Significance
Fruit set, which is triggered by the phytohormone gibberellin (GA), is the developmental transition of ovaries into fruits. Our multiomics approaches revealed that PROCERA-dependent GA responses rewired central carbon metabolism, predominantly under transcriptional control. The kinetic analysis approach used in this study enabled us to construct a carbon flux model of the earliest processes that occur during fruit set. The model revealed that fruit set coincided with the temporal changes in sugar compartmentalization due to the coordinated actions of the enzymes and tonoplastic carriers and highlighted that fructokinase likely contributed to early ovary growth by pulling fructose out of the vacuole to feed the downstream pathways for biosynthesis of cell wall components and energy provision.
Keywords: fruit set, gibberellin, metabolic enzymes, tomatoes, parthenocarpy
Abstract
Fruit set is the process whereby ovaries develop into fruits after pollination and fertilization. The process is induced by the phytohormone gibberellin (GA) in tomatoes, as determined by the constitutive GA response mutant procera. However, the role of GA on the metabolic behavior in fruit-setting ovaries remains largely unknown. This study explored the biochemical mechanisms of fruit set using a network analysis of integrated transcriptome, proteome, metabolome, and enzyme activity data. Our results revealed that fruit set involves the activation of central carbon metabolism, with increased hexoses, hexose phosphates, and downstream metabolites, including intermediates and derivatives of glycolysis, the tricarboxylic acid cycle, and associated organic and amino acids. The network analysis also identified the transcriptional hub gene SlHB15A, that coordinated metabolic activation. Furthermore, a kinetic model of sucrose metabolism predicted that the sucrose cycle had high activity levels in unpollinated ovaries, whereas it was shut down when sugars rapidly accumulated in vacuoles in fruit-setting ovaries, in a time-dependent manner via tonoplastic sugar carriers. Moreover, fruit set at least partly required the activity of fructokinase, which may pull fructose out of the vacuole, and this could feed the downstream pathways. Collectively, our results indicate that GA cascades enhance sink capacities, by up-regulating central metabolic enzyme capacities at both transcriptional and posttranscriptional levels. This leads to increased sucrose uptake and carbon fluxes for the production of the constituents of biomass and energy that are essential for rapid ovary growth during the initiation of fruit set.
Fruit set marks the transition of ovaries into fruits and is generally induced by pollination and then fertilization. Pollination is usually essential for the induction of fruit set, as it stimulates the accumulation of plant growth regulators within fertilized ovaries, including plant hormones like auxin and gibberellin (GA). Gibberellins are tetracyclic diterpenoid hormones that stimulate aspects of plant development, such as root and shoot elongation, seed germination, flower transition, and fruit set and expansion (1). They stimulate these processes by disrupting proteins from the DELLA family of negative regulators (2, 3). The tomato genome contains a single copy of the DELLA homolog called PROCERA—and its loss-of-function mutant, procera (pro)—induces most of the GA responses; it has been consistently found that ∼95% of the GA response genes in leaf tissues are regulated by PROCERA (4, 5). However, the metabolic responses induced by GAs are poorly understood.
The roles of plant hormones are widely accepted in the regulation of fruit set initiation, especially auxin and GA, that act as positive regulatory signals triggering rapid ovary growth. Both auxin and GA are involved in the active cell division phase, whereas the cell expansion phase is dominated by GA in fruit-setting ovaries, and cell growth intensity is dependent on auxin and gibberellin dose and signaling (6). Furthermore, the exogenous application of these hormones onto ovaries or genetic mutations in the negative regulatory genes of these hormone cascades, such as INDOLE-3 ACETIC ACID 9 (IAA9) or PROCERA, can induce pollination-independent fruit set, termed parthenocarpy (7, 8). Although fruit set is induced by both auxin and GA, it generally requires GA biosynthesis or its signaling cascades; thus GA most likely acts downstream of auxin‐mediated signaling during fruit set (9, 10). To decipher fruit set mechanisms, extensive transcriptome studies have been conducted using young ovaries to shed light on their molecular pathways. The main findings from these studies are highlighted by changes in mRNA transcripts associated with, for example, hormone metabolism and signaling, specific families of transcription factors (TFs) (for instance, MADS box and homeobox), photosynthesis, cell division and expansion, and the metabolism of cell walls and carbohydrates (11, 12). However, how these transcriptome fluctuations affect metabolic pathways, particularly the sugar and central metabolism pathways, is poorly understood. Their associations have been implicated with genetic evidence that the silencing of sucrose (Suc) cleavage genes, such as SUCROSE SYNTHASE 1 (SuSy1) or LYCOPERSICUM INVERTASE5 (LIN5), results in reduced fruit set efficiency, as well as the fact that the down-regulation of IAA9, encoding a negative regulatory protein of auxin responses, induces parthenocarpy associated with the accumulation of high levels of the major sugars and hexose phosphates in fruit-growing ovaries (12–14). Additionally, organic compound polyamines in ovaries have been shown to play important roles in early ovary growth, as growth regulators or nitrogen sources (15). These results have led to speculation about whether activated sugar and carbohydrate metabolism and subsequent downstream metabolic pathways were critical for fruit set initiation, but current biochemical evidence is limited.
Systems biology approaches have been widely applied to decipher the fundamental mechanisms of fruit development and ripening in tomato, by assessing their transcripts, proteins, metabolites, and enzymes (16–18). These analyses have identified subsets of components that specifically fluctuate during fruit development and ripening. Furthermore, kinetic modeling analysis, integrating both tonoplastic carriers and sugar metabolizing enzymes, has revealed the tight correlations between sugar uptake and vacuolar expansion and enabled the precise prediction of metabolic behaviors (19). However, the earliest stages of fruit development, including fruit set initiation, have yet to be investigated with this systematic approach. To address this, multiomics approaches were carried out and comprehensive distributions of the molecular and metabolic components were determined. A kinetic model to calculate sugar fluxes and partitioning in young fruit-setting ovaries was also constructed. Our results shed light on the currently unknown role of GAs in rewiring the metabolic status of ovaries for high carbohydrate metabolizing activities. These activities allow for active metabolic fluxes and the generation of energy and components that are essential for rapid ovary growth.
Results
Comparison of Ovary Growth in Wild Type and pro during Fruit Set.
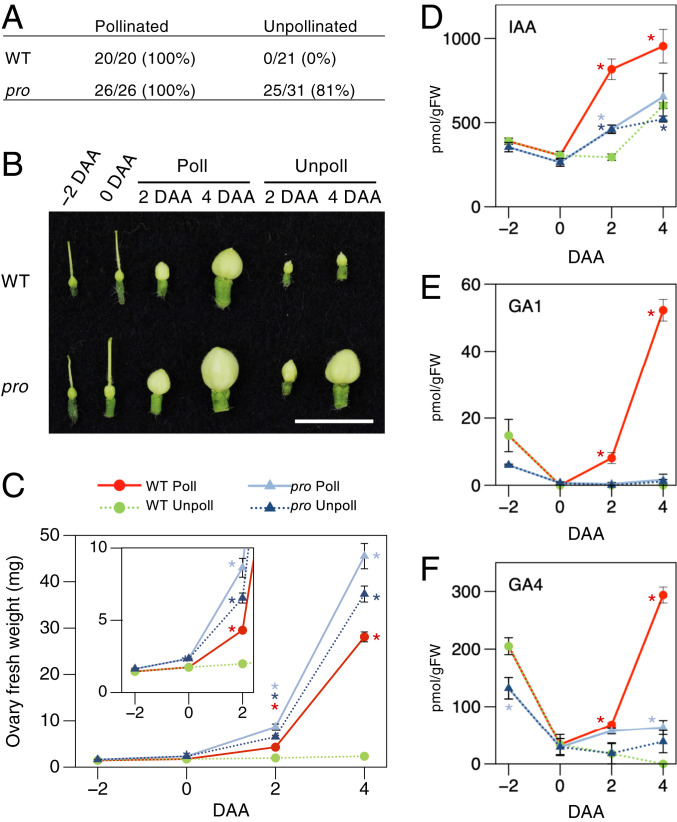
Ovary growth for the wild-type (WT) tomato and the procera mutant (pro) during fruit set, in the defined conditions of this investigation, was assessed. The ovary weight and histology of the ovary wall were nearly equivalent for the WT and pro, both −2 and 0 d after anthesis (DAA) (Fig. 1 A–C and SI Appendix, Fig. S1). The weight of the WT ovaries increased greatly with pollination 4 DAA, with a 100% fruit set rate, unlike the nongrowing unpollinated WT ovaries that showed little change. In contrast, the unpollinated pro ovaries had an 81% pollination-independent fruit set rate, through parthenocarpy; hand pollination increased this to 100%, and there were concomitant increases in ovary weight by 4 DAA. Fruit-growing ovaries (pollinated WT or both pollinated and unpollinated pro ovaries) showed drastic increases in the cell layers and size, due to intense cell division and strong cell expansion, respectively, between 0 and 4 DAA (SI Appendix, Fig. S1). In the pollinated WT ovaries, cell division that commenced by 2 DAA resulted in a slight increase in ovary weight, whereas cell enlargement likely contributed to a rapid increase in ovary weight between 2 and 4 DAA. Unpollinated pro ovaries showed similar cell division activity, but their cell enlargement was more prominent than the pollinated WT. Pollination of the pro further activated cell proliferation 2 DAA, which contributed to thicker ovary walls.
Fig. 1.
Fruit set in WT tomato and the GA response mutant procera (pro). (A) Pollination- and parthenocarpy-dependent fruit set rate. Number of developed fruits per attempt is also shown. (B and C) Representative pictures (B) and weights (C) of pollinated and unpollinated ovaries from −2 to 2 DAA (Inset) or 4 DAA. Values in C are mean ± SEs of six replicates, each one defined as the average ovary weight obtained by measuring a mixture of 5 to 10 ovaries. (D–F) Endogenous levels of IAA (D) and bioactive GAs (E and F). Values in D–F are mean ± SEs of three replicates. (Scale bar in B, 1 cm.) Asterisks in C–F indicate significant differences from nongrowing unpollinated WT ovaries (Student's t test, P < 0.05). DAA, days after anthesis; Poll, pollination; Unpoll, unpollinated.
Changes in Hormonal Dynamics during Fruit Set.
Hormonal dynamics in the ovaries, coupled with the associated hormone transcript profiles, were investigated. In contrast to nongrowing unpollinated WT ovaries, the levels of auxin (indole acetic acid or IAA) in the pollinated ovaries rapidly increased 2 DAA and persisted until 4 DAA. While the levels of bioactive GAs (GA1 and GA4) were also stimulated by pollination 2 DAA, a notable increase was observed between 2 and 4 DAA (Fig. 1 D–F). The levels of auxin and bioactive GAs agreed with active cell division persisting until 4 DAA, and the notable cell expansion between 2 and 4 DAA. Transcriptome profiling by RNA sequencing (RNA-Seq) analysis revealed that in the fruit-growing WT ovaries, the hormone levels correlated with the transcripts of their key metabolism genes—i.e., TAR1, toFZY2, and toFZY5 for auxin, and SlGA20ox1 and SlGA20ox2 for GA (20) (SI Appendix, Fig. S2 and Dataset S1). In contrast, the levels of bioactive GAs in the pro ovaries were always lower than in the WT, indicating negative feedback regulations in their biosynthetic genes due to the constitutive GA response (21) (Fig. 1 E and F and SI Appendix, Fig. S2B). Both the pollinated and unpollinated pro ovaries accumulated lower amounts of auxin and transcripts encoding auxin biosynthesis genes than in the pollinated WT (Fig. 1D and SI Appendix, Fig. S2A), suggesting that the GA response counteracts auxin biosynthesis in the tomato ovary. Nevertheless, the pro ovaries showed auxin-dependent responses for some auxin signaling genes, such as the down-regulation of ARF5 and ARF7, which were also suppressed in the pollinated WT ovaries (SI Appendix, Fig. S2A) and are known to act as repressors of fruit set initiation (9, 22, 23). Unpollinated pro ovary growth through parthenocarpy is thus unlikely to be related to the absolute levels of those hormones, but rather associated with the constitutive GA responses underlying the GA-regulatory PROCERA network, in which there is some auxin signaling cascade cross-talk.
Transcript and Metabolite Profiling and Integrated Network Analysis of Fruit Set.
Besides RNA-Seq, metabolomic analysis by gas chromatography-time-of flight-mass spectrometry (GC-TOF-MS) and high-performance liquid chromatography coupled with photodiode array detector (LC-PDA) analyses were performed to examine and compare the dynamics of the transcript and metabolite profiles during fruit setting. The GC-TOF-MS analysis systematically detected 252 metabolites as unique peaks, and the LC-PDA analysis quantified six major hydrophobic pigments including carotenoids and chlorophylls (Dataset S2). A principal component analysis (PCA) revealed that the transcriptomes and metabolomes clearly discriminated between fruit-growing and nongrowing ovaries (SI Appendix, Fig. S3). Profiles obtained for the WT and pro were strongly correlated −2 DAA, but less so after anthesis. However, both the transcript and metabolite profiles in the fruit-growing ovaries had clear clusters corresponding to each time point, indicating that these ovaries largely share transcript and metabolite profiles, and therefore parthenocarpy can mimic pollination-dependent molecular and biochemical mechanisms.
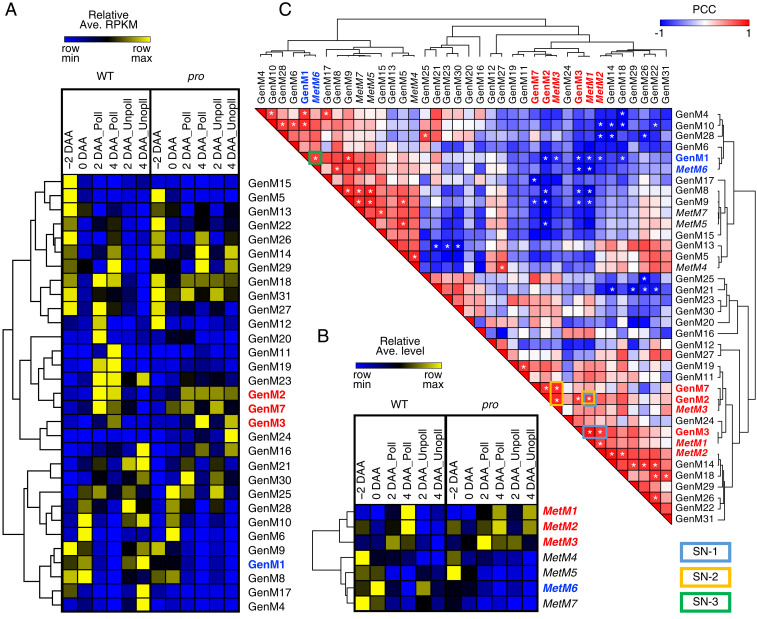
To reveal the biochemical and regulatory networks of fruit set, we applied a weighted gene coexpression network analysis (WGCNA) using 12,322 transcripts that showed a high variation among sample types and 101 identified or provisionally identified metabolites from the GC-TOF-MS and LC-PDA analyses (SI Appendix, Materials and Methods and Datasets S3 and S4). This analysis is based on pairwise correlations between the levels of each transcript or metabolite and it identified a total of 31 transcriptional gene expression modules (GenM1 to GenM31) and seven metabolite modules (MetM1 to MetM7) (Fig. 2 A and B and Datasets S3 and S4). The module eigengene (MEgene) and eigenmetabolite (MEmetabolite) values, which are considered the first principal components of the profiles in the individual modules (24), summarized the accumulation patterns of the clustered transcripts and metabolites and the intermodular similarities (Fig. 2 A and B and SI Appendix, Figs. S4 and S5). Several significant correlations appeared to be present in the module correlation map (Pearson correlation coefficient [PCC] > 0.75, P < 0.05; Fig. 2C). The highest correlation between transcript–metabolite modules was found between GenM3 and MetM1 (PCC = 0.943), and while GenM3 also showed a significant correlation with MetM2 (PCC = 0.806), together with the GenM2–MetM1 module correlation (PCC = 0.811), these created a subnetwork (SN-1) associated with high levels of transcripts and metabolites after anthesis, which were maintained until 4 DAA in the fruit-growing ovaries. We also found significant correlations between MetM3 and GenM7 (PCC = 0.850) and GenM2 (PCC = 0.856), which created another subnetwork (SN-2) associated with early responses 2 DAA in the fruit-growing ovaries. SN-1 and SN-2 were part of a fruit-growing transcript–metabolite network with the significantly connected GenM2 and MetM1 (PCC = 0.811) as the intersubnetwork hubs (Fig. 3). When focused on transcriptome correlation, both GenM2 and GenM3 showed strong negative correlations with the largest transcript module GenM1 (PCC = −0.943 and −0.867, respectively), which showed a clear negative association with fruit-growing ovaries (Fig. 2 A and C).
Fig. 2.
Network analysis across pollinated and unpollinated ovaries in WT tomato and procera (pro) mutants. (A and B) Heat map showing averaged relative reads per kilobase of the exons per million mapped reads (RPKM) of genes (A) and relative levels of metabolites (B) clustered in the individual WGCNA gene transcript (GenM) and metabolite (MetM) modules. (C) PCC of each pair of GenM and MetM module. Asterisks indicate pairs with PCC > 0.75 or < −0.75 (P < 0.05). Fruit set associated GenM–MetM subnetworks are indicated by blue (SN-1), orange (SN-2), or green (SN-3) boxes, which correspond to positive associations with fruit set until 4 DAA, 2 DAA, and a negative association with fruit set, respectively. Among the modules consisting of these subnetworks, those positively and negatively associated with fruit set are indicated by bold red and blue, respectively. Metabolite modules are indicated by italic. DAA, days after anthesis; Poll, pollinated; Unpoll, unpollinated.
Fig. 3.
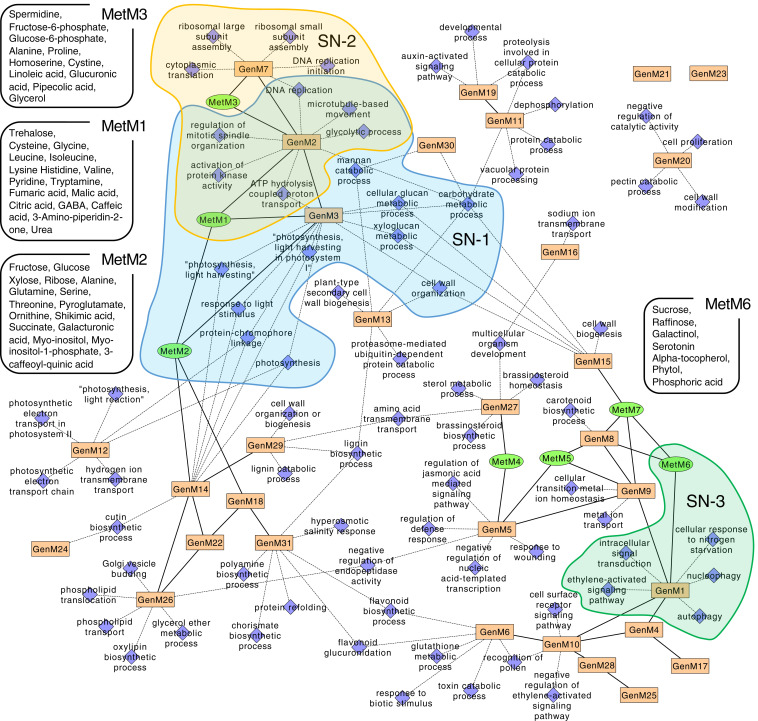
Integrated network of transcript and metabolite modules. The orange rectangles and green circles represent nodes of coexpression of the WGCNA transcript (GenM) and metabolite (MetM) modules, respectively. Blue squares connected by dashed lines to transcript modules represent the nodes from the top five GO terms enriched in each module (false discovery rate [FDR]-adjusted P value <0.05, hypergeometric test). Edges with solid lines represent intermodular correlation (PCC > 0.75, P < 0.05). The identified metabolites included in fruit set-associated modules with kME > 0.7 are shown. Fruit set-associated GenM–MetM subnetworks (SN-1, -2, and -3; Fig. 2C) and the metabolites included in the subnetworks are also shown.
To determine the functional associations of the module networks, we constructed an interconnection map for the transcript modules with the gene ontology (GO) terms that exhibited the lowest P values (Fig. 3 and Dataset S5). GenM2 and GenM3 were found to enrich the GO terms related to carbohydrate metabolism and/or energy production, such as “carbohydrate metabolic process,” “glycolytic process,” “xyloglucan metabolic process,” “cell wall organization,” and “ATP synthesis coupled protein transport” (Dataset S5), and these correlated to form metabolite modules MetM1 to 3, that included major intermediates and derivatives of the carbohydrate and sugar metabolism (Fig. 3). The metabolites enriched in the MetM3 module showed strong increases 2 DAA in the fruit-growing ovaries, especially fructose-6-phosphate (F6P) and glucose-6-phosphate (G6P), which are involved in glycolysis and Suc and starch metabolism. The MetM3 module also contained the polyamine spermidine that promotes fruit set through the modulation of GA metabolism in tomato (15). The MetM2 module included fructose (Fru) and glucose (Glc) as well as derivatives and intermediates of the gamma-aminobutyric acid (GABA) biosynthetic pathway that partially bypasses the tricarboxylic acid cycle (TCA) cycle, including 5-oxoproline (pyroglutamate), ornithine, and glutamine, as well as four amino acids (alanine, serine, threonine, and glutamine). The MetM2 module also contained shikimic acid, which originates from phosphoenolpyruvate (glycolysis) and erythrose-4-phosphate (oxidative/reductive pentose phosphate pathway) and is the precursor of aromatic amino acids and phenylpropanoids, which are used to generate pigments, hormones, and cell wall components including lignin (25). These data indicate that both central carbon and nitrogen metabolism are rapidly activated in fruit-growing ovaries. The MetM1 module, which peaked 4 DAA (SI Appendix, Fig. S5), included seven amino acids and three organic acids (citric acid, malic acid, and fumaric acid), which are intermediates of the TCA cycle and vacuolar storage compounds, and therefore play an important role in the energy and osmotic status of pericarp cells (26). These data also suggested a continuous activation of the central metabolism between 0 and 4 DAA. Matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) analysis confirmed that some of the major metabolites described above, including hexoses (m/z = 179.1, [M-H]−), GABA (m/z = 102.1, [M-H]−), citric acid/isocitric acid (m/z = 191.1, [M-H]−), malic acid (m/z = 133.0, [M-H]−), and fumaric acid (m/z = 115.0, [M-H]−), accumulated in rapidly growing maternal tissues, such as the pericarp and placenta, in growing ovaries 4 DAA, rather than the ovules, regardless of whether the pollination/fertilization was dependent or independent of fruit set (SI Appendix, Fig. S6). Taken together, transcriptomes associated with central metabolism pathways, spanning sugar and carbohydrate metabolism, glycolysis, and the TCA cycle, were correlated with their corresponding metabolomes that could have a major biochemical impact on the expansive growth of the ovary during fruit set initiation.
In contrast to those metabolite modules, the MetM6 module showed a clear negative association with the fruit-growing ovaries, and included metabolites known to be accumulated in senescing organs such as serotonin, raffinose, and alpha-tocopherol (27–29). Thus, GA signaling reduced the level of senescence-related metabolites, thereby suppressing the progression of ovary senescence. The GenM1 module, that was positively correlated with MetM6 (PCC = 0.779), enriched senescence-related GO terms such as “ethylene-activated signaling pathway” and “autophagy,” and these created a subnetwork (SN-3) negatively associated with fruit set (Figs. 2C and 3). This agreed with the notion that ethylene signaling and ovary senescence counteracted fruit set initiation (30, 31).
Functional Analysis of a Transcriptional Hub Negatively Associated with Fruit Set.
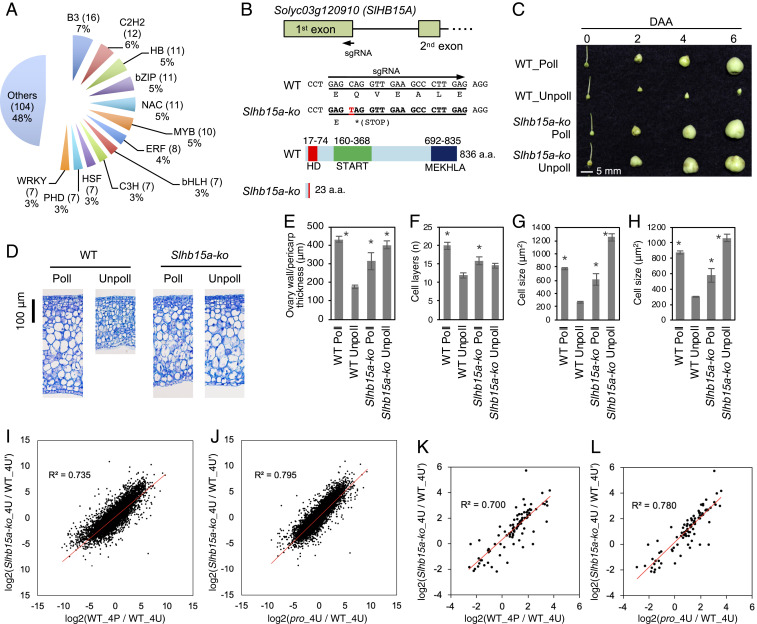
Interestingly, GenM1, the largest transcript module, showed a negative association with fruit set, which suggested a potential importance for negative functional networks in fruit set (Fig. 2 and SI Appendix, Fig. S4). The fact that the inverse correlation of GenM1 with the modules that were positively associated with fruit set, including the next largest transcript modules GenM2 (PCC = −0.943) and GenM3 (PCC = −0.867), as well as metabolite modules MetM1 (PCC = −0.830), MetM2 (PCC = −0.802), and MetM3 (PCC = −0.782), suggested that GenM1 was a hub module of the fruit set network (Figs. 2C and 3). TFs potentially play important roles in the regulation of transcriptional networks. We found that there were five families that accounted for over 25% of the TF members in the GenM1 module (Fig. 4A). Among the members of these families, a class III homeodomain-leucine zipper gene (Solyc03g120910)—a homolog of the Arabidopsis CORONA (CNA)—that is known to be associated with carpel cell development (32) was identified as a central hub gene (module membership [kME] = 0.922; Dataset S3), and its homologous gene in tomatoes is called Solanum lycopersicum HOMEOBOX 15A (SlHB15A) (33). To examine the function of SlHB15A in fruit set, we generated putative knockout plants by incorporating the stop codon within the first exon, using a modified CRISPR-Cas9 system that allowed for single base substitutions (34) and confirmed that the genome edit resulted in a homozygous mutation devoid of T-DNA integration (Fig. 4B). The putative SlHB15A-knockout (Slhb15a-ko) plants showed efficient parthenocarpy, with similar cytology to pro-induced parthenocarpy, in that active cell enlargement rather than cell division, largely contributed to rapid ovary growth (Fig. 4 C–H and SI Appendix, Fig. S1). Moreover, the parthenocarpic ovary transcriptome was obtained by RNA-Seq. At 4 DAA, the Slhb15a-ko was highly similar to that of the pollinated WT (Fig. 4I, SI Appendix, Materials and Methods, and Dataset S6) and parthenocarpic pro (Fig. 4J). Furthermore, a high similarity of the transcripts of the 92 selected genes associated with the central metabolism (SI Appendix, Fig. S7) was observed between the transcriptome of parthenocarpic Slhb15a-ko and that of the pollinated WT (Fig. 4K) and parthenocarpic pro (Fig. 4L). Thus, our results demonstrated that the network construction analysis makes it possible to identify key gene hubs. SlHB15A likely coordinates the regulation of central metabolism pathways by PROCERA in fruit set.
Fig. 4.
Targeted mutagenesis of the homolog of Arabidopsis CNA and its effect on ovary growth and transcriptome. (A) The family of transcription factors that are included in the GenM1 module. (B) Target sites specified by sgRNA and the edited genome and amino acid sequences in the putative knockout plant (Slhb15a-ko)—with a stop codon at the 24th amino acid altered using the modified CRISPR-Cas9 system (target-activation induced cytidine deaminase [Target-AID]). The three functional domains (homeodomain [HD], START, and MEKHLA), their positions on the SlHB15A protein, and the putative truncated protein structure in Slhb15a-ko are shown. (C) Parthenocarpy exhibited in Slhb15a-ko. (D) Representative sections of the ovary walls 4 DAA for Poll and Unpoll. (E–H) The thickness (E), number of cell layers (F), and mean cell size of internal (G) and external (H) mesocarp in the ovary wall 4 DAA. Values are means ± SEs of three independent ovaries. Asterisks in E–H indicate significant differences from nongrowing unpollinated WT ovaries (Student’s t test, P < 0.05). (I–L) Transcript correlations between parthenocarpic Slhb15a-ko ovaries and pollinated WT ovaries (I and K) or parthenocarpic pro ovaries 4 DAA (J and L). Scatterplots show the log2 fold changes of 18,775 commonly expressed genes (I and J) or 92 selected genes associated with central metabolism shown in SI Appendix, Fig. S7 (K and L) between unpollinated WT ovaries and pollinated WT, unpollinated Slhb15a-ko, or unpollinated pro ovaries. WT_4U and WT_4U′ indicate different batches of unpollinated WT ovaries analyzed together with pollinated WT and unpollinated procera (pro) ovaries or unpollinated Slhb15a-ko, respectively. DAA, days after anthesis; 4P, 4 DAA with pollination; 4U (4U′), 4 DAA without pollination; Poll, pollination; Unpoll, unpollinated.
Correlations between Transcripts and Proteins during Fruit Set.
We analyzed the proteome using label-free shotgun proteomics with the ovaries depicted in Fig. 1B. A total of 1,343 proteins were systematically detected from three replicates of each ovary sample derived from pollinated or unpollinated WT and unpollinated pro plants (Dataset S7). A PCA assessed the similarities of the protein profiles and revealed the clusters of proteins associated with the ovary growth stages. The results were similar to those of the transcripts and metabolites, as the fruit-growing ovaries were closely related and were discriminated from the unpollinated WT ovaries (SI Appendix, Fig. S8).
Abundance correlations between the transcripts and proteins were significantly positive for all sample types (SI Appendix, Table S1). Correlations between the individual transcripts and the corresponding proteins were determined and compared across different MapMan functional categories (35) (SI Appendix, Fig. S9). Overall, positive correlations were observed, except for the transport-related proteins. High correlations during both pollination-dependent and pollination-independent fruit set were observed among several functional categories that were related to central metabolism, such as “carbohydrate metabolism,” “glycolysis/gluconeogenesis,” “TCA cycle and mitochondrial electron transfer chain,” “cell wall,” and “nitrogen and amino acid metabolism.” This suggests that the reprogramming of central metabolism that occurs during early fruit growth is in large part under the control of transcription.
Integration of Metabolic Data into an Enzyme-Based Model of Fruit Setting.
The activities of 36 enzymes across the central metabolism during fruit set were quantitatively measured (SI Appendix, Fig. S10). In total, 18 showed higher activity in the fruit-growing ovaries when compared with unpollinated WT ovaries 4 DAA (Student’s t test, P < 0.05), indicating that fruit set eventually impacted the activities of many enzymes across central metabolism. Moreover, we identified genes whose transcript abundances were correlated with the corresponding protein abundances in the central metabolism pathways (SI Appendix, Fig. S7). For instance, transcript abundances of the fructokinase (FK; FRK1 and FRK2), ADP glucose pyrophosphorylase (AGPase) small submit 1 (AGPS1), fructose-6-phosphate 1-phosphotransferase (PFP; two PFP family members), fructose-bisphosphate (FBP)-aldolase (FBA7 and FBA8), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GAPC2), enolase (PGH1), and malic enzyme (ME; two ME family members), were significantly associated with their corresponding protein abundances (0.805 < Spearman correlation coefficient [SCC] < 0.874; P < 0.05). Significant correlations were also found between the protein abundances and enzyme activities in FRK1, FRK2, AGPS1, FBA7, FBA8, and an ME family member (0.762 < SCC < 0.881; P < 0.05; SI Appendix, Table S2). These results reinforce the idea that these pathways are strongly transcriptionally controlled during fruit set.
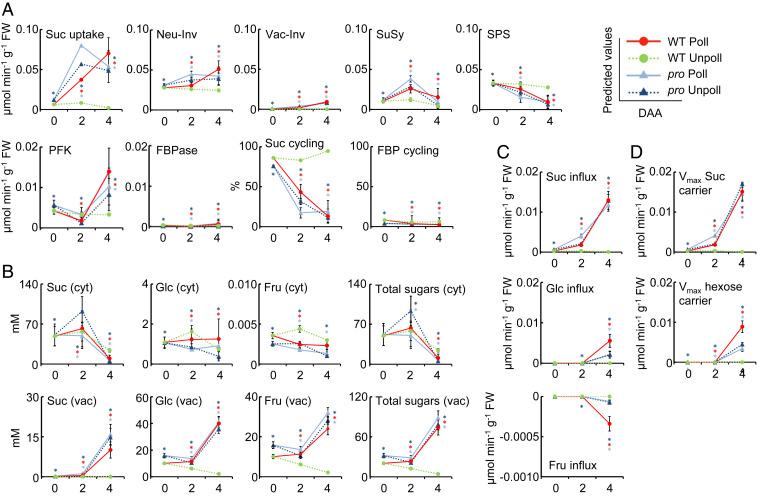
A kinetic model of sugar metabolism was constructed by integrating enzyme activity, subcellular compartmentation, and growth data, in which the carbon supply relied on a symplastic phloem unloading Suc (19, 36). We also integrated the absolute levels of seven major metabolites that were measured (SI Appendix, Fig. S11A). The parameterization of the core model was specific to each line (WT and pro), to each condition (with or without pollination), and to each time point (0, 2, and 4 DAA) (Fig. 1C and SI Appendix, Table S3). To optimize the model, we parameterized five unknown parameters (e.g., the Suc uptake flux [the capacity of the ovaries to import Suc] and the capacity of the tonoplastic sugar carriers) that were not experimentally obtained. The optimization process allowed us to determine the best values for these unknown parameters and allowed for the simulations to match the measurements (SI Appendix, Fig. S12 and Table S4). We found a parameter setting that enabled a good fit for the Suc, Glc, and Fru contents, the Suc-to-hexoses and Glc-to-Fru ratios (SI Appendix, Fig. S13), and, to some extent, the content of sugar phosphates (SI Appendix, Fig. S14). The effects of unknown parameters on the modeling results were determined by calculating their sensitivity coefficients, that showed how the model variables (fluxes and concentrations) were sensitive to parameterization. Among the five unknown parameters, the Suc uptake flux showed the highest coefficient, suggesting that it was the most important factor for model fitness, regardless of the genotype (SI Appendix, Fig. S15). The model was then cross-validated using a different dataset obtained from WT ovaries 4 DAA, both with and without pollination (SI Appendix, Table S5). Using this dataset and the five unknown parameters optimized with the first dataset (SI Appendix, Table S4), we found a reasonable consistency between the measured and simulated sugar contents with a linear correlation (P = 0.0097; SI Appendix, Fig. S16A). This correlation was further improved by optimizing the value of the Suc uptake flux (P = 0.0068; SI Appendix, Fig. S16B). This illustrated that the carbon input was the most influential parameter in the generic enzyme-based sugar model.
The Kinetic Model Predicted that Enzyme Flux Patterns Were Growth Dependent.
To compare the enzyme flux patterns, two-dimension hierarchical clustering was performed on the fluxes together with the relative growth rate (RGR) (SI Appendix, Table S3). On the first dimension, ovaries were classified in three clusters related to the ovary growth status—namely, nongrowing unpollinated WT and anthesis (0 DAA) ovaries (cluster I), those growing 2 DAA (cluster II), and ovaries 4 DAA (pollinated WT and un- or pollinated pro, cluster III) (SI Appendix, Fig. S17). On the second dimension, clustering categorized variables in two main clusters related to metabolic activity with respect to the ovary growth status. For instance, ovaries that were not growing or were in anthesis, were characterized by a low metabolic activity—i.e., low glycolysis (FBP-aldolase), low fluxes through kinases (FK, glucokinase [GK], phosphofructokinase [PFK]), and low sucrolytic activity (vacuolar acid invertase [Vac-Inv] and neutral invertase [Neu-Inv])—while high fluxes were through sucrose-phosphate synthase (SPS), resulting in a low hexose content and high Suc content (SI Appendix, Fig. S11A). By contrast, ovaries growing 4 DAA (cluster III), regardless of genotype, exhibited high metabolic activity—i.e., high glycolysis, high fluxes through kinases, and high sucrolytic activity while low SPS flux, which was associated with high hexose and low Suc contents. The ovaries growing 2 DAA exhibited an intermediate pattern with some variables being classified in the same cluster as those growing 4 DAA and those that were not growing or in anthesis (SI Appendix, Fig. S17). Overall, the clustering analysis of the measured and modeled metabolic data did not enable the separation of the WT and pro ovaries, on one side, and the pollinated and unpollinated pro, on the other. This strongly suggests that the metabolic rewiring that occurs during fruit setting is related more to ovary growth than to pollination or hormone status (Fig. 1).
Fruit Setting Shuts Down Suc Cycle and Induces Vacuolar Sugar Accumulation.
The kinetic model predicted that fruit setting of the pro ovary, both pollinated and not, and that of the pollinated WT, were associated with an increase in Suc uptake that was linked to growth initiation (Fig. 5A). In this model, imported Suc was subsequently cleaved, mainly in the cytosol, by Neu-Inv and to some extent by SuSy. However, before fruit setting, even though Suc uptake flux was low, SPS flux was high, thus making the so-called Suc cycle highly active—i.e., about 80% of the cleaved Suc was resynthesized 0 DAA, regardless of the genotype. It is worth noting that fruit setting drastically shut down this cycle, mainly by decreasing the SPS flux, in such a way that only 10 to 20% of the cleaved Suc was resynthesized 4 DAA. Fluxes of PFK—that catalyze the first step of glycolysis by the irreversible conversion of F6P to F1,6BP—were elevated, whereas those of the cytoplasmic fructose 1,6-bisphosphatase (FBPase)—that catalyzes the opposite direction of the carbon reaction through gluconeogenesis—were much lower in the fruit-setting ovaries (SI Appendix, Fig. S17). Thus, the FBP futile cycle was almost inactive (below 10%), regardless of the genotype and growth status (Fig. 5A).
Fig. 5.
Modeling of sugar metabolism in WT tomato and procera (pro) mutant ovaries during fruit set. Models were parameterized according to the data in SI Appendix, Table S3 and further optimized to fit the experimental data. (A) Fluxes of Suc uptake and Suc interconverting enzymes, expressed in µmol min−1 g−1 FW. The percentage of Suc cycling within the cytosol was calculated from the ratio between Suc synthesis and cleavage. The percentage of FBP cycling was calculated from the ratio between synthesis and hydrolysis. (B) Concentrations of each sugar within the cytosol (cyt) and the vacuole (vac), expressed in mM. The total sugar concentrations (Fru + Glc + Suc) in each compartment (cyt and vac) are also shown. (C and D) Sugar transport fluxes across the tonoplast (C) and transport capacities (Vmax) of the Suc and hexose carriers (D), expressed in µmol min−1 g−1 FW. The x axis represents DAA. Data are means ± SDs of the 200 best fits. Asterisks indicate significant differences from Student’s t tests (P < 0.05) between the WT and pro ovaries 0 DAA, and between unpollinated WT (dotted light green line) and fruit-growing ovaries (pollinated WT [continuous red line], unpollinated pro [dotted blue line], and pollinated pro [continuous sky-blue line]) ovaries 2 and 4 DAA. Neu-Inv, neutral invertase; Vac-Inv, vacuolar acid invertase; SuSy, sucrose synthase; SPS, sucrose-phosphate synthase; PFK, ATP phosphofructokinase; FBPase, fructose 1,6-bisphosphatase; Fru, fructose; Glc, glucose; Suc, sucrose; FBP, fructose 1,6-bisphosphate.
Concomitantly, fruit setting induced a reallocation of Suc within the ovary cells. In nongrowing and anthesis ovaries, Suc, in contrast to Glc and Fru, was predicted to be predominantly localized to the cytosol (Fig. 5B), thus promoting the cytosolic Suc cycle, as described above. Between 2 and 4 DAA, the concentration of Suc decreased in the cytosol and that of Glc, Fru, and to a lesser extent Suc, increased in the vacuole. The increased levels of hexoses in the vacuole were correlated with the flux of Vac-Inv (Fig. 5A). Summarizing the contribution of these soluble sugars in each compartment, the cytosolic sugar concentration followed a mirror-shaped evolution pattern compared to the vacuolar concentrations. Indeed, whereas most of the sugars were accumulated, mainly as Suc, in the cytosol of the ovaries 0 and 2 DAA, most of the Suc and hexoses were localized to the vacuole in the growing ovaries 4 DAA (Fig. 5B). Overall, this sugar compartmentalization builds up an osmotic potential difference across the tonoplast of about 60 to 80 mOsm 4 DAA (calculated as the difference between the total sugar concentration in the vacuole minus that in the cytosol). The question was then raised as to what the mechanisms and regulations of this sugar reallocation upon fruit setting were.
Sugar Reallocation Is Concomitant with Tonoplast Carrier Induction.
The modeling approach considered the activity of two proton-coupled tonoplastic carriers, one specific to Suc and the other to both Fru and Glc (hexose), and enabled the calculation of sugar transport fluxes across the tonoplast at a steady state (Fig. 5C). Whereas the sugar exchanges between the vacuole and cytosol were small in nongrowing ovaries, upon fruit setting, Suc, and to a lesser extent Glc, were actively imported into the vacuole. In contrast, Fru transport remained extremely low, regardless of the ovary growth stage. However, a slight efflux, indicated by negative influx values, occurred from the vacuole 4 DAA in the growing ovaries. Fig. 5D presents time courses of the vacuolar carrier’s activities, as predicted by the model. Upon fruit setting, the Vmax of both the Suc and hexose tonoplast carriers increased with time with each following a specific pattern, whereas the Suc carrier was up-regulated 2 and 4 DAA, and the hexose carrier was mainly up-regulated 4 DAA. The earlier up-regulation of the Suc carrier is possibly due to a high demand for Suc, for hexose production by Vac-Inv. In contrast, in nongrowing and anthesis ovaries, Vmax values of both the Suc and hexose tonoplast carriers were low and remained constant. The fact that similar profiles were noted for both the pollinated WT and pro and for the unpollinated pro, suggests that the underlying induction mechanisms may not be triggered by the pollination itself, but rather by growth initiation. Overall, these data suggest that tonoplast carriers are involved in the early metabolic events that cause sugars to accumulate in the vacuoles.
Vacuolar Sugar Accumulation Is Kinetically Controlled by Suc Transport.
To decipher the regulation of sugar accumulation in the vacuole during fruit setting, control coefficients were calculated according to the principles of metabolic control analysis (MCA) (37). Such a coefficient can determine how much (in terms of percentage) the vacuolar sugars would increase (positive sign) or decrease (negative sign) if a given parameter was increased by 1%. Most of the negative control was exerted by the RGR on the vacuolar sugars, thus illustrating the dilution effect of growth on sugar storage (SI Appendix, Fig. S18). Crucially, although the flux in the Vac-Inv and Vmax of the hexose carrier were both correlated with the hexose levels in the vacuole (Fig. 5 A and D), neither the Vac-Inv nor the hexose carrier exerted any significant control (SI Appendix, Fig. S18). Most of the positive controls were shared between the fluxes of Suc uptake and the capacity of the vacuoles to transport it (Suc carrier Vmax).
In nongrowing ovaries (unpollinated WT), the Suc carrier was the major controlling step. However, upon fruit setting, its control coefficient decreased for the pollinated WT and for the pro (pollinated or not) ovaries, whereas the control exerted by the Suc uptake increased concomitantly (SI Appendix, Fig. S18). The Fig. 5D and SI Appendix, Fig. S18 together, show that the lower control strength exerted by the vacuolar Suc carrier in the fruit-growing ovaries could be the consequence of the up-regulation of this carrier upon fruit setting. It is possible that the transcript abundances of the Suc carriers increased with fruit set, and this led to an increase in Suc entry into the vacuole, whereas saturated levels of transcripts or proteins of this carrier no longer acted as limiting factors for vacuolar sugar concentrations. Whether the transcript levels of some (putative) sugar carriers increased upon fruit setting was then considered. Despite the important role of the sugar carrier proteins on fruit set initiation, the transcript abundances of most of these genes did not exhibit good associations with fruit set (SI Appendix, Fig. S19), possibly because the transporter protein levels showed a relatively low correlation with the transcripts (SI Appendix, Fig. S9). However, in this framework, it is worth noting that such an adaptation of the carrier’s capacity to satisfy metabolic needs, would make the growing ovaries more sensitive to the changes in the symplastic Suc availability. This model prediction is in line with the high fruit abortion levels that are usually observed when carbon availability is decreased (e.g., when tomato plants are grown in shaded conditions) (17).
Induction of Fructokinase Controls the Fru Concentration and Glc-to-Fru Ratio within the Cytosol.
In plant cells, FK and GK activities are crucial for initiating cytosolic glycolysis by phosphorylating the hexoses that are either liberated by Neu-Inv or exported by the vacuole. To realistically model sugar metabolism, parametrization must consider the kinetic properties of FK and GK isozymes that are known to be specifically expressed in young tomato fruits (38, 39). Modeling the phosphorylation of Fru and Glu in a steady state showed that the FK and GK fluxes increased in parallel upon fruit setting (SI Appendix, Fig. S20 A and B), in correlation with the Suc uptake (Fig. 5A). However, the ratio between the FK and GK fluxes (∼1.2 to 2.0, depending on the ovary stage; SI Appendix, Fig. S20C) emphasizes a pivotal role for FK in feeding the downstream catabolic (glycolysis) and anabolic (carbohydrate synthesis—i.e., cell wall components and starch) pathways. FK has been shown to be associated, together with invertase, with starch synthesis in pollen (40). Indeed, we observed remarkable starch accumulation in the plastids of fruit-growing ovaries, but not in the unpollinated WT ovaries (SI Appendix, Fig. S11 A and C), suggesting rapid and intense carbon fluxes into the starch synthesis, that are in line with the fact that Suc metabolism acts as a source of starch accumulation and vice versa (41). Overall, the flux through FK, in contrast to that of GK, was found to be correlated with its Vmax (SI Appendix, Fig. S20D), though only a few percentage points of the catalytic capacity (at most 10%) were readily used for metabolic activity (i.e., the flux-to-Vmax ratio for FK varied between 0.05 and 0.1, SI Appendix, Fig. S20E). These data suggest that the bursts of metabolic activity that occur upon fruit setting were, at least in part, triggered by the induction of FK. One of the metabolic markers of such a regulation is the elevated Glc-to-Fru ratio that was both measured and predicted in the growing versus nongrowing ovaries (SI Appendix, Figs. S11B and S21).
The role of FK induction in this phenomenon has been further investigated by looking at the control coefficients exerted by this enzyme on the sugar concentrations and fluxes of enzymes and tonoplastic carriers. Regardless of the physiological state of the ovary, FK exerted a high level of control on the Glc-to-Fru ratio within the cytosol (SI Appendix, Fig. S22A), the cytosolic Fru concentrations, (SI Appendix, Fig. S22B), and, to some extent, the vacuolar Fru efflux via the Fru carrier (SI Appendix, Fig. S22 B and C). This suggests that the pivotal intermediate that is regulated by FK is the cytosolic Fru. This is in line with the fact that the affinity of FRK2 (the most abundant isozyme in tomato fruit-growing ovaries, SI Appendix, Fig. S7A) for Fru is usually very high, within the same range as the affinity of hexose kinases for Glc (39, 40).
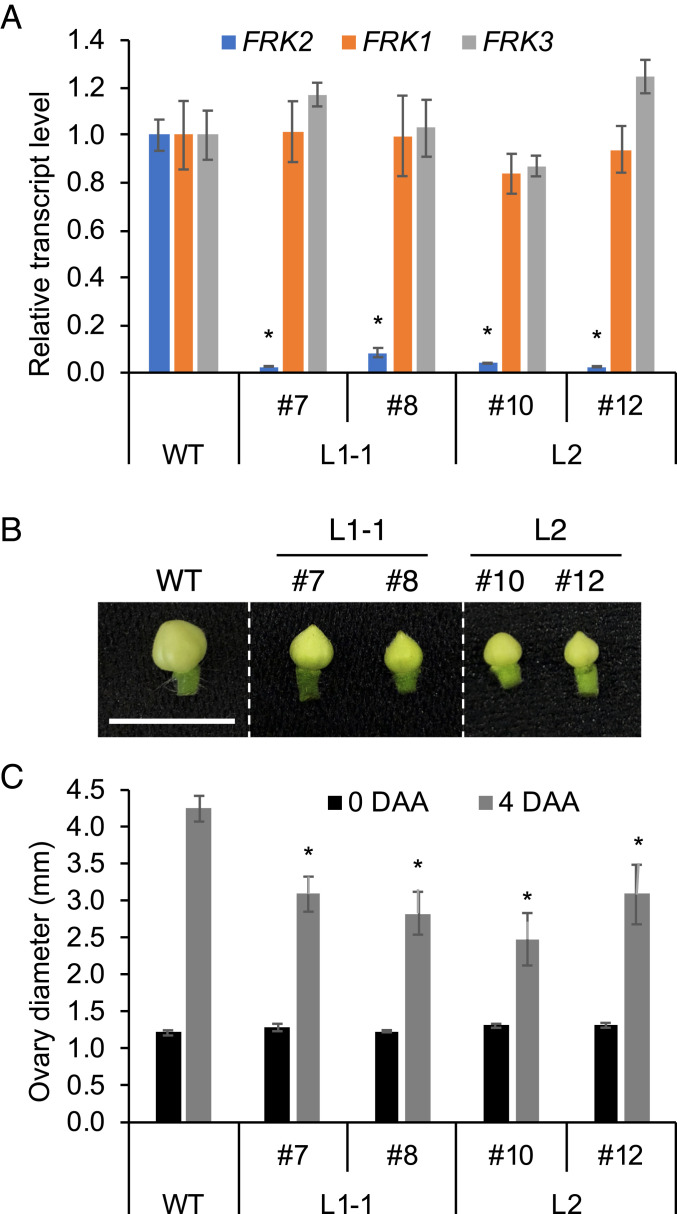
To confirm the pivotal role of FK during fruit set, the FRK2-silenced lines were generated by an RNA interference (RNAi) approach. Two independent lines (L1-1 and L2) were selected in which the FRK2 transcript level was significantly reduced compared to WT (although not for the other two isozymes, FRK1 and FRK3) (Fig. 6A). Then, two sibling plants derived from each line were obtained. A total of four plants (#7 and #8 for L1-1, and #10 and #12 for L2) were used for the measurement of ovary growth upon pollination. Although ovary sizes of WT and FRK2-silenced plants were equivalent at anthesis, the ovary size 4 DAA in the FRK2-silenced lines was significantly decreased by 27 to 42% when compared to the WT (Fig. 6 B and C). These results demonstrate the important role of FRK2 in ovary growth during fruit set.
Fig. 6.
Silencing of FRK2 suppressed ovary growth during fruit set. (A) Expression analysis of three FK isozyme genes in the leaves of four individual plants (#7, #8, #10, and #12) derived from two independent transformed lines (L1-1 and L2). CAC gene was used as a reference and the expression levels of FRK genes were normalized to those of the WT. Values are mean ± standard error of three independent leaves. (B) Representative pictures and (C) diameter of the ovaries at 4 DAA, which were pollinated at 0 DAA with WT pollen. Diameter of unpollinated ovaries at 0 DAA are also shown in C. Values in C are mean ± standard errors of six replicates. (Scale bar in B, 1 cm.) Asterisks in A and C indicate significant differences from the corresponding WT (Student’s t test, P < 0.05).
Discussion
Both Pollination-Dependent and -Independent Fruit Set Rewires the Central Metabolism Pathway with Increased GA Sensitivity.
The primary purpose of this research was to identify the fundamental biochemical networks and regulations of fruit set, using multiomics and kinetic modeling, and the GA hypersensitive mutant pro, as GA is believed to be the most epistatic hormone inducing fruit set (30). The integral analysis was able to define the transcripts, proteins, enzymes, and metabolites that were consistently impacted by both pollination and pro-induced parthenocarpy and highlighted that the central metabolism was consistently rewired (Figs. 2 and 3 and SI Appendix, Fig. S17). High metabolic activity was evidenced by increased enzyme activities (SI Appendix, Fig. S10) and fluxes (SI Appendix, Fig. S17) in carbohydrate metabolism, glycolysis, and the TCA cycle, and by the up-regulation of the associated central metabolites, including hexoses, hexose phosphates, and organic and amino acids (Figs. 3 and 7 and SI Appendix, Figs. S6 and S11, and Dataset S4). In particular, the early responsive MetM3 module (SI Appendix, Fig. S5) contained the product of FK, F6P, whose levels were associated with FK activity and flux (Fig. 3 and SI Appendix, Figs. S10 and S17). Furthermore, FK activity was correlated with the protein abundances of the two isoforms (FRK1 and FRK2), which were found in the MetM3-correlating transcript module GenM2 (Fig. 2, SI Appendix, Fig. S7A and Table S2, and Dataset S3), and with the corresponding transcript abundances (SI Appendix, Fig. S7A). These synchronous transcript, protein, enzyme, and metabolite fluctuations indicate tight and functional regulation of FK by the transcript levels, due to the initiation of fruit set, that was induced by GA signaling. Furthermore, FK showed a high catalytic capacity (SI Appendix, Fig. S10), that was also apparent from its low flux-to-Vmax ratio (SI Appendix, Fig. S20). Considering that high hexose phosphate levels coupled with low ATP-to-ADP ratios caused high fluxes in glycolysis (17), FK likely plays an important role in driving the metabolic rewiring that occurs in early phases of fruit setting (i.e., 2 DAA). Indeed, this study demonstrated that specific silencing of FRK2 was associated with the suppression of ovary growth (Fig. 6). Notably, transgenic tomato plants in which three FK isoform genes (FRK1, FRK2, and FRK3) were simultaneously silenced, had reduced plant biomasses and increased flower abortion (42), and transgenic tomato plants in which cell wall invertase was enhanced, had improved fruit set efficiency associated with increased FK activity, under long-term, moderate heat-stress conditions (43). Taken together with the synchronous regulation as described above, we propose that FK is at least partially important for inducing fruit set as a driving force to feed the downstream pathways for biosynthesis of cell wall components and energy provision.
Fig. 7.
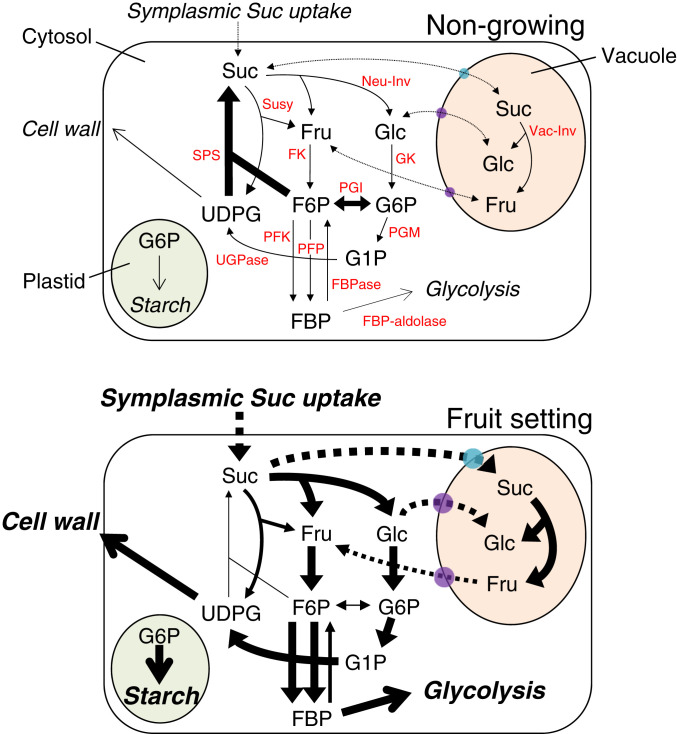
Model-assisted overview of the metabolic and compartment shifts of carbohydrates during tomato fruit set, as controlled by pollination and PROCERA. A fruit set model was constructed from the data for the enzyme fluxes and metabolite levels that were kinetically calculated, as shown in Fig. 5. Suc and hexose carriers are indicated with sky blue- and purple-colored circles, respectively. The enzyme fluxes and tonoplastic activities are correlated with the size of their circles, while the levels of metabolite accumulation are correlated with the size of their letter. Vacuole and plastid compartments are highlighted by orange and green, respectively. Arrows indicate reactions, while dashed arrows indicate translocations between the cytosol and plastid or vacuole. In nongrowing ovaries (i.e., at anthesis and unpollinated WT), the Suc cycle, as indicated by the SPS and SuSy fluxes, was highly active, leading to relatively high Suc content in the ovary. In the fruit-growing ovaries, significant accumulations of starch were observed with increased fluxes of Suc uptake via the symplast, Neu-Inv, FK, and GK at 2 DAA. Such fluxes were further activated 4 DAA, where fluxes of Vac-Inv, PGM, UGPase, PFK, cell wall synthesis, and glycolysis were also activated. Output fluxes are italicized. Neu-Inv, neutral invertase; Vac-Inv, vacuolar acid invertase; SuSy, sucrose synthase; SPS, sucrose-phosphate synthase; FK, fructokinase; GK, glucokinase; PGI, phosphoglucoisomerase; PGM, phosphoglycerate mutase; UGPase, UDP-glucose pyrophosphorylase; PFK, ATP phosphofructokinase; FBPase, fructose 1,6-bisphosphatase; Fru, fructose; Glc, glucose; Suc, sucrose; F6P, fructose-6-phosphate; G6P, glucose-6-phosphate; G1P, glucose-1-phosphate; UDPG, uracil-diphosphate glucose; FBP, fructose 1,6-bisphosphate.
The WGCNA identified that MetM1 and MetM2, both of which correlated with GenM2 or GenM3 and peaked 4 DAA (SN-1), contained hexose and many derivatives and intermediates of the glycolysis and TCA cycles (Figs. 2 and 3, SI Appendix, Fig. S5, and Dataset S4). This suggests that fruit set shifts the emphasis to the cleavage of Suc by 4 DAA, rapidly providing an energy source and building blocks for the production of biomass, such as the components of the cell wall that are critical for rapid growth and the development of sink organs (fruit-setting ovaries) (Fig. 7) (24, 25). This metabolic activation most likely allows for the rapid increases of ovary biomass between 2 and 4 DAA (Fig. 1C). Collectively, considering that bioactive GA levels also peaked 4 DAA and were correlated with ovary growth (Fig. 1 and SI Appendix, Fig. S1), it is suggested that levels of bioactive GAs or GA signaling pathways, determine sink capacities during fruit setting by transcriptionally elevating the subset of genes related to central metabolism (SI Appendix, Fig. S7).
Sugar Partitioning and Fluxes during Fruit Set.
Fruit growth is highly dependent on carbon availability from the phloem’s unloading of photosynthetic assimilates from source organs. In tomato, Suc unloading into the sink organ, followed by its degradation into hexoses, causes the generation of turgor pressure gradients between the sink and source organs, creating the osmotic potential for Suc unloading into the sink, and this is likely to be responsible for the degree of fruit growth (44). In this study, the kinetic model predicted the mode of sugar flux and the spatial and temporal distributions of sugars, as well as their metabolic regulation during fruit set (Figs. 5 and 7). In this model, Suc uptake into the ovary cytoplasm rapidly induced 2 DAA, and Suc was subsequently cleaved by the sugar interconversion enzymes Neu-Inv and SuSy (Fig. 5A). By 4 DAA, the resulting Glc and a large proportion of the unloaded Suc had been transported into the vacuoles via the tonoplastic carrier proteins, where Vac-Inv produces hexose, which increases the total sugar content in the vacuoles (Figs. 5B and 7). Together with the organic acids (such as malate) or amino acids, this may decrease the osmotic potential of the vacuole, triggering water flow into this compartment and contributing to cell expansion via vacuole expansion. Large water flow across tonoplast likely occurs between 4 and 10 DAA in tomato fruits, until the vacuole volume fraction reaches its maximal value—e.g., about 80% of the cell volume (19).
It is of note that, in contrast to Glc, the resulting Fru is deposited within the cytosol and is most likely directly used by the FK for the generation of glycolytic fluxes. Outflux of the vacuolar Fru to the cytosol is likely due to the high demand of Fru by FK and results in a high Glc-to-Fru ratio in the vacuoles of growing ovaries (Fig. 5 B and C and SI Appendix, Fig. S21). In contrast, the Suc biosynthesis through both Suc and FBP cycling appears to be nearly inactive in fruit-growing ovaries (Figs. 5A and 7). These observations suggest that Suc uptake by ovaries is highly dependent on phloem unloading and is maintained with the high fluxes of the temporal and spatial sugar partitioning, as well as the metabolic rewiring. In this context, the activation of metabolism during fruit setting is concomitant with the shutdown of the ATP-dissipating Suc cycle (45, 46), further indicating that energy production becomes a priority; this is consistent with the fact that the activities of the TCA cycle-related enzymes and metabolites were up-regulated in fruit-growing ovaries (Fig. 3 and SI Appendix, Figs. S10 and S11). The metabolic rewiring is most likely the key process of fruit set, which is in line with the evidence that the decline of the steady state of central metabolism impairs fruit set efficiency, or ovary growth (Fig. 6) (13, 14).
In summary, our results show that GA signaling induces a significant reprogramming of the central metabolism (i.e., metabolites in MetM1 to 3) via the expression of genes encoding pathway enzymes (i.e., genes in GenM1 to 3 and GenM7; Figs. 2–4 and SI Appendix, Fig. S7). Modeling Suc metabolism showed that this reprogramming leads to a strong increase in Suc uptake by 2 DAA (Fig. 5A), followed on one hand by the reallocation of sugars to the vacuole by 4 DAA (Fig. 5B), leading to decreases in the osmotic potential within this compartment, promoting vacuole growth at the expanse of the cytoplasm. However, on the other hand it leads to degradation that provides energy and building blocks for growth (Fig. 7). Fruit-setting ovaries thus shows high Suc metabolizing activities and maintain a low osmotic potential, while also maintaining a turgor pressure gradient against the source organs, which acts as a driving force for further phloem unloading. Therefore, GA-regulatory central metabolism most likely determines the sink capacity of fruit-setting ovaries. Such metabolic rewiring induced by the GA cascade is caused by depression of the PROCERA, at least in part through mediating the down-regulation of SlHB15A, a member of the negative module GenM1 (Figs. 2 and 4). Furthermore, the strong and early increase in FK capacity appeared as a key event counteracting sugar trapping in the vacuoles (Fig. 5 and SI Appendix, Figs. S15, S18, and S20), which appeared to be important for ovary growth during fruit set (Fig. 6).
The fact that GA sensitivity was already present in the pro mutant at anthesis indicates that a switch enabling fruit growth at this stage occurs upstream of the GA signaling. It will be interesting to investigate this switch in the future, for example, via mutagenesis of a genotype harboring the pro allele. Ultimately, a better understanding of fruit set metabolism will lead to new strategies for production, and in particular, the possibility of breeding for parthenocarpic fruits, and an increased control of fruit survival during the early stages of development.
Materials and Methods
Plant Materials, Growth Condition, and Experimental Procedures.
This study used the tomato variety Micro-Tom WT and the procera (pro) mutant of the Micro-Tom. Both plant types were grown in a photoperiod of 16 h light at 25 °C (light)/20 °C (dark), under fluorescent lights at 300 μmol m−2 s−1, with a nutrient solution (Ohtsuka house 1 and 2, OAT Agrio Co., Ltd) with an electrical conductivity (EC) of 1.6 dS m−1. The genome edited Slhb15a-ko, the transgenic FRK2-silenced plants, and WT used for their comparison were also grown under the same condition as described above.
The details of the experimental procedures used for cytology, hormone profiling, transcriptome, proteome, metabolome, enzyme analysis, network analysis, vector construction, kinetic modeling analysis, MALDI-MSI, and qRT-PCR, are shown in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI), grants 15KK0273 and 221S0002; the Program to Disseminate Tenure Tracking System; the Japan Society for the Promotion of Science (JSPS) bilateral program to T.A.; and grants from the JSPS to Y. Shinozaki. (16J00582 and 19K23672) and K.E. (16J00797). Y.G. and B.P.B. acknowledge Cédric Cassan for technical help and funding from PHENOME (ANR-11-INBS-012) and Fruit integrative modelling for a unified selection system (ANR-15-CE20-0009-01). We thank the National BioResource Project-Tomato for providing the tomato research material (TOMJPF0001). We also thank M. Okada, Y. Iida, Y. Fujimori, N. Inage, K. S. Miyamoto, R. Masuda, N. Ito, and K. Ito at the Gene Research Center and Y. Mitani at RIKEN for technical assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. Z.B.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011859117/-/DCSupplemental.
Data Availability.
Nucleotide sequence data have been deposited in DNA Data Bank of Japan (DDBJ) Sequence Read Archive (DRA010267). All study data are included in the article and supporting information.
References
- 1.Yamaguchi S., Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59, 225–251 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Sun T. P., Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol. 154, 567–570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueguchi-Tanaka M., Nakajima M., Motoyuki A., Matsuoka M., Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 58, 183–198 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Bassel G. W., Mullen R. T., Bewley J. D., procera is a putative DELLA mutant in tomato (Solanum lycopersicum): Effects on the seed and vegetative plant. J. Exp. Bot. 59, 585–593 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Livne S. et al., Uncovering DELLA-independent gibberellin responses by characterizing new tomato procera mutants. Plant Cell 27, 1579–1594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong M., Mariani C., Vriezen W. H., The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 60, 1523–1532 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Carrera E., Ruiz-Rivero O., Peres L. E., Atares A., Garcia-Martinez J. L., Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 160, 1581–1596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martí C. et al., Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 52, 865–876 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Hu J., Israeli A., Ori N., Sun T. P., The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 30, 1710–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrani J. C., Ruiz-Rivero O., Fos M., García-Martínez J. L., Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 56, 922–934 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Vriezen W. H., Feron R., Maretto F., Keijman J., Mariani C., Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 177, 60–76 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Wang H. et al., Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21, 1428–1452 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Aoust M. A., Yelle S., Nguyen-Quoc B., Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 11, 2407–2418 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanor M. I. et al., RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol. 150, 1204–1218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fos M. et al., Polyamine metabolism is altered in unpollinated parthenocarpic pat-2 tomato ovaries. Plant Physiol. 131, 359–366 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osorio S. et al., Systems biology of tomato fruit development: Combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 157, 405–425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biais B. et al., Remarkable reproducibility of enzyme activity profiles in tomato fruits grown under contrasting environments provides a roadmap for studies of fruit metabolism. Plant Physiol. 164, 1204–1221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belouah I. et al., Modeling protein destiny in developing fruit. Plant Physiol. 180, 1709–1724 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauvoit B. P. et al., Model-assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion. Plant Cell 26, 3224–3242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattison R. J. et al., Comprehensive tissue-specific transcriptome analysis reveals distinct regulatory programs during early tomato fruit development. Plant Physiol. 168, 1684–1701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olszewski N., Sun T. P., Gubler F., Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 14 (suppl.), S61–S80 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S. et al., Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci. Rep. 8, 2971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong M., Wolters-Arts M., Feron R., Mariani C., Vriezen W. H., The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 57, 160–170 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Langfelder P., Horvath S., WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tohge T., Watanabe M., Hoefgen R., Fernie A. R., Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 4, 62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernie A. R., Carrari F., Sweetlove L. J., Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 7, 254–261 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Kang K., Kim Y. S., Park S., Back K., Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol. 150, 1380–1393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L. et al., Comprehensive investigation of tobacco leaves during natural early senescence via multi-platform metabolomics analyses. Sci. Rep. 6, 37976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M. et al., Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 162, 1290–1310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinozaki Y. et al., Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 83, 237–251 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Shinozaki Y., Ezura H., Ariizumi T., The role of ethylene in the regulation of ovary senescence and fruit set in tomato (Solanum lycopersicum). Plant Signal. Behav. 13, e1146844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green K. A., Prigge M. J., Katzman R. B., Clark S. E., CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17, 691–704 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q. et al., Domain-specific expression of meristematic genes is defined by the LITTLE ZIPPER protein DTM in tomato. Commun. Biol. 2, 134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimatani Z. et al., Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 35, 441–443 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Thimm O., et al. , MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37, 914–939 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Damon S., Hewitt J., Nieder M., Bennett A. B., Sink metabolism in tomato fruit : II. Phloem unloading and sugar uptake. Plant Physiol. 87, 731–736 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuster S., Heinrich R., The definitions of metabolic control analysis revisited. Biosystems 27, 1–15 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Claeyssen E., Rivoal J., Isozymes of plant hexokinase: Occurrence, properties and functions. Phytochemistry 68, 709–731 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Kanayama Y. et al., Tomato fructokinases exhibit differential expression and substrate regulation. Plant Physiol. 117, 85–90 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granot D., David-Schwartz R., Kelly G., Hexose kinases and their role in sugar-sensing and plant development. Front. Plant Sci. 4, 44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streb S., Zeeman S. C., Starch metabolism in Arabidopsis. Arabidopsis Book 10, e0160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein O. et al., The tomato plastidic fructokinase SlFRK3 plays a role in xylem development. New Phytol. 209, 1484–1495 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Liu Y. H., Offler C. E., Ruan Y. L., Cell wall invertase promotes fruit set under heat stress by suppressing ROS-independent cell death. Plant Physiol. 172, 163–180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osorio S., Ruan Y. L., Fernie A. R., An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 5, 516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen-Quoc B., Foyer C. H., A role for “futile cycles” involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J. Exp. Bot. 52, 881–889 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Amthor J. S. et al., Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 31, 297–314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nucleotide sequence data have been deposited in DNA Data Bank of Japan (DDBJ) Sequence Read Archive (DRA010267). All study data are included in the article and supporting information.