When humans vocalize along with rhythmic arm movements, the recorded voice shows the same rhythmic pattern as these movements (1). When others listen to this voice, their rhythmic movements synchronize with those of the unseen vocalizer (1). Pouw et al.’s (1) outstanding multimodal research connects voice and gesture, and—we suggest—even more so breathing and movement.
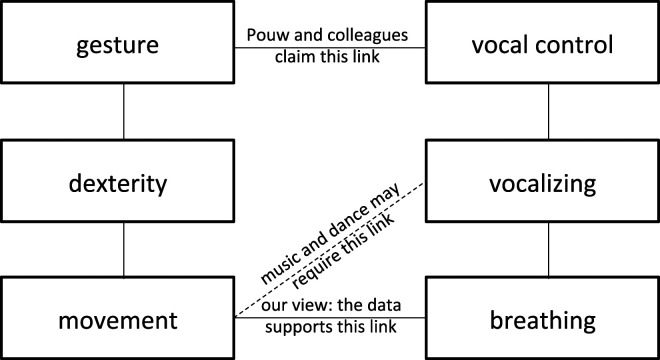
Pouw et al. (1) report rhythmic modulation of fundamental frequency, which is the rate at which vocal folds vibrate. They attribute this rhythmicity to voice modulation. However, many mechanisms can give the voice a rhythmic character; the most common one is breathing. Try exhaling while rhythmically moving your arm; the sound of your breath will become rhythmic. Could breathing alone explain Pouw et al.’s results (1, 2) (Fig. 1)? Their data may show that muscular contractions for movement propagate to the ribcage and diaphragm, potentially affecting breathing. This could explain stronger synchronization with (larger) arm than (smaller) wrist movements. We propose that frequency modulation of the voice while moving may have a neuroanatomical basis. In fact, the larynx motor area at the dorsal end of the orofacial division of motor cortex integrates laryngeal and respiratory control (3, 4). To counteract rhythmic muscular tension induced by movement, this area may contract the vocal folds rhythmically. These compensatory contractions would generate the high-frequency peaks seen in Pouw et al.’s data (1).
Fig. 1.
Alternative interpretations of Pouw et al.’s data (1). Gesture requires dexterity, which requires movement (Left). Vocal control requires vocalizing, which requires breathing (Right). While the authors interpret their data as supporting a potential evolutionary link between gesture and vocal control (Top), we consider that linking joint rhythmic movement with breathing (Bottom) is more parsimonious.
Pouw et al. (1) attribute their findings to how humans integrate gesture and vocalization. They report that better synchronization correlates with increased frequency modulation of the voice. While breathing and vocalizing are rather likely coinvolved (5), we consider that similar results could emerge without vocalizations. Had Pouw et al. asked participants to breathe while moving, would breathing spectrograms also contain rhythmic regularities? Could other listeners synchronize their movements to recordings of breathing alone? Likewise, we wonder whether movements other than gestures could explain Pouw et al.’s data (1). Physiology suggests that gestures are not strictly needed for rhythmic sounds to emerge. For instance, the tracheal sounds produced in human sleep contain the regular rhythm of the heartbeat (6). This suggests that rhythmic sound production in a phonatory apparatus can, in principle, emerge without voice or motor control.
Pouw et al. (1) conclude that “gestural movements may thus have evolved as an embodied innovation for vocal control,” that is, we were gestural apes before becoming vocal apes. There is controversial support for this intriguing “theremin model of speech control” (Fig. 2). Indeed, evidence from other primates suggests we evolved movement precision and dexterity (7, 8) before direct corticobulbar projections for enhanced laryngeal control (9). Why, then, have all other dexterous primates not evolved similar capacities for embodied vocal control? Until these questions are answered, Pouw et al.’s (1) data are key to formulate evolutionary hypotheses connecting breathing control and interactive rhythms in movement (Fig. 1).
Fig. 2.
Playing theremin is an alternative example of how movement flexibility can affect sound production. Here, the player moves her hands around the musical instrument to shape the sounds it produces. What we call the “theremin model of speech control” is based on the idea that, while the vocal tract of participants in ref. 1 provides a sound flow (much like the depicted theremin), hand movements of participants in ref. 1 can modulate and control this flow (similar to what the theremin player does). In a (highly dexterous) musical duet of two theremin performers, the movements of players might also synchronize by sound alone, akin to the voice-mediated movement synchrony in Pouw et al.’s experiment (1), but without enhanced vocal control. Image credit: Wikimedia Commons/What's On the Air Company.
Footnotes
The authors declare no competing interest.
References
- 1.Pouw W., Paxton A., Harrison S. J., Dixon J. A., Acoustic information about upper limb movement in voicing. Proc. Natl. Acad. Sci. U.S.A. 117, 11364–11367 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardy B. G., Hoffmann C. P., Moens B., Leman M., Dalla Bella S., Sound-induced stabilization of breathing and moving. Ann. N. Y. Acad. Sci. 1337, 94–100 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Belyk M., Brown S., The origins of the vocal brain in humans. Neurosci. Biobehav. Rev. 77, 177–193 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Belyk M., et al. , Human specific neurophenotype integrates laryngeal and respiratory components of voice motor control. PsyArXiv:0.31234/osf.io/pc4uh (29 May 2020).
- 5.Pouw W., Harrison S. J., Gibert N. E., Dixon J. A., Energy flows in gesture-speech physics: The respiratory-vocal system and its coupling with hand gestures. PsyArXiv: 10.31234/osf.io/c7456 (27 November 2019). [DOI] [PubMed]
- 6.Kalkbrenner C., et al. , Apnea and heart rate detection from tracheal body sounds for the diagnosis of sleep-related breathing disorders. Med. Biol. Eng. Comput. 56, 671–681 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Bortoff G. A., Strick P. L., Corticospinal terminations in two new-world primates: Further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J. Neurosci. 13, 5105–5118 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemon R. N., Neural control of dexterity: What has been achieved? Exp. Brain Res. 128, 6–12 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Simonyan K., The laryngeal motor cortex: Its organization and connectivity. Curr. Opin. Neurobiol. 28, 15–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]