Highlights
-
•
Neprilysin (NEP) participates in development and functions of most body organs
-
•
It is an important brain neuropeptidase which cleaves amyloid β (Aβ) peptide
-
•
NEP dysregulation leads to development of various diseases of old age
-
•
Regulation of NEP expression and activity is an important therapeutic target
Keywords: Ageing, AICD, Alzheimer’s disease, Aβ peptide, amyloid-degrading enzyme, CALLA, cancer, CD10, cognitive behaviour, cortex, diabetes, embryogenesis, endopeptidase 24.11, enkephalinase, epigenetic regulation, HDACs, hippocampus, memory, hypertension, neprilysin, NEP inhibitors olfactory bulbs, prenatal hypoxia, striatum
Abstract
Neprilysin (NEP) is an integral membrane-bound metallopeptidase with a wide spectrum of substrates and physiological functions. It plays an important role in proteolytic processes in the kidney, cardiovascular regulation, immune response, cell proliferation, foetal development etc. It is an important neuropeptidase and amyloid-degrading enzyme which makes NEP a therapeutic target in Alzheimer’s disease (AD). Moreover, it plays a preventive role in development of cancer, obesity and type-2 diabetes. Recently a role of NEP in COVID-19 pathogenesis has also been suggested. Despite intensive research into NEP structure and functions in different organisms, changes in its expression and regulation during brain development and ageing, especially in age-related pathologies, is still not fully understood. This prevents development of pharmacological treatments from various diseases in which NEP is implicated although recently a dual-acting drug sacubitril-valsartan (LCZ696) combining a NEP inhibitor and angiotensin receptor blocker has been approved for treatment of heart failure. Also, various natural compounds capable of upregulating NEP expression, including green tea (EGCG), have been proposed as a preventive medicine in prostate cancer and AD. This review summarizes the existing literature and our own research on the expression and activity of NEP in normal brain development, ageing and under pathological conditions.
1. Introduction
1.1. Structure and general properties
Neprilysin (NEP, EC 3.4.24.11, neutral endopeptidase, CD10 and CALLA) is a type II integral membrane protein residing in the plasma membrane with the major part of it, including the active site, located in the extracellular space. As such it is considered as an ectoenzyme providing proteolytic cleavage of various substrates outside cells. It is exclusively a zinc-dependent enzyme with the Zn-binding domain in the extracellular C-terminal part of the molecule (Fulcher and Kenny, 1983). It exists predominantly as a noncovalently associated homodimer although in some species it was shown to exist as a monomer and a recombinant form of NEP is soluble in aqueous medium (Liu et al., 2010).
Originally NEP was discovered in rat kidney brush border membranes and later purified from rabbit kidney where it constitutes approximately 4% of all proteins of the renal brush border membrane (Kerr and Kenny, 1974). NEP was also shown to be an important brain enzyme cleaving enkephalins and, hence, named enkephalinase. However, after further discovery of a wide range of its substrates NEP was given a more general name - endopeptidase-24.11 based on its enzyme classification number (EC3.4.24.11) (Matsas et al., 1983). In the current literature it is mostly referred to as neprilysin, neutral endopeptidase or NEP. Importantly, NEP was also identified as a leukocyte cell surface antigen, the common acute lymphoblastic leukemia antigen (CALLA or CD10) (Letarte et al., 1988).
NEP has a pH optimum of 6.0 and is inhibited by zinc-chelating reagents (Kerr and Kenny, 1974). A variety of synthetic inhibitors designed to date are widely used for studying NEP properties and also in the clinics. The most widely used NEP inhibitors are thiorphan ([dl-3-mercapto-2-benzylpropanoyl]-glycine) with a K i in the nanomolar range (Roques et al., 1982) and phosphoramidon, although the latter is less specific and inhibits a range of metalloendopeptidases (Komiyama et al., 1975). Comparative analysis suggested that NEP from the kidney and brain have similar sensitivity both to thiorphan and phosphoramidon (Fulcher et al., 1982).
NEP was shown to be heavily glycosylated with five or six N-linked glycosylation sites depending on the species (Fulcher et al., 1983;Lafrance et al., 1994). It can also be sialylated and hyposialylation of the enzyme was found to lead to myopathy (Broccolini et al., 2008). The cytoplasmic domain of NEP can be phosphorylated by casein kinase II (CKII) and its interaction with tyrosine-phosphorylated Lyn kinase blocks focal adhesion kinase signaling (Ganju et al., 1996). Although the C-terminus of NEP contains a putative prenylation CAAX motif this posttranslational modification has not been detected in an in vivo assay (Santos et al., 2002). However, NEP myristoylation at the N-terminal site (Gly2) affects the membrane targeting of the enzyme (Zheng et al., 2010). There are other NEP posttranslational modifications which regulate its binding to various target molecules. For example, phosphorylation of Ser6 in the NEP molecule by casein kinase 2 inhibits its interaction with PTEN (phosphatase and tensin homologue deleted on chromosome 10) which acts as a regulator of the cell cycle (Siepmann et al., 2010).
The human NEP gene is located on chromosome 3 spanning more than 80 kb. It exists in a single copy and is composed of 24 exons. The NEP gene is highly conserved among mammalian species (D’Adamio et al., 1989). NEP gene expression is controlled through two distinct promoters whose influence differs between cell types, although both promoters show similar characteristics and activity (Belyaev et al., 2009). Three distinct NEP mRNAs have been identified in human and rat which differ only in their 5’- noncoding regions (Li et al., 1995a,1995b).
Up to now the list of NEP substrates and its functions continues to grow. It includes a range of peptides up to 40-50 amino acids in length, although the efficiency of hydrolysis declines with increasing length of the peptide (Bayes-Genis et al., 2016). The most efficiently hydrolysed substrates, apart from enkephalins are tachykinins such as substance P, bradykinin, endothelins, atrial natriuretic peptide family, somatostatin, adrenomedullin, members of the vasoactive intestinal peptide family, glucagon, thymopentin etc. This makes NEP inhibitors effective in the treatments of pain, inflammation and cardiovascular disease (Roques and Beaumont, 1990). Moreover, the discovery that NEP can cleave amyloid-β peptide (Aβ) made it one of the major amyloid-degrading enzymes and a target in Alzheimer’s disease (AD) pathogenesis (for review see Nalivaeva et al., 2012a).
1.2. Distribution and biological roles
NEP is a widely distributed enzyme and thiorphan- and phosphoramidon-sensitive activities with the appropriate specificity have been identified in diverse species including the nematode worm, Caenorhabditis elegans, and the fly, Drosophila melanogaster (Turner et al., 2001; Bland et al., 2008). NEP cDNA cloning suggests that rat, rabbit and human enzymes contain 742, 750 and 742 amino acid residues, respectively (Malfroy et al., 1987; Devault et al., 1987; Malfroy et al., 1988). The similarity of rat NEP to the human enzyme makes it a good model for studying its biology and functions. Three NEP cDNAs isolated in rats have different organ and brain cell distribution suggesting that NEP expression has a specific cell-type dependent mechanism (Li et al., 1995a,1995b).
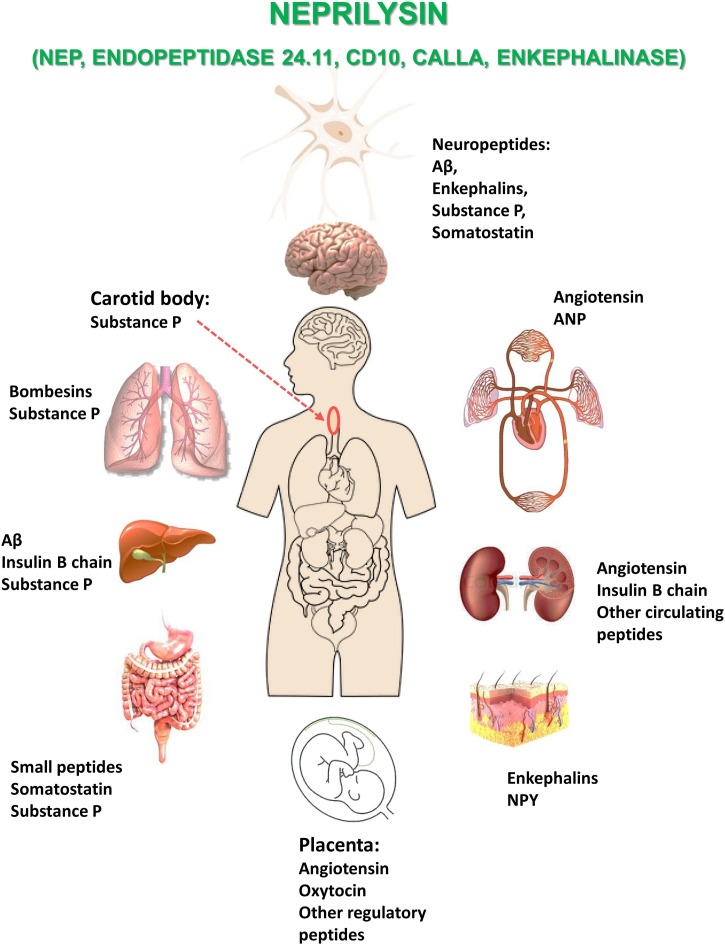
Apart from already mentioned NEP expressing tissues it is also abundant in lungs where it plays an important role in lung development and functions via hydrolysing bombesin-like peptides (Sunday et al., 1992) and NEP levels are indicative of lung dysfunctions (Wick et al., 2011). NEP in the lung may play a role in the airways by modulating responses to inflammatory neuropeptides, for example substance P, which constricts the airway smooth muscle (Borson, 1991).
In vivo NEP labelling by 3H-HACBO-Gly in rats has also revealed its abundance in liver, adipose tissue in the neck region, flat bones of the skull, the mandibula, the vertebrae, the long bones of the limbs, articular cartilages and synoviae, while lower labelling was found in the intestine, the glomeruli and submaxillary glands (Sales et al., 1991). NEP is also expressed in the carotid body where it participates in oxygen level sensing (Kumar et al., 1990) and reticular cells of the lymph nodes (Bowes and Kenny, 1986). NEP activity is also identical to skin fibroblast elastase and thus may play a role in skin ageing (Morisaki et al., 2010). NEP may participate in bone metabolism both through its presence on the surface of human osteoblasts and by its ability to metabolize osteogenic peptides (Ruchon et al., 2000).
Like many other metallopeptidases, NEP is also present in the reproductive system. In the testis, a new soluble form of NEP has been identified which plays an important role in sperm formation and the processes related to fertility (Ghaddar et al., 2000). In the ovary NEP regulates follicle maturation, ovulation and ovarian blood flow (Zappulla and DesGroseillers, 2001). NEP is also expressed in placenta where it was shown to hydrolyse oxytocin but not vasopressin (Johnson et al., 1984) and in foetal membranes suggesting its role in the materno-foetal interface (Imai et al., 1994).
NEP is expressed in stem cells and has been used as a marker of stem cells in many tissues (for review see Maguer-Satta et al., 2011). It is also implicated in the differentiation of immature cells in bone marrow (Torlakovic et al., 2005).
In blood plasma soluble circulating NEP was shown not only to degrade natriuretic peptides but also was suggested as a marker and target in therapy of heart failure (Bayes-Genis et al., 2016). There are clinical data suggesting that the heart is a major source of soluble circulating NEP (Arrigo et al., 2018). NEP was also found in animal and human cerebrospinal and amniotic fluids (Spillantini et al., 1990).
In the brain NEP is present in much lower abundance than in the kidney and lungs but its expression was confirmed for such brain structures as the gray matter, caudate-putamen, striatum, globus pallidus, olfactory tubercle, nucleus interpeduncularis, substantia nigra, pia mater and the ependymal lining of the central canal and the spinal cord (dorsal root ganglia and nerve roots) (Matsas et al., 1986) as well as in the choroid plexus (Bourne and Kenny, 1990). In the human, NEP is distributed rather heterogeneously among brain regions with the highest levels being found in the globus pallidus and pars reticulata of the substantia nigra (Llorens et al., 1982). NEP is primarily located on neuronal cells, especially in the striatonigral pathway and in much lower amounts in the hippocampus (Barnes et al., 1988). The enzyme has also been found on Schwann cells in the peripheral nervous system (Barnes et al., 1995). In mouse brain immunocytochemical analysis has revealed NEP presence in the stratum pyramidale and stratum lacunosum-moleculare of the CA1-3 fields of the hippocampus and in the molecular layer of the dentate gyrus (Fukami et al., 2002). Confocal double immunofluorescence analyses confirmed NEP localization along axons and at the synapses suggesting that after synthesis in the soma, NEP is axonally transported to the synapses. More recent studies have demonstrated that in human brain NEP is enriched in GABAergic neurons and that NEP mRNA can be detected mainly in the parvalbumin-expressing interneurons where NEP protein is localized in the perisomatic synapses (Pacheco-Quinto et al., 2016). These data confirmed NEP protein expression in the GABAergic and metabotropic glutamate 2/3 receptor-positive neurons but not in catecholaminergic or cholinergic neurons (Fukami et al., 2002).
The ability of NEP to catabolise Aβ at multiple sites was first demonstrated in vitro (Howell et al., 1995) which was later confirmed in vivo with intracerebrally administered Aβ into the rat brain parenchyma (Iwata et al., 2000). Further, the correlation between the levels of Aβ peptide in the brain and NEP activity was demonstrated both in NEP knockout mice (Iwata et al., 2001), in rats chronically administered with the NEP inhibitor thiorphan (Zou et al., 2006) and in AD brain (Carpentier et al., 2002). This made NEP a very important target in AD studies.
2. NEP and foetal development
NEP gene knockout is not lethal, and animals appeared developmentally normal (Lu et al., 1995). However, NEP null mice are highly sensitive to endotoxic shock which confirms an important role of NEP in proinflammatory peptide metabolism. Moreover, NEP gene deletion results in an opioid-related increase in thermo-nociceptive threshold in mice suggesting that NEP selectively controls opioid biosynthesis in the hypothalamus and spinal cord (Saria et al., 1997). NEP deficient mice also demonstrate increased consumption of alcohol (Siems et al., 2000). NEP knockout mice also show enhanced aggressive behaviour and altered locomotor activity correlating with compromised NEP enkephalinase activity which cannot be fully compensated by enhanced activities of other enkephalin-degrading enzymes (Fischer et al., 2000).
The importance of NEP for normal foetal development was studied both in animals and humans. NEP mRNA expression and enzymatic activity were detected in foetal bodies early in embryogenesis with progressive major appearance of NEP in the kidney and lungs, and with transient or enhanced expression observed during development of the heart and the major blood vessels, the intestine, the bones, the genital tubercle and sensory organs (Dutriez et al., 1992). Detailed analysis of cellular and tissue distribution of NEP mRNA and enzyme during rat embryonic development reported its first detection on embryonic day 10 (E10) in the gut lining and then, at E12, in the notochord, otocyst, medial and lateral nasal processes, mesonephros, heart and neuroepithelium. Later, by E14 and E16, NEP was also detected in a wide range of craniofacial structures, choroid plexus, tongue and perichondrium. Although NEP was detectable in the craniofacial vasculature at E12 and E14, this was no longer apparent at E16. Importantly, distribution of NEP mRNA matched quite closely localization of the protein detected by immunocytochemistry at all investigated stages of rat embryonic development (Spencer-Dene et al., 1994). Injections of NEP inhibitors, phosphoramidon and thiorphan, to rat embryos on E9.5 and E10.5 resulted in asymmetric developmental abnormalities associated with a haematoma and the severity of the effect was dependent on the inhibitor doses (ibid). The developmental profile of NEP in numerous tissues strongly suggests an important regulatory role of NEP in ontogenesis of various organs.
The peak of CD10/NEP transcript levels in human foetus was identified at 11-13 weeks of gestation and localized to epithelial cells and mesenchyme of developing airways suggesting that NEP plays an important role in lung development (Sunday et al., 1992). It was later confirmed that NEP regulates foetal lung growth and maturation by mediating endogenous bombesin-like peptides (King et al., 1997). In human kidney NEP (CD10) expression was detected starting from early stages of embryogenesis increasing with maturation of the foetuses which suggests that it plays an important role in renal embryogenesis (Faa et al., 2012) (Fig. 1, Fig. 2 ).
Fig. 1.
NEP distribution in various organs and allocation of its main substrates.
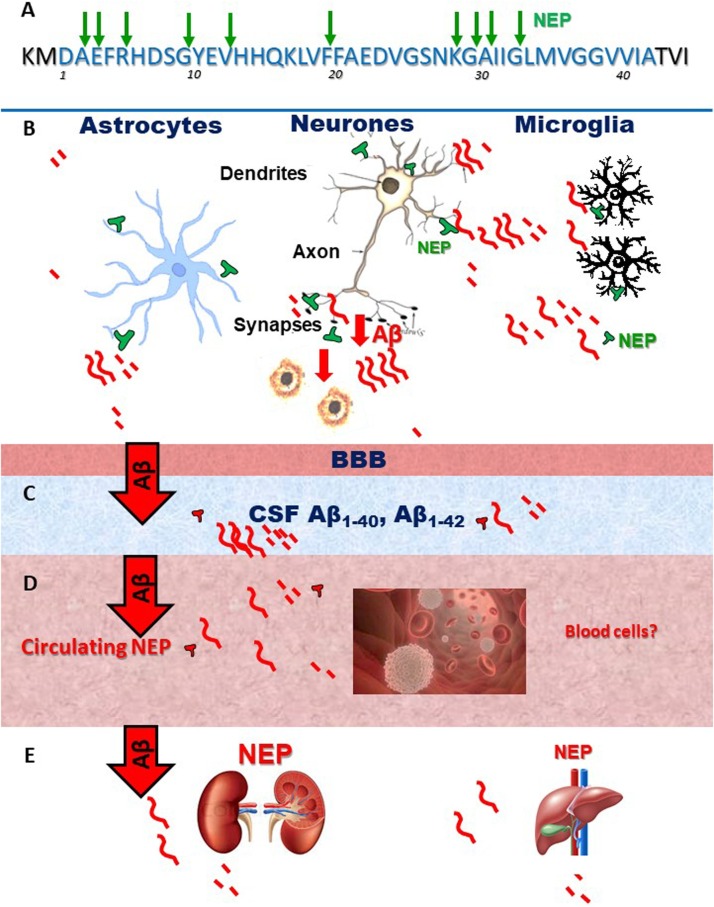
Fig. 2.
Sites of NEP action on Aβ and sites of Aβ clearance in the brain and periphery.
A – diagram of Aβ sequence and the sites cleaved by NEP.
B- Schematic presentation of brain localization of NEP.
C – E – Aβ which is not cleaved by brain NEP is transported via BBB and the glymphatic system into the CSF, blood and peripheral organs (kidney and liver) where it can be degraded by circulating or resident NEP.
In fibroblasts NEP activity was found to increase during foetal to adult transition although in fibroblasts from a centenarian donor it was comparable to that in the cells from young donors (Kletsas et al., 1998). Adrenocorticotropin hormone (a regulatory peptide that can be cleaved by NEP) was shown to increase NEP enzymic activity in foetal cells and young adult donors but decrease in adults and old donors suggesting that ageing affects NEP activity in this type of cell. NEP was also detected in growing ligaments of calf foetuses (Johnson et al., 1990).
NEP also plays an important role in early thymic development and maturation of T lymphocytes as shown in both in vivo and in vitro studies in mice (Guérin et al., 1997). Although the amino acid sequence of CD10 expressed in lymphocytes is almost identical to NEP, its enzymatic activity was never detected biochemically in normal lymphocytes (Leoni and Losa, 1996).
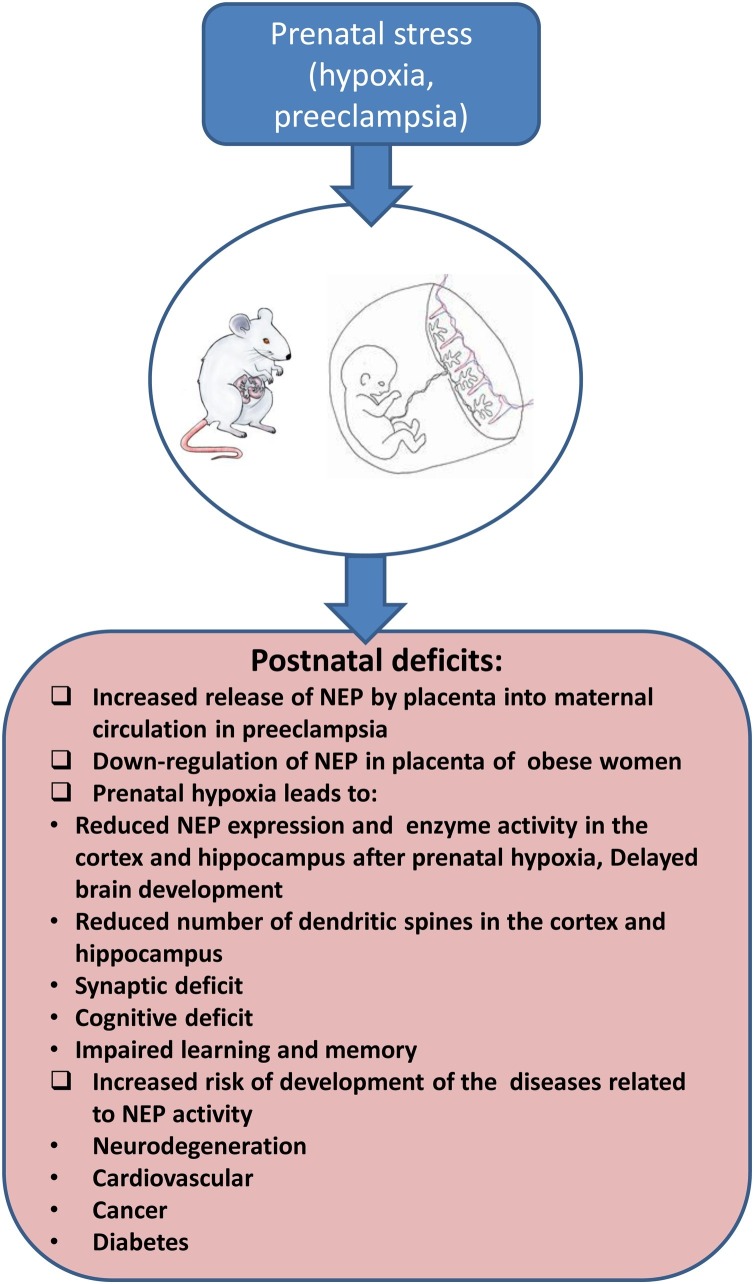
The status of the maternal organism plays an important role in NEP expression and activity in the developing foetuses and offspring. Increased NEP expression in placenta was observed in such conditions as pre-eclampsia suggesting that it may be involved in regulation of peptide activity at the foetal-maternal interface, and as such could be implicated in the developmental changes observed in the growing foetus under such pathological conditions (Li et al., 1995a,1995b). It was also shown that placenta releases active NEP into the maternal circulation at significantly greater levels in preeclampsia which can also predispose to various complications both in the maternal organism and the foetus including hypertension and heart failure (Gill et al., 2019) (Fig. 3 ). On the other hand, maternal obesity was found to downregulate NEP expression in foetal-placental endothelium and in the umbilical cord blood which also may alter the balance of vasoactive peptides and affect foetal development and predispose the offspring to various metabolic diseases in later life (Weiß et al., 2020).
Fig. 3.
Role of prenatal stress in development of NEP-related pathologies.
A significant amount of experimental, epidemiological and clinical studies suggest that gestational factors play a crucial role in development of all organ functions in postnatal life. Such adverse factors as prenatal hypoxia, preeclampsia, maternal diet or drugs of abuse have a negative effect on the foetus and increase its vulnerability to later development of various disorders (for review see Nalivaeva et al., 2018). Changes in NEP expression and activity during pregnancy affect the balance of a variety of regulator peptides. Prenatal hypoxia also affects levels of expression of NEP in the brain and blood plasma of the offspring predisposing them to development of neurodegenerative and cardiovascular diseases, cancer and diabetes.
3. NEP in development of the nervous system
In the central nervous system, the external layer of the olfactory bulbs is the only structure prominently labelled in the foetal rat brain before birth while other structures enriched with NEP in the adult brain, such as the nigrostriatal tract, were shown to become progressively labelled after birth (Dutriez et al., 1992). In murine embryonic brain NEP expression was reported in the nigro-striatal axis, globus pallidus and nucleus accumbens (Dauch et al., 1993). NEP protein levels in rat brain cortex were found to be the highest during the first two weeks after birth declining significantly by the end of the first month of postnatal development. On the contrary, in the striatum, NEP protein levels reached their peak by the end of the first month and stayed at this level practically during all life (Nalivaeva et al., 2004). Several studies from our laboratory have demonstrated that prenatal hypoxia in rats results in a significant reduction of NEP mRNA and protein levels and its enzyme activity in the cortex and hippocampus of rat offspring which correlates with a reduced number of dendritic spines in these brain structures and cognitive impairment of animals during postnatal development and can predispose to development of neurodegeneration, including AD, in later life (Fig. 3) (Nalivaeva et al., 2018; Zhuravin et al., 2019). On the other hand, NEP levels in postnatal life were shown to be dependent on the environment in which animals have been raised and elevated NEP activity was reported in the brains of mice raised in an "enriched environment" which inversely correlated with the amyloid burden (Lazarov et al., 2005).
Analysis of NEP expression in the peripheral nervous system has demonstrated that from E16 to maturity in rat sciatic nerve NEP expression declined both in the levels of immunoreactive protein and enzyme activity in the Schwann cells suggesting age-dependent down-regulation of its expression in this particular type of cell since in non-myelin-forming cells in the sciatic nerve NEP levels were retained in adulthood (Kioussi et al., 1992). The significant increase in NEP expression by Schwann cells after axonal damage suggests that this enzyme plays an important role in nerve development and regeneration (Kioussi et al., 1995). A loss-of-function NEP mutation in humans is also linked with development of Charcot-Marie-Tooth disease characterised by late-onset axonal neuropathy (Higuchi et al., 2016). These patients had decreased or lack of NEP protein expression in the peripheral nerves correlated with muscle weakness, atrophy, and sensory disturbance in the lower extremities although they did not demonstrate symptoms of dementia.
4. NEP and brain functions
Being an important and promiscuous neuropeptidase, NEP participates in various brain functions moderated by its corresponding substrates. This includes NEP roles in metabolism of sensory and inflammatory neuropeptides such as tachykinins and neurokinins (Turner and Nalivaeva, 2007) and pain perception (Fischer et al., 2002). By terminating the effects of neuropeptides in the basal ganglia NEP also participates in movement regulation (Chen et al., 2020) and loss of NEP expression results in altered locomotor activity (Fischer et al., 2000).
Experiments either with NEP knockout mice or NEP inhibitor injections have demonstrated that NEP plays certain roles in cognitive function. NEP knockout mice demonstrated increased aggressive behaviour in the resident-intruder paradigm (Fischer et al., 2000) as well as considerably weaker conditioned taste aversion which extinguished faster than in the age-matched controls (Madani et al., 2006). Rats continuously injected with thiorphan via an osmo-pump during 4 weeks displayed significant cognitive dysfunction in the ability to discriminate in the object recognition test and spatial memory in the water maze test, but not in other hippocampus-dependent learning and memory tasks (Mouri et al., 2006). Single microinjections of phosphoramidon or thiorphan into rat sensorimotor cortex led to disruption of short-term memory tested in a two-level radial maze test (Dubrovskaya et al., 2010).
Studies performed in 5XFAD mice modelling AD demonstrated that NEP deficiency aggravates the behavioural and neuropathological phenotype demonstrating impairment in spatial working memory (Hüttenrauch et al., 2015). Intracranial injections of thiorphan together with Aβ42 to aged rhesus monkeys accelerated development of AD phenotype with significant intraneuronal accumulation of endogenous Aβ in the basal ganglia, cortex and hippocampus, accompanied by neuronal atrophy and loss (Li et al., 2010). On the contrary, overexpression of the human NEP gene in 5XFAD mice under control of the αCaMKII promoter was shown to ameliorate the AD-like phenotype in aged animals (Devi and Ohno, 2015). It also was reported that, in the absence of the NEP gene, other neuropeptides e.g. glucagon-like peptide 1 and galanin could substitute for NEP activity and support the processes involved in learning in aged NEP-deficient mice (Walther et al., 2009).
5. NEP in the ageing brain
As already mentioned above, NEP expression and activity decrease with age in some peripheral tissues and in brain structures of experimental animals (Kletsas et al., 1998; Nalivaeva et al., 2004; Zhuravin et al., 2019). There are also several studies reporting that in human brain, and especially in the hippocampus, which is vulnerable to AD pathology, NEP steady-state levels diminish as a function of age (Caccamo et al., 2005). Reduced NEP protein levels were reported in the temporal and frontal cortex of normally aged and AD brains (Hellström-Lindahl et al., 2006) while another study confirmed that NEP immunoreactivity was decreased in AD but not in other pathological ageing (Wang et al., 2005). More detailed analysis showed that NEP mRNA and protein levels, as well as enzyme activity were decreased in AD brain compared to normal controls with no cognitive impairment (Wang et al., 2010). Comparative mRNA analysis in high plaque areas of AD brains also demonstrated reduced NEP expression compared to age-matched controls while in the periphery they were much higher suggesting that in the periphery the capacity of NEP to degrade Aβ is stronger than in the brain and explaining the lack of Aβ deposits in the peripheral organs (Yasojima et al., 2001). Although another group reported no differences in NEP mRNA levels in the temporal lobe of AD patients they noted that the changes in the somatostatin system in this brain area might impair NEP regulation causing a deficit in its activity leading to Aβ accumulation (Gahete et al., 2010). There are also data, demonstrating that NEP protein levels do not significantly change in adult human brain with advanced ageing from 40 to over 60 years old and in AD and that NEP activity is higher in the homogenates from advanced age and AD samples. However, such a discrepancy is most likely to be attributed to the methodological approach used in this study (Miners et al., 2010).
Since amyloid clearance and NEP deficit play an important role in development of late-onset sporadic AD (Farris et al., 2007), more studies of NEP developmental profile and regulation in various brain areas both in non-transgenic animals and humans using state of the art unified techniques are still needed for a better understanding of its role in AD pathogenesis and preventions.
6. Role of NEP in diseases
6.1. Cancer
The identity of NEP (CD10) as the common acute lymphoblastic leukemia antigen CALLA (LeTarte et al., 1988; Le Bien and McCormack, 1989) and its transient expression on the surface of certain hematopoietic cells makes CD10 an important marker for some types of lymphomas (de Jong et al., 2007) and is widely used for diagnosis of certain forms of childhood leukemia (Cutrona et al., 2002). More recently NEP was also reported as a marker for primary central nervous system lymphoma (Yang and Liu, 2017).
Apart from lymphomas, NEP has been implicated in the progression of various types of cancers due to its ability to inactivate such bioactive peptides as endothelin-1 (ET-1), angiotensin-II, and bombesin which participate in neoplastic transformation. The most studied types of cancer in which NEP expression and activity are affected by tumour progression include prostate (Papandreou et al., 1998, Usmani et al., 2000), kidney (Ahn et al., 2013; Erin et al., 2016), lungs (Shipp et al., 1991; Goldstein et al., 2017), breast (Louhichi et al., 2018), endometrium (Pekonen et al., 1995) and cervix (Terauchi et al., 2005). In the majority of cases NEP has tumour suppressive action and its loss contributes to cancer progression (Sumitomo et al., 2005). However, in colorectal cancer, CD10 expression was shown to increase with tumour progression (Jang et al., 2013). NEP overexpression was also reported in melanomas (Velazquez et al., 2007).
Mechanisms of NEP gene regulation were studied in great detail in prostate cancer (Usmani et al., 2000; Hong et al., 2012) where NEP contributes to androgen-independent progression of the disease through the loss of ability to metabolise mitogenic peptides, such as endothelin-1. Down-regulation of NEP expression in prostate cancer was shown to be attributed to extensive hypermethylation of its gene promoter region (Usmani et al., 2000). NEP gene transcription in the prostate is up-regulated by androgens (Shen et al., 2000) as well as by estrogen through a hormone-responsive element (Xiao et al., 2009). Although in neuronal cells the NEP gene is regulated by the amyloid-precursor protein (APP) intracellular domain (AICD) (Belyaev et al., 2010) and APP is expressed in prostate, there is no evidence that a similar AICD-dependent mechanism is also involved in NEP gene regulation in this tissue (Hong et al., 2012) although histone acetylation in the region of the NEP gene promoter may facilitate partial re-activation of NEP expression in advanced prostate cancer cells. Recently it was shown that long noncoding RNA (lncRNA) within mRNA sequences of APP is progressively activated in prostate cancer promoting its development by competitive binding to miR218 (Shi et al., 2020) although its involvement in NEP expression has not yet been investigated.
Up-regulation of NEP activity in cancer cell lines was also achieved by treatment with Epilobium extracts and polyphenols which also inhibited cell proliferation suggesting that this herbal remedy can be efficient in cancer cells with dysregulated NEP expression and activity (Kiss et al., 2006). Re-expression of NEP via an inducible tetracycline-regulatory gene expression system in mice resulted in inhibition of tumour formation and progression in their prostates (Dai et al., 2001) which opens new avenues in prostate cancer therapy.
6.2. Cardiovascular diseases
Taking into account a very important role of NEP in inactivating the natriuretic peptides in vivo and its participation in the renin-angiotensin system (RAS), much effort has been paid to the design of potential NEP inhibitors for the treatment of certain forms of cardiovascular and renal diseases. Moreover, circulating plasma NEP was shown to be a predictor of heart failure (Bayes-Genis et al., 2016, especially in patients on dialysis (Claus et al., 2020). However, the observations that loss of NEP activity can promote accumulation of Aβ in the brain (Newell et al., 2003) or enhance the activity of mitogenic peptides (Dawson et al., 2004) urged caution for the chronic application of NEP inhibitors in humans (Campbell, 2017). Currently a novel angiotensin receptor/neprilysin inhibitor (ARNI) sacubitril/valsartan has been approved for treatment of hypertension and prevention of heart failure in elderly hypertensive patients (for review see Kario, 2018). While there is no doubt that hypertension should be treated for prevention of cardiovascular diseases as well as of stroke and vascular dementia, the role of NEP inhibitors as antihypertensive agents in AD pathogenesis remains unclear and needs further investigation (Lebouvier et al., 2020).
6.3. Obesity and Diabetes
Recent studies have revealed that NEP is involved in the development of obesity and type 2 diabetes. NEP-deficient mice become obese even under a normocaloric diet at the age of 6-7 months (Becker et al., 2010). In humans, a direct correlation between NEP activity, body mass index (BMI) and insulin resistance was observed in subjects with multiple cardiovascular risk factors. NEP protein production in human adipocytes increased during cell differentiation, and plasma and adipose tissue levels of NEP were increased in obese insulin-resistant mice (Standeven et al., 2010). It was also demonstrated that NEP accelerates adipogenesis through enhancement of insulin-mediated PI3K-Akt activation (Kim et al., 2017). Treatment of obese mice (Berlin fat mice) with green tea significantly reduced body fat and prevented its further accumulation. This correlated with increased peripheral (in the kidney and intestine) NEP expression and downregulation of orexigens (Muenzner et al., 2016). This effect of green tea was not observed in obese NEP-knockout mice. NEP deficient mice are also characterised by increased islet β-cell mass when fed on a high fat diet which suggests a role for NEP in modulating metabolism of this type of cells (Parilla et al., 2018). Elevated NEP activity was also observed in the vitreous of patients with diabetic retinopathy which inversely correlated with Aβ levels (Hara et al., 2006). Hyperlipidemia and hyperglycemia associated with type 2 diabetes cause delayed diabetic wound healing and were found to be associated with increased NEP activity in human microvascular endothelial cells which might impair normal response of the tissues to the injury (Muangman et al., 2003). Topical application of thiorphan to the wounds of diabetic mice, on the contrary, improved wound healing (Spenny et al., 2002).
In patients with diabetes NEP levels in urine are increased and could be used as early biomarkers to predict the incidence or progression of chronic kidney disease in individuals with type 2 diabetes (Gutta et al., 2018).
In a monkey model of streptozotocin-induced type 1 diabetes a decrease in NEP mRNA levels were observed in the hippocampus which correlated with increased levels of Aβ in this brain structure suggesting a link between diabetes and AD pathogenesis (Morales-Corraliza et al., 2016).
Accumulating data suggest that NEP plays an important role in glucose metabolism and clinical studies with a dual angiotensin receptor-neprilysin inhibitor (ARNi) demonstrated that such treatment improved insulin sensitivity and glycaemic control in patients with type 2 diabetes (Seferovic et al., 2017). This suggests that NEP inhibitors in combination with angiotensin II receptor blockers might be a useful therapeutic approach for treatment of type 2 diabetes (Esser and Zraika, 2019). However, considering the promiscuous nature of NEP and its involvement in normal homeostasis and development of a range of diseases, this should be done with significant care and preliminary laboratory research.
6.4. Immune system diseases
Involvement of NEP in development of immunodeficiency can be anticipated based on its involvement in development and maturation of haemopoietic cells and pathogenesis of lymphomas. In HIV patients an increased ratio of CD10-positive B cells has been reported suggesting B cell activation and immaturity (Martínez-Maza et al., 1987). Although NEP enzymatic activity was not reported in normal lymphocytes, it was detectable in HIV + cells (Leoni and Losa, 1996). However, in the brain of HIV patients the increase of a viral transactivating transcription factor (Tat) correlated with reduced NEP activity and reported accumulation of Aβ in the brain of HIV-infected patients (Rempel and Pulliam, 2005). Later it was reported that NEP hydrolyses the Tat protein and the peptides derived from it inhibit NEP activity competitively and reversibly leading to increased Aβ levels (Daily et al., 2006).
Although no direct correlation was reported between Aβ42 and NEP activity in the CSF of HIV patients (Mothapo et al., 2015) the changes in NEP/Aβ ratio was found to be dependent on HIV subtype suggesting higher Aβ deposit in HIV1-C infected patients than in HIV1-B (de Almeida et al., 2018).
6.5. Alzheimer’s disease
As documented above NEP, being the major amyloid-degrading enzyme in the brain (Iwata et al., 2000, 2001), plays a significant role in AD pathogenesis, in particular, of the late onset sporadic form (for review see Nalivaeva et al., 2012a). A recent meta-analysis of NEP levels in AD demonstrated both that NEP expression and activity are decreased in the cortex of elderly AD patients (Zhang et al., 2017). NEP inhibition or gene silencing in animal models recapitulate the pathological hallmarks of AD detected in human brains with accumulation of amyloid peptide and cognitive impairment (Hanson et al., 2011; Li et al., 1995a,1995b). Interestingly, NEP gene knockout in APPSw mice modelling AD led to the changes in expression of more than 600 proteins including some which were not previously reported to be linked to AD but associated with increased Aβ levels (Nilsson et al., 2015). These mice also exhibited enhanced amyloid pathology compared with only APP transgenic ones and showed impaired learning and memory in the Morris water maze test (Mohajeri and Wolfer, 2009).
Numerous studies have demonstrated the efficacy of NEP up-regulation for lowering Aβ levels in the brain, including lentiviral or adenoviral NEP gene transfer (Marr et al., 2003; Carty et al., 2013). By this approach it was shown that NEP gene transfer was able to abolish increased Aβ levels at the presynaptic sites in young transgenic mice (Iwata et al., 2004). Moreover, up-regulation of peripheral NEP in experimental animals (in the skeletal muscles or plasma) could also reduce Aβ levels in the brain and ameliorate AD pathology (Liu et al., 2009; Liu et al., 2010). There are also numerous pharmacological approaches for increasing NEP expression and activity recently reviewed in (Nalivaeva and Turner, 2019). Some of them are based on the endogenous feedback mechanism for upregulating the NEP gene by the APP C-terminal fragment AICD which is formed alongside Aβ in the amyloidogenic APP proteolytic processing in neuronal cells (Pardossi-Piquard et al., 2005; Belyaev et al., 2010). This mechanism involves competitive binding of AICD and histone deacetylases (HDACs) to the promoter region of the NEP gene which, in the cells with low levels of NEP expression, can be activated by HDAC inhibitors (Belyaev et al., 2009). On the other hand, there is convincing evidence that DNA methylation of the NEP promoter is not involved in AD pathogenesis (Nagata et al., 2018).
Our work in cell and animal models has demonstrated that NEP expression and activity is reduced after hypoxia and that this downregulation is related to increased cleavage of AICD by caspases activated by hypoxia (Kerridge et al., 2015; Kozlova et al., 2015). Intracranial injections to rats, subjected to prenatal hypoxia, of a caspase-3 inhibitor (Z-DEVD-FMK or Ac-DEVD-CHO) at the age of three weeks reduced caspase activity and restored AICD levels and NEP protein expression (Vasilev et al., 2016; Kozlova et al., 2015). Moreover, administration of an HDAC inhibitor, sodium valproate, or the antioxidant EGCG were shown to increase NEP expression and activity in rats subjected to prenatal hypoxia (Nalivaeva et al., 2012b; Zhuravin et al., 2019).
One of the substrates of NEP, somatostatin (Barnes et al., 1995), which plays an important role in the hippocampus facilitating memory processes (Eyre and Bartos, 2019), was suggested to upregulate NEP expression (Saito et al., 2005). Somatostatin levels decline in the brain with ageing (Hayashi et al., 1997) and are consistently reduced in the brain and CSF of AD patients (Burgos-Ramos et al., 2008) which together with reduced NEP activity may trigger and promote Aβ accumulation leading to late-onset AD. Incubation of primary cortical neurons with somatostatin was shown to upregulate NEP activity (Saito et al., 2005). Moreover, i.c.v. injection of a selective somatostatin receptor-subtype agonist to 12-month SAMP8 mice not only increased NEP activity in the cortical tissue but also enhanced their learning and memory (Sandoval et al., 2012).
Screening 25 curcumin analogues for their ability to upregulate NEP activity it was shown that only four of them (namely dihydroxylated curcumin, monohydroxylated demethoxycurcumin, and mono- and di-hydroxylated bisdemethoxycurcumin) increased NEP activity while curcumin itself did not have this effect (Chen et al., 2016). Although such pharmacological approaches can be beneficial for NEP-dependent prevention of Aβ accumulation and development of late onset AD, treatments have to start rather early even before disease manifestation (El-Amouri et al., 2008). Further works on the role of HDAC modifications in AD pathology demonstrated that HDAC1 SUMOylation might be an intrinsic defence mechanism protecting neuronal cells against Aβ toxicity which opens a new avenue in therapeutic strategy in AD prevention (Tao et al., 2017).
In relation to AD pathology it is important to mention another NEP-like endopeptidase termed neprilysin-2 (NEP2) whose expression in the brain is restricted to developing and differentiating areas (Facchinetti et al., 2003) and which has different subcellular localization and substrate preference from NEP (Whyteside and Turner, 2008) but contributes to Aβ metabolism in vivo (Hafez et al., 2011; Huang et al., 2012). It was demonstrated that NEP2 activity is reduced in individuals with mild cognitive impairment and in AD patients compared to non-impaired individuals (Huang et al., 2012). NEP2 was suggested to be even more selective as a therapeutic target than NEP since it has a more restricted substrate specificity (Whyteside and Turner, 2008).
There are reports on a biallelic polymorphism in the 3'UTR of the NEP gene associated with an increased risk for AD in an age-dependent manner adjusting for sex and ApoE status (Clarimón et al., 2003). Also, a single nucleotide polymorphism (SNP) in the NEP gene correlating with development of AD was reported in Finnish patients (Helisalmi et al., 2004). However, studies in Swedish patients failed to demonstrate significant differences in the distribution of promoter polymorphisms between AD cases and controls (Lilius et al., 2003) as well as in a cohort from a Southern Chinese community (Fu et al., 2009). Nevertheless, analysis of NEP SNP rs1816558 in two Han Chinese cohorts demonstrated significant association with AD after adjustment for APOEε4 allele while NEP mRNA levels were substantially increased during AD progression (Wang et al., 2016).
Reduced CSF NEP activity levels have been shown to occur in early AD, suggesting that altered CSF NEP activity levels may also be predictive of the progression of dementia (Maetzler et al., 2010). There are also reports on reduced NEP activity in blood plasma in individuals with mild cognitive impairment and AD patients (Zhuravin et al., 2015), which are in line with studies showing that NEP is reduced in CSF in prodromal AD (Maruyama et al. 2005).
6.6. COVID-19
The recent ongoing pandemic caused by the new coronavirus (SARS-CoV2) has raised the question about a possible role of NEP in the pathogenesis of the complications developed following the viral infection. Despite intensive search for potential drug candidates and development of effective vaccines there is still no treatment to prevent the infection or reduce the severity of the disease progression. The most severe complications of SARS-CoV2 infection are observed in the lungs and heart where the viral receptor, angiotensin-converting enzyme-2 (ACE2), a metallopeptidase determining together with its homologues ACE and NEP the effectiveness of the RAS, is expressed (Tipnis et al., 2000; Rice et al., 2006). Taking into account the role of NEP in the lung RAS and its ability to convert angiotensin I to the protective angiotensin-(1-7) (Rice et al., 2006), it might be protective against the inflammatory reactions and fibrosis caused by viral infections in the lungs (for review see El Tabaa and El Tabaa, 2020; Manolis et al., 2020).
7. NEP as a therapeutic target
As already discussed above, development of NEP inhibitors for controlling blood pressure has been compromised by the facts that chronic NEP inhibition might result in angio-oedema, bronchial reactivity, inflammation, cancer, and could predispose to polyneuropathy and AD (for review see Campbell, 2017). Despite these concerns, in recent years interest in this strategy has been revived leading to the development of sacubitril-valsartan (LCZ696), a combined NEP inhibitor and angiotensin receptor blocker, whose use for the treatment of heart failure was approved in 2015. The dual cardioprotective action of the drug appears to have significant advantages in the management of heart failure (the PARADIGM-HF phase III trial) (Seferovic et al., 2017; Kario, 2018).
NEP inhibition was also suggested for treatment of corneal injury and corneal wound healing (Genova et al., 2018). Another proposed area for NEP inhibitor application was female sexual arousal disorders although no significant progress in this area has been achieved (Angulo, 2010).
NEP inhibitors may also be suitable for pain management because of their effects on nociception (Aykan et al., 2019; Ramírez-Sánchez et al., 2019). Oral administration of a dual enkephalinase (NEP/ANP) inhibitor PL37 was shown to suppress completely osteosarcoma-induced thermal hyperalgesia in mice via activation of opioid receptors (Menéndez et al., 2008). Apart from synthetic NEP inhibitors there are some natural endogenous NEP inhibitors, including the stress-response peptide, sialorphin (Rougeot et al., 2003) and “opiorphins”, which possess strong analgesic properties (Wisner et al., 2006). Some studies have also suggested that opiorphins promote sperm motility which adds another important role to the list of NEP functions and therapeutic avenues (Bosler et al., 2014). Role of NEP in development and modulation of pain, stress response, addiction, sexuality, and food intake has recently been reviewed in (Roques, 2018).
Although some areas of medicine are seeking compounds to inhibit NEP there is clearly an urgent need to upregulate NEP expression and activity for treatment of other groups of diseases such as cancer and AD (Fig. 4 ). Various pharmacological and epigenetic approaches have been developed to up-regulate NEP expression (for review see Nalivaeva and Turner, 2019). However, for designing strategies to treat each particular type of pathology it is not only necessary to develop specific drugs but also to acquire a deeper knowledge on the specificity of NEP regulation by endogenous and exogenous factors at the levels of its gene expression, mRNA translation, protein post-translational modification and local modulation of its activity. Such knowledge could help us to use this rather unique enzyme for the benefit of human health.
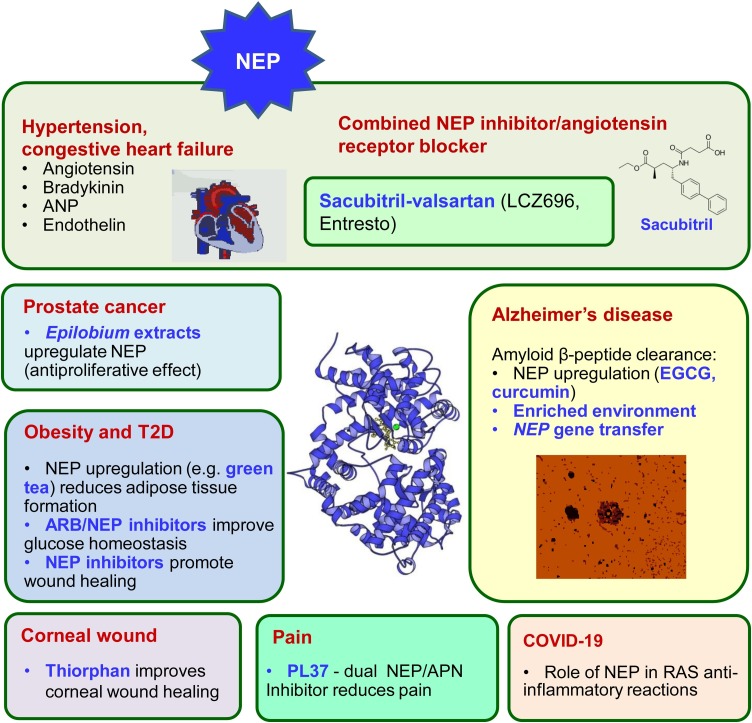
Fig. 4.
NEP dysregulation leads to various diseases of old age and creates a platform for designing therapeutic targets.
The involvement of NEP in diverse disease conditions, its relevant substrates and inhibitors, and strategies for its upregulation are highlighted, alongside the enzyme 3D structure.
Declaration of Competing Interest
Authors declare no conflict of interest.
Acknowledgements
Supported by RFBR (19-015-00232) and Russian Federation state budget (assignment АААА-А18-118012290373-7).
References
- Ahn S., Kwon G.Y., Cho Y.M., Jun S.Y., Choi C., Kim H.J., Park Y.W., Park W.S., Shim J.W. Acquired cystic disease-associated renal cell carcinoma: further characterization of the morphologic and immunopathologic features. Med. Mol. Morphol. 2013;46:225–232. doi: 10.1007/s00795-013-0028-x. [DOI] [PubMed] [Google Scholar]
- Angulo J. Neutral endopeptidase inhibition: could it have a role in the treatment of female sexual arousal disorder? Br. J. Pharmacol. 2010;160:48–50. doi: 10.1111/j.1476-5381.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo M., Vodovar N., Nougué N., Sadoune M., Pemberton C.J., Ballan P., Ludes P.-O., Gendron N., Carpentier A., Cholley B., Bizouarn P., Cohen-Solal A., Singh J.P., Szymonifka J., Latremouille C., Samuel J.-L., Launay J.-M., Pottecher J., Richards A.M., Truong Q.A., Smadja D.M., Mebazaa A. The heart regulates the endocrine response to heart failure: cardiac contribution to circulating neprilysin. Eur. Heart J. 2018;39:1794–1798. doi: 10.1093/eurheartj/ehx679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykan D.A., Koca T.T., Yaman S., Eser N. Angiotensin converting enzyme and neprilysin inhibition alter pain response in dexhamethasone-induced hypertensive rats. Pharmacol. Rep. 2019;71:306–310. doi: 10.1016/j.pharep.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Barnes K., Doherty S., Turner A.J. Endopeptidase-24.11 is the integral membrane peptidase initiating degradation of somatostatin in the hippocampus. J. Neurochem. 1995;64:1826–1832. doi: 10.1046/j.1471-4159.1995.64041826.x. [DOI] [PubMed] [Google Scholar]
- Barnes K., Matsas R., Hooper N.M., Turner A.J., Kenny A.J. Endopeptidase-24.11 is striosomally ordered in pig brain and, in contrast to aminopeptidase N and peptidyl dipeptidase A (‘angiotensin converting enzyme’) is a marker for a set of striatal efferent fibres. Neuroscience. 1988;27:799–817. doi: 10.1016/0306-4522(88)90184-4. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A., Barallat J., Richards A.M. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J. Am. Coll. Cardiol. 2016;68:639–653. doi: 10.1016/j.jacc.2016.04.060. [DOI] [PubMed] [Google Scholar]
- Becker M., Siems W.E., Kluge R., Gembardt F., Schultheiss H.P., Schirner M., Walther T. New function for an old enzyme: NEP deficient mice develop late-onset obesity. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev N.D., Nalivaeva N.N., Makova N.Z., Turner A.J. Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 2009;10:94–100. doi: 10.1038/embor.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev N.D., Kellett K.A., Beckett C., Makova N.Z., Revett T.J., Nalivaeva N.N., Hooper N.M., Turner A.J. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a β-secretase-dependent pathway. J. Biol. Chem. 2010;285:41443–41454. doi: 10.1074/jbc.M110.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland N.D., Pinney J.W., Thomas J.E., Turner A.J., Isaac R.E. Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol. Biol. 2008;8:16. doi: 10.1186/1471-2148-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borson D.B. Roles of neutral endopeptidase in airways. Am. J. Physiol. 1991;260:L212–L225. doi: 10.1152/ajplung.1991.260.4.L212. [DOI] [PubMed] [Google Scholar]
- Bosler J.S., Davies K.P., Neal-Perry G.S. Peptides in seminal fluid and their role in infertility: a potential role for opiorphin inhibition of neutral endopeptidase activity as a clinically relevant modulator of sperm motility: a review. Reprod. Sci. 2014;21:1334–1340. doi: 10.1177/1933719114536473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne A., Kenny A.J. The hydrolysis of brain and atrial natriuretic peptides by porcine choroid plexus is attributable to endopeptidase-24.11. Biochem. J. 1990;271:381–385. doi: 10.1042/bj2710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes M.A., Kenny A.J. Endopeptidase-24.11 in pig lymph nodes. Purification and immunocytochemical localization in reticular cells. Biochem. J. 1986;236:801–810. doi: 10.1042/bj2360801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccolini A., Gidaro T., De Cristofaro R., Morosetti R., Gliubizzi C., Ricci E., Tonali P.A., Mirabella M. Hyposialylation of neprilysin possibly affects its expression and enzymatic activity in hereditary inclusion-body myopathy muscle. J. Neurochem. 2008;105:971–981. doi: 10.1111/j.1471-4159.2007.05208.x. [DOI] [PubMed] [Google Scholar]
- Burgos-Ramos E., Hervás-Aguilar A., Aguado-Llera D., Puebla-Jiménez L., Hernández-Pinto A.M., Barrios V., Arilla-Ferreiro E. Somatostatin and Alzheimer’s disease. Mol. Cell. Endocrinol. 2008;286:104–111. doi: 10.1016/j.mce.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Caccamo A., Oddo S., Sugarman M.C., Akbari Y., LaFerla F.M. Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol. Aging. 2005;26:645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Campbell D.J. Long-term neprilysin inhibition - implications for ARNIs. Nat. Rev. Cardiol. 2017;14:171–186. doi: 10.1038/nrcardio.2016.200. [DOI] [PubMed] [Google Scholar]
- Carpentier M., Robitaille Y., DesGroseillers L., Boileau G., Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2002;61:849–856. doi: 10.1093/jnen/61.10.849. [DOI] [PubMed] [Google Scholar]
- Carty N., Nash K.R., Brownlow M., Cruite D., Wilcock D., Selenica M.L., Lee D.C., Gordon M.N., Morgan D. Intracranial injection of AAV expressing NEP but not IDE reduces amyloid pathology in APP+PS1 transgenic mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.T., Chen Z.T., Hou W.C., Yu L.C., Chen R.P. Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer’s disease. Sci. Rep. 2016;6:29760. doi: 10.1038/srep29760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.Y., Xue Y., Chen H., Chen L. The globus pallidus as a target for neuropeptides and endocannabinoids participating in central activities. Peptides. 2020;124 doi: 10.1016/j.peptides.2019.170210. [DOI] [PubMed] [Google Scholar]
- Clarimón J., Muñoz F.J., Boada M., Tàrraga L., Sunyer J., Bertranpetit J., Comas D. Possible increased risk for Alzheimer’s disease associated with neprilysin gene. J. Neural. Transm. (Vienna) 2003;110:651–657. doi: 10.1007/s00702-002-0807-3. [DOI] [PubMed] [Google Scholar]
- Claus R., Berliner D., Bavendiek U., Vodovar N., Lichtinghagen R., David S., Patecki M., Launay J.M., Bauersachs J., Haller H., Hiss M., Balzer M.S. Soluble neprilysin, NT-proBNP, and growth differentiation factor-15 as biomarkers for heart failure in dialysis patients (SONGBIRD) Clin. Res. Cardiol. 2020 doi: 10.1007/s00392-020-01597-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona G., Tasso P., Dono M., Roncella S., Ulivi M., Carpaneto E.M., Fontana V., Comis M., Morabito F., Spinelli M., Frascella E., Boffa L.C., Basso G., Pistoia V., Ferrarini M. CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL. Br. J. Cancer. 2002;86:1776–1785. doi: 10.1038/sj.bjc.6600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamio L., Shipp M.A., Masteller E.L., Reinherz E.L. Organization of the gene encoding common acute lymphoblastic leukemia antigen (neutral endopeptidase 24.11): multiple miniexons and separate 5’ untranslated regions. Proc. Natl. Acad. Sci. USA. 1989;86:7103–7107. doi: 10.1073/pnas.86.18.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Shen R.Q., Sumitomo M., Goldberg J.S., Geng Y.P., Navarro D., Xu S., Koutcher J.A., Garzotto M., Powell C.T., Nanus D.M. Tumor-suppressive effects of neutral endopeptidase in androgen-independent prostate cancer cells. Clin. Cancer Res. 2001;7:1370–1377. PMID: 11350908. [PubMed] [Google Scholar]
- Daily A., Nath A., Hersh L.B. Tat peptides inhibit neprilysin. J. Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- Dauch P., Masuo Y., Vincent J.P., Checler F. A survey of the cerebral regionalization and ontogeny of eight exo- and endopeptidases in murines. Peptides. 1993;14:593–599. doi: 10.1016/0196-9781(93)90150-f. [DOI] [PubMed] [Google Scholar]
- Dawson L.A., Maitland N.J., Turner A.J., Usmani B.A. Stromal-epithelial interactions influence prostate cancer cell invasion by altering the balance of metallopeptidase expression. Br. J. Cancer. 2004;90:1577–1582. doi: 10.1038/sj.bjc.6601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida S.M., Tang B., Ribeiro C.E., Rotta I., Vaida F., Piovesan M., Batistela Fernandes M.S., Letendre S., Potter M., Ellis R.J., HIV Neurobehavioral Research Center (HNRC) Group Neprilysin in the Cerebrospinal Fluid and Serum of Patients Infected With HIV1-Subtypes C and B. J. Acquir. Immune Defic. Syndr. 2018;78:248–256. doi: 10.1097/QAI.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D., Rosenwald A., Chhanabhai M., Gaulard P., Klapper W., Lee A., Sander B., Thorns C., Campo E., Molina T., Norton A., Hagenbeek A., Horning S., Lister A., Raemaekers J., Gascoyne R.D., Salles G., Weller E., Lunenburg Lymphoma Biomarker Consortium Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications--a study from the Lunenburg Lymphoma Biomarker Consortium. J. Clin. Oncol. 2007;25:805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N.G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A., Roques B.P., Crine P., Boileau G. Amino acid sequence of rabbit kidney neutral endopeptidase-24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987;6:1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L., Ohno M. A combination Alzheimer’s therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice. Mol. Brain. 2015;8:19. doi: 10.1186/s13041-015-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovskaya N.M., Nalivaeva N.N., Plesneva S.A., Feponova A.A., Turner A.J., Zhuravin I.A. Changes in the Activity of Amyloid-Degrading Metallopeptidases Leads to Disruption of Memory in Rats. Neurosci. Behav. Physiol. 2010;40:975–980. doi: 10.1007/s11055-010-9355-8. [DOI] [PubMed] [Google Scholar]
- Dutriez I., Salès N., Fournié-Zaluski M.C., Roques B.P. Pre- and post-natal ontogeny of neutral endopeptidase 24-11 (’enkephalinase’) studied by in vitro autoradiography in the rat. Experientia. 1992;48:290–300. doi: 10.1007/bf01930479. [DOI] [PubMed] [Google Scholar]
- El-Amouri S.S., Zhu H., Yu J., Marr R., Verma I.M., Kindy M.S. Neprilysin: an enzyme candidate to slow the progression of Alzheimer’s disease. Am. J. Pathol. 2008;172:1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Tabaa M.M., El Tabaa M.M. Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost. Biochem Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erin N., İpekçi T., Akkaya B., Özbudak İ.H., Baykara M. Neuropeptide Levels as well as Neprilysin Activity Decrease in Renal Cell Carcinoma. Cancer Microenviron. 2016;9:141–147. doi: 10.1007/s12307-016-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N., Zraika S. Neprilysin inhibition: a new therapeutic option for type 2 diabetes? Diabetologia. 2019;62:1113–1122. doi: 10.1007/s00125-019-4889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre M.D., Bartos M. Somatostatin-Expressing Interneurons Form Axonal Projections to the Contralateral Hippocampus. Front. Neural. Circuits. 2019;13:56. doi: 10.3389/fncir.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faa G., Gerosa C., Fanni D., Nemolato S., Marinelli V., Locci A., Senes G., Mais V., Van Eyken P., Iacovidou N., Monga G., Fanos V. CD10 in the developing human kidney: immunoreactivity and possible role in renal embryogenesis. J. Matern. Fetal. Neonatal. Med. 2012;25:904–911. doi: 10.3109/14767058.2011.599457. [DOI] [PubMed] [Google Scholar]
- Facchinetti P., Rose C., Schwartz J.C., Ouimet T. Ontogeny, regional and cellular distribution of the novel metalloprotease neprilysin 2 in the rat: a comparison with neprilysin and endothelin-converting enzyme-1. Neuroscience. 2003;118:627–639. doi: 10.1016/s0306-4522(02)01002-3. [DOI] [PubMed] [Google Scholar]
- Farris W., Schütz S.G., Cirrito J.R., Shankar G.M., Sun X., George A., Leissring M.A., Walsh D.M., Qiu W.Q., Holtzman D.M., Selkoe D.J. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am. J. Pathol. 2007;171:241–251. doi: 10.2353/ajpath.2007.070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H.S., Zernig G., Schuligoi R., Miczek K.A., Hauser K.F., Gerard C., Saria A. Alterations within the endogenous opioid system in mice with targeted deletion of the neutral endopeptidase (’enkephalinase’) gene. Regul. Pept. 2000;96:53–58. doi: 10.1016/s0167-0115(00)00200-7. [DOI] [PubMed] [Google Scholar]
- Fischer H.S., Zernig G., Hauser K.F., Gerard C., Hersh L.B., Saria A. Neutral endopeptidase knockout induces hyperalgesia in a model of visceral pain, an effect related to bradykinin and nitric oxide. J. Mol. Neurosci. 2002;18:129–134. doi: 10.1385/JMN:18:1-2:129. [DOI] [PubMed] [Google Scholar]
- Fu Y., Li A.F., Shi J.J., Tang M.N., Guo Y.B., Zhao Z.H. Lack of association of neprilysin gene polymorphisms with Alzheimer’s disease in a southern Chinese community. Int. Psychogeriatr. 2009;21:354–358. doi: 10.1017/S1041610208008338. [DOI] [PubMed] [Google Scholar]
- Fukami S., Watanabe K., Iwata N., Haraoka J., Lu B., Gerard N.P., Gerard C., Fraser P., Westaway D., St George-Hyslop P., Saido T.C. Aβ-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Aβ pathology. Neurosci. Res. 2002;43:39–56. doi: 10.1016/s0168-0102(02)00015-9. [DOI] [PubMed] [Google Scholar]
- Fulcher I.S., Chaplin M.F., Kenny A.J. Endopeptidase-24.11 purified from pig intestine is differently glycosylated from that in kidney. Biochem. J. 1983;215:317–323. doi: 10.1042/bj2150317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I.S., Kenny A.J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem. J. 1983;211:743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I.S., Matsas R., Turner A.J., Kenny A.J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem. J. 1982;203:519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete M.D., Rubio A., Durán-Prado M., Avila J., Luque R.M., Castaño J.P. Expression of Somatostatin, cortistatin, and their receptors, as well as dopamine receptors, but not of neprilysin, are reduced in the temporal lobe of Alzheimer’s disease patients. J. Alzheimers Dis. 2010;20:465–475. doi: 10.3233/JAD-2010-1385. [DOI] [PubMed] [Google Scholar]
- Ganju R.K., Shpektor R.G., Brenner D.G., Shipp M.A. CD10/neutral endopeptidase 24.11 is phosphorylated by casein kinase II and coassociates with other phosphoproteins including the lyn src-related kinase. Blood. 1996;88:4159–4165. doi: 10.1182/blood.V88.11.4159.bloodjournal88114159. [DOI] [PubMed] [Google Scholar]
- Genova R.M., Meyer K.J., Anderson M.G., Harper M.M., Pieper A.A. Neprilysin inhibition promotes corneal wound healing. Sci. Rep. 2018;8:14385. doi: 10.1038/s41598-018-32773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaddar G., Ruchon A.F., Carpentier M., Marcinkiewicz M., Seidah N.G., Crine P., Desgroseillers L., Boileau G. Molecular cloning and biochemical characterization of a new mouse testis soluble-zinc-metallopeptidase of the neprilysin family. Biochem. J. 2000;347:419–429. doi: 10.1042/0264-6021:3470419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M., Motta-Mejia C., Kandzija N., Cooke W., Zhang W., Cerdeira A.S., Bastie C., Redman C., Vatish M. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension. 2019;73:1112–1119. doi: 10.1161/HYPERTENSIONAHA.119.12707. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Trivedi M., Speth R.C. Alterations in Gene Expression of Components of the Renin-Angiotensin System and Its Related Enzymes in Lung Cancer. Lung Cancer Int. 2017;2017 doi: 10.1155/2017/6914976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin S., Mari B., Maulon L., Belhacène N., Marguet D., Auberger P. CD10 plays a specific role in early thymic development. FASEB J. 1997;11:376–381. doi: 10.1096/fasebj.11.5.9141505. [DOI] [PubMed] [Google Scholar]
- Gutta S., Grobe N., Kumbaji M., Osman H., Saklayen M., Li G., Elased K.M. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. Am. J. Physiol. Renal. Physiol. 2018;315:F263–F274. doi: 10.1152/ajprenal.00565.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez D., Huang J.Y., Huynh A.M., Valtierra S., Rockenstein E., Bruno A.M., Lu B., DesGroseillers L., Masliah E., Marr R.A. Neprilysin-2 is an important β-amyloid degrading enzyme. Am. J. Pathol. 2011;178:306–312. doi: 10.1016/j.ajpath.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L.R., Hafez D., Svitak A.L., Burns R.B., Li X., Frey W.H., 2nd, Marr R.A. Intranasal phosphoramidon increases β-amyloid levels in wild-type and NEP/NEP2-deficient mice. J. Mol. Neurosci. 2011;43:424–427. doi: 10.1007/s12031-010-9460-8. [DOI] [PubMed] [Google Scholar]
- Hara H., Oh-hashi K., Yoneda S., Shimazawa M., Inatani M., Tanihara H., Kiuchi K. Elevated neprilysin activity in vitreous of patients with proliferative diabetic retinopathy. Mol. Vis. 2006;12:977–982. PMID: 16943769. [PubMed] [Google Scholar]
- Hayashi M., Yamashita A., Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 1997;749:283–289. doi: 10.1016/S0006-8993(96)01317-0. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E., Ravid R., Nordberg A. Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiol. Aging. 2006;29:210–221. doi: 10.1016/j.neurobiolaging.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Helisalmi S., Hiltunen M., Vepsäläinen S., Iivonen S., Mannermaa A., Lehtovirta M., Koivisto A.M., Alafuzoff I., Soininen H. Polymorphisms in neprilysin gene affect the risk of Alzheimer’s disease in Finnish patients. J. Neurol. Neurosurg. Psychiatry. 2004;75:1746–1748. doi: 10.1136/jnnp.2004.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y., Hashiguchi A., Yuan J., Yoshimura A., Mitsui J., Ishiura H., Tanaka M., Ishihara S., Tanabe H., Nozuma S., Okamoto Y., Matsuura E., Ohkubo R., Inamizu S., Shiraishi W., Yamasaki R., Ohyagi Y., Kira J., Oya Y., Yabe H., Nishikawa N., Tobisawa S., Matsuda N., Masuda M., Kugimoto C., Fukushima K., Yano S., Yoshimura J., Doi K., Nakagawa M., Morishita S., Tsuji S., Takashima H. Mutations in MME cause an autosomal-recessive Charcot-Marie-Tooth disease type 2. Ann. Neurol. 2016;79:659–672. doi: 10.1002/ana.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Beckett C., Belyaev N.D., Turner A.J. The impact of amyloid precursor protein signalling and histone deacetylase inhibition on neprilysin expression in human prostate cells. Int. J. Cancer. 2012;130:775–786. doi: 10.1002/ijc.26028. [DOI] [PubMed] [Google Scholar]
- Howell S., Nalbantoglu J., Crine P. Neutral endopeptidase can hydrolyze β-amyloid (1-40) but shows no effect on β-amyloid precursor protein metabolism. Peptides. 1995;16:647–652. doi: 10.1016/0196-9781(95)00021-b. [DOI] [PubMed] [Google Scholar]
- Huang J.Y., Hafez D.M., James B.D., Bennett D.A., Marr R.A. Altered NEP2 expression and activity in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012;28:433–441. doi: 10.3233/JAD-2011-111307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttenrauch M., Baches S., Gerth J., Bayer T.A., Weggen S., Wirths O. Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015;44:1291–1302. doi: 10.3233/JAD-142463. [DOI] [PubMed] [Google Scholar]
- Imai K., Kanzaki H., Fujiwara H., Maeda M., Ueda M., Suginami H., Mori T. Expression and localization of aminopeptidase N, neutral endopeptidase, and dipeptidyl peptidase IV in the human placenta and fetal membranes. Am. J. Obstet. Gynecol. 1994;170:1163–1168. doi: 10.1016/s0002-9378(94)70115-6. [DOI] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Watanabe K., Seikiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.-J., Hama E., Sekine-Aizawa Y., Saido T.C. Identification of the major Aβ1-42 degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nature Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H., Saido T.C. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N., Mizukami H., Shirotani K., Takaki Y., Muramatsu S., Lu B., Gerard N.P., Gerard C., Ozawa K., Saido T.C. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-β peptide in mouse brain. J. Neurosci. 2004;24:991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.R., Gray L.D., Youngblood E., Sullivan J. Neutral endopeptidase from nuchal ligament of fetal calves. J. Cell. Biochem. 1990;43:243–254. doi: 10.1002/jcb.240430305. [DOI] [PubMed] [Google Scholar]
- Johnson A.R., Skidgel R.A., Gafford J.T., Erdös E.G. Enzymes in placental microvilli: angiotensin I converting enzyme, angiotensinase A, carboxypeptidase, and neutral endopeptidase ("enkephalinase") Peptides. 1984;5:789–796. doi: 10.1016/0196-9781(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Jang T.J., Park J.B., Lee J.I. The Expression of CD10 and CD15 Is Progressively Increased during Colorectal Cancer Development. Korean J. Pathol. 2013;47:340–347. doi: 10.4132/KoreanJPathol.2013.47.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kario K. The Sacubitril/Valsartan, a First-in-Class, Angiotensin Receptor Neprilysin Inhibitor (ARNI): Potential Uses in Hypertension, Heart Failure, and Beyond. Curr. Cardiol. Rep. 2018;20:5. doi: 10.1007/s11886-018-0944-4. [DOI] [PubMed] [Google Scholar]
- Kerr M.A., Kenny A.J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem. J. 1974;137:477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge C., Kozlova D.I., Nalivaeva N.N., Turner A.J. Hypoxia Affects Neprilysin Expression Through Caspase Activation and an APP Intracellular Domain-dependent Mechanism. Front. Neurosci. 2015;9:426. doi: 10.3389/fnins.2015.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Han D., Byun S.H., Kwon M., Cho S.J., Koh Y.H., Yoon K. Neprilysin facilitates adipogenesis through potentiation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Mol. Cell. Biochem. 2017;430:1–9. doi: 10.1007/s11010-017-2948-6. [DOI] [PubMed] [Google Scholar]
- King K.A., Hua J., Torday J.S., Drazen J.M., Graham S.A., Shipp M.A., Sunday M.E. CD10/neutral endopeptidase 24.11 regulates fetal lung growth and maturation in utero by potentiating endogenous bombesin-like peptides. J. Clin. Invest. 1997;91:1969–1973. doi: 10.1172/JCI116417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C., Crine P., Matsas R. Endopeptidase-24.11 is suppressed in myelin-forming but not in non-myelin-forming Schwann cells during development of the rat sciatic nerve. Neuroscience. 1992;50:69–83. doi: 10.1016/0306-4522(92)90382-c. [DOI] [PubMed] [Google Scholar]
- Kioussi C., Mamalaki A., Jessen K., Mirsky R., Hersh L.B., Matsas R. Expression of endopeptidase-24.11 (common acute lymphoblastic leukaemia antigen CD10) in the sciatic nerve of the adult rat after lesion and during regeneration. Eur. J. Neurosci. 1995;7:951–961. doi: 10.1111/j.1460-9568.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Kiss A., Kowalski J., Melzig M.F. Induction of neutral endopeptidase activity in PC-3 cells by an aqueous extract of Epilobium angustifolium L. and oenothein B. Phytomedicine. 2006;13:284–289. doi: 10.1016/j.phymed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kletsas D., Caselgrandi E., Barbieri D., Stathakos D., Franceschi C., Ottaviani E. Neutral endopeptidase-24.11 (NEP) activity in human fibroblasts during development and ageing. Mech. Ageing Dev. 1998;102:15–23. doi: 10.1016/s0047-6374(98)00003-7. [DOI] [PubMed] [Google Scholar]
- Komiyama T., Aoyagi T., Takeuchi T., Umezawa H. Inhibitory effects of phosphoramidon on neutral metalloendopeptidases and its application on affinity chromatography. Biochem. Biophys. Res. Commun. 1975;65:352–357. doi: 10.1016/s0006-291x(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Kozlova D.I., Vasylev D.S., Dubrovskaya N.M., Nalivaeva N.N., Tumanova N.L., Zhuravin I.A. Role of caspase-3 in regulation of the amyloid-degrading neuropeptidase neprilysin level in the rat cortex after hypoxia. J. Evol. Biochem. Physiol. 2015;51:480–484. doi: 10.1134/S0022093015060046. [DOI] [PubMed] [Google Scholar]
- Kumar G.K., Runold M., Ghai R.D., Cherniack N.S., Prabhakar N.R. Occurrence of neutral endopeptidase activity in the cat carotid body and its significance in chemoreception. Brain Res. 1990;517:341–343. doi: 10.1016/0006-8993(90)91047-k. [DOI] [PubMed] [Google Scholar]
- Lafrance M.H., Vézina C., Wang Q., Boileau G., Crine P., Lemay G. Role of glycosylation in transport and enzymic activity of neutral endopeptidase-24.11. Biochem. J. 1994;302:451–454. doi: 10.1042/bj3020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O., Robinson J., Tang Y.P., Hairston I.S., Korade-Mirnics Z., Lee V.M., Hersh L.B., Sapolsky R.M., Mirnics K., Sisodia S.S. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Le Bien T.W., McCormack R.T. The common acute lymphoblastic leukaemia antigen (CD10) – emancipation from a functional enigma. Blood. 1989;73:625–634. [PubMed] [Google Scholar]
- Lebouvier T., Chen Y., Duriez P., Pasquier F., Bordet R. Antihypertensive agents in Alzheimer’s disease: beyond vascular protection. Expert Rev. Neurother. 2020;20:175–187. doi: 10.1080/14737175.2020.1708195. [DOI] [PubMed] [Google Scholar]
- Leoni L.M., Losa G.A. Changes in membrane enzymes and glycosphingolipids in lymphocytes from HIV-1--infected and noninfected intravenous drug users. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;11:188–197. doi: 10.1097/00042560-199602010-00011. [DOI] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J.B.L., Onizuka R.J., Quackenbush E.J., Jongeneel C.V., McInnes R.R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J. Exp. Med. 1988;168:1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Booze R.M., Hersh L.B. Tissue-specific expression of rat neutral endopeptidase (neprilysin) mRNAs. J. Biol. Chem. 1995;270:5723–5728. doi: 10.1074/jbc.270.11.5723. [DOI] [PubMed] [Google Scholar]
- Li X.M., Moutquin J.M., Deschênes J., Bourque L., Marois M., Forest J.C. Increased immunohistochemical expression of neutral metalloendopeptidase (enkephalinase; EC 3.4.24.11) in villi of the human placenta with pre-eclampsia. Placenta. 1995;16:435–445. doi: 10.1016/0143-4004(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Li W., Wu Y., Min F., Li Z., Huang J., Huang R. A nonhuman primate model of Alzheimer’s disease generated by intracranial injection of amyloid-β42 and thiorphan. Metab. Brain Dis. 2010;25:277–284. doi: 10.1007/s11011-010-9207-9. [DOI] [PubMed] [Google Scholar]
- Lilius L., Forsell C., Axelman K., Winblad B., Graff C., Tjernberg L. No association between polymorphisms in the neprilysin promoter region and Swedish Alzheimer’s disease patients. Neurosci. Lett. 2003;337:111–113. doi: 10.1016/s0304-3940(02)01300-9. [DOI] [PubMed] [Google Scholar]
- Liu Y., Studzinski C., Beckett T., Guan H., Hersh M.A., Murphy M.P., Klein R., Hersh L.B. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol. Ther. 2009;17:1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Studzinski C., Beckett T., Murphy M.P., Klein R.L., Hersh L.B. Circulating neprilysin clears brain amyloid. Molec. Cell. Neurosci. 2010;45:101–107. doi: 10.1016/j.mcn.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens C., Malfroy B., Schwartz J.C., Gacel G., Roques B.P., Roy J., Morgat J.L., Javoy-Agid F., Agid Y. Enkephalin dipeptidyl carboxypeptidase (enkephalinase) activity: selective radioassay, properties, and regional distribution in human brain. J Neurochem. 1982;39:1081–1089. doi: 10.1111/j.1471-4159.1982.tb11500.x. [DOI] [PubMed] [Google Scholar]
- Louhichi T., Saad H., Dhiab M.B., Ziadi S., Trimeche M. Stromal CD10 expression in breast cancer correlates with tumor invasion and cancer stem cell phenotype. BMC Cancer. 2018;18:49. doi: 10.1186/s12885-017-3951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Gerard N.P., Kolakowski L.F. Jr., Bozza M., Zurakowski D., Finco O., Carroll M.C., Gerard C. Neutral endopeptidase modulation of septic shock. J. Exp. Med. 1995;181:2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R., Poirier R., Wolfer D.P., Welzl H., Groscurth P., Lipp H.P., Lu B., El Mouedden M., Mercken M., Nitsch R.M., Mohajeri M.H. Lack of neprilysin suffices to generate murine amyloid-like deposits in the brain and behavioral deficit in vivo. J. Neurosci. Res. 2006;84:1871–1878. doi: 10.1002/jnr.21074. [DOI] [PubMed] [Google Scholar]
- Maetzler W., Stoycheva V., Schmid B., Schulte C., Hauser A.K., Brockmann K., Melms A., Gasser T., Berg D. Neprilysin activity in cerebrospinal fluid is associated with dementia and amyloid-β42 levels in Lewy body disease. J. Alzheimers Dis. 2010;22:933–938. doi: 10.3233/JAD-2010-101197. [DOI] [PubMed] [Google Scholar]
- Maguer-Satta V., Besançon R., Bachelard-Cascales E. Concise review: neutral endopeptidase (CD10): a multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells. 2011;29:389–396. doi: 10.1002/stem.592. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Kuang W.J., Seeburg P.H., Mason A.J., Schofield P.R. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase) FEBS Lett. 1988;229:206–210. doi: 10.1016/0014-5793(88)80828-7. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P.R., Kuang W.J., Seeburg P.H., Mason A.J., Henzel W.J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem. Biophys. Res. Commun. 1987;144:59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Manolis A.S., Manolis T.A., Manolis A.A., Melita H. The Controversy of Renin-Angiotensin-System Blocker Facilitation Versus Countering COVID-19 Infection. J Cardiovasc Pharmacol. 2020 doi: 10.1097/FJC.0000000000000894. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Marr R.A., Rockenstein E., Mukherjee A., Kindy M.S., Hersh L.B., Gage F.H., Verma I.M., Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. PMCID: PMC6742010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Maza O., Crabb E., Mitsuyasu R.T., Fahey J.L., Giorgi J.V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 1987;138:3720–3724. PMID: 2953790. [PubMed] [Google Scholar]
- Maruyama M., Higuchi M., Takaki Y., Matsuba Y., Tanji H., Nemoto M., Tomita N., Matsui T., Iwata N., Mizukami H., Muramatsu S., Ozawa K., Saido T.C., Arai H., Sasaki H. Cerebrospinal fluid neprilysin is reduced in prodromal Alzheimer’s disease. Ann. Neurol. 2005;57:832–842. doi: 10.1002/ana.20494. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I.S., Kenny A.J., Turner A.J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc. Natl. Acad. Sci. USA. 1983;80:3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A.J., Turner A.J. An immunohistochemical study of endopeptidase-24.11 ("enkephalinase") in the pig nervous system. Neuroscience. 1986;18:991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Menéndez L., Hidalgo A., Meana A., Poras H., Fournié-Zaluski M.C., Roques B.P., Baamonde A. Inhibition of osteosarcoma-induced thermal hyperalgesia in mice by the orally active dual enkephalinase inhibitor PL37. Potentiation by gabapentin. Eur J Pharmacol. 2008;596:50–55. doi: 10.1016/j.ejphar.2008.07.043. [DOI] [PubMed] [Google Scholar]
- Miners J.S., van Helmond Z., Kehoe P.G., Love S. Changes with age in the activities of β-secretase and the Aβ-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 2010;20:794–802. doi: 10.1111/j.1750-3639.2010.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri M.H., Wolfer D.P. Neprilysin deficiency-dependent impairment of cognitive functions in a mouse model of amyloidosis. Neurochem. Res. 2009;34:717–726. doi: 10.1007/s11064-009-9919-6. [DOI] [PubMed] [Google Scholar]
- Morales-Corraliza J., Wong H., Mazzella M.J., Che S., Lee S.H., Petkova E., Wagner J.D., Hemby S.E., Ginsberg S.D., Mathews P.M. Brain-Wide Insulin Resistance, Tau Phosphorylation Changes, and Hippocampal Neprilysin and Amyloid-β Alterations in a Monkey Model of Type 1 Diabetes. J. Neurosci. 2016;36:4248–4258. doi: 10.1523/JNEUROSCI.4640-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki N., Moriwaki S., Sugiyama-Nakagiri Y., Haketa K., Takema Y., Imokawa G. Neprilysin is identical to skin fibroblast elastase: its role in skin aging and UV responses. J. Biol. Chem. 2010;285:39819–39827. doi: 10.1074/jbc.M110.161547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothapo K.M., Stelma F., Janssen M., Kessels R., Miners S., Verbeek M.M., Koopmans P., van der Ven A. Amyloid β-42 (Aβ-42), neprilysin and cytokine levels. A pilot study in patients with HIV related cognitive impairments. J. Neuroimmunol. 2015;282:73–79. doi: 10.1016/j.jneuroim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Mouri A., Zou L.B., Iwata N., Saido T.C., Wang D., Wang M.W., Noda Y., Nabeshima T. Inhibition of neprilysin by thiorphan (i.c.v.) causes an accumulation of amyloid β and impairment of learning and memory. Behav. Brain Res. 2006;168:83–91. doi: 10.1016/j.bbr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Muangman P., Spenny M.L., Tamura R.N., Gibran N.S. Fatty acids and glucose increase neutral endopeptidase activity in human microvascular endothelial cells. Shock. 2003;19:508–512. doi: 10.1097/01.shk.0000055815.40894.16. [DOI] [PubMed] [Google Scholar]
- Muenzner M., Tappenbeck N., Gembardt F., Rülke R., Furkert J., Melzig M.F., Siems W.E., Brockmann G.A., Walther T. Green tea reduces body fat via upregulation of neprilysin. Int. J. Obes. (Lond). 2016;40:1850–1855. doi: 10.1038/ijo.2016.172. [DOI] [PubMed] [Google Scholar]
- Nagata K., Mano T., Murayama S., Saido T.C., Iwata A. DNA methylation level of the neprilysin promoter in Alzheimer’s disease brains. Neurosci. Lett. 2018;670:8–13. doi: 10.1016/j.neulet.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Nalivaeva N.N., Belyaev N.D., Zhuravin I.A., Turner A.J. The Alzheimer’s amyloid-degrading peptidase, neprilysin: can we control it? Int. J. Alzheimers Dis. 2012;2012 doi: 10.1155/2012/383796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva N.N., Belyaev N.D., Lewis D.I., Pickles A.R., Makova N.Z., Bagrova D.I., Dubrovskaya N.M., Plesneva S.A., Zhuravin I.A., Turner A.J. Effect of sodium valproate administration on brain neprilysin expression and memory in rats. J. Mol. Neurosci. 2012;46:569–577. doi: 10.1007/s12031-011-9644-x. [DOI] [PubMed] [Google Scholar]
- Nalivaeva N.N., Fisk L., Kochkina E.G., Plesneva S.A., Zhuravin I.A., Babusikova E., Dobrota D., Turner A.J. Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann. N. Y. Acad. Sci. 2004;1035:21–33. doi: 10.1196/annals.1332.002. [DOI] [PubMed] [Google Scholar]
- Nalivaeva N.N., Turner A.J. Targeting amyloid clearance in Alzheimer’s disease as a therapeutic strategy. Br. J. Pharmacol. 2019;176:3447–3463. doi: 10.1111/bph.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva N.N., Turner A.J., Zhuravin I.A. Role of Prenatal Hypoxia in Brain Development, Cognitive Functions, and Neurodegeneration. Front Neurosci. 2018;12:825. doi: 10.3389/fnins.2018.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A.J., Sue L.I., Scott S., Rauschkolb P.K., Walker D.G., Potter P.E., Beach T.G. Thiorphan-induced neprilysin inhibition raises amyloid β levels in rabbit cortex and cerebrospinal fluid. Neurosci. Lett. 2003;350:178–180. doi: 10.1016/s0304-3940(03)00902-9. [DOI] [PubMed] [Google Scholar]
- Nilsson P.1, Loganathan K., Sekiguchi M., Winblad B., Iwata N., Saido T.C., Tjernberg L.O. Loss of neprilysin alters protein expression in the brain of Alzheimer’s disease model mice. Proteomics. 2015;15:3349–3355. doi: 10.1002/pmic.201400211. [DOI] [PubMed] [Google Scholar]
- Papandreou C.N., Usmani B., Geng Y.P., Bogenrieder T., Freeman R., Wilk S., Finstad C.L., Reuter V.E., Powell C.T., Scheinberg D., Magill C., Scher H.I., Albino A.P., Nanus D.M. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nature Med. 1998;4:50–57. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- Pacheco-Quinto J., Eckman C.B., Eckman E.A. Major amyloid-β-degrading enzymes, endothelin-converting enzyme-2 and neprilysin, are expressed by distinct populations of GABAergic interneurons in hippocampus and neocortex. Neurobiol. Aging. 2016;48:83–92. doi: 10.1016/j.neurobiolaging.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi-Piquard R., Petit A., Kawarai T., Sunyach C., Alves da Costa C., Vincent B., Ring S., D’Adamio L., Shen J., Müller U., St George Hyslop P., Checler F. Presenilin-dependent transcriptional control of the Aβ-degrading enzyme neprilysin by intracellular domains of βAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Parilla J.H., Hull R.L., Zraika S. Neprilysin Deficiency Is Associated With Expansion of Islet β-Cell Mass in High Fat-Fed Mice. J. Histochem. Cytochem. 2018;66:523–530. doi: 10.1369/0022155418765164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen F., Nyman T., Ammälä M., Rutanen E.M. Decreased expression of messenger RNAs encoding endothelin receptors and neutral endopeptidase 24.11 in endometrial cancer. Br. J. Cancer. 1995;71:59–63. doi: 10.1038/bjc.1995.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Sánchez M., Prieto I., Segarra A.B., Martínez-Cañamero M., Banegas I., de Gasparo M. Enkephalinase regulation. Vitam. Horm. 2019;111:105–129. doi: 10.1016/bs.vh.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Rempel H.C., Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid β. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- Roques B.P. Contribution of Delta-Opioid Receptors to Pathophysiological Events Explored by Endogenous Enkephalins. Handb Exp Pharmacol. 2018;247:53–70. doi: 10.1007/164_2016_17. [DOI] [PubMed] [Google Scholar]
- Roques B.P., Beaumont A. Neutral endopeptidase-24.11 inhibitors: from analgesics to antihypertensives. Trends Pharmacol. Sci. 1990;11:245–249. doi: 10.1016/0165-6147(90)90252-4. [DOI] [PubMed] [Google Scholar]
- Roques B.P., Fournié-Zaluski M.C., Florentin D., Waksman G., Sassi A., Chaillet P., Collado H., Costentin J. New enkephalinase inhibitors as probes to differentiate "enkephalinase" and angiotensin-converting-enzyme active sites. Life Sci. 1982;31:1749–1752. doi: 10.1016/0024-3205(82)90201-6. [DOI] [PubMed] [Google Scholar]
- Rougeot C., Messaoudi M., Hermitte V., Rigault A.G., Blisnick T., Dugave C., Desor D., Rougeon F. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc. Natl. Acad. Sci. USA. 2003;100:8549–8554. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchon A.F., Marcinkiewicz M., Ellefsen K., Basak A., Aubin J., Crine P., Boileau G. Cellular localization of neprilysin in mouse bone tissue and putative role in hydrolysis of osteogenic peptides. J. Bone Min. Res. 2000;15:1266–1274. doi: 10.1359/jbmr.2000.15.7.1266. [DOI] [PubMed] [Google Scholar]
- Saito T., Iwata N., Tsubuki S., Takaki Y., Takano J., Huang S.M., Suemoto T., Higuchi M., Saido T.C. Somatostatin regulates brain amyloid β peptide Aβ42 through modulation of proteolytic degradation. Nat. Med. 2005;11:434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- Sales N., Dutriez I., Maziere B., Ottaviani M., Roques B.P. Neutral endopeptidase 24.11 in rat peripheral tissues: comparative localization by’ ex vivo’ and’ in vitro’ autoradiography. Regul. Pept. 1991;33:209–222. doi: 10.1016/0167-0115(91)90215-3. [DOI] [PubMed] [Google Scholar]
- Sandoval K.E., Farr S.A., Banks W.A., Crider A.M., Morley J.E., Witt K.A. Somatostatin receptor subtype-4 agonist NNC 26-9100 decreases extracellular and intracellular Aβ₁−₄₂ trimers. Eur. J. Pharmacol. 2012;683:116–124. doi: 10.1016/j.ejphar.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A.N., Wulfanger J., Helbing G., Blosz T., Langner J., Riemann D. Two C-terminal cysteines are necessary for proper folding of the peptidase neprilysin/CD10. Biochem. Biophys. Res. Commun. 2002;295:423–427. doi: 10.1016/s0006-291x(02)00675-7. [DOI] [PubMed] [Google Scholar]
- Saria A., Hauser K.F., Traurig H.H., Turbek C.S., Hersh L., Gerard C. Opioid-related changes in nociceptive threshold and in tissue levels of enkephalins after target disruption of the gene for neutral endopeptidase (EC 3.4.24.11) in mice. Neurosci. Lett. 1997;234:27–30. doi: 10.1016/s0304-3940(97)00660-5. [DOI] [PubMed] [Google Scholar]
- Seferovic J.P., Claggett B., Seidelmann S.B., Seely E.W., Packer M., Zile M.R., Rouleau J.L., Swedberg K., Lefkowitz M., Shi V.C., Desai A.S., McMurray J.J.V., Solomon S.D. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R.Q., Sumitomo M., Dai J., Hardy D.O., Navarro D., Usmani B., Papandreou C.N., Hersh L.B., Shipp M.A., Freedman L.P., Nanus D.M. Identification and characterization of two androgen response regions in the human neutral endopeptidase gene. Mol. Cell. Endocrinol. 2000;170:131–142. doi: 10.1016/s0303-7207(00)00326-9. [DOI] [PubMed] [Google Scholar]
- Shi X., Zhang W., Nian X., Lu X., Li Y., Liu F., Wang F., He B., Zhao L., Zhu Y., Ren S., Sun Y. The previously uncharacterized lncRNA APP promotes prostate cancer progression by acting as a competing endogenous RNA. Int. J. Cancer. 2020;146:475–486. doi: 10.1002/ijc.32422. [DOI] [PubMed] [Google Scholar]
- Shipp M.A., Tarr G.E., Chen C.Y., Switzer S.N., Hersh L.B., Stein H., Sunday M.E., Reinherz E.L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc. Natl. Acad. Sci. USA. 1991;88:10662–10666. doi: 10.1073/pnas.88.23.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems W., Maul B., Krause W., Gerard C., Hauser K.F., Hersh L.B., Fischer H.S., Zernig G., Saria A. Neutral endopeptidase and alcohol consumption, experiments in neutral endopeptidase-deficient mice. Eur. J. Pharmacol. 2000;397:327–334. doi: 10.1016/s0014-2999(00)00222-3. [DOI] [PubMed] [Google Scholar]
- Siepmann M., Kumar S., Mayer G., Walter J. Casein kinase 2 dependent phosphorylation of neprilysin regulates receptor tyrosine kinase signaling to Akt. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spenny M.L., Muangman P., Sullivan S.R., Bunnett N.W., Ansel J.C., Olerud J.E., Gibran N.S. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10:295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- Spencer-Dene B., Thorogood P., Nair S., Kenny A.J., Harris M., Henderson B. Distribution of, and a putative role for, the cell-surface neutral metallo-endopeptidases during mammalian craniofacial development. Development. 1994;120:3213–3226. doi: 10.1242/dev.120.11.3213. PMID: 7720564. [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., Sicuteri F., Salmon S., Malfroy B. Characterization of endopeptidase 3.4.24.11 ("enkephalinase") activity in human plasma and cerebrospinal fluid. Biochem. Pharmacol. 1990;39:1353–1356. doi: 10.1016/0006-2952(90)90012-a. [DOI] [PubMed] [Google Scholar]
- Standeven K.F., Hess K., Carter A.M., Rice G.I., Cordell P.A., Balmforth A.J., Lu B., Scott D.J., Turner A.J., Hooper N.M., Grant P.J. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 2010;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo M., Shen R., Nanus D.M. Involvement of neutral endopeptidase in neoplastic progression. Biochim. Biophys. Acta. 2005;1751:52–59. doi: 10.1016/j.bbapap.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Sunday M.E., Hua J., Torday J.S., Reyes B., Shipp M.A. CD10/neutral endopeptidase 24.11 in developing human fetal lung. Patterns of expression and modulation of peptide-mediated proliferation. J. Clin. Invest. 1992;90:2517–2525. doi: 10.1172/JCI116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao C.C., Hsu W.L., Ma Y.L., Cheng S.J., Lee E.H. Epigenetic regulation of HDAC1 SUMOylation as an endogenous neuroprotection against Aβ toxicity in a mouse model of Alzheimer’s disease. Cell Death Differ. 2017;24:597–614. doi: 10.1038/cdd.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi M., Kajiyama H., Shibata K., Ino K., Mizutani S., Kikkawa F. Anti-progressive effect of neutral endopeptidase 24.11 (NEP/CD10) on cervical carcinoma in vitro and in vivo. Oncology. 2005;69:52–62. doi: 10.1159/000087476. [DOI] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde E., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Torlakovic E., Tenstad E., Funderud S., Rian E. CD10+ stromal cells form B-lymphocyte maturation niches in the human bone marrow. J. Pathol. 2005;205:311–317. doi: 10.1002/path.1705. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Isaac R.E., Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Nalivaeva N.N. New insights into the roles of metalloproteinases in neurodegeneration and neuroprotection. Int. Rev. Neurobiol. 2007;82:113–135. doi: 10.1016/S0074-7742(07)82006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani B.A., Shen R., Janeczko M., Papandreou C.N., Lee W.H., Nelson W.G., Nelson J.B., Nanus D.M. Methylation of the neutral endopeptidase gene promoter in human prostate cancers. Clin. Cancer Res. 2000;6:1664–1670. PMID: 10815884. [PubMed] [Google Scholar]
- Vasilev D.S., Dubrovskaya N.M., Nalivaeva N.N., Zhuravin I.A. Regulation of caspase-3 content and activity in rat cortex in norm and after prenatal hypoxia. Neurochem. J. 2016;10:144–150. doi: 10.1134/S1819712416020100. [DOI] [Google Scholar]
- Velazquez E.F., Yancovitz M., Pavlick A., Berman R., Shapiro R., Bogunovic D., O’Neill D., Yu Y.L., Spira J., Christos P.J., Zhou X.K., Mazumdar M., Nanus D.M., Liebes L., Bhardwaj N., Polsky D., Osman I. Clinical relevance of neutral endopeptidase (NEP/CD10) in melanoma. J. Transl. Med. 2007;5:2. doi: 10.1186/1479-5876-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T., Albrecht D., Becker M., Schubert M., Kouznetsova E., Wiesner B., Maul B., Schliebs R., Grecksch G., Furkert J., Sterner-Kock A., Schultheiss H.P., Becker A., Siems W.E. Improved learning and memory in aged mice deficient in amyloid β-degrading neutral endopeptidase. PLoS One. 2009;4:e4590. doi: 10.1371/journal.pone.0004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.S., Lipton R.B., Katz M.J., Davies P., Buschke H., Kuslansky G., Verghese J., Younkin S.G., Eckman C., Dickson D.W. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J. Neuropathol. Exp. Neurol. 2005;64:378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- Wang H.Z., Bi R., Zhang D.F. Neprilysin Confers Genetic Susceptibility to Alzheimer’s Disease in Han Chinese. Mol. Neurobiol. 2016;53:4883–4892. doi: 10.1007/s12035-015-9411-z. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang R., Chen L., Bennett D.A., Dickson D.W., Wang D.S. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer’s brain. J. Neurochem. 2010;115:47–57. doi: 10.1111/j.1471-4159.2010.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß E., Berger H.M., Brandl W.T., Strutz J., Hirschmugl B., Simovic V., Tam-Ammersdorfer C., Cvitic S., Hidden U. Maternal Overweight Downregulates MME (Neprilysin) in Feto-Placental Endothelial Cells and in Cord Blood. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21030834. pii: E834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyteside A.R., Turner A.J. Human neprilysin-2 (NEP2) and NEP display distinct subcellular localisations and substrate preferences. FEBS Lett. 2008;582:2382–2386. doi: 10.1016/j.febslet.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Wick M.J., Buesing E.J., Wehling C.A., Loomis Z.L., Cool C.D., Zamora M.R., Miller Y.E., Colgan S.P., Hersh L.B., Voelkel N.F., Dempsey E.C. Decreased Neprilysin and Pulmonary Vascular Remodeling in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2011;183:330–340. doi: 10.1164/rccm.201002-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner A., Dufour E., Messaoudi M., Nejdi A., Marcel A., Ungeheuer M.N., Rougeot C. Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. USA. 2006;103:17979–17984. doi: 10.1073/pnas.0605865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z.M., Sun L., Liu Y.M., Zhang J.J., Huang J. Estrogen regulation of the neprilysin gene through a hormone-responsive element. J. Mol. Neurosci. 2009;39:22–26. doi: 10.1007/s12031-008-9168-1. [DOI] [PubMed] [Google Scholar]
- Yang X.L., Liu Y.B. Advances in Pathobiology of Primary Central Nervous System Lymphoma. Chin. Med. J. (Engl). 2017;130:1973–1979. doi: 10.4103/0366-6999.211879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasojima K., McGeer E.G., McGeer P.L. Relationship between β amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- Zappulla J.P., DesGroseillers L. Neutral endopeptidase is expressed on the follicular granulosa cells of rabbit ovaries. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2001;129:863–870. doi: 10.1016/s1096-4959(01)00390-6. [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu D., Wang Y., Huang H., Zhao Y., Zhou H. Meta-analysis of expression and function of neprilysin in Alzheimer’s disease. Neurosci. Lett. 2017;657:69–76. doi: 10.1016/j.neulet.2017.07.060. [DOI] [PubMed] [Google Scholar]
- Zheng R., Horiguchi A., Iida K., Lee J., Shen R., Goodman O.B. Jr., Nanus D.M. Neutral endopeptidase is a myristoylated protein. Mol. Cell. Biochem. 2010;335:173–180. doi: 10.1007/s11010-009-0253-8. [DOI] [PubMed] [Google Scholar]
- Zou L.B., Mouri A., Iwata N., Saido T.C., Wang D., Wang M.W., Mizoguchi H., Noda Y., Nabeshima T. Inhibition of neprilysin by infusion of thiorphan into the hippocampus causes an accumulation of amyloid β and impairment of learning and memory. J. Pharmacol. Exp. Ther. 2006;317:334–340. doi: 10.1124/jpet.105.095687. [DOI] [PubMed] [Google Scholar]
- Zhuravin I.A., Dubrovskaya N.M., Vasilev D.S., Kozlova D.I., Kochkina E.G., Tumanova N.L., Nalivaeva N.N. Regulation of Neprilysin Activity and Cognitive Functions in Rats After Prenatal Hypoxia. Neurochem. Res. 2019;44:1387–1398. doi: 10.1007/s11064-019-02796-3. [DOI] [PubMed] [Google Scholar]
- Zhuravin I.А., Nalivaeva N.N., Kozlova D.I., Kochkina E.G., Fedorova Y.B., Gavrilova S.I. The activity of blood serum cholinesterases and neprilysin as potential biomarkers of mild-cognitive impairment and Alzheimer’s disease. Zh. Nevrol. Psikhiatr. Im. S.S. Korsakova. 2015;115:110–117. doi: 10.17116/jnevro2015115112110-117. [DOI] [PubMed] [Google Scholar]