY New York, OK Oklahoma. LCI lifestyle change intervention, QALY quality adjusted life year, ICER incremental cost-effectiveness ratio, CE cost-effectiveness, WTP willingness to pay
Return on Investment From a Health Care System and Medicaid Perspective
From a healthcare system perspective, the break-even point (the point where cost savings from prevented type 2 diabetes and diabetes complications offset initial program investments) would be 15 years and the 25-year ROI would be US$548. From a narrow Medicaid perspective, the break-even point would be delayed to 24 years and the 25-year ROI would decrease to US$27 (Fig. 1d and Online Appendix A-R-Table 3 for state-specific estimates).
Population Cost, Health, and Health Equity Impact
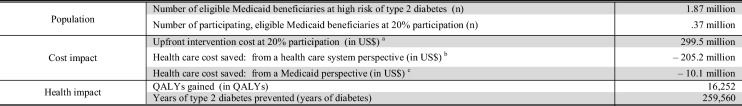
Assuming that 20% (i.e. 0.37 million) of the 1.87 million eligible Medicaid beneficiaries in the eight analyzed states participate in a LCI, one could expect that upfront investments of US$300 million would lead to savings of US$205 million and US$10 million from a healthcare system and narrow Medicaid perspective over a 25-year time horizon (Table 4). Owing to variance in per-person ROI and population size, the cost and health impact differs substantially between the states (Online Appendix A-R-Table 4).
Table 4.
The 25-year population-level health and cost impact assuming a 20% DPP-like LCI participation in eligible Medicaid beneficiaries: sum of 8 states
QALY quality-adjusted life year
aSum of costs for referral and intervention
bSum of costs for referral and intervention minus savings for prevention of treatment for type 2 diabetes and complications assuming that all savings from preventing type 2 diabetes and its complications in the US Healthcare System are captured
cSum of costs for referral and intervention minus savings for prevention of treatment for type 2 diabetes and complications assuming that only savings from preventing type 2 diabetes and its complications in the Medicaid system are captured. It is assumed that beneficiaries are insured for 72% of their time under Medicaid until they turn 65 years
Furthermore, with a 20% LCI participation in eligible Medicaid beneficiaries, one could expect that the average 25-year cumulative diabetes incidence in the general US adult population would decrease by 0.02%, from 27.30 to 27.28%. Due to their overrepresentation in Medicaid, type 2 diabetes incidence reductions in Non-Hispanic black (− 0.04%), Hispanic (− 0.02%), and low-income adults (− 0.05%) would be higher than in white (0.01%) and non-low-income adults (0.00%). This would decrease the difference in the cumulative type 2 diabetes incidence between whites and non-Hispanic blacks, between whites and Hispanics, and between adults ≥ 138% FPL and adults < 138% FPL at the population level by 0.31%, 0.13%, and 1.07% in relative terms (Online Appendix A-R-Table 5).
Discussion
Offering DPP-like LCI to Medicaid beneficiaries at high risk of type 2 diabetes may lower the morbidity burden from type 2 diabetes and its complications in low-income populations and the healthcare cost in state Medicaid programs. We used the best available data from eight US states and a simulation model to analyze the health and economic consequences of paying for DPP-like LCI in Medicaid programs. According to our data, 18% of non-disability-based adult Medicaid beneficiaries could profit from LCIs—almost half of them are below the age of 45 years, and two-thirds are Hispanic or non-Hispanic black. Implementing DPP-like LCI is likely to be a highly cost-effective or dominant strategy in the long term, but irrespective of LCI uptake, its impact on population health and health equity is expected to be small. Furthermore, due the fragmentation of the US healthcare system, the anticipated long-term cost savings from a narrow Medicaid perspective are much lower than from a general healthcare system perspective.
Facing economic pressure, on the one hand, and encouraging data on expected cost savings from LCI in its beneficiaries, on the other, the Centers for Medicare and Medicaid Services (CMS) recently decided to pay for in-person DPP-like LCI in Medicare [15, 21, 26]. With around 23 million people aged 65 years and older who have ‘pre-diabetes’ and may be eligible for DPP-LCIs, this was a landmark in chronic disease prevention in the USA [3]. However, despite promising data on the feasibility and effectiveness of LCI in Medicaid beneficiaries, only a few Medicaid programs currently pay for DPP-like LCI [17, 50, 51]. This is the first study that comprehensively addresses policy relevant economic questions such as the short- and long-term budgetary impact of a program implementation in state Medicaid programs.
Various previous studies have analyzed and described the cost-effectiveness of the DPP or DPP-like LCI in different populations with increased diabetes risk. The within-trial cost-effectiveness analyses of the original DPP and DPP-Outcome studies reported ICERs of US$27,000/QALY and US$10,000/QALY over a 3- and 10-year time horizon, respectively [52, 53]. Noteworthy, with intervention costs of around US$2250 over 3 years and weight loss of around 6% after 1-year follow-up, the costs and weight loss effect in this efficacy trial was higher compared to our model assumptions. A recent systematic review showed that studies that modelled the life-time cost effectiveness of individual and group-based diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk, reported ICERs ranging between negative values that indicate dominance and US$20,000/QALY [22]. However, the socio-demographic and contextual factors of populations insured under Medicaid differ substantially from the general population and data on the cost effectiveness of DPP-like LCI in the Medicaid population at high risk for diabetes are scarce. The only other economic evaluation in the Medicaid population that we are aware of showed that a community-based DPP-like LCI for Montana Medicaid beneficiaries is cost effective at an ICER of US$39,500/QALY over a 20-year time horizon [54]. This estimate is similar to findings for the least cost-effective state in our analysis (Alabama, 20-year ICER=US$17,000/QALY). Notably, 1-year weight loss was comparable (around 2 kg) and upfront costs per participant in this study (US$940) were slightly higher than in ours (US$800). However, the model only captured healthcare costs related to diabetes complications, but not costs related to routine diabetes care, which might lead to an underestimation of actual cost savings.
Our analyses show that there is heterogeneity in the cost effectiveness between Medicaid programs, but that paying for LCIs is likely to be a cost-effective or dominant strategy in all eight analyzed states and is cost effective even under most conservative assumptions. We identified four influential drivers that have the potential to make the intervention more cost-effective in the real world. First, the cost of delivering the intervention, second, the relative risk reduction achieved by the intervention, third, the level of diabetes risk of eligible participants, and forth, the costs of treating diabetes and complications. Some of those factors can be altered or influenced: for example, investments in the delivery, referral, and reimbursement infrastructure, as currently ongoing in the Medicare DPP, could increase efficiency and reduce delivery costs in the long term. Further, tailoring the programs to the young and diverse eligible Medicaid population and addressing competing priorities such as childcare, transport, and mobility that naturally exist in these predominantly employed populations has the potential to improve the reach and effectiveness of LCIs. Also the use of virtual or telehealth DPP-like LCI versions may increase effectiveness and reach [55–57]. In addition, expected advances in this technology sector raise hope that virtual or telehealth versions might become less costly in future. Finally, applying selective strategies and concentrating on even higher risk segments with even higher HbA1c or FPG may be a strategy to improve the cost effectiveness and per-person ROI. In contrast, the costs of treating diabetes and complications can be hardly influenced by policy makers; however, given the trend of rising costs for medication and treatment, which is not captured by our model, the real-world, long-term savings of LCI per se are likely to be higher than our results suggest [58, 59].
With 18% of Medicaid beneficiaries who could benefit from LCIs and an expected 25-year ROI of US$548 per participant, the expected long-term savings of DPP-like LCIs from a healthcare system perspective would be substantial. However, owing to Medicaid population turnover, a substantial proportion of future savings would not be absorbed by Medicaid programs, but by beneficiaries, private insurance, or the Medicare program (compare Fig. 1d). As some of anticipated savings that occur beyond age 65 are expected to be absorbed by Medicare [27], CMS could consider maximizing health benefits and its overall cost savings through mechanisms that encourage state Medicaid programs to cover DPP-like LCI interventions.
Our analyses further suggest that paying for DPP-like LCI in state Medicaid programs could not only be cost effective but also reduce health disparities, at least modestly. However, the numbers also indicate that individual-level approaches for vulnerable high-risk adults have a very small population health impact and need to be complemented by effective population-wide policies to substantially improve health on a population level and diminish health disparities [60, 61]. Several US and international examples show the potential of these approaches in reducing important risk factors for diabetes [62–65].
Some limitations should be considered in the interpretation of our study results. Our study is based on a simulation model, and the results are thus influenced by model assumptions and input parameters that are likely to vary in the real world. For example, we used Medicaid data from the years 2008–2012 to populate our model with demographic and economic estimates, although with state Medicaid expansions in 2014 the population’s characteristics and expenditure might have changed between 2012 and 2018 [66]. As there is no evidence from randomized studies on the effectiveness of DPP-like LCI in the Medicaid population, we had to extrapolate from observational data on pre–post LCI weight loss to the expected long-term diabetes incidence reduction. Furthermore, to date, all the long-term clinical trials that have data on the reduction in diabetes incidence have been limited to people with impaired glucose tolerance, leaving open the question of whether the level of risk reduction extends to the full segment of the population with our high-risk definition. However, in light of the robustness of our results toward variations in our crucial model assumptions, the aforementioned limitations are unlikely to change the general conclusions of our study.
Conclusion
This study provides detailed state-specific data on the size and characteristics of people at high risk of type 2 diabetes, and on the short- and long-term health and cost impact of DPP-like LCI in eight state Medicaid programs. Whereas the health and economic implications of paying for DPP-like LCI in other Medicaid programs remains unknown, the results from our eight highly heterogeneous study states indicate that paying for DPP-like LCI is probably a highly cost-effective policy in most state Medicaid programs. As population health impact is small there is a need to complement high-risk lifestyle approaches by alternative population-based prevention policies.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
Open Access funding provided by Projekt DEAL.
Author Contributions
Michael Laxy had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. ML designed the study, analyzed the model input data, executed the simulation analyses and drafted the manuscript. PZ co-designed the study and commented on drafts of the manuscript. BPN supported the analysis of the model input data and commented on drafts of the manuscript. HS supported the execution of simulation analyses and commented on drafts of the manuscript. MKA co-designed the study and commented on drafts of the manuscript. AA commented on drafts of the manuscript. EG co-designed the study and commented on drafts of the manuscript.
Data Availability
Data from the NHANES are freely available from https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Data from the NHIS are freely available from https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.html. Data from the MEPS are freely available from https://meps.ahrq.gov/data_stats/download_data_files.jsp. Data from the ARIC study can be obtained for eligible projects through individual requests from https://biolincc.nhlbi.nih.gov/studies/aric/. Data from the CARDIA study can be obtained for eligible projects through individual requests from https://biolincc.nhlbi.nih.gov/studies/cardia/?q=cardia. Medicaid Analytic eXtract (MAX) files can be accessed for eligible projects through individual data requests through https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/MAXGeneralInformation.html.
Compliance with Ethical Standards
Funding
The authors received no funding for this work. Michael Laxy was funded by The Commonwealth Fund through a Harkness Fellowship for Health Care Policy and Practice while conducting the study.
Competing interests
The authors declare that no competing interests exist.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care, 2013. 36(4): p. 1033-1046. [DOI] [PMC free article] [PubMed]
- 2.Dieleman JL, et al. Us spending on personal healthcare and public health, 1996-2013. JAMA. 2016;316(24):2627–2646. doi: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017 Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 19 Jan 2018
- 4.The Henry Kaiser Family Foundation. Status of State Action on the Medicaid Expansion Decision. https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 10 Dec 2018.
- 5.Ng BP, et al. Medical Expenditures Associated With Diabetes Among Adult Medicaid Enrollees in Eight States. Prev Chronic Di. 2018;15:E116. doi: 10.5888/pcd15.180148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Group, D.P.P.R. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann RT, et al. A Randomized Comparative Effectiveness Trial for Preventing Type 2 Diabetes. Am J Public Health. 2015;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 10.Haw JS, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808–1817. doi: 10.1001/jamainternmed.2017.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. ThLancet Diabetes Endocrinology. 3(11): p. 866–75. [DOI] [PMC free article] [PubMed]
- 12.Centers for Medicare and Medicaid. Fact Sheet: Final Policies for the Medicare Diabetes Prevention Program Expanded Model in the Calendar Year 2018 Physician Fee Schedule Final Rule. https://innovation.cms.gov/Files/fact-sheet/mdpp-cy2018fr-fs.pdf. Accessed 8 Jan 2018
- 13.THE 6|18 INITIATIVE EVIDENCE SUMMARY. Prevent Type 2 Diabetes. https://www.cdc.gov/sixeighteen/docs/6-18-evidence-summary-diabetes.pdf. Accessed 19 Jan 2018
- 14.Albright A. The National Diabetes Prevention Program: from Research to Reality. Diabetes care & education newsletter. 2012;33(4):4–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Alva ML, et al. Impact Of The YMCA Of The USA Diabetes Prevention Program On Medicare Spending And Utilization. Health Aff (Millwood) 2017;36(3):417–424. doi: 10.1377/hlthaff.2016.1307. [DOI] [PubMed] [Google Scholar]
- 16.National Association of Chronic Disease Directors. Medicaid Coverage for the National Diabetes Prevention Program Demonstration Project. Retrieved from http://www.chronicdisease.org/page/Medicaid_NDPP. Accessed 8 Apr 2018.
- 17.Centers for Medicare and Medicaid. Medicaid Incentives for Diabetes Prevention of Chronic Disease. https://downloads.cms.gov/files/cmmi/mipcd-finalevalrpt.pdf. Accessed 8 Jan 2018.
- 18.Carpenedo D, et al. Can an Adapted Version of the Diabetes Prevention Program (DPP) Be Delivered Effectively to Adult Medicaid Beneficiaries? In: Poster presentation at the ADA meeting 2014.
- 19.Siminerio LM, et al. The National Diabetes Education Program at 20 Years: lessons Learned and Plans for the Future. Diabetes Care. 2018;41(2):209–218. doi: 10.2337/dc17-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ely EK, et al. A National Effort to Prevent Type 2 Diabetes: participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–1341. doi: 10.2337/dc16-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo X, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save US$5.7 billion in 25 years. Health Aff (Millwood) 2012;31(1):50–60. doi: 10.1377/hlthaff.2011.1115. [DOI] [PubMed] [Google Scholar]
- 22.Li R, et al. Economic Evaluation of Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: a Systematic Review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):452–460. doi: 10.7326/M15-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuo X, et al. Cost-effectiveness of alternative thresholds of the fasting plasma glucose test to identify the target population for type 2 diabetes prevention in adults aged >/=45 years. Diabetes Care. 2013;36(12):3992–3998. doi: 10.2337/dc13-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman WH, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142(5):323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoerger TJ, Segel JE, Zhang P, Sorensen SW. Validation of the CDCRTI Diabetes Cost-Effectiveness Model. RTI Press Methods Report. Research Triangle Park, NC: RTI Press, 2009, Accessed on 01/19/2018, retrieved from 10.3768/rtipress.2009.mr.0013.0909. [DOI]
- 26.Medicare Diabetes Prevention Program (MDPP) Expanded Model Fact Sheet. https://innovation.cms.gov/Files/x/MDPP_Overview_Fact_Sheet.pdf. Accessed on 19 Jan 2018.
- 27.Ndumele CD, Sommers BD, Trivedi AN. The ACA’s 65th Birthday Challenge: moving from Medicaid to Medicare. J Gen Intern Med. 2015;30(11):1704–1706. doi: 10.1007/s11606-015-3328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MACStats: Medicaid and CHIP Data Book. Retrieved from https://www.macpac.gov/wp-content/uploads/2015/12/MACStats-Medicaid-CHIP-Data-Book-December-2017.pdf. Accessed 6 Jul 2017.
- 29.Medicaid Expansion Enrollment. Retrieved from https://www.kff.org/health-reform/state-indicator/medicaid-expansion-enrollment/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 6 Jul 2018.
- 30.Health MDo. Medicaid and National Diabetes Prevention Program (DPP) Briefing .Maryland Medicaid Advisory Committee. Retrieved from https://mmcp.health.maryland.gov/Documents/MMAC/2018/02_February/MMAC %20DPP %20%20Presentation %20Feb %2018.pdf. Last accessed on 04/25/2018.
- 31.Ackermann RT, et al. Translating the Diabetes Prevention Program into the community The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amundson HA, et al. Translating the diabetes prevention program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ. 2009;35(2):1–209. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- 33.Hall DL, et al. Translation of the diabetes prevention program to ethnic communities in the United States. J Immigr Minor Health. 2016;18(2):479–489. doi: 10.1007/s10903-015-0209-x. [DOI] [PubMed] [Google Scholar]
- 34.Mau MK, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Pilot project. Prog Community Health Partnersh. 2010;4(1):7–16. doi: 10.1353/cpr.0.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brokaw S, et al., Montana Diabetes Prevention Program Workgroup. Do Financial Incentives Make a Difference in Outcomes for Medicaid Members in the Diabetes Prevention Program (DPP)? In: Posterpresentation: ADA Meeting 2016.
- 36.Directors NAoCD. National Diabetes Prevention Program Coverage Toolkit. Retrieved from https://coveragetoolkit.org/. Accessed 25 Apr 2018.
- 37.Association AM. and CfDCa. Prevention, Prevent Diabetes STAT Toolkit. Retrieved from https://preventdiabetesstat.org/toolkit.html. Accessed 25 Apr 2018.
- 38.Prevention CfDCa. National Diabetes PRevention Program. Requirements for CDC Recognition. Retrieved from https://www.cdc.gov/diabetes/prevention/lifestyle-program/requirements.html. Accessed 25 Apr 2018.
- 39.Clarke PM, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47(10):1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 40.Goff DC, Jr, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 41.Diabetes Prevention Program Research G 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoerger TJ, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. Adults. Diabetes Care. 2007;30(11):2874–2879. doi: 10.2337/dc07-0885. [DOI] [PubMed] [Google Scholar]
- 43.Hemmingsen B, et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12:Cd003054. doi: 10.1002/14651858.CD003054.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balk EM, et al. Combined diet and physical activity promotion programs to prevent type 2 diabetes among people at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–451. doi: 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudaliar U, et al. Cardiometabolic risk factor changes observed in diabetes prevention programs in us settings: a systematic review and meta-analysis. PLoS Med. 2016;13(7):e1002095. doi: 10.1371/journal.pmed.1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoerger TJ, et al. Financial incentives for Medicaid beneficaries increase use of preventive services and lower expenditures? Evidence from 10 States. In: American Health Economic Association Meeting, Atlanta, June 2018. https://ashecon.confex.com/ashecon/2018/webprogram/Paper5947.html.
- 47.Sanders GD, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 48.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the US$50,000-per-QALY Threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 49.Ku L, Steinmetz E. Bridging the gap: continuity and quality of coverage in medicaid. http://ccf.georgetown.edu/wp-content/uploads/2013/09/GW-Continuity-Report-9-10-13.pdf. Accessed 1 Aug
- 50.Montana Diabetes Prevention Program. Retrieved from http://www.astho.org/Programs/Health-Systems-Transformation/Montana-Diabetes-Prevention-Program/. Accessed last 6 Feb 2018.
- 51.Services CDoHC. Diabetes prevention program established for medi-cal. http://files.medi-cal.ca.gov/pubsdoco/newsroom/newsroom_26479.asp. Accessed 25 Apr 2018.
- 52.Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care, 2003; 26(9): 2518–23. [DOI] [PMC free article] [PubMed]
- 53.The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention. An intent-to-treat analysis of the DPP/DPPOS, 201;. 35(4): 723–30. [DOI] [PMC free article] [PubMed]
- 54.Todd G, et al. Cost‐Effectiveness of a community‐based diabetes prevention program with participation incentives for medicaid beneficiaries. Health Serv Res. 53(6): 4704–24 [DOI] [PMC free article] [PubMed]
- 55.Block G, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. doi: 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wieland LS, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database of Syste Rev. 2012;8:CD007675. doi: 10.1002/14651858.CD007675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grock S, et al. A Review of Technology-Assisted Interventions for Diabetes Prevention. Curr Diab Rep. 2017;17(11):107. doi: 10.1007/s11892-017-0948-2. [DOI] [PubMed] [Google Scholar]
- 58.Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care, 2018; 41(5): 917–28. [DOI] [PMC free article] [PubMed]
- 59.Ozieh MN, et al. Trends in health care expenditure in U.S. adults with diabetes: 2002–2011. Diabetes Care. 2015;38(10):1844–1851. doi: 10.2337/dc15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cookson R, et al. Using cost-effectiveness analysis to address health equity concerns. Value Health. 2017;20(2):206–212. doi: 10.1016/j.jval.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregg EW, et al. Modeling the impact of prevention policies on future diabetes prevalence in the United States: 2010–2030. Popul Health Metrics. 2013;11(1):18. doi: 10.1186/1478-7954-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colchero MA, et al. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ, 2016. 352. [DOI] [PMC free article] [PubMed]
- 63.Falbe J, et al. Impact of the Berkeley excise tax on sugar-sweetened beverage consumption. Am J Public Health. 2016;106(10):1865–1871. doi: 10.2105/AJPH.2016.303362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gittelsohn J, Trude A. Diabetes and obesity prevention: changing the food environment in low-income settings. Nutri Rev. 2017;75(suppl_1):62–69. doi: 10.1093/nutrit/nuw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu S, Madsen K. Effectiveness and equity of sugar-sweetened beverage taxation. PLOS Med. 2017;14(6):e1002327. doi: 10.1371/journal.pmed.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonisse L, et al. The Effects of Medicaid Expansion under the ACA: Updated Findings from a Literature Review. Retrieved from https://www.kff.org/health-reform/state-indicator/medicaid-expansion-enrollment/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 6 July 2018, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the NHANES are freely available from https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Data from the NHIS are freely available from https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.html. Data from the MEPS are freely available from https://meps.ahrq.gov/data_stats/download_data_files.jsp. Data from the ARIC study can be obtained for eligible projects through individual requests from https://biolincc.nhlbi.nih.gov/studies/aric/. Data from the CARDIA study can be obtained for eligible projects through individual requests from https://biolincc.nhlbi.nih.gov/studies/cardia/?q=cardia. Medicaid Analytic eXtract (MAX) files can be accessed for eligible projects through individual data requests through https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/MAXGeneralInformation.html.