Abstract
Glioma causes significant mortality across the world and the most aggressive type of brain cancer. The incidence of glioma is believed to increase in the next few decades and hence more efficient treatment strategies need to be developed for management of glioma. Herein, we examined the anticancer effects of Indirubin against a panel of human glioma cells and attempted to explore the underlying mechanisms. The results of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay showed that Indirubin could inhibit the growth of all the glioma cells but the lowest IC50 of 12.5 µM was observed against the U87 and U118 glioma cells. Additionally, the cytotoxic effects of Indirubin were comparatively negligible against the normal astrocytes with an IC50 of > 100 µM. Investigation of mechanism of action, revealed that Indirubin exerts growth inhibitory effects on the U87 and U118 glioma cells by autophagic and apoptotic cell death. Annexin V/PI staining assay showed that apoptotic cell percentage increased dose dependently. Apoptosis was associated with increase in Bax decrease in Bcl-2 expressions. Additionally, the expression of autophagic proteins such as LC3II, ATG12, ATG15 and Beclin 1 was also increased. Wound heal assay showed that Indirubin caused remarkable decrease in the migration of the U87 and U118 cells indicative of anti-metastatic potential of Indirubin. Taken together, these results suggest that Indirubin exerts potent anticancer effects on glioma cells and may prove essential in the management of glioma.
Keywords: Indirubin, Glioma, Autophagy, Apoptosis
Introduction
Gliomas include all tumors of glial origin and accounts for about 77% of all the primary tumors of brain (Ohgaki and Kleihues 2005). Considered to be among the most destructive human cancers, gliomas cause tremendous human mortality throughout the globe (Ostrom et al. 2014). Surgery followed by chemo- and radiotherapy is generally employment for the management of gliomas (Malmer et al. 2001). Despite improvements in treatment, the average survival still remains 16 months for grade four gliomas (Lenting et al. 2017). Therefore, there is need for the identification of biomarkers for early detection and exploration of novel therapeutic targets for efficient treatment of gliomas (Schwartzbaum et al. 2006). Over the years, the use of plant derived drugs has gained huge attention owing to their potency and lower side effects. Moreover, it is important to note that even many of the chemically synthesized drugs are derivatives of herbal isolated drugs (Cragg and Newman 2005). Although the use of herbal extracts dates back to times immemorial, but use of pure isolated compounds started only in the nineteenth century. Since, then a wide array of molecules have been isolated, evaluated and used for the treatment of several diseases and disorders (Shoeb 2006). Indirubin is a important plant metabolite commonly isolated from several plant species which include but are not limited to Baphicacanthus cusia, Indigofera suffruticosa and Polygonum tinctorium (Wu et al. 2008). Indirubin is valuable natural product with wide array of pharmacological properties (Nam et al. 2005). The anticancer properties of Indirubin are well reported in literature. Indirubin has been reported to exert anticancer effects on several cancer cells types by modulation of VEGFR2-mediated JAK/STAT3 signaling cascade (Zhang et al. 2011). Indirubin has also been shown to modulate the STAT3 signaling pathway to inhibit the growth of the ovarian cancer cells (Chen et al. 2018). Similarly, Indirubin has been reported to halt the growth of renal cancer cells by promoting apoptosis (Perabo et al. 2011). Nonetheless, the anticancer effects of Indirubin have not been extensively studied on the glioma cells. This study was therefore carried out to elucidate the anticancer effects of Indirubin on the human glioma cells in vitro. We for the first time report that Indirubin suppresses the proliferation of human glioma cells via induction of apoptosis and autophagy. Moreover, it also suppresses the migration of the human glioma cells. Taken together these results suggest that Indirubin may prove to be beneficial lead molecule for the development of chemotherapy for glioma.
Materials and methods
Cell cultures
The normal astrocytes and the glioma cell lines (U-87, U-118, M059K and Hs 683) were obtained from the ATCC, USA. The cells were cultured in RPMI 1640 (Thermo Scientific) medium supplemented with ampicillin and streptomycin (100 U/mL each) and 10% FBS at 37 °C in a humidified incubator containing 5% CO2.
Cell proliferation assay
The glioma cells and astrocytes were cultured with different doses of indirubin for 24 h at 37 °C in a 96-well plate a the density of 3 × 103 cells/well. Untreated cells served as control. Following 24-h cell culturing, each well of the plate was inoculated with 10 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent. The plate was again incubated for 4 h at 37 °C. The resultant formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The absorbance of the formazan solution in each well was read in a spectrophotometer at 450 nm and the values were used for determination of percentage cell proliferation.
Acridine orange and ethidium bromide staining assay
To investigate the effects indirubin on apoptosis in U87 and U118 cells cancer cells, the cells (0.6 × 106) were cultured in six well plates separately for 12 h and then treated with different doses of indirubin for 24 h. This was followed by washed with cold PBS buffer and fixation with ethyl alcohol. The cells were subsequently stained using a solution of acridine orange and ethidium bromide. The stained cells were examined under a fluorescent microscope for changes in nuclear morphology to assess the levels of apoptosis.
Annexin V/PI staining assay
ApoScan kit was used to determine the apoptotic U87 and U118 cell percentage. In brief, Indirubin (0, 6.2, 12.5 and 25 µM) treated U87 and U118 cells (5 × 105 cells per well) were incubated for 24 h. This was followed by the staining of these cells with annexin V-FITC or PI. The percentage of apoptotic U87 and U118 cells at each concentration was then determined by flow cytometery.
Electron microscopy
The induction of autophagy in Indirubin treated glioma cells was assessed by electron microscopy. In brief, the colorectal U87 and U118 cancer cells were treated with 00, 6.2, 12.5 and 25 µM Indirubin for 24 h. The cells were collected by trypsinization and subjected washing which was followed by fixation in glutaraldehyde (2%) in phosphate buffer (0.1 M). The cells were then post-fixed in osmium tetroxide (1%). This was followed by the treatment of the cells with ethanol and embedding in resin. The thin section were then cut with the help of an ultramicrotome and subjected to electron microscopy.
Wound-healing assay
After treatment of the U87 and U118 cells with Indirubin, the medium was removed and cells were subjected to PBS washing. A sterile pipette tip was employed to scratch a wound in each well and cells were subjected washing again and a picture was taken. The plates were subjected to culturing at 24 h and a picture was taken again under an inverted microscope (Leica, Germany). The wound width was compared by taking the initial and final pictures into consideration.
Western blotting
The indirubin treated U87 and U118 cells were digested in RIPA lysis buffer containing protease inhibitors. The cell lysates were centrifuged, and the total proteins content of the supernatants were determined using the Lowry method. From all samples, equal amounts of proteins were loaded and subjected to SDS-polyacrylamide gel electrophoresis, followed by blotting to polyvinylidene membranes. After transferring the gel contents to the membranes, the membranes were serially incubated with primary and secondary antibodies. Finally, high performance chemiluminescence reagent was used for obtaining the protein bands. Actin was used as the control in the western blotting studies.
Results
Indirubin decreases the viability of glioma cells
The MTT assay was employed to assess the effects of Indirubin (Fig. 1a) on the viability of a panel of glioma cells. Although Indirubin inhibited the growth of all the glioma cells (Table 1), more profound growth inhibitory effects were observed on the U87 and U118 glioma and cells with IC50 range from 12.5 to 25 µM. Indirubin caused a significant depletion in the viability of the U87 and U118 cells. The effects of Indirubin on the viability of the U87 and U118 cells were concentration dependent. The IC50 of Indirubin again the U87 and U118 glioma cells was found to be 12.5 µM (Fig. 1b). Interestingly, the effects of Indirubin on the normal astrocytes were less and an IC50 of > 100 µM was reported for Indirubin against these normal astrocytes (Table 1; Fig. 1b).
Fig. 1.
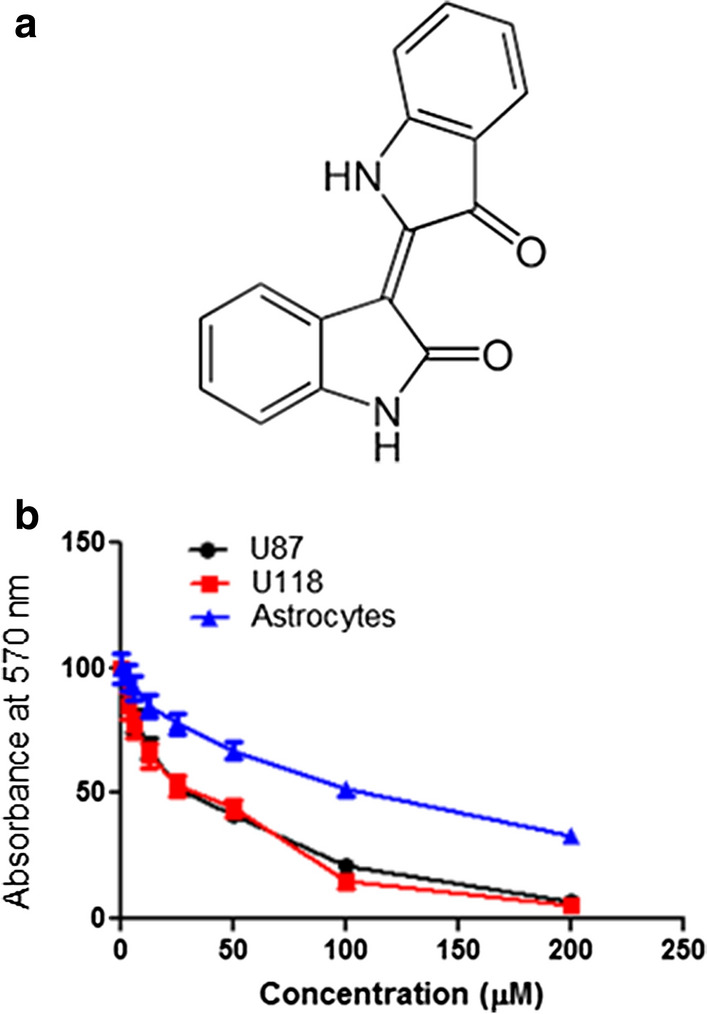
a Chemical structure of Indirubin, b MTT assays showing the effects of different concentrations of Indirubin on glioma U87 and U118 cells and normal human astrocytes. The experiments were performed in triplicate and expressed as mean ± SD (p < 0.05)
Table 1.
Anticancer activity of Indirubin against different glioma cell lines expressed as IC50
| S. no | Cell line | IC50 (µM) |
|---|---|---|
| 1 | Hs 683 | 25 |
| 2 | U-87 | 12.5 |
| 3 | M059K | 25 |
| 4 | U-118 | 12.5 |
| 5 | Astrocytes | > 100 |
Indirubin prompts apoptosis in the glioma cells
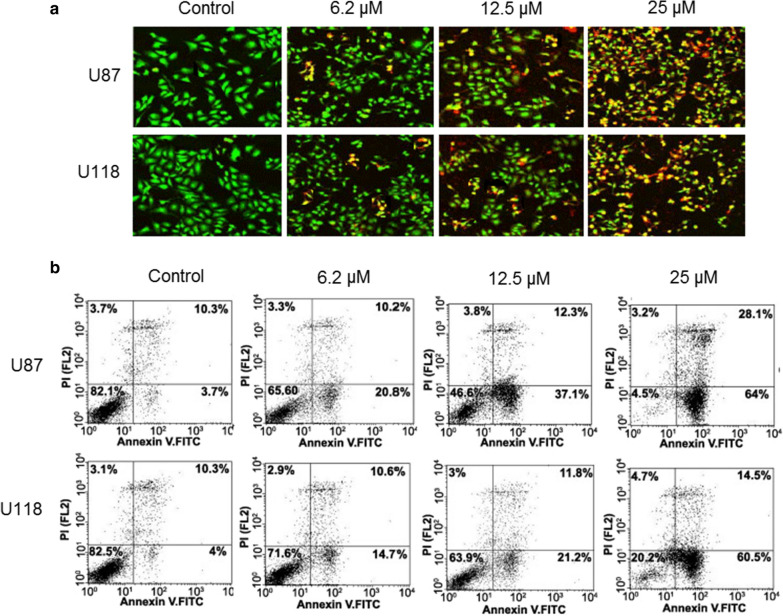
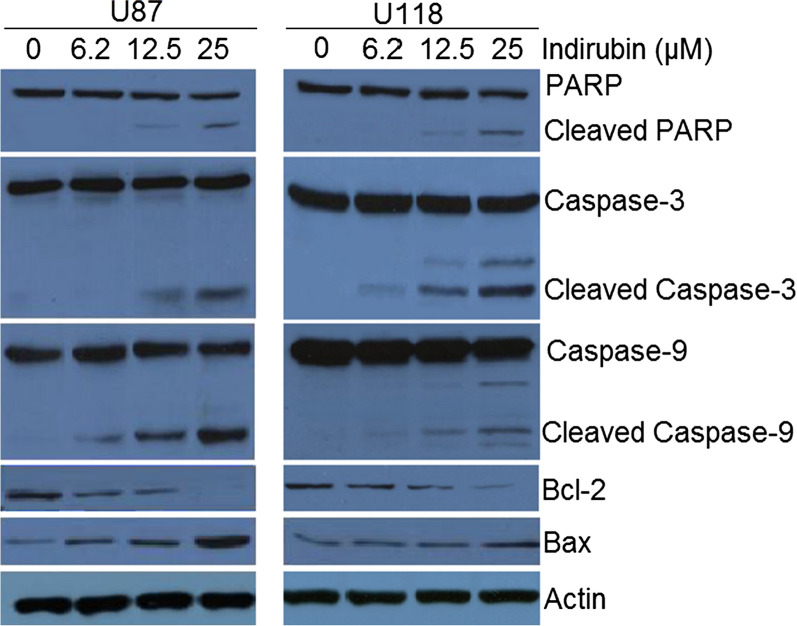
To ascertain the underlying mechanism for the growth inhibitory property of Indirubin, the U87 and U118 cells were treated with different doses of Indirubin and then stained with a solution of AO and EB. The results of showed that Indirubin caused nuclear fragmentation of both the U87 and U118 cells characteristic of apoptosis (Fig. 2a). Annexin V/PI staining was done to estimate the apoptotic cell percentage at different concentrations of Indirubin. It was found that percentage of apoptosis cells increased both in case of U87 and U118 cells with increase in the dosage of Indirubin (Fig. 2b). The apoptotic cell percentage was 3.7, 20.8, 37.1 and 64% for U87 cells and 4, 14.7, 21.2 and 60.5% for U118 cells at the Indirubin concentrations of 0, 6.2, 12.5 and 50 µM. Next the western blot analysis of the Indirubin treated U87 and U118 cells was performed to determine the expression of the apoptosis related proteins. It was found that in both U87 and U118 cells, the cleavage of PARP, caspase-3 and Caspase-9 was increased upon Indirubin treatment. Furthermore, the Indirubin caused increase of Bax and decrease of Bcl-2 expression in the glioma U87 and U118 cells, confirming the apoptotic cell death (Fig. 3).
Fig. 2.
a AO/EB staining showing the induction of apoptosis at in U87 and U118 cells. b Annexin V/PI staining showing the percentage of apoptotic U87 and U118 cells. The experiments were performed in triplicate
Fig. 3.
Western blots showing the effects of Indirubin on expression of apoptosis related proteins in U87 and U118 cells. The experiments were performed in triplicate
Autophagy inducing effects of Indirubin on the glioma cells
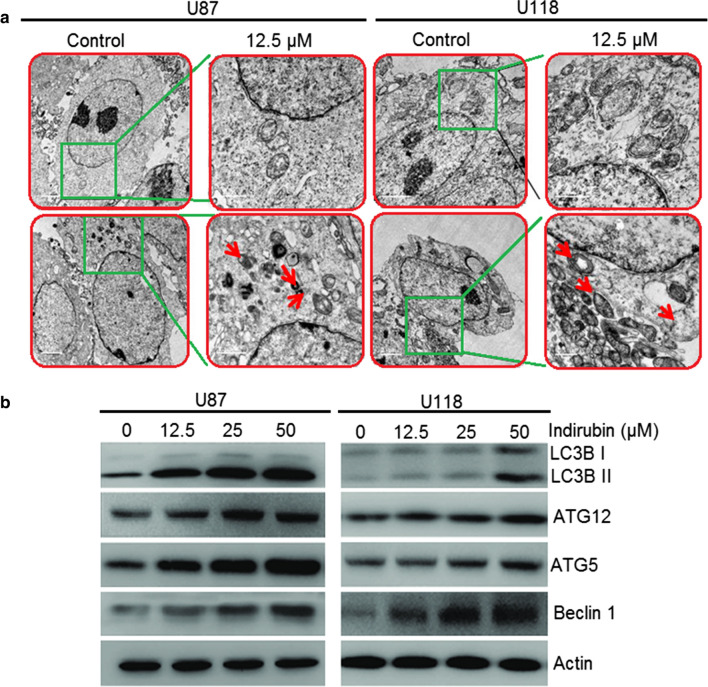
Next, electron microscopic analysis of the Indirubin treated U87 A and U118 cells was also performed. It was observed that Indirubin causes the development of the autophagic vesicles or autophagosomes in the U87 and U118 cells which are the hallmarks of autophagy (Fig. 4a). Moreover, Indirubin also caused increase in the protein levels of LC3B-II, ATG-5 and 12 as well as Beclin-1, indicative of autophagy. Nonetheless, no apparent effects were observed on the protein expression level of LC3B-I (Fig. 4b).
Fig. 4.
a Electron microscopic analysis of the U87 and U118 glioma cells showing the induction of autophagy (Arrows depict autophagosomes), b Effect of Indirubin on the expression of autophagy related proteins. The experiments were performed in triplicate
Indirubin suppresses the migration of glioma cells
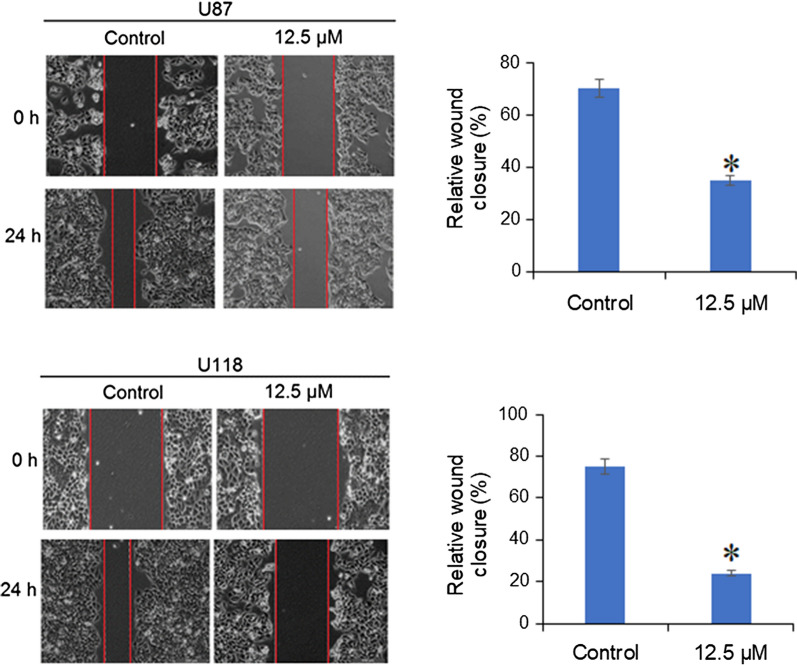
The effects Indirubin of the migration potential of the U87 and U118 glioma cells was assessed by wound heal assay. The results showed that the treatment of U87 and U118 cells with Indirubin for 24 h caused reduction in the migration of these cells as evident from the wound width (Fig. 5).
Fig. 5.
Effect of Indirubin at IC50 (25 µM) on the migration of the U87 and U118 glioma cells as depicted by the wound heal assay. The experiments were performed in triplicate
Discussion
Gliomas cause tremendous mortality and are among the most aggressive tumors. The limited availability of the reliable and efficient therapeutic targets/agents hurdles the treatment of glioma (Peckham-Gregory et al. 2018). The unsatisfactory clinical outcome, flawed treatment strategies and adverse effects on the existing drugs form some major hurdles in the glioma. In addition, emergence of chemoresistance and frequent relapse makes it more difficult to treat glioma (Ohgaki and Kleihues 2005; Ostrom et al. 2014). Herein, we report that Indirubin, an important constituent of several plant species, exerts growth inhibitory effects on the human glioma cells. The anticancer were of were also found to exhibit a dose-dependent pattern. These observations are in agreement with previous studies wherein Indirubin has been shown to cause the inhibition of the growth of the breast cancer cells (Paitoon et al. 2007). Additionally, it was observed that Indirubin exhibits negligible cytotoxicity on the normal human astrocytes suggesting that Indirubin acts specifically on the cancer cells. DAPI and annexin staining were performed to ascertain the mechanism behind the growth inhibitory effects of Indirubin. The results showed that Indirubin caused apoptosis of the U87 and U118 glioma cells and was also accompanied with enhancement of Bax and depletion of Bcl-2 expression. Moreover, the cleavage of PARP caspase 3 and 9 was also activated. Numerous studies have previously shown that Indirubin induces apoptosis on the human cancer cells, for instance, Indirubin and its derivatives have been reported to trigger apoptosis in human lung cancer cells by inhibiting cyclin dependent kinases (Nam et al. 2012). In myelogenous leukemia cells, Indirubin blocks STAT5 to induce apoptotic cell death (Perabo et al. 2011). Similarly, in lung adenocarcinoma cells, Indirubin induces apoptosis by regulating the NF-kB signal transduction (Sethi et al. 2006). Autophagy is degradation process that eliminates the defective proteins and organelles and plays an important role in inhibition of tumorigenesis (Kondo et al. 2005). Although, studies have also shown that Indirubin derivatives trigger autophagy in cancer cells (Lee et al. 2013) there is not any report about the autophagy inducing properties of Indirubin. Herein we for the first time showed that Indirubin induced autophagy in the U87 and U118 glioma cells which was also associated with upregulation of LC3B II, ATG12, ATG5 and Beclin, which are the biomarker proteins for autophagy (Cao et al. 2016). The effects of Indirubin were also investigated on the migration of the glioma cells by wound heal assay and it was found that Indirubin suppressed the migration of the glioma U87 as well as the U118 cells. These results are in agreement with previous studies wherein Indirubin has been shown to suppress the migration and invasion of the brain cancer cells (Williams et al. 2011). Taken together, the findings of the present study indicate that Indirubin exerts remarkable anticancer effects on the human glioma cells by induction of autophagy and apoptosis in vitro. Besides Indirubin also triggers inhibits the migration of glioma cells. Taken together, Indirubin may prove a potential lead molecule and warrants further investigations.
Acknowledgements
All the author of this manuscript is thankful to China–Japan Union Hospital of Jilin University, 130033, China to conduct the presented protocol.
Authors’ contributions
ZL and ZG designed the protocol of the study. ZL, HW, JW and LH performed the experimental work and collect the data for presented study. ZL and HW involve in the statistical analysis. ZG supervised the work and drafted the manuscript, although all author contributes for the preparation of manuscript. All authors read and approved the final manuscript.
Funding
National Science Foundation of China (No. 30672159).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13568-024-01711-6"
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/2/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s13568-024-01711-6
References
- Cao QH, Liu F, Yang ZL, Fu XH, Yang ZH, Liu Q, Wang L, Wan XB. Fan XJ (2016) Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Trans Res. 2016;8:3831. [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang J, Wu J, Zheng Q, Hu J. Indirubin suppresses ovarian cancer cell viabilities through the sTaT3 signaling pathway. Drug Des Dev Ther. 2018;12:3335. doi: 10.2147/DDDT.S174613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Grönberg H. Genetic epidemiology of glioma. Br J Cancer. 2001;84:4–29. doi: 10.1054/bjoc.2000.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Lee MY, Liu YW, Chen MH, Wu JY, Ho HY, Wang QF, Chuang JJ. Indirubin-3’-monoxime promotes autophagic and apoptotic death in JM1 human acute lymphoblastic leukemia cells and K562 human chronic myelogenous leukemia cells. Oncol Rep. 2013;29:2072–2078. doi: 10.3892/or.2013.2334. [DOI] [PubMed] [Google Scholar]
- Leenders W. Glioma: experimental models and reality. Acta Neuropathol. 2017;133:263–82. doi: 10.1007/s00401-017-1671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, Jove R. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Scuto A, Yang F, Chen W, Park S, Yoo HS, Konig H, Bhatia R, Cheng X, Merz KH, Eisenbrand G. Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling. Mol Oncol. 2012;6:276–283. doi: 10.1016/j.molonc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR. The epidemiology of glioma in adults: a “state of the science” review. Neurooncology. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitoon A, Supachok S, Suree P, Shui-Tein C. Simple purification of indirubin from Indigofera tinctoria Linn. and inhibitory effect on MCF-7 human breast cancer cells. Chiang Mai J Sci. 2007;34:329–337. [Google Scholar]
- Peckham-Gregory EC, Montenegro RE, Stevenson DA, Viskochil DH, Scheurer ME, Lupo PJ, Schiffman JD. Evaluation of racial disparities in pediatric optic pathway glioma incidence: results from the Surveillance, Epidemiology, and End Results Program, 2000–2014. Cancer Epidemiol. 2018;54:90–94. doi: 10.1016/j.canep.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perabo FG, Landwehrs G, Frössler C, Schmidt DH, Mueller SC (2011) Antiproliferative and apoptosis inducing effects of indirubin-3′-monoxime in renal cell cancer cells. In: Urologic oncology: seminars original investigations, vol 29, Elsevier. pp 815–820 [DOI] [PubMed]
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Rev Neurol. 2006;2:494. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-κB signaling pathway. J Biol Chem. 2006;281:23425–23435. doi: 10.1074/jbc.M602627200. [DOI] [PubMed] [Google Scholar]
- Shoeb M. Anti-cancer agents from medicinal plants. Bangladesh J Pharmacol. 2006;1:35–41. [Google Scholar]
- Williams SP, Nowicki MO, Liu F, Press R, Godlewski J, Abdel-Rasoul M, Kaur B, Fernandez SA, Chiocca EA, Lawler SE. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011;71:5374–5380. doi: 10.1158/0008-5472.CAN-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QW, Ge ZL, Gao Y, Zhang L. Inhibitory effect of indirubin on growth of some cancer cells and its mechanism. Tianjin J Traditional Chinese Med. 2008;25:55–58. [Google Scholar]
- Zhang X, Song Y, Wu Y, Dong Y, Lai L, Zhang J, Lu B, Dai F, He L, Liu M, Yi Z. Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell. Int J Cancer. 2011;129:2502–2511. doi: 10.1002/ijc.25909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.