Abstract
Brain plasticity is dynamically regulated across the life span, peaking during windows of early life. Typically assessed in the physiological range of milliseconds (real time), these trajectories are also influenced on the longer timescales of developmental time (nurture) and evolutionary time (nature), which shape neural architectures that support plasticity. Properly sequenced critical periods of circuit refinement build up complex cognitive functions, such as language, from more primary modalities. Here, we consider recent progress in the biological basis of critical periods as a unifying rubric for understanding plasticity across multiple timescales. Notably, the maturation of parvalbumin-positive (PV) inhibitory neurons is pivotal. These fast-spiking cells generate gamma oscillations associated with critical period plasticity, are sensitive to circadian gene manipulation, emerge at different rates across brain regions, acquire perineuronal nets with age, and may be influenced by epigenetic factors over generations. These features provide further novel insight into the impact of early adversity and neurodevelopmental risk factors for mental disorders.
Keywords: critical period, gamma oscillations, circadian clock, parvalbumin
Neural circuits are shaped by experience during critical periods (CPs) of development, allowing individuals to uniquely adapt their behaviors to the surrounding environment. The common occurrence of these plastic windows across species and systems speaks to their fundamental importance in ensuring future fitness. Most clearly observed for the organization of primary sensory areas, including auditory, visual, and somatosensory processing in mammals (1–3), CPs are also evident for complex multimodal integration in barn owls (4), filial imprinting in geese (5), and the generation of vocal behavior in humans and songbirds (6, 7), to name a few. The emergence of these windows for distinct domains occurs at different times over development; properly staggered CPs may be essential for the acquisition of higher-order functions such as language (8). The expected experience must then coincide with the CP for proper circuit wiring to occur. Thus, normal brain development requires temporal alignment between intrinsic maturational programs and environmental input.
Brain plasticity at any given moment multiplexes the history of the individual (through both genetic and epigenetic factors in the long term), the fluctuating cortical milieu (factors accumulating on a daily basis), and more rapid alterations in brain state (acute neuromodulatory tone). Even slight variation along any of these parameters may collectively inject individual differences in plasticity. These may be further magnified in the higher cognitive capacities of humans built upon a series of sequential CPs. By considering how plasticity is regulated at multiple timescales during development, we can better understand how the history of the system constrains or enables current and future plasticity. Such a perspective may hold several merits, including the identification of potential biomarkers of CP state and possible transgenerational consequences on human brain development.
Based on recent cellular insight into the biological basis of CPs, we propose a novel framework for reflecting on their regulation across multiple timescales. Description of any biological process at this level attempts to capture the temporal dynamics of the process, specifically the duration of the phenomenon and the sampling rate at which observations must be collected to capture these dynamics. Our appreciation of the system is typically limited by experimental design and set by theoretical as well as technical constraints (9). If too narrow a time window is taken, long-lasting changes to system responsiveness may be missed. However, lengthening the considered time window at the expense of sampling rate means subtleties in temporal dynamics of the response are lost. Here, we consider CP regulation across the following timescales (Fig. 1): 1) rapid, moment-by-moment shifts in circuit physiology; 2) gradual molecular events controlling the maturation of cortical circuits dictating CP onset and closure in early life; and 3) epigenetic modifications over the life span (or across generations) that set the baseline level of plasticity.
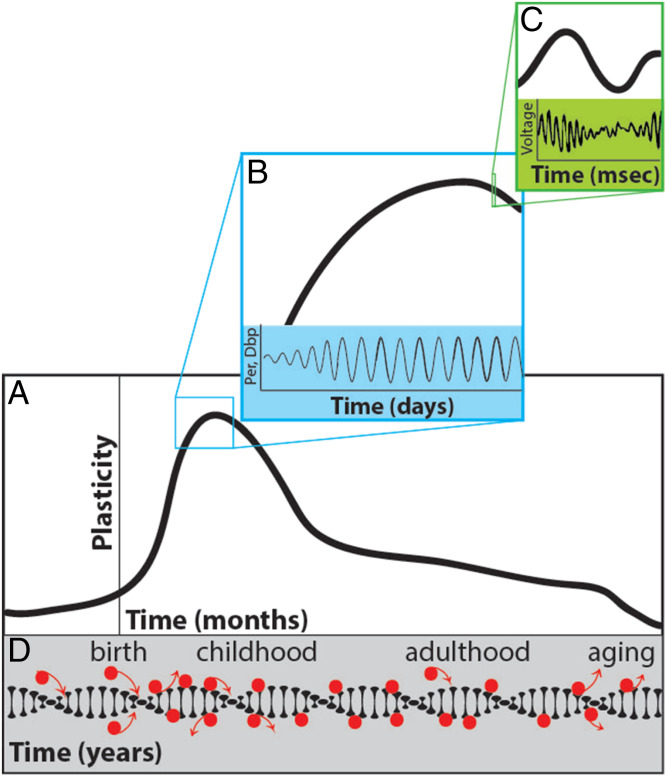
Fig. 1.
CP regulation across multiple timescales. (A) Schematic representing a CP in early development. The onset of plasticity is controlled by the maturation of inhibitory cells in cortex. (B) The emergent oscillatory expression of circadian genes within PV inhibitory cells contributes to their maturation and the beginning of CP plasticity. (C) Altered experience during CPs induces a transient, rapid rise in gamma oscillatory activity, which is hypothesized to enable circuit plasticity. (D) Dynamic methylation rates (red circles) and chromatin stability across the life span may regulate levels of plasticity during CPs, adulthood, and aging.
Parvalbumin Circuits as a Timing Hub
Maturation of inhibitory interneurons drives CP trajectories in primary sensory areas (10). Specifically, fast-spiking, parvalbumin-expressing (PV) cells are well poised to control cortical activity—they form a richly interconnected network, extend lateral inhibition onto nearby pyramidal cells, influence action potential firing and their backpropagation into dendrites, and orchestrate rhythmic oscillations (11–14). Inhibitory circuit formation typically lags behind that of excitatory pyramidal cells (15) with interneuron migration from the ganglionic eminences continuing for several months postnatally in the human brain (16). However, once mature, fast-spiking cells are the first to respond to sensory deprivation (17–19), preceding a much slower homeostatic process that restores circuit balance (20, 21). Likewise, the bulk of CP circuit rewiring lies downstream of the dynamic excitatory–inhibitory (E/I) balance that triggers it, including the pruning of dendritic spines by proteases (22) and unsilencing of excitatory synaptic transmission by PSD-95 (23, 24).
Maturation and maintenance of PV cells is exquisitely sensitive to experience and cell-extrinsic factors (10). Without experience, the number and strength of PV synapses onto pyramidal cells is reduced, PV cell membrane properties remain immature, and perineuronal net (PNN) accumulation is delayed (25–27). Neural activity drives largely non–cell-autonomous factors, including modification of cell adhesion molecules and release of developmental regulators such as orthodenticle homeobox 2 (Otx2), neuronal pentraxins (NARP), neurotrophins (BDNF), and neuregulins (28–32). Notably, PV circuits emerge at different times across brain regions (33), contributing to the sequential nature of CPs (Fig. 2).
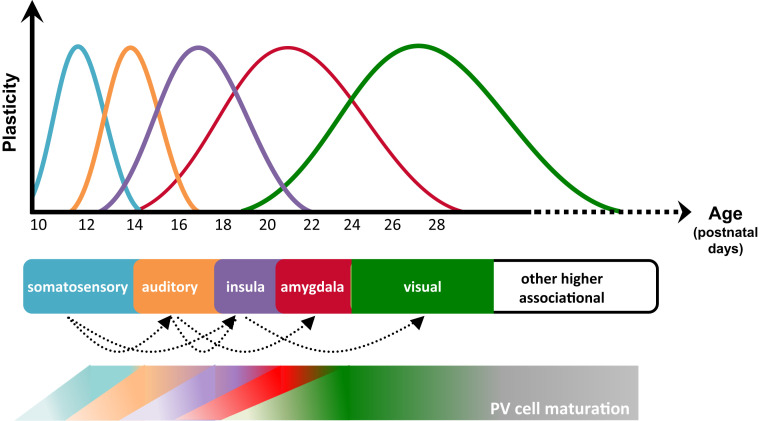
Fig. 2.
Sequential CP plasticity is observed in the mouse brain. Time windows for peak plasticity in barrel cortex (173, 174), auditory cortex (1), insular cortex (154), amygdala (175), visual cortex (30, 31, 75), and higher cognitive areas (176, 177) are staggered in register with the respective emergence of PV circuits (178, 179). Similarly, hierarchical development of monkey visual cortical regions is correlated with PV circuit maturation (33).
Molecular manipulations of PV maturation and maintenance factors confirm CP timing itself to be plastic. Intrinsic membrane properties, an increase in PV and GABA content, altered innervation patterns, and shifts in receptor composition underlie the characteristic fast-spiking behavior and strong feedforward inhibition by recurrent PV networks (11, 25, 26, 28, 29, 34). With age, PV cells are gradually enwrapped by PNNs, which both envelop synaptic boutons (35) and create microenvironments in the extracellular milieu for factors such as Otx2 (30, 36, 37). Removal of PNNs (38–40) or Otx2 in adulthood (41, 42) resets PV cells to a juvenile state, transiently enabling CP plasticity anew. Consequently, the brain may toggle between a plastic or nonplastic state to varying degree as a function of PNN/PV cell integrity across the life span.
The dynamic interaction of cell-intrinsic maturational programs with cell-extrinsic factors is exemplified by inhibitory neuron transplantation studies. Inserting newly born inhibitory cells from the medial ganglionic eminence into the visual cortex of adult mice reopens CP plasticity (43, 44). This new CP emerges only once the transplanted cells reach an age that corresponds with the opening of CP plasticity in the visual system of the donor, suggesting that intrinsic maturational programs drive the timing of plasticity. Nonetheless, they are also modified by the host’s cortical environment—transplanted cells become innervated (43) and rapidly enwrapped by PNNs (45).
Altering the balance of local circuit inhibition to excitation through drugs, sensory experience, stress, nutrition, genetic, or epigenetic factors can shift the timing of CP windows in both animals and humans (46–51). In the visual cortex, mistimed ocular dominance plasticity disrupts the binocular matching of orientation tuning (52). Likewise, progressive misalignment between expected input and periods of circuit plasticity across modalities may impact their later integration in the etiology of complex cognitive disorders (see below) (53).
Milliseconds to Minutes—The Role of Neuronal Oscillations
Rhythmic neuronal activity acutely captures both the state of ongoing network dynamics and shifts in these dynamics as they occur in real time (54). The same inhibitory circuits that track CP timing (PV cells) generate moment-by-moment oscillatory activity in the brain (13, 14). Here, we outline the relevance of such rhythms as a potential biomarker of plasticity level in the circuit, allowing more precise determination of CP trajectories and individual differences. In addition, acute shifts in network dynamics brought about by neuromodulators may control the level of circuit plasticity in real time, providing one route to enhance plasticity in adulthood.
Shortly after oscillations in the electroencephalogram (EEG) were first described by Berger (55), alterations in rhythmic activity across development were noted (56). The EEG power spectrum shifts dramatically over the first few years of life. These changes reflect differences in conduction speed, synapse number/strength, cellular composition, and maturity. The EEG waveform further integrates the level of circuit maturation with neuronal activity driven by an individuals’ environment. This is manifest clearly during non-REM sleep: slow-wave amplitude increases gradually over development and peaks in early childhood (57, 58). Increases in slow-wave amplitude have been linked to daily waking experience (59, 60) thought to reflect activity-driven strengthening of cortical connections (61)—not just limited to experience immediately preceding sleep, but also capturing the cumulative experiences of that cortical region. For example, dark-reared kittens and mice fail to show the developmental increase in slow-wave power, specifically in the visual cortex (62, 63).
Early deprivation likewise alters development of the EEG power spectrum in humans. In a randomized controlled trial of foster care as an intervention for early institutionalization, the Bucharest Early Intervention Project (BEIP) has consistently reported for children who were randomly assigned to 1) continued institutional care show dramatic reductions in EEG power across frequency bands relative to those never institutionalized, and 2) foster care intervention shows increased EEG power in most frequency bands if placement occurs before ∼2 y of age (64–67). These changes signal dramatic shifts in the trajectory of brain development for these children, which may impact later plasticity. Indeed, the BEIP team has recently demonstrated that EEG power at age 8 predicts performance on a number of tests of executive function at ages 12 and 16 (68). Global changes in EEG power indicate abnormal brain development, while changes in particular frequency bands may reflect more subtle circuit imbalances.
PV cells preferentially fire at 40 Hz, and their activity can drive cortical gamma (40–80 Hz) rhythms (13, 69). According to the pyramidal-interneuronal gamma network model, oscillatory activity in the gamma frequency band is generated by reverberation of activity between pyramidal cells that periodically excite interneurons, which in turn induce synchronized silencing of the pyramidal neurons (14). Thus, the strength and coherence of activity in the gamma band reflects the E/I balance in the circuit. Computational modeling has demonstrated increases in gamma frequency and coherence with the maturation of PV cell electrophysiological properties (70). In humans, induced gamma activity increases in power and coherence with age, reflecting the maturational increase in inhibitory circuit strength and precision (71). More directly, multimodal imaging studies using both EEG and positron emission tomography technology have correlated gamma power and GABA concentrations (72).
In rodents, changes in gamma rhythm have been observed in relation to CP plasticity. As animals enter the CP, baseline gamma power in the visual cortex increases. This increase is blocked by dark rearing, a manipulation that disrupts the experience-dependent maturation of inhibitory circuitry in the visual cortex (63). During the CP, eyelid closure drives a robust, transient peak in gamma power (73). Both the generation of this oscillatory activity (13, 14) and CP plasticity are dependent on the level of inhibition in the circuit. Juvenile mice lacking the GABA synthetic enzyme GAD65 have constitutively reduced inhibitory tone and fail to enter a CP for sensory plasticity (74, 75). In the adult cat visual cortex, stimulus-dependent modifications of orientation selectivity are observed only if electrical stimulation of the brainstem arousal system successfully induces strong synchronized gamma-oscillations (76). In adult rodents, removal of the PNNs surrounding PV cells reopens plasticity (39, 40), by resetting inhibitory circuits to a juvenile state (41), and restores the peak in gamma power induced by eye closure (73).
While the evidence linking gamma oscillations and plasticity in humans is less direct, malleable oscillatory activity in the gamma frequency range is associated with language acquisition and processing. Infants form phonemic categories via a process of perceptual narrowing to their native tongue, during a CP in the first year of life (8). At 6 mo, when environmental input is actively shaping phonemic maps, exposure to a native phonemic contrast leads to a rise in low gamma power; this rise is not observed after infants reach 1 y of age (77, 78). Interestingly, the timing of both phoneme category formation and speech-induced gamma activity are maturationally constrained. Premature infants do not show accelerated CP timing despite having 3 mo of additional extrauterine language experience (79). Similarly, premature infants do not show an elevation in gamma power in response to the rhythmical structure of their native language when compared to their age-matched peers, despite earlier environmental exposure (80). These findings are consistent with the animal work and suggest language-driven gamma activity may contribute to acoustic mapping in infancy.
During this CP, the infant’s sensitivity to acoustic detail facilitates formation of phonemic maps. Acoustic training boosts their ability to discriminate a variety of prelinguistic cues (81) as well as timbre (82). Additionally, active training (as opposed to passive exposure to the same sounds) yields an elevation in gamma power over the left auditory cortex when infants detect a difference between two sounds (83). While this study only looked at gamma power in response to the stimuli after the training period, the cortical response over the course of training would be of great interest.
Abnormal gamma oscillations have been observed in developmental disorders, which are marked by E/I imbalance (84). For example, among a sample of infants at high risk for developing autism by virtue of having at least one older sibling with autism, EEG power trajectories during the first postnatal year at delta and gamma frequency best differentiated those receiving an eventual autism diagnosis (85). Reduced beta and gamma activity at 3 mo of age is likewise predictive of which high-risk infants would subsequently be diagnosed with autism at 3 y of age (86).
Beyond progressive development, oscillatory activity in the gamma frequency range also captures rapid shifts in E/I balance driven by state changes. Locomotion disinhibits the cortical circuit via basal forebrain mediated activation of upper layer interneurons, resulting in a rise in gamma power (87, 88). These inhibitory cells expressing vasoactive intestinal peptide (VIP) preferentially innervate PV cells (89), supporting real-time disinhibition in circuit dynamics. Exposure to visual stimuli while running restores vision in amblyopic mice past their CP (90). Increased attention is also associated with enhanced gamma power, which may be further linked to perception (91). In the example above, those infants who received active as opposed to passive auditory training showed more mature neuronal responses to the acoustic stimuli, as well as improved performance on the discrimination task (81), and were also the only group to show enhanced gamma oscillatory activity in response to the deviant tone (83).
Both attention and the maturation of inhibition at CP onset may enable plasticity by increasing the evoked “signal-to-noise” ratio, shifting learning cues from internally generated activity to external sources (92). Indeed, intrinsic activity (e.g., retinal waves) is essential for the initial maturation of neural circuits across systems, setting a scaffold upon which external input can eventually be played. Neural oscillations and entrainment in the gamma range may drive such attentional shifts (91, 93) and hence selectively modulate plasticity level in the circuit (Fig. 1C). Together, these data suggest that oscillatory activity may not only indicate the plastic state of the cortex but may play a more active role enabling circuit plasticity.
Days to Months—The Role of Circadian Genes
To ensure alignment between optimal plastic state and salient inputs, an intrinsic timing mechanism might orchestrate CPs to arise at particular periods and places across brain regions. Circadian gene expression may play a role in this process (Fig. 1B). Circadian clocks are endogenous timers that regulate temporal sequences of gene expression, physiological events, and behavior (94). They also read out ambient timing cues such as light–dark and temperature cycles and respond to social signals. The possibility that circadian clocks contribute to developmental trajectories is intriguing because clocks provide what CPs require: integration of environmental patterns with sequences of molecular/physiological events. We consider how some of these mechanisms, including circadian regulation of neuronal maturation, differentiation, and metabolic stability, may impact the level of plasticity in the circuit.
The transcriptional/translational feedback loops that compose the circadian clock oscillate in most mammalian cells (94). While clock components begin to oscillate before birth in the suprachiasmatic nucleus (SCN) (the master physiological clock located in the ventral hypothalamus), oscillatory patterns of circadian gene (Per1, Per2, and Dbp) expression do not emerge until 2 wk postnatally in the mouse visual cortex. This increase in rhythmic profile coincides with the CP for vision. Genetic deletion of either CLOCK or BMAL disrupts this rhythmicity and is sufficient to delay PV cell maturation and CP onset (95). The conditional deletion of CLOCK from PV cells alone is sufficient to delay CP opening, suggesting CLOCK may serve as a cell-intrinsic timer. While the mechanisms by which CLOCK and BMAL support PV cell maturation are currently unknown, a role in cellular metabolism or differentiation may contribute.
The PV cells’ high firing rate and mitochondrial composition make them uniquely susceptible to oxidative stress (96). Circadian genes, including CLOCK:BMAL complex, regulate cellular metabolism and oxidative balance (94). Global deletion of BMAL in mice elevates reactive oxygen species (97) and ultimately premature aging and death (98). The CLOCK:BMAL1 complex regulates mitochondrial metabolism and antioxidant protein expression, thus influencing reactive oxygen species load (99). Mice rendered vulnerable to oxidative stress by PV cell-specific disruption of glutathione (antioxidant) synthesis show prolonged CP plasticity (100)—consistent with their destabilized PNNs and PV cell metabolic processes altering plasticity levels (101).
In this light, CLOCK is well poised to integrate cell-intrinsic metabolic programs with signals from the cortical milieu. Otx2 accumulation is an important cell-extrinsic PV cell maturation and maintenance factor (30, 41, 42). Through a positive-feedback loop, internalized Otx2 promotes PV cell enwrapment by PNNs. These, in turn, foster Otx2 uptake from the cortical milieu and protect fast-spiking cells from oxidative stress (96). Notably, the CLOCK promotor region contains an Otx2 binding site (37). Synchronizing circadian regulation of metabolism with activity-dependent signals such as Otx2 would ensure the concomitant development of protective mechanisms. CLOCK knockout mice have fewer PNNs (95), and whether reduced Otx2 accumulation conversely alters oscillatory expression of circadian genes would be of interest.
Circadian control of cell cycle and differentiation may also ultimately impact PV cell number, which is reduced in CLOCK knockouts. In the hippocampus, expression of Per2 and Bmal1 varies with the circadian cycle (102). Quiescent hippocampal stem cells are thus driven to enter the cell cycle and proliferate as granule cells, which replenish mossy fiber input into CA3 throughout life. Two distinct PV cell populations delineated by their birthdays play differential roles in regulating plasticity in this circuit (103). Circadian driven changes in neural differentiation could bias one population over the other to influence plasticity.
As CP timing differs across brain regions (Fig. 2), does each modality run at different clock speeds or require fewer turns of the dial? A functional clock at the circuit level depends upon population synchrony between multiple neurons in the network. In the SCN, such synchrony is influenced by neuropeptidergic signaling. VIP mediates input to the SCN from the visual system and also coordinates timing of clock cells (104). Likewise, a yet-to-be-identified peptide may play a similar role within cortical areas, coordinating the activity of circuits to regulate sequential CPs. In this regard, it is intriguing that VIP-expressing cortical interneurons are involved in cortical state regulation and adult plasticity (87). VIP cells are activated by cholinergic and serotonergic fibers and receive long-range intracortical input (105), making them good candidates to integrate information across brain regions. Kobayashi et al. (95) established that CLOCK genes impact CP timing in visual cortex unrelated to the SCN, which is devoid of PV cells. Of course, the triggering role on PV cell maturation, PNN development, and physiological inhibition could reflect an independent function of CLOCK as a transcription factor or epigenetic regulator (histone acetyltransferase). However, a circadian hypothesis is sensible and raises several intriguing and experimentally tractable questions. For example, regarding physiological rhythms discussed above, evoked gamma power cycles throughout the day, reflecting shifts in local E/I balance (106). Transcranial magnetic stimulation in humans reveals changes in cortical excitability that vary with relative clock time (107). The CP framework thus provides a focus on a role for circadian clocks in neuronal metaplasticity.
Within a Life Span and across Generations—The Role of Chromatin Remodeling
Epidemiological data gathered from the Dutch Hunger Winter—and more recently the Quebec Ice Storm—suggest that in utero exposure to environmental catastrophes can contribute to obesity, cardiovascular disease, and cognitive deficits in the offspring (108, 109). Adverse experiences during infancy (e.g., impoverished institutional care in orphanages, childhood trauma, and maternal postpartum depression), perturb growth, stress responsiveness, and nervous system and intellectual development, increasing the risk for mental health disorders (110–112). Moreover, by 2 mo of age, infants born into low-resource/high-stress homes already show a dramatic reduction in EEG power that varies as a function of maternal perceived stress (113). Animal studies have in many ways successfully modeled the influence of stressors experienced during gestation and infancy on metabolism, physiology, nervous system development, and behavior of these offspring in adulthood (e.g., refs. 114–117).
Conditions in early life influence the baseline and developmental trajectory of a number of physiological systems. Epigenetic modifications driven by neural activity, in particular, modulate DNA accessibility, altering gene expression profiles and thereby brain plasticity levels. Baseline chromatin configuration is constantly being updated by environmental exposures over the life span (118), serving as an integrative hub (Fig. 1D). Shifts in the pattern and stability of chromatin marks may explain how early experience “gets under the skin.” Multiple modifications (e.g., phosphorylation, acetylation, methylation) may combinatorially regulate gene expression as seen in memory formation (119), or cognitive decline associated with aging (120). During CPs, chromatin state is highly sensitive to environmental input. We postulate that this sensitivity underlies the heightened plasticity present during these developmental windows. Both early life experience and transgenerational influences can alter the level of this sensitivity, leading to individual differences in baseline plasticity.
Increased levels of histone acetylation have been observed in juvenile animals during CPs (121), and chromatin state regulates the level of neuronal plasticity in the circuit. At this age, visual stimulation triggers a robust rise in histone acetylation, which is no longer observed in adult animals (47, 121). Environmental enrichment (EE) instead leads to precocious visual system development, including early closure of the CP for ocular dominance and accelerated binocular matching (122). This early life experience drives increased histone acetylation, and the effects of EE are mimicked by treatment with histone deacetylase (HDAC) inhibitors, suggesting a surge in acetylation underlies the environmental effects (122). Pharmacologically increasing histone acetylation in adulthood with HDAC inhibitors is in fact sufficient to restore plasticity in both animal models and humans (46, 47, 123, 124). Alternative exposures that reopen CP plasticity in adulthood have also been tied to increases in histone acetylation, including EE and fluoxetine treatment (49, 121, 122). In contrast, environmental conditions that delay circuit development, such as sensory deprivation, result in histone deacetylation and a concomitant reduction of Bdnf and Pvalb gene expression (125).
Chromatin methylation patterns are also altered by neural activity (126), enabling early environmental changes to prime the system for future physiological and behavioral demands. Recent work reveals DNA methylation also occurs at non-CpG cytosines (127). Neurons preferentially methylate non-CpG cytosines (mCH) during postnatal development with a divergence of methylation patterns in cortex occurring by the third postnatal week to yield early differences in gene expression by subtype (128). Methylation in PV cells might be especially dynamic during early postnatal development, as they show higher genome-wide mCH levels compared to excitatory (CamKIIα) or other inhibitory (VIP) cells (129). Intriguingly, the Gadd45b DNA demethylase is regulated by potent, experience-dependent PV cell maintenance factors, such as BDNF and Otx2 (130, 131). In the visual cortex, increases in Otx2 lead to age-dependent changes in Gadd45b expression: Otx2 infusion in juvenile animals triggers a rise, while in adult animals the same treatment lowers Gadd45b. Viral expression of Gadd45b in adult mice then alters the methylation status of several plasticity genes, and reactivates visual plasticity (131).
Strikingly, cortical plasticity is not only influenced by an animal’s life experiences but may also be modified by that of the parents. This occurs via parental behavior during the offspring’s early postnatal life, the in utero environment during gestation, or modification of the parental or fetal germ cells. In both hippocampus and neocortex, enriched parental experience (EE) alters gene expression and enhances plasticity not only in the parents but also in their offspring (132–134). Conversely, preconceptional stress to the paternal lineage exerts epigenetic modifications in the paternal germline that influence the behavior, neuroanatomy, and physiology of future generations (135–138). Stressful postnatal experiences, such as fragmented maternal care, impair memory performance and hippocampal plasticity in both the pups and their future offspring (139). Indeed, the impact of early traumatic experiences is consistently more robust across generations (140, 141). The effects of stress exposure on offspring can be mitigated by parental EE (142, 143) or by exposing the paternal lineage to behavioral interventions like extinction training (144).
Such findings suggest an integration of environmental experiences at the level of the germline. Raising mice in EE extends visual cortical plasticity into adulthood (145)—a feature that can remarkably be transmitted to nonenriched offspring, primarily from enriched mothers (146). Whether these effects arise from epigenetic modifications to the germline or are instead passed down to their progeny by changes in maternal behavior remains unclear (147). In the case of paternal influences, recent studies have pointed to a role for RNA found in sperm whereby paternal stress or diet might exert an influence on offspring (141, 148–150). Nevertheless, definitive mechanisms of intergenerational influence by parental environments on future generations are still hotly debated. Recent genetic engineering technology (e.g., CRISPR/Cas9) now enables us to test whether epigenetic modification of specific genetic loci using cell-specific promoters might configure set points of physiology and behavior: in the germline (to affect embryonic development and adult behavior) or during development (to affect adult behavior).
Consequences for Mental Illness and Brain Injury
Multimodal processes may be particularly susceptible to shifted CP timing, as the mis-alignment between two systems may prevent their proper integration. Schizophrenia and autism spectrum disorders (ASDs) may serve to illustrate. The unification of multisensory information into a single percept is indeed disrupted in many individuals with ASD (151). Language processing requires the integration of auditory, visual, tactile, and motor information and is vulnerable in several developmental disorders. Deficits in audiovisual speech integration have been reported for individuals with ASD and are correlated with multisensory temporal acuity (152, 153). Multisensory integration deficits have also been described in several mouse models of ASD (154). Low MET expression, an autism gene, yields a “timing mosaic” in the cortex due to accelerated maturation of AMPA/NMDA receptor ratio and precocious CP closure (155).
Prefrontal PV cell disruption is well documented in schizophrenia (156, 157), and symptom onset usually begins in adolescence, a time when gamma oscillatory power is normally reduced during development (65, 158). Recent magnetoencephalography studies have revealed transiently elevated gamma power in clinical high-risk subjects at psychosis onset (159). Prolonged plasticity and restructuring may render prefrontal circuits unstable (160). Interestingly, circadian disruption is associated with worse prognosis in adolescents at risk for psychosis (161). Patients show reduced expression of CLOCK and a loss of rhythmic expression of CRY1 and PER2, revealing circadian clock disruption (162). As noted above, disrupted circadian function may accumulate redox burden in PV cells, perturbing physiology and plastic windows, which destabilizes circuitry (101).
While psychotic symptoms typically appear in adolescence, exposures during a prenatal period increase the risk (163). What underlies the latent circuit changes that lead to disease vulnerability only later in life remains unknown. Divergent methylation and histone modifications between prenatal and adult brain tissue cause differences in gene expression (164). Likewise, the timing of cortical development may help to inform recovery from early brain injury. Once believed simply to be “earlier the better,” postinjury plasticity (e.g., anomalous connections, failure of neuron pruning, dendritic growth and gliogenesis, neurogenesis, synaptogenesis, and changes in gene expression) exhibits CPs for recovery (165). Thus, outcome is good for injuries during neurogenesis (in utero), peak synaptogenesis, or adolescence, but outcome is poor if injured during the period of neuronal migration or the beginning of synaptogenesis. Notably, early exposure to GABAergic drugs (e.g., prenatal benzodiazepines) shifts the CP earlier for good recovery from perinatal injury, just as it leads to precocious onset of sensory CPs (above).
Conclusion
The term “critical period” refers to a time when specific regions undergo heightened brain plasticity. This refinement occurs in response to ambient cues, tuning the circuit to best represent environmental stimuli and to associate distinct modalities so that meaning can be extracted and processed from sensory primitives. Thus, human language as an example depends upon a sequence of CP events within and between various brain regions that support the developing individual to interact successfully with the world around them. We have argued that full understanding of the mechanisms underlying such CPs involves the identification and investigation of child and brain development across several timescales (Fig. 1), focusing on the PV circuit hub for CP timing.
New evidence gathered here suggests classical cellular timing mechanisms (e.g., chromatin remodeling, circadian clocks, redox balance, neuronal oscillations) may mark CP trajectories, while “brake-like” factors such as PNNs or dampened neuromodulation may actively keep them closed (51). This offers several routes for future investigation. For example, prefrontal PV cells in control of attention may help to explain the focused plastic episodes in the adult brain or sleep regulation of higher cortical plasticity during human adolescence. Another aspect is that structures and circuits laid down during early CPs of heightened plasticity can be preserved over a lifetime and even lie dormant until needed later (166–169). Perhaps experience-driven changes occurring early in life are embedded in the epigenome, altering the baseline level of circuit plasticity that may stretch across generations.
The biological basis of CPs is likely to provide key insights into disorders or early life adversity (170). Developmental or environmental imbalances to the synaptic E/I ratio [as described in ASD (171, 172)] would lead to shifts in CP timing that further disrupt circuit development (50). Precise mapping of CP timing in different brain areas will be of utmost importance for tracking and correcting aberrant trajectories (Fig. 2). Shifts in CP timing would signify that the same environmental input at a given time results in different outcomes between individuals. Molecular (e.g., PNN imaging) or epigenetic (e.g., exposome) analysis combined with brain signatures (e.g., oscillatory activity) by advances in novel wearable technologies and machine learning will allow us to better connect the timescales of child brain development.
Data Availability
There are no data associated with this manuscript.
Acknowledgments
All authors are members of the Canadian Institute for Advanced Research Child Brain Development network. This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery Grant 81103 (to J.F.W.), National Institute of Mental Health Grant P50MH094271, and World Premier International Research Center Initiative–International Research Center for Neurointelligence (to T.K.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.B.S. is a guest editor invited by the Editorial Board.
References
- 1.Barkat T. R., Polley D. B., Hensch T. K., A critical period for auditory thalamocortical connectivity. Nat. Neurosci. 14, 1189–1194 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesel T. N., Hubel D. H., Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963). [DOI] [PubMed] [Google Scholar]
- 3.Belford G. R., Killackey H. P., The sensitive period in the development of the trigeminal system of the neonatal rat. J. Comp. Neurol. 193, 335–350 (1980). [DOI] [PubMed] [Google Scholar]
- 4.Knudsen E. I., Experience alters the spatial tuning of auditory units in the optic tectum during a sensitive period in the barn owl. J. Neurosci. 5, 3094–3109 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz K. Z., The companion in the bird’s world. Auk 54, 245–273 (1937). [Google Scholar]
- 6.Immelmann K., “Song development in the zebra finch and other estrilidid finches” in Bird Vocalizations, Hinde R. A., Ed. (Cambridge University Press, Cambridge, UK, 1969), pp. 61–74. [Google Scholar]
- 7.Doupe A. J., Kuhl P. K., Birdsong and human speech: Common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Werker J. F., Hensch T. K., Critical periods in speech perception: New directions. Annu. Rev. Psychol. 66, 173–196 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Marom S., Neural timescales or lack thereof. Prog. Neurobiol. 90, 16–28 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Takesian A. E., Hensch T. K., Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Hu H., Gan J., Jonas P., Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 345, 1255263 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Packer A. M., Yuste R., Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: A canonical microcircuit for inhibition? J. Neurosci. 31, 13260–13271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal V. S., Zhang F., Yizhar O., Deisseroth K., Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartos M., Vida I., Jonas P., Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Lodato S., et al. , Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 69, 763–779 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paredes M. F., et al. , Extensive migration of young neurons into the infant human frontal lobe. Science 354, aaf7073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazaki-Sugiyama Y., Kang S., Câteau H., Fukai T., Hensch T. K., Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462, 218–221 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Aton S. J., et al. , Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 110, 3101–3106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhlman S. J., et al. , A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko M., Stellwagen D., Malenka R. C., Stryker M. P., Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hengen K. B., Torrado Pacheco A., McGregor J. N., Van Hooser S. D., Turrigiano G. G., Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell 165, 180–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mataga N., Mizuguchi Y., Hensch T. K., Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44, 1031–1041 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Huang X., et al. , Progressive maturation of silent synapses governs the duration of a critical period. Proc. Natl. Acad. Sci. U.S.A. 112, E3131–E3140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H., et al. , Early seizures prematurely unsilence auditory synapses to disrupt thalamocortical critical period plasticity. Cell Rep. 23, 2533–2540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katagiri H., Fagiolini M., Hensch T. K., Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53, 805–812 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Chattopadhyaya B., et al. , Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Q., Miao Q. L., Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 32, 352–363 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Huang Z. J., et al. , BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Di Cristo G., et al. , Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 10, 1569–1577 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama S., et al. , Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Gu Y., et al. , Neuregulin-dependent regulation of fast-spiking interneuron excitability controls the timing of the critical period. J. Neurosci. 36, 10285–10295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y., et al. , Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 79, 335–346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condé F., Lund J. S., Lewis D. A., The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Res. Dev. Brain Res. 96, 261–276 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Okaty B. W., Miller M. N., Sugino K., Hempel C. M., Nelson S. B., Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci. 29, 7040–7052 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigal Y. M., Bae H., Bogart L. J., Hensch T. K., Zhuang X., Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging. Proc. Natl. Acad. Sci. U.S.A. 116, 7071–7076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Favuzzi E., et al. , Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95, 639–655.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Sakai A., et al. , Genome-wide target analysis of Otx2 homeoprotein in postnatal cortex. Front. Neurosci. 11, 307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balmer T. S., Perineuronal nets enhance the excitability of fast-spiking neuorns. eNeuro 3, ENEURO.0112-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzorusso T., et al. , Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Pizzorusso T., et al. , Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl. Acad. Sci. U.S.A. 103, 8517–8522 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beurdeley M., et al. , Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 32, 9429–9437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spatazza J., et al. , Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 3, 1815–1823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Southwell D. G., Froemke R. C., Alvarez-Buylla A., Stryker M. P., Gandhi S. P., Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y., Stryker M. P., Alvarez-Buylla A., Espinosa J. S., Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc. Natl. Acad. Sci. U.S.A. 111, 18339–18344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradshaw K. P., Figueroa Velez D. X., Habeeb M., Gandhi S. P., Precocious deposition of perineuronal nets on parvalbumin inhibitory neurons transplanted into adult visual cortex. Sci. Rep. 8, 7480 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gervain J., et al. , Valproate reopens critical-period learning of absolute pitch. Front. Syst. Neurosci. 7, 102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putignano E., et al. , Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron 53, 747–759 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Maya Vetencourt J. F., et al. , The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Maya Vetencourt J. F., Tiraboschi E., Spolidoro M., Castrén E., Maffei L., Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 33, 49–57 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Weikum W. M., Oberlander T. F., Hensch T. K., Werker J. F., Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc. Natl. Acad. Sci. U.S.A. 109 (suppl. 2), 17221–17227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morishita H., Miwa J. M., Heintz N., Hensch T. K., Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B. S., Feng L., Liu M., Liu X., Cang J., Environmental enrichment rescues binocular matching of orientation preference in mice that have a precocious critical period. Neuron 80, 198–209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeBlanc J. J., Fagiolini M., Autism: A “critical period” disorder? Neural Plast. 2011, 921680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardin J. A., Snapshots of the brain in action: Local circuit operations through the lens of γ oscillations. J. Neurosci. 36, 10496–10504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berger H., Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr. Nervenkr. 87, 527–570 (1929). [Google Scholar]
- 56.Smith J. R., The electroencephalogram during normal infancy and childhood: 1. Rhythmic activities present in the neonate and their subsequent development. Pedagog. Semin. J. Genet. Psychol. 52, 431–453 (1938). [Google Scholar]
- 57.Esser S. K., Hill S. L., Tononi G., Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep 30, 1617–1630 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huber R., Born J., Sleep, synaptic connectivity, and hippocampal memory during early development. Trends Cogn. Sci. (Regul. Ed.) 18, 141–152 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Huber R., Ghilardi M. F., Massimini M., Tononi G., Local sleep and learning. Nature 430, 78–81 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Huber R., et al. , Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9, 1169–1176 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Huber R., et al. , Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J. Neurosci. 28, 7911–7918 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyamoto H., Katagiri H., Hensch T., Experience-dependent slow-wave sleep development. Nat. Neurosci. 6, 553–554 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Chen G., Rasch M. J., Wang R., Zhang X. H., Experience-dependent emergence of beta and gamma band oscillations in the primary visual cortex during the critical period. Sci. Rep. 5, 17847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall P. J., Fox N. A.; Bucharest Early Intervention Project Core Group , A comparison of the electroencephalogram between institutionalized and community children in Romania. J. Cogn. Neurosci. 16, 1327–1338 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Marshall P. J., Reeb B. C., Fox N. A., Nelson C. A. 3rd, Zeanah C. H., Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev. Psychopathol. 20, 861–880 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderwert R. E., Marshall P. J., Nelson C. A. 3rd, Zeanah C. H., Fox N. A., Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One 5, e11415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanderwert R. E., Zeanah C. H., Fox N. A., Nelson C. A. 3rd, Normalization of EEG activity among previously institutionalized children placed into foster care: A 12-year follow-up of the Bucharest Early Intervention Project. Dev. Cogn. Neurosci. 17, 68–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wade M., Fox N. A., Zeanah C. H., Nelson C. A., Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc. Natl. Acad. Sci. U.S.A. 116, 1808–1813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardin J. A., et al. , Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doischer D., et al. , Postnatal differentiation of basket cells from slow to fast signaling devices. J. Neurosci. 28, 12956–12968 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uhlhaas P. J., et al. , The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. U.S.A. 106, 9866–9871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kujala J., et al. , Gamma oscillations in V1 are correlated with GABAA receptor density: A multi-modal MEG and Flumazenil-PET study. Sci. Rep. 5, 16347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lensjø K. K., Lepperød M. E., Dick G., Hafting T., Fyhn M., Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J. Neurosci. 37, 1269–1283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fagiolini M., Hensch T. K., Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Kalish B. T., Barkat T. R., Diel E. E., Zhang E. J., Greenberg M. E., Hensch T. K., Single-nucleus RNA sequencing of mouse auditory cortex reveals critical period triggers and brakes. Proc. Natl. Acad. Sci. U.S.A. 117, 11744–11752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galuske R. A. W., Munk M. H. J., Singer W.. Relation between gamma oscillations and neuronal plasticity in the visual cortex. Proc. Natl. Acad. Sci. USA 116, 23317–23325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ortiz-Mantilla S., Hämäläinen J. A., Musacchia G., Benasich A. A., Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J. Neurosci. 33, 18746–18754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortiz-Mantilla S., Hämäläinen J. A., Realpe-Bonilla T., Benasich A. A., Oscillatory dynamics underlying perceptual narrowing of native phoneme mapping from 6 to 12 months of age. J. Neurosci. 36, 12095–12105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peña M., Werker J. F., Dehaene-Lambertz G., Earlier speech exposure does not accelerate speech acquisition. J. Neurosci. 32, 11159–11163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peña M., Pittaluga E., Mehler J., Language acquisition in premature and full-term infants. Proc. Natl. Acad. Sci. U.S.A. 107, 3823–3828 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benasich A. A., Choudhury N. A., Realpe-Bonilla T., Roesler C. P., Plasticity in developing brain: Active auditory exposure impacts prelinguistic acoustic mapping. J. Neurosci. 34, 13349–13363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trainor L. J., Lee K., Bosnyak D. J., Cortical plasticity in 4-month-old infants: Specific effects of experience with musical timbres. Brain Topogr. 24, 192–203 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Musacchia G., et al. , Active auditory experience in infancy promotes brain plasticity in theta and gamma oscillations. Dev. Cogn. Neurosci. 26, 9–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gogolla N., et al. , Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabard-Durnam L. J., et al. , Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat. Commun. 10, 4188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levin A. R., Varcin K. J., O’Leary H. M., Tager-Flusberg H., Nelson C. A., EEG power at 3 months in infants at high familial risk for autism. J. Neurodev. Disord. 9, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu Y., Kaneko M., Tang Y., Alvarez-Buylla A., Stryker M. P., A cortical disinhibitory circuit for enhancing adult plasticity. eLife 4, e05558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niell C. M., Stryker M. P., Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hioki H., et al. , Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J. Neurosci. 33, 544–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaneko M., Stryker M. P., Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fries P., Reynolds J. H., Rorie A. E., Desimone R., Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Toyoizumi T., et al. , A theory of the transition to critical period plasticity: Inhibition selectively suppresses spontaneous activity. Neuron 80, 51–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lakatos P., Karmos G., Mehta A. D., Ulbert I., Schroeder C. E., Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320, 110–113 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Takahashi J. S., Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobayashi Y., Ye Z., Hensch T. K., Clock genes control cortical critical period timing. Neuron 86, 264–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cabungcal J. H., et al. , Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 110, 9130–9135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Musiek E. S., et al. , Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kondratov R. V., Kondratova A. A., Gorbacheva V. Y., Vykhovanets O. V., Antoch M. P., Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 20, 1868–1873 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Putker M., O’Neill J. S., Reciprocal control of the circadian clock and cellular redox state—a critical appraisal. Mol. Cells 39, 6–19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morishita H., Cabungcal J. H., Chen Y., Do K. Q., Hensch T. K., Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry 78, 396–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Do K. Q., Cuenod M., Hensch T. K., Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr. Bull. 41, 835–846 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bouchard-Cannon P., Mendoza-Viveros L., Yuen A., Kærn M., Cheng H. Y., The circadian molecular clock regulated adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 5, 691–673 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Donato F., Chowdhury A., Lahr M., Caroni P., Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 85, 770–786 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Aton S. J., Herzog E. D., Come together, right...now: Synchronization of rhythms in a mammalian circadian clock. Neuron 48, 531–534 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wall N. R., et al. , Brain-wide maps of synaptic input to cortical interneurons. J. Neurosci. 36, 4000–4009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chellappa S. L., et al. , Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep. 6, 33661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ly J. Q. M., et al. , Circadian regulation of human cortical excitability. Nat. Commun. 7, 11828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bygren L. O., Intergenerational health responses to adverse and enriched environments. Annu. Rev. Public Health 34, 49–60 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Dancause K. N., et al. , Disaster-related prenatal maternal stress influences birth outcomes: Project ice storm. Early Hum. Dev. 87, 813–820 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Sheridan M. A., Fox N. A., Zeanah C. H., McLaughlin K. A., Nelson C. A. 3rd, Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl. Acad. Sci. U.S.A. 109, 12927–12932 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehta D., et al. , Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. U.S.A. 110, 8302–8307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heim C., et al. , Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 3, 41 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pierce L. J., et al. , Association of perceived maternal stress during the perinatal period and EEG in 2-month-old infants. JAMA Pediatr. 173, 561–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mueller B. R., Bale T. L., Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morgan C. P., Bale T. L., Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 31, 11748–11755 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Radford E. J., et al. , In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345, 1255903 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anway M. D., Cupp A. S., Uzumcu M., Skinner M. K., Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fraga M. F., et al. , Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U.S.A. 102, 10604–10609 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jenuwein T., Allis C. D., Translating the histone code. Science 293, 1074–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Penner M. R., Roth T. L., Barnes C. A., Sweatt J. D., An epigenetic hypothesis of aging-related cognitive dysfunction. Front. Aging Neurosci. 2, 9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vierci G., Pannunzio B., Bornia N., Rossi F. M., H3 and H4 lysine acetylation correlates with developmental and experimentally induced adult experience-dependent plasticity in the mouse visual cortex. J. Exp. Neurosci. 10 (suppl. 1), 49–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baroncelli L., et al. , Experience affects critical period plasticity in the visual cortex through an epigenetic regulation of histone post-translational modifications. J. Neurosci. 36, 3430–3440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silingardi D., Scali M., Belluomini G., Pizzorusso T., Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur. J. Neurosci. 31, 2185–2192 (2010). [DOI] [PubMed] [Google Scholar]
- 124.Yang E. J., Lin E. W., Hensch T. K., Critical period for acoustic preference in mice. Proc. Natl. Acad. Sci. U.S.A. 109 (suppl. 2), 17213–17220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koh D. X., Sng J. C., HDAC1 negatively regulates Bdnf and Pvalb required for parvalbumin interneuron maturation in an experience-dependent manner. J. Neurochem. 139, 369–380 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Guo J. U., et al. , Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat. Neurosci. 14, 1345–1351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aristizabal M. J., et al. , Biological embedding of experience: A primer on epigenetics. Proc. Natl. Acad. Sci. U.S.A. 117, 23261–23269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stroud H., et al. , Early-life gene expression in neurons modulates lasting epigenetic states. Cell 171, 1151–1164.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mo A., et al. , Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron 86, 1369–1384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Labonté B., et al. , Gadd45b mediates depressive-like role through DNA demethylation. Sci. Rep. 9, 4615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Apulei J., et al. , Non-cell autonomous OTX2 homeoprotein regulates visual cortex plasticity through Gadd45b/g. Cereb. Cortex 29, 2384–2395 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Arai J. A., Li S., Hartley D. M., Feig L. A., Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J. Neurosci. 29, 1496–1502 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benito E., et al. , RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 23, 546–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mychasiuk R., et al. , Parental enrichment and offspring development: Modifications to brain, behavior and the epigenome. Behav. Brain Res. 228, 294–298 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Rodgers A. B., Morgan C. P., Bronson S. L., Revello S., Bale T. L., Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33, 9003–9012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodgers A. B., Morgan C. P., Leu N. A., Bale T. L., Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. U.S.A. 112, 13699–13704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dickson D. A., et al. , Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Transl. Psychiatry 8, 101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harker A., Raza S., Williamson K., Kolb B., Gibb R., Preconception paternal stress in rats alters dendritic morphology and connectivity in the brain of developing male and female offspring. Neuroscience 303, 200–210 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Bohacek J., et al. , Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol. Psychiatry 20, 621–631 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Franklin T. B., Linder N., Russig H., Thöny B., Mansuy I. M., Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 6, e21842 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gapp K., et al. , Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gapp K., et al. , Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology 41, 2749–2758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCreary J. K., et al. , Environmental intervention as a therapy for adverse programming by ancestral stress. Sci. Rep. 6, 37814 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aoued H. S., et al. , Reversing behavioral, neuroanatomical, and germline influences of intergenerational stress. Biol. Psychiatry 85, 248–256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sale A., et al. , Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 10, 679–681 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Kalogeraki E., Yusifov R., Löwel S., Transgenerational transmission of enhanced ocular dominance plasticity from enriched mice to their non-enriched offspring. eNeuro 6, ENEURO.0252-18.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Champagne F. A., Meaney M. J., Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 121, 1353–1363 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Chen Q., et al. , Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Gapp K., et al. , Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol. Psychiatry, 10.1038/s41380-018-0271-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma U., et al. , Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Foss-Feig J. H., et al. , An extended multisensory temporal binding window in autism spectrum disorders. Exp. Brain Res. 203, 381–389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith E. G., Bennetto L., Audiovisual speech integration and lipreading in autism. J. Child Psychol. Psychiatry 48, 813–821 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Stevenson R. A., et al. , Keeping time in the brain: Autism spectrum disorder and audiovisual temporal processing. Autism Res. 9, 720–738 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Gogolla N., Takesian A. E., Feng G., Fagiolini M., Hensch T. K., Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 83, 894–905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen K., Ma X., Nehme A., Wei J., Cui Y., Cui Y., Yao D., Wu J., Anderson T., Ferguson D., Levitt P., Qiu S., Time-delimited signaling of MET receptor tyrosine kinase regulates cortical circuit development and critical period plasticity. Mol. Psychiatry, 10.1038/s41380-019-0635-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hashimoto T., et al. , Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 23, 6315–6326 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dienel S. J., Lewis D. A., Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol. Dis. 131, 104208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Uhlhaas P. J., Singer W., Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Grent-’t-Jong T., et al. , Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife 7, e37799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fuhrmann D., Knoll L. J., Blakemore S. J., Adolescence as a sensitive period of brain development. Trends Cogn. Sci. (Regul. Ed.) 19, 558–566 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Lunsford-Avery J. R., et al. , Adolescents at clinical-high risk for psychosis: Circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophr. Res. 189, 37–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johansson A. S., Owe-Larsson B., Hetta J., Lundkvist G. B., Altered circadian clock gene expression in patients with schizophrenia. Schizophr. Res. 174, 17–23 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Brown A. S., Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 72, 1272–1276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li M., et al. ; BrainSpan Consortium ; PsychENCODE Consortium ; PsychENCODE Developmental Subgroup , Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362, eaat7615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kolb B., Mychasiuk R., Muhammad A., Gibb R., Brain plasticity in the developing brain. Prog. Brain Res. 207, 35–64 (2013). [DOI] [PubMed] [Google Scholar]
- 166.Knudsen E. I., Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science 279, 1531–1533 (1998). [DOI] [PubMed] [Google Scholar]
- 167.Hofer S. B., Mrsic-Flogel T. D., Bonhoeffer T., Hübener M., Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lindenberger U., “Plasticity beyond early development: Hypotheses and questions” in Emergent Brain Dynamics: Prebirth to Adolescence, Benasich A. A., Ribary U., Eds. (The MIT Press, Cambridge, MA, 2018), pp. 207–223. [Google Scholar]
- 169.Yang G., Pan F., Gan W. B., Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cameron J. L., Eagleson K. L., Fox N. A., Hensch T. K., Levitt P., Social origins of developmental risk for mental and physical illness. J. Neurosci. 37, 10783–10791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rubenstein J. L., Merzenich M. M., Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nelson S. B., Valakh V., Excitatory/Inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lendvai B., Stern E. A., Chen B., Svoboda K., Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000). [DOI] [PubMed] [Google Scholar]
- 174.Stern E. A., Maravall M., Svoboda K., Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron 31, 305–315 (2001). [DOI] [PubMed] [Google Scholar]
- 175.Gogolla N., Caroni P., Lüthi A., Herry C., Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261 (2009). [DOI] [PubMed] [Google Scholar]
- 176.Pattwell S. S., et al. , Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U.S.A. 109, 16318–16323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bicks L. K., et al. , Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat. Commun. 11, 1003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.del Río J. A., de Lecea L., Ferrer I., Soriano E., The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res. Dev. Brain Res. 81, 247–259 (1994). [DOI] [PubMed] [Google Scholar]
- 179.Wang X. J., Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat. Rev. Neurosci. 21, 169–178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data associated with this manuscript.