Abstract
Sirtuins are the class III of histone deacetylases whose deacetylate of histones is dependent on nicotinamide adenine dinucleotide (NAD+). Among seven sirtuins, SIRT1 plays a critical role in modulating a wide range of physiological processes, including apoptosis, DNA repair, inflammatory response, metabolism, cancer, and stress. Neuroinflammation is associated with many neurological diseases, including ischemic stroke, bacterial infections, traumatic brain injury, Alzheimer's disease (AD), and Parkinson's disease (PD). Recently, numerous studies indicate the protective effects of SIRT1 in neuroinflammation-related diseases. Here, we review the latest progress regarding the anti-inflammatory and neuroprotective effects of SIRT1. First, we introduce the structure, catalytic mechanism, and functions of SIRT1. Next, we discuss the molecular mechanisms of SIRT1 in the regulation of neuroinflammation. Finally, we analyze the mechanisms and effects of SIRT1 in several common neuroinflammation-associated diseases, such as cerebral ischemia, traumatic brain injury, spinal cord injury, AD, and PD. Taken together, this information implies that SIRT1 may serve as a promising therapeutic target for the treatment of neuroinflammation-associated disorders.
1. Introduction
Inflammation is a physiological response of the immune system to harmful infectious and noninfectious stimuli. In response to such stimuli, macrophages, immune cells, and vascular cells take concerted reactions to maintain or restore the integrity of tissue [1]. However, a sustained inflammatory response can be harmful to the body [2]. Inflammatory responses that are centralized in the central nervous system (CNS) are referred to as neuroinflammation [3]. Neuroinflammation has been implicated as the pathogenesis in multiple neurological diseases, including ischemic stroke, bacterial infections, traumatic brain injury, and neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), age-related dementia, and Huntington's disease (HD) [4–6].
Due to the existence of the blood-brain barrier (BBB), the peripheral immune cells and molecules have difficulty entering the CNS. Hence, resident innate immune cells of the CNS need to deal directly with various toxins, pathogens, and tissue damage [1]. Pattern recognition receptors (PRRs) have an essential effect on the recognition of pathogen-associated molecular patterns (PAMPs) and derived danger-associated molecular patterns (DAMPs) during inflammatory conditions. There are four families of PRRs, namely, Toll-like receptors (TLRs), NOD-like receptors (NLRs), retinoid acid-inducible gene-1- (RIG-1-) like receptors (RLRs), and C-type lectin receptors (CLRs) [7]. In CNS, these PRRs are mainly expressed in microglia, astrocytes, endothelial cells, dendritic cells (DCs), and oligodendrocytes [8, 9]. There are several innate immune cells in the brain, including astrocytes, microglia, macrophages, natural killer (NK) cells, mast cells, and oligodendrocytes [10]. Among these cells, microglia are the primary innate immune cells, and they constitute 10-15% of all the glial cells. Under pathological conditions, microglia are activated by PAMPs and characterized by rapid proliferation and production of proinflammatory cytokines [11]. Acute neuroinflammation is a key response to clear pathogens and to repair tissue damage. However, if acute neuroinflammation remains unresolved, it can lead to chronic inflammation and neurodegeneration [12].
Sirtuins are the class III of histone deacetylases and are nicotinamide adenine dinucleotide- (NAD+-) dependent enzymes. The family consists of seven members in mammals, namely, silent information regulator 1 (SIRT1), SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7 [13]. The sirtuins are epigenetic modulators involved in many physiologic activities, such as genomic stabilization, cancer, stress response [14], apoptosis, metabolism, senescence, proliferation [15], and inflammation [16]. Among all sirtuins, SIRT1 is the first sirtuin to be recognized and is well studied [17]. Many studies have indicated that SIRT1 could regulate the inflammation response in multiple tissues and cells [16]. An increasing number of studies have reported that SIRT1 was closely linked with neuroinflammation [18]. However, the mechanistic pathways involving SIRT1 and neuroinflammation remain poorly defined. Hence, the focus of this review is to summarize the latest advances regarding the potential beneficial roles of SIRT1 in the regulation of neuroinflammation. This study may serve as a useful foundation for the design of a drug for future neuroinflammation and neurodegenerative disease treatments.
2. SIRT1 Structure
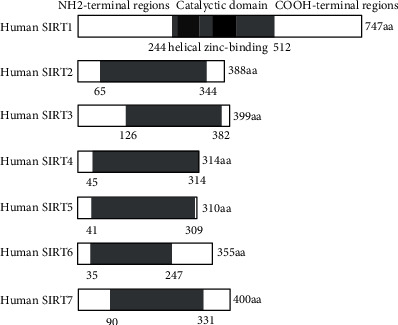
Sirtuins contain a conserved catalytic domain, NAD+ binding domains, and flanking variable NH2- and COOH-terminal regions [19, 20] (Figure 1). The diversity of sirtuin amino acid sequences contribute to their cellular localization, catalysis activity, and function. The human sirtuins are divided into four classes. SIRT1, SIRT2, and SIRT3 are class I showing close homology to yeast Sir2. SIRT4 is class II, and SIRT5 is class III. SIRT6 and SIRT7 belong to class IV sirtuins [21] Among sirtuins, SIRT1 has the largest extensions of terminal regions. The SIRT1 protein (747 amino acids) is made up of the conserved catalytic core (244–512 residues), the COOH-terminal region (1–180 residues), and the NH2-terminal region (513–747 residues) [22]. SIRT1 protein contains the nuclear localization signal (KRKKRK) in 41-46 residues [19]. Thus, it is easy to understand that SIRT1 is identified as a nuclear protein. However, numerous evidences indicated that SIRT1 is localized in both the nucleus and cytoplasm in several cells [23–25]. Under certain conditions, SIRT1 can shuttle between the nucleus and cytoplasm [26]. The nuclear import and export sequences in the NH2-terminal region of SIRT1 have been identified as a regulatory mechanism of nucleocytoplasmic shuttling of SIRT1[27]. Aside from SIRT1, the other six sirtuins have distinct subcellular localizations. SIRT2 is primarily located in the cytoplasm [28], but it can shuttle from the cytoplasm to the nucleus [29]. SIRT3, SIRT4, and SIRT5 are generally identified as mitochondrial proteins. Recent studies indicated that SIRT3 is not exclusive in mitochondria. SIRT3 primarily localizes in the nucleus and translates to the mitochondria after ultraviolet radiation or etoposide treatment [30]. Apart from SIRT1, SIRT6 and SIRT7 also show nuclear localization. SIRT6 is a chromatin-bound protein, whereas SIRT7 is concentrated in the nucleolus [31].
Figure 1.
3. Catalytic Mechanism of SIRT1
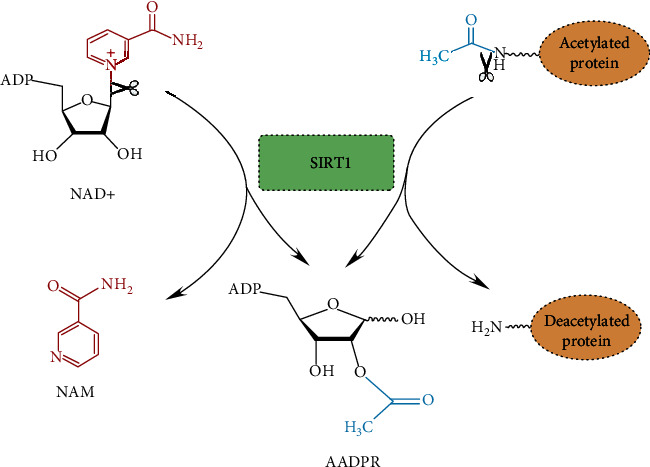
As the histone deacetylases, mammalian sirtuins share a conserved catalytic domain. For human SIRT1, the catalytic core consists of two domains. The larger NAD+-binding domain composes of a Rossmann fold, and the smaller domain consists of a helical (269–324 residues) and a zinc-binding module (362–419 residues) [32]. On the basis of the three-dimensional crystal structures of a sirtuin protein, the catalytic pocket between large and small domains is frequently divided into three active sites: the site A is involved in binding of the adenine-ribose part of NAD, the site B is involved in binding of the nicotinamide-ribose portion, and the site C is the deep location of the NAD-binding pocket [33]. The molecular mechanism of the deacetylation reaction is complicated. Several studies have reported the possible mechanism of the reaction. Briefly, the catalytic reaction begins with forming the ternary complex, which is mediated by NAD+, and an acetylated substrate both binds to the sirtuin enzyme [34]. In this deacetylation process, the glycosidic bond of NAD+-linking nicotinamide and ADP-ribose moiety is cleaved, and the free nicotinamide is released. Then, the acetyl moiety is transferred to ADP-ribose and produces acetyl-ADP-ribose (2′-O-acetyl-ADP-ribose, AADPR) and deacetylated protein [35] (Figure 2). Although human sirtuins belong to histone deacetylases, it should be pointed out that SIRT1, SIRT2, SIRT3, and SIRT7 display deacetylase activity, whereas the deacetylase activities of SIRT4–6 are considered to be weak. SIRT4 has ADP-ribosyltransferase and lipoamidase activity [36, 37]. SIRT5 possesses strong desuccinylation and depropanediylation activity [38]. SIRT6 was initially reported to have deacetylase activity on specific histone acetyl peptides H3 K9 and H3 K56 [39]. A recent study found that SIRT6 has demyristoylation activity by regulating the hydrolysis of fatty acylation of lysine residues [40].
Figure 2.
4. SIRT1 Substrates and Function
SIRT1 is the most well-studied member of the sirtuin family. As a deacetylate histone, it is easy to understand that SIRT1 can regulate the deacetylation of the acetyl-lysine of histone substrate. A previous study confirmed that SIRT1 deacetylates histone H4 at lysine 16 (K16), histone H3 at lysine 9 (K9), and histone H1 at lysine 26 (K26) [41]. In addition to the deacetylate of histones, SIRT1 could catalyze the deacetylation of many nonhistone substrates. A growing number of evidence indicate that SIRT1 can deacetylate the lysine residues of a variety of substrates, such as p53, nuclear factor kappa B (NF-κB), Ku70, FOXO1, FOXO3, FOXO4, hypoxia-inducible factor-1a (HIF-1α), HIF-2α, proliferator activated receptor γ coactivator 1α (PGC-1α), and signal transducer and activator of transcription 3 (STAT3) (Table 1). Thus, this wide range of targets reflects that SIRT1 has a strong capacity to regulate multiple physiological functions.
Table 1.
The substrates of SIRT1.
| Substrate | Lysine site | Function | Reference |
|---|---|---|---|
| p53 | K382 | Apoptosis and senescence | [42, 43] |
| NF-κB p65 | K310 | Apoptosis and inflammation | [45] |
| Ku70 | K539, K542, K544, K533, and K556 | DNA repair | [48] |
| FOXO1 | K222, K245, K248, K262, K265, K274, and K294 | Apoptosis | [50] |
| FOXO3 | K242, K259, K271, K290, and K569 | Cell cycle arrest, oxidative stress | [50] |
| FOXO4 | K186, K189, and K408 | Oxidative stress | [50] |
| HIF-1α | K674 | Cell invasion | [53] |
| HIF-2α | K385, K685, and K741 | Cell survival | [53] |
| PGC-1α | K13 | Mitochondrial biogenesis and metabolism | [58] |
| STAT3 | K685, K679, K707, and K709 | Gluconeogenesis and cell proliferation | [60] |
p53 is the first nonhistone target of SIRT1; deacetylation of p53 at lysine 382 (K382) results in the repression of p53-dependent apoptosis in H1299 cells in response to DNA damage [42, 43]. As a tumor suppressor, the functional and transcriptional activities of p53 are blocked by the deacetylation of SIRT1 [44]. Thus, SIRT1 provides a prosurvival response following DNA damage. NF-κB is an important transcription factor that regulates the transcription of many genes. SIRT1 can deacetylate the p65 subunit of NF-κB at lysine 310. It is interesting that deacetylation of p65 augments TNFα-induced apoptosis in non-small-cell lung cancer (NSCLC) cells. A possible hypothesis is the different apoptotic signaling and stimulations [45]. In addition to the apoptosis role, the deacetylation of p65 has been shown to regulate the inflammation response [46]. Ku70 is a DNA repair protein; SIRT1 can deacetylate and activate Ku70 by direct interaction. The deacetylation of Ku70 increases the DNA repair capacity [47, 48]. The Forkhead box O (FOXO) transcription factor family is a subclass of Forkhead transcription factors, including four members FOXO1, FOXO3, FOXO4, and FOXO6. Many evidences suggest that the deacetylation of FOXO1, FOXO3, and FOXO4 can be mediated by SIRT1 [49, 50]. For example, the activation of FOXO1 mediated by SIRT1 induces the apoptosis of prostate cancer cells [51]. Deacetylation of FOXO3 by SIRT1 potentiates the ability of FOXO3-induced cell cycle arrest and inhibits cell apoptosis mediated by FOXO3 in response to oxidative stress [52]. In addition, the hyperacetylation and transcriptional activity of FOXO4 can be mediated by peroxide stress, while deacetylation of FOXO4 by SIRT1 enhances the protective effect against oxidative stress [53]. Hypoxia-inducible factors are transcription factors that are critical in the regulation of metabolism under hypoxia. Previous studies have found that both HIF-1α and HIF-2α can be deacetylated by SIRT1 [54]. The deacetylation of HIF-1α by SIRT1 enhances the expression of HIF-1α target genes (VEGF, GLUT1, and MMP2) and then results in the promotion of cell invasion [55]. SIRT1 augments the expression of HIF-2α target gene erythropoietin (prosurvival factor) by deacetylation of HIF-2α [56]. PGC-1α is a transcriptional coactivator with multiple functions, including mitochondrial biogenesis and glucose and fatty acid metabolism [57]. Deacetylation of PGC-1α by SIRT1 enhances the effects of PGC-1α on fatty acid oxidation and gluconeogenesis genes in response to caloric restriction [58, 59]. STAT3 is a cellular signal transcription factor that mediates many cellular functions [60]. SIRT1-mediated deacetylation of STAT3 is involved in the regulation of gluconeogenic genes [61] and cell proliferation [62]. In addition to these highlighted substrates, numerous SIRT1 substrates have been discovered [63]. Taken together, these multiple substrates indicate that SIRT1 participates in the regulation of multiple physiological functions, including apoptosis, DNA repair, inflammatory response, metabolism, cancer, and stress.
5. SIRT1 Activator Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural polyphenol found in a variety of plant species, which is a natural antibiotic compound synthesized by plants [64]. Numerous studies have shown that resveratrol has many functions, including antioxidant capacity, anti-inflammation, neuroprotection, and anticancer [65]. Many studies confirm that resveratrol is a natural activator of SIRT1. Previous experiments have shown that resveratrol doubled the catalytic rate of SIRT1 at about 11 μM [66]. Dozens of studies report that the anti-inflammatory effects of resveratrol are dependent on SIRT1 [67–70]. However, recent evidence shows that resveratrol may activate SIRT1 activity indirectly. One mechanism in which resveratrol activates SIRT1 involves cAMP and AMPK [71]. Another mechanism is that resveratrol activates Lamin A, which is a protein activator of SIRT1 [72]. Several studies have shown that resveratrol regulated neuroinflammation through SIRT1. A recent study indicated that resveratrol inhibits neuroinflammatory response in aged rats after anesthesia and surgery by activation of SIRT1 [73]. Similarly, resveratrol inhibited the expressions of inflammatory factors in LPS-induced activation of microglia cells by upregulation of SIRT1 [74].
6. SIRT1 Distribution in CNS
Previous studies have confirmed the distribution of SIRT1 in a wide variety of mouse organs, including the brain, spinal cord, eyes, heart, liver, kidney, lung, and testis [75, 76]. It is also widely expressed in several human tissues and organ, including the brain [77], liver [78], heart [79], skeletal muscle [80], pancreas [81], and adipose tissue [82]. It is interesting that the high levels of SIRT1 expression are observed in the embryonic brain, spinal cord, and heart [75]. An anatomical study of the rodent and human nervous systems indicated that the distribution of SIRT1 is localized in the areas of the hippocampus, prefrontal cortex, and basal ganglia [83]. In addition to these regions, a mouse anatomical study revealed that SIRT1 is also expressed in the hypothalamus and cerebellum [84]. Moreover, this study also indicated that SIRT1 mRNA is mainly expressed in neurons [84]. Recent studies had reported that several cellular types (mouse astrocyte, microglia, and oligodendrocytes) express SIRT1 proteins [85, 86]. Taken together, these studies suggest that SIRT1 is widely expressed in CNS.
7. SIRT1 and Neuroinflammation
The innate immune system is the first line of defense against infection and injury and plays critical roles in the maintenance of brain homeostasis. It has been shown that astrocytes, microglia, macrophages, NK cells, mast cells, and oligodendrocytes serve as the innate immune cells of the brain [11]. Among these cells, microglia are mainly innate immune cells that are derived from the embryonic yolk sac [87]. Under physiological conditions, microglia constantly survey their microenvironments and present in the resting state with small cellular bodies and slender branching [88]. Under pathological conditions, microglia are activated and converted into amoeboid reactive cells, with the production of proinflammatory factors [89]. Overactivation of microglia has been implicated to the development of neuroinflammation [90]. In addition to microglia, astrocytes also are key innate immune cells involved in the regulation of neuroinflammation [91]. Astrocytes became reactive astrocytes with significant changes in morphology, function, and gene expression in response to CNS injuries and diseases [92]. A previous study suggests that the increase of inflammatory factor (TNF-α, IL-1β, and iNOS) gene expression is observed in both primary microglia and astrocytes after treatment with LPS [85]. Growing evidence supports a role for SIRT1 in the regulation of inflammatory responses in CNS. For example, the SIRT1 activator decreases the secretion of TNF-α and IL-6 in microglial BV2 cells by LPS stimulation, while the SIRT1 inhibitor increases the inflammatory factors release [93]. Another study found that SIRT1 inhibition upregulates the gene expression levels of TNF-α and IL-1β and ROS production in astrocytes after stimulation with LPS [94]. However, the molecular mechanisms involving SIRT1 and neuroinflammation are still unclear. Emerging potential regulatory mechanisms of SIRT1 in the process of neuroinflammation may involve the NF-κB pathway, Toll-like receptor pathway, NLRP3 inflammasome pathway, and MAPK signal pathway (Figure 3). These signal pathways will be discussed in the following paragraphs.
Figure 3.

7.1. SIRT1 and NF-κB
The transcription factor NF-κB family regulates the expression of multiple genes involved in the inflammatory process [95]. It has been shown that the NF-κB family has five subunits: p65 (also RelA), p50, p52, c-Rel, and RelB. The relative pathway mediated by NF-κB complex p65/p50 is commonly defined as the canonical NF-κB pathway. Generally, NF-κB complexes are sequestered by NF-κB inhibitory proteins (IκBs) in the cytoplasm. Once the pathway is activated, the IκB proteins are activated by phosphorylation and its subsequent degradation, resulting in the nuclear translocation of NF-κB complexes and then regulation of target gene expression [96]. A growing number of studies have shown that SIRT1-mediated NF-κB participates in the regulation of inflammation response and diseases. As described previously, SIRT1 has demonstrated the capacity of the deacetylating p65 subunit [45]. Thus, it is easy to understand that SIRT1 mediates the inflammation process by directly regulating the NF-κB pathway. Bone marrow-derived macrophages (BMDMs) from myeloid cell-specific SIRT1 knockout mice result in the hyperacetylation of NF-κB and the increase of proinflammatory cytokines [97]. Activation of SIRT1 by resveratrol inhibits TNF-α-induced inflammation responses in 3T3 fibroblasts by deacetylating the Lys310 residue of the p65 subunit [70]. Similarly, resveratrol decreases the TNF-α-mediated inflammation responses in human chondrocytes by deacetylating NF-κB p65 [98].
A number of studies demonstrated that the SIRT1 and NF-κB pathways are involved in neuroinflammation. Accumulating studies indicate that the decrease of SIRT1 and the activation of the NF-κB pathway participate in the progress of neuroinflammation and diseases. A recent study found that microglial SIRT1 deficiency aggravates memory deficits and increases IL-1β transcription in aging mice [99]. High levels of acetylation of NF-κB/RelA in primary cortical neurons following oxygen-glucose deprivation (OGD) have been found [100]. Moreover, several neural cell experiments have demonstrated that SIRT1 activator resveratrol suppresses inflammatory cytokines via inhibition of NF-κB transcriptional activity [101, 102]. Furthermore, many animal models indicated that SIRT1 activator resveratrol inhibits the inflammatory cytokines and improves the survival of the neuronal cell by regulating the acetylation of NF-κB p65 [73, 103–105] (Table 2). In addition to the direct regulation of the NF-κB pathway by deacetylating the p65 subunit, some studies demonstrated that SIRT1 could indirectly regulate the NF-κB pathway by other targets, such as PPARα [46, 106]. Taken together, SIRT1 can suppress the neuroinflammation involved in regulating NF-κB signaling by deacetylation of the p65 subunit and inhibition of NF-κB transcriptional activity.
Table 2.
SIRT1 regulate neuroinflammation involved in the NF-κB pathway.
| Chemical agents | Models | Involved mechanisms | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | Rat astrocytes induced by amyloid-beta (Aβ) | Upregulation of SIRT1 decreases the nuclear translocation of NF-κB p65 | Suppression of inflammatory cytokines (TNF-α, IL-1β, and MCP-1) | Zhao et al. [101] |
| Resveratrol | N9 microglia cell lines induced by amyloid-beta (Aβ) | Upregulation of SITRT1 decreases the nuclear translocation of NF-κB p65 | Suppression of inflammatory cytokines (IL-1β, IL-6, and NO) | Zhao et al. [101] |
| Resveratrol | Primary glial from rat cortices induced by amyloid-beta (Aβ) | Upregulation of SIRT1 inhibits NF-κB p65 by deacetylating Lys310 residue | Suppression of iNOS and EGFP expression; improvement of the survival of MAP2-positive neurons | Chen et al. [102] |
| Resveratrol | Cerebral ischemia mouse model; primary cortical neurons induced by oxygen-glucose deprivation (OGD) | The high levels of acetylation of NF-κB p65 occur in mouse and cell models; resveratrol deacetylated the Lys310 in primary neurons after OGD | Suppression of LDH expression and improvement of the survival of neuronal cell | Lanzillotta et al. [103] |
| Resveratrol | Postoperative cognitive dysfunction (POCD) rat model | Upregulation of SIRT1 decreases the expression of acetyl-NF-κB p65 in the hippocampus | Suppression of inflammatory cytokines (TNF-α, IL-1β, and IL-6) | Yan et al. [73] |
| Resveratrol | LPS-induced depressive-like behaviors mouse model | Upregulation of SIRT1 decreases the expression of NF-κB in the hippocampus | Suppression of LPS-induced depression-like behaviors and the overactivation of microglia | Liu et al. [104] |
| Resveratrol | Alzheimer's disease rat model induced by ovariectomized (OVX)+D-galactose (D-gal) | Upregulation of SITRT1 decreases the expression of NF-κB p65 in the hippocampus | Suppression of insoluble amyloid-beta (Aβ) and MMP-9; increase of the tight junction protein claudin-5 | Zhao et al. [105] |
7.2. SIRT1 and Toll-Like Receptors
Several toll-like receptors (TLRs) are widely expressed in the immune system of the brain, including microglia, astrocytes, and neurons [6]. As membrane receptors, TLRs recognize several molecules, such as lipopolysaccharide (LPS), heat shock proteins, high-mobility group protein B1 (HMGB1), and peptidoglycan. After activation, TLRs recruit the various downstream molecules, such as myeloid differentiation factor-88 (MyD88), TIR-domain-containing adaptor protein (TIRAP), TNFR-associated factor 6 (TRAF6), and subsequent regulation of activation of NF-κB, leading to the transcription of inflammatory cytokines [107]. It has been confirmed that the TLR pathway is involved in the inflammation response and diseases of CNS. TLR4 knockout inhibits the expression of inflammatory factors IL-1β and TNF-α and improves the survival rate after induction of intracerebral hemorrhage (ICH) compared with wild-type (WT) mice [108]. TLR4 deficiency suppresses the inflammatory factors IL-1β and TNF-α and improves cognitive dysfunction in β2-microglobulin- (B2M-) induced age-related cognitive decline compared with WT mice [109]. Moreover, several studies have demonstrated that SIRT1 regulates neuroinflammation via inhibition of the TLR pathway. There is a recent study of neuroinflammation induced by excessive ethanol (EtOH), and the study found that the SIRT1 activator resveratrol decreases the inflammatory cytokines IL-1β and TNF-α and improves the spatial reference memory by inhibition of the TLR2-MyD88-NF-κB signal pathway [110]. Similarly, resveratrol suppresses the proinflammatory cytokines IL-1β and TNF-α, inhibiting the activation of matrix metalloproteinase-9 (MMP-9) and MMP-2 by downregulation of the TLR-4-NF-κB signaling pathway in trigeminal neuralgia mice [111]. Another study has shown that resveratrol attenuates the cytokines IL-1β, IL-6, and TNF-α by the TLR4-MyD88-NF-κB pathway in hypoxic-ischemic brain injury mice [112]. An experimental subarachnoid hemorrhage model has shown that resveratrol inhibits the proinflammatory cytokines IL-1β, IL-6, and TNF-α and ameliorates neurological behavior impairment by the TLR4-MyD88-NF-κB pathway [113].
7.3. SIRT1 and NLRP3 Inflammasome
Inflammasomes are cytosolic molecular complexes that sense a variety of stimuli and subsequently trigger and activate inflammatory events to participate in innate immune defenses. Generally, inflammasome complexes consist of a cytosolic sensor (NLR protein or AIM2-like receptor), an apoptosis-associated speck-like protein containing CARD (ASC), and a pro-caspase-1. Once the cytosolic sensor NLR is in response to intracellular signals, this leads to the formation of the inflammasome complex, regulation of activation of caspase-1 and subsequently activated caspase-1 cleavage pro-IL-1β and pro-IL-18, and release of the proinflammatory cytokines IL-1β and IL-18 [114]. To date, five inflammasome complexes have been found: NLRP1, NLRP3, NLRC4, AIM2, and pyrin inflammasome [115]. In CNS, several cells have been found in the expression of inflammasome components, including microglia, neurons, and astrocytes [116]. Accumulating evidence indicates that activation of the NLRP3 inflammasome is involved in neuroinflammation and neurodegenerative pathologies, and the SIRT1 activator resveratrol can regulate neuroinflammation via inhibition of this pathway. In a cell experiment, resveratrol suppresses the release of inflammatory cytokines IL-1β, IL-6, and TNF-α in amyloid-beta- (Aβ-) induced BV2 microglial cells by inhibition of the NLRP3 and NF-κB signaling pathways [117]. Similarly, another study found that resveratrol attenuates ATP and LPS-induced NLRP3 inflammasome activation in BV2 microglial cells. The animal experiment of this study also showed that resveratrol suppresses the inflammatory cytokine IL-1β and activation of microglia in the hippocampus of the sepsis-associated encephalopathy mice by the NLRP3 signaling pathway [118]. Furthermore, many researchers put their interests in the investigation between SIRT1 activator resveratrol and several animal models of brain injury, including subarachnoid hemorrhage, traumatic brain injury, middle cerebral artery occlusion, and cognitive impairment model. All these studies suggest that the SIRT1 activator resveratrol inhibits the inflammatory cytokines IL-1β, IL-18, and TNF-α by inhibiting the NLRP3 inflammasome signaling pathway [119–123] (Table 3).
Table 3.
SIRT1 regulate neuroinflammation involved in the NLRP3 inflammasome pathway.
| Chemical agents | Models | Involved mechanisms | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | BV2 microglia cell lines induced by amyloid-beta (Aβ) | Upregulation of SIRT1 inhibits TXNIP/TRX/NLRP3 and NF-κB signaling pathway | Suppression of inflammatory cytokines (IL-1β, IL-6, and TNF-α) | Feng and Zhang [117] |
| Resveratrol | BV2 microglia cell lines induced by ATP and LPS | Upregulation of SIRT1 inhibits NLRP3 inflammasome signaling pathway | Suppression of inflammatory cytokine IL-1β | Sui et al. [118] |
| Resveratrol | Sepsis-associated encephalopathy mouse model induced by cecal ligation and puncture (CLP) | Upregulation of SIRT1 inhibits NLRP3 inflammasome signaling pathway | Suppression of inflammatory cytokine IL-1β, activation of microglia in the hippocampus | Sui et al. [118] |
| Resveratrol | Estrogen deficiency-induced depression-like behavior mouse model | Upregulation of SIRT1 inhibits NLRP3 inflammasome and NF-κB signaling pathway | Suppression of inflammatory cytokines (IL-1β and IL-18) and activation of microglia in the hippocampus | Liu et al. [119] |
| Resveratrol | Subarachnoid hemorrhage rat model | Upregulation of SIRT1 inhibits NLRP3 inflammasome signaling pathway | Suppression of inflammatory cytokines (IL-1β, IL-18, and TNF-α), microglia activation, and neutrophil infiltration | Zhang et al. [120] |
| Resveratrol | Isoflurane-induced cognitive impairment mouse model | Upregulation of SIRT1 inhibits the expression of NLRP3 inflammasome | Suppression of inflammatory cytokines ( IL-1β and TNF-α) in the hippocampus | Li et al. [121] |
| Resveratrol | Traumatic brain injury rat model | Upregulation of SIRT1 inhibits NLRP3 inflammasome signaling pathway | Suppression of inflammatory cytokines ( IL-1β and IL-18) and ROS | Zou et al. [122] |
| Resveratrol | Middle cerebral artery occlusion (MCAO) rat model | Resveratrol inhibits NLRP3 inflammasome signaling pathway by upregulated autophagy | Suppression of inflammatory cytokines (IL-1β and IL-18) | He et al. [123] |
7.4. SIRT1 and Mitogen-Activated Protein Kinase
The activation of the mitogen-activated protein kinase (MAPK) pathway in the innate immune response has been widely studied. The stimulation of PRRs on the cell surface can activate the members of MAPK subfamilies, including p38 kinase, Jun N-terminal kinase (JNK), and extracellular signal-regulated kinases (ERK), in conjunction with the NF-κB pathway and many transcription factors of proinflammatory cytokines [124]. It has been found that MAPK pathways contribute to the pathology of several CNS diseases [125]. A growing body of evidence suggests that SIRT1 regulates neuroinflammation involved in MAPK pathways, especially the p38 MAPK pathway. In the study of primary cortical astrocytes, upregulation of SIRT1 by resveratrol or adenoviral vectors inhibits the astrocyte activation after stimulation of IL-1β via inhibition of the MAPK signaling pathway [126]. Another study showed that resveratrol suppresses the inflammatory cytokines IL-1β and TNF-α in hypoxia-induced cytotoxicity in BV2 microglial cells by inhibition of the MAPK and NF-κB signaling pathways [127]. Similarly, resveratrol-enriched rice attenuates several inflammatory cytokines in LPS-activated BV2 microglial cells by suppression of MAPK signaling pathways and NF-κB translocation [128]. There is a study on the neuron cells that found that resveratrol decreases the inflammatory cytokine TNF-α and neuron damage in the hippocampus of an alcohol-induced neurodegeneration rat through the p38 MAPK pathway [129]. Moreover, the SIRT1 inhibitor sirtinol promotes the neuronal apoptosis in injured-side cortexes of a traumatic brain injury rat model by the activation of the p38 MAPK pathway [130]. However, there is an opposite conclusion that the SIRT1 inhibitor salermide or SIRT1 siRNA promotes apoptotic neuronal death by inhibition of the ERK1/2 MAPK pathway [131] (Table 4).
Table 4.
SIRT1 regulate neuroinflammation involved in the MAPK pathway.
| Chemical agents | Models | Involved mechanisms | Effect | Reference |
|---|---|---|---|---|
| Resveratrol or adenoviral vectors | IL-1β-stimulated primary cortical astrocyte model | Overexpression of SIRT1 or resveratrol inhibits the MAPK pathway | Suppression of the astrocyte activation | Li et al. [126] |
| Resveratrol | Hypoxia-induced cytotoxicity in BV2 microglial cells | Resveratrol inhibits ERK and JNK MAPK signaling pathways and the NF-κB pathway | Suppression of inflammatory cytokines (IL-1β and TNF-α) | Zhang et al. [127] |
| Resveratrol-enriched rice (RR) | LPS-activated BV2 microglial cells | RR inhibits MAPK signaling pathways and NF-κB translocation | Suppression of inflammatory cytokines (iNOS, COX-2, TNF-α, IL-1β, and IL-6) | Subedi et al. [128] |
| Resveratrol | Alcohol-induced neurodegeneration rat and SH-SY5Y cells | Resveratrol inhibits the activation of the p38 MAPK pathway | Suppression of inflammatory cytokine TNF-α and reduction of alcohol-induced neuron damage in the hippocampus | Gu et al. [129] |
| Sirtinol | Traumatic brain injury (TBI) rat model | Sirtinol exacerbate the activation of the p38 MAPK pathway | Promotion of TBI-induced mitochondrial damage and neuronal apoptosis in injured-side cortexes | Yang et al. [130] |
| Salermide and SIRT1 siRNA | Primary cortical neurons induced by scratch injury | Salermide and SIRT1 siRNA inhibit the activation of the ERK1/2 pathway | Promotion of apoptotic neuron death | Zhao et al. [131] |
| Salermide | Traumatic brain injury (TBI) mice model | Salermide inhibits the activation of the ERK1/2 pathway | Promotion of apoptotic neuron death in injured-side cortex | Zhao et al. [131] |
8. SIRT1 and CNS Diseases
Numerous studies have shown that SIRT1 was implicated in a variety of CNS diseases. The pharmacological SIRT1 activator or inhibitor participant in the regulation of neuroinflammation and neurodegenerative diseases has been reported. Moreover, accumulating studies indicate that synthetic drugs and natural compounds mediate CNS diseases by regulating SIRT1. The following sections discuss the effect of SIRT1 on CNS disorders, including cerebral ischemia, traumatic brain injury, spinal cord injury, AD, and PD.
8.1. Cerebral Ischemia
Cerebral ischemia is the most common neurological disease due to the sudden reduction or cessation of blood flow to the brain, which leads to infarction and neuronal dysfunction. Increasing evidence suggests that SIRT1 is a promising target for the treatment of cerebral ischemia injury. In an animal model study, several studies have shown that the SIRT1 activator resveratrol provides a neuroprotective effect in the middle cerebral artery occlusion model [132–136]. One of these studies suggested that SIRT1 exerts the anti-inflammatory and antiapoptotic effects on cerebral ischemic injury by inhibition of acetylation of p53 and NF-κB [130]. Apart from resveratrol, a large number of natural compounds have reported the neuroprotective benefits for cerebral ischemic by activation of SIRT1, while the mechanisms of these compounds that regulate cerebral ischemic are different. The mainly regulatory mechanisms include anti-inflammatory, antiapoptotic, and antioxidative effects [137, 138] (Table 5). For example, rosuvastatin was found to provide a neuroprotective effect against ischemic stroke by inhibiting the NF-κB signaling pathway through the activation of SIRT1 [139]. Salvianolic acid B derived from danshen has a protective effect against ischemic stroke through the activation of SIRT1, leads to an increase of Bcl-2 and a decrease of Bax expression by inhibiting the acetylation of FOXO1, and finally inhibits the apoptosis [140]. Another study has shown that alpha-lipoic acid reduced the ischemic brain damage by the improvement of oxidative damage through the SIRT1/PGC-1α pathway [141].
Table 5.
Synthetic drugs and natural compounds regulate ischemic stroke by involving in SIRT1 in animal models.
| Chemical agents | Models | Involved pathway | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | Transient MCAO mice | SIRT1-Akt/ERK/p38/PGC-1α | Antioxidative, antiapoptotic, and anti-inflammatory effects | Shin et al. [132] |
| Activator 3, sirtinol | pMCAO mice | SIRT1/p53/NF-κB p65 | Neuroprotective effect | Hernández-Jiménez et al. [133] |
| Resveratrol | MCAO mice | SIRT1-Akt/HO-1 | Antiapoptotic effects | Hermann et al. [134] |
| Resveratrol | MCAO mice | SIRT1-BDNF | Neuroprotective effect | Koronowski et al. [135] |
| Resveratrol | MCAO rats | cAMP/AMPK/SIRT1 | Neuroprotective effect | Wan et al. [136] |
| Magnolol | MCAO rats | SIRT1/FOXO1/Bcl-2/Bax | Anti-inflammatory and antiapoptotic effects | Kou et al. [137] |
| Nampt | MCAO rats | Nampt/SIRT1/AMPK | Neuroprotective effect | Wang et al. [138] |
| PEA-OXA | MCAO rats | SIRT1/NF-κB/Bcl-2/Bax | Anti-inflammatory and antiapoptotic effects | Fusco et al. [211] |
| Liraglutide | MCAO mice | SIRT1/ICDH/α-KGDH/SDH | Improvement of mitochondrial enzyme activity | He et al. [212] |
| Rosuvastatin | Cerebral ischemic stroke rat model | SIRT1/NF-κB p65 | Neuroprotective effect | Yan and Zhu [139] |
| Salvianolic acid B | MCAO rats | SIRT1/FOXO1/Bcl-2/Bax | Anti-inflammatory and antiapoptotic effects | Lv et al. [140] |
| Alpha-lipoic acid | pMCAO rats | SIRT1/PGC-1α | Antioxidative effect and improvement of neurological deficit and brain edema | Fu et al. [141] |
| Arctigenin | MCAO rats | SIRT1/NLRP3 inflammasome | Anti-inflammatory effects | Zhang et al. [213] |
| CDP-choline | MCAO rats | SIRT1 | Neuroprotective effect | Hurtado et al. [214] |
| Curcumin | MCAO rats | SIRT1/p53/Bcl-2/Bax | Anti-inflammatory effects | Miao et al. [215] |
| Icariin | MCAO mice | SIRT1/PGC-1α | Improvement of neurological scores and brain edema | Zhu et al. [216] |
pMCAO: permanent middle cerebral artery occlusion; PEA-OXA: N-palmitoylethanolamide-oxazoline.
8.2. Traumatic Brain Injury
Traumatic brain injury (TBI) is the neurological dysfunction caused by physical force. In addition to this direct insult, secondary injury is driven by a cascade of the inflammatory response, BBB disruption, and metabolic changes [142]. Currently, several studies have demonstrated that the activator of SIRT1 contributes to the improvement of TBI animal models by the involvement of NLRP3 inflammasome and MAPK pathways [122, 126, 143]. Similarly, some studies have shown that an inhibitor of SIRT1 exacerbated the brain damage of TBI models by activation of p38 and ERK1/2 MAPK pathways [130, 131]. Besides, several studies have demonstrated that natural compounds protect against TBI by activation of SIRT1. For example, berberine has reported the neuroprotective effect in a severe TBI mouse model by inhibition of the p38 MAPK pathway through the activation of SIRT1 [144]. Polydatin derived from Polygonum cuspidatum ameliorated the damage of TBI through SIRT1-mediated improvement of endoplasmic reticulum stress and mitochondrial injury [145]. Another study has demonstrated that omega-3 polyunsaturated fatty acids (ω-3 PUFA) reduced the neuronal apoptosis in TBI rats through the promotion of autophagy by SIRT1-mediated deacetylation of beclin-1 [146]. They also found that ω-3 PUFA could inhibit the inflammatory response in the TBI model by SIRT1-mediated deacetylation of HMGB1 and NF-κB [147] (Table 6).
Table 6.
Synthetic drugs and natural compounds regulate traumatic brain injury by involving in SIRT1 in animal models.
| Chemical agents | Models | Involved pathway | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | TBI rats | SIRT1/NLRP3 inflammasome | Anti-inflammatory effects | Zou et al. [122] |
| Resveratrol | TBI mice | SIRT1/MAPK | Suppression of the astrocyte activation | Li et al. [126] |
| Resveratrol | TBI mice | SIRT1/autophagy | Neuroprotective effect | Zhang et al. [143] |
| Sirtinol | TBI rats | SIRT1/p38 | Exacerbations of neuronal damage | Yang et al. [130] |
| Salermide | TBI mice | SIRT1/ERK1/2 | Promotion of apoptotic neuron | Zhao et al. [131] |
| Berberine | TBI mice | SIRT1/p38 | Neuroprotective effect | Wang and Zhang [144] |
| Polydatin | TBI rats | SIRT1/p38/p-PERK/XBP-1/ATF6 | Antiapoptotic effects | Li et al. [145] |
| ω-3 PUFA | TBI rats | SIRT1/beclin-1 | Antiapoptotic effects | Chen et al. [146] |
| ω-3 PUFA | TBI rats | SIRT1/HMGB1/NF-κB | Anti-inflammatory effects | Chen et al. [147] |
8.3. Spinal Cord Injury
Spinal cord injury (SCI) is a devastating neurological disorder which leads not only to motor and sensory deficits but also to a wide range of other organ dysfunctions, such as respiratory and bowel issues, bladder dysfunction, and osteoporosis [148]. Posttraumatic inflammation plays a critical role in the pathogenesis of SCI [149]. Numerous studies have shown that SIRT1 exerts a neuroprotective effect in the animal model of SCI. For instance, SIRT1 agonist CAY10602 inhibited cell apoptosis through the p53 signaling pathway in the SCI rat model [150]. Pretreatment of resveratrol had a neuroprotective effect on spinal cord neuron apoptosis by regulating autophagy via the activation of the SIRT1/AMPK pathway [151, 152]. A study has found that the SIRT1 activator of SRT1720 ameliorated inflammatory response and microglial activation through regulation of the Wnt/β-catenin pathway [153]. Consistent with this study, another study had confirmed that SRT1720 suppressed the inflammatory reactions. In addition, SIRT1 knockout mice led to severe motor function, neuronal survival, and inflammatory response than WT mice after SCI [154].
8.4. Alzheimer's Disease (AD)
Alzheimer's disease (AD) is the most common neurodegenerative disease that leads to the decline of cognitive function and memory. AD is characterized by the pathological accumulation of amyloid-β (Aβ), neurofibrillary tangles (NFTs), and phosphorylated tau protein [155]. In addition, inflammation is thought to exacerbate the pathology of AD [156]. One study has reported that resveratrol provides a beneficial effect on AD rats by downregulation of NF-κB p65, Aβ, and MMP-9 [105]. Another study has shown that resveratrol ameliorated the Aβ-induced cognitive deficits in AD mice by upregulation of SIRT1 and downregulation of NF-κB/IL-1β/NLRP3 [157]. However, numerous evidences indicated the beneficial effects of SIRT1 in AD by regulating the expression of Aβ and tau protein. Some research has suggested that resveratrol suppressed the Aβ-induced neuronal apoptosis by activation of SIRT1 pathways [158–160]. Moreover, a recent study has reported that the upregulation of SIRT1 promoted the degradation of Aβ in primary astrocytes [161]. In addition, a study suggested that SIRT1 could suppress the spread of pathogenic tau by deacetylation of tau in tauP301S transgenic mice [162]. Furthermore, accumulating evidence has demonstrated that the SIRT1 agonist resveratrol has a protective effect on multiple animal models of AD [163–168]. For example, the treatment of resveratrol improved learning and memory and suppressed neural apoptosis in the Tg2576 mouse model of AD [163]. Similarly, resveratrol has been found to decrease Aβ levels and improve learning and memory in APP/PS1 AD mice [166]. In addition, another AD model study was induced by Aβ1–42, and this study has shown that resveratrol ameliorated the spatial, learning, and memory deficits by regulating SIRT1 signaling pathways [168]. Furthermore, overexpression of SIRT1 in the hippocampus improved learning and memory and reduced the expression of Aβ and tau in the 3xTg AD mouse model [169]. Apart from resveratrol, several natural compounds have reported the beneficial effects of the regulation of SIRT1 in AD animal studies [170–175] (Table 7).
Table 7.
Synthetic drugs and natural compounds regulate Alzheimer's disease by involving in SIRT1 in animal models.
| Chemical agents | Models | Involved pathway | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | OVX- and D-gal- induced AD rat model | SIRT1/NF-κB p65 | Suppression of Aβ and MMP-9 | Zhao et al. [105] |
| Resveratrol | AD mouse model induced by Aβ1–42 | SIRT1/AMPK/PGC-1α/NLRP3/NF-κB | Improvement of cognitive deficits | Qi et al. [157] |
| Resveratrol | Tg2576 mouse AD model | SIRT1 | Improvement of learning and memory, suppression of neural apoptosis | Wang et al. [163] |
| Resveratrol | Diabetes and AD rat model | SIRT1 | Improvement of memory deficits | Ma et al. [164] |
| Resveratrol | APP/PS1 AD mice | SIRT1 | Decrease of senile plaques and antioxidant effects | Dong et al. [165] |
| Resveratrol | APP/PS1 AD mice | SIRT1/ERK1/2 | Improvement of learning and memory and decrease of Aβ | Cao et al. [166] |
| Resveratrol | APP/PS1 AD mouse model | SIRT1/AMPK | Improvement of memory loss and decrease of amyloid burden | Porquet et al. [167] |
| Resveratrol | AD rat model induced by Aβ1–42 | SIRT1/CREB | Improvement of spatial, learning, and memory | Wang et al. [168] |
| — | 3xTg AD mouse model | SIRT1/BDNF | SIRT1-transgenic mice improve cognitive behavior and decrease Aβ and tau pathology | Corpas et al. [169] |
| SLAB51 | 3xTg AD mouse model | SIRT1 | Antioxidant effects | Bonfili et al. [170] |
| 24-OH | hTau mice | SIRT1 | Decrease of hyperphosphorylated tau protein | Testa et al. [171] |
| Acetylshikonin | D-galactose-induced AD mouse model | SIRT1/p53/p21 | Improvement of cognitive impairment and hippocampus senescence | Li et al. [172] |
| Melatonin | Aβ-induced AD mouse model | SIRT1/TFAM | Improvement of memory and hippocampal cell damage | Ansari Dezfouli et al. [173] |
| Dihydromyricetin | Aβ-induced AD rat model | AMPK/SIRT1 | Improvement of cognitive function and suppression of inflammatory responses and cell apoptosis | Sun et al. [174] |
| Salidroside | D-gal-induced AD rat model | SIRT1/NF-κB | Suppression of inflammatory responses | Gao et al. [175] |
D-gal: D-galactose; OVX: ovariectomized; Aβ: amyloid-β; APP: amyloid precursor protein; PS1: presenilin 1.
8.5. Parkinson's Disease (PD)
Parkinson's disease (PD) is the second most common neurological disorder and is characterized by the loss of dopaminergic (DA) neurons. Aggregation of alpha-synuclein (α-syn) of neurons is another pathological feature of PD [176]. Increasing evidence supports the important role of neuroinflammation in the pathogenesis of PD [177]. Accumulating evidence supports the hypothesis that SIRT1 has beneficial effects on different models of PD. Three neurotoxins, including rotenone, 6-hydroxydopamine (6-OHDA), and 1-methyl-4-phenylpyridinium (MPP+), are extensively used in cellular models of PD [178, 179]. Several in vitro studies have found that resveratrol ameliorated neuronal apoptosis in the rotenone-induced SH-SY5Y cell model of PD by regulation of SIRT1 pathways [180, 181]. A recent study has reported that the upregulation of SIRT1 protected SH-SY5Y cells from rotenone by inhibition of NF-κB [182]. In addition, some natural compounds, including epigallocatechin-3-gallate (EGCG), echinacoside (ECH), and salidroside, have reported the neuroprotective effects on MPP+-treated cellular models of PD by regulating SIRT1 pathways [183–185]. Furthermore, some recent studies indicate that the neuroprotective role of SIRT1 in the animal model of PD. For example, activation SIRT1 by resveratrol alleviated the loss of dopaminergic neurons and promoted the degradation of α-synuclein by deacetylation of LC3 in the MPTP-induced mouse model [186]. SIRT1 knockout worsens movement function in a mouse PD model induced by MPTP [187]. However, a recent study suggested that SIRT1-transgenic mice fail to alleviate the loss of nigrostriatal dopamine neurons in the MPTP-induced mouse model [188]. A list of studies of treatments for PD that target SIRT1 is shown in Table 8.
Table 8.
Synthetic drugs and natural compounds regulate Parkinson's disease by involving in SIRT1 in cell and animal models.
| Chemical agents | Models | Involved pathway | Effect | Reference |
|---|---|---|---|---|
| Resveratrol | 6-OHDA-treated SH-SY5Y cells | SIRT1/BMAL1 | Antioxidant effects | Wang et al. [179] |
| Resveratrol | Rotenone-induced SH-SY5Y cells | SIRT1/p53 | Suppression of neural apoptosis | Feng et al. [180] |
| Resveratrol | Rotenone-induced SH-SY5Y cells | AMPK/SIRT1/autophagy | Suppression of neural apoptosis and promotion of the degradation of α-synuclein | Wu et al. [181] |
| Oxyresveratrol | 6-OHDA-treated SH-SY5Y cells | SIRT1/JNK | Neuroprotective effects | Chao et al. [217] |
| DCHC | 6-OHDA-treated PC-12 cells | SIRT1 | Suppression of neural apoptosis | Tsai et al. [218] |
| SIRT1 viral plasmid | Rotenone-treated SH-SY5Y cells | SIRT1/NF-κB | Increase of cell survival and decrease of α-synuclein aggregates | Singh et al. [182] |
| EGCG | MPP+-treated PC12 cells | SIRT1/PGC-1α | Suppression of oxidative stress | Ye et al. [183] |
| ECH | MPP+-treated PC12 cells | SIRT1/FOXO1/autophagy | Increase of cell survival | Chen et al. [184] |
| Salidroside | MPP+-treated SH-SY5Y cells | SIRT1/MAPK | Suppression of neural apoptosis and oxidative stress | Wang et al. [185] |
| Resveratrol | MPTP-induced mouse model | SIRT1/PGC-1α | Neuroprotective effects | Mudò et al. [219] |
| Resveratrol | MPTP-induced mouse model | SIRT1/LC3 | Protect the loss of dopaminergic neurons and promote the degradation of α-synuclein | Guo et al. [186] |
| — | MPTP-induced mouse model | SIRT1 | SIRT1 knockout mice worsen movement function | Zhang et al. [187] |
| — | MPTP-induced mouse model | SIRT1 | SIRT1-transgenic mice fail to alleviate loss of nigrostriatal dopamine neurons | Kitao et al. [188] |
MPP+: 1-methyl-4-phenyl-pyridine; DCHC: 3-(2,4-dichlorophenyl)-7-hydroxy-4H-chromen-4-one; EGCG: epigallocatechin-3-gallate; 6-OHDA: 6-hydroxydopamine; ECH: echinacoside; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
9. SIRT1 and Brain Aging
Aging is a physiological phenomenon in which the body function declines in a time-dependent fashion. It has been widely reported that brain aging is characterized by the presence of cognitive changes [189]. Aging is a major risk factor for some neurodegenerative diseases, such as AD [190] and PD [191]. Some studies have shown that the inflammatory process and oxidative stress contribute to the cognitive changes in aged brains [192, 193]. Gene expression studies have shown the age-related gene expression changes in the human brain. The downregulation genes which include those related to memory and learning, calcium signaling, and mitochondrial function are downregulated. In addition, the upregulation genes include DNA repair, oxidative stress, and inflammatory response [194–196]. The chronic inflammatory status was shown to link with aging and age-related diseases closely [197]. The major characteristics of the aging process are a progressive increase in proinflammatory status. This phenomenon was firstly called “inflamm-aging” [198, 199]. As discussed in the previous section, SIRT1 plays an important role in the progression of neurodegenerative diseases, such as AD and PD. Increasing evidence shows that SIRT1 is involved in the regulation of aging and lifespan. Brain-specific Sirt1-overexpressing (BRASTO) transgenic mice were shown to have increased lifespans [200], and pharmacologic SIRT1 activator SRT1720 was shown to have lifespan benefits in mice [201]. Growing evidence indicates that SIRT1 can be a connecting link among inflammatory response, oxidative stress, and aging. A recent study has shown that SIRT1-knockout zebrafish resulted in chronic inflammation, oxidative injury, and decreased lifespan [202]. In addition, SIRT1 can regulate oxidative stress, inflammation, and senescence in the progression of COPD [203]. These studies suggest that SIRT1 may be a potential target for aging-associated disorders.
10. SIRT1 and Clinical Study
On the basis of the above research, a large body of cellular and animal studies indicates the neuroprotective effect of SIRT1 in neuroinflammation and neurodegenerative diseases. In addition, there are an increasing number of clinical studies to explore the effect of SIRT1 on inflammatory diseases. For example, treating ulcerative colitis subjects with resveratrol for six weeks decreased serum inflammatory markers TNF-α and hs-CRP compared to placebo [204]. Another randomized controlled clinical trial demonstrated that treatment with resveratrol for 12 weeks led to the reduction of serum ALT, TNF-α, IL-6, and hs-CRP in nonalcoholic fatty liver disease patients [205]. Moreover, there are several clinical trials in the area of CNS diseases. AD subjects who received resveratrol for 52 weeks were able to decrease the expression of cerebrospinal fluid MMP-9 and modulate the inflammatory markers and adaptive immunity [206]. However, another clinical trial of AD indicates that resveratrol has no beneficial effects [207]. In addition, treatment with resveratrol for 26weeks improved memory performance and hippocampal functional connectivity in healthy older adults [208]. In contrast, one recent clinical trial indicates that resveratrol fails to improve verbal memory in older adults [209]. A randomized interventional study showed that resveratrol improved the resting-state functional connectivity (RSFC) of the hippocampus and reduced the serum glycated hemoglobin A1c (HbA1c) in patients with mild cognitive impairment (MCI) [210]. These findings suggest that SIRT1 may be the potential target treatment of neuroinflammation and neurodegenerative disorders. However, in order to explore the therapeutic benefit of SIRT1 on CNS diseases, larger sample clinical trials with longer duration need to be performed in the future.
11. Conclusions and Perspectives
In this review, we summarized the latest evidence of the beneficial roles of SIRT1 in the regulation of neuroinflammation and neurodegenerative diseases. As one of the most well-studied sirtuins, SIRT1 can regulate multiple biological functions, including apoptosis, DNA repair, inflammatory response, metabolism, cancer, and stress. We focused on the regulatory effect of SIRT1 in the inflammation response of CNS. Numerous researches indicate that mechanisms of SIRT1 modulate inflammatory reactions in CNS by many different molecules and pathways. Several potential modulatory pathways were summarized in this text, including NF-κB, TLRs, NLRP3 inflammasome, and MAPK pathways. The neuroprotective effects of SIRT1 activation have been reported in in vivo and in vitro models of neuroinflammation-associated diseases. Moreover, some clinical trials have reported the neuroprotective effects of the SIRT1 activator resveratrol. Although a large number of studies demonstrated the benefic effects of the pharmacological activator of SIRT1 in neurological disorders. Some questions still need to be answered. For example, the biological functions of SIRT1 are complex and wide. The precise mechanisms of SIRT1 by deacetylation, which targets the inflammatory process of CNS diseases, are unclear. In addition, the evidence of anti-inflammatory and neuroprotective effects of SIRT1 from clinical studies is not enough. Therefore, further investigations are needed in the precise targets of SIRT1, which will contribute to developing treatment strategies for neuroinflammation-related diseases.
Data Availability
The data used to support the findings of the study can be available from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Xanthos D. N., Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nature Reviews Neuroscience. 2014;15(1):43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 2.Meneses G., Cárdenas G., Espinosa A., et al. Sepsis: developing new alternatives to reduce neuroinflammation and attenuate brain injury. Annals New York Academy Sciences. 2019;1437(1):43–56. doi: 10.1111/nyas.13985. [DOI] [PubMed] [Google Scholar]
- 3.DiSabato D. J., Quan N., Godbout J. P. Neuroinflammation: the devil is in the details. Journal Neurochemistry. 2016;139(Supplement 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladak A. A., Enam S. A., Ibrahim M. T. A review of the molecular mechanisms of traumatic brain injury. World Neurosurgery. 2019;131:126–132. doi: 10.1016/j.wneu.2019.07.039. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz M., Deczkowska A. Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunology. 2016;37(10):668–679. doi: 10.1016/j.it.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. Journal Neuroimmunology. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm I., Nyúl-Tóth Á., Kozma M., Farkas A. E., Krizbai I. A. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. American Journal Physiology Heart Circulatory Physiology. 2017;313(5):H1000–H1012. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 8.Deerhake M. E., Biswas D. D., Barclay W. E., Shinohara M. L. Pattern recognition receptors in multiple sclerosis and its animal models. Frontiers Immunology. 2019;10:p. 2644. doi: 10.3389/fimmu.2019.02644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampron A., Elali A., Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and its environment. Neuron. 2013;78(2):214–232. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson J., Nutma E., van der Valk P., Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subhramanyam C. S., Wang C., Hu Q., Dheen S. T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Seminars Cell Developmental Biology. 2019;94:112–120. doi: 10.1016/j.semcdb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Dokalis N., Prinz M. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Seminars Immunopathology. 2019;41(6):699–709. doi: 10.1007/s00281-019-00764-1. [DOI] [PubMed] [Google Scholar]
- 13.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochemical Journal. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel T., Deng C. X., Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carafa V., Rotili D., Forgione M., et al. Sirtuin functions and modulation: from chemistry to the clinic. Clinical Epigenetics. 2016;8(1):p. 61. doi: 10.1186/s13148-016-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendes K. L., Lelis D. F., Santos S. H. S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Reviews. 2017;38:98–105. doi: 10.1016/j.cytogfr.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacological Sciences. 2012;33(9):494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Anoopkumar-Dukie S., Arora D., Davey A. K. Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases. European Journal Pharmacology. 2020;867, article 172847 doi: 10.1016/j.ejphar.2019.172847. [DOI] [PubMed] [Google Scholar]
- 19.Frye R. A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochemical Biophysical Research Communications. 1999;260(1):273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H., Schoonjans K., Auwerx J. Sirtuin functions in health and disease. Molecular Endocrinology. 2007;21(8):1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 21.Frye R. A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochemical Biophysical Research Communications. 2000;273(2):793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A., Chauhan S. How much successful are the medicinal chemists in modulation of SIRT1: a critical review. European Journal Medicinal Chemistry. 2016;119:45–69. doi: 10.1016/j.ejmech.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Jin Q., Yan T., Ge X., Sun C., Shi X., Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. Journal Cellular Physiology. 2007;213(1):88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 24.Moynihan K. A., Grimm A. A., Plueger M. M., et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metabolism. 2005;2(2):105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Stünkel W., Peh B. K., Tan Y. C., et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnology Journal. 2007;2(11):1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 26.Yanagisawa S., Baker J. R., Vuppusetty C., et al. The dynamic shuttling of SIRT1 between cytoplasm and nuclei in bronchial epithelial cells by single and repeated cigarette smoke exposure. PLoS One. 2018;13(3, article e0193921) doi: 10.1371/journal.pone.0193921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanno M., Sakamoto J., Miura T., Shimamoto K., Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. Journal Biological Chemistry. 2007;282(9):6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 28.North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Molecular Cell. 2003;11(2):437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T., Hiratsuka M., Osaki M. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. 2007;26(7):945–957. doi: 10.1038/sj.onc.1209857. [DOI] [PubMed] [Google Scholar]
- 30.Scher M. B., Vaquero A., Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Development. 2007;21(8):920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michishita E., Park J. Y., Burneskis J. M., Barrett J. C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Molecular Cellular Biology. 2005;16(10):4623–4635. doi: 10.1091/mbc.e05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davenport A. M., Huber F. M., Hoelz A. Structural and functional analysis of human SIRT1. Journal Molecular Biology. 2014;426(3):526–541. doi: 10.1016/j.jmb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min J., Landry J., Sternglanz R., Xu R.-M. Crystal structure of a SIR2 homolog-NAD complex. Cell. 2001;105(2):269–279. doi: 10.1016/S0092-8674(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 34.Borra M. T., Langer M. R., Slama J. T., Denu J. M. Substrate specificity and kinetic mechanism of the Sir2 family of NAD+-dependent histone/protein deacetylases. Biochemistry. 2004;43(30):9877–9887. doi: 10.1021/bi049592e. [DOI] [PubMed] [Google Scholar]
- 35.Wojcik M., Mac-Marcjanek K., Wozniak L. A. Physiological and pathophysiological functions of SIRT1. Mini Reviews Medicinal Chemistry. 2009;9(3):386–394. doi: 10.2174/1389557510909030386. [DOI] [PubMed] [Google Scholar]
- 36.Haigis M. C., Mostoslavsky R., Haigis K. M., et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 37.Min Z., Gao J., Yu Y. The roles of mitochondrial SIRT4 in cellular metabolism. Frontiers Endocrinology. 2019;9:p. 783. doi: 10.3389/fendo.2018.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L., Ma X., He Y., et al. Sirtuin 5: a review of structure, known inhibitors and clues for developing new inhibitors. Science China Life Sciences. 2017;60(3):249–256. doi: 10.1007/s11427-016-0060-7. [DOI] [PubMed] [Google Scholar]
- 39.Michishita E., McCord R. A., Berber E., et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H., Khan S., Wang Y., et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaquero A., Scher M., Lee D., Erdjument-Bromage H., Tempst P., Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Molecular Cell. 2004;16(1):93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Vaziri H., Dessain S. K., Eaton E. N., et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 43.Luo J., Nikolaev A. Y., Imai S., et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 44.Ong A. L. C., Ramasamy T. S. Role of sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Research Reviews. 2018;43:64–80. doi: 10.1016/j.arr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Yeung F., Hoberg J. E., Ramsey C. S., et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO Journal. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Jeong J., Juhn K., Lee H., et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Experimental Molecular Medicine. 2007;39(1):8–13. doi: 10.1038/emm.2007.2. [DOI] [PubMed] [Google Scholar]
- 48.Cohen H. Y., Lavu S., Bitterman K. J., et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Molecular Cell. 2004;13(5):627–638. doi: 10.1016/S1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 49.Tia N., Singh A. K., Pandey P., Azad C. S., Chaudhary P., Gambhir I. S. Role of forkhead box O (FOXO) transcription factor in aging and diseases. Gene. 2018;648:97–105. doi: 10.1016/j.gene.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 50.Tikhanovich I., Cox J., Weinman S. A. Forkhead box class O transcription factors in liver function and disease. Journal Gastroenterology Hepatology. 2013;28:125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y., Hou H., Haller E. M., Nicosia S. V., Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO Journal. 2005;24(5):1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunet A., Sweeney L. B., Sturgill J. F., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 53.van der Horst A., Tertoolen L. G., de Vries-Smits L. M., Frye R. A., Medema R. H., Burgering B. M. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2SIRT1. Journal Biological Chemistry. 2004;279(28):28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 54.Yoon H., Shin S. H., Shin D. H., Chun Y. S., Park J. W. Differential roles of Sirt1 in HIF-1α and HIF-2α mediated hypoxic responses. Biochemical Biophysical Research Communications. 2014;444(1):36–43. doi: 10.1016/j.bbrc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Joo H. Y., Yun M., Jeong J., et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochemical Biophysical Research Communications. 2015;462(4):294–300. doi: 10.1016/j.bbrc.2015.04.119. [DOI] [PubMed] [Google Scholar]
- 56.Dioum E. M., Chen R., Alexander M. S., et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324(5932):1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 57.Lagouge M., Argmann C., Gerhart-Hines Z., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 58.Gerhart-Hines Z., Rodgers J. T., Bare O., et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO Journal. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 60.Hu Y. S., Han X., Liu X. H. STAT3: a potential drug target for tumor and inflammation. Current Topics Medicinal Chemistry. 2019;19(15):1305–1317. doi: 10.2174/1568026619666190620145052. [DOI] [PubMed] [Google Scholar]
- 61.Nie Y., Erion D. M., Yuan Z., et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nature Cell Biology. 2009;11(4):492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Zhu W., Li J., Liu M., Wei M. Resveratrol suppresses the STAT3 signaling pathway and inhibits proliferation of high glucose-exposed HepG2 cells partly through SIRT1. Oncology Reports. 2013;30(6):2820–2828. doi: 10.3892/or.2013.2748. [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa T., Guarente L. SnapShot: sirtuins, NAD, and aging. Cell Metabolism. 2014;20(1):192–192.e1. doi: 10.1016/j.cmet.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Xia N., Förstermann U., Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19(10):16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jardim F. R., de Rossi F. T., Nascimento M. X. Resveratrol and brain mitochondria: a review. Molecular neurobiology. 2018;55(3):2085–2101. doi: 10.1007/s12035-017-0448-z. [DOI] [PubMed] [Google Scholar]
- 66.Howitz K. T., Bitterman K. J., Cohen H. Y. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 67.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5):p. 946. doi: 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X. L., Li T., Li J. H., Miao S. Y., Xiao X. Z. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. 2017;22(9):p. 1529. doi: 10.3390/molecules22091529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadeghi A., Seyyed Ebrahimi S. S., Golestani A., Meshkani R. Resveratrol ameliorates palmitate-induced inflammation in skeletal muscle cells by attenuating oxidative stress and JNK/NF-κB pathway in a SIRT1-independent mechanism. Journal of cellular biochemistry. 2017;118(9):2654–2663. doi: 10.1002/jcb.25868. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X., Liu Q., Wang M., et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PloS One. 2011;6(11):p. e27081. doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park S. J., Ahmad F., Philp A., et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu B., Ghosh S., Yang X., et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell metabolism. 2012;16(6):738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Yan J., Luo A., Gao J., et al. The role of SIRT1 in neuroinflammation and cognitive dysfunction in aged rats after anesthesia and surgery. American Journal Translational Research. 2019;11(3):1555–1568. [PMC free article] [PubMed] [Google Scholar]
- 74.Li L., Sun Q., Li Y., et al. Overexpression of SIRT1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. Journal of Molecular Neuroscience. 2015;56(4):858–867. doi: 10.1007/s12031-015-0526-5. [DOI] [PubMed] [Google Scholar]
- 75.Sakamoto J., Miura T., Shimamoto K., Horio Y. Predominant expression of Sir2α, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain1. FEBS Letter. 2004;556(1-3):281–286. doi: 10.1016/S0014-5793(03)01444-3. [DOI] [PubMed] [Google Scholar]
- 76.Ogawa T., Wakai C., Saito T., et al. Distribution of the longevity gene product, SIRT1, in developing mouse organs. Congenital Anomalies. 2011;51(2):70–79. doi: 10.1111/j.1741-4520.2010.00304.x. [DOI] [PubMed] [Google Scholar]
- 77.Cao K., Dong Y. T., Xiang J., et al. Reduced expression of SIRT1 and SOD-1 and the correlation between these levels in various regions of the brains of patients with Alzheimer's disease. Journal Clinical Pathology. 2018;71(12):1090–1099. doi: 10.1136/jclinpath-2018-205320. [DOI] [PubMed] [Google Scholar]
- 78.Al-Bahrani R., Tuertcher D., Zailaie S., et al. Differential SIRT1 expression in hepatocellular carcinomas and cholangiocarcinoma of the liver. Annals Clinical Laboratory Science. 2015;45(1):3–9. [PubMed] [Google Scholar]
- 79.Tanno M., Kuno A., Yano T., et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. Journal Biological Chemistry. 2010;285(11):8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goh K. P., Lee H. Y., Lau D. P., Supaat W., Chan Y. H., Koh A. F. Y. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. International Journal Sport Nutrition Exercise Metabolism. 2014;24(1):2–13. doi: 10.1123/ijsnem.2013-0045. [DOI] [PubMed] [Google Scholar]
- 81.Tavano F., Pazienza V., Fontana A., et al. SIRT1 and circadian gene expression in pancreatic ductal adenocarcinoma: effect of starvation. Chronobiology International. 2014;32(4):497–512. doi: 10.3109/07420528.2014.1003351. [DOI] [PubMed] [Google Scholar]
- 82.Jorge A., Jorge G., Paraíso A., et al. Brown and white adipose tissue expression of IL6, UCP1 and SIRT1 are associated with alterations in clinical, metabolic and anthropometric parameters in obese humans. Experimental Clinical Endocrinology Diabetes. 2017;125(3):163–170. doi: 10.1055/s-0042-119525. [DOI] [PubMed] [Google Scholar]
- 83.Zakhary S. M., Ayubcha D., Dileo J. N., et al. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec. 2010;293(6):1024–1032. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramadori G., Lee C. E., Bookout A. L., et al. Brain SIRT1: anatomical distribution and regulation by energy availability. Journal Neuroscience. 2008;28(40):9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kannan V., Brouwer N., Hanisch U. K., Regen T., Eggen B. J., Boddeke H. W. Histone deacetylase inhibitors suppress immune activation in primary mouse microglia. Journal Neuroscience Research. 2013;91(9):1133–1142. doi: 10.1002/jnr.23221. [DOI] [PubMed] [Google Scholar]
- 86.Prozorovski T., Ingwersen J., Lukas D., et al. Regulation of sirtuin expression in autoimmune neuroinflammation: Induction of SIRT1 in oligodendrocyte progenitor cells. Neuroscience Letters. 2019;704:116–125. doi: 10.1016/j.neulet.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Ginhoux F., Greter M., Leboeuf M., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 89.Qin L., Wu X., Block M. L., et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lull M. E., Block M. L. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colombo E., Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunology. 2016;37(9):608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Zamanian J. L., Xu L., Foo L. C., et al. Genomic analysis of reactive astrogliosis. Journal Neuroscience. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye J., Liu Z., Wei J., et al. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neuroscience Letters. 2013;553:72–77. doi: 10.1016/j.neulet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Y., Takeuchi H., Sonobe Y., et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. Journal Neuroimmunology. 2014;269(1-2):38–43. doi: 10.1016/j.jneuroim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 95.DiDonato J. A., Mercurio F., Karin M. NF-κB and the link between inflammation and cancer. Immunological Reviews. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 96.Gasparini C., Feldmann M. NF-κB as a target for modulating inflammatory responses. Current Pharmaceutical Design. 2012;18(35):5735–5745. doi: 10.2174/138161212803530763. [DOI] [PubMed] [Google Scholar]
- 97.Schug T. T., Xu Q., Gao H., et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Molecular Cellular Biology. 2010;30(19):4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moon M. H., Jeong J. K., Lee Y. J., Seol J. W., Jackson C. J., Park S. Y. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21(3):470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 99.Cho S. H., Chen J. A., Sayed F., et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. Journal Neuroscience. 2015;35(2):807–818. doi: 10.1523/JNEUROSCI.2939-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanzillotta A., Pignataro G., Branca C., et al. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiology Disease. 2013;49:177–189. doi: 10.1016/j.nbd.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 101.Zhao H., Wang Q., Cheng X., et al. Inhibitive effect of resveratrol on the inflammation in cultured astrocytes and microglia induced by Aβ1–42. Neuroscience. 2018;379:390–404. doi: 10.1016/j.neuroscience.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 102.Chen J., Zhou Y., Mueller-Steiner S., et al. SIRT1 protects against microglia-dependent Amyloid-β toxicity through inhibiting NF-κB signaling. Journal Biological Chemistry. 2005;280(48):40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 103.Lanzillotta A., Sarnico I., Ingrassia R., et al. The acetylation of RelA in Lys310 dictates the NF-κB-dependent response in post-ischemic injury. Cell Death Disease. 2010;1(11):p. e96. doi: 10.1038/cddis.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L., Zhang Q., Cai Y., et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7(35):56045–56059. doi: 10.18632/oncotarget.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao H. F., Li N., Wang Q., Cheng X. J., Li X. M., Liu T. T. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Planavila A., Iglesias R., Giralt M., Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovascular Research. 2011;90(2):276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 107.Kay E., Scotland R. S., Whiteford J. R. Toll-like receptors: role in inflammation and therapeutic potential. Biofactors. 2014;40(3):284–294. doi: 10.1002/biof.1156. [DOI] [PubMed] [Google Scholar]
- 108.Fei X., He Y., Chen J., et al. The role of Toll-like receptor 4 in apoptosis of brain tissue after induction of intracerebral hemorrhage. Journal Neuroinflammation. 2019;16(1):p. 234. doi: 10.1186/s12974-019-1634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong Q., Zou Y., Liu H., et al. Toll-like receptor 4 deficiency ameliorates β2-microglobulin induced age-related cognition decline due to neuroinflammation in mice. Molecular Brain. 2020;13(1):p. 20. doi: 10.1186/s13041-020-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qi B., Shi C., Meng J., Xu S., Liu J. Resveratrol alleviates ethanol-induced neuroinflammation in vivo and in vitro: Involvement of TLR2-MyD88-NF-κB pathway. International Journal Biochemistry Cell Biology. 2018;103:56–64. doi: 10.1016/j.biocel.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 111.Yin Y., Guo R., Shao Y., et al. Pretreatment with resveratrol ameliorate trigeminal neuralgia by suppressing matrix metalloproteinase-9/2 in trigeminal ganglion. International Immunopharmacology. 2019;72:339–347. doi: 10.1016/j.intimp.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 112.Le K., Daliv E. C., Wu S., et al. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: a possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. International Immunopharmacology. 2019;75, article 105779 doi: 10.1016/j.intimp.2019.105779. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X.-S., Li W., Wu Q., et al. Resveratrol attenuates acute inflammatory injury in experimental subarachnoid hemorrhage in rats via inhibition of TLR4 pathway. International Journal Molecular Sciences. 2016;17(8):p. 1331. doi: 10.3390/ijms17081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rathinam V. A., Fitzgerald K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165(4):792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanneganti T. D. The inflammasome: firing up innate immunity. Immunological Reviews. 2015;265(1):1–5. doi: 10.1111/imr.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Albornoz E. A., Woodruff T. M., Gordon R. Inflammasomes in CNS diseases. Experientia Supplementum. 2018;108:41–60. doi: 10.1007/978-3-319-89390-7_3. [DOI] [PubMed] [Google Scholar]
- 117.Feng L., Zhang L. Resveratrol suppresses Aβ-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biology. 2019;38(8):874–879. doi: 10.1089/dna.2018.4308. [DOI] [PubMed] [Google Scholar]
- 118.Sui D.-M., Xie Q., Yi W.-J., et al. Resveratrol protects against sepsis-associated encephalopathy and inhibits the NLRP3/IL-1β axis in microglia. Mediators Inflammation. 2016;2016, article 1045657:1–10. doi: 10.1155/2016/1045657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu T., Ma Y., Zhang R., et al. Resveratrol ameliorates estrogen deficiency-induced depression- and anxiety-like behaviors and hippocampal inflammation in mice. Psychopharmacology. 2019;236(4):1385–1399. doi: 10.1007/s00213-018-5148-5. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X., Wu Q., Zhang Q., et al. Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of NLRP3 inflammasome activation. Frontiers Neuroscience. 2017;11:p. 611. doi: 10.3389/fnins.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X. M., Zhou M. T., Wang X. M., Ji M. H., Zhou Z. Q., Yang J. J. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. Journal Molecular Neuroscience. 2014;52(2):286–293. doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 122.Zou P., Liu X., Li G., Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Molecular Medicine Reports. 2018;17(2):3212–3217. doi: 10.3892/mmr.2017.8241. [DOI] [PubMed] [Google Scholar]
- 123.He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. International Immunopharmacology. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 124.Arthur J. S., Ley S. C. Mitogen-activated protein kinases in innate immunity. Nature Reviews Immunology. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 125.Kim E. K., Choi E.-J. Compromised MAPK signaling in human diseases: an update. Archives Toxicology. 2015;89(6):867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 126.Li D., Liu N., Zhao H.-H., et al. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. Journal Neuroinflammation. 2017;14(1):p. 67. doi: 10.1186/s12974-017-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Yuan L., Zhang Q., et al. Resveratrol attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. International Immunopharmacology. 2015;28(1):578–587. doi: 10.1016/j.intimp.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 128.Subedi L., Baek S.-H., Kim S. Y. Genetically engineered resveratrol-enriched rice inhibits neuroinflammation in lipopolysaccharide-activated BV2 microglia via downregulating mitogen-activated protein kinase-nuclear factor kappa B signaling pathway. Oxidative Medicine Cellular Longevity. 2018;2018, article 8092713:1–14. doi: 10.1155/2018/8092713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu X., Cai Z., Cai M., et al. AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol-induced neurodegeneration by resveratrol. Molecular Medicine Reports. 2018;17(4):5402–5408. doi: 10.3892/mmr.2018.8482. [DOI] [PubMed] [Google Scholar]
- 130.Yang H., Gu Z. T., Li L., et al. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacologica Sinica. 2017;38(2):168–181. doi: 10.1038/aps.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Y., Luo P., Guo Q., et al. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Experimental Neurology. 2012;237(2):489–498. doi: 10.1016/j.expneurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Shin J. A., Lee K. E., Kim H. S., Park E. M. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochemical Research. 2012;37(12):2686–2696. doi: 10.1007/s11064-012-0858-2. [DOI] [PubMed] [Google Scholar]
- 133.Hernandez-Jimenez M., Hurtado O., Cuartero M. I., et al. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44(8):2333–2337. doi: 10.1161/STROKEAHA.113.001715. [DOI] [PubMed] [Google Scholar]
- 134.Hermann D. M., Zechariah A., Kaltwasser B., et al. Sustained neurological recovery induced by resveratrol is associated with angioneurogenesis rather than neuroprotection after focal cerebral ischemia. Neurobiology Disease. 2015;83:16–25. doi: 10.1016/j.nbd.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 135.Koronowski K. B., Dave K. R., Saul I., et al. Resveratrol preconditioning induces a novel extended window of ischemic tolerance in the mouse brain. Stroke. 2015;46(8):2293–2298. doi: 10.1161/STROKEAHA.115.009876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wan D., Zhou Y., Wang K., Hou Y., Hou R., Ye X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Research Bulletin. 2016;121:255–262. doi: 10.1016/j.brainresbull.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 137.Kou D. Q., Jiang Y. L., Qin J. H., Huang Y. H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacological Reports. 2017;69(4):642–647. doi: 10.1016/j.pharep.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 138.Wang P., Xu T. Y., Guan Y. F., et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Annals Neurology. 2011;69(2):360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- 139.Yan L., Zhu T. Effects of rosuvastatin on neuronal apoptosis in cerebral ischemic stroke rats via Sirt1/NF-kappa B signaling pathway. European Review Medical Pharmacological Sciences. 2019;23(12):5449–5455. doi: 10.26355/eurrev_201906_18214. [DOI] [PubMed] [Google Scholar]
- 140.Lv H., Wang L., Shen J., et al. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Research Bulletin. 2015;115:30–36. doi: 10.1016/j.brainresbull.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Fu B., Zhang J., Zhang X., et al. Alpha-lipoic acid upregulates SIRT1-dependent PGC-1α expression and protects mouse brain against focal ischemia. Neuroscience. 2014;281:251–257. doi: 10.1016/j.neuroscience.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 142.Needham E. J., Helmy A., Zanier E. R., Jones J. L., Coles A. J., Menon D. K. The immunological response to traumatic brain injury. Journal Neuroimmunology. 2019;332:112–125. doi: 10.1016/j.jneuroim.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Z., Li D., Xu L., Li H. P. Sirt1 improves functional recovery by regulating autophagy of astrocyte and neuron after brain injury. Brain Research Bulletin. 2019;150:42–49. doi: 10.1016/j.brainresbull.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 144.Wang J., Zhang Y. Neuroprotective effect of berberine agonist against impairment of learning and memory skills in severe traumatic brain injury via Sirt1/p38 MAPK expression. Molecular Medicine Reports. 2018;17(5):6881–6886. doi: 10.3892/mmr.2018.8674. [DOI] [PubMed] [Google Scholar]
- 145.Li L., Tan H. P., Liu C. Y., et al. Polydatin prevents the induction of secondary brain injury after traumatic brain injury by protecting neuronal mitochondria. Neural Regeneration Research. 2019;14(9):1573–1582. doi: 10.4103/1673-5374.255972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen X., Pan Z., Fang Z., et al. Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. Journal Neuroinflammation. 2018;15(1):p. 310. doi: 10.1186/s12974-018-1345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen X., Chen C., Fan S., et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. Journal Neuroinflammation. 2018;15(1):p. 116. doi: 10.1186/s12974-018-1151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bloom O., Herman P. E., Spungen A. M. Systemic inflammation in traumatic spinal cord injury. Experimental Neurology. 2020;325, article 113143 doi: 10.1016/j.expneurol.2019.113143. [DOI] [PubMed] [Google Scholar]
- 149.Wang S., Smith G. M., Selzer M. E., Li S. Emerging molecular therapeutic targets for spinal cord injury. Expert Opinion Therapeutic Targets. 2019;23(9):787–803. doi: 10.1080/14728222.2019.1661381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu X., Zhang S., Zhao D., et al. SIRT1 inhibits apoptosis in in vivo and in vitro models of spinal cord injury via microRNA-494. Journal Molecular Medicine. 2019;43(4):1758–1768. doi: 10.3892/ijmm.2019.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yan P., Bai L., Lu W., Gao Y., Bi Y., Lv G. Regulation of autophagy by AMP-activated protein kinase/sirtuin 1 pathway reduces spinal cord neurons damage. Iranian Journal Basic Medical Sciences. 2017;20(9):1029–1036. doi: 10.22038/IJBMS.2017.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao H., Chen S., Gao K., et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 153.Lu P., Han D., Zhu K., Jin M., Mei X., Lu H. Effects of sirtuin 1 on microglia in spinal cord injury: involvement of Wnt/β-catenin signaling pathway. Neuroreport. 2019;30(13):867–874. doi: 10.1097/WNR.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 154.Chen H., Ji H., Zhang M., et al. An agonist of the protective factor SIRT1 improves functional recovery and promotes neuronal survival by attenuating inflammation after spinal cord injury. Journal Neuroscience. 2017;37(11):2916–2930. doi: 10.1523/JNEUROSCI.3046-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yan Y., Yang H., Xie Y., Ding Y., Kong D., Yu H. Research progress on Alzheimer's disease and resveratrol. Neurochemical Research. 2020;45(5):989–1006. doi: 10.1007/s11064-020-03007-0. [DOI] [PubMed] [Google Scholar]
- 156.Heppner F. L., Ransohoff R. M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Neuroscience. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 157.Qi Y., Shang L., Liao Z., et al. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metabolic Brain Disease. 2019;34(1):257–266. doi: 10.1007/s11011-018-0348-6. [DOI] [PubMed] [Google Scholar]
- 158.Feng X., Liang N., Zhu D., et al. Resveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS One. 2013;8(3):p. e59888. doi: 10.1371/journal.pone.0059888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ai Z., Li C., Li L., He G. Resveratrol inhibits β-amyloid-induced neuronal apoptosis via regulation of p53 acetylation in PC12 cells. Molecular Medicine Reports. 2015;11(4):2429–2434. doi: 10.3892/mmr.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang J., Feng X., Wu J., et al. Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by β-amyloid through activation of SIRT1/Akt1 pathway. Biofactors. 2014;40(2):258–267. doi: 10.1002/biof.1149. [DOI] [PubMed] [Google Scholar]
- 161.Li M. Z., Zheng L. J., Shen J., et al. SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes. Neural Regeneration Research. 2018;13(11):2005–2013. doi: 10.4103/1673-5374.239449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Min S.-W., Sohn P. D., Li Y., et al. SIRT1 deacetylates tau and reduces pathogenic tau spread in a mouse model of tauopathy. Journal Neuroscience. 2018;38(15):3680–3688. doi: 10.1523/JNEUROSCI.2369-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X., Ma S., Yang B., et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer's disease. Behavioural Brain Research. 2018;339:297–304. doi: 10.1016/j.bbr.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma X. R., Sun Z. K., Han X., et al. Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer's disease. Frontiers Neuroscience. 2020;13:p. 1400. doi: 10.3389/fnins.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dong Y.-T., Cao K., Tan L.-C., et al. Stimulation of SIRT1 attenuates the level of oxidative stress in the brains of APP/PS1 double transgenic mice and in primary neurons exposed to oligomers of the amyloid-β peptide. Journal Alzheimers Disease. 2018;63(1):283–301. doi: 10.3233/JAD-171020. [DOI] [PubMed] [Google Scholar]
- 166.Cao K., Dong Y. T., Xiang J., et al. The neuroprotective effects of SIRT1 in mice carrying the APP/PS1 double-transgenic mutation and in SH-SY5Y cells over-expressing human APP670/671 may involve elevated levels of α7 nicotinic acetylcholine receptors. Aging. 2020;12(2):1792–1807. doi: 10.18632/aging.102713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Porquet D., Griñán-Ferré C., Ferrer I., et al. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer's disease. Journal Alzheimers Disease. 2014;42(4):1209–1220. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- 168.Wang R., Zhang Y., Li J., Zhang C. Resveratrol ameliorates spatial learning memory impairment induced by Aβ 1–42 in rats. Neuroscience. 2017;344:39–47. doi: 10.1016/j.neuroscience.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 169.Corpas R., Revilla S., Ursulet S., et al. SIRT1 overexpression in mouse hippocampus induces cognitive enhancement through proteostatic and neurotrophic mechanisms. Molecular Neurobiology. 2017;54(7):5604–5619. doi: 10.1007/s12035-016-0087-9. [DOI] [PubMed] [Google Scholar]
- 170.Bonfili L., Cecarini V., Cuccioloni M., et al. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Molecular Neurobiology. 2018;55(10):7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Testa G., Staurenghi E., Giannelli S., et al. A silver lining for 24-hydroxycholesterol in Alzheimer's disease: the involvement of the neuroprotective enzyme sirtuin 1. Redox Biology. 2018;17:423–431. doi: 10.1016/j.redox.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Q., Zeng J., Su M., He Y., Zhu B. Acetylshikonin from Zicao attenuates cognitive impairment and hippocampus senescence in d-galactose-induced aging mouse model via upregulating the expression of SIRT1. Brain Research Bulletin. 2018;137:311–318. doi: 10.1016/j.brainresbull.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 173.Ansari Dezfouli M., Zahmatkesh M., Farahmandfar M., Khodagholi F. Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal sirtuin-1 signaling pathway. Physiology Behavior. 2019;204:65–75. doi: 10.1016/j.physbeh.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 174.Sun P., Yin J.-B., Liu L.-H., et al. Protective role of dihydromyricetin in Alzheimer's disease rat model associated with activating AMPK/SIRT1 signaling pathway. Bioscience Reports. 2019;39(1) doi: 10.1042/bsr20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao J., Zhou R., You X., et al. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer's disease via SIRT1/NF-κB pathway. Metabolic Brain Disease. 2016;31(4):771–778. doi: 10.1007/s11011-016-9813-2. [DOI] [PubMed] [Google Scholar]
- 176.Kalampokini S., Becker A., Fassbender K., Lyros E., Unger M. M. Nonpharmacological modulation of chronic inflammation in Parkinson's disease: role of diet interventions. Parkinsons Disease. 2019;2019, article 7535472:1–12. doi: 10.1155/2019/7535472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hirsch E. C., Vyas S., Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism related disorders. 2012;18(Supplement 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 178.Falkenburger B. H., Saridaki T., Dinter E. Cellular models for Parkinson's disease. Journal Neurochemistry. 2016;139:121–130. doi: 10.1111/jnc.13618. [DOI] [PubMed] [Google Scholar]
- 179.Wang Y., Lv D., Liu W., et al. Disruption of the circadian clock alters antioxidative defense via the SIRT1-BMAL1 pathway in 6-OHDA-induced models of Parkinson's disease. Oxidative Medicine and Cellular Longevity. 2018;2018:11. doi: 10.1155/2018/4854732.4854732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Feng Y., Liu T., Dong S. Y., et al. Rotenone affects p53 transcriptional activity and apoptosis via targeting SIRT1 and H3K9 acetylation in SH-SY5Y cells. Journal Neurochemistry. 2015;134(4):668–676. doi: 10.1111/jnc.13172. [DOI] [PubMed] [Google Scholar]
- 181.Wu Y., Li X., Zhu J. X., et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19(3):163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Singh P., Hanson P. S., Morris C. M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson's disease. BMC Neuroscience. 2017;18(1):p. 46. doi: 10.1186/s12868-017-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ye Q., Ye L., Xu X., et al. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complementary Medicine Therapies. 2012;12(1):p. 82. doi: 10.1186/1472-6882-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen C., Xia B., Tang L., et al. Echinacoside protects against MPTP/MPP (+)-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metabolic Brain Disease. 2019;34(1):203–212. doi: 10.1007/s11011-018-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang C. Y., Sun Z. N., Wang M. X., Zhang C. SIRT1 mediates salidroside-elicited protective effects against MPP (+) -induced apoptosis and oxidative stress in SH-SY5Y cells: involvement in suppressing MAPK pathways. Cell Biology International. 2018;42(1):84–94. doi: 10.1002/cbin.10864. [DOI] [PubMed] [Google Scholar]
- 186.Guo Y.-J., Dong S.-Y., Cui X.-X., et al. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Molecular Nutrition Food Research. 2016;60(10):2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Q., Zhang P., Qi G. J., et al. Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson's disease models. Biochimica Biophysica Acta General Subjects. 2018;1862(6):1443–1451. doi: 10.1016/j.bbagen.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 188.Kitao Y., Ageta-Ishihara N., Takahashi R., Kinoshita M., Hori O. Transgenic supplementation of SIRT1 fails to alleviate acute loss of nigrostriatal dopamine neurons and gliosis in a mouse model of MPTP-induced parkinsonism. F1000Res. 2015;4:p. 130. doi: 10.12688/f1000research.6386.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Erdő F., Denes L., de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. Journal Cerebral Blood Flow Metabolism. 2016;37(1):4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Friedrich M. J. Researchers probe the aging brain in health and disease. JAMA. 2014;311(3):231–232. doi: 10.1001/jama.2013.284609. [DOI] [PubMed] [Google Scholar]
- 191.Collier T. J., Kanaan N. M., Kordower J. H. Aging and Parkinson's disease: different sides of the same coin. Movement Disorders. 2017;32(7):983–990. doi: 10.1002/mds.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.von Bernhardi R., Eugenín-von Bernhardi L., Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Frontiers Aging Neuroscience. 2015;7:p. 124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Berr C., Balansard B., Arnaud J., Roussel A. M., Alpérovitch A. Cognitive decline is associated with systemic oxidative stress: the EVA Study. Journal American Geriatrics Society. 2000;48(10):1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 194.Erraji-Benchekroun L., Underwood M. D., Arango V., et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biological Psychiatry. 2005;57(5):549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 195.Cribbs D. H., Berchtold N. C., Perreau V., et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. Journal Neuroinflammation. 2012;9(1):p. 179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cornejo F., von Bernhardi R. Age-dependent changes in the activation and regulation of microglia. Advances Experimental Medicine Biology. 2016;949:205–226. doi: 10.1007/978-3-319-40764-7_10. [DOI] [PubMed] [Google Scholar]
- 197.Bektas A., Schurman S. H., Sen R., Ferrucci L. Aging, inflammation and the environment. Experimental Gerontology. 2018;105:10–18. doi: 10.1016/j.exger.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xia S., Zhang X., Zheng S., et al. An update on inflamm-aging: mechanisms, prevention, and treatment. Journal Immunology Research. 2016;2016, article 8426874:1–12. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Franceschi C., Bonafè M., Valensin S., et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Annals New York Academy Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 200.Satoh A., Brace C. S., Rensing N., et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metabolism. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mitchell S. J., Martin-Montalvo A., Mercken E. M., et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Reports. 2014;6(5):836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim D. H., Jung I. H., Kim D. H., Park S. W. Knockout of longevity gene Sirt1 in zebrafish leads to oxidative injury, chronic inflammation, and reduced life span. PloS One. 2019;14(8, article e0220581) doi: 10.1371/journal.pone.0220581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Conti V., Corbi G., Manzo V., Pelaia G., Filippelli A., Vatrella A. Sirtuin 1 and aging theory for chronic obstructive pulmonary disease. Analytical Cellular Pathology. 2015;2015:8. doi: 10.1155/2015/897327.897327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Samsami-Kor M., Daryani N. E., Asl P. R., Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Archives Medical Research. 2015;46(4):280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 205.Faghihzadeh F., Adibi P., Rafiei R., Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutrition Research. 2014;34(10):837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 206.Moussa C., Hebron M., Huang X., et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. Journal Neuroinflammation. 2017;14(1):p. 1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Turner R. S., Thomas R. G., Craft S., et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Witte A. V., Kerti L., Margulies D. S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. Journal Neuroscience. 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huhn S., Beyer F., Zhang R., et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults - a randomized controlled trial. Neuroimage. 2018;174:177–190. doi: 10.1016/j.neuroimage.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 210.Köbe T., Witte A. V., Schnelle A., et al. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Frontiers Neuroscience. 2017;11:p. 105. doi: 10.3389/fnins.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fusco R., Scuto M., Cordaro M., et al. N-Palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. International Journal Molecular Sciences. 2019;20(19):p. 4845. doi: 10.3390/ijms20194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.He W., Wang H., Zhao C., Tian X., Li L., Wang H. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. Journal Cellular Physiology. 2020;235(3):2986–3001. doi: 10.1002/jcp.29204. [DOI] [PubMed] [Google Scholar]
- 213.Zhang S., Jiang L., Che F., Lu Y., Xie Z., Wang H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochemical Biophysical Research Communications. 2017;493(1):821–826. doi: 10.1016/j.bbrc.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 214.Hurtado O., Hernández-Jiménez M., Zarruk J. G., et al. Citicoline (CDP-choline) increases sirtuin1 expression concomitant to neuroprotection in experimental stroke. Journal Neurochemistry. 2013;126(6):819–826. doi: 10.1111/jnc.12269. [DOI] [PubMed] [Google Scholar]
- 215.Miao Y., Zhao S., Gao Y., et al. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: the possible role of Sirt1 signaling. Brain Research Bulletin. 2016;121:9–15. doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 216.Zhu H. R., Wang Z. Y., Zhu X. L., Wu X. X., Li E. G., Xu Y. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1α expression in experimental stroke. Neuropharmacology. 2010;59(1-2):70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 217.Chao J., Yu M. S., Ho Y. S., Wang M., Chang R. C. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radical Biology Medicine. 2008;45(7):1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 218.Tsai K. L., Cheng Y. Y., Leu H. B., et al. Investigating the role of Sirt1-modulated oxidative stress in relation to benign paroxysmal positional vertigo and Parkinson's disease. Neurobiology Aging. 2015;36(9):2607–2616. doi: 10.1016/j.neurobiolaging.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 219.Mudò G., Mäkelä J., Di Liberto V., et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cellular Molecular Life Sciences. 2012;69(7):1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the study can be available from the corresponding author.


