Abstract
When used for the evaluation of drug efficacy against Psoroptes ovis, the diagnostic performance of different sampling strategies for a mite count reduction test (MCRT) remains unclear. In the present study, a novel simulation framework was constructed that accounted for relevant biological features of P. ovis infestations in cattle and that was parameterized with field data from 16 farms (154 animals). Second, this framework was applied to explore the impact of study specific factors (number of animals, number of sampled lesions, and number of scrapings per lesion) and biological factors (mite infestation intensity and size of lesions) on the diagnostic performance of MCRT. Its outcome provided a basis to determine the diagnostic performance of MCRT when it was applied according to the World Association for the Advancement in Veterinary Parasitology (WAAVP) and the European Medicine Agency (EMA) guidelines, and to formulate recommendations to ensure a good diagnostic performance of the MCRT. For both guidelines, the MCRT allowed to correctly detect (power 80%) reduced and normal efficacy when the therapeutic efficacy was <70%, and ≥95%, respectively. The results highlighted a reliable diagnostic performance of the MCRT when performed as recommended by WAAVP and EMA for the detection of normal drug efficacy. When used for the detection of reduced efficacy, therapeutic efficacies between 70% and 90% could not be detected with sufficient reliability. The diagnostic performance can be improved by increasing the total number of skin scrapings (increasing the number of animals, number of sampled lesions and/or number of samples per lesion). In order to help researchers and veterinarians to optimize the design of the MCRT to their field settings, the findings were translated into a simple tool.
Keywords: Acaricide resistance –Psoroptes ovis–cattle–mite count reduction test–monitoring drug efficacy
Graphical abstract

Highlights
-
•
Simulation framework for drug efficacy evaluation against Psoroptes ovis.
-
•
WAAVP and EMA guidelines reliable for detection of normal efficacy; reduced efficacy unreliable.
-
•
The total number of samples taken per farm and mite infestation intensity increase reliability.
-
•
Summarizing tool for a wide variety of sampling strategies with resulting diagnostic performance.
1. Introduction
The mite Psoroptes ovis causes an exudative dermatitis in beef cattle and sheep, predominantly localized on the back of the animal. When left untreated, lesions can generalize and even result in death of the animal (Kirkwood, 1986; Losson et al., 1999; Bridi et al., 2001; Van Den Broek and Huntley, 2003; Fischer and Walton, 2014). The resulting financial losses and decline in animal welfare can be substantial, due to reduced growth and leather quality (Lonneux et al., 1998; Rehbein et al., 2003, 2016). In cattle, the disease is geographically limited to Europe and certain parts of North and South America (Sarre et al., 2012), whereas in sheep, psoroptic mange is endemic in Europe, Africa, North and South America (ADAS, 2008).
Acaricides are at the centre of mange treatment, with the macrocyclic lactones, pyrethroids, amitraz and phoxim as possible active compounds. However, due to the development of resistance, the therapeutic efficacy can be reduced. This can lead to treatment failure and consequently important animal welfare issues and more financial losses due to additional treatment costs and continuing production losses. At the time of writing, resistance in P. ovis in cattle has only been reported for the macrocyclic lactones (Lonneux et al., 1998; Genchi et al., 2008; Lekimme et al., 2010; Sarre et al., 2015; Lifschitz et al., 2018; van Mol et al., 2020).
The reduction in mite counts following drug administration (mite count reduction test; MCRT) is used to measure the therapeutic efficacy of acaricides, and guidelines on how to best design the MCRT are provided by the World Association for the Advancement of Veterinary Parasitology (WAAVP) and the European Medicines Agency (EMA) (Committee for medicinal products for veterinary use(CVMP), 2005; Vercruysse et al., 2006). Unfortunately, both guidelines provide quite different recommendations. For example, they both agree on mite count reduction of at least 90% to define a normal drug efficacy, but they differ in number of animals (6 vs. 15), number of skin scrapings per animal (≥6 if large lesions or ≥2 if small lesions vs. 3 without any specification of the size of lesions), size of the sampled skin surface (3 cm × 3 cm vs. no recommendations) and number of lesions sampled (no recommendations vs. 3).
There is little compliance to these guidelines, as shown by the diversity in sampling strategies applied to assess drug efficacy under field conditions (number of animals included: 6 to 15; number of skin scrapings per animal: 1 to 6; surface of scraping: 4 cm2– 9 cm2; Lonneux and Losson, 1992; Lonneux et al., 1997; Bridi et al., 2001; Rehbein et al., 2002; Committee for medicinal products for veterinary use(CVMP), 2005; Vercruysse et al., 2006; Genchi et al., 2008; Lekimme et al., 2010; Hamel et al., 2014; Sarre et al., 2015; Lifschitz et al., 2018; van Mol et al., 2020). This lack in standardisation jeopardises a direct comparison of results across studies and a reliable assessment of acaricide resistance.
More generally, it is not clear how these different study specific factors affect the final conclusions, and this lack of insights hampers any evidence-based guidance on how to optimize the diagnostic accuracy of MCRT to correctly detect normal and reduced efficacy. Confronted with a similar challenge to detect reduced efficacy against gastrointestinal nematodes by means of a faecal egg count reduction test (FECRT), researchers recently performed a series of simulation studies that were designed to assess the impact of both study specific factors (the number of animals and the sensitivity of the diagnostic method used) and biological factors (the intensity and distribution of infections among the animals) on the diagnostic accuracy of FECRT (Levecke et al., 2012, 2018). Overall, these studies confirmed that the diagnostic accuracy of FECRT is affected by a complex interplay of the aforementioned factors, and that the diagnostic value of FECRT to detect anthelmintic resistance early is limited.
Although such a simulation study is also primordial to formulate more evidence-based recommendations for MCRT, such a simulation framework that accounts for all the biological features of mite infestations in cattle is lacking. The only simulation studies performed for P. ovis were a Leslie-matrix simulation to study the population growth in sheep under favourable conditions and a model to simulate the pathogenicity of biological agents for the control of P. ovis (Wall et al., 1999; Rose and Wall, 2009). This lack of P. ovis simulations is mainly because there are little insights on the biological distribution of mite counts at the level of farms, individual animals and skin surfaces (with and without lesions), yet these are of utmost importance to reliably mimic field conditions. For example, Sarre et al. (2015) and van Mol et al. (2020) reported details on the distribution of mite counts, reporting both mean and range of mite counts across farms, whereas others divided their mite counts in different arbitrary categories (Lonneux and Losson, 1992; Genchi et al., 2008; Lekimme et al., 2010; Lifschitz et al., 2018). P. ovis mites are distributed throughout the entire lesion and not restricted to the periphery of the lesion, as is the case in sheep (Losson et al., 1999), but a more detailed description of the distribution within lesions is still lacking.
In contrast to FECRT, where counting eggs in stool does not affect the worm burden, skin scrapings imply removal of the mites. Hence, taking skin scrapings prior to drug administration may already induce a significant reduction in mite counts at follow-up, which may potentially hide an underlying reduced therapeutic efficacy.
The overall aim of the present study was to develop recommendations for surveys on acaricide resistance in P. ovis. To this end, a simulation framework was first constructed that accounts for the most important biological features of P. ovis infestations. Second, this framework was applied to explore the impact of both study specific factors (number of animals, number of sampled lesions and number of scrapings per lesion) and biological factors (intensity of mite infestation and size of lesions) on the diagnostic performance of MCRT. Based on the outcome of the simulation study, the diagnostic performance of MCRT was determined when it is applied according to the existing WAAVP and EMA guidelines, and recommendations were formulated to ensure a good diagnostic performance of the MCRT. Finally, a simple supporting tool was developed that will help researchers and veterinarians to further optimize the design of the MCRT to their own field settings.
2. Material and methods
2.1. Construction of a simulation framework for MCRT accounting for the biological features of psoroptic mange
To mimic the field condition as much as possible, a simulation framework was constructed that accounts for both the variation in mite counts at different levels (Level 1: farm, Level 2: animal and Level 3: lesion), the difference in mite counts across lesions and non-lesions, the variation in size of lesions, and the removal of mites by taking skin scrapings. The parameterisation of these variables was based on re-analyses of field data (van Mol et al., 2020) and collection of new field data.
Subsequently, the MCRT was mimicked in an iterative process, during which it was verified whether the MCRT allowed for a correct classification of the true underlying drug efficacy (TDE) under varying scenarios for both infestation intensities (mean mite counts at farm level) and sampling strategy (number of animals, number of sampled lesions, surface of lesion and number of scrapings per lesion).
All simulations and statistical analyses were performed in R (R Core Team, 2018). The script for the simulation framework can be found in Supplementary Info I1.
2.1.1. General simulation framework
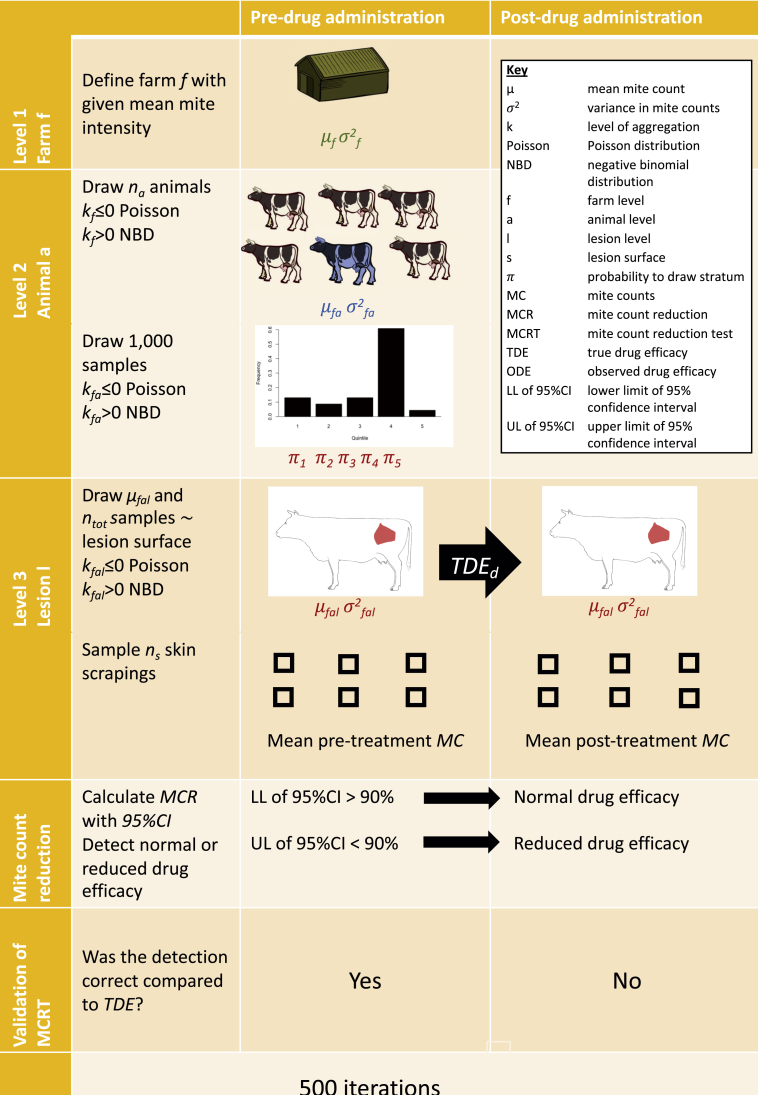
A representation of the general simulation framework is shown in Fig. 1. Each iteration starts with virtually counting the mite counts in skin scrapings of 3 cm by 3 cm from lesions prior to drug administration. To this end, a farm f was defined (Level 1), where the mean and variance in mite counts across individuals equalled μf and σ2f, respectively. In order to get to the level of individual animal a (Level 2), a subset of na non-zero mite counts were randomly selected within this virtual farm and assigned to na animals, each mite count corresponding to the mean mite counts/9 cm2 for each individual animal a (μfa). These mite counts were drawn either from a negative binomial distribution with mean μf and an aggregation kf (= μf2/(σ2f - μf)) in case of kf > 0 or from a Poisson distribution (λf = μf) in case of kf ≤ 0.
Fig. 1.
General overview of the simulation framework for mite counts. On a given farm f (Level 1) with predetermined mean (μf), variation (σf) in mite counts and level of aggregation (kf), a number of animals (na) with their respective mean (μfa), variation (σfa) in mite counts and level of aggregation (kfa) were drawn (Level 2) from a negative binomial distribution (NBD) or Poisson distribution. Subsequently, 1000 skin areas were generated per animal and divided over their respective quintiles, each quintile representing a different level of mite infestation. From these quintiles, nllesions were proportionally (π1 - π5) drawn with their respective mean (μfal), variation (σfal) in mite counts and level of aggregation (kfal) (Level 3). Then, nsskin scrapings were sampled from these lesions with a surface equal to Sfal. Post-drug administration mite counts (MC) within each lesion were generated by multiplying the pre-drug administration by 1 – TDEd(= the true underlying drug efficacy of the drug d) and nsskin scrapings were sampled from the same lesion. The observed drug efficacy (ODE), consisting of the mite count reduction (MCR) and the 95% confidence intervals (95%CI), were calculated, resulting in the mite count reduction test (MCRT). Depending on the preferred definition of normal drug efficacy, MCR≥90% or MCR≥95%, efficacy was classified as either normal (lower limit (LL) of 95%CI was at least 90% or 95%) or reduced (upper limit (UL) of 95%CI was less than 90% or 95%). Finally, the outcome of the MCRT was compared to the true drug efficacy of the given mite population.
For each animal a, the variation in mite counts (σ2fa) was then defined as a function of μfa and a random subset of 1000 mite counts was drawn from either a negative binomial distribution (kfa >0) or a Poisson distribution (kfa ≤0). Then, these mite counts were grouped into 5 strata based on the quintiles, each stratum representing a level of mite infestation. In order to get the distribution on the level of individual lesion l (Level 3), a random subset of nl mite counts was proportionally selected across the different strata applying a multinomial distribution with probabilities π1 - π5 to select mite counts in strata 1 to 5, respectively. This methodology allowed to differentiate skin areas with more mite counts (lesions) from those with lower mite counts (healthy skin areas). Each of these mite counts was assigned to nl lesions within an animal a, and they corresponded to the mean mite counts/9 cm2 within these lesions (μfal).
For each lesion l, the variation in mite counts within each lesion (σ2fal) was then defined as a function of μfal, and a random subset of ntot mite counts was drawn from either a negative binomial distribution (kfal >0) or a Poisson distribution (kfal ≤0). These mite counts corresponded with the ntot skin areas of 9 cm2 that can be scraped within a lesion with a surface Sfal. For example, in a Sfal of 180 cm2 the ntot equals 20 (= 180 cm2/9 cm2). Finally, a predetermined subset of ns scrapings was randomly selected from these ntot mite counts, resulting in na x nl x ns pre-drug administration mite counts across na animals of farm f.
In a second step, the post-drug administration mite counts were defined when a drug d with efficacy of TDEd is administered to all na animals. For the mite counts following drug administration, it was assumed that skin scrapings are taken from the same lesions, but that the skin area sampled within a lesion l could be different. This implies that there is already a reduction in mite counts solely due to the removal of mites prior to the drug administration. To account for this, the mite counts for each 9 cm2 within each lesion l of animal a were first generated by multiplying these counts by 1 – TDEd. Subsequently, the mite counts of skin areas that were already sampled prior to drug administration were put to zero. By doing so, it was assumed that mites do not move across the animals’ skin. Although this does not happen in reality, this assumption hugely simplified the data generation process.
In a third step, the MCR and the corresponding 95% confidence intervals (95%CI) were calculated. The MCR was calculated as: MCR (%) = 100 x (1 - (arithmetic mean of pre-drug administration mite counts/arithmetic mean of post-drug administration mite counts)). For the 95%CI, a previously described methodology (Levecke et al., 2015, 2018) was used. Based on both MCR and the corresponding 95%CI, resulting in the MCRT, the therapeutic efficacy of the drug was classified into normal and reduced. A drug was classified as normal when the lower limit of the 95%CI did not include 90%, and as reduced when the upper limit did not include 90%.
2.1.2. Parameterisation of the simulation framework at farm, animal and lesion level
In order to parameterise the simulation framework for P. ovis, values for both the mean (μf) and the variance (σ2f) in mite counts were imputed at the farm level (Level 1). At the animal level (Level 2), the variance in mite counts across skin areas (σ2fa), the probabilities π1 to π5, and the variation in skin surface were imputed. At the level of the lesion (Level 3), the variance in mite counts within a lesion (σ2fal) was required.
2.1.2.1. Parameterisation at farm level with re-analyses of field data
To determine the mean (μf) and variance (σ2f) of mite counts at farm level, the mite count data collected during a series of published acaricide drug efficacy trials were re-analysed. This dataset included mite counts of 154 animals from 16 farms (7–13 animals per farm) based on 3 scrapings of 9 cm2 per animal taken from a varying number of lesions per animal (1–3) or predilection locations (withers, midback or tail-base) when lesions were considered too small (van Mol et al., 2020). To cover a wide range of possible mean mite counts at farm level (μf) into the simulation, the 5th, 50th and the 95th percentile of the mean mite counts/9 cm2 were determined at these 16 farms (per farm the mean mite counts at the individual level was averaged). For the variance in mite counts between individuals of the same farm (σ2f), a linear regression model was built with the log transformed mean mite counts at the farm level (μf) as independent variable and the log transformed variance in mean mite counts across individuals at the farm level (σ2f) as dependent variable.
2.1.2.2. Parameterisation at the individual animal level with new field data
A new field study was designed to characterise the variance in mite counts within an individual animal (σ2fa), difference in mite counts across both lesions and healthy skin areas (probabilities π1 to π5) and the individual variation in skin surface of the back. In this study, 15 animals (≥2 years old) across three farms with confirmed psoroptic mange were included. In total, 27 skin scrapings (9 cm2) were collected per animal, of which 9 samples were taken from each of the 3 regions of the back (the withers, the midback and the tail base region). If lesions were present in a region, the samples were taken randomly within a lesion. When no lesions were present in a region, the samples were taken from predefined locations. The lesions were sketched on a silhouette and sampling locations were recorded (see Supplementary Fig. 1). The length from withers to tail-base and the width at the shoulders and tuber coxae was measured for all animals with a tape measure. Skin scrapings were transported and stored at room temperature. Within five days after collection of the skin scrapings, the P. ovis mites (living and unresponsive intact) were counted as previously described (van Mol et al., 2020). Per individual animal, the mean mite counts at the sampling area (μsa) were averaged, which was either a lesion or healthy skin.
For the variance in mite counts within individuals of the same farm (σ2fa), a linear regression model was built with the log transformed mean of the averaged mite counts across the lesions and healthy skin areas (~μfa) as independent variable and the log transformed variance in the averaged mite counts across the lesions and healthy skin areas (~σ2fa) as dependent variable. For this analysis, only animals for which μfa > 0 were included.
To determine the probabilities π1 to π5, the averaged mite counts were stratified across the lesions and healthy skin areas combined based on their quintiles, resulting in 5 levels of mite infestation. Subsequently, the proportion of averaged mite counts of lesions that fell in each of these 5 levels of mange infestation were determined. For this analysis, only lesions and healthy skin sampling areas for which 9 scrapings were available were included.
To impute the surface of the lesions, the mean, smallest and largest length from the withers to the tail base and averaged width (at the shoulders and tuber coxae) across the different animals was determined. Subsequently, these values were multiplied by each other (smallest length x smallest width; mean length x mean width; longest length x widest width), resulting in three values for total surface of the back (small, medium and large animals). To also allow for variation in size of lesions within an animal, the total surface of the back was arbitrarily divided into 12 equal parts, ranging from 1/12 to 12/12 of the back of an animal.
2.1.2.3. Parameterisation at lesion level
To determine the variation in mite counts across lesions, a linear regression model was built with the log transformed mean mite counts in lesions and healthy sampling areas combined (~μfal) as independent variable and the log transformed variance in mean mite counts across lesions and healthy skin sampling areas (~σ2fal) as dependent variable. For this analysis, lesions and healthy skin sampling areas were only included for which 9 scrapings were available.
2.1.3. Impact of number of skin scrapings on post-drug administration mite counts
As a result of the skin scrapings, a fraction of the total mite population on an animal will always be removed. This removal of mites may potentially hide an underlying reduced therapeutic efficacy through overestimation of the reduction. Since this fraction increases as more skin scrapings are collected per animal, it will be important to minimize the number of scrapings to the strict minimum. Therefore, a separate simulation study was performed in order to explore the impact of the number of skin scrapings on post-drug administration mite counts. In this simulation study, the first step of the aforementioned simulation framework was applied that was subsequently parameterized based on the methodology described in Section 2.1.1. For each iteration, the proportion of the total number of mites that were removed was determined.
In this simulation, μf (the mite infestation at the farm level) was fixed to the 50th percentile of the mean mite counts at the 16 aforementioned farms, na (number of animals) to 6 and nl (number of lesions) to 1. The variation in surface of the lesion varied from 1/12 to 12/12 of the back. To also explore the individual variation in size of the animal, three different values in cm2 were imputed for 1/12. These absolute values for surface correspond with the smallest, medium and largest measured surface of a back on the animals (See Section 2.1.1.2.). The number of scrapings of 9 cm2 per lesion (ns) was set at 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30, resulting in 432 unique combinations (1 μf x 1 na x 1 nl x 12 parts of the back x 3 absolute surfaces for 1/12 of a back x 12 ns).
2.2. Application of simulation framework to explore the impact of both study specific and biological factors on the diagnostic performance of MCRT
To gain insights in the impact of both study specific and biological factors on the diagnostic performance of MCRT, a final simulation study was performed. In this simulation study, the complete simulation framework was applied that was subsequently parameterized based on the methodology described in Section 2.1.1.). In total, the impact was explored of the mean mite infestation at the level of farms (μf = 5th, 50th and 95th percentile of the mean mite counts at the 16 aforementioned farms), number of animals (na = 6, 10, 15 and 20) and TDEd (= 70.00, 72.50, 75.00, 77.50, 80.00, 81.25, 82.50, 83.75, 85.00, 86.25, 87.50, 88.75, 90.00, 91.25, 92.50, 93.75, 95.00, 96.25, 97.50 and 98.75). For the number of scrapings (ns) and the surface sizes of the lesions (Sfal), which was based on both the proportion of the back and the individual size of the individual animal, our selection was based on the outcome of the separate simulation described in Section 2.1.2. This was done because it would be redundant to include combinations of ns and Sfal, where high proportion of the mites are already removed prior to drug administration. Therefore, only those combinations of ns and Sfal, were considered for which the proportion of mites removed was less than 10% in half of the iterations. A total of 627 combination fulfilled these requirements, resulting in a total of 7524 unique combinations (3 μf x 4 na x 627 ns - Sfal) for each of the 20 values of TDEd
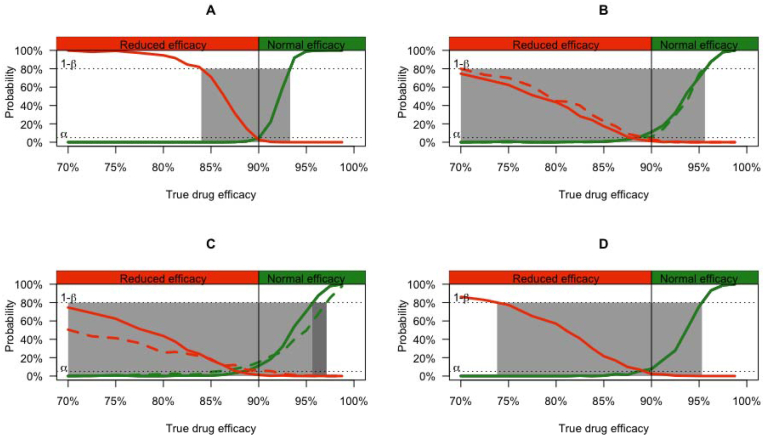
For each of these 7524 combinations, the TDEd was determined for which a normal and reduced efficacy could be reliably detected. In case of a normal drug efficacy (TDEd ≥90%), this corresponded with the lowest possible TDEd for which type I error (α; probability of reduced efficacy (TDEd <90%) falsely classified as normal) and type II errors (β; probability of classifying normal efficacy (TDEd ≥90%) as reduced) are not higher than 5% and 20%, respectively. In case of a reduced drug efficacy (TDEd <90%), this corresponded with the highest possible TDEd for which α (probability of normal efficacy (TDEd ≥90%) falsely classified as reduced) and β (probability of classifying reduced efficacy (TDEd <90%) as normal) are not higher than 5% and 20%. A general graphical representation of this analysis is shown in Fig. 4, Panel A. In this example, the lowest possible TDEd ≥90% that can reliably be detected is 92.5%, the highest TDEd <90% that could be detected is 83.8%. For both these TDEd, α and β are not higher than 5% and 20%, respectively. For any value TDEd between 83.8% and 92.5%, the detection of normal and reduced efficacy remained unreliable. This range of TDEd is referred to as the grey zone.
Fig. 4.
The diagnostic performance of a mite count reduction test to detect reduced and normal drug efficacy against P. ovis across a selection of combinations. Panel A represents a general format of the results of the simulation of a given combination. The true underlying drug efficacy of drug d (TDEd) is given in the x-axis, with the reduced drug efficacy threshold (90%) represented by a vertical line. The probability (or proportion of iterations) is given in the y-axis. The green line represents the probability of classifying the efficacy of drug d as normal based on the mite count reduction test (MCRT), while the red line represents the probability of classifying the efficacy of drug d as reduced based on the MCRT. Type I (α) and type II errors (β) were set at 5% and 20% respectively. The grey area indicates the range of TDEdfor which α ≥5% or β ≥20. Panel B and C illustrate the WAAVP guidelines (Panel B: 6 animals and 6 skin scrapings per animal; Panel C: 2 skin scrapings per animal), and Panel D the EMA guideline (15 animals and 3 skin scrapings per animal). In each Panel, the green line depicts the probability of classifying the efficacy of drug d as normal based on the MCRT, while the red line represents the probability of classifying the efficacy of drug d as reduced based on the MCRT. In Panels B and C, the solid line represents multiple skin scrapings (Panel B: 6; Panel C: 2) taken from 1 lesion, whereas the dotted line indicates that 1 skin scraping was collected from multiple lesions (Panel B: 6; Panel C: 2). Type I (α) and type II errors (β) were set at 5% and 20%, respectively. The grey area indicates the range of TDEdfor which α ≥5% or β ≥20%. In these Figures, it was assumed that the mean infestation at the farm equalled 15.4 mites/9 cm2, all animals where of medium size (back=4380 cm2) and that only lesions covering 1/12 of the back where sampled. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Parameterisation of the simulation framework
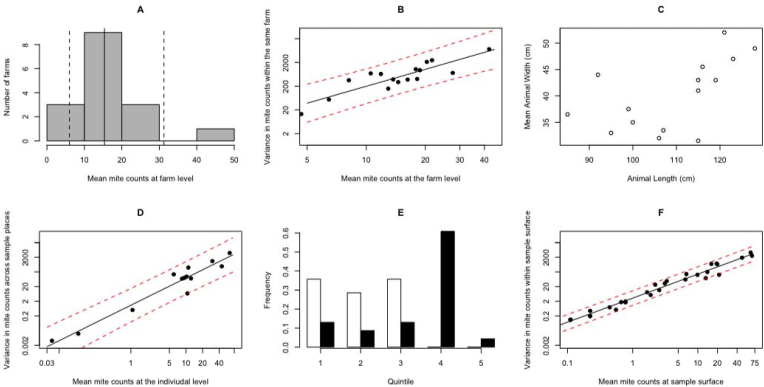
The parameterisation of the simulation framework is illustrated by Fig. 2. The mean mite counts at the farm level (μf) ranged from 4.7 to 42.3 mites/9 cm2, with the majority within the range of 10–20 mites/9 cm2 of skin scrapings (Fig. 2, panel A). The 5th-, 50th- and 95th-quantiles of μf equalled 6.0, 15.4 and 31.3 mites/9 cm2 of skin scrapings, respectively.
Fig. 2.
The parameterisation of a simulation framework for P. ovis mite counts. Panel A describes the distribution of mean mite counts per 9 cm2across 16 farms, whereas the full vertical line represents the median, and the vertical dashed lines indicate the 5th(left of median) and the 95thpercentile (right of median). Panel B illustrates the output of the linear regression model with the variance in mite counts at farm level (σ2f) as dependent variable and mean mite counts at farm level (μf) as independent variable. The black dots represent the data, the straight black line the estimated σ2fas function of μfand the red dashed lines represent the 95% prediction intervals. Panel C illustrates the distribution of the length and the width. The length of the individual animals is given on the horizontal axis, whereas the average width of an animal is given on the vertical axis. Panel D illustrates the output of the linear regression model with the variance in mite counts at animal level (σ2fa) as dependent variable and mean mite counts at animal level (μfa) as independent variable. The black dots represent the data, the straight black line the estimated σ2fas function of μfand the red dashed lines represent the 95% prediction intervals. Panel E describes the distribution of the mite counts found in lesions (black bars) and healthy skin areas (white bars) over 5 levels of mite infestation. Panel F illustrates the output of the linear model with the variance in mite counts at lesion level (σ2fal) as dependent variable and mean mite counts at lesion level (μfal) as independent variable. The black dots represent the data, the straight black line the estimated σ2fas function of μfand the red dashed lines represent the 95% prediction intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To estimate the variances at the different levels (farm: σ2f, animal: σ2fa and lesion: σ2fa), linear regression models were built with the corresponding mean at the different levels as independent variable (farm: μf, animal: μfa and lesion: μfal). The equations below represent the output of these models at the level of farm (Eq (1)), animal (Eq (2)) and lesion (Eq (3)). The graphical representation of the estimations and the corresponding 95% prediction intervals are provided in Panels B (farm), D (animal) and F (lesions) of Fig. 2. The raw data for the analysis is provided in Supplementary Info I2.
| Eq (1): log(σ2f) = 2.376 x log (μf) - 0.181 + εf ~ normal(0,0.7512) |
| Eq (2): log(σ2fa) = 1.855 x log (μfa) + 0.073 + εfa ~ normal(0,1.1232) |
| Eq (3): log(σ2fal) = 1.637 x log (μfal) – 1.186 + εfal ~ normal(0,0.5452) |
Across the lesions (n = 24) and the healthy skin areas (n = 14), the 20th, 40th, 60th and 80th percentile of the mean mite counts equalled 0.0, 0.4, 2.3 and 13.7 mites/9 cm2, resulting in 5 levels of mite infestations: 0.0, 0.0–0.4, 0.4–2.3, 2.3–13.7, ≥13.7 mites/9 cm2. Across these levels of mite infestation, the mite counts from lesions were mainly found at the higher levels of infestation (Fig. 2, Panel E). Approximately 60% of all the mite counts in lesions were classified in at least the fourth level of mite infestation (mean mite counts/9 cm2 ≥ 2.3), whereas for healthy skin areas, all values for the mite counts fell in the first three levels of mite infestation. For lesions, the probabilities across the five levels of mite infestation were 16.7% (π1), 8.3% (π2), 12.5% (π3), 58.3% (π4) and 4.2% (π5). The mean length was 109 cm (smallest = 85 cm and largest = 128 cm) and the mean width was 40 cm (smallest = 30.5 cm and largest = 52 cm). Subsequently, this resulted in a mean surface of the back of 4380 cm2 and corresponding minimum and maximum surfaces of 2592 cm2 and 6656 cm2. The size of 1/12th of a back across 15 animals from 3 farms varied from 216 to 555 cm2, corresponding with 24 and 62 skin scrapings of 9 cm2, respectively.
3.2. Impact of number of skin scrapings on post drug administration mite counts
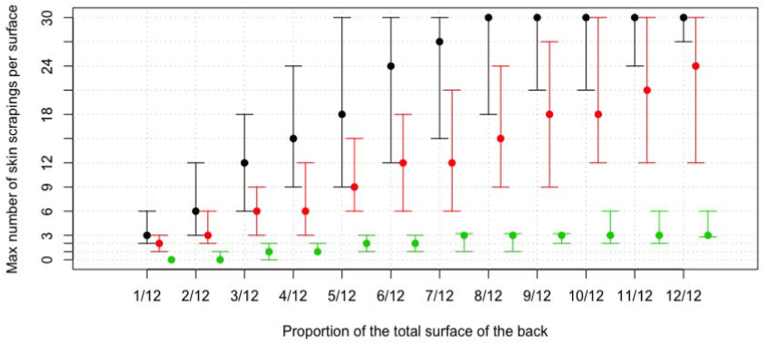
Fig. 3 illustrates the impact of the number of skin scrapings (size of 9 cm2) on post-drug administration mite counts. For a clear presentation of the data, and alignment with the selection of number of scrapings and the size of the lesions in the final simulation, the highest number of scrapings was determined that resulted in a mite reduction not exceeding 1%, 5% and 10% in at least half of the iterations. Overall, more samples can be sampled per lesion when the lesions covered more skin surface (1/12 vs. 12/12 and small vs. large animals) and when a less stringent mite reduction cut-off was applied (1% vs. 10%). When a mite reduction cut-off of 10% was assumed, one can take the maximum number (n = 30) of skin scrapings from lesions covering 5/12 of the back of large animals, whereas for medium sized animals this is only possible when lesions cover 8/12 of the back. For small animals, the highest number of scrapings was 27 and this was only possible when the lesion covered the entire back (12/12). For a 5% mite reduction cut-off, similar trends were observed, yet collection of 30 skin scrapings per lesion was only possible for large animals (from lesions covering 10/12 of the back onwards). For medium and small animals, the highest possible number of scrapings for lesions covering the entire back was 24 and 12, respectively. For a 1% mite reduction cut-off, the highest possible number of scrapings did not exceed 6, regardless of the size of the lesions. Supplementary Table T2 provides the highest possible number of skin scrapings across the different absolute surfaces of lesions (1/12 vs. 12/12 and small vs. large animals) that were included in the final simulation.
Fig. 3.
The maximum number of skin scrapings for different surfaces of lesions and mite reduction thresholds. The maximum number of scrapings of 9 cm2 for different surfaces of lesions that resulted in a mite reduction not exceeding 1% (green lines), 5% (red lines) and 10% (black lines) in at least half of the iterations. The size of the lesions is expressed as a proportion of an animal's back (1/12 to 12/12). The dot represents a medium sized animal (surface back = 4380 cm2), with the top and bottom whiskers representing a large (surface back = 6656 cm2) and small animal (surface back = 2592 cm2), respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Application of the simulation framework to explore the impact of both study specific and biological factors on the diagnostic performance of MCRT
3.3.1. The diagnostic performance of MCRT when it is applied according to the existing WAAVP and EMA guidelines
Fig. 4, Panels B, C and D illustrate the diagnostic performance of MCRT when it is applied according to both the WAAVP (panels B: 6 skin scrapings per animal, i.e. either 6 skin scrapings from 1 lesion or 1 skin scraping from 6 lesions and Panel C: 2 skin scrapings per animal, i.e. either 2 skin scrapings from 1 lesion or 1 skin scraping from 2 lesions), and EMA guideline (Panel D: 15 animals; 3 skin scrapings per animal taken from 3 lesions). Across the different panels, it was assumed that the mean infestation at the farm equalled 15.4 mites/9 cm2, all animals were of medium size (total surface of the back = 4380 cm2) and that only lesions covering 2/12 of the back were sampled. When applying the WAAVP guidelines, a reduced efficacy of 70% could not be reliably detected, although the type II error (β) was only slightly above 20% when 6 skin scrapings were collected per animal. When applying the EMA guidelines, MCRT was able to reliably detect reduced efficacies up to 72.5%. The detection of normal drug efficacy was similar between both guidelines, the MCRT generally being able to reliably detect normal drug efficacy when the TDEd was at least 95%. Only for 2 skin scrapings (1 scraping from 2 lesions), the TDEd for which the type II error was less than 20% equalled 97.5%.
3.3.2. Guidance to optimize the diagnostic performance of the MCRT
In Supplementary Figs. S2 and S3 the impact of the different study specific factors is graphically summarized (number of animals, number of lesions per animal, number of skin scrapings per lesion and surface of the lesions (proportion of the back covered and the size of the animals)) on the diagnostic performance of MCRT to detect reduced (TDEd <90%) and normal drug efficacy (TDEd ≥90%), respectively. Generally, the diagnostic accuracy increased (narrow grey zone) as a function of increasing number and size of animals, number of lesions sampled, the number of skin scrapings per lesion and intensity of mite infestation. For varying surface of sampled lesion, the diagnostic accuracy of MCRT remained largely unchanged.
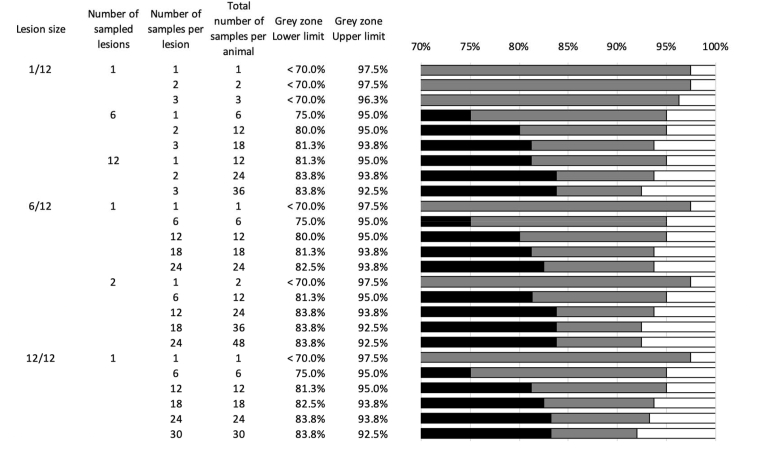
In Fig. 5, the different options to design a MCRT were presented in farms with an average mite infestation (μf = 15.4 mites/9 cm2) in 10 medium sized animals (total surface of the back = 4380 cm2). For farms of this type, we determined the diagnostic accuracy of MCRT depending on the size of the lesions (1/12 vs. 6/12 vs. 12/12 of the animal's back) and the number of skin scrapings. Based on the width of the grey zones, it is clear that collecting 1 sample per scraping is not recommended. Changing parameters that increase the number of scrapings per animal, as number of scrapings per lesion or number of sampled lesions narrow the width of the grey zone. The possible changes and resulting grey zones can be identified in Fig. 5. In Supplementary Info I3, the diagnostic performance for each of the 7524 combinations (3 levels of mite infestation on farm level x 4 numbers of animals x 627 numbers and sizes of lesions) was summarized. To ensure oversight of the different combinations, this was converted into a supporting tool that allows veterinarians/researchers to identify the MCRT design that best fits their purpose. The sliders can be used to select only the scenarios with the fixed parameters. For every combination with the chosen parameters, the corresponding grey zone will be given.
Fig. 5.
The diagnostic performance of MCRT for a farm with an average mite infestation in 10 medium sized animals. The grey zone of the different combinations is illustrated through the grey horizontal bar. The TDE values with a type II error <20% for the detection of normal and reduced efficacy are depicted by a white and black bar respectively. All combinations have a fixed mite infestation intensity (50th percentile = 15.4 mites/9 cm2) and a fixed number (n = 10) of medium sized (total surface back = 4380 cm2) animals.
4. Discussion
Recent reports on macrocyclic lactone resistance and the likelihood of the development of resistance against other acaricides, highlight the need for monitoring the therapeutic efficacy of acaricides (Lifschitz et al., 2018; van Mol et al., 2020). However, the absence of evidence-based guidelines and the wide variation in sampling strategies currently being implemented make it difficult to directly compare results of different studies and to optimize the diagnostic accuracy of MCRT to correctly detect normal and reduced efficacy. In the present study, a unique simulation framework was constructed that accounts for the most relevant biological features of P. ovis infestations. Second, this framework was applied to explore the impact of both study-specific and biological factors on the diagnostic performance of the MCRT. The outcome of the simulation study provided a basis to determine the diagnostic performance of the MCRT when it is applied according to the existing WAAVP and EMA guidelines, and to make recommendations on how to ensure a good diagnostic performance of the MCRT while minimizing the number of animals and samples needed. Finally, a simple tool was developed that will help researchers and veterinarians to optimize the design of the MCRT to their own field settings.
4.1. First description of mite distribution across farms, individual animals and lesions
This is one of the first studies to give a detailed description of the distribution of P. ovis in naturally infested cattle between and within both animals and lesions. Most studies either limited the mite counts to 100 mites per animal (Lonneux et al., 1997) or classified mite counts into arbitrary categories (Genchi et al., 2008; Lekimme et al., 2010; Lifschitz et al., 2018; Lonneux and Losson, 1992). To our knowledge, only Sarre et al. (2015) reported the mean, minimum and maximum mite counts observed in two farms, but no variance.
The distribution of P. ovis follows a negative binomial distribution, when the aggregation value is positive. This means that a minority of the animals on a farm contain the majority of the mites. This is also observed in for gastrointestinal nematodes infections (Levecke et al., 2012). The same distribution is also observed within an animal and lesion, where a minority of the lesions or sampling locations within a lesion contain the majority of the mites. However, low mean mite counts within a farm, animal or lesion follow a Poisson distribution, due to the high correlation between mean mite count and the variance.
4.2. A novel and mite specific simulation framework
A novel simulation framework for mimicking of field efficacy trials was described that accounts for a series of mite specific features and that is parameterized using field data at farm, animal and lesion level. The use of a simulation has the advantage to generate a lot of data in a relative short time and with low cost. It would be impossible to collect the same amount of data through actual farm visits, as the simulation framework generated 72,240,000 farm visits. In comparison, Belgium had 3089 beef cattle farms in 2017 (Danckaert et al., 2018).
Similar work has been done with the FECRT (Levecke et al., 2012, 2018), but the simulation framework for P. ovis differs in mite specific biological features and sampling methodology. The mite specific parameters were quantified through the analysis of field data. The sampling methodology in P. ovis is based on the collection and counting of the parasite itself rather than the eggs that are dispersed by the parasite. A consequence is that a proportion of the parasite population is removed due to sampling. The framework also identified a maximum recommended number of samples per given lesion surface. A 1% reduction cut-off resulted in a limited number of possible sampling strategies, as only 3 samples could be taken from a medium sized animal when more than half of its back is covered with lesions. Therefore, a minimum reduction of mite numbers through sampling should always be accepted. Because of the possibility of false positive detections of normal efficacy, the framework allowed for a maximum number of samples with a 50% probability to give a 10% reduction.
The use of the simulation framework has possibilities beyond the application in this paper. For example, validation of diagnostic methodologies, detection limit of mite infestation intensity in asymptomatic carriers or expansion of the range of the parameters affecting the MCRT.
4.3. The simulation framework comes with its limitations
While the simulation framework is valuable to gain insights into to diagnostic performance of MCRT, it can only be as good as the assumptions made. Consequently, it is important that the results of the presented simulations are interpreted with some caution. An overview of all assumptions and their potential impact on the interpretation of the results is given in Supplementary Info I4. In the following paragraphs, the most important limitations are discussed.
The parameterisation of the simulation framework was based on field data with given minimum and maximum values for mite infestation intensity, animal size, lesion size and number of lesions, and hence the findings should not be extrapolated to values other than those incorporated in the simulation study. For example, the effect of mite infestation intensities outside the range of this study on the diagnostic performance of the MCRT was excluded from the simulation framework. The mite counts in the present study can be considered low or medium compared to other studies (Lonneux and Losson, 1992; Sarre et al., 2015; Lifschitz et al., 2018). However, it would be expected that the diagnostic performance of the MCRT on a farm will benefit from higher mite infestation intensity.
Another limitation of the framework is the exclusion of dynamics in the parameters, such as the size of the mite populations, lesions and animals, which could bring additional variation in the sampling results. For example, it was assumed that there is no growth of the mite population. However, there could be an increase in the population after treatment, due to successful reproduction of resistant individuals. This could result in an underestimation of the reduction. More research is needed to know the full impact population dynamics. Wall et al. (1999) have simulated population growth in sheep in a favourable environment, but no data are available for population growth of P. ovis in cattle.
The diagnostic performance of the MCRT based on the WAAVP and EMA guidelines detects normal efficacy reliably, but may fail to detect reduced efficacy.
In order to detect normal efficacy with sufficient power (type II error <20%), the underlying TDE needs to be at least 95% for both guidelines. In this simulation framework, the lowest possible limit of the grey zone for the detection of normal drug efficacy was 92.5%, and hence this small difference shows an acceptable performance of the guidelines for the detection of normal drug efficacy.
In a best-case scenario, MCRT based on the WAAVP guidelines can only reliably detect resistance when the underlying TDE is 70% or less. Other scenarios could not detect resistance in our dataset with a type II error less than 20%. The EMA recommendations performed slightly better, but still resulted in a wide grey zone [74%–95%].
A difference in the methodology of the simulation framework is the incorporation of the 95%CI to account for the present uncertainty, while both guidelines only used the observed mean reduction. The 95%CI have been used for multiple methodologies in the FECRT. The inclusion of an intermediate class of acaricide susceptibility based on the 95%CI acknowledged the uncertainty in the FECRT (Levecke et al., 2018). This methodology has also been used for the MCRT in P. ovis by van Mol et al. (2020).
The reliability of the detection of the therapeutic drug efficacy increases with the total number of samples per farm.
In order to reduce the type II error (increase the power) of MCRT, it is recommended to increase the number of total samples collected per farm. This can be achieved through the number of animals, the number of sampled lesions and the number of samples per lesion. The possibility to change multiple parameters opens up for a more flexible use of parameters. For example, if the number of animals is limited on a farm more samples can be taken per animal until their number is limited by the lesion's size or when lesions are small on a farm, more animals can be included.
The total number of skin scrapings per farm is restricted by biological factors and field settings. The lesion size is the limiting factor of the maximum number of samples taken per lesion, due to the removal of mites. In order to keep the costs of a drug efficacy trial within its limits it should be noted that the counting of the mites in the skin scrapings is a labour and time-consuming procedure. Thus, an increase in the total samples per farm will result in a higher labour cost.
The mite infestation intensity also had an impact on the grey zone of the MCRT. Even though it is not always an option, pre-treatment screening of the mite infestation intensity of the animals and selection of animals with a high mean mite count is recommended. It should be noted that the selection of animals with larger lesions will not provide a higher mite infestation intensity, due to the poor correlation between mite counts and lesions extent (van Mol et al., 2020). The size of a lesion did not have a direct effect on the type I and II errors of the MCRT, but should not be neglected. This is because more scrapings can be collected from larger lesions before the reduction in mite numbers caused by the sampling becomes too big. Thus, including animals with larger lesions increases the allowed maximum number of samples per lesion.
Even though the grey zone can be reduced through optimisation of the sampling parameters, it is important to note that there was always a grey zone around the threshold, even when the best possible scenario of 20 large animals with 30 samples taken from each of the 12 lesions was executed.
A tool to guide farmers/veterinarians in designing MCRT based on their operational resources and field settings.Whereas every setting for a MCRT is unique, it could be difficult to find the right balance between a sampling strategy with the highest diagnostic performance and available resources. Therefore, the table with all generated scenarios (Supplementary Info I3) was translated into a simple supporting tool that generates the grey zones of possible sampling strategies for the fixed parameters of the field setting. Selection of the desired parameters with the sliders in the file excludes all scenarios that are not eligible within the study set-up. The user can then quickly identify the best combination of parameters for his/her setting based on the corresponding grey zone.
Due to the presence of a grey zone for every combination and the limits of every field setting to alter the sampling strategies, an acceptable grey zone needs to be aspired. A tentative acceptable window is between TDE values of 80% and 95%. In the example used in Fig. 5, this means that combinations that result in 12 samples per animal would be recommended.
The use of a mite count reduction of 90% as normal efficacy threshold can be a matter of debate, due to the aggressive nature of the disease and the rapid growth of the population (Kirkwood, 1986; Losson et al., 1999; Wall et al., 1999; Bridi et al., 2001; Fischer and Walton, 2014). In order to facilitate a possible increase of the threshold, the grey zones with a 95% normal efficacy threshold have been determined for all included combinations of parameters and can be found in Supplementary Info I3.
5. Conclusion
A novel and mite specific simulation model was developed that allowed to gain insights into the use of the MCRT for the detection of acaricide resistance in P. ovis in cattle. The current WAAVP and EMA guidelines performed well for the detection of normal drug efficacy, but poorly for reduced drug efficacy. The diagnostic performance of the MCRT can be improved by increasing the total number of skin scrapings on a farm (e.g. by increasing the number of animals, number of sampled lesions and number of samples per lesion). In order to help researchers and veterinarians to further optimize the design of the MCRT to their own field settings, the findings were translated into a simple calculation tool.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the farmers for participation in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.09.002.
Supplementary Fig. S1. Silhouette of a cattle back with the three regions of the back, i.e. withers, midback and tail base region. If lesions were absent in a region, the samples were taken from the predefined locations on the figure. This figure was also used to sketch the lesions and to record the location of the samples within a lesion.
Supplementary Fig. S2. Determination of the effect of different parameters on the detection of a reduced drug efficacy. The bar charts give the distribution of the lower limits of the grey zone over the intervals in function of the parameter. The lower limit of the grey zone is the true underlying drug efficacy that is correctly classified as reduced with a power of less than 80%. The parameters observed were mite infestation intensity, the number of animals, the size of the animals, the size of the lesions, the number of sampled lesions and the number of samples per lesion.
| Farm | μf | σ2f | kf |
|---|---|---|---|
| 1 | 18.15 | 402.03 | 0.86 |
| 2 | 13.70 | 387.10 | 0.50 |
| 3 | 14.53 | 296.72 | 0.75 |
| 4 | 27.57 | 722.32 | 1.09 |
| 5 | 8.15 | 356.20 | 0.19 |
| 6 | 20.33 | 2103.44 | 0.20 |
| 7 | 10.53 | 692.65 | 0.16 |
| 8 | 4.67 | 13.33 | 2.51 |
| 9 | 17.87 | 1037.07 | 0.31 |
| 10 | 21.63 | 2479.82 | 0.19 |
| 11 | 16.22 | 379.36 | 0.72 |
| 12 | 42.27 | 7196.00 | 0.25 |
| 13 | 11.87 | 654.84 | 0.22 |
| 14 | 18.75 | 943.17 | 0.38 |
| 15 | 12.93 | 156.74 | 1.16 |
| 16 | 6.45 | 54.63 | 0.86 |
| Animal size | Size relative to back |
Lesion surface (cm2) | Maximum number of samples |
||
|---|---|---|---|---|---|
| 10% | 5% | 1% | |||
| Small | 1/12 | 216 | 2 | 1 | 0 |
| 2/12 | 432 | 3 | 2 | 0 | |
| 3/12 | 648 | 6 | 3 | 0 | |
| 4/12 | 864 | 9 | 3 | 1 | |
| 5/12 | 1080 | 9 | 6 | 1 | |
| 6/12 | 1296 | 12 | 6 | 1 | |
| 7/12 | 1512 | 15 | 6 | 1 | |
| 8/12 | 1728 | 18 | 9 | 1 | |
| 9/12 | 1944 | 21 | 9 | 2 | |
| 10/12 | 2160 | 21 | 12 | 2 | |
| 11/12 | 2376 | 24 | 12 | 2 | |
| 12/12 | 2592 | 27 | 12 | 3 | |
| Medium | 1/12 | 365 | 3 | 2 | 0 |
| 2/12 | 730 | 6 | 3 | 0 | |
| 3/12 | 1095 | 12 | 6 | 1 | |
| 4/12 | 1460 | 15 | 6 | 1 | |
| 5/12 | 1825 | 18 | 9 | 2 | |
| 6/12 | 2190 | 24 | 12 | 2 | |
| 7/12 | 2555 | 27 | 12 | 3 | |
| 8/12 | 2920 | 30 | 15 | 3 | |
| 9/12 | 3285 | 30 | 18 | 3 | |
| 10/12 | 3650 | 30 | 18 | 3 | |
| 11/12 | 4015 | 30 | 21 | 3 | |
| 12/12 | 4380 | 30 | 24 | 3 | |
| Large | 1/12 | 555 | 6 | 3 | 0 |
| 2/12 | 1109 | 12 | 6 | 1 | |
| 3/12 | 1664 | 18 | 9 | 2 | |
| 4/12 | 2219 | 24 | 12 | 2 | |
| 5/12 | 2773 | 30 | 15 | 3 | |
| 6/12 | 3328 | 30 | 18 | 3 | |
| 7/12 | 3883 | 30 | 21 | 3 | |
| 8/12 | 4437 | 30 | 24 | 3 | |
| 9/12 | 4992 | 30 | 27 | 3 | |
| 10/12 | 5547 | 30 | 30 | 6 | |
| 11/12 | 6101 | 30 | 30 | 6 | |
| 12/12 | 6656 | 30 | 30 | 6 | |
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adas An evidence base for new legislation and guidance for implementation of a compulsory treatment period for sheep scab. 2008. https://www.webarchive.org.uk/wayback/archive/20180516214411/http://www.gov.scot/Publications/2008/07/17113358/0 Wolverhampton.
- Bridi A.A., Carvalho L.A., Cramer L.G., Barrick R.A. Efficacy of a long-acting formulation of ivermectin against Psoroptes ovis (Hering, 1838) on cattle. Vet. Parasitol. 2001;97:277–283. doi: 10.1016/s0304-4017(01)00434-4. [DOI] [PubMed] [Google Scholar]
- Committee for medicinal products for veterinary use CVMP Guideline on specific efficacy requirements for ectoparasiticides in cattle. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-specific-efficacy-requirements-ectoparasiticides-cattle_en.pdf
- Danckaert S., Demuynck E., de Regt E., De Samber J., Lambrechts G., Lenders S., Vanhee M., Vervloet D., Vermeyen V., Vrints G. VLEESVEE. 2018. https://lv.vlaanderen.be/sites/default/files/attachments/gr_201807_lara2018_hst8_1.pdf
- Fischer K., Walton S. Parasitic mites of medical and veterinary importance – is there a common research agenda? Int. J. Parasitol. 2014;44:955–967. doi: 10.1016/j.ijpara.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Genchi C., Alvinerie M., Forbes A., Bonfanti M., Genchi M., Vandoni S., Innocenti M., Sgoifo Rossi C.A. Comparative evaluation of two ivermectin injectable formulations against psoroptic mange in feedlot cattle. Vet. Parasitol. 2008;158:110–116. doi: 10.1016/j.vetpar.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Hamel D., Joachim A., Löwenstein M., Pfister K., Silaghi C., Visser M., Winter R., Yoon S., Cramer L., Rehbein S. Treatment and control of bovine sarcoptic and psoroptic mange infestation with ivermectin long-acting injectable (IVOMEC® GOLD) Parasitol. Res. 2014;114:535–542. doi: 10.1007/s00436-014-4215-z. [DOI] [PubMed] [Google Scholar]
- Kirkwood A.C. History, biology and control of sheep scab. Parasitol. Today. 1986;2:302–307. doi: 10.1016/0169-4758(86)90124-9. [DOI] [PubMed] [Google Scholar]
- Lekimme M., Farnir F., Maréchal F., Losson B. Failure of injectable ivermectin to control psoroptic mange in cattle. Vet. Rec. 2010;167:575–576. doi: 10.1136/vr.c4906. [DOI] [PubMed] [Google Scholar]
- Levecke B., Dobson R.J., Speybroeck N., Vercruysse J., Charlier J. Novel insights in the faecal egg count reduction test for monitoring drug efficacy against gastrointestinal nematodes of veterinary importance. Vet. Parasitol. 2012;188:391–396. doi: 10.1016/j.vetpar.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Levecke B., Anderson R.M., Berkvens D., Charlier J., Devleesschauwer B., Speybroeck N., Vercruysse J., Van Aelst S. Mathematical inference on helminth egg counts in stool and its applications in mass drug administration programmes to control soil-transmitted helminthiasis in public health. Adv. Parasitol. 2015;87:193–247. doi: 10.1016/bs.apar.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Levecke B., Kaplan R.M., Thamsborg S.M., Torgerson P.R., Vercruysse J., Dobson R.J. How to improve the standardization and the diagnostic performance of the fecal egg count reduction test? Vet. Parasitol. 2018;253:71–78. doi: 10.1016/j.vetpar.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Lifschitz A., Fiel C., Steffan P., Cantón C., Muchiut S., Dominguez P., Lanusse C., Alvarez L. Failure of ivermectin efficacy against Psoroptes ovis infestation in cattle: integrated pharmacokinetic-pharmacodynamic evaluation of two commercial formulations. Vet. Parasitol. 2018;263:18–22. doi: 10.1016/j.vetpar.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Lonneux J.F., Losson B.J. Field efficacy of injectable and pour-on moxidectin in cattle naturally infested with Psoroptes ovis ( Acarina: psoroptidae ) Vet. Parasitol. 1992;45:147–152. doi: 10.1016/0304-4017(92)90037-a. [DOI] [PubMed] [Google Scholar]
- Lonneux J.-F., Nguyen T.Q., Losson B.J. Efficacy of pour-on and injectable formulations of moxidectin and ivermectin in cattle naturally infected with Psoroptes ovis: parasitological, clinical and serological data. Vet. Parasitol. 1997;69:319–330. doi: 10.1016/s0304-4017(96)01090-4. [DOI] [PubMed] [Google Scholar]
- Lonneux J.F., Nguyen T.Q., Detry J., Farnir F., Losson B.J. The relationship between parasite counts, lesions, antibody titres and daily weight gains in Psoroptes ovis infested cattle. Vet. Parasitol. 1998;76:137–148. doi: 10.1016/s0304-4017(97)00220-3. [DOI] [PubMed] [Google Scholar]
- Losson B.J., Lonneux J.F., Lekimme M. The pathology of Psoroptes ovis infestation in cattle with a special emphasis on breed difference. Vet. Parasitol. 1999;83:219–229. doi: 10.1016/s0304-4017(99)00059-x. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- Rehbein S., Martin A.E., Winter R., Maciel A.E. Efficacy of a new long-acting formulation of ivermectin and other injectable avermectins against induced Psoroptes ovis infestations in cattle. Parasitol. Res. 2002;88:1061–1065. doi: 10.1007/s00436-002-0713-5. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Visser M., Winter R., Trommer B., Matthes H.F., Maciel A.E., Marley S.E. Productivity effects of bovine mange and control with ivermectin. Vet. Parasitol. 2003;114:267–284. doi: 10.1016/s0304-4017(03)00140-7. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Visser M., Meyer M., Lindner T. Ivermectin treatment of bovine psoroptic mange: effects on serum chemistry, hematology, organ weights, and leather quality. Parasitol. Res. 2016;115:1519–1528. doi: 10.1007/s00436-015-4885-1. [DOI] [PubMed] [Google Scholar]
- Rose H., Wall R. Pathogenicity of biological control agents for livestock ectoparasites: a simulation analysis. Med. Vet. Entomol. 2009;23:379–386. doi: 10.1111/j.1365-2915.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- Sarre C., De Bleecker K., Deprez P., Levecke B., Charlier J., Vercruysse J., Claerebout E. Risk factors for Psoroptes ovis mange on Belgian blue farms in northern Belgium. Vet. Parasitol. 2012;190:216–221. doi: 10.1016/j.vetpar.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Sarre C., Geurden T., Vercruysse J., De Wilde N., Casaert S., Claerebout E. Evaluatie van twee intensieve behandelingsschema's tegen Psoroptes ovis-schurft bij Belgisch witblauwe runderen op negen Vlaamse rundveebedrijven. Vlaams Diergeneeskd Tijdschr. 2015;84:311–317. [Google Scholar]
- Van Den Broek A.H., Huntley J.F.A. Sheep Scab : the disease , pathogenesis and control. J. Comp. Pathol. 2003;128:79–91. doi: 10.1053/jcpa.2002.0627. [DOI] [PubMed] [Google Scholar]
- van Mol W., De Wilde N., Casaert S., Chen Z., Vanhecke M., Duchateau L., Claerebout E. Resistance against macrocyclic lactones in Psoroptes ovis in cattle. Parasites Vectors. 2020;13(127):1–9. doi: 10.1186/s13071-020-04008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse J., Rehbein S., Holdsworth P.A., Letonja T., Peter R.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of acaricides against (mange and itch) mites on ruminants. Vet. Parasitol. 2006;136:55–66. doi: 10.1016/j.vetpar.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Wall R., Smith K.E., Berriatua E., French N.P. Simulation analysis of the population dynamics of the mite, Psoroptes ovis, infesting sheep. Vet. Parasitol. 1999;83:253–264. doi: 10.1016/s0304-4017(99)00062-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.