Abstract
The collection of fungal spores by honey bees, Apis mellifera, can be classified as active or passive, the latter when spores are associated with pollen, nectar or honey dew. While low quality and shortage of pollen have been raised as hypotheses for fungal spore collection, the nutritional value of fungal spores for honey bees is poorly understood. Here we investigated the effect of consumption of fungal spores on survival, ovarian activation and the development of the hypopharyngeal glands (HPGs) in honey bee workers. Two pollen diets (Eucalyptus sp. pollen and a multifloral pollen) supplemented or not with spores of Botrytis cinerea, Cladosporium sp. or Colletotrichum acutatum were used. Consumption of diets that contained fungal spores increased the longevity of honey bee workers but had no significant effect on the development of their HPGs and ovaries. This demonstrates that fungal spores may have nutritional value for honey bees and that the consumption of fungal spores may compensate for nutritional imbalances of poor-quality pollen diets.
Subject terms: Ecology, Microbiology, Zoology
Introduction
Honey bees, Apis mellifera, are primary pollinators of crops worldwide1, 2 and without these pollinators, the yields of many fruit, seed and nut crops would decrease by more than 90%3. Hence, factors that affect bee health, such as the Varroa mite, associated viruses, pesticide use, and nutritional stress, are causes of concern4–6. Nutritional stress in honey bee hives can arise either through a lack of floral resources, for example due to drought, urbanisation or planting of crops that do not provide adequate food for bees. While nutrient deficiency can be obvious during pollination events in monocultures7–9, even polyfloral scenarios may be composed of combinations of floral resources that are either unhealthy due to the presence of certain toxic components or are lacking in certain amino acids10.
Spore collection has been described as ‘incidental’ or ‘the collection in the lieu of pollen’ (sic)11, 12, where the term ‘incidental’ refers to passive acquisition on the body, while ‘the collection in lieu of pollen’ refers to the occasional active collection by the bees. The use of these terms differs slightly from the historical classification of pollen-collecting behaviour of bees into ‘active’ and’ incidental’13–18. In the former case, active pollen collection refers to the purposeful uptake of pollen directly from anthers or other floral surfaces, while incidental pollen collection refers to pollen that accumulates on bees as they forage for nectar. To achieve clarity with regard to the use of these terms, we suggest that both pollen and spore collection be classified as ‘active’ and ‘passive’.
Shaw11 suggested that bees might be motivated to collect fungal spores due to (a) lack of floral resources, (b) the chemical composition of spores (i.e. nutrients, amino acids and steroids), (c) attractants such as colour or odour, and (d) because certain spores may resemble pollen grains. However, none of these hypotheses has been experimentally tested. Based on the fact that honey bees actively collect fungal spores as sole load, it has been suggested that the bees might obtain some nutritional benefit from their consumption11. Seemingly contrary to this suggestion, detrimental effects of consumption of pure spores of a rust fungus have been experimentally demonstrated19. However, bee bread, which is consumed by workers, is unlikely to consist solely of spores, as field-collected spore pellets become mixed with pollen during storage in the hive. Therefore, the health effects of consumption of fungal spores are unclear.
To investigate the functional significance of spore collection, we experimentally tested the effects of consumption of fungal spores in combination with pollen on bee health. We investigated whether diets of pollen plus fungal spores negatively or positively affected survival of workers, ovary activation and development of hypopharyngeal glands (HPGs). Both worker survival and HPG development are related to hive health. HPGs constitute a paired organ, composed of numerous vesicles or acini connected to a duct, in the head of workers20. Nurse bees feed the larvae with secretions from these glands21. The volume of the acini can serve as an indicator of the nutritional status of a colony21, as diets6 and fungicides22 have been observed to affect HPG development. Similarly, the nutritional value of forage has been linked to ovarian activation23. However, the impact of fungal spores on ovarian activation in workers has not been investigated. Here we investigate the effect of consumption of spores of three common fungi, Botrytis cinerea, Cladosporium sp. and Colletotrichum acutatum, in association with Eucalyptus sp. pollen (EP) and multifloral pollen (MP) on longevity, the volume of acini in the HPGs and ovarian activation in honey bee workers. These fungi were chosen because honey bee workers have been observed to actively collect spores of Cladosporium spp.24 and because Botrytis cinerea and Colletotrichum acutatum, important pathogens of a number of crops, have been demonstrated to be vectored by honey bees12, 25.
Results
Establishment of the spore concentration in the diet
Honey bee workers did not consume diets solely comprising spores of any of the three species of fungi tested. In addition, workers did not consume diets comprising EP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum at weight ratios of 5:1 and 10:1. There was no significant difference in the diet consumption rate among the treatments involving the monofloral EP with or without spores of B. cinerea, Cladosporium sp. or C. acutatum at the ratio of 20:1 (F = 2.34, df = 3, P = 0.07, Table S1). Diet consumption rates during the 7-day experiments in which the effects on the hypopharyngeal glands and ovaries were assessed, were similar among the treatments (F = 0.005, df = 3, P = 0.99). There was no significant interaction between treatments and cages. Hence, as the ratio of 20:1 of pollen to spores allowed assessment of the effects of spore consumption in association with EP, it was used for further assessments.
Effects of spore consumption on the survival of workers
In an initial experiment in which several ratios of pollen to spores were examined, the survival curves for the workers fed with the monofloral EP with and without fungal spores differed significantly among the treatments (log-rank test and Bonferroni correction: X2 = 32, df = 3, P < 0.05; Fig. 1, Table 1). Workers fed on EP as the sole diet died sooner than bees that consumed the mixture of pollen and fungal spores. Overall, the maximum survival of the bees that consumed EP only, or EP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum was 39, 40, 39 and 46 days, respectively.
Figure 1.
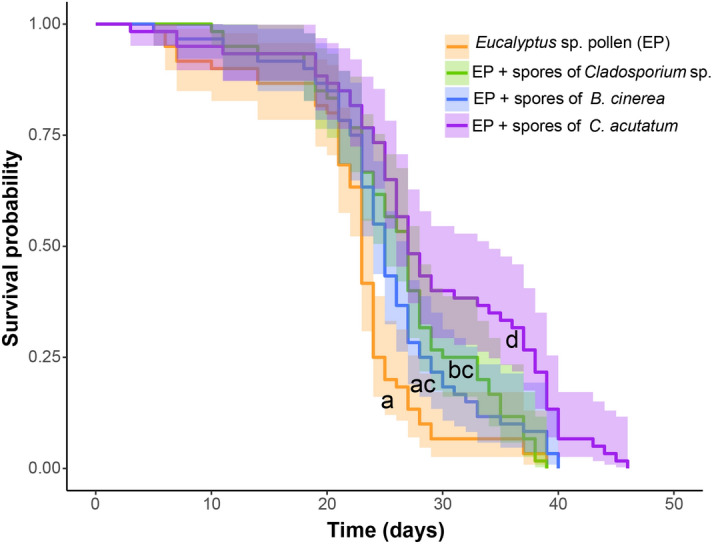
Survival probability of honey bee workers fed Eucalyptus sp. pollen alone or supplemented with spores of Botrytis cinerea, Cladosporium sp. or Colletotrichum acutatum at a mass ratio of 20:1 (Mean age at death ± standard error for the treatments in the above order were: 22.43 ± 0.90, 25.31 ± 0.92, 26.5 ± 0.89 and 29.1 ± 1.21). Shaded areas represent 95% confidence intervals. Lower case letters indicate significant differences among the diets (Kaplan–Meier method, log-Rank test P < 0.05 and Bonferroni correction).
Table 1.
P values of the survival analysis for both experiments by the Kaplan–Meier method, log rank test and Bonferroni correction.
| EP | EP + spores of B. cinerea | EP + spores of Cladosporium sp. | EP + spores of C. acutatum | MP | MP + spores of B. cinerea | MP + spores of Cladosporium sp. | |
|---|---|---|---|---|---|---|---|
| Eucalyptus sp. pollen only | |||||||
| EP + spores of B. cinerea | n.s | – | – | – | – | – | – |
| EP + spores of Cladosporium sp. | 0.02 | n.s | – | – | – | – | – |
| EP + spores of C. acutatum | 2.5 × 10–6 | 0.01 | 0.02 | – | – | – | – |
| Eucalyptus sp. and multifloral pollen | |||||||
| EP + spores of B. cinerea | 0.001 | – | – | – | – | – | – |
| EP + spores of Cladosporium sp. | 4.2 × 10–5 | n.s | – | – | – | – | – |
| EP + spores of C. acutatum | 0.002 | n.s | n.s | – | – | – | – |
| MP | n.s | n.s | 0.0005 | 0.03 | – | – | – |
| MP + spores of B. cinerea | n.s | n.s | 0.004 | n.s | n.s | – | – |
| MP + spores of Cladosporium sp. | 0.005 | n.s | n.s | n.s | n.s | n.s | – |
| MP + spores of C. acutatum | 0.01 | n.s | n.s | n.s | n.s | n.s | n.s |
EP = Eucalyptus sp. pollen, MP = Multifloral pollen, n.s. – Not significant.
Similar results were obtained in the subsequent comparative experiment using the two pollen diets (i.e. EP and MP) with or without spores of B. cinerea, Cladosporium sp. or C. acutatum. The survival curves differed significantly between the treatments (log-rank test and Bonferroni correction: X2 = 53.8, df = 7, P < 0.05; Fig. 2; Table 1). Survival of workers that were fed with the pollen-only diets was significantly shorter than of those that consumed the mixtures of pollen and fungal spores, except for the combination of the MP with spores of B. cinerea. Overall, the maximum survival of workers that were fed EP only and EP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum was 25, 28, 43 and 40 days, respectively. The maximum survival of workers that were fed MP only and MP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum was 29, 27, 30 and 36 days, respectively. In most cases, the survival of bees that were fed MP was shorter than the bees fed on EP. This corresponds with the generally poorer nutritious value of the multifloral pollen (Table 2). There were no significant differences among the treatments: EP, MP and MP supplemented with spores of B. cinerea; EP with spores of B. cinerea and all of the diets with the exception of EP alone; EP supplemented with spores of Cladosporium sp., MP with spores of Cladosporium sp. and MP with spores of C. acutatum; EP supplemented with spores of C. acutatum, MP supplemented with spores of either B. cinerea, Cladosporium sp. or C. acutatum; or MP supplemented with spores of either B. cinerea, Cladosporium sp. C. acutatum. There was no significant difference among the diets involving the EP and the MP with or without fungal spores in terms of amount consumed per bee (F = 0.91, df = 7, P = 0.49, Table S1).
Figure 2.
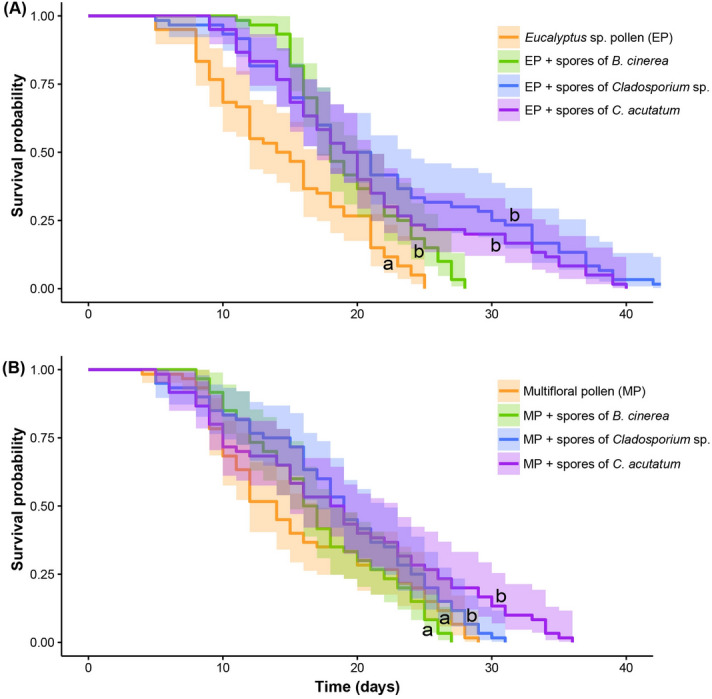
Survival of honey bee workers fed on pollen of Eucalyptus sp. (A) or multifloral sources (B) with or without spores of Botrytis cinerea, Cladosporium sp. or Colletotrichum acutatum at the mass ration of 20:1 (Mean age at death ± standard error for the treatments in the above order were: 14.80 ± 0.74, 19.55 ± 0.56, 22.2 ± 1.25 and 20.8 ± 1.09, 15.81 ± 0.89, 17.1 ± 0.71, 18.66 ± 0.90, 18.78 ± 1.15). Shaded areas represent 95% confidence intervals. Lower case letters indicate significant differences between the diets (Kaplan–Meier method, log-Rank test P < 0.05 and Bonferroni correction).
Table 2.
Nutritional composition and amino acid profile of the pollen samples (mean ± standard deviation).
| Composition (g/100 g) | Pollen diet | |
|---|---|---|
| Eucalyptus sp. | Multifloral | |
| Ash | 2.55 ± 0.13 | 1.91 ± 0.10 |
| Carbohydrates | 61.20 ± 3.06 | 64.40 ± 3.22 |
| Fat | 5.30 ± 0.33 | 6.80 ± 0.42 |
| Protein | 28.60 ± 0.92 | 20.70 ± 0.66 |
| Sodium | 14.00 ± 0.35 | 14.00 ± 0.35 |
| Sugars | 39.00 ± 4.29 | 38.00 ± 4.18 |
| Alanine | 1.11 ± 0.00 | 0.81 ± 0.06 |
| Arginine | 1.80 ± 0.04 | 0.85 ± 0.05 |
| Aspartic acid | 2.25 ± 0.00 | 1.84 ± 0.13 |
| Glutamic acid | 2.72 ± 0.02 | 1.82 ± 0.12 |
| Glycine | 1.03 ± 0.00 | 0.71 ± 0.05 |
| Histidine | 0.71 ± 0.01 | 0.51 ± 0.04 |
| Isoleucine | 1.02 ± 0.00 | 0.73 ± 0.05 |
| Leucine | 1.74 ± 0.00 | 1.25 ± 0.09 |
| Lysine | 1.39 ± 0.01 | 1.05 ± 0.08 |
| Methionine | 0.45 ± 0.01 | 0.33 ± 0.02 |
| Phenylalanine | 1.06 ± 0.01 | 0.74 ± 0.05 |
| Proline | 3.20 ± 0.01 | 1.85 ± 0.13 |
| Serine | 1.05 ± 0.01 | 0.78 ± 0.06 |
| Threonine | 0.89 ± 0.00 | 0.66 ± 0.05 |
| Tyrosine | 0.53 ± 0.03 | 0.39 ± 0.03 |
| Valine | 1.22 ± 0.00 | 0.85 ± 0.05 |
Effects of diets on the development of HPGs and ovaries
Neither the volume of the acini in the HPGs nor the ovary activation of workers that consumed EP only or EP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum differed significantly among the treatments (HPGs—F = 0.24, df = 3, P = 0.26, Fig. S1; ovaries—F = 1.66, df = 3, P = 0.17). For all treatments, the acini appeared normal in shape.
Gamma irradiation and nutritional factors of pollen samples
The plated suspensions of gamma-irradiated pollen did not yield any microorganisms, whereas non-irradiated monofloral and multifloral pollen yielded approximately 105 CFU of viable fungi/mL of suspension (i.e. gram of pollen) and sporadic bacterial colonies. The nutritional content of the pollen samples (i.e. amino acids, ash, lipids and protein) and the microorganisms naturally associated with them varied between the pollen samples, except for carbohydrates, sugar and sodium levels (Table 2). Pesticide residues were not detected in the samples (Table S2).
Discussion
Our results demonstrate that consumption of spores of B. cinerea, Cladosporium sp. and C. acutatum in association with pollen can extend the lifespan of honey bee workers but had no effect on the volume of the acini in the HPGs or activation of the ovaries. HPG development and ovary activation are known to be influenced by the quality and quantity of protein ingested by honey bee workers6, 21, 26, 27. Although fungal spores generally have a low protein content28, spores could conceivably serve as a source of additional nutrients for bees when combined with pollen. This is supported by our data, as bees that fed on pollen supplemented with spores survived longer than those that did not. In addition, the diets with fungal spores contained 5% less pollen than the EP-only diet. As the development of HPGs and ovary activation were similar in diets that did and did not contain spores, the nutritional value of spores may be equal to the value of the EP used in our experiments. It would have been informative to investigate whether spore consumption had a positive effect on these traits in combination with MP. Unfortunately, we did not assess this, as, we did not, a priori, expect the MP to be less nutritious than the EP. The differences between the first and subsequent experiments with respect to the survival of honey bee workers that were fed EP may be due to condition or genetic variation between worker cohorts29.
Pollens of different plant species vary greatly in protein content, amino acid composition, lipid, starch, vitamin and mineral content. This can affect individual bee health and colony longevity, physiology, and resistance to or tolerance of disease6, 30, 31. The quality of pollen for bees can be classified32 according to their levels of crude protein (c.p.) as excellent (> 25% c.p.), average (20–25% c.p.), and poor (< 20%). Our finding that EP was richer in c.p. than the MP was unexpected, but it may explain the finding that workers fed with EP diets typically survived longer than those on the MP diets.
Furthermore, the MP contained the species Arctotheca calendula and Echium plantagineum which belong to the families Asteraceae and Boraginaceae, respectively. The pollen of some species of the Asteraceae and Boraginaceae contains pyrrolizidine alkaloids33, 34, plant secondary compounds which confer protection from herbivory35. These alkaloids possess chemical properties that interfere with their digestion by non-specialized bees, including A. mellifera36. Thus, if pyrrolizidine alkaloids were present in the pollen of A. calendula and E. plantagineum this might, in part, explain the difference in survival rates between EP and MP. Nevertheless, in both experiments, the survivorship of workers was, overall, extended by the consumption of spores of B. cinerea, Cladosporium sp. or C. acutatum combined with pollen. The consumption of spores of Cladosporium sp. and C. acutatum in association with either pollen sample used in our experiments provided the longest lifespan, substantiating the hypothesis that nutritional benefits can be obtained by the collection of fungal spores by honey bees.
In addition to the nutritional contribution of pollen and fungal spores tested to the health and survival of honey bee workers, the microorganisms naturally present in the pollen samples, i.e. fungal spores and bacterial cells, could be a source of nutrients that enriches the quality of the diets consumed by the bees. Our results highlight the importance of knowing the composition of the microbiota associated with food sources fed to bees to understand their role and impact on the health and lifespan of bees. Thus, in our gamma-irradiated diets these dead microorganisms may have played a role in the nutritive value of the pollen, a factor that has so far been overlooked in other honey bee feeding experiments6, 20, 25. However, it was not possible to determine the nutrient composition of the spores of the three fungi used to supplement the pollen diets due to the challenges of producing sufficient quantities of spores for experimentation and limited resources.
During our experiments, honey bees failed to consume a diet consisting solely of spores of any of the three species of fungi used, nor did they consume spores of the cereal rust fungus, Puccinia graminis (data not shown). Comparable results have been reported by Schmidt et al.19, in that honey bee workers in cages consumed negligible amounts of spores of Uromyces sp. However, in contrast to our experiments, even those small amounts of spores of Uromyces sp. were toxic, causing rapid mortality. When spores are collected by bees in the field, it is likely that they are mixed with pollen from different sources and various microorganisms during the formation of bee bread. This would dilute the risk of any possible toxic effects and could enrich the nutritional quality of the food stored in the hives.
Honey bees have been observed to actively collect spores of Cladosporium sp.37, and our results show that these spores can significantly increase the longevity of the workers. Cell walls of Cladosporium sp. contain β-1,3–1,6-glucan, a member of β-D-glucose polysaccharides37, 38 which are known to be involved in the immune stimulatory activity in many vertebrate and invertebrate species39. Although the effects of β-glucan depend on the target organism and the dose administered39, it has been shown to extend the lifespan of honey bee workers40. In addition, extracts of the mushroom Agaricus brasiliensis, which is rich in glucans, have been shown to slightly increase the strength of experimentally fed honey bee colonies41. Hence, the β-glucans contained in the cell walls of higher fungi, including B. cinerea and C. acutatum, might have triggered immune responses in the workers that may have contributed to increasing their survival.
In summary, this study provides evidence that consumption of spores of some fungal species can extend the lifespan of honey bee workers, presumably by providing nutritional benefits. This supports the notion that foraging honey bee workers are likely to actively collect fungal spores. Further research is needed to identify specific interactions between honey bees and different species and strains of fungi; and to investigate the impact of the consumption of fungal spores on the learning, memory, resistance to and tolerance of diseases of bees. Furthermore, it needs to be established whether bee colonies preferentially collect fungal spores when other nutrient sources are in low supply, and whether β-glucans are responsible for the extended longevity of the workers.
Material and methods
Pollen samples
Two pollen mixtures were used in the experiments. The first pollen sample was composed mainly of Eucalyptus sp. and was collected in Nannup, Western Australia. During the collection of this pollen the main species in flower was E. baxteri. The second pollen sample was collected in Adelaide, South Australia, and consisted of a mixture of Arctotheca calendula (Cape weed), Eucalyptus sp., Echium plantagineum (salvation Jane) and Oxalis pes-caprae (soursob). Pollen grains were morphologically identified by the acetolysis method described by Erdtman42 and glycerine slides were prepared as described in Kearns and Inouye43. Photographs showing polar and equatorial views of the pollen grains at 400 × magnification were obtained using a Leica DM750 optical microscope coupled to an ICC50W camera (Leica, Germany) and compared with the APSA44 reference key. Both EP and MP samples were obtained from the same beekeeper by placing a pollen trap in front of the hive entrances, subsequently dried and stored at − 20 °C after arrival at the laboratory.
Gamma irradiation and nutritional quality of pollen samples used in the experiments
Pollen samples were sterilised by subjecting them to gamma irradiation of 25 KGy45, 46 at Steritech (Dandenong, Victoria). To ascertain effectiveness, the pollen samples were plated before and after irradiation. For each pollen sample, 1 g was diluted in 0.9 ml of sterile water containing 0.01% (v/v) Tween 20, the suspension was mixed by vortex and a tenfold serial dilution was performed. After mixing each dilution by vortex for 1 min, aliquots of 0.1 ml of the dilutions 1:100 and 1:1,000 were spread onto three replicate plates of potato dextrose agar (PDA, Difco) in 9-cm diameter Petri dishes and incubated at 25 °C under white fluorescent light with 12-h photoperiod. Observations were made 36 h post-plating to determine the number of colony forming units (CFU).
Nutritional quality and pesticide residues of the pollen samples were tested at Agrifood Technology (Werribee, Victoria). Duplicates of each pollen sample was assessed for ash, carbohydrate, lipid, protein, sodium and sugar contents. The protein (N × 6.25) content was determined by the Kjeldahl method47 and total lipids were analysed after the disruption of pollen walls by hydrolysis with hydrochloric acid. The presence of pesticide residues in the two pollen diets was assessed via gas and liquid chromatography with a limit of quantification of 0.01 mg/kg and a limit of detection of 0.005 mg/kg. Amino acid profiles were determined by analysis of duplicate samples at the Australian Proteome Analysis Facility (Sydney, New South Wales).
Fungal material and spore production
Three fungal species were used in the bioassays: (i) Botrytis cinerea (CBS140599, Filamentous Fungi Database, Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, GenBank accession number: KX710078 which corresponds to the 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence); (ii) Colletotrichum acutatum (EU670080, European Molecular Biology Laboratory, which corresponds to the Glomerella acutata strain CSL-1689 internal transcribed spacer 1, partial sequence; 5.8S ribosomal RNA gene, complete sequence; and internal transcribed spacer 2, partial sequence) and (iii) Cladosporium sp. (CBS146925, Filamentous Fungi Database, Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, GenBank accession number: MK402112 which corresponds to internal transcribed spacer 1, partial sequence; 5.8S ribosomal RNA gene, complete sequence; and internal transcribed spacer 2, partial sequence). These fungal species had been isolated from grapes, almond, and bee bread, respectively. The first two isolates were obtained from the Plant Pathology laboratory and the third from a honey bee hive located at the School of Agriculture, Food and Wine, University of Adelaide, South Australia, Australia. The Cladosporium isolate, which was not identified to species level, shares 100% sequence similarity with C. cladosporioides, C. halotolerans, C. sphaerospermum, and C. parahalotolerans48, 49. Mycelia were cultured on PDA in 9-cm diameter Petri plates for 10 to 14 days and incubated as described above. Cultures were established in consecutive weeks to generate spores of similar age.
Spores were harvested by flooding 10–14 day-old cultures with 15 mL of sterile reverse osmosis (RO) water containing 0.01% (v/v) Tween 20 and dislodging spores from the mycelia using a sterile plastic spreader. The resulting suspensions were filtered through two layers of sterile cheesecloth to remove hyphal fragments50. Spore suspensions from three plates of the same fungus were combined in a sterile 50-mL tube and then centrifuged at 4,500 rpm, 25 °C for 10 min. The supernatant was removed and the remaining suspensions (approximately 0.3–0.4 mL) were placed in sterile lids of 2 mL cryogenic tubes (Simport, Canada) in a biohazard laminar flow cabinet and left to dry overnight. Spores in lids were kept in sterile sealed Petri dishes at 4 °C until diet preparation.
Bee rearing and feeding
Experiments were conducted with newly emerged workers collected from three hives during March of 2018 and 2019. Colonies were maintained in a bee enclosure located at the Waite Campus, University of Adelaide and were regularly inspected for symptoms of diseases. Workers were obtained by placing single frames with late stage pupae in an incubator at 34 °C and 50–70% relative humidity6. The bees emerging from these three frames were collected every 24 h in a jar, mixing them to ensure that any results could not be attributed to genetic differences. From these collections, bees were randomly placed in metal cages (6.5 × 8.5 × 4.5 cm; Small-Life Supplies, England) in groups of 10 individuals and fed with gamma-irradiated pollen and fungal spores offered in a sterile cryogenic lid; and sterile 50% (w/v) sucrose solution provided in a 5-mL syringe.
Pollen was mixed with sterile RO water at a mass ratio of 7:3 and subsequently with fungal spores. To establish the optimal concentration of spores, workers were offered spores of each fungus as a sole diet, or in a mix with EP at a weight ratio of 5:1, 10:1 and 20:1. In a subsequent experiment the effects of fungal spores were tested in mixes with EP and MP, mixed separately with spores of each fungus at a mass ratio of 20:1. Diets were freshly prepared every day, measured and provided ad libitum. The rates of diet consumption per bee per day were assessed. At the beginning of the experiments, approximately 10–15 mg of diet per bee per day was offered as in our preliminary experiments workers consumed less than 10 mg of diet on a daily basis. Towards the end of the experiments, the provision of food was adjusted because the workers consumed less than 2 mg per bee per day. Caged bees were incubated as described above. Each treatment consisted of six replications. Dead bees were removed from the cages every day until 100% mortality was reached for every cage.
Hypopharyngeal gland development and ovary activation
To assess whether fungal spores can affect the development of the HPGs, workers were fed with EP with or without spores of B. cinerea, Cladosporium sp. or C. acutatum at a mass ratio of 20:1. In the same manner as for the longevity experiment, groups of ten newly emerged workers were placed in cages and reared for 7 days. On day 8, bees were placed in 1.5-mL Eppendorf tubes and stored at − 80 °C until dissection. The paired HPG were dissected in 100 μL of physiological saline (0.9% NaCl) and stained with a drop of methyl blue dissolved in Ringer’s solution20. Glands were mounted on slides and examined as described above. The development of HPGs was assessed by measuring the length and width of 30 randomly chosen acini per bee using the Leica Acquire 3.4.1 software. The total number of bees assessed for diets of EP, or EP supplemented with spores of B. cinerea, Cladosporium sp. or C. acutatum was 59, 58, 57 and 58, respectively. The volume of each acinus was calculated by applying the following equation: 1/6 × 3.14 x length x width251.
The ovaries of five bees per cage were dissected under an binocular dissecting microscope and their development was classified into five stages according to Pernal and Currie25 as; 0: undeveloped ovaries, 1: slight enlargement, early stages of differentiation; 2: slight development (presence of distinct cells leading to swellings and constrictions), 3: moderate development (egg volume exceeding that of the nutritive follicle), 4: highly developed (presence of fully formed eggs and ovaries with mature oocytes). The effects on HPG development and ovary activation were investigated for the combination of spores with EP only.
Statistical analysis
All statistical analyses were executed in R version 3.2.152. The survival data were analysed using the Kaplan–Meier method and log-Rank test. Bonferroni correction was applied to the comparison sets to limit the risk that significant differences would be obtained by chance53. The HPG volume data were subjected to analysis of variance followed by Tukey’s honestly significant difference (HSD) test, when appropriate. Diet consumption and ovary activation data were compared using a generalized linear model (GLM) with a Gaussian family distribution and Tukey’s HSD test, when appropriate. Normality and homoscedasticity assumptions were tested by QQ plot and Shapiro test, and by Bartlett test, respectively. Only the acini volume data required transformation [log(x)] prior to analyses of variance.
Supplementary information
Acknowledgements
The first author was supported by the Adelaide Scholarships International (ASI).
Author contributions
J.B.P.: designed and performed experiment and data analyses, and wrote the paper. E.S.S., K.H.: experimental design, technical assistance, and editing of the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72758-1.
References
- 1.Gallai N, Salles J-M, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- 2.Klein D, Vaissiere BE, Cane JH, Stefan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southwick EE, Southwick L. Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 1992;85:621–633. doi: 10.1093/jee/85.3.621. [DOI] [Google Scholar]
- 4.Brodschneider R, Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010;41:278–294. doi: 10.1051/apido/2010012. [DOI] [Google Scholar]
- 5.Smart M, Pettis J, Rice N, Browning Z, Spivak M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE. 2016;11:e0152685. doi: 10.1371/journal.pone.0152685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet J-L, Cedric A. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE. 2013;8:e72016. doi: 10.1371/journal.pone.0072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simone-Finstrom M, Li-Byarlay H, Huang MH, Strand MK, Rueppel O, Tarpy DR. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016;6:32023. doi: 10.1038/srep32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vere N, Jones LE, Gilmore T, Moscrop J, Lowe A, Smith D, Hegarty MJ, Creer S, Ford CR. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Sci. Rep. 2017;7:42838. doi: 10.1038/srep42838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn K, Xie X, Riddle J, Pettis J, Huang ZY. Effects of long distance transportation on honey bee physiology. Psyche A J. Entomol. 2012;2012:1–9. doi: 10.1155/2012/193029. [DOI] [Google Scholar]
- 10.Vaudo AD, Tooker JF, Grozinger CM, Patch HM. Bee nutrition and floral resource restoration. Curr. Opin. Insect. Sci. 2015;10:133–141. doi: 10.1016/j.cois.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Shaw DE. The incidental collection of fungal spores by bees and the collection of spores in lieu of pollen. Bee World. 1990;71:158–176. doi: 10.1080/0005772X.1990.11099059. [DOI] [Google Scholar]
- 12.Shaw DE. Bees and fungi, with special reference to certain plant pathogens. Aust. Plant Pathol. 1999;28:269–282. doi: 10.1071/ap99044. [DOI] [Google Scholar]
- 13.Parker, R. L. The collection of pollen by the honey bee. Memoir: Cornell University Agricultural experiment Station98, 1–55 (1926).
- 14.Doull, K. M. An analysis of bee behaviour as it relates to pollination in The Indispensable pollinators (ed Warren L.O.). Report 9th Pollination Conference 12-15 (Hot Springs, Arkansas 1970).
- 15.Inouye DW, Gill DE, Dudash MR, Fenster CB. A model and lexicon for pollen fate. Am. J. Bot. 1994;81:1517–1530. doi: 10.1002/j.1537-2197.1994.tb11462.x. [DOI] [Google Scholar]
- 16.Westerkamp CP. Pollen in bee flower relations. Some considerations on melittophily. Botanica. Acta. 1996;109:325–332. doi: 10.1111/j.1438-8677.1996.tb00580.x. [DOI] [Google Scholar]
- 17.Thorp RW. The collection of pollen by bees. Plant Syst. Evol. 2000;222:211–223. doi: 10.1007/BF00984103. [DOI] [Google Scholar]
- 18.Portman ZM, Tepedino VJ. Convergent evolution of pollen transport mode in two distantly related bee genera (Hymenoptera: Andrenidae and Melittidae) Apidologie. 2017;48:461–472. doi: 10.1007/s13592-016-0489-8. [DOI] [Google Scholar]
- 19.Schmidt JO, Thoenes SC, Levin MD. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 1987;80:176–183. doi: 10.1093/aesa/80.2.176. [DOI] [Google Scholar]
- 20.Renzi MT, et al. Combined effect of pollen quality and thiamethoxam on hypopharyngeal gland development and protein content in Apis mellifera. Apidologie. 2016;47:779–788. doi: 10.1007/s13592-016-0435-9. [DOI] [Google Scholar]
- 21.Hrassnigg, N. & Crailsheim, K. Adaptation of hypopharyngeal gland development to the brood status of honeybee (Apis mellifera L.) colonies. J Insect Physiol44, 929–939 (1998). [DOI] [PubMed]
- 22.Heylen K, Gobin B, Arckens L, Huybrechts R, Billen J. The effects of four crop protection products on the morphology and ultrastructure of the hypopharyngeal gland of the European honeybee, Apis mellifera. Apidologie. 2011;42:103–116. doi: 10.1051/apido/2010043. [DOI] [Google Scholar]
- 23.Hoover SE, Higo HA, Winston ML. Worker honey bee ovary development: seasonal variation and the influence of larval and adult nutrition. J. Comput. Physiol. B. 2006;176:55–63. doi: 10.1007/s00360-005-0032-0. [DOI] [PubMed] [Google Scholar]
- 24.Modro AFH, Silva IC, Message D, Luz CFP. Saprophytic fungus collection by africanized bees in Brazil. Neotrop. Entomol. 2009;38:434–436. doi: 10.1590/S1519-566X2009000300022. [DOI] [PubMed] [Google Scholar]
- 25.Gasparoto MCG, et al. Honeybees can spread Colletotrichum acutatum and C. gloeosporioides among citrus plants. Plant Pathol. 2017;66:777–782. doi: 10.1111/ppa.12625. [DOI] [Google Scholar]
- 26.Brouwers E. Measurement of hypopharyngeal gland activity in the honeybee. J. Apic. Res. 1982;21:193–198. doi: 10.1080/00218839.1982.11100541. [DOI] [Google Scholar]
- 27.Pernal SF, Currie RW. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.) Apidologie. 2000;31:387–409. doi: 10.1051/apido:2000130. [DOI] [Google Scholar]
- 28.Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu. Rev. Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 29.Page RE, Jr, Peng CY-S. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 2001;36:695–711. doi: 10.1016/S0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt LS, Schmidt JO, Rao H, Wang W, Xu L. Feeding preference and survival of young worker honey bees (Hymenoptera: Apidae) fed rape, sesame, and sunflower pollen. J. Econ. Entomol. 1995;88:1591–1595. doi: 10.1093/jee/88.6.1591. [DOI] [Google Scholar]
- 31.Goulson D, Nicholls E, Botias C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 32.Kleinschmidt G, Kondos A, Harden J, Turner J. Colony management for eucalypt honey flows. Aust. Beekeeper. 1974;75:261–264. [Google Scholar]
- 33.Anke, S., Niemüller, D., Moll, S., Hänsch, R. & Ober, D. Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from differential tissue expression of homospermidine synthase. Plant Physiol.136, 4037–4047 (2004). [DOI] [PMC free article] [PubMed]
- 34.Boppré M, Colegate SM, Edgar JA. Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen. J. Agr. Food. Chem. 2005;53:594–600. doi: 10.1021/jf0484531. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann, T. & Ober, D. Biosynthesis and metabolism of pyrrolizidine alkaloids in plants and specialized insect herbivores in Topics in Current Chemistry: Biosynthesis—Aromatic Polyketides, Isoprenoids, Alkaloids (ed Vederas J.C. Leeper F.J.) 207–243 (Springer, Berlin 2000).
- 36.Praz CJ, Müller A, Dorn S. Specialized bees fail to develop on non-host pollen: do plants chemically protect their pollen. Ecology. 2008;89:795–804. doi: 10.1890/07-0751.1. [DOI] [PubMed] [Google Scholar]
- 37.San-Blas G, Guanipa O, Moreno B, Pekerar S, San-Blas F. Cladosporium carrionii and Hormoconis resinae (C. resinae): cell wall and melanin studies. Curr. Microbiol. 1996;32:11–16. doi: 10.1007/s002849900003. [DOI] [PubMed] [Google Scholar]
- 38.Szaniszlo, P., Cooper, B. & Voges, H. S. Chemical compositions of the hyphal walls of three chromomycosis agents. Sabouraudia: J Med Vet Mycol10, 94–102 (1972). [DOI] [PubMed]
- 39.Soltanian S, Stuyven E, Cox E, Sorgeloos P, Bossier P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009;35:109–138. doi: 10.1080/10408410902753746. [DOI] [PubMed] [Google Scholar]
- 40.Mazzei M, et al. Effect of 1, 3–1, 6 β-glucan on natural and experimental deformed wing virus infection in newly emerged honeybees (Apis mellifera ligustica) PLoS ONE. 2016;11:e0166297. doi: 10.1371/journal.pone.0166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevanovic J, et al. The effect of Agaricus brasiliensis extract supplementation on honey bee colonies. Anais da Academia Brasileira de Ciências. 2018;90:219–229. doi: 10.1590/0001-3765201820150182. [DOI] [PubMed] [Google Scholar]
- 42.Erdtman G. Handbook of Palynology: Morphology, Taxonomy, Ecology. Copenhagen: Munksgaard; 1969. [Google Scholar]
- 43.Kearns, C. A. & Inouye, D. W. Techniques for pollination biologists. (University Press of Colorado, 1993).
- 44.APSA. Australasian Pollen and Spore Atlas, <https://apsa.anu.edu.au/> (2007).
- 45.Graystock P, et al. Hygienic food to reduce pathogen risk to bumblebees. J. Invert. Pathol. 2016;136:68–73. doi: 10.1016/j.jip.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Meeus I, Mosalanejad H, Niu J, de Graaf DC, Wackers F, Smagghe G. Gamma irradiation of pollen and eradication of Israeli acute paralysis virus. J. Invert. Pathol. 2014;121:74–77. doi: 10.1016/j.jip.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Bradstreet RB. Kjeldahl method for organic nitrogen. Anal. Chem. 1954;26:185–187. doi: 10.1021/ac60085a028. [DOI] [Google Scholar]
- 48.National Center for Biotechnology Information (NCBI)[Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]–[October 2019]. Available from: https://www.ncbi.nlm.nih.gov/
- 49.Bensch K, Groenewald J, Meijer M, Dijksterhuis J, Jurjević Z, Andersen B, Houbraken J, Crous PW, Samson RA. Cladosporium species in indoor environments. Stud. Mycol. 2018;89:177–301. doi: 10.1016/j.simyco.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheuerell SJ, Mahaffee WF. Variability associated with suppression of gray mold (Botrytis cinerea) on geranium by foliar applications of nonaerated and aerated compost teas. Plant Dis. 2006;90:1201–1208. doi: 10.1094/PD-90-1201. [DOI] [PubMed] [Google Scholar]
- 51.Preston FW. The volume of an egg. AOS. 1974;91:132–138. doi: 10.2307/4084667. [DOI] [Google Scholar]
- 52.R Core Team. R: A Language and Environment for Statistical Computing. v. 1.2.1335 (Vienna, Austria; 2019).
- 53.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


