Abstract
Survival following liver transplantation has changed dramatically owing to improvement in surgical techniques, peri-operative care and optimal immunosuppressive therapy. Post-Liver transplant (LT) de novo or recurrent viral infection continues to cause major allograft dysfunction, leading to poor graft and patient survival in untreated patients. Availability of highly effective antiviral drugs has significantly improved post-LT survival. Patients transplanted for chronic hepatitis B infection should receive life-long nucleos(t)ide analogues, with or without HBIg for effective viral control. Patients with chronic hepatitis C should be commenced on directly acting antiviral (DAA) drugs prior to transplantation. DAA therapy for post-LT recurrent hepatitis C infection is associated with close to 100% sustained virological response (SVR), irrespective of genotype. De novo chronic Hepatitis E infection is an increasingly recognised cause of allograft dysfunction in LT recipients. Untreated chronic HEV infection of the graft may lead to liver fibrosis and allograft failure. CMV and EBV can reactivate leading to systemic illness following liver transplantation. With COVID-19 pandemic, post-transplant patients are at risk of SARS-Co-V2 infection. Majority of the LT recipients require hospitalization, and the mortality in this population is around 20%. Early recognition of allograft dysfunction and identification of viral aetiology is essential in the management of post-LT de novo or recurrent infections. Optimising immunosuppression is an important step in reducing the severity of allograft damage in the treatment of post-transplant viral infections. Viral clearance or control can be achieved by early initiation of high potency antiviral therapy.
Keywords: Liver transplantation, de novo infection, Recurrent viral infection, Hepatitis B, Hepatitis C, COVID-19, Hepatitis E, CMV, EBV, HSV, HHV-6
Introduction
Liver transplantation (LT) is the only curative therapy for patients with decompensated cirrhosis, with an one year and 5-year survival of around 90% and 70%, respectively [1]. Survival following LT has improved over the years from a 1 and 5 year survival of 70% and 50%, to 90% and 70%, respectively. Significant improvement in surgical techniques, peri-operative care and immunosuppression therapy has translated in to better survival. However, increasing complexity of patients selection and expansion of indications for liver transplantation may influence post-transplant outcomes. Optimal immunosuppression is essential to maintain the balance between rejection and infection in the post-transplant period. Recurrent viral infection in the post-transplant has been a major obstacle in the previous era causing severe allograft dysfunction leading to graft failure and death.
There exists a wide geographical heterogeneity in the burden of chronic liver disease (CLD) across the globe, which may influence the indication and outcome of LT. Around 350 million people are affected by Hepatitis B infection (HBV) worldwide, with a higher prevalence in Asia and Africa (0.7–22.3%) compared to the West [2]. Likewise, around 200 million people suffer from chronic hepatitis C infection (HCV) globally, more common in central Asia and Japan [3]. Approximately, 80% of HCV infected patients develop chronic liver disease and 16–20% patients develop cirrhosis over 20 years depending on the host, viral and environmental factors [4]. Hepatic decompensation occurs in 3–6% annually and 15–20% in these patients over 3–5 years [4,5]. Chronic Hepatitis C infection is the most common indications for liver transplantation around the world.
Considering the long term immunosuppressive state, post-transplant patients are at a significant risk of ‘de novo’ viral infections, defined as a new onset viral illness in the absence of previous exposure. The source of de novo infection could be environmental or donor derived at the time of transplantation. In addition, there remains a significant risk of disease reactivation leading to ‘recurrence’ particularly in patients transplanted for viral hepatitis. Development of de novo or recurrent viral hepatitis can damage the liver allograft leading to a considerable impairment of graft and patient survival. Understanding the natural history of viral hepatitis in the LT recipients and the evolution of antiviral therapy has changed long term survival of these patients remarkably.
In this review, we focus on the prevalence, natural history and clinical outcomes of hepatitis C (rHCV), hepatitis B (rHBV), HEV and other viral infections such as CMV, HSV and EBV in LT recipients. In addition, given the current COVID-19 pandemic, we discuss impact of SARS-CoV-2 in the liver allograft and their outcomes.
Hepatitis C
Recurrent Hepatitis C (rHCV) infection of the liver allograft is universal in untreated patients transplanted for this indication. Allograft colonisation by viral particles occurs at the time of portal reperfusion inducing liver damage as early as 72 hours post-LT. A study on HCV viral kinetics demonstrated an initial sharp decline in the RNA levels within 24 h of reperfusion followed by a rapid increase to nearly 20 fold higher than the pre-transplant values during the first week [6]. Untreated rHCV may progress with necroinflammation, leading to allograft injury and fibrosis. In a study to evaluate the natural course of 183 HCV liver transplanted patients using protocol liver biopsies, fibrosis score progressed from 1.2 to 2.2 over a 10 year period. Post-LT patients with severe fibrosis at 1 year due to rHCV were associated with poor survival. Furthermore, donor age >33 years and HCV genotype 1 or 4 developed were associated with rapid fibrosis [7].
Post-transplant immunosuppression accelerates rHCV related liver damage with fibrosis progression at the rate of 0.3%–0.8% per year leading to cirrhosis and graft loss in 20–40% of patients within 5 years [8,9]. Up to 40% patients with rHCV develop hepatic decompensation within a year of cirrhosis [10]. These patients follow an accelerated course with a three year survival of only 10% following hepatic decompensation unlike 60% in those with native cirrhotic liver. Over all patients transplanted for HCV have a lower 3 and 5 year survival of 73% and 67%, respectively [11].
Rarely (<10%), rHCV can present in a severe form, Fibrosing cholestatic hepatitis (FCH), characterized by bilirubin >6 mg/dl, alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) >5x upper limit normal, high HCV RNA typically occurring in the first 6 months post-LT. Liver histology characterised by severe cholestasis, hepatocyte ballooning spotty necrosis and Kupffer cell hypertrophy. Progressive FCH is associated with severe allograft dysfunction leading to graft failure leading to death within 12 months. In summary, HCV LT recipients had worst long term survival until 5 years ago.
HCV viral clearance described as sustained virological response (SVR) defined as undetectable HCV RNA at 24 weeks following antiviral therapy, but recently reduced to 12 weeks (SVR12). SVR has a major implication in disease progression in rHCV. Similar to chronic hepatitis C in pre-transplant patients, achieving SVR in post-transplant patients results in stability of the disease or even regression of fibrosis in 75% of patients [12]. Whereas, failure to achieve SVR results in worsening of fibrosis. Unlike, in patients with chronic liver disease, fibrosis in rHCV progresses at faster pace. In patients untreated rHCV, fibrosis score 2 progress rapidly to cirrhosis and graft failure than patients with absent or mild fibrosis [13]. In a 10 year follow up Italian study of 358 LT patients for HCV, patients who did not achieve SVR had the worst 10-year survival 39.8% compared to 84.7% in those with SVR [14].
Risk factors for rHCV
A number of studies evaluated recipient, donor and virus related risk factors associated with rHCV. Advanced recipient age, diabetes mellitus, severe liver disease (Child Pugh >10), IL-28B polymorphism, high HCV RNA >107 IU/ml, ischemic/reperfusion injury, CMV, donor age >65 years, cold ischaemic time over 8 h and warm ischemia over 90 min, marginal graft, DCD donor, higher immunosuppression in particular high dose corticosteroids for acute cellular rejection, use of anti-thymocyte globulin were significantly associated with rHCV in the liver allograft [15,16].
Role of immunosuppression in rHCV
Calcineurin inhibitors (CNI) such as tacrolimus and cyclosporine are the most commonly used immunosuppressive drugs in LT recipients. Ideal immunosuppressive regimen in HCV LT recipients to avoid rejection and at the same time to reduce the risk of rHCV is not established. Corticosteroid therapy was considered to be the main drug associated with increased risk of rHCV. In particular, use of steroid boluses and rapid tapering has been shown to reactivate hepatitis C in the graft [17]. Various immunosuppressive modifications were adopted in the past to reduce rHCV of the allograft. Low dose slow steroid tapering has shown to reduce rHCV in the graft. A study by Berenguer et al., showed 29% rHCV in patients with CNI and tapering steroids over 9–12 months compared to 48% in the historical controls [18].
Studies attempted to eliminate corticosteroids using interleukin (IL-2) receptor antagonist induction regimens to reduce rHCV. In a prospective randomised multi-centre trial evaluation dacluzimab induction followed by tacrolimus and mycophenolate mofetil (MMF) (n = 153) vs standard treatment showed similar rates of rHCV, 33% vs 40% (P not significant) [19]. Similarly, a double blinded comparative study showed no difference in histological rHCV in patients receiving basiliximab vs steroid-cyclosporine (41% vs 37%) based immunosuppression [20].
Cyclosporine has been shown to inhibit HCV virus, by blocking cyclophyllin, a protein that aides viral binding and replication in vitro. However, human studies failed to demonstrate clinical benefit. A meta-analysis of nine studies with 1180 HCV transplanted patients on cyclosporine vs tacrolimus showed no difference in histological HCV recurrence (RR = 0.92, 95% CI: 0.71–1.19, P = 0.51), allograft loss due to rHCV (RR1.62, 95% CI: 0.64–4.07, P = 0.31) and mortality (RR = 0.98, 95% CI: 0.77–1.25, P = 0.87) [21].
Donation after circulatory death (DCD) organ and rHCV
With increasing demand for cadaveric organs, DCD organ harvest has been increasingly utilized. Studies showed increased rHCV in patients undergoing LT from a DCD compared to donation after brain death (DBD) liver, probably related to ischaemic reperfusion injury [22]. Interestingly, a large case control study on HCV patients who received DCD liver showed no significant differences in the HCV RNA titres (P = 0.7), severe rHCV with fibrosis (8% vs 15%, P = 0.38) and graft loss (5% vs 9%, P = 0.6) between the two groups at 12 months post-transplant [23].
Diagnosis
Previously, interpretation of abnormal LFTs in patients transplanted for HCV infection was difficult to distinguish between a rejection episode and rHCV infection, in the absence of liver biopsy. Therefore, management of graft dysfunction in HCV transplant recipients was a challenge. This led to the development of non-invasive markers of liver fibrosis. Fibroscan using transient elastography technique plays a major role in the assessment of disease severity in these patients [24].
Liver histology is definitive in the diagnosis and management of rHCV. This shows variable severities of portal tract lymphocytic infiltration, interface hepatitis, ductular reaction, lobular activity with mononuclear inflammatory infiltrate, necroinflammatory foci and apoptotic bodies. Nodular lymphoid aggregates may be present. FCH is characterised histologically by irregular portal expansion, portal fibrosis with immature pericellular/sinusoidal fibrous bands, extensive hepatocyte ballooning and degeneration, bile ductular reaction, marked canalicular and intracellular bilirubinostasis, and mild to moderate mononuclear inflammation. Confluent or bridging necrosis may be present. Varying stages of fibrosis depending on the disease severity.
HCV treatment in decompensated cirrhosis awaiting LT
Pre-LT HCV RNA level strongly predicts post-transplant rHCV severity. Therefore, treatment should be aimed at making the patient virus free prior to liver transplantation. SVR has consistently shown to improve several aspects of HCV related complications. Achieving SVR in patients awaiting liver transplantation has shown to decrease the rate of disease progression, symptomatic improvement, reduction in MELD score and importantly it improves post-transplant survival [25,26].
Treatment of HCV in the previous era
A decade ago, the treatment of HCV in patients with decompensated cirrhosis was nearly impossible. In the absence of advanced liver disease, the use of Peg-IFN and ribavirin achieved 50–70% SVR depending on the genotype. Unfortunately, both these drugs are contraindicated in patients with decompensated cirrhosis due to higher adverse events, like anaemia and sepsis. Subsequent introduction of first generation directly acting antiviral (DAA) therapy, protease inhibitors telaprevir and boceprevir achieved SVR around 70% given with Peg-IFN and ribavirin. However, protease inhibitors were effective only against HCV genotype 1 and similarly these were contraindicated in advanced liver disease. Both these drugs were discontinued in 2015.
Treatment of HCV in patients with decompensated cirrhosis changed significantly following the introduction of Sofosbuvir, the second generation DAA with high efficacy, shorter treatment course, better safety profile and importantly interferon free regime.
Sofosbuvir with ribavirin for up to 48 weeks in 61 HCV HCC patients awaiting liver transplantation, showed an undetectable HCV RNA at the time of LT was associated with 70% SVR12 in the post-transplant period. Moreover, patients with undetectable HCV RNA at least 4 weeks prior to LT never developed rHCV. Adverse events leading to drug discontinuation was noted in 2 patients (sepsis and kidney injury) which was unrelated to the drug [27]. The success of this therapy changed the perspective of HCV management in patients undergoing liver transplantation.
Subsequent studies were conducted in HCV patients with decompensated cirrhosis with second generation DAA. SOLAR-1 was a phase 2 open label multi-centre study on 12 or 24 weeks of ledipasvir (NS5A polymerase inhibitor), sofosbuvir and ribavirin in patients with advanced cirrhosis (Child Pugh B or C, n = 108). SVR12 for Child Pugh B was 87% and 89%, and for Child Pugh C was 86% and 87%, with 12 and 24 weeks treatment irrespective of previous therapy. Adverse events occurred in 23% of patients but only 4% discontinued treatment [28]. Similarly, SOLAR-2 study was conducted across European and New Zealand sites on HCV Child Pugh B (n = 56) and Child Pugh C (n = 51) with SVR12 of 87% and 96%, and 85% and 78% for 12 and 24 weeks, respectively. Adverse events occurred in 11–50% of patients, predominantly in Child Pugh C, including 3 (12%) patients death. These two studies clearly demonstrated high SVR rates in patients with 12 weeks of this combination therapy with effective HCV clearance in patients with decompensated cirrhosis. With the higher viral eradication rates, MELD score improved in these patients over a period of time; however, in many of the study centres patients have undergone liver transplantation due to shorter waiting times in different parts of the study sites [29]. Data from our liver unit showed a significant decline in patients transplanted for chronic HCV from 2009 to 2019 (17.1% vs 10.1%, P = 0.002).
ALLY-1, an open labelled prospective, multi-centre, phase 3 study evaluated the use of 12 weeks daclatasvir 60 mg daily in combination with sofosbuvir 400 mg twice a day and weight based ribavirin in patients with decompensated cirrhosis (n = 60) with an SVR12 of 83% [30]. Patients with Child Pugh A and B had a higher SVR12 than C, 94%, 94% and 56%, respectively. Likewise, genotype 1b had a better SVR12 than genotype 1a (100% vs 76%). In patients with decompensated cirrhosis, MELD score improved by −0.3, −0.3 and −0.9 across Child Pugh A, B and C patients, respectively. Viral relapse occurred in 10 patients in decompensated cirrhosis, and were successfully treated with 24 weeks combination therapy. Anaemia and fatigue developed in around 20% patients predominantly attributed to ribavirin.
Higher rates of SVR12 with DAA has shown to reduce HCV related death by 74% [31]. Achieving SVR12 has shown to reduce the chance of liver disease progression with improvement in portal pressure [32]. In several cases there were significant improvement in MELD score leading to delisting of patients waitlisted for LT. In a study involving 409 patients with Child B and C cirrhosis, the mean MELD declined by 0.85 within 6 months compared to untreated patients (P <0.0001) and encountered reduced episodes of hepatic decompensation (3.7% vs 10%, P= 0.009) in patients with SVR [33]. Importantly, a cohort study from SRTR database showed 32% reduction in the HCV LT waitlist after they cleared HCV with DAA therapy [34].
Post LT rHCV management
In the previous decade, management of post-transplant rHCV has been posed several difficulties. Introduction of Peg-IFN and ribavirin in the treatment of rHCV slightly improved clinical outcome. Unfortunately, these drugs were associated with significant side effects including graft rejection. Therefore, treatment was recommended only in patients with moderate and severe rHCV. In an earlier study by Berenguer et al., HCV LT recipients were treated with 48 weeks of either IFN and ribavirin (n = 31) or Peg IFN and ribavirin (n = 58) in a median time of 16 months from transplantation. SVR was achieved in 16% and 48% with IFN and Peg-IFN, respectively. Patients who achieved SVR had a clear survival benefit (5-year patient survival 93% vs 69%). Unfortunately, 23 (26%) patients developed hepatic decompensation and 18 (20.2%) patients had allograft loss following the therapy [35].
Telaprevir and boceprevir, the first generation protease inhibitor, introduced in 2010 for the treatment of chronic hepatitis C. Although not approved for post-transplant patients, these drugs were studied in the treatment of rHCV infection. In combination with Peg-IFN and ribavirin, these drugs achieved around 50% SVR. Unfortunately, these drugs were poorly tolerated, predominantly due to adverse effects such as transfusion dependent anaemia. Moreover, profound inhibition of cytochrome P450-3A4 enzyme by these drugs increased CNI trough levels leading to toxicity, with cyclosporine level increase by 4–6 fold and tacrolimus trough levels by 70 fold [36]. Treatment of rHCV in the previous era showed disappointing results.
Second generation DAA has completely changed the outlook of post-transplant rHCV infection [37]. First multicentre open label prospective pilot study was conducted across US and European centres evaluated the safety and efficacy of 24 weeks sofosbuvir and ribavirin combination therapy in post-transplant rHCV. In 40 patients who received therapy (40% biopsy proven cirrhosis) the SVR12 was high at 70%. Median time from liver transplantation to therapy was 4.3 years (range 1–10 years). Virological relapse occurred in 30% of patients. Fatigue, diarrhoea and anaemia occurred in 30%, 28% and 20% of patients [38].
Initial studies with sofosbuvir and ribavirin combination therapy for post-transplant rHCV showed poor drug tolerance, however, the main adverse event was anaemia related to ribavirin in 62% of patients, and subsequent hepatic decompensation related to the low haemoglobin [39].
SOLAR 1 and SOLAR 2 also evaluated the efficacy of the combination of sofosbuvir and Ledipasvir also included post liver transplantation rHCV patients.
SOLAR-1 study involving 12 or 24 weeks of ledipasvir, sofosbuvir and ribavirin in post-LT rHCV (n = 229), the SVR12 was 96%, 96%, 86%, 60%, 100% in patients with no cirrhosis, Child A, Child B, Child C and FCH patients with 12 weeks and, 98%, 96%, 88%, 75% and 100% for 24 weeks therapy. Adverse events such as mild hyperbilirubinemia and anaemia however, mostly managed by reducing median dose of ribavirin to 600 mg daily [38].
SOLAR-2 trial evaluated 226 post-transplant rHCV patients with ledipasvir sofosbuvir combination however, recipients with Child Pugh score above 13 were excluded. SVR12 was 93%, 100% and 95% in patients with no cirrhosis, Child A and Child B cirrhosis with 12 weeks therapy and 100%, 96%, 100% with 24 weeks therapy. These drugs were well tolerated with 5% adverse events, 2% discontinuation rates, and 2% viral relapse rates [40]. The success of SOLAR trial with all oral DAA in this challenging population opened the avenue for patients post LT rHCV.
These two studies clearly demonstrated high SVR rates in patients with 12 weeks of this combination therapy with effective HCV clearance post-transplant rHCV, than the previous generation drugs.
ALLY-1 study also recruited patients with post-transplant rHCV infection (n = 53) to assess the efficacy of 12 weeks daclatasvir 60 mg daily in combination with sofosbuvir 400 mg twice a day and weight based ribavirin. SVR12 was 94% in LT recipients. HCV virus relapsed in 3 post-transplant patients and were subsequently treated with 24 weeks of daclatasvir, sofosbuvir and ribavirin. There was no significant drug interaction with CNI requiring dose adjustments [30]. Interestingly, a real world analysis of daclatasvir and sofosbuvir showed 87–100% SVR12 in patients with Child B and C cirrhosis, and 58% of decompensated cirrhotic patients showed improvement in MELD score [41].
A large multicentre study on daclatasvir in combination with sofosbuvir or simeprevir with or without ribavirin for 24 weeks was carried out on 97 LT recipients with severe rHCV including 37% patients with FCH and 31% with cirrhosis. SVR12 with daclatasvir plus sofosbuvir was 91% and dactlasvir plus simepravir was 72%. There was a significant improvement in Child Pugh score from 6.8 to 5.7 (P < 0.001) and MELD score from 12.1 to 9.7 (P < 0.001) following SVR. However, these scores worsened in 13% patients despite antiviral therapy [42].
Introduction of pangenotypic Velpatasvir-sofosbuvir combination further simplified HCV therapy with maximal efficacy. A phase 3 study on 624 patients with HCV increased SVR to 99% including in cirrhotic patients [43].
Monitoring for rHCV
Careful regular monitoring of liver function is essential in patients undergoing LT for HCV. Higher ALT was shown to accelerate disease progression and cirrhosis. ALT>100 U/L was shown to predict cirrhosis at 5 years due to rHCV (35% vs 6%). Similarly, serum bilirubin >3.5 mg/dl was strongly associated with development of cirrhosis following rHCV [44,45]. Practice of protocol liver biopsies at regular intervals post transplantation were carried out in the past. Presence of moderate to severe lobular inflammation was associated with disease progression (30% vs 0.10%) compared to no or minimal inflammation over 5 years post transplantation.
HCV RNA titres has a direct correlation with the severity of post-transplant rHCV infection. In a study by Shakel et al., [46]. Peak HCV RNA in the first year of untreated patients was associated with poor patient survival. A level of less than 107, 107-108 and >108 U/L was associated with a median survival of 89, 71 and 12 months, respectively.
Hepatitis C positive donors
HCV positive (HCV+) donors has been utilized to curb cadaver organ demand. However, the risk of graft reinfection was a threat to the recipients who are already HCV positive, in particular when donor age >50 years. Recipient selection for HCV+ donors should be carried out with care [[47], [48], [49]]. Alvaro et al., compared patients transplanted for HCV receiving HCV + liver donors (n = 13) vs HCV- donors (n = 130). These donors were carefully evaluated based on normal liver function tests (LFT), normal macroscopic appearance at the time of harvest, frozen biopsy at the time of donation showing mild inflammation and no to mild fibrosis. Histologically, severe rHCV was detected in 23.1% and 22.3% (P = 0.46), with no differences in 1-,3-, and 5-year graft (P = 0.35) and patient survival (P = 0.25) [50]. In a recent innovative approach non-liver recipients of HCV positive donors were prescribed a short 7 day course of newer DAA Glecaprevir/pibrentasvir along with ezetimibe (HCV entry inhibitor) showed 100% viral clearance at week 12 [51].
In summary, long term survival of HCV LT recipients has improved dramatically following second generation DAA. With current SVR close 100%, excellent safety profile DAA therapy are increasingly used in decompensated cirrhosis and for post-transplant rHCV treatment.
Treating HCV infection in patients with decompensated cirrhosis with DAA is associated with significant improvement in MELD score and may subsequently help reducing transplant waitlist. There was a debate whether to treat these patients while waiting for transplant or to treat in the post-transplant period, because some studies observed an increased risk of HCC following SVR.
In the management of rHCV, the timing of antiviral therapy is not well established. Most experts recommend starting DAA after first 3 months with stable immunosuppression onboard. A study by Pellicelli et al., showed significant adverse events including hepatic decompensation and 25% mortality in those with advanced disease following treatment with daclatasvir and sofosbuvir for post-transplant rHCV [52]. Similarly, Forns et al., provided compassionate access sofosbuvir and ribavirin to 104 patients with severe rHCV with life expectancy less than 12 months. SVR was 59% and much higher (73%) in patients with severe rHCV. However, severe adverse event occurred in 47% of patients including 13% mortality [53]. Therefore, it is advisible to commence antiviral therapy prior to hepatic decompensation. Post-transplant rHCV management previously included allograft biopsy to assess the severity of rHCV before commencing anti-viral therapy. However, the necessity of biopsy may be arguable with the currently available DAA. Abnormal liver enzymes in the presence of high HCV RNA level may suffice to commence DAA.
Retransplantation for graft failure secondary to rHCV had much worse outcomes. Retransplantation for rHCV related graft failure is associated with prolonged hospitalization, increased cost and reduced survival. However, outcome and patients selection differed amongst various studies. A multi-centred study from the US showed 1 and 3 year survival of HCV retransplantation was 69% and 49% with no difference in survival compared to other indications [54]. However, the currently available DAA, the need for retransplantation likely to have reduced.
Hepatitis B
Hepatitis B is a major cause of chronic liver disease across the world affecting 350 million population, with higher prevalence in Asian and African countries despite universal vaccination. Hepatitis B was considered an absolute contraindication in the initial years of liver transplantation due to early graft reinfection and poor survival benefit (less than 50%, 2 year survival) [55,56]. FCH was in fact first reported in patients with severe rHBV occurred in 25% of transplant recipients, characterized by rapidly progressive disease with cholestasis, hepatocyte ballooning leading to severe allograft failure and death.
Subsequent evolution of antiviral drugs use in pre-and post-transplant setting and introduction of HBIg in 1980s in the post-operative period has improved the clinical outcomes with better long term survival. Factors associated with rHBV includes pre-transplant HBeAg status and high HBV DNA levels (>20,000 IU/ml). Interestingly, HBV presenting as acute liver failure has lower recurrence rates in the post-transplant period due to low DNA level [57].
Diagnosis of rHBV is established by high HBV DNA with or without abnormal LFTs. Recurrent chronic HBV infection has a more aggressive course with more rapid progression of fibrosis and more severe activity. Liver histology is characterized by lymphoplasmacytic portal inflammation, portal fibrosis, interface activity, bile ductular reaction, lobular disarray, lobular spotty or confluent necrosis and Kupffer cell hyperplasia. Ground glass hepatocytes or sanded-appearing nuclei may be seen. Immunohistochemistry demonstrates HBV surface and core antigen. Occasionally, atypical histological patterns of rHBV have been described. Liver histology of FCH shows periportal expansion with fibrosis extending as thin perisinusoidal strands for varying distances into the liver lobule, along with bile ductular structures with or without an identifiable lumen. Liver parenchyma shows prominent hepatocyte ballooning, bilirubinostasis and immunopositivity for HBcAg (nuclear and cytoplasmic) and to a variable degree for HBsAg.
Treatment aimed at reducing HBV DNA viral load was associated with better outcomes in the post-transplant period. In addition, hepatitis B immunoglobulin (HBIg) provides an adequate cover to minimize viral transmission in the perioperative period.
HBV therapy in the previous era consisted of use of lamivudine monotherapy, subsequently adefovir was added particularly in patients with lamivudine resistance. In 2012, with the introduction of nucleos(t)ide analogue drugs with high genetic barrier to resistance, entecavir and subsequently tenofovir showed better viral suppression. Patients with decompensated cirrhosis with or without HCC should be commenced on antiviral therapy prior to liver transplantation, and should be continued for life following liver transplantation to minimize Hepatitis B reactivation. Passive immunization with HBIg given around the time of transplantation has shown beneficial effects in previous studies. Most experts recommend to maintain HBsAb titres 100–500 U/L in the first few months following liver transplantation. Initial high dose HBIg is usually administered at the anhepatic phase, and subsequent daily maintenance dose for a week. Further doses depends on the anti-HBs antibody titres. Combination of HBIg and nucleos(t)ide analogue is the most commonly used regimen to minimize recurrent Hepatitis B infection of the liver allograft. However, the dose and route of administration was not clearly established, moreover, the exorbitant cost, unavailability in several countries were major limiting factors of HBIg. Several studies were conducted using various protocols in the usage of HBIg. Interestingly, combination of lamivudine plus intramuscular HBIg up to 800 IU/day for a week followed by monthly injections was associated with 1 and 5 year HBV recurrence rate of 1% and 4%, respectively [58]. Later studies found that patients transplanted for Hepatitis B may not require long term HBIg. In a study of 58 post-LT patients HBIg was discontinued at 1 year and with the commencement of entecavir or tenofovir, was associated with low rHBV rates (8.6%) [59]. A similar study showed, low recurrence rate of hepatitis B following nucleos(t)ide therapy and HBIg withdrawal [60]. Subsequent studies showed that long term HBIg may not be necessary in the presence of potent antiviral therapy. Teperman et al., studied 40 post-LT patients with 24 weeks HBIg plus tenofovir-emtricitabine and then randomised to tenofovir-emtricitabine vs tenofovir-emtricitabine with HBIg. This study found no HBV recurrence in both the group [61]. Later studies found post-transplant prophylaxis with nucleos(t)ide analogues with complete elimination of HBIg. In a study of 75 liver transplant recipients for Hepatitis B received nucleos(t)ide therapy and no HBIg was given in patients with HBV DNA <2000 IU/ml. The recurrence rate was low at 8% and resolved after changing antiviral therapy [62]. A larger study published in 2013, on 362 post-liver transplant patients without HBIg showed HBV recurrence at 3 years were 17% and 0% with lamivudine and entecavir, respectively.
Thus, most liver transplant centres adopted lifelong entecavir or tenofovir to reduce viral relapse following liver transplantation, particularly in low risk patients such as those with decompensated cirrhosis with low viral load (<100 copies/ml) and those with ALF presentation. Low dose and short course HBIg with antivirals may be useful in patients with high HBV DNA and HBeAg positive patients.
Tenofovir alafenamide (TAF) is the most recently introduced formulation of tenofovir, to overcome renal and bone related adverse events due to tenofovir disoproxil fumarate. The efficacy is similar to its prodrug tenofovir disoproxil fumarate with a good safety profile. It is recommended in patients with chronic kidney disease and osteoporosis. Data in post-transplant patients with TAF is scarce. Small case series found switching from TDF to TAF improved renal profile measured by eGFR +2.5 ml/min/1.73 m2 compared to +0.29 with TDF over 48 weeks. In addition, there was no significant drug interaction with immunosuppressants [63].
HBcAb positive donors
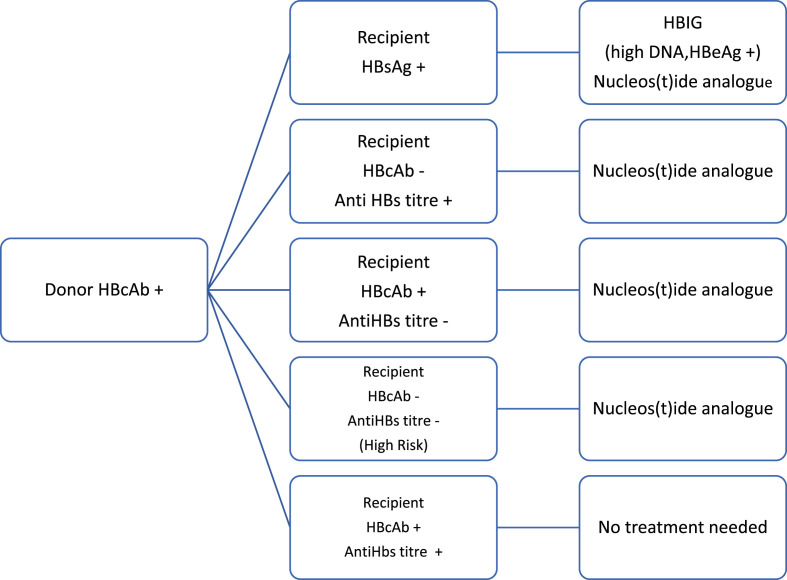
HBsAg negative, HBcAb positive organs have been utilized for transplantation to expand organ pool. However, these recipients are at risk of disease transmission due to de novo hepatitis B affecting the graft. Highest risk of de novo infection was observed in hepatitis B naïve recipients receiving HBcAb positive donors as compared to HBsAg negative HBcAb positive and HBsAb positive recipients (48% vs 15%) without antiviral therapy [63]. In a study of 40 liver transplant patients receiving HBcAb graft, de novo hepatitis B occurred in 5 (12.5%) patients, with more occurrence in the HBcAb negative HBsAb negative compared to HBcAb positive HBsAb negative (50% vs 4.2%, P = 0.049) in the absence of antiviral therapy [65]. Therefore, long term anti-viral prophylaxis is necessary in these patients to prevent de novo HBV infection. The rate of de novo HBV infection in HBSAg naïve patients receiving HBcAb positive liver showed similar rates of allograft infection irrespective of lamivudine monotherapy or lamivudine with HBIg combination (3.6% vs 2.7%) [64,66] indicating that oral antiviral therapy alone sufficient to prevent de novo Hepatitis B infection. Currently, most centres use entecavir or tenofovir long term in the prevention of de novo hepatitis B infection. Management of patients receiving Hepatitis B core antibody positive donor liver is illustrated in Fig. 1 .
Fig. 1.
Treatment of liver recipient with hepatitis B core positive donor.
Hepatitis B core antibody recipient receiving hepatitis B negative liver graft
HBsAg negative, HBcAb positive, HBV DNA negative patients undergoing liver transplantation are at extremely low risk of hepatitis B reactivation [67]. These patients should receive double dose hepatitis B vaccine prior to liver transplantation and any abnormality in the liver enzymes should trigger HBV DNA analysis for reactivation.
With high genetic barrier for resistance currently preferred drug for post-LT patients is either entecavir or tenofovir. Renal function should be monitored regularly in patients receiving tenofovir, particularly in the post-transplant where CNI can also affect the kidney function. Entecavir is the most commonly used antiviral drug due to its excellent ling term efficacy and safety profile.
Hepatitis D
Hepatitis D is an incomplete RNA virus requires Hepatitis B for its replication. HDV occurs in 5% patients with Hepatitis B infection either as a co-infection or superinfection. HDV-HBV can lead to severe liver disease with rapid progression to cirrhosis and hepatic decompensation or acute liver failure. However, post-transplant outcomes are similar or better than HBV monoinfected patients. Due to effective control of HBV with currently available antiviral drugs against HBV, HDV is rarely an issue encountered in LT recipients [68].
Non-HBV patients undergoing LT should be adequately vaccinated against Hepatitis B, preferably while on the waiting list. Patients with advanced liver disease may produce suboptimal response to vaccination. Hence, double dose vaccination with 40 mcg at standard intervals recommended.
All patients undergoing liver transplantation for chronic HBV liver disease should be commenced on antiviral therapy if not already. Choice of antiviral therapy is one with high genetic barrier of resistance (entecavir or tenofovir). Routine use of HBIg is no longer recommended in post-transplant patients. HBV DNA should be checked in patients with post-transplant graft dysfunction.
COVID-19
The current pandemic corona virus disease (COVID-19) is caused by a new strain of corona virus named as SARS-CoV-2, with 80% similar phylogenetic homology to previous SARS-CoV [69]. It is a single positive stranded RNA virus belonging to beta coronavirus genus and is a chimeric variant of bat coronavirus [70]. This disease initially started as a zoonotic infection from a sea food market of Wuhan in December 2019 causing potentially fatal pneumonia, with person to person droplet spread nuclei resulting in rapid increase in infections across the world earning it a pandemic status by March 11, 2020 [71]. SARS-CoV-2 uses angiotensin converting enzyme (ACE2) receptors for cell entry as it has similar receptor binding domain to that of SARS-CoV [72]. ACE2 is abundantly expressed in alveolar type II cells and less commonly in bronchial epithelial cells of lungs. ACE2 is also expressed in stratified epithelial cells of oral and esophageal mucosa, enterocytes of small intestine and colon, liver, myocardial cells, vascular endothelium and smooth muscle cells [73]. SARS-CoV-2 has 10 to 20-fold higher affinity than SARS-CoV to ACE2 receptors. This disease can manifest with mild respiratory or gastrointestinal symptoms to interstitial pneumonia, acute respiratory distress syndrom (ARDS) and diffuse thromboembolic events leading on to multiorgan failure and death. Over all the case fatality of COVID-19 ranges from 5.65% to 15% with geographic heterogenicity [70].
Liver & SARS-CoV-2
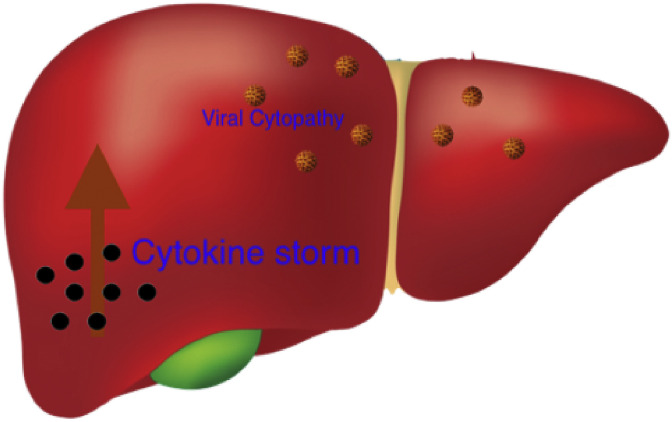
Hepatic involvement occurs in 14–53% of patients with COVID-19, particularly in severe cases [74]. ACE2 receptor is highly expressed in cholangiocytes (59.7%) and vascular endothelial cells. Interestingly, only 2% hepatocytes express ACE2 and no expression observed in sinusoidal endothelial cells [73]. Liver involvement in COVID-19 is probably to the direct viral cytopathic effect and also related increased cytokine storm associated with severe disease (Fig. 2 ).
Fig. 2.
Liver Injury in SARS-CoV-2 infection.
In a recent multicentre study on COVID-19 patients with underlying cirrhosis (n = 50), 97% required hospitalization and 71% required respiratory support. The number of patients with MELD>15 increased from 13% to 26% (P = 0.037) and acute on chronic liver failure (ACLF) occurred in 28% of patients, with a 30-day mortality of 34% [75]. SARS-CoV-2 in patients with decompensated cirrhosis is associated with higher mortality [76]. Analysis of APASL COVID-19 Liver injury spectrum study (APCOLIS) on 228 patients (43 cirrhotics), hepatic decompensation occurred in 9% and ACLF in 11.6% of patients. Child Pugh score >9 predicted higher mortality (ROC 0.94, HR 19.2). In patients with decompensated cirrhosis, mortality was 33% compared to 16.3% in compensated cirrhosis [77]. Based on a recent survey from European LT registry, the crude incidence of COVID-19 in patients awaiting liver transplantation was 1.05% (0.5–20%) with a crude death rate of 18% [78].
Post-LT de novo COVID-19
LT recipients are more susceptible for SARS-CoV-2 infection, owing to their immunosuppressive state and associated comorbidities. Incidence of COVID-19 in post-LT patients was 0.34% (0.1–4.8%), with a crude death rate of 15% [78]. Initial analysis on 90 solid organ transplant recipients with COVID-19 showed that 76% of patients required hospitalization with 18% mortality [79]. Earlier, Donato et al., from Italy reported COVID-19 incidence of 1.25% in 640 post LT patients of which three-fourths developed only mild disease [80].
Similar to non-transplant COVID-19 patients, Becchetti et al., observed fever (79%), cough (55%) and gastrointestinal symptoms (33%) in the majority of LT recipients. Risk factors such as hypertension (56%) and diabetes (37%) were observed in these cohort. ARDS developed in 19% with a case fatality rate of 12–17% [81]. An interim analysis from COVID-Hep registry showed 23% mortality in a cohort of 39 LT patients. Interestingly, mortality was not influenced by time from transplant or immunosuppressive drugs [82]. A study from Spanish society for liver transplantation reported 111 recipients with COVID-19, where 86.5% required hospitalization for their symptoms of whom of 19.8% required respiratory support and 10.8% patients required intensive care. MMF was an independent predictor of severe COVID-19; whereas tacrolimus appeared protective. Allograft function showed mildly elevated liver enzymes in 14.7% patients and severe graft dysfunction were observed in 2.7% patients. Mortality in post-LT patients with COVID-19 was 18%.
A largest multicentric international COVID-19 registry analysis of 151 post-LT and 627 chronic liver disease patients showed higher ICU admission (28% vs 8%, p < 0.0001), invasive ventilation (20% vs 5%, (p < 0.0001) in post-transplant group. Interestingly, mortality was less in LT recipients (19% vs 27%, p = 0.046) [83].
Drugs used in COVID-19 treatment can cause liver injury and drug interactions. Remdesivir, was initially considered in the management of COVID-19 but a recent randomized controlled trial failed to show clinical benefit in COVID-19 treatment. Unfortunately, 10–13% of patients developed drug induced liver injury [84]. This may lead to allograft dysfunction in LT recipients. Similarly, lopinovir-ritonovir inhibits cytochrome P450 leading to significant drug interaction particularly CNI trough level in post LT patients.
Based on expert opinions, international bodies recommends against stopping immunosuppression particularly CNI. However, MMF may be dose reduced at the time of COVID-19 illness, particularly in those with severe lymphopenia, superadded bacterial or fungal infection.
Hepatitis E (HEV)
HEV is a single stranded RNA virus; four genotypes have been described. Genotype 1 and 2 spreads through faeco-oral contamination, a self-limiting illness, prevalent in Asian and African countries. Genotype 3 and 4 are acquired as a zoonotic infection through the consumption of undercooked swine, boars, deer and, camel meat and milk recently recognised in the western world [85]. Post-LT HEV infection is an increasingly recognised cause of chronic allograft dysfunction, predominantly genotype 3 and 4. A surveillance study from the US identified 22% HEV seroprevalence amongst blood donors. This may cause transfusion related transmission in liver transplant recipeints [86]. A study by Koning et al., identified HEV seroprevalence of 42% in patients transplanted for HCV. Surprisingly, none of these patients developed HEV reactivation [87]. This may be due to the protective effect of Peg-IFN used in the treatment of HCV.
Post-LT HEV infection
Clinical presentation of acute hepatitis E infection in the post-transplant patients is similar to the non-transplant setting, however, chronic presentation defined as detectable HEV RNA or HEV IgM in the serum for 6 months, and is exclusively observed in the post-transplant patients [88].
Chronic HEV in liver transplant recipients was first recognised by Kamar et al. These patients continued to have allograft dysfunction following an acute infection and persistent positive HEV RNA in serum or stool for 10–24 months [89]. Exact prevalence of HEV seroprevalence in LT recipients is unknown. Based on HEV RNA or HEV IgG level, a retrospective analysis of frozen sera showed 1%–16% seroprevalence of HEV in LT recipients [90]. In a French study, HEV seroprevalence (HEV IgG) was observed in 12.9% of patients during pre-LT evaluation, interestingly one third of these patients became HEV negative in the post-transplant period [91]. Studies show that over all seroprevalence is low in transplant recipients compared to the general population, but given the immunosuppressive state the rates of chronic infection remains high, 50–65%, leading to significant graft fibrosis and failure [92]. Most of these infections are de novo chronic HEV infection. Rare cases of donor derived HEV have been described, confirmed by higher levels of HEV RNA with similar phylogenetic sequence in the stored donor liver tissue [93].
Clinical features of chronic HEV include a modest increase in ALT between 100 and 300 IU/L, unlike acute viral hepatitis. Liver histology is characterized by portal fibrosis, variable portal inflammation and interface hepatitis. In severe cases, increased lobular hepatitis and progressive graft fibrosis [88]. Factors associated with chronic HEV disease progression were studied. A multi-centre European study on post LT patients showed tacrolimus usage independently predicted chronic HEV infection (OR 1.87, P = 0.04). However, more studies are clearly required to evaluate the exact role of immunosuppression in chronic HEV infection [94].
Post-LT chronic HEV causes allograft dysfunction, leading to chronic liver disease, cirrhosis and death. Diagnosis of HEV in the post-transplant period is difficult due to the heterogeneity in the sensitivity of HEV IgM detection; HEV RNA quantification is more reliable however, it is not widely available and there are variations in the assays.
Treatment of Chronic HEV alleviates allograft dysfunction. Lowering immunosuppression as in other post-transplant viral infections may lower HEV RNA level. Although no treatment has been firmly established, ribavirin has been found useful in post-LT chronic HEV infection, with a clearance rate of 78% [95]. However, around 20% of patients can be viremic leading to disease progression. Recently, an invitro study showed inhibitory effect of sofosbuvir on HEV genotype 3 replication as an add on therapy to ribavirin [96]. Unfortunately, a phase 2 study of 9 post-transplant chronic HEV patients (n = 9), sofosbuvir 400 mg once a day for 24 weeks failed to clear the virus. Surprisingly, patients on MMF but not mTOR inhibitor showed higher viral suppression [97].
Cytomegalovirus (CMV)
Cytomegalovirus is a ubiquitous large DNA herpes virus that belongs to Betaherpesvirinae subfamily. It infects 60–100% of adult population and usually causes asymptomatic illness. CMV similar to other herpes infection causes latent infection in hematopoietic cells particularly in myeloid lineage.
CMV is the most common infection in post-transplant setting with the highest risk first 3 months correlating with higher levels of immunosuppression [98]. CMV occurs in various forms in post-transplant setting either as a de novo primary infection or as a reactivation [99]. CMV serology done in donor and recipients pre-transplant helps to determine the individual risk for reactivation or de novo infection.
CMV infection can be latent when the virus is in a non-replicating phase. Active viral replication as in reactivation or active primary infection can present as any of asymptomatic with no clinical manifestations, viral syndrome like illness or tissue invasive CMV disease with histopathological evidence of CMV in end organs as illustrated in Table 1 .
Table 1.
CMV disease clinical presentation: EBV, Epstein Barr Virus; PTLD, Post-transplant Lymphoproliferative Disorders.
| Viral like Syndrome | Tissue invasive Disease | Indirect effects |
|---|---|---|
| Fever | Gastritis | Acute rejection |
| Malaise | Esophagitis | Chronic rejection |
| Neutropenia | Enteritis | Opportunistic infections – bacterial, fungal and viral. |
| Thrombocytopenia | Colitis | EBV/PTLD |
| Hepatitis | Diabetes mellitus – new onset | |
| Pneumonitis | Vascular endothelial damage and thrombosis. | |
| Retinitis | HCV recurrence |
CMV donor positive recipient negative (D+/R-) are at highest risk (44-65%) of de novo CMV infection, whereas donor and recipient positve (D+/R+), and donor negative recipient positive (D-/R+) carry moderate risk (8-19%) and those with donor and recipient negative (D-/R-) carry lower risk of CMV infection. The latter group may acquire the disease as a primary infection or through blood transfusion [[100], [101], [102]].
The most common clinical manifestation of CMV disease in post-LT situation is viral like syndrome (60%) with bone marrow suppression with fever, leukopenia and thrombocytopenia. 70% of tissue invasive CMV present as GI manifestation [103]. CMV being a potent up regulator of alloantigen increases the risk of acute rejection [[104], [105], [106]]. Incidence of HAT and other vascular thrombosis are common with post-transplant CMV infection [107].
Diagnostic strategies and follow-up testing
CMV serology, CMV DNA quantitative and qualitative analysis, pp65 antigenemia, culture and histopathology are the available modalities for diagnosis of CMV infection or CMV disease. Due to impaired ability to mount antibody response in immunosuppressed state serology is not an useful test to monitor or diagnose CMV infection in post-transplant setting [107]. Likewise, blood culture has limited utility in this situation [108].
Measuring CMV DNA from whole blood is more sensitive and allows early detection. Level of CMV DNA determines the risk and severity of the disease [109,110]. Negative PCR in blood does not exclude invasive CMV disease especially in presence of clinical symptoms, where concomitant immunohistochemical testing for pp65 antigen is useful in diagnosis [111]. A rise of CMV titre by factor 3 (>0.5 log copies/ml) within 1 week is an indication for initiation of treatment [112]. WHO released international reference standard for CMV DNA quantification should be used to calibrate the commercially available assays.
A weekly PCR based testing is necessary in case of preemptive treatment strategy. Monthly testing for CMV DNA PCR followed by 3 monthly testing is recommended for patients on antiviral therapy in their first year.
Preventive strategies
The two approaches followed in the management of CMV are pre-emptive and prophylactic antiviral treatment most importantly during the first month after LT. The choice of approach is based on the risk benefit ratio, drug related toxicities and risk of developing CMV disease determined by sero-status of the donor and recipient. The International CMV consensus guidelines recommend 3 months antiviral prophylaxis for high risk patients (D+/R-) [113]. In a recent randomized multicentric trial where 205 CMV negative recipients received CMV positive liver, the occurence of CMV disease with end organ damage was reduced in premptive therapy group (9%) recieving Valganciclovir 900 mg BD until 2 negative tests a week apart when compared to prophylactic antivirals for 100 days (19%) at the end of 12 months post transplant. Whereas allograft rejection (28% vs 25%), graft loss (2% vs 2%) opportunistic infections (25% vs 27%) do not differ significantly in both the groups [114]. Hence preemptive therapy with valgancyclovir can be considered in high risk individuals [114].
Pre-emptive therapy warrants weekly CMV DNA monitoring for first 3 months and advocating therapy at the time of early viral detection thereby pre-empting the occurrence of CMV disease. The expected viremia is high in pre-emptive therapy as reported by Onor et al., 4.9% had significant viremia in prophylaxis group as against 50% in preemptive group. The outcomes of therapy were similar in both groups [115].
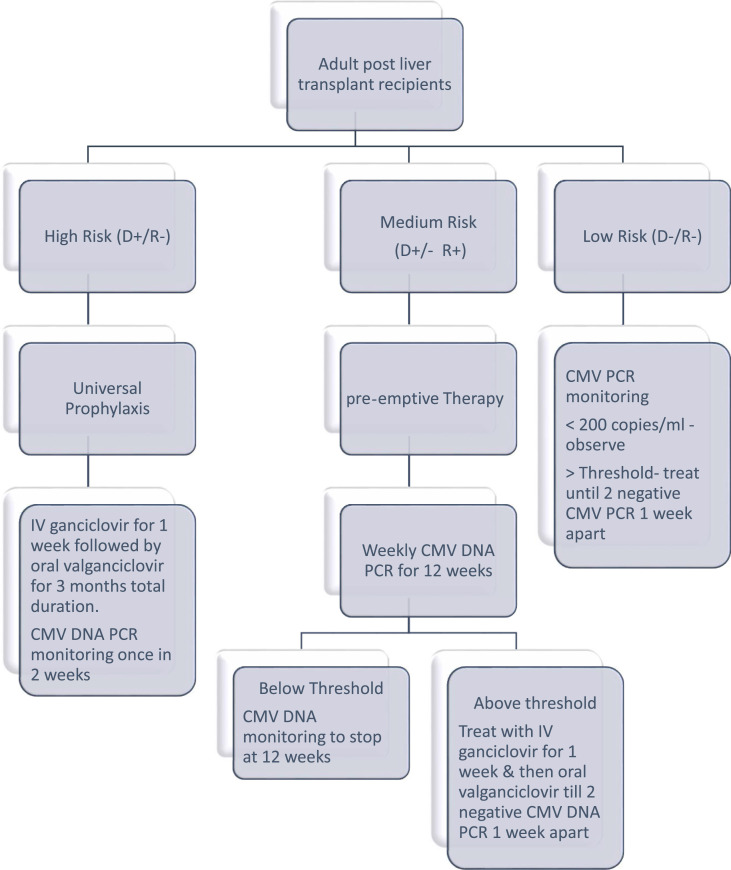
The current recommendations are 3–6 months of prophylaxis with valganciclovir 900 mg/day orally for high risk recipients and for 3 months in moderate risk recipients. Glomerular filtration rate (GFR) dependent dose modification is required in patients renal insufficiency. IV ganciclovir is given at a dose of 5 mg/kg body weight per day. At these doses both the drugs can cause hematological toxicities which can limit duration of CMV prophylaxis or need dose adjustments. Universal prophylaxis is more cost effective and does not need a strict testing regime [116] (Fig. 3 ).
Fig. 3.
CMV Prophylaxis in Post liver transplant recipients. CMV, Cytomegalo virus; DNA, Deoxyribonucliec acid; PCR, Polymerase chain reaction; D+, Donor positive status; R+, Recipient positive status; R-, Recipient negative; D-, Donor negative.
Treatment of CMV disease
DNA polymerase inhibitor such as ganciclovir 3–5 mg/kg body weight twice daily (intravenous) or oral valganciclovir 900 mg twice daily is the recommended treatment for CMV disease. Intravenous route is preferred for life threatening CMV infection [117]. These drugs are excreted through the kidneys and hence regular monitoring of renal function is recommended for nephrotoxicity. In addition, although no direct drug interaction documented with CNI, patients should be observed for nephrotoxicity (Table 2 ).
Table 2.
Antiviral drugs for cytomegalovirus infection.
| Drug | Prophylaxis | Treatment | Toxicity |
|---|---|---|---|
| Ganciclovir | 5 mg/kg IV once daily Or 1 gram thrice daily oral |
5 mg/kg IV twice daily | Nephrotoxicity Bone marrow suppression Poor oral bioavailability |
| Valganciclovir | 900 mg oral once daily | 900 mg oral twice daily | Bone marrow suppression leucopenia |
| Foscarnet | Not recommended | 60 mg/kg IV thrice daily Or 90 mg/kg IV twice daily |
Second line drug Nephrotoxicity |
| Cidofovir | Not recommended | 5 mg/kg IV once weekly for 2 weeks followed by once in 2 weeks | Third line drug Nephrotoxicity |
Foscarnet is a viral DNA polymerase inhibitor used as a second line agent particularly useful in patients with ganciclovir resistance (UL 97). Cidofovir have been tried in difficult to treat CMV. Both these drugs have been used off label in post-transplant setting.
Leflunomide used as immunosuppressive in rheumatoid arthritis is also tried in gancyclovir resistant CMV infection with mixed results [118]. Letermovir is a recently approved drug for stem cell recipients which is devoid of myelosuppression and minimal drug to drug interactions, not subjected to UL97 and UL54 mediated resistance. It is yet to be approved for use in post LT patients [119]. Treatment should be continued till two samples are negative for CMV DNA one week apart.
Late onset CMV disease occurs in 25% of patients after completion of prophylaxis. Especially in (D+/R-) recipients. Whereas the incidence is reduced to 8.3% in recipients recieving 3-6 months pre-emptive treatment. Some studies report mitigation of this by extending the prophylaxis to 200 days, but data are insufficient [120]. Hence surveillance after prophylaxis need to be advocated as an alternative when prolonged drug exposure is not warranted and especially in high risk individuals [121].
CMV & immunosuppression
Immunosuppression increases the risk of CMV reactivation Studies show MMF but not CNI increases the risk and severity of CMV infection [122]. Interestingly, everolimus, a mTOR inhibitor has been found to have a virostatic effects on CMV [123,124]. Therefore, it is better to avoid MMF in post-LT patients with CMV and maintain low tacrolimus trough level.
Persistence of CMV DNA beyond 6 weeks despite antiviral therapy indicates drug resistance which entails resistance testing for UL-97 & UL54 gene mutation. Resistance mutation occurs in both genes for ganciclovir and only in UL54 for Cidofovir and Foscarnet [116,125]. In summary, CMV is the most common infection in post tranplant period. CMV negative recipents recieving CMV positive liver allogarft have high risk of CMV infection. Prophylactic therapy with valgancyclovir 900 mg OD for 3 months for all high and moderate risk recipients is the current recommendation. Premeptive therapy can also be done after close CMV DNA monitoring in first 3 months after transplant. Immunosupression dose reduction or modification is necessary while treating post transplant CMV.
Epstein Barr virus (EBV)
Epstein Barr virus also known as human herpes virus 4 (HHV4) is a DNA virus belonging to herpes virus family. EBV infection is most commonly acquired in childhood with 90% seropositivity in adulthood. Acute de novo EBV infections are self-limited and resolves with supportive care. The virus remains latent in B cells for life and reactivates in conditions with decreased immunity [126]. EBV reactivation occurs due to reduced activity of cytotoxic T-cells. Post-LT, immunosuppression supresses memory T cell function causing proliferation of EBV mediated B cells in 10% of patients. Usually asymptomatic but rarely uncontrolled proliferation and clonal transformation of EBV infected B cells results in post-transplant lymphoproliferative disorders (PTLD) with an incidence of 4.7% in children and 1% in adult LT recipients [[127], [128], [129]]. Incidence of PTLD is highest in first year following LT. About 90% of PTLD are associated with EBV infection. In a large SRTR database analysis, 383 (0.95%) out of 40,437 LT recipients developed PTLD. This study revealed that recipient EBV seronegative status as a significant risk factor for PTLD, with an adjusted HR 3.49 (<0.0001) [130]. However, in the paediatric transplant recipients PTLD is caused by activation of persistent donor B lymphocytes after engraftment.
EBV serostatus is routinely determined by serological assays, VCA (EBV viral capsid antigen), EA (early antigen) and EBNA (Epstein Barr nuclear antigen) [131] (Table 3 ). These are useful only in pre-transplant setting and may disappear in the post-transplant period. EBV DNA viral load monitoring is required in the post-LT period to assess viral reactivation [132].
Table 3.
EBV specific markers and interpretation. VCA, Viral capsid antigen; EA, early antigen; EBNA, Epstein Barr nuclear antigen.
| EBV Negative status | Primary Infection | Past Infection | Reactivated infection | |
|---|---|---|---|---|
| VCA IgM | Negative | Positive | Negative | Negative |
| VCA IgG | Negative | Positive | Positive | Positive |
| EA IgG | Negative | Negative | Negative | Positive |
| EBNA IgG | Negative | Negative | Positive | Positive |
| EBNA IgM | Negative | Positive | Negative | positive |
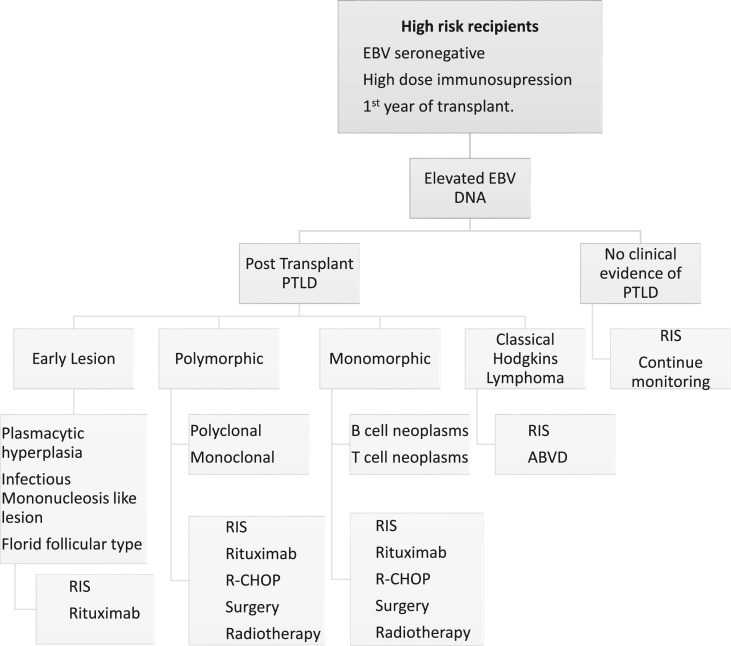
According to WHO, PTLD has been classified in to early, polymorphic, monomorphic and classic Hodgkin’s lymphoma types [132]. Early PTLD is always associated with EBV and further subclassified into infectious mononucleosis like PTLD, plasmacytic and florid follicular type. Early PTLD is characterized by abnormal B cell production due to reactive hyperplasia but no formation of tumor cells and hence these 3 subclasses of PTLD are known as non destructive PTLD. Polymorphic, monomorphic and classic Hodgkins lymphoma types broadly grouped as destructive PTLD. Polymorphic PTLD has both B & T cell proliferation, tumor cell formation is present but it does not qualify to be classified as lymphoma. 90% of polymorphic PTLD are associated with EBV. Monomorphic PTLD is the most common type representing 50% of cases, with a clinical presentation of a diffuse large B cell lymphoma or occasionally T cell lymphoma. Only 50% of monomorphic PTLD are associated with EBV, whereas 90% of classic Hodgkin -like PTLD is also associated with EBV [133]. Lymphnodes, gastrointestinal tract, central nervous system, liver, lung, tonsils and salivary glands are the most common locations for PTLD. Clinical presentation vary from local asymptomatic lesions to wide spread disease with multiorgan failure. In LT recipients chronic fever, night sweats, anorexia and weight loss (B symptoms) occur in classical lymphomas [134]. PTLD features a bimodal clinical distribution, with an early disease occurring within 12 months following transplantation affecting liver allograft and a ‘delayed’ presenting as Hodgkin’s lymphoma. High EBV viral load is associated with the severity of PTLD and therefore serial monitoring of EBV DNA level is recommended in these patients [132]. Other investigations includes a whole body positron emission tomography (PET-CT) and tissue histology. There are no definitive established treatment for PTLD. A pre-emptive reduction of immunosuppressive medications has shown to regress PTLD with reduction of EBV DNA by partially restoring EBV specific cellular immunity [135]. Age > 50 years, advanced stage of the disease with larger lesions (> 7 cm) may show complete lack of response to reduction in immunosuppression. Rituximab, an anti-CD20 monoclonal antibody, weekly for 4 weeks may be required in patients who do not respond to the initial immunosuppression reduction [136]. Patients with aggressive tumor often requires cytotoxic chemotherapy as used in other lymphomas [137]. Localised lesions can be surgically removed or can be given radiation therapy. EBV specific cytotoxic T lymphocytes are being used as immunotherapy but cumbersome and expensive [131,137] (Fig. 4 ). One year survival with PTLD is 56.5% in adult LT recipients. Hepatic, neurological, bone marrow involvement are associated with poor prognosis [138].
Fig. 4.
Management of post-transplant PTLD. RIS, reduction in immunosuppression; R-CHOP, Rituximab combined cyclophosphamide/doxorubicin/vincristine/prednisolone; ABVD, adriamycin/bleomycin/vinblastine/dacarbazine
Herpes simplex virus (HSV)
Herpes simplex virus type 1 and type 2 belong to alpha herpesvirus subfamily of Herpesviridae family. Humans are the only known reservoir for herpes simplex viruses [139]. With high prevalence in the general population, most adults are seropositive by 5th decade [140]. Post-transplant HSV occurs primarily as a reactivation of latent infection and in few as a de novo including donor derived infection [141]. Mucocutaneous lesions make up the majority of HSV infection in post-transplant period of which 85% are orolabial followed by anogenital infections. Most lesions are mild occurring in the oral cavity posterior pharynx, chin and neck. Anogenital typically involves glans and shaft of penis in males and, vulva, labia and vaginal introitus in women [142]. A study of HSV infection post-LT patients revealed 91% were limited to local infections; however, 9% had fatal disseminated disease [143].
HSV oesophagitis can be difficult to distinguish from other causes of post-transplant oesophagitis such as candida and CMV infections. Presence of concurrent orolabial infection may point towards HSV. Pneumonitis caused by HSV in LT recipients leads to significant mortality and morbidity [144]. Antiviral prophylaxis during first month post-transplant may reduce incidence of infection/reactivation. Acyclovir is the drug of choice for the treatment of HSV infections [145]. Valacyclovir and ganciclovir can also be used as alternative therapy [146]. Mucocutaneous infection usually need treatment for 7–14 days. Visceral infections need a prolonged course of antivirals [147].
Varicella Zoster
Varicella Zoster, that causes chicken pox remains latent in the dorsal root ganglia. This can reactivate following immunosuppression leading to Herpes zoster, characterized by painful vesicular rash along the dermatomal distribution, usually restricted to one side. The 1-year incidence is 3% whereas the 5- and 10-year incidences are 14% and 18% respectively [148]. In a study of 209 consecutive LT recipients 12% (25) developed zoster infection in a median time of about 23 months from the time of transplantation [149]. Reduction of immunosuppression is the first step in the management of Herpes zoster, particularly temporarily withholding anti-metabolites like azathioprine and mycophenolate mofetil. Antivirals such as valacyclovir, acyclovir and ganciclovir are effective in the treatment of herpes zoster. These drugs should be given for at least 2 weeks along with pain killers like gabapentin [150]. Post herpetic neuralgia occurs in one third of patients with persistent long standing pain.
Human herpes virus 6
Human Herpes Virus 6 (HHV-6) belongs to beta herpesviridae subfamily under Roseolovirus genus. This is a common name for 2 similar viruses HHV-6A and HHV-6B, the latter accounting in most cases [151]. Asymptomatic exposure to HHV-6 occur in childhood such that 90–95% adult population are seropositive [152]. The circular DNA of the virus integrates with the host genome and may remain latent for several years in the mononuclear cells [153,154]. HHV-6 infection occurs as a result of reactivation in the post-transplant state. Occasionally, donor derived infections or rarely through blood products have been reported [155].
Incidence of HHV6 varies between 14% and 82% [156], commonly occurring in the first 2–8 weeks after liver transplantation. Sporadic cases were reported as early as 10 days and rarely after 5 years following liver transplantation [157]. In a prospective study of 51 LT patients, 11 (21.5%) developed HHV-6B reactivation, of which 4 had fever and abdominal pain [158]. Reactivation rates are less when in patients receiving gancyclovir prophylaxis against CMV.
Most HHV-6 reactivation occurs as an asymptomatic infection with low viral load [159] and they usually do not require treatment. Clinically, HHV-6 reactivation presents with fever, skin rash and raised liver enzymes [160,161].
Histologically, HHV-6 hepatitis may mimic acute cellular rejection with elevated liver enzymes, portal lymphocytic infiltration and confluent periportal necrosis [155,160]. In 170 LT patients with graft hepatitis, high levels of intrahepatic HHV-6 DNA and HHV antigenemia were significantly associated with decreased graft survival [162]. HHV-6 encephalitis though rare in LT recipients may occur within 4–6 weeks of transplantation. HHV-6A is a neurotrophic virus and is detected in plasma as well as CSF. Brain imaging shows characteristic signal intensity in medial temporal lobes involving amygdala and hippocampi. HHV-6 infection may also present as post-transplant colitis [163]. It may present as a co-infection with CMV where HHV-6 antigenemia precedes CMV antigenemia [164]. In an analysis of 45 LT recipients 23 (51.1%) had CMV infection and 12 (26.7%) HHV-6 infection [165]. HHV-6 infection associated with higher incidence of opportunistic infections, invasive fungal infections and mycobacterial disease [160,166]. HLA-DR15 positivity in donor liver biopsy predisposes the graft to HHV-6 infection and subsequent rejection [167]. HHV-6 is highly cell bound and the rates of PCR positivity are comparitively less in plasma (33%) and whole blood (19%). Liver biopsy may show two distinct patterns of liver injury. Recipients with high viral load may show severe periportal inflammation and necrosis, whereas those with low viral load have lobular activity with mild portal inflammation [168]. Biopsy remains the gold standard in diagnosing end organ damage. HHV-6 IgM antibodies appear by 2–4 weeks of primary infections reactivations, whereas IgG antibody titres increase 4 fold by 4–6 weeks.
Ganciclovir and valganciclovir are the most common drugs used for treatment of HHV-6 infection in LT patients [161,169]. Few patients exhibit ganciclovir resistance. Cidofovir can be used but these patients needs close monitoring for nephrotoxicity. Foscarnet is the most selective in vitro inhibitor of HHV-6, and is preferred in patients with underlying anemia [170].
To summarise, HHV-6 infection after LT is rare, but its reactivation has found to be has associated with significant increase in graft failure, mortality, hepatitis C progression and CMV disease [171]. HHV infections in LT patients can be treated successfully with CMV antivirals ganciclovir, Cidofovir and foscarnet.
Conclusion
Post-LT viral infections can cause significant allograft dysfunction. Early recognition, diagnosis and systematic approach can ameliorate the infective process and preserve allograft function. Newly evolving less familiar viral infections such as COVID-19 should also be considered in the differential diagnosis of post-LT viral infections. Management of post-transplant viral infection has improved over the last decade due to significant changes in immunosuppression protocols, and availability of effective anti-viral drugs with high genetic barrier for resistance. These high potency anti-viral drugs have translated in to better long-term allograft and patient survival.
Practice points
-
•
Patients with decompensated cirrhosis due to chronic Hepatitis B or hepatitis C infection should be commenced on high efficacy antiviral therapy
-
•
Post-liver transplant patients with recurrent hepatitis C infection should be treated with 12 weeks of second generation directly acting antiviral therapy
-
•
Carefully selected HCV positive donor liver can be used for transplantation.
-
•
Recurrent Hepatitis B can effectively controlled with Entecavir or Tenofovir without the need for HBIg
-
•
Mortality of liver transplant recipients with COVID-19 is around 20%.
-
•
Post-liver transplant chronic HEV can lead to graft fibrosis and failure
-
•
CMV infection causes significant allograft dysfunction and systemic illness. MMF increases the risk of CMV
-
•
90% of PTLD cases are associated with EBV infection.
Research agenda
-
•
Pathogenesis of COVID-19 in post-liver transplant patients requires larger studies.
-
•
Ideal immunosuppressive regimen to reduce the chance of recurrent or de novo viral infection needs to be studied.
-
•
Developing vaccines to prevent de novo viral infections in post-transplant patients will be a crucial milestone.
Funding source
None.
Declaration of competing interest
None.
References
- 1.Trotter J.F. Liver transplantation around the world. Curr Opin Organ Transplant. 2017 Apr;22(2):123–127. doi: 10.1097/MOT.0000000000000392. PMID: 28151809. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004 Mar;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. PMID: 14996343. [DOI] [PubMed] [Google Scholar]
- 3.Petruzziello A., Marigliano S., Loquercio G., Cozzolino A., Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016 Sep 14;22(34):7824–7840. doi: 10.3748/wjg.v22.i34.7824. PMID: 27678366; PMCID: PMC5016383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thein H.H., Yi Q., Dore G.J., Krahn M.D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008 Aug;48(2):418–431. doi: 10.1002/hep.22375. PMID: 18563841. [DOI] [PubMed] [Google Scholar]
- 5.Westbrook R.H., Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014 Nov;61(1 Suppl):S58–S68. doi: 10.1016/j.jhep.2014.07.012. Epub 2014 Nov 3. PMID: 25443346. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Retortillo M., Forns X., Feliu A., Moitinho E., Costa J., Navasa M., Rimola A., Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002 Mar;35(3):680–687. doi: 10.1053/jhep.2002.31773. PMID: 11870384. [DOI] [PubMed] [Google Scholar]
- 7.Neumann U.P., Berg T., Bahra M., Seehofer D., Langrehr J.M., Neuhaus R., Radke C., Neuhaus P. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J Hepatol. 2004 Nov;41(5):830–836. doi: 10.1016/j.jhep.2004.06.029. PMID: 15519657. [DOI] [PubMed] [Google Scholar]
- 8.Berenguer M., Ferrell L., Watson J., et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32(4):673–684. doi: 10.1016/s0168-8278(00)80231. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner R.H., Rakela J., Ishitani M.B., Mulligan D.C., Spivey J.R., Steers J.L., Krom R.A. Recent advances in liver transplantation. Mayo Clin Proc. 2003 Feb;78(2):197–210. doi: 10.4065/78.2.197. PMID: 12583530. [DOI] [PubMed] [Google Scholar]
- 10.Berenguer M., Prieto M., Rayón J.M., Mora J., Pastor M., Ortiz V., Carrasco D., San Juan F., Burgueño M.D., Mir J., Berenguer J. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000 Oct;32(4 Pt 1):852–858. doi: 10.1053/jhep.2000.17924. PMID: 11003634. [DOI] [PubMed] [Google Scholar]
- 11.Berenguer M., Prieto M., Rayón J.M., et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32(4 Pt 1):852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 12.Berenguer M., Aguilera V., Rubín A., Ortíz C., Jimenez M., Prieto M. Comparison of two non-contemporaneous HCV-liver transplant cohorts: strategies to improve the efficacy of antiviral therapy. J Hepatol. 2012 Jun;56(6):1310–1316. doi: 10.1016/j.jhep.2011.12.031. Epub 2012 Feb 5. PMID: 22314429. [DOI] [PubMed] [Google Scholar]
- 13.Blasco A., Forns X., Carrión J.A., García-Pagán J.C., Gilabert R., Rimola A., Miquel R., Bruguera M., García-Valdecasas J.C., Bosch J., Navasa M. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006 Mar;43(3):492–499. doi: 10.1002/hep.21090. PMID: 16496308. [DOI] [PubMed] [Google Scholar]
- 14.Gitto S., Belli L.S., Vukotic R., Lorenzini S., Airoldi A., Cicero A.F.G., Vangeli M., Brodosi L., Panno A.M., Di Donato R., Cescon M., Grazi G.L., De Carlis L., Pinna A.D., Bernardi M., Andreone P. Hepatitis C virus recurrence after liver transplantation: a 10-year evaluation. World J Gastroenterol. 2015;21(13):3912–3920. doi: 10.3748/wjg.v21.i13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche B., Samuel D. Risk factors for hepatitis C recurrence after liver transplantation. J Viral Hepat. 2007 Nov;14(Suppl 1):89–96. doi: 10.1111/j.1365-2893.2007.00920.x. PMID: 17958649. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M., Wiesner R. Natural history and management of hepatitis C infection after liver transplantation. Semin Liver Dis. 2004;24(Suppl 2):79–88. doi: 10.1055/s-2004-832932. [DOI] [PubMed] [Google Scholar]
- 17.Samonakis D.N., Germani G., Burroughs A.K. Immunosuppression and HCV recurrence after liver transplantation. J Hepatol. 2012 Apr;56(4):973–983. doi: 10.1016/j.jhep.2011.06.031. Epub 2011 Sep 29. PMID: 21963518. [DOI] [PubMed] [Google Scholar]
- 18.Berenguer M., Aguilera V., Prieto M., San Juan F., Rayón J.M., Benlloch S., Berenguer J. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006 Apr;44(4):717–722. doi: 10.1016/j.jhep.2006.01.005. Epub 2006 Feb 6. PMID: 16487616. [DOI] [PubMed] [Google Scholar]
- 19.Klintmalm G.B., Washburn W.K., Rudich S.M., et al. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transplant. 2007;13(11):1521–1531. doi: 10.1002/lt.21182. [DOI] [PubMed] [Google Scholar]
- 20.Filipponi F., Callea F., Salizzoni M., Grazi G.L., Fassati L.R., Rossi M., Risaliti A., Burra P., Agnes S., De Carlis L., Valente U., Ferrara R., Pisati R. Double-blind comparison of hepatitis C histological recurrence Rate in HCV+ Liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation. 2004 Nov 27;78(10):1488–1495. doi: 10.1097/01.tp.0000140881.07208.4e. PMID: 15599313. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z., Chen Y., Tao R., Xv J., Meng J., Yong X. Tacrolimus-based versus cyclosporine-based immunosuppression in hepatitis C virus-infected patients after liver transplantation: a meta-analysis and systematic review. PLoS One. 2014 Sep 8;9(9) doi: 10.1371/journal.pone.0107057. PMID: 25198195; PMCID: PMC4157850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watt K.D., Lyden E.R., Gulizia J.M., McCashland T.M. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transplant. 2006 Jan;12(1):134–139. doi: 10.1002/lt.20583. PMID: 16382465. [DOI] [PubMed] [Google Scholar]
- 23.Tao R., Ruppert K., Cruz R.J., Jr., Malik S.M., Shaikh O., Ahmad J., DiMartini A., Humar A., Fontes P.A., de Vera M.E. Hepatitis C recurrence is not adversely affected by the use of donation after cardiac death liver allografts. Liver Transplant. 2010 Nov;16(11):1288–1295. doi: 10.1002/lt.22168. PMID: 21031544. [DOI] [PubMed] [Google Scholar]
- 24.Foucher J., Chanteloup E., Vergniol J., Castéra L., Le Bail B., Adhoute X., Bertet J., Couzigou P., de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006 Mar;55(3):403–408. doi: 10.1136/gut.2005.069153. Epub 2005 Jul 14. PMID: 16020491; PMCID: PMC1856085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrion A.F., Khaderi S.A., Sussman N.L. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transplant. 2016 Mar;22(3):279–280. doi: 10.1002/lt.24383. PMID: 26663608. [DOI] [PubMed] [Google Scholar]
- 26.Bunchorntavakul C., Reddy K.R. Treat chronic hepatitis C virus infection in decompensated cirrhosis - pre- or post-liver transplantation? the ironic conundrum in the era of effective and well-tolerated therapy. J Viral Hepat. 2016;23:408–418. doi: 10.1111/jvh.12534. [DOI] [PubMed] [Google Scholar]
- 27.Curry M.P., Forns X., Chung R.T., Terrault N.A., Brown R., Jr., Fenkel J.M., et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015 Jan;148(1):100–107.e1. doi: 10.1053/j.gastro.2014.09.023. Epub 2014 Sep 28. PMID: 25261839. [DOI] [PubMed] [Google Scholar]
- 28.Charlton M., Gane E., Manns M.P., Brown R.S., Jr., Curry M.P., Kwo P.Y., et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015 Jan;148(1):108–117. doi: 10.1053/j.gastro.2014.10.001. Epub 2014 Oct 7. PMID: 25304641. [DOI] [PubMed] [Google Scholar]
- 29.Mutimer D. Pre- and post-transplant treatment of viral hepatitis C. Dig Dis. 2017;35(4):347–350. doi: 10.1159/000456586. Epub 2017 May 3. PMID: 28468002. [DOI] [PubMed] [Google Scholar]
- 30.Poordad F., Schiff E.R., Vierling J.M., Landis C., Fontana R.J., Yang R., et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016 May;63(5):1493–1505. doi: 10.1002/hep.28446. Epub 2016 Mar 7. PMID: 26754432; PMCID: PMC5069651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backus L.I., Belperio P.S., Shahoumian T.A., Mole L.A. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69(2):487–497. doi: 10.1002/hep.29408. [DOI] [PubMed] [Google Scholar]
- 32.Lens S., Alvarado-Tapias E., Mariño Z., et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153(5) doi: 10.1053/j.gastro.2017.07.016. 1273-1283.e1. [DOI] [PubMed] [Google Scholar]
- 33.Foster G.R., Irving W.L., Cheung M.C., et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Flemming J.A., Kim W.R., Brosgart C.L., Terrault N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berenguer M., Palau A., Aguilera V., Rayón J.M., Juan F.S., Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008 Mar;8(3):679–687. doi: 10.1111/j.1600-6143.2007.02126.x. PMID: 18294165. [DOI] [PubMed] [Google Scholar]
- 36.Jothimani D., Chandy G., Conjeevaram H. A new era in the treatment of chronic hepatitis C infection. Indian J Gastroenterol. 2012;32:71–79. doi: 10.1007/s12664-012-0254-5. [DOI] [PubMed] [Google Scholar]
- 37.Jothimani D., Govil S., Rela M. Management of post liver transplantation recurrent hepatitis C infection with directly acting antiviral drugs: a review. Hepatol Int. 2016 Sep;10(5):749–761. doi: 10.1007/s12072-016-9744-3. [DOI] [PubMed] [Google Scholar]
- 38.Charlton M., Everson G.T., Flamm S.L., Kumar P., Landis C., Brown R.S., Jr., et al. SOLAR-1 investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015 Sep;149(3):649–659. doi: 10.1053/j.gastro.2015.05.010. Epub 2015 May 15. PMID: 25985734. [DOI] [PubMed] [Google Scholar]
- 39.Patel N., Bichoupan K., Ku L., Yalamanchili R., Harty A., Gardenier D., et al. Hepatic decompensation/serious adverse events in post-liver transplantation recipients on sofosbuvir for recurrent hepatitis C virus. World J Gastroenterol. 2016;22(9):2844–2854. doi: 10.3748/wjg.v22.i9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manns M., Samuel D., Gane E.J., Mutimer D., McCaughan G., Buti M., et al. SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016 Jun;16(6):685–697. doi: 10.1016/S1473-3099(16)00052-9. Epub 2016 Feb 18. Erratum in: Lancet Infect Dis. 2016 Jun;16(6):636. PMID: 26907736. [DOI] [PubMed] [Google Scholar]
- 41.Welzel T.M., Petersen J., Ferenci P. Safety and efficacy of daclatasvir plus sofosbuvir with or without ribavirin for the treatment of chronic HCV genotype 3 infection: interim results of a multicenter European compassionate use program. Hepatology. 2015;62(suppl 1):225A–226A. [Google Scholar]
- 42.Fontana R.J., Brown R.S., Jr., Moreno-Zamora A., Prieto M., Joshi S., Londoño M.C., et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transplant. 2016 Apr;22(4):446–458. doi: 10.1002/lt.24416. PMID: 26890629. [DOI] [PubMed] [Google Scholar]
- 43.Feld J.J., Jacobson I.M., Hézode C., Asselah T., Ruane P.J., Gruener N., et al. ASTRAL-1 investigators. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015 Dec 31;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. Epub 2015 Nov 16. PMID: 26571066. [DOI] [PubMed] [Google Scholar]
- 44.Prieto M., Berenguer M., Rayón J.M., Córdoba J., Argüello L., Carrasco D., et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999 Jan;29(1):250–256. doi: 10.1002/hep.510290122. PMID: 9862874. [DOI] [PubMed] [Google Scholar]
- 45.Rosen H.R., Gretch D.R., Oehlke M., Flora K.D., Benner K.G., Rabkin J.M., et al. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation. 1998 May 15;65(9):1178–1182. doi: 10.1097/00007890-199805150-00006. PMID: 9603164. [DOI] [PubMed] [Google Scholar]
- 46.Shackel N.A., Jamias J., Rahman W., Prakoso E., Strasser S.I., Koorey D.J., et al. Early high peak hepatitis C viral load levels independently predict hepatitis C-related liver failure post-liver transplantation. Liver Transplant. 2009 Jul;15(7):709–718. doi: 10.1002/lt.21747. PMID: 19562704. [DOI] [PubMed] [Google Scholar]
- 47.Arenas J.I., Vargas H.E., Rakela J. The use of hepatitis C-infected grafts in liver transplantation. Liver Transplant. 2003 Nov;9(11):S48–S51. doi: 10.1053/jlts.2003.50252. PMID: 14586895. [DOI] [PubMed] [Google Scholar]
- 48.Khapra A.P., Agarwal K., Fiel M.I., Kontorinis N., Hossain S., Emre S., et al. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transplant. 2006 Oct;12(10):1496–1503. doi: 10.1002/lt.20849. PMID: 16964597. [DOI] [PubMed] [Google Scholar]
- 49.Ballarin R., Cucchetti A., Spaggiari M., Montalti R., Di Benedetto F., Nadalin S., et al. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011 Jun 15;91(11):1265–1272. doi: 10.1097/TP.0b013e318219eb8f. PMID: 21478815. [DOI] [PubMed] [Google Scholar]
- 50.Álvaro E., Abradelo M., Fuertes A., Manrique A., Colina F., Alegre C., Calvo J., García M., García-Sesma A., Cambra F., Sanabria R., Moreno E., Jimenez C. Liver transplantation from anti-hepatitis C virus-positive donors: our experience. Transplant Proc. 2012 Jul-Aug;44(6):1475–1478. doi: 10.1016/j.transproceed.2012.05.012. PMID: 22841188. [DOI] [PubMed] [Google Scholar]
- 51.Feld J.J., Cypel M., Kumar D., Dahari H., Pinto Ribeiro R.V., Marks N., et al. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: a phase 3, single-centre, open-label study. Lancet Gastroenterol Hepatol. 2020 Jul;5(7):649–657. doi: 10.1016/S2468-1253(20)30081-9. Epub 2020 May 6. Erratum in: Lancet Gastroenterol Hepatol. 2020 Jul;5(7):e6. PMID: 32389183; PMCID: PMC7391837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellicelli A.M., Montalbano M., Lionetti R., Durand C., Ferenci P., D’Offizi G., et al. Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig Liver Dis. 2014 Oct;46(10):923–927. doi: 10.1016/j.dld.2014.06.004. Epub 2014 Jul 3. PMID: 24997638. [DOI] [PubMed] [Google Scholar]
- 53.Forns X., Charlton M., Denning J., McHutchison J.G., Symonds W.T., Brainard D., et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015 May;61(5):1485–1494. doi: 10.1002/hep.27681. Epub 2015 Mar 20. PMID: 25557906. [DOI] [PubMed] [Google Scholar]
- 54.McCashland T., Watt K., Lyden E., Adams L., Charlton M., Smith A.D., et al. Retransplantation for hepatitis C: results of a U.S. multicenter retransplant study. Liver Transplant. 2007 Sep;13(9):1246–1253. doi: 10.1002/lt.21322. PMID: 17763405. [DOI] [PubMed] [Google Scholar]
- 55.O’Grady G., Smith H.M., Davies S.E., Daniels H.M., Donaldson P.T., Tan K.C., et al. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol. 1992;14:104–111. doi: 10.1016/0168-8278(92)90138-f. [DOI] [PubMed] [Google Scholar]
- 56.Todo S., Demetris A.J., Van Thiel D., Teperman L., Fung J.J., Starzl T.E. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991 Apr;13(4):619–626. PMID: 2010156; PMCID: PMC2972630. [PMC free article] [PubMed] [Google Scholar]
- 57.Samuel D., Muller R., Alexander G., Fassati L., Ducot B., Benhamou J.P., Bismuth H. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993 Dec 16;329(25):1842–1847. doi: 10.1056/NEJM199312163292503. PMID: 8247035. [DOI] [PubMed] [Google Scholar]
- 58.Gane E.J., Angus P.W., Strasser S., Crawford D.H., Ring J., Jeffrey G.P., et al. Australasian Liver Transplant Study Group Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007 Mar;132(3):931–937. doi: 10.1053/j.gastro.2007.01.005. Epub 2007 Jan 5. PMID: 17383422. [DOI] [PubMed] [Google Scholar]
- 59.Fernández I., Loinaz C., Hernández O., Abradelo M., Manrique A., Calvo J., et al. Tenofovir/entecavir monotherapy after hepatitis B immunoglobulin withdrawal is safe and effective in the prevention of hepatitis B in liver transplant recipients. Transpl Infect Dis. 2015 Oct;17(5):695–701. doi: 10.1111/tid.12434. Epub 2015 Oct 3. PMID: 26257166. [DOI] [PubMed] [Google Scholar]
- 60.Cholongitas E., Vasiliadis T., Antoniadis N., Goulis I., Papanikolaou V., Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis. 2012 Oct;14(5):479–487. doi: 10.1111/j.1399-3062.2012.00741.x. Epub 2012 May 25. PMID: 22624695. [DOI] [PubMed] [Google Scholar]
- 61.Teperman L.W., Poordad F., Bzowej N., Martin P., Pungpapong S., Schiano T., et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transplant. 2013 Jun;19(6):594–601. doi: 10.1002/lt.23628. Epub 2013 Apr 9. PMID: 23447407. [DOI] [PubMed] [Google Scholar]
- 62.Wadhawan M., Gupta S., Goyal N., Taneja S., Kumar A. Living related liver transplantation for hepatitis B-related liver disease without hepatitis B immune globulin prophylaxis. Liver Transplant. 2013 Sep;19(9):1030–1035. doi: 10.1002/lt.23692. Epub 2013 Aug 18. PMID: 23788470. [DOI] [PubMed] [Google Scholar]
- 63.Sripongpun P., Mannalithara A., Kwo P.Y., Kim W.R. Potential benefits of switching liver transplant recipients to tenofovir alafenamide prophylaxis. Clin Gastroenterol Hepatol. 2020 Mar;18(3):747–749. doi: 10.1016/j.cgh.2019.05.057. Epub 2019 Jun 11. PMID: 31271737. [DOI] [PubMed] [Google Scholar]
- 64.Saab S., Waterman B., Chi A.C., Tong M.J. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transplant. 2010 Mar;16(3):300–307. doi: 10.1002/lt.21998. PMID: 20209589. [DOI] [PubMed] [Google Scholar]
- 65.Han J.H., Kim D.G., Na G.H., Kim E.Y., Lee S.H., Hong T.H., You Y.K., Choi J.Y., Yoon S.K. De novo hepatitis B virus infection developing after liver transplantation using a graft positive for hepatitis B core antibody. Ann Surg Treat Res. 2015 Sep;89(3):145–150. doi: 10.4174/astr.2015.89.3.145. Epub 2015 Aug 24. PMID: 26366384; PMCID: PMC4559617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu A.S., Vierling J.M., Colquhoun S.D., Arnaout W.S., Chan C.K., Khanafshar E., et al. Transmission of hepatitis B infection from hepatitis B core antibody--positive liver allografts is prevented by lamivudine therapy. Liver Transplant. 2001 Jun;7(6):513–517. doi: 10.1053/jlts.2001.23911. PMID: 11443579. [DOI] [PubMed] [Google Scholar]
- 67.Maguire D., Heaton N.D., Smith H.M. Failure of reactivation of hepatitis B after liver transplantation in hepatitis B surface antigen-negative, core antibody-positive recipients. Transplantation. 2002 Feb 15;73(3):481–482. doi: 10.1097/00007890-200202150-00027. PMID: 11884950. [DOI] [PubMed] [Google Scholar]
- 68.Mederacke I., Filmann N., Yurdaydin C., et al. Rapid early HDV RNA decline in the peripheral blood but prolonged intrahepatic hepatitis delta antigen persistence after liver transplantation. J Hepatol. 2012;56(1):115–122. doi: 10.1016/j.jhep.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 69.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. Epub 2020 Feb 3. PMID: 32015507; PMCID: PMC7095418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus10.1002/jmv.25688. Epub.2020 Feb 7. PMID: 31994738; PMCID: PMC7166400. [DOI] [PMC free article] [PubMed]
- 71.World Health Organization Situation report - 120 ; coronavirus disease 2019 (COVID-19) 19 may 2020. www.who.int
- 72.Zhou C. medRxiv; 2020. Evaluating new evidence in the early dynamics of the novel coronavirus COVID-19 outbreak inWuhan, China with real time domestic traffic and potential asymptomatic transmissions. [DOI] [Google Scholar]
- 73.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may CauseLiver damage after 2019-nCoV infection. BioRxiv Feb. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 74.Jothimani D., Venugopal R., Abedin M.F., Kaliamoorthy I., Rela M. COVID-19 and the liver. J Hepatol. 2020 Jun 15 doi: 10.1016/j.jhep.2020.06.006. S0168-8278(20)30377-9. Epub ahead of print. PMID: 32553666; PMCID: PMC7295524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iavarone M., D’Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020 Jun 9 doi: 10.1016/j.jhep.2020.06.001. S0168-8278(20)30365-2. Epub ahead of print. PMID: 32526252; PMCID: PMC7280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rela M, Patil V, Narasimhan G, Jothimani D. COVID-19 in Decompensated cirrhosis. Hepatology International. 2020 doi: 10.1007/s12072-020-10092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarin S.K., Choudhury A., Lau G.K., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) [published online ahead of print, 2020 Jul 4] Hepatol Int. 2020:1–11. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polak W.G., Fondevila C., Karam V., Adam R., Baumann U., Germani G., et al. Impact of COVID-19 on liver transplantation in europe: alert from an early survey of European liver and intestine transplantation association and European liver transplant registry. Transpl Int. 2020 Jul 1 doi: 10.1111/tri.13680. 10.1111/tri.13680. Epub ahead of print. PMID: 32609908; PMCID: PMC7361228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020 Jul;20(7):1800–1808. doi: 10.1111/ajt.15941. Epub 2020 May 10. PMID: 32330343; PMCID: PMC7264777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donato M.F., et al. Clin Gastroenterol Hepatol. 2020 Apr 22 doi: 10.1016/j.cgh.2020.04.041. S1542-3565(20)30538-3. [Epub ahead of print] [DOI] [Google Scholar]
- 81.Becchetti C., Zambelli M.F., Pasulo L COVID-LT group, et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020 Jun;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. Epub 2020 Apr 9. PMID: 32278366; PMCID: PMC7146678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020 Jul;5(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. Epub 2020 Apr 25. PMID: 32339474; PMCID: PMC7182520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236) doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee G.H., Tan B.H., Teo E.C., Lim S.G., Dan Y.Y., Wee A., et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016 Feb;150(2):355–357. doi: 10.1053/j.gastro.2015.10.048. e3. Epub 2015 Nov 6. PMID: 26551551. [DOI] [PubMed] [Google Scholar]
- 86.Meng X.J., Wiseman B., Elvinger F., Guenette D.K., Toth T.E., Engle R.E., Emerson S.U., Purcell R.H. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koning L., Charlton M.R., Pas S.D., Heimbach J.K., Osterhaus A.D., Watt K.D., et al. Prevalence and clinical consequences of Hepatitis E in patients who underwent liver transplantation for chronic Hepatitis C in the United States. BMC Infect Dis. 2015 Sep 2;15:371. doi: 10.1186/s12879-015-1103-9. PMID: 26328802; PMCID: PMC4557757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamar N., Selves J., Mansuy J.M., Ouezzani L., Péron J.M., Guitard J., et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008 Feb 21;358(8):811–817. doi: 10.1056/NEJMoa0706992. PMID: 18287603. [DOI] [PubMed] [Google Scholar]
- 89.Hoerning A., Hegen B., Wingen A.M., Cetiner M., Lainka E., Kathemann S., et al. Prevalence of hepatitis E virus infection in pediatric solid organ transplant recipients--a single-center experience. Pediatr Transplant. 2012 Nov;16(7):742–747. doi: 10.1111/j.1399-3046.2012.01740.x. Epub 2012 Jun 28. PMID: 22738211. [DOI] [PubMed] [Google Scholar]
- 90.Haagsma E.B., Niesters H.G., van den Berg A.P., Riezebos-Brilman A., Porte R.J., Vennema H., et al. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transplant. 2009 Oct;15(10):1225–1228. doi: 10.1002/lt.21819. PMID: 19790147. [DOI] [PubMed] [Google Scholar]
- 91.Legrand-Abravanel F., Kamar N., Sandres-Saune K., Lhomme S., Mansuy J.M., Muscari F., et al. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis. 2011 Jan;17(1):30–37. doi: 10.3201/eid1701.100527. PMID: 21192851; PMCID: PMC3298369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verna E.C. Hepatitis viruses and liver transplantation: evolving trends in antiviral management. Clin Liver Dis. 2014 Aug;18(3):575–601. doi: 10.1016/j.cld.2014.05.002. PMID: 25017077. [DOI] [PubMed] [Google Scholar]
- 93.Schlosser B., Stein A., Neuhaus R., Pahl S., Ramez B., Krüger D.H., et al. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012 Feb;56(2):500–502. doi: 10.1016/j.jhep.2011.06.021. Epub 2011 Jul 26. PMID: 21798217. [DOI] [PubMed] [Google Scholar]
- 94.Kamar N., Garrouste C., Haagsma E.B., Garrigue V., Pischke S., Chauvet C., et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011 May;140(5):1481–1489. doi: 10.1053/j.gastro.2011.02.050. Epub 2011 Feb 24. PMID: 21354150. [DOI] [PubMed] [Google Scholar]
- 95.Kamar N., Izopet J., Tripon S., Bismuth M., Hillaire S., Dumortier J., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014 Mar 20;370(12):1111–1120. doi: 10.1056/NEJMoa1215246. PMID: 24645943. [DOI] [PubMed] [Google Scholar]
- 96.Dao Thi V.L., Debing Y., Wu X., Rice C.M., Neyts J., Moradpour D., et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016 Jan;150(1):82–85. doi: 10.1053/j.gastro.2015.09.011. e4. Epub 2015 Sep 25. PMID: 26408347. [DOI] [PubMed] [Google Scholar]
- 97.Cornberg M., Pischke S., Müller T., Behrendt P., Piecha F., Benckert J., et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E - the HepNet SofE pilot study. J Hepatol. 2020 Sep;73(3):696–699. doi: 10.1016/j.jhep.2020.05.020. Epub 2020 Jul 2. PMID: 32624195. [DOI] [PubMed] [Google Scholar]
- 98.Razonable R.R., Emery V.C. 11th annual meeting of the IHMF (international herpes management forum). Management of CMV infection and disease in transplant patients. 27-29 february 2004. Herpesviridae. 2004 Dec;11(3):77–86. PMID: 15960905. [PubMed] [Google Scholar]
- 99.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016 Feb;64(2):433–485. doi: 10.1016/j.jhep.2015.10.006. Epub 2015 Nov 17. PMID: 26597456. [DOI] [PubMed] [Google Scholar]
- 100.Gane E., Saliba F., Valdecasas G.J., O’Grady J., Pescovitz M.D., Lyman S., et al. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected] Lancet. 1997 Dec 13;350(9093):1729–1733. doi: 10.1016/s0140-6736(97)05535-9. Erratum in: Lancet 1998 Feb 7;351(9100):454. PMID: 9413463. [DOI] [PubMed] [Google Scholar]
- 101.Razonable R.R., van Cruijsen H., Brown R.A., Wilson J.A., Harmsen W.S., Wiesner R.H., Smith T.F., Paya C.V. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis. 2003 Jun 1;187(11):1801–1808. doi: 10.1086/375194. Epub 2003 May 9. PMID: 12751039. [DOI] [PubMed] [Google Scholar]
- 102.Singh N., Wannstedt C., Keyes L., Wagener M.M., Cacciarelli T.V. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transplant. 2005 Jun;11(6):700–704. doi: 10.1002/lt.20417. PMID: 15915496. [DOI] [PubMed] [Google Scholar]
- 103.Paya C.V., Hermans P.E., Wiesner R.H., Ludwig J., Smith T.F., Rakela J., et al. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989 Nov;160(5):752–758. doi: 10.1093/infdis/160.5.752. PMID: 2553824. [DOI] [PubMed] [Google Scholar]
- 104.Razonable R.R., Paya C.V. Infections and allograft rejection - intertwined complications of organ transplantation. Swiss Med Wkly. 2005 Oct 1;135(39–40):571–573. doi: 10.4414/smw.2005.10984. PMID: 16333768. [DOI] [PubMed] [Google Scholar]
- 105.O’Grady J.G., Alexander G.J., Sutherland S., Donaldson P.T., Harvey F., Portmann B., et al. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988 Aug 6;2(8606):302–305. doi: 10.1016/s0140-6736(88)92356-2. PMID: 2899720. [DOI] [PubMed] [Google Scholar]
- 106.Noack K.B., Wiesner R.H., Batts K., van Hoek B., Ludwig J. Severe ductopenic rejection with features of vanishing bile duct syndrome: clinical, biochemical, and histologic evidence for spontaneous resolution. Transplant Proc. 1991 Feb;23(1 Pt 2):1448–1451. PMID: 1989260. [PubMed] [Google Scholar]
- 107.Pastacaldi S., Teixeira R., Montalto P., Rolles K., Burroughs A.K. Hepatic artery thrombosis after orthotopic liver transplantation: a review of nonsurgical causes. Liver Transplant. 2001 Feb;7(2):75–81. doi: 10.1053/jlts.2001.22040. PMID: 11172388. [DOI] [PubMed] [Google Scholar]
- 108.Humar A., Mazzulli T., Moussa G., Razonable R.R., Paya C.V., Pescovitz M.D., et al. Valganciclovir Solid Organ Transplant Study Group. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R- transplant recipients. Am J Transplant. 2005 May;5(5):1065–1070. doi: 10.1111/j.1600-6143.2005.00797.x. PMID: 15816887. [DOI] [PubMed] [Google Scholar]
- 109.Razonable R.R., Humar A., AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013 Mar;13(Suppl 4):93–106. doi: 10.1111/ajt.12103. PMID: 23465003. [DOI] [PubMed] [Google Scholar]
- 110.Humar A., Paya C., Pescovitz M.D., Dominguez E., Washburn K., Blumberg E., et al. Clinical utility of cytomegalovirus viral load testing for predicting CMV disease in D+/R- solid organ transplant recipients. Am J Transplant. 2004 Apr;4(4):644–649. doi: 10.1111/j.1600-6143.2004.00391.x. PMID: 15023158. [DOI] [PubMed] [Google Scholar]
- 111.Sun H.Y., Cacciarelli T.V., Wagener M.M., Singh N. Preemptive therapy for cytomegalovirus based on real-time measurement of viral load in liver transplant recipients. Transpl Immunol. 2010 Aug;23(4):166–169. doi: 10.1016/j.trim.2010.06.013. Epub 2010 Jul 4. PMID: 20609386. [DOI] [PubMed] [Google Scholar]
- 112.Breda G., Almeida B., Carstensen S., Bonfim C.M., Nogueira M.B., Vidal L.R., Almeida S.M., Raboni S.M. Human cytomegalovirus detection by real-time PCR and pp65-antigen test in hematopoietic stem cell transplant recipients: a challenge in low and middle-income countries. Pathog Glob Health. 2013 Sep;107(6):312–319. doi: 10.1179/2047773213Y.0000000114. PMID: 24188241; PMCID: PMC4001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kotton C.N., Kumar D., Caliendo A.M., Huprikar S., Chou S., Danziger-Isakov L., et al. The transplantation society international CMV consensus group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018 Jun;102(6):900–931. doi: 10.1097/TP.0000000000002191. PMID: 29596116. [DOI] [PubMed] [Google Scholar]
- 114.Singh N., Winston D.J., Razonable R.R., Lyon G.M., Silveira F.P., Wagener M.M., et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. J Am Med Assoc. 2020 Apr 14;323(14):1378–1387. doi: 10.1001/jama.2020.3138. PMID: 32286644; PMCID: PMC7157180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Onor I.O., Todd S.B., Meredith E., Perez S.D., Mehta A.K., Marshall Lyon G., et al. Evaluation of clinical outcomes of prophylactic versus preemptive cytomegalovirus strategy in liver transplant recipients. Transpl Int. 2013 Jun;26(6):592–600. doi: 10.1111/tri.12101. Epub 2013 Apr 16. PMID: 23590709. [DOI] [PubMed] [Google Scholar]
- 116.Yadav S.K., Saigal S., Choudhary N.S., Saha S., Kumar N., Soin A.S. Cytomegalovirus infection in liver transplant recipients: current approach to diagnosis and management. J Clin Exp Hepatol. 2017 Jun;7(2):144–151. doi: 10.1016/j.jceh.2017.05.011. Epub 2017 May 22. PMID: 28663679; PMCID: PMC5478971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grayson M.L., Cosgrove S.E., Crowe S., Hope W., McCarthy J.S., Mills J., Mouton J.W., Paterson D.L. CRC Press; Boca Raton, FL, USA: 2017. Kucers’ the Use of antibiotics: a clinical Review of antibacterial, antifungal, antiparasitic, and antiviral drugs; three volume set. [Google Scholar]
- 118.Eid A.J., Razonable R.R. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010 May 28;70(8):965–981. doi: 10.2165/10898540-000000000-00000. PMID: 20481654. [DOI] [PubMed] [Google Scholar]
- 119.Ligat G., Cazal R., Hantz S., Alain S. The human cytomegalovirus terminase complex as an antiviral target: a close-up view. FEMS Microbiol Rev. 2018 Mar 1;42(2):137–145. doi: 10.1093/femsre/fuy004. PMID: 29361041; PMCID: PMC5972660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Humar A., Lebranchu Y., Vincenti F., Blumberg E.A., Punch J.D., Limaye A.P., et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010 May;10(5):1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. Epub 2010 Mar 26. PMID: 20353469. [DOI] [PubMed] [Google Scholar]
- 121.Boillat Blanco N., Pascual M., Venetz J.P., Nseir G., Meylan P.R., Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. 2011 Jan 27;91(2):251–255. doi: 10.1097/TP.0b013e318200b9f0. PMID: 21099744. [DOI] [PubMed] [Google Scholar]
- 122.Sarmiento J.M., Dockrell D.H., Schwab T.R., Munn S.R., Paya C.V. Mycophenolate mofetil increases cytomegalovirus invasive organ disease in renal transplant patients. Clin Transplant. 2000 Apr;14(2):136–138. doi: 10.1034/j.1399-0012.2000.140206.x. PMID: 10770418. [DOI] [PubMed] [Google Scholar]
- 123.Herzer K., Strassburg C.P., Braun F., Engelmann C., Guba M., Lehner F., et al. Selection and use of immunosuppressive therapies after liver transplantation: current German practice. Clin Transplant. 2016 May;30(5):487–501. doi: 10.1111/ctr.12708. Epub 2016 Mar 31. PMID: 26855333. [DOI] [PubMed] [Google Scholar]
- 124.Malvezzi P., Jouve T., Rostaing L. Use of everolimus-based immunosuppression to decrease cytomegalovirus infection after kidney transplant. Exp Clin Transplant. 2016 Aug;14(4):361–366. doi: 10.6002/ect.2015.0292. Epub 2016 Apr 4. PMID: 27041365. [DOI] [PubMed] [Google Scholar]
- 125.Lurain N.S., Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010 Oct;23(4):689–712. doi: 10.1128/CMR.00009-10. PMID: 20930070; PMCID: PMC2952978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Straus S.E., Cohen J.I., Tosato G., Meier J. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993 Jan 1;118(1):45–58. doi: 10.7326/0003-4819-118-1-199301010-00009. PMID: 8380053. [DOI] [PubMed] [Google Scholar]
- 127.Abu-Elmagd K.M., Mazariegos G., Costa G., Sol- tys K., Bond G., Sindhi R., et al. Lymphoproliferative disorders and de novo malignancies in intestinal and multivisceral recipients: improved outcomes with new out- looks. Transplantation. 2009;88:926–934. doi: 10.1097/TP.0b013e3181b7509c. [DOI] [PubMed] [Google Scholar]
- 128.Kamdar K.Y., Rooney C.M., Heslop H.E. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011 Jun;16(3):274–280. doi: 10.1097/MOT.0b013e3283465715. PMID: 21467936; PMCID: PMC3167800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burra P., Buda A., Livi U., Rigotti P., Zanus G., Calabrese F., et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol. 2006 Oct;18(10):1065–1070. doi: 10.1097/01.meg.0000231752.50587.ae. PMID: 16957512. [DOI] [PubMed] [Google Scholar]
- 130.Dharnidharka V.R., Lamb K.E., Gregg J.A., Meier-Kriesche H.U. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR National Registry Data in the United States. Am J Transplant. 2012 Apr;12(4):976–983. doi: 10.1111/j.1600-6143.2011.03893.x. Epub 2012 Jan 6. PMID: 22226225. [DOI] [PubMed] [Google Scholar]
- 131.Parker A., Bowles K., Bradley J.A., Emery V., Featherstone C., Gupte G., et al. Haemato-oncology task force of the British committee for standards in haematology and British transplantation society. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS guidelines. Br J Haematol. 2010 Jun;149(5):675–692. doi: 10.1111/j.1365-2141.2010.08161.x. Epub 2010 Apr 16. PMID: 20408847. [DOI] [PubMed] [Google Scholar]
- 132.Tsai D.E., Douglas L., Andreadis C., Vogl D.T., Arnoldi S., Kotloff R., et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008 May;8(5):1016–1024. doi: 10.1111/j.1600-6143.2008.02183.x. Epub 2008 Feb 29. PMID: 18312608. [DOI] [PubMed] [Google Scholar]
- 133.Swerdlow S.H., World Health Organization . fourth ed. International Agency for Research on Cancer; Lyon, France: 2008. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer. [Google Scholar]
- 134.Dotti G., Fiocchi R., Motta T., Mammana C., Gotti E., Riva S., et al. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002 Oct 27;74(8):1095–1102. doi: 10.1097/00007890-200210270-00007. PMID: 12438953. [DOI] [PubMed] [Google Scholar]
- 135.Koch D.G., Christiansen L., Lazarchick J., Stuart R., Willner I.R., Reuben A. Posttransplantation lymphoproliferative disorder--the great mimic in liver transplantation: appraisal of the clinicopathologic spectrum and the role of Epstein-Barr virus. Liver Transplant. 2007 Jun;13(6):904–912. doi: 10.1002/lt.21152. PMID: 17539010. [DOI] [PubMed] [Google Scholar]
- 136.Allen U.D., Preiksaitis J.K., AST Infectious Diseases Community of Practice Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013 Mar;13(Suppl 4):107–120. doi: 10.1111/ajt.12104. MID: 23465004. [DOI] [PubMed] [Google Scholar]
- 137.Trappe R., Oertel S., Leblond V., Mollee P., Sender M., Reinke P., et al. German PTLD Study Group, European PTLD Network Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012 Feb;13(2):196–206. doi: 10.1016/S1470-2045(11)70300-X. Epub 2011 Dec 13. PMID: 22173060. [DOI] [PubMed] [Google Scholar]
- 138.Zimmermann H., Trappe R.U. Therapeutic options in post-transplant lymphoproliferative disorders. Ther Adv Hematol. 2011 Dec;2(6):393–407. doi: 10.1177/2040620711412417. PMID: 23556105; PMCID: PMC3573421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith J.S., Robinson N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002 Oct 15;186(Suppl 1):S3–S28. doi: 10.1086/343739. PMID: 12353183. [DOI] [PubMed] [Google Scholar]
- 140.Corey L. In: Mandell, douglas, and bennett’s principles and practice of infectious diseases. sixth ed. Mandell G.L., Bennett J.E., Dolin R., editors. Elsevier Churchill Livingstone; Philadelphia: 2005. Herpes simplex virus; pp. 1762–1780. [Google Scholar]
- 141.Singh N., Dummer J.S., Kusne S., Breinig M.K., Armstrong J.A., Makowka L., et al. Infections with cytomegalovirus and other herpesviruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988 Jul;158(1):124–131. doi: 10.1093/infdis/158.1.124. PMID: 2839576; PMCID: PMC2976493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gnann J.W. In: Transplant infections. second ed. Bowden R.A., Ljungman P., Paya C.V., editors. Lippincott, Williams, and Wilkins; Philadelphia: 2003. Herpes simplex virus and varicella-zoster virus infections in hematopoietic stem cell or solid organ transplantation; pp. 350–366. [Google Scholar]
- 143.Kusne S., Dummer J.S., Singh N., Iwatsuki S., Makowka L., Esquivel C., et al. Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine (Baltim) 1988 Mar;67(2):132–143. doi: 10.1097/00005792-198803000-00006. PMID: 3280944; PMCID: PMC2979316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel R., Paya C.V. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997 Jan;10(1):86–124. doi: 10.1128/CMR.10.1.86-124.1997. PMID: 8993860; PMCID: PMC172945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shepp D.H., Newton B.A., Dandliker P.S., Flournoy N., Meyers J.D. Oral acyclovir therapy for mucocutaneous herpes simplex virus infections in immunocompromised marrow transplant recipients. Ann Intern Med. 1985 Jun;102(6):783–785. doi: 10.7326/0003-4819-102-6-783. PMID: 2986508. [DOI] [PubMed] [Google Scholar]
- 146.Soul-Lawton J., Seaber E., On N., Wootton R., Rolan P., Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995 Dec;39(12):2759–2764. doi: 10.1128/aac.39.12.2759. PMID: 8593015; PMCID: PMC163025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wilck M.B., Zuckerman R.A., AST Infectious Diseases Community of Practice Herpes simplex virus in solid organ transplantation. Am J Transplant. 2013 Mar;13(Suppl 4):121–127. doi: 10.1111/ajt.12105. PMID: 23465005. [DOI] [PubMed] [Google Scholar]
- 148.Hamaguchi Y., Mori A., Uemura T., Ogawa K., Fujimoto Y., Okajima H., et al. Incidence and risk factors for herpes zoster in patients undergoing liver transplantation. Transpl Infect Dis. 2015 Oct;17(5):671–678. doi: 10.1111/tid.12425. Epub 2015 Sep 21. PMID: 26201686. [DOI] [PubMed] [Google Scholar]
- 149.Herrero J.I., Quiroga J., Sangro B., Pardo F., Rotellar F., Alvarez-Cienfuegos J., Prieto J. Herpes zoster after liver transplantation: incidence, risk factors, and complications. Liver Transplant. 2004 Sep;10(9):1140–1143. doi: 10.1002/lt.20219. PMID: 15350004. [DOI] [PubMed] [Google Scholar]
- 150.Pergam S.A., Limaye A.P., AST Infectious Diseases Community of Practice Varicella zoster virus in solid organ transplantation. Am J Transplant. 2013 Mar;13(Suppl 4):138–146. doi: 10.1111/ajt.12107. Suppl 4. PMID: 23465007; PMCID: PMC5331930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ablashi D., Agut H., Alvarez-Lafuente R., Clark D.A., Dewhurst S., DiLuca D., et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014 May;159(5):863–870. doi: 10.1007/s00705-013-1902-5. Epub 2013 Nov 6. PMID: 24193951; PMCID: PMC4750402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Bolle L., Naesens L., De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005 Jan;18(1):217–245. doi: 10.1128/CMR.18.1.217-245.2005. PMID: 15653828; PMCID: PMC544175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kondo K., Kondo T., Okuno T., Takahashi M., Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991 Jun;72(Pt 6):1401–1408. doi: 10.1099/0022-1317-72-6-1401. PMID: 1646280. [DOI] [PubMed] [Google Scholar]
- 154.Leong H.N., Tuke P.W., Tedder R.S., Khanom A.B., Eglin R.P., Atkinson C.E., et al. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of UK blood donors. J Med Virol. 2007 Jan;79(1):45–51. doi: 10.1002/jmv.20760. PMID: 17133548. [DOI] [PubMed] [Google Scholar]
- 155.Feldstein A.E., Razonable R.R., Boyce T.G., Freese D.K., El-Youssef M., Perrault J., et al. Prevalence and clinical significance of human herpesviruses 6 and 7 active infection in pediatric liver transplant patients. Pediatr Transplant. 2003 Apr;7(2):125–129. doi: 10.1034/j.1399-3046.2003.00028.x. PMID: 12654053. [DOI] [PubMed] [Google Scholar]
- 156.Emery V.C., Clark D.A. In: Human herpesviruses: biology, therapy, and immunoprophylaxis. Arvin A., Campadelli-Fiume G., Mocarski E., et al., editors. Cambridge University Press; Cambridge: 2007. HHV-6A, 6B, and 7: persistence in the population, epidemiology and transmission.https://www.ncbi.nlm.nih.gov/books/NBK47441/ [Chapter 49]. Available from: [PubMed] [Google Scholar]
- 157.Lautenschlager I., Höckerstedt K., Linnavuori K., Taskinen E. Human herpesvirus-6 infection after liver transplantation. Clin Infect Dis. 1998 Mar;26(3):702–707. doi: 10.1086/514592. PMID: 9524848. [DOI] [PubMed] [Google Scholar]
- 158.Lautenschlager I., Linnavuori K., Höckerstedt K. Human herpesvirus-6 antigenemia after liver transplantation. Transplantation. 2000 Jun 27;69(12):2561–2566. doi: 10.1097/00007890-200006270-00015. PMID: 10910277. [DOI] [PubMed] [Google Scholar]
- 159.Ohashi M., Sugata K., Ihira M., Asano Y., Egawa H., Takada Y., et al. Human herpesvirus 6 infection in adult living related liver transplant recipients. Liver Transplant. 2008 Jan;14(1):100–109. doi: 10.1002/lt.21304. PMID: 18161770. [DOI] [PubMed] [Google Scholar]
- 160.Humar A., Kumar D., Caliendo A.M., Moussa G., Ashi-Sulaiman A., Levy G., Mazzulli T. Clinical impact of human herpesvirus 6 infection after liver transplantation. Transplantation. 2002 Feb 27;73(4):599–604. doi: 10.1097/00007890-200202270-00021. PMID: 11889438. [DOI] [PubMed] [Google Scholar]
- 161.Potenza L., Luppi M., Barozzi P., Rossi G., Cocchi S., Codeluppi M., et al. HHV-6A in syncytial giant-cell hepatitis. N Engl J Med. 2008 Aug 7;359(6):593–602. doi: 10.1056/NEJMoa074479. PMID: 18687640. [DOI] [PubMed] [Google Scholar]
- 162.Pischke S., Gösling J., Engelmann I., Schlue J., Wölk B., Jäckel E., et al. High intrahepatic HHV-6 virus loads but neither CMV nor EBV are associated with decreased graft survival after diagnosis of graft hepatitis. J Hepatol. 2012 May;56(5):1063–1069. doi: 10.1016/j.jhep.2011.12.017. Epub 2012 Jan 13. PMID: 22245897. [DOI] [PubMed] [Google Scholar]
- 163.Revest M., Minjolle S., Veyer D., Lagathu G., Michelet C., Colimon R. Detection of HHV-6 in over a thousand samples: new types of infection revealed by an analysis of positive results. J Clin Virol. 2011 May;51(1):20–24. doi: 10.1016/j.jcv.2011.02.001. Epub 2011 Mar 3. PMID: 21376662. [DOI] [PubMed] [Google Scholar]
- 164.Härmä M., Höckerstedt K., Lyytikäinen O., Lautenschlager I. HHV-6 and HHV-7 antigenemia related to CMV infection after liver transplantation. J Med Virol. 2006 Jun;78(6):800–805. doi: 10.1002/jmv.20626. PMID: 16628583. [DOI] [PubMed] [Google Scholar]
- 165.Sampaio A.M., Guardia A.C., Milan A., Sasaki A.N., Andrade P.D., Bonon S.H., et al. Co-infection and clinical impact of human herpesvirus 5 and 6 in liver transplantation. Transplant Proc. 2012 Oct;44(8):2455–2458. doi: 10.1016/j.transproceed.2012.07.034. PMID: 23026619. [DOI] [PubMed] [Google Scholar]
- 166.Rogers J., Rohal S., Carrigan D.R., Kusne S., Knox K.K., Gayowski T., et al. Human herpesvirus-6 in liver transplant recipients: role in pathogenesis of fungal infections, neurologic complications, and outcome. Transplantation. 2000 Jun 27;69(12):2566–2573. doi: 10.1097/00007890-200006270-00016. PMID: 10910278. [DOI] [PubMed] [Google Scholar]
- 167.Guardia A.C., Stucchi R.S., Milan A., Costa S.C., Boin Ide F. Human herpesvirus-6 and cytomegalovirus DNA in liver donor biopsies and their correlation with HLA matches and acute cellular rejection. Braz J Infect Dis. 2014 Mar-Apr;18(2):220–224. doi: 10.1016/j.bjid.2013.07.007. Epub 2013 Nov 22. PMID: 24275367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Buyse S., Roque-Afonso A.M., Vaghefi P., Gigou M., Dussaix E., Duclos-Vallée J.C., et al. Acute hepatitis with periportal confluent necrosis associated with human herpesvirus 6 infection in liver transplant patients. Am J Clin Pathol. 2013 Sep;140(3):403–409. doi: 10.1309/AJCP0FWI2XAHECBJ. PMID: 23955460. [DOI] [PubMed] [Google Scholar]
- 169.Montejo M., Ramon Fernandez J., Testillano M., Valdivieso A., Aguirrebengoa K., Varas C., Olaizola A., De Urbina J.O. Encephalitis caused by human herpesvirus-6 in a liver transplant recipient. Eur Neurol. 2002;48(4):234–235. doi: 10.1159/000066172. PMID: 12422078. [DOI] [PubMed] [Google Scholar]
- 170.Pritchett J.C., Naesens L., Montoya J. In: Human herpesviruses HHV-6A, HHV-6B & HHV-7: diagnosis and clinical management. third ed. Flamand L., Lautenschlager I., Krueger G., Ablashi D., editors. Elsevier; Oxford, UK: 2014. Treating HHV-6 infections; p. 311-320. [Google Scholar]
- 171.Phan T.L., Lautenschlager I., Razonable R.R., Munoz F.M. HHV-6 in liver transplantation: a literature review. Liver Int. 2018 Feb;38(2):210–223. doi: 10.1111/liv.13506. Epub 2017 Jul 29. PMID: 28650593. [DOI] [PubMed] [Google Scholar]