Abstract
The majority of essential oils obtained from vascular plants have been demonstrated to be effective in treating fungal and bacterial infections. Among others, Salvia hydrangea is an endemic half-shrub belonging to the Lamiaceae family that has been widely used from ancient times in Iranian traditional medicine. The aim of this study was to compare the composition and antimicrobial properties of essential oils obtained from leaves or flowers of this plant, collected from the Daran region of Iran during June 2018. The oils were obtained using Clevenger apparatus, their composition was evaluated by means of gas chromatography/mass spectrometry (GC/MS) and the antimicrobial properties were assayed by measuring inhibition halos, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The yield of leaf oil was ~ 0.25% and that of flower oil was ~ 0.28%. Oil composition was affected by the part of the plants used: the most abundant bioactives contained in leaf essential oil were (+)-spathulenol (16.07%), 1,8-cineole (13.96%), trans-caryophyllene (9.58%), β-pinene (8.91%) and β-eudesmol (5.33%) and those in flower essential oil were caryophyllene oxide (35.47%), 1,8-cineole (9.54%), trans-caryophyllene (6.36%), β-eudesmol (4.11%), caryophyllenol-II (3.46%) and camphor (3.33%). Both the oils showed a significant inhibitory and lethal effect on the Gram-negative bacteria Pseudomonas aeruginosa (MIC ~ 16 µg/mL), Shigella dysenteriae and Klebsiella pneumoniae (MIC ~ 62 µg/mL). Therefore, the essential oils obtained from both leaves and flowers of S. hydrangea may have potential application as bactericidal agents against some bacteria.
Subject terms: Environmental biotechnology, Bioinorganic chemistry
Introduction
Essential oils are mainly composed of aromatic and volatile compounds and can be obtained from different parts of plants, especially the leaves and flowers1. Indeed, in plants, they are either secreted directly from the protoplasm by the degradation of cell membrane and resin materials or by the hydrolysis of some glycosides2. In particular, glycosides produced by different species of plants and stored in different organs have a strict relationship with biosynthesis, metabolism and biological activity and are mostly affected by the environmental climatic conditions3. Essential oils are usually rich in terpenes, sesquiterpenes, esters, aldehydes, phenols, ethers and peroxides3,4; they are mostly colorless or yellowish, less dense than water and soluble in organic solvents5. They are widely used in various industries, including the food and cosmetic industries among others6. Moreover, since ancient times, they have been widely used for the treatment of different disorders thanks to their well-known antioxidant, antimicrobial and antifungal properties7–9. Currently, essential oils have been proposed and tested in alternative medicine, especially as antimicrobial and antifungal products. Indeed, there is increased demand for safe and effective plant-derived bioactives as an alternative to antimicrobial synthetic drugs because their widespread and continuous use has led to the modification of microbes that have become resistant, thus reducing the therapeutic effect of these drugs10. Essential oils derived from plants have demonstrated promising antimicrobial therapeutic effects, which are generally accompanied by reduced side effects11. They have been screened and used in pharmacology, herbal pharmacology, medical microbiology and phytopathology12 especially because of their known insecticidal, antifungal, anti-parasitic, antibacterial, antiviral, antioxidant and cytotoxic properties13. These activities are related to the lipophilic nature of the hydrocarbon skeleton and of the functional groups of the bioactives. Indeed, the hydrophobic properties of the bioactives facilitate their interaction with bacteria and entrance into the cell, where they can exert their therapeutic effect. Rod-shaped cells and Gram-positive bacteria seem to be more sensitive than Gram-negative bacteria to these bioactives5,14.
Lamiaceae Martinov is one of the largest plant families in the world (~ 252 genera and 6700 taxa) and has the main differentiation center in the Mediterranean and Irano-Turanian biogeographic regions15–18. The majority of Lamiaceae produce terpenes and a wide variety of other compounds, which are mainly stored in the epidermal glands of leaves, stems and reproductive organs19.
One of the most important genera of Lamiaceae is Salvia L. with about 900 species worldwide and more than 70 species in Iran, 17 of which are endemic and exclusive to Iran20,21. Salvia, the name of which is derived from the word salwar which means healer, has been traditionally used as an anti-toxin and a restorative, aimed at strengthening the health and extending the longevity of both humans and soul22. The essential oils of Salvia species contain various bioactives such as terpenoids, steroids, flavonoids and polyphenols among others23 and their concentration varies as a function of the part of the plant used24. Indeed, many Salvia species and their essential oils are commonly used in pharmaceutical and cosmetic products or used as additives for foods (seasonings and flavors)25,26.
Previous studies reported that the main components contained in the essential oils from S. hydrangea DC. ex Benth. depend on the part of the plant used and the collection zone. Caryophyllene oxide and β-caryophyllene10,27; and β-caryophyllene, 1,8-cineole, α-pinene and caryophyllene oxide28 have been identified by different authors. Naphthalene, 1,8-cineole, camphor and α-terpineol are the main bioactives contained in plants at 2000 m above sea level, while 1,8-cineole, camphor, β-pinene, naphthalene and α-amorphene are those contained in plants at 1100 m above sea level29. Camphor, α-humulene30, α-pinene, 1,8-cineole, trans-caryophyllene and camphene31 have been found in the essential oil from aerial parts of S. hydrangea and 1,8-cineole, caryophyllene oxide, α-pinene and β-pinene32 are the major compounds detected in oil obtained only from the leaves of this plant.
The oil obtained from S. hydrangea flowers shows an in vitro anti-malarial effect due to the presence of high levels of pentacyclic triterpenes (mainly oleanic acid) that inhibit the growth of the malaria pathogen33. The essential oil from the aerial parts of S. hydrangea is effective against different bacteria10,28,30.
The present study aimed to investigate essential oil from both leaves and flowers of Iranian S. hydrangea. To this purpose, the chemical composition of oils has been determined and compared. Moreover, the variations in yield and antimicrobial activity as a function of the composition have been evaluated.
Materials and methods
Plant material
To select the sampling region, at first, habitats of the plant were identified through field surveys. Then, Daran region, located in Isfahan, Iran was selected (longitude: E 46° 49ʹ 02ʺ; latitude: N 36° 54ʹ 170ʺ). To sample the studied plant, in June 2018, coinciding with flowering, three points were selected randomly from Daran region. At each point, leaves and flowers of S. hydrangea were collected randomly from different plants (100 plants at each point). The specimens were transferred to the laboratory after being harvested and then exposed to free air to dry. One sample of the whole plant was also collected and pressed. The specimens were identified and recorded in the herbarium of the University of Kashan.
Isolation of essential oils
After complete drying, the samples were ground using a small electric mill. Each dried plant was weighed (100 g) and subjected to the extraction process by means of water distillation using Clevenger apparatus (made in Germany) for 5 h. The essential oil was dried by anhydrous sodium sulfate and after filtration was stored in dark bottles at 4 °C until use for further studies. Essential oil yield was calculated based on weight percent (w/w). This process was repeated three times for the oil from each plant part.
Gas chromatography/mass spectrometry (GC–MS) analyses
The main bioactives contained in the essential oils were determined by means of GC–MS, using an Agilent 6890 chromatograph coupled with an N-5973 mass spectrometer. A capillary column (HP-5MS) with a 5% methylphenylsiloxane static phase (length 30 m, internal diameter 0.25 mm, static layer thickness 0.25 μm) and ionization energy of 70 eV was used. The temperature for the analyses was first set at 60 °C and then increased at a rate of 3 °C/min up to 246 °C. The injector and detector temperatures were maintained at 250 °C, the volume of the injected sample was 1 µL and the helium carrier gas was maintained at a flow rate of 1.5 mL/min. Identification of chemical components was based on analysis of the chromatograms obtained for each oil, by means of evaluating the retention indices (RI) in comparison with standards of n-alkane mixtures (C8–C20) and the mass spectral data of each peak using a computer library (Wiley-14 and NIST-14 Mass Spectral Library), and comparison of the results with those contained in the literature34.
Bacterial strains tested
Twelve microorganisms, provided by the Iranian Research Organization for Science and Technology (IROST), were used to evaluate the antimicrobial activity of the essential oils: three Gram-positive bacteria, Staphylococcus epidermidis (ATCC 12228), Staphylococcus aureus (ATCC 29737) and Bacillus subtilis (ATCC 6633), and six Gram-negative bacteria, Klebsiella pneumoniae (ATCC 10031), Shigella dysenteriae (PTCC 1188), Pseudomonas aeruginosa (ATCC 27853), Salmonella paratyphi-A serotype (ATCC 5702), Proteus vulgaris (PTCC 1182) and Escherichia coli (ATCC 10536). Fungal strains were used as well: Aspergillus niger (ATCC 16404), Aspergillus brasiliensis (PTCC 5011) and Candida albicans (ATCC 10231). Bacterial strains were cultured overnight at 37 °C in nutrient agar and fungi were cultured overnight at 30 °C in Sabouraud dextrose agar.
Agar diffusion method
This procedure was performed according to CLSI standards: 6.0 mm diameter well plates containing Müller Hinton agar were prepared and 100 µL of bacterial suspension with a half-McFarland turbidity equivalent in culture medium were cultured. The essential oils were dissolved in dimethyl-sulfoxide (DMSO) at a concentration of 30 mg/mL; 10 μL (equivalent to 300 μg) of each oil was poured into the wells. The plates were incubated at 37 °C for 24 h for bacterial strains and 48 h and 72 h at 30 °C for yeast and fungi, respectively, and antimicrobial activity was evaluated for each microorganism by measuring the diameter of the inhibition halo (in millimeters), according to an antibiogram ruler. To evaluate the repeatability of the results, three replicates were performed for each essential oil and each strain. DMSO was used as a negative control. Gentamicin (10 µg/disk) and rifampin (5 µg/disk) for bacteria and nystatin (100 I.U.) for yeast were used as standard drugs for positive control in the same conditions as tested oils.
Minimum inhibitory concentration (MIC)
The minimum concentration able to inhibit the growth of bacteria and yeast was calculated by means of a microdilution method and for fungal strains was calculated by agar dilution assay. Essential oils (2000 μg/mL) were dissolved in a mixture of tryptic soy broth medium and DMSO and then opportunely diluted, using the same mixture, to reach different concentrations (1000, 500, 250, 125, 62.5, 31.25, and 15.63 mg/mL).
Sterile 96-well microplates were filled with 95 µL of culture medium, 5 µL of bacterial suspension with 0.5 McFarland dilution and 100 µL of the essential oil at different concentrations. Then, plates were incubated at 37 °C for 24 h for bacterial strains and 48 h at 30 °C for yeast. The MIC was determined by means of the improvement of opacity or the change in color. The MIC was the lowest concentration of an antimicrobial that inhibited visible growth (absence of turbidity).
Minimum bactericidal concentration (MBC)
To determine the minimum concentration able to kill the bacteria, the same microdilution method described above was used. After 24 h of incubation with both bacteria and oils at different concentrations, 5 µL of the content of each well was inoculated with nutrient agar medium and incubated at 37 °C for 24 h for bacterial strains. After incubation, the colony-forming units were enumerated. The MBC was the lowest concentration able to effectively reduce the growth of microorganisms (99.5%).
Statistical analysis
Statistical analysis was performed using SPSS software. First, the normality of the statistical variables was investigated using a Kolmogorov–Smirnov test. After that, to ensure the normality of the data, the variance was analyzed using one-way analysis of variance (ANOVA). Comparison of the means was performed using a Duncan test with a probability level of 5% error.
Results and discussion
Chemical composition of essential oils
The leaves or flowers of S. hydrangea were separately distilled by steam, resulting in two light yellow essential oils. The yield from leaves was ~ 0.25% and that from flowers was ~ 0.28%, lower than those obtained in previous studies based on extraction of the aerial parts of this plant, but it can be connected to both the species used and climatic growth conditions28–31. Indeed, secondary metabolites are generally synthetized in plants as they represent the most important defense mechanisms against pathogens; the amount produced along with the quality may vary as a function of habitat, the organ in which they are produced and climate conditions35,36. These differences are most likely due to differences in chemotype, which are also connected to environmental and climate conditions37. GC/MS analyses showed that the chemical composition of essential oils was similar even though oil from leaves contained 39 components and that from flowers only 27. They represent 99.96% and 97.85% of all the compounds, respectively (Table 1). The results obtained for flower oil are in agreement with previous studies in which the number of compounds in the aerial parts of this plant varied from 33 to 5428,30,31. Differently, Ghannadi et al.32 identified 13 compounds in the essential oil obtained from the leaves of this plant species, which represent a small part of those detected in this study. As previously underlined, this difference may be due to growth, genetic and environmental factors38.
Table 1.
Bioactives contained in essential oils from leaves and flowers of S. hydrangea.
| No. | Compound (%) | RIa | Relative percentage | Molecular formula | |
|---|---|---|---|---|---|
| Leaf | Flower | ||||
| 1 | α-Thujene | 863.546 | 0.45 ± 0.00u | – | C10H16 |
| 2 | α-Pinene | 871.921 | 3.61 ± 0.00h | 1.78 ± 0.01m | C10H16 |
| 3 | Camphene | 888.669 | 2.53 ± 0.00j | 0.83 ± 0.00q | C10H16 |
| 4 | β-Pinene | 914.238 | 8.91 ± 0.00d | 4.98 ± 0.00d | C10H16 |
| 5 | β-Myrcene | 920.198 | 0.62 ± 0.01t | – | C10H16 |
| 6 | α-Terpinene | 942.715 | 0.31 ± 0.00wx | – | C10H16 |
| 7 | 1,8-Cineole | 957.615 | 13.96 ± 0.00b | 9.54 ± 0.00b | C10H18O |
| 8 | 1,3,6-Octatriene | 966.556 | 0.42 ± 0.02uv | – | C8H12 |
| 9 | γ-Terpinene | 977.152 | 0.67 ± 0.00t | 0.52 ± 0.00t | C10H16 |
| 10 | α-Terpinolene | 1001.587 | 0.27 ± 0.00x | 1.28 ± 0.00o | C10H16 |
| 11 | Linalool | 1013.756 | 2.24 ± 0.06k | 1.70 ± 0.02m | C10H18O |
| 12 | Camphor | 1046.031 | 4.06 ± 0.00f | 3.33 ± 0.00g | C10H16O |
| 13 | Borneol | 1065.343 | 3.89 ± 0.00g | 0.96 ± 0.02p | C10H18O |
| 14 | α-Terpinen-4-ol | 1070.105 | 0.95 ± 0.01qr | 0.85 ± 0.02q | C10H18O |
| 15 | α-Terpineol | 1081.481 | 1.12 ± 0.02o | – | C10H18O |
| 16 | (−)-Bornyl acetate | 1136.298 | 1.76 ± 0.01m | 1.90 ± 0.02l | C12H20O2 |
| 17 | Thymol | 1157.932 | 1.59 ± 0.01n | – | C10H14O |
| 18 | β-Bourbonene | 1198.317 | 3.20 ± 0.07i | 2.05 ± 0.00j | C15H24 |
| 19 | cis-Jasmone | 1211.611 | 0.68 ± 0.01t | – | C11H16O |
| 20 | trans-Caryophyllene | 1221.563 | 9.58 ± 0.00c | 6.36 ± 0.00c | C15H24 |
| 21 | β-Cubebene | 1224.881 | 0.95 ± 0.01qr | – | C15H24 |
| 22 | γ-Cadinene | 1233.175 | 0.53 ± 0.01u | – | C15H24 |
| 23 | β-Farnesene | 1236.255 | 1.02 ± 0.00pq | 0.89 ± 0.03q | C15H24 |
| 24 | α-Humulene | 1239.810 | 0.72 ± 0.01t | 0.59 ± 0.00s | C15H24 |
| 25 | α-Amorphene | 1253.791 | 1.18 ± 0.00o | – | C15H24 |
| 26 | β-Selinene | 1258.767 | 0.37 ± 0.00vw | – | C15H24 |
| 27 | β-Bisabolene | 1268.009 | 0.80 ± 0.00s | 0.59 ± 0.01s | C15H24 |
| 28 | δ-Cadinene | 1277.488 | 0.63 ± 0.01t | – | C15H24 |
| 29 | Caryophyllene oxide | 1297.156 | 0.46 ± 0.00u | 35.47 ± 0.00a | C15H24O |
| 30 | (+) Spathulenol | 1318.886 | 16.07 ± 0.00a | – | C15H24O |
| 31 | Calarene | 1323.170 | 0.83 ± 0.00rs | – | C15H24 |
| 32 | (−)-Humulene epoxide II | 1331.234 | 1.06 ± 0.04op | 1.97 ± 0.00k | C15H24O |
| 33 | Widdrene | 1343.825 | 1.45 ± 0.07n | – | C15H24 |
| 34 | Adamantane | 1348.184 | – | 2.22 ± 0.00i | C10H16 |
| 35 | Isoaromadendrene epoxide | 1348.910 | 1.82 ± 0.02m | – | C15H24O |
| 36 | β-Eudesmol | 1357.584 | 5.33 ± 0.00e | 4.11 ± 0.00e | C15H26O |
| 37 | Valencene | 1359.806 | – | 1.39 ± 0.02n | C15H24 |
| 38 | Valeranone | 1365.859 | 1.81 ± 0.06m | 3.32 ± 0.02g | C15H24O |
| 39 | Caryophyllenol-II | 1368.523 | 2.03 ± 0.01l | 3.46 ± 0.00f | C15H24O |
| 40 | Calamenene | 1375.786 | 0.68 ± 0.00t | – | C15H22 |
| 41 | Phthalic acid | 1462.468 | – | 0.70 ± 0.04r | C8H6O4 |
| 42 | Palmitic acid | 1515.526 | 0.90 ± 0.02r | 2.98 ± 0.00h | C16H32O2 |
| 43 | p-Cymene | 1583.157 | – | 2.06 ± 0.01j | C10H14 |
| 44 | trans-Oleic acid | 1600.831 | – | 2.02 ± 0.02j | C18H34O2 |
| Total | 99.96 | 97.85 | |||
| Monoterpenes hydrocarbons | 17.37 | 13.65 | |||
| Oxygenated monoterpenes | 27.81 | 16.38 | |||
| Sesquiterpenes hydrocarbons | 21.94 | 11.87 | |||
| Oxygenated sesquiterpenes | 28.58 | 48.33 | |||
| Others | 3.76 | 7.6 | |||
aRetention indices (RIs) relative to n-alkanes (C6–C40) on the same methyl silicone capillary column. Values with different letters are statistically different (Duncan, p ≤ 0.05).
The most representative volatile constituents of S. hydrangea essential oils were oxygenated sesquiterpenes (28.58% in leaf oil and 48.33% in flower oil), in accordance with previous results39. Oxygenated monoterpenes were also present but in lower amounts (27.81% in leaf oil and 16.38% in flower oil), but these results were not in agreement with those obtained by Kotan et al.30, who detected a higher amount of these compounds. These differences may be related to the timing of plant collection and the ecological conditions38.
The ANOVA results showed that there was a significant difference between the mean of the components obtained for each of the essential oils of S. hydrangea flowers and leaves (P ≤ 0.05). The most abundant components in the essential oil from leaves were (+)-spathulenol (16.07%), 1,8-cineole (13.96%), trans-caryophyllene (9.58%), β-pinene (8.91%), β-eudesmol (5.33%), camphor (4.06%) and α-pinene (3.61%) (Table 1 and Fig. 1), in accordance with the findings of Ghannadi et al.32. However, (+)-spathulenol, β-eudesmol, trans-caryophyllene and camphor were detected for the first time in this study. Again, habitat and climate changes may affect the composition of the extractive products, as growth and growth stages, farming and genetic characteristics may vary significantly40.
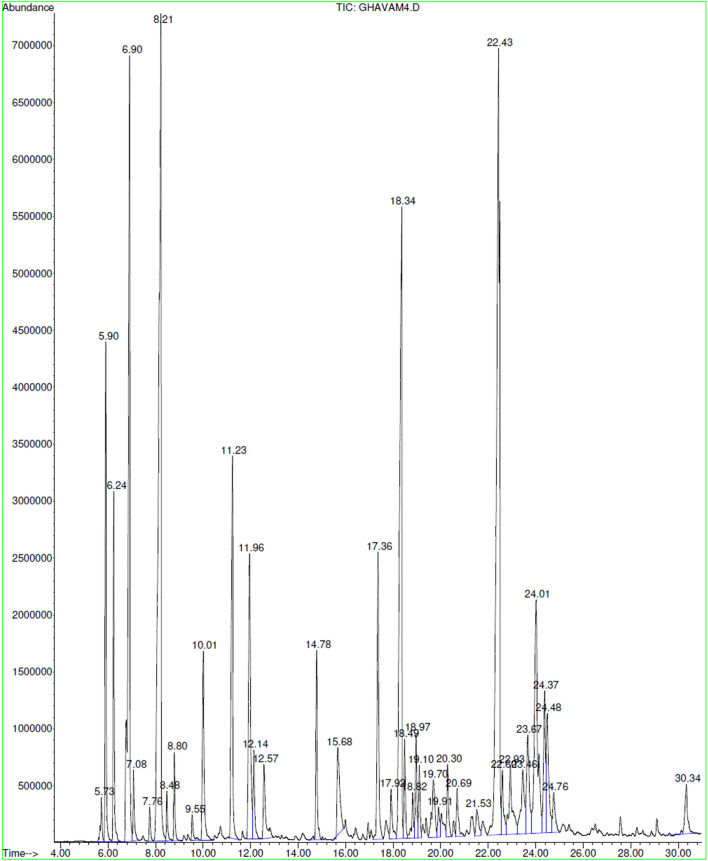
Figure 1.
Representative GC–MS chromatogram of essential oil from leaves of S. hydrangea.
The most abundant component in the essential oil from leaves was spathulenol, an alcoholic sesquiterpene with a primary skeleton similar to that of azulene. It has antibacterial and antifungal properties along with anti-inflammatory and anti-cancer activity and is also considered an inducer of apoptosis41. Spathulenol can be also used as a pesticide.
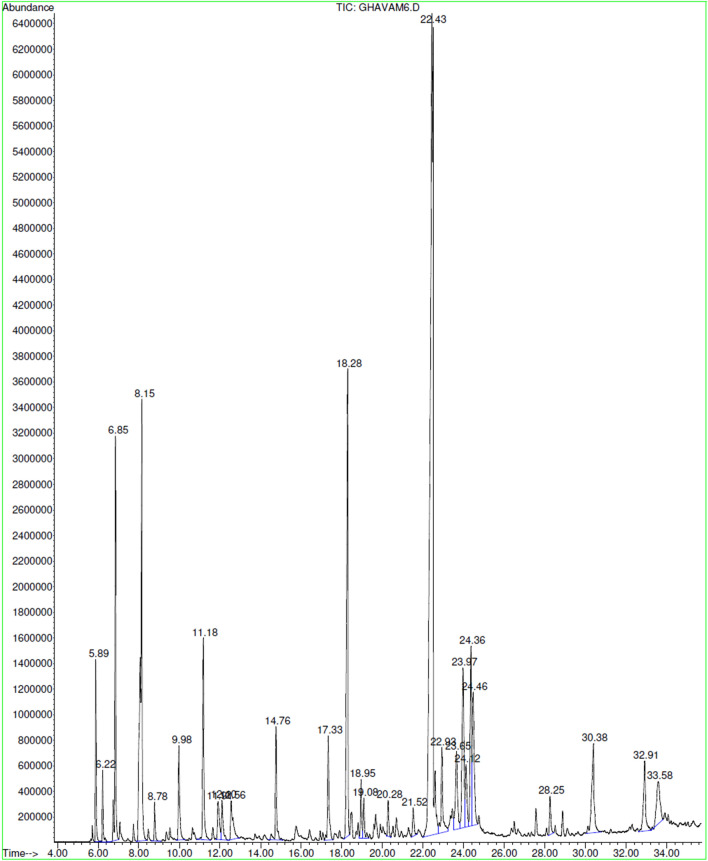
Caryophyllene oxide (35.47%), 1,8-cineole (9.54%), trans-caryophyllene (6.36%), β-eudesmol (4.11%), caryophyllenol-II (3.46%) and camphor (3.33%) were the most abundant compounds in the oil from S. hydrangea flowers (Table 1; Fig. 2). Caryophyllene oxide (55.4%) was also the most abundant component detected from the epigean parts of this plant in a previous study39, although other studies showed different results, as the amount of this component varied from 25.427 to 8.6%28. Caryophyllene oxide inhibits abnormal fluid accumulation in the intercellular spaces of both healthy and tumor tissues42.
Figure 2.
Representative GC–MS chromatogram of essential oil from flowers of S. hydrangea.
1,8-Cineole (9.54%) was the second most abundant bioactive in this essential oil, as reported previously by other authors, with some differences in the amount detected: 18.08%31, 15.2%28 and 9.45% (at elevations of 1100 m above sea level29). 1,8-Cineole has been successfully used in pharmaceutical and cosmetic fields thanks to its anti-parasitic and antifungal activity and insect-repellent properties. Moreover, it has been used as a key component in topical mouthwashes thanks to its analgesic properties43,44.
Trans-caryophyllene was detected in a high amount as well (6.36%) and it was the third main component of flower oil. This result is in agreement with that obtained previously by Mahdiyan et al.31, who detected a higher amount (17.38%) of this bioactive as it was also the third most abundant in their extractive products.
The amount of β-eudesmol detected (4.11%) was slightly lower than that found in the essential oil previously prepared (5.22%) by Ebrahimi et al.29. This small difference may be connected to the different habitat and altitude in which the same plant was grown. β-Eudesmol has been used in traditional medicine mainly because of its diuretic, anti-hypertensive, antipyretic, antiseptic and antimicrobial properties45–47.
Caryophyllene-II was detected for the first time in this study in the essential oil obtained from the flowers. A small amount of camphor was detected. Differently, it was the second main bioactive detected in the essential oil obtained by Ebrahimi et al.29; in particular, the amount was higher (12.06%) when the plant was cultivated at 1100 m above sea level and lower (5.71%) when cultivated at 2000 m above sea level. These differences confirm the key role of the environment (soil chemical composition and physiographic factors such as altitude) on the genetic and non-genetic variations of plants along with the production of secondary metabolites35,36,48. Camphor is a monoterpene, widely used in traditional and modern medicine thanks to its antimicrobial properties and beneficial effects on the cardiovascular system. Moreover, it has been used as a topical anti-itching treatment, insect repellent, anti-inflammatory and analgesic49,50. It is well accepted by patients as it has a bitter taste. In addition, its low solubility in aqueous solvents makes it an excellent candidate to be delivered in nanocarriers51.
Antimicrobial activity
Essential oils are traditionally used as antibacterial and antifungal agents in natural medicine. The increasing interest of modern society and the pharmaceutical industry for medicinal plants, makes crucial the scientific studies aimed at confirming these effects and founding new therapeutic agents52.
In this study, considering their promising composition, the antibacterial and antifungal activity of essential oils from S. hydrangea leaves or flowers was assayed (Table 2). The ANOVA results showed that there was a significant difference between the mean inhibition halos obtained on treating different microorganisms with the essential oil of flowers and leaves of S. hydrangea and antibiotics (P ≤ 0.05). The essential oil from S. hydrangea leaves was especially active against Gram-positive bacteria including Bacillus subtilis, Staphylococcus epidermidis and Staphylococcus aureus as the inhibition halo was large irrespective of the concentration used (~ 9.50 mm), even if it was significantly lower than that obtained with rifampin (19, 44 and 21 mm) and gentamicin (30, 39 and 27 mm). Similarly, Kotan et al.30 reported a weak antibacterial effect of the essential oil against Staphylococcus aureus (8 mm inhibition halo), while Sonboli et al.28 and Asadollahi et al.39, detected a better antibacterial activity, especially against Bacillus subtilis and Staphylococcus epidermidis which showed inhibition halos of 17 and 16 mm, respectively.
Table 2.
Inhibition halo (IH) diameter, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) obtained on treating microorganisms with the essential oils from leaves and flowers of S. hydrangea.
| Microorganism | Leaves | Flowers | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rifampin | Gentamicin | Nystatin | ||||||||||
| IH (mm) | MIC (µg/mL) | MBC (µg/mL) | IH (mm) | MIC (µg/mL) | MBC (µg/mL) | IH (mm) | MIC (µg/mL) | IH (mm) | MIC (µg/mL) | IH (mm) | MIC (µg/mL) | |
| Shigella dysenteriae | ND | 62.5 | 62.5 | ND | 62.5 | 125 | 9 ± 0.00b | 15.63 | 17 ± 0.01a | 3.90 | NA | NA |
| Pseudomonas aeruginosa | ND | 15.63 > | 15.63 > | ND | 15.63 > | 15.63 > | ND | 31.25 | 20 ± 0.00a | 7.81 | NA | NA |
| Bacillus subtilis | 9.50 ± 0.00 c | 250 | > 1000 | ND | 31.25 | 31.25 | 19 ± 0.00b | 31.25 | 30 ± 0.02a | 3.90 | NA | NA |
| Staphylococcus epidermidis | 9.50 ± 0.00d | 1000 | 1000 | 10.67 ± 1.15c | 250 | 250 | 44 ± 0.01a | 1.95 | 39 ± 0.00b | 1.95 | NA | NA |
| Escherichia coli | ND | 1000 | 1000 | ND | 31.25 | 125 | 10 ± 0.00b | 15.63 | 23 ± 0.02a | 31.25 | NA | NA |
| Staphylococcus aureus | 9.50 ± 0.50 c | 500 | > 1000 | ND | 125 | 125 | 21 ± 0.01b | 31.25 | 27 ± 0.03a | 1.95 | NA | NA |
| Klebsiella pneumoniae | ND | 62.50 | 62.5 | ND | 62.5 | 62.5 | 8 ± 0.00b | 15.63 | 17 ± 0.03a | 3.90 | NA | NA |
| Proteus vulgaris | ND | 250 | 250 | ND | 125 | 125 | 8 ± 0.00b | 15.63 | 24 ± 0.04a | 15.63 | NA | NA |
| Salmonella paratyphi-A | ND | 125 | 125 | ND | 62.5 | 125 | 8 ± 0.01b | 15.63 | 18 ± 0.01a | 3.90 | NA | NA |
| Candida albicans | ND | 250 | 250 | ND | 1000 | 1000 | NA | NA | NA | NA | 33 ± 0.01a | 125 |
| Aspergillu sniger | ND | > 2000 | > 2000 | ND | 2000 | 2000 | NA | NA | NA | NA | 27 ± 0.00a | 31.2 |
| Aspergillus brasiliensis | ND | > 2000 | > 2000 | ND | 2000 | 2000 | NA | NA | NA | NA | 30 ± 0.01a | 31.2 |
NA indicates no activity and ND indicates not determined. Mean values ± standard deviations of three cultures were reported (n = 3).
Values with different letters are statistically different (Duncan, p ≤ 0.05).
The essential oil from S. hydrangea flowers did not show any inhibition halo, except against the Gram-positive Staphylococcus epidermidis (~ 11 mm). It was significantly lower in comparison with those obtained after treatment with rifampin (~ 44 mm) and gentamicin (~ 39 mm).
The antibacterial activity of essential oils, especially that of the essential oil obtained from leaves, seems to be mainly related to the presence of two monoterpenes: α-pinene and β-pinene. The first is widely used in the manufacture of insecticides, sprays and disinfectants. Further, it has anti-inflammatory, antibacterial and anti-cancer properties, along with spasmolytic and skin redness properties. β-Pinene is generally used to manufacture aromatic oil and as a monomer in the production of terpene resins; moreover, it has anti-inflammatory and antibacterial activity. Both monoterpenes (α- and β-pinene) have antimicrobial activity against Gram-positive and Gram-negative bacteria, especially Escherichia coli, Staphylococcus bacteria53,54, Staphylococcus aureus and Bacillus subtilis55,56.
Essential oil from leaves or flowers of S. hydrangea was also effective in inhibiting the growth of bacteria. MIC values varied from 15.63 to 2000 µg/mL as a function of the organism tested and the oil used. The lowest MIC was found against Pseudomonas aeruginosa (15.63 μg/mL) irrespective of the oil used. This value was half that provided by rifampin (31.25 μg/mL) and double that for gentamicin (7.81 µg/mL). Moreover, these results are encouraging as in previous studies any inhibitory effect was detected by using essential oil from the aerial part of the same plant28,39.
The highest MIC (2000 µg/mL) was detected for Aspergillus brasiliensis and Aspergillus niger, which showed a high resistance to both oils. Inhibition of the growth of Gram-negative bacteria such as Staphylococcus epidermidis and Escherichia coli was low for leaf oil (MIC = 1000 µg/mL), but always higher than that obtained by Sonboli et al.28 and Asadollahi et al.39, who found an MIC of 1500 µg/mL against Escherichia coli. Surprisingly, the MIC (31.2 µg/mL) of flower oil against Escherichia coli, which is the main diarrhea-causing agent in humans, was significantly higher in comparison with the corresponding oil from leaves. It was similar to that provided by gentamicin (31.25 µg/mL), but significantly lower than that for rifampin (15.63 µg/mL).
The MIC value of S. hydrangea essential oils was the same against the Gram-negative bacteria Shigella dysenteriae (62.5 µg/mL), Pseudomonas aeruginosa (15.63 µg/mL) and Klebsiella pneumoniae (62.5 µg/mL), probably because of the similar composition of the two oils. However, the differences detected in the composition of the oils may be responsible for the greater inhibitory effect of the essential oil obtained from flowers against Gram-positive bacteria like Bacillus subtilis, Staphylococcus epidermidis and Staphylococcus aureus and Gram-negative bacteria such as Escherichia coli, Proteus vulgaris and Salmonella paratyphi-A. On the contrary, efficacy against Candida albicans was higher when the oil from leaves was used (MIC 250 µg/mL versus 1000 µg/mL provided by flower oil), especially when compared to the commercial antifungal nystatin (125 µg/mL).
In general, the ability of these essential oils to inhibit the growth of different bacterial strains was lower than that of both rifampin and gentamicin used as controls.
Essential oils from both leaves and flowers showed very low or no effectiveness against Gram-negative bacteria, in agreement with previous results28.
Other studies have shown that Gram-negative bacteria are more resistant to plant-derived essential oils in comparison with Gram-positive bacteria, probably because of the different composition of the lipopolysaccharide membrane. Indeed, the cell wall structure of Gram-negative bacteria is more complex than that of Gram-positive bacteria57,58. Due to the similar composition of oils from leaves and flowers, as reported above, the antibacterial activity can be attributed to the monoterpenes α- and β-pinene. However, since the amount of these two compounds was lower in the oil from flowers (Table 1), the inhibition of Staphylococcus epidermidis was also lower.
The MBC value of essential oils from both leaves and flowers varied from 15.63 to 2000 µg/mL. The results underline that the MBC values provided by the oil from leaves were always equal to the MIC values except for that for Bacillus subtilis. Differently, MBC values provided by flower oil were higher than MIC values against Gram-negative bacteria (Shigella dysenteriae, Escherichia coli, Proteus vulgaris and Salmonella paratyphi-A), while they were similar for all the other microorganisms treated. To the best of our knowledge, the MBC has been investigated for the first time in this work, thus no comparison can be made with previous studies.
The essential oils from S. hydrangea leaves and flowers have a significant inhibitory and lethal effect, especially against the Gram-negative bacterium Pseudomonas aeruginosa for which the lowest MIC and MBC values were found (15.63 µg/mL). This bacterium may cause opportunistic and often nosocomial infections. This efficacy may be specific to the essential oils from the leaves and flowers of S. hydrangea. Indeed, the activity of the genus Salvia against Pseudomonas aeruginosa is strictly dependent on the species used. Many species of the genus Salvia do not show any activity against this bacterium and a significant effect has only been reported for Salvia mirzayanii Rech.f. & Esfand.59. Similarly, the antimicrobial effect against the bacteria Shigella dysenteriae and Klebsiella pneumoniae, which may cause diarrhea and pneumonia, may vary as a function of the species used. In general, all Salvia species have significant antibacterial activity against both Gram-positive and Gram-negative bacteria (Bacillus, Klebsiella, Pseudomonas and Salmonella) and yeasts (Candida and Aspergillus). Therefore, the antimicrobial properties of Salvia against food spoilage and food-borne pathogens suggest its use as a natural preservative in food applications60,61.
As reported above, the antibacterial activity of essential oils obtained from different species of Salvia is mainly connected to their composition, especially oxygenated monoterpenes, which are present in high amounts62,63. They act by destroying the microbial cytoplasmic wall, improving its permeability and allowing the passage of large protons and ions. However, it is difficult to attribute the antibacterial effect to a specific compound because the essential oils obtained for different species may contain different mixtures of bioactives64. Further, due to the complexity of the composition of the essential oils, it is also difficult to explain the mechanism of action of these blends, but is important to underline that the wide variety of composition is a positive factor that may limit the development of resistance which is otherwise very common for synthetic drugs. In light of this, essential oils may represent a valid alternative to avoid the multidrug resistance of many pathogens, or they could be used in combination with antimicrobials to improve their effectiveness against different infectious diseases65. In addition, several studies have suggested that their delivery into ad hoc formulated carriers may improve their efficacy66.
Conclusions
The main component of the two essential oils obtained from leaves and flowers of S. hydrangea were spathulenol, 1,8-cineole, trans-caryophyllene, β-pinene, β-eudesmol, camphor, α-pinene and caryophyllene oxide. Both oils had significant inhibitory and lethal effects on the Gram-negative bacterium Pseudomonas aeruginosa, that is not common among different Salvia species. In addition, both were able to inhibit Shigella dysenteriae and Klebsiella pneumoniae, which are responsible for diarrhea and pneumonia in humans. Overall, the results underline the potential application of these oils for future development of new therapeutic systems and drug adjuvants, while additional tests should be performed to evaluate their effectiveness as food preservatives. This is a very interesting prospect, which has gained large interest in the last two decades due to awareness concerning the toxicity, ineffectiveness, antibiotic resistance and adverse effects provided by the widespread use of synthetic drugs and food preservatives.
Author contributions
M.G. was the supervisor, designer of the hypotheses, and responsible for all the steps (laboratory, statistical analysis, data analysis, etc.) and wrote the text of the article. M.L.M. helped with statistical analysis of data and to corrected and wrote part of the text. M.M. interpretaded of part of data, substantively revised the text and edited English language. G.B. identified and approved the study plant and edited the text.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharifi-Rad J, et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules. 2017;1:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao V. Phytochemicals A Global Perspective of Their Role in Nutrition and Health. Croatia: InTech; 2012. p. 548. [Google Scholar]
- 3.Omidbeigy R. Approaches to the Production and Processing of Medicinal Plants Vol II. Stockholm: Designers Publishing; 1995. p. 424. [Google Scholar]
- 4.Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;12:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;3:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 7.-Buchbauer, G., Jäger, W., Jirovetz, L., Ilmberger, J. & Dietrich, H. Therapeutic Properties of Essential Oils and Fragrances Vol 525, 159–165 (ACS Symposium Series, 1993).
- 8.Dagli N, Dagli R, Mahmoud RS, Baroudi K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015;5:335. doi: 10.4103/2231-0762.165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edris AE. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phyther. Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 10.Abdollahi A, Koohpayeh SA, Najafipoor S, Mansoori Y, Abdollahi S. Evaluation of drug resistance and Staphylococcal cassette chromosome (SCCmec) types among methicillin-resistant Staphylococcus aureus (MRSA) J. Alborz Health. 2013;1:47–52. [Google Scholar]
- 11.Meshkibaf MH, Abdollahi A, Fsihi Ramandi M, Adnani Sadati SJ, Moravvej A. Antibacterial effects of hydro-alcoholic extracts of Ziziphora tenuior, Teucrium polium, Berberis corcorde and Stachys inflata. Koomesh. 2011;4:240–245. [Google Scholar]
- 12.Defera DJ, Ziogas BN, Polission MG. GC/MS analysis of essential oils from some Greek aromatic plants and their fungitoxity on Penicillum digitatum. J. Agric. Food Chem. 2000;48:2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 13.Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. Determination of the chemical composition and antioxidant activity of the EO of Artemisia dracunculus and the antifungal and antibacterial activities of Artemisia absinthium and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- 14.Khorshidian N, Yousefi M, Khanniri E, Mortazavian AM. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Merg. Technol. 2018;45:62–72. doi: 10.1016/j.ifset.2017.09.020. [DOI] [Google Scholar]
- 15.Cantino PD, Harley RM, Wagstaff SJ. Genera of Labiatae: Status and classification. In: Harley RM, Reynolds T, editors. Advances in Labiate Science. Kew: Royal Botanic Gardens; 1992. pp. 511–522. [Google Scholar]
- 16.Cantino PD, Olmstead RG, Wagstaff SJ. A comparison of phylogenetic nomenclature with the current system: A botanical case study. Syst. Biol. 1997;46:313–331. doi: 10.1093/sysbio/46.2.313. [DOI] [Google Scholar]
- 17.Kadereit JW. The Families and Genera of Vascular Plants Vol 7. Berlin: Lamiales; 2004. pp. 1–219. [Google Scholar]
- 18.Bendiksby M, Thorbek L, Scheen AC, Lindqvist C, Ryding O. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon. 2011;2:471–484. doi: 10.1002/tax.602015. [DOI] [Google Scholar]
- 19.Baghalian K, Naghdibadi H. Essential Plants. 1. New York: Anders Publishing; 2000. [Google Scholar]
- 20.Hedge IC. Notes on some cultivated species of Salvia. J. R. Hortic. Soc. 1960;85:451–545. [Google Scholar]
- 21.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran: Farhang Moaser Press; 1996. p. 594. [Google Scholar]
- 22.Zargari A. Medicinal Plants. 7. Tehran: Tehran University Publication; 2012. p. 121. [Google Scholar]
- 23.Shirota O, Nagamatsu K, Sekita S. Neoclerodane diterpenes from the hallucinogenic Sage Salvia divinorum. J. Nat. Prod. 2006;69:1782–1786. doi: 10.1021/np060456f. [DOI] [PubMed] [Google Scholar]
- 24.Lakušić BS, Ristić MS, Slavkovska VN, Stojanović DL, Lakušić DV. Variations in EO yields and compositions of Salvia officinalis (Lamiaceae) at different developmental stages. Bot. Serbica. 2013;2:127–139. [Google Scholar]
- 25.Lambert Ortiz E. Encyclopedia of Herbs, Spices and Flavouring. London: Dorling Kinderslei; 1996. [Google Scholar]
- 26.Demirci B, Baser KHC, Yildiz B, Bahcecioglu Z. Composition of essential oils of six endemic Salvia spp. from Turkey. Flav. Frag J. 2003;18:116–121. doi: 10.1002/ffj.1173. [DOI] [Google Scholar]
- 27.Barazandeh M. Volatile constituents of the oil of Salvia hydrangea DC. ex Benth. from Iran. J. Essent. Oil Res. 2004;16:1–20. doi: 10.1080/10412905.2004.9698634. [DOI] [Google Scholar]
- 28.Sonboli A, Kanani M, Yousefzadi M, Mojarad M. Chemical composition and antibacterial activity of the EO of Salvia hydrangea from two localities of Iran. Iran. J. Med. Plants. 2009;2:20–2837. [Google Scholar]
- 29.Ebrahimi M, Ranjbar S. Essential oils of Salvia hydrangea DC. ex Benth. from Kiasar-Hezarjarib regions, Iran-impact of environmental factors as quality determinants. J. Med. Plants. By-prodt. 2016;2:159–167. [Google Scholar]
- 30.Kotan R, Kordali S, Cakir A, Kesdek M, Kaya Y, Kilic H. Antimicrobial and insecticidal activities of EO isolated from Turkish Salvia hydrangea. Biochem. Syst. Ecol. 2008;36:360–368. doi: 10.1016/j.bse.2007.12.003. [DOI] [Google Scholar]
- 31.Mahdiyan F, Ghasemi Pirbalouti A, Malekpoor F. Qualitative and quantitative changes in the EO of sage (Salvia hydrangea DC. Ex Benth.) as affected by different drying methods. J. Herbal Drug. 2016;4:269–274. [Google Scholar]
- 32.Ghannadi A, Samsam-Shariat H, Moattar F. Composition of the leaf oil of Salvia hydrangeaDC. ex Benth. grown in Iran preview access options. J. Essential Oil Res. 1999;11:745–746. doi: 10.1080/10412905.1999.9712010. [DOI] [Google Scholar]
- 33.Sairafianpour M, Bahreininejad B, Witt M, Ziegler HL, Jaroszewski JW, Staerk D. Terpenoids of Salvia hydrangea: Two new, rearranged 20-norabietanes and the effect of oleanolic acid on erythrocyte membrane. Planta Med. 2003;69:846. doi: 10.1055/s-2003-43212. [DOI] [PubMed] [Google Scholar]
- 34.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Quadruple Mass Spectroscopy. Carol Stream: Allured Publishing Cropration; 2007. p. 804. [Google Scholar]
- 35.Zargoosh Z, Ghavam M, Bacchetta G, Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019;9:16021. doi: 10.1038/s41598-019-52605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moradi H, Ghavam M, Tavili A. Study of antioxidant activity and some herbal compounds of Dracocephalum kotschyi Boiss. in different ages of growth. Biotech. Rep. 2020;25:00408. doi: 10.1016/j.btre.2019.e00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yavari AR, Nazeri V, Sefidkon F, Hassani ME. Evaluation of some ecological factors, morphological traits and EO productivity of Thymus migricus Klokov & Desj-Shost. Iran. J. Med. Aro Plants. 2010;2:227–238. [Google Scholar]
- 38.Omidbeigy R. Production and Processing of Medicinal Plants Vol 1. Mashhad: Behnashr Publication; 2005. p. 347. [Google Scholar]
- 39.Asadollahi M, Firuzi O, Heidary Jamebozorgi F, Alizadeh M, Jassbi AR. Ethnopharmacological studies, chemical composition, antibacterial and cytotoxic activities of essential oils of eleven Salvia in Iran. J. Herbal Med. 2018;20:100–250. [Google Scholar]
- 40.Millauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- 41.Taherkhani M, Masoudi Sh, Fatah Elahi R, Baradari T, Rustayian A. Identify the compounds in the essential oils of two plants from the Apiaceae family, Reichenb Torilis leptophylla and Boiss Thecocarpus meifolius, and study their antibacterial properties. Iran. J. Chem. Chem. Eng. 2012;31:70–65. [Google Scholar]
- 42.Jaimand, K. & Rezaei, MB. Essential Oil, Distillers, Test Methods and Inhibition Index in EO Analysis. First Edition. 350 (Publication of the Medicinal Plants Association, 2006).
- 43.Francisco JC, Sivik B. Solubility of three monoterpenes, their mixtures and eucalyptus leaf oils in dense carbon dioxide. J. Super Fluid. 2002;23:11–19. doi: 10.1016/S0896-8446(01)00131-0. [DOI] [Google Scholar]
- 44.Boland DJ, Brophy JJ, House AP. Eucalyptus Leaf Oil Use Chemistry, Distillation and Marketing. Melbourne: Inkatta Press; 1991. p. 252. [Google Scholar]
- 45.Kusuma IW, Ogawa T, Itoh K, Tachibana S. Isolation and identification of an antifungal sesquiterpene alcohol from Amboyna wood. Pak. J. Biol. Sci. 2004;7:1735–1740. doi: 10.3923/pjbs.2004.1735.1740. [DOI] [Google Scholar]
- 46.Ding HY, Wu YC, Lin HC. Phytochemical and pharmacological studies on Chinese changzhu. J. Chin. Chem. Soc. 2000;47:561–566. doi: 10.1002/jccs.200000075. [DOI] [Google Scholar]
- 47.Marinho CGS, Della Lucia TMC, Guedes RNC, Ribeiro MMR, Lima ER. β-eudesmol-induced aggression in the leaf-cutting ant Atta sexdens rubropilosa. Entomol. Exp. Appl. 2005;117:89–93. doi: 10.1111/j.1570-7458.2005.00338.x. [DOI] [Google Scholar]
- 48.Hasani J, NikBaher Z. Evaluation of ecological needs different species of thyme in Kurdistan Habitats. Echo J. Medil Plant. 2014;1:22–342014. [Google Scholar]
- 49.Majruhi AA. Study of variation in quantity and quality of the EO of Zhumeria majdae Rech. F. at different growth stages. J. Med. Plants. 2008;29:107–113. [Google Scholar]
- 50.Majdjabari T, Rustaiyan A, Vatanpuor H. Study the ingredients of in essential oil. Tanacetum khorassanicum (Krasch) Parsat. J. Med. Plants. 2003;6:15–20. [Google Scholar]
- 51.Manconi M, et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces. 2018;171:115–122. doi: 10.1016/j.colsurfb.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Baptista-Silva S, Borges S, Ramos OL, Pintado M, Bruno S. The progress of essential oils as potential therapeutic agents: A review. J. Essential Oil Res. 2020;4:279–295. doi: 10.1080/10412905.2020.1746698. [DOI] [Google Scholar]
- 53.Mohammadi N, Ghasemi A, Aghabarari B, Hamehi B. EOmixtures, anti-bacterial and antioxidant activity of EO of Nigella sativa L. different ecotypes in different habitats Iran. Ecol. Chem. J. Med Plants. 2016;4:58–68. [Google Scholar]
- 54.Dhar P, et al. Synthesis, antimicrobial evaluation, and structure activity relationship of α-pinene derivatives. J. Agric. Food Chem. 2014;16:3548–3552. doi: 10.1021/jf403586t. [DOI] [PubMed] [Google Scholar]
- 55.Gilsic S, Milojeij S, Dimitrjvi J, Orlovij A, Skala D. Antimicrobial activity of the EO and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007;4:311–320. doi: 10.2298/JSC0704311G. [DOI] [Google Scholar]
- 56.Leite AM, et al. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Ciên Farma. 2007;43:121–126. [Google Scholar]
- 57.Elshafie HS, Ghanney N, Mang SM, Ferchichi A, Camele I. An in Vitro attempt for controlling severe phytopathogens and human pathogens using essential oils from Mediterranean plants of genus Schinus. J. Med. Food. 2016;3:1–8. doi: 10.1089/jmf.2015.0093. [DOI] [PubMed] [Google Scholar]
- 58.Vikram A, Tripathi DN, Ramarao P, Jena GB. Evaluation of streptozotocin genotoxicity in rats from different ages using the micronucleus assay. Regul. Toxicol. Pharmacol. 2007;49:238–244. doi: 10.1016/j.yrtph.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Moshefim MH, Mehrabini M, Zolhasb H. Investigating the antimicrobial effects of Iranian sage and Azerbaijani sage extracts on six Gram-positive and Gram-negative microbial strains. J. Kerman Uni. Med. Sci. 2004;2:109–118. [Google Scholar]
- 60.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- 61.Sharifi-Rad M, et al. Salvia spp. Plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018;80:242–263. doi: 10.1016/j.tifs.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Norouzi-Arasi H, et al. Volatile constituents and antimicrobial activities of Salvia suffruticosa Montbr. & Auch. ex Benth. from Iran. Flavour Fragr. J. 2005;20:633–636. doi: 10.1002/ffj.1514. [DOI] [Google Scholar]
- 63.Tepe B, Donmez E, Unlu M. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha and Salvia multiculis. J. Food Chem. 2004;84:519–525. doi: 10.1016/S0308-8146(03)00267-X. [DOI] [Google Scholar]
- 64.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejcyk PP. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: Elucidation of the mechanism of action. Gene Pharm. 2008;24:1123–1131. doi: 10.1016/0306-3623(93)90359-6. [DOI] [PubMed] [Google Scholar]
- 65.Salem N, et al. Variation in chemical composition of Eucalyptus globulus EO under phonological stages and evidence synergism with antimicrobial standards. Ind. Crops Prod. 2018;124:115–125. doi: 10.1016/j.indcrop.2018.07.051. [DOI] [Google Scholar]
- 66.Usach I, et al. Comparison between citral and pompia essential oil loaded in phospholipid vesicles for the treatment of skin and mucosal infections. Nanomat (Basel Switzerland) 2020;2:286. doi: 10.3390/nano10020286. [DOI] [PMC free article] [PubMed] [Google Scholar]