Cell-based treatments of central nervous system (CNS) disorders are an emerging paradigm in experimental medicine. Given the anatomical and functional complexity of the mammalian brain, the tempting concept of simply replacing lost or critically affected brain tissue by stem cell transplants appeared difficult to realise. Therapeutic approaches now try to support endogenous regeneration and reorganisation, as well as to prevent secondary damage. This is achieved by paracrine and other so-called bystander effects. Preclinical research revealed a broad spectrum of such mechanisms for two of the most frequently investigated cell populations: mesenchymal stem cells (MSCs) and neural stem cells (NSCs). Although therapeutically relevant systemic effects of MSC and NSC transplantation have been identified, targeted delivery of cells to the brain tissue is considered important to achieve an optimal outcome in CNS disorders.
Clinical trials are subject to a number of logistical and medical requirements. These ensure maximum patient safety and the best possible standard of treatment, but cause significant design differences when compared to preclinical studies using stem cells [1]. For instance, cell populations are predominantly applied intravenously in clinics although more targeted approaches such as stereotaxic transplantation are often more effective in animal experiments. On the other hand, repeated stereotaxic cell administration to place stem cell deposits in the target area is not only logistically challenging, but may increase the risk of secondary brain damage in humans [2]. Alternative approaches such as intra-arterial (carotid) stem cell administration may achieve more targeted cell delivery to the brain while being combined with state-of-the-art recanalization approaches in stroke.
However, intra-carotid administration of relatively large cell populations such as MSCs bears the risk of secondary cerebral vessel occlusion and microlesions [3]. To overcome these limitations, stem cell populations with enhanced migratory abilities are required. These cells would be able to reach the target tissue in greater numbers after systemic or intranasal administration and would egress from blood vessels more swiftly. Two recent publications in this issue of EBioMedicine report significant progress in exactly that direction (Fig. 1).
Fig. 1.
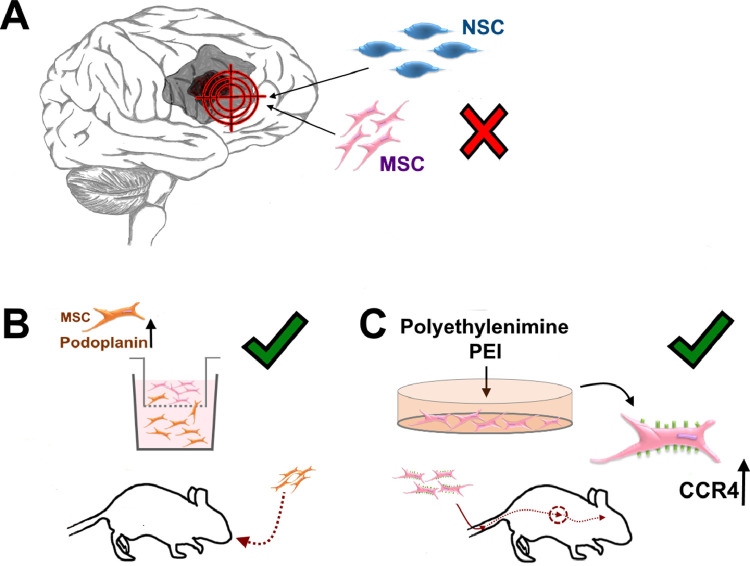
Mechanisms to increase stem cell migration abilities. The targeted delivery of neural stem cells (NSCs) and mesenchymal stem cells (MSCs) to damaged brain areas is a promising tool to improve post-lesional regeneration and reorganization, and to prevent secondary damage. (A) Current state of the art delivery of cell delivery through stereotaxic transplantation or intra-carotid administration can cause complications and bears the risk of additional brain damage. (B) MSC, modified to express enhanced levels of podoplanin (PDPN), showed significantly increased migration abilities. The MSCs migrated faster and over longer distances, which resulted in enhanced therapeutic abilities in an animal model of Alzheimer's disease after intranasal application. In a glioblastoma mouse model, MSCs also migrated more efficiently after stereotactic transplantation into the contralateral hemisphere (not shown) (C) Another promising approach is the pre-treatment of MSCs with polyethylenimine (PEI) that increased CCR4 surface expression and blocked adhesion receptors. This was shown to prevent pulmonary MSC filtering and increased cell homing to the brain without changing MSC viability or therapeutically relevant functions.
The first manuscript [4] describes the isolation of highly migrating NSC and MSC subpopulations from murine and human cell populations. These subpopulations (sNSC and sMSC, respectively) were able to migrate faster and over longer distances. Detailed transcriptome analyses of these populations revealed an increased expression of podoplanin (PDPN), a factor crucial for regulating stem cell migratory abilities, in the cells. The cells showed superior therapeutic abilities when applied in in vitro models of glioblastoma and an animal model of Alzheimer's disease. Importantly, superior migration abilities were induced by PDPN mRNA transfection allowing for transient modification of the cells. The second manuscript [5] reported that pre-treatment with the cationic molecule polyethylenimine (PEI) increases CCR4 expression on MSCs. Subsequent suppression of adhesion molecule receptors effectively prevented pulmonary filtering, a well-known phenomenon after intravenous delivery of large cell populations such as MSCs, and increased cell homing to the brain. MSC viability and therapeutically essential functions such as immunomodulation and differentiation potential remained unchanged. PEI-modified MSCs were successfully applied in animal models of cerebrovascular disorders, brain excitotoxicity and malignant CNS neoplasm. Those findings are highly relevant to both basic science and clinical medicine for the following reasons.
First, these approaches might be used to augment therapeutically relevant mechanisms of other adult cell populations such as mononuclear cells (MNCs) for which a direct cell-cell interaction in the therapeutically targeted brain tissue is required [6]. The concepts may also help to improve therapeutic application of other cell populations that rely on long-distance targeted migration within the brain such as glial-restricted progenitor cells being used to treat white matter damage. Bone marrow-derived MNC therapies for CNS disorders are already in early-stage clinical evaluation and upcoming, efficacy-oriented studies might benefit from these findings.
Second, the reported applications are well in line with current recommendations in the field of translational stem cell research. For instance, increased homing and vessel egress abilities increase comparability with recanalization approaches in stroke, a central requirement for future cytoprotective therapies in stroke [7]. In parallel, compatibility with cell labelling techniques based on ferromagnetic nanoparticles provide excellent options for non-invasive cell tracking in animal models and human patients that can be utilized for companion diagnostics and monitoring.
Third, described cell selection processes and PEI pre-treatment are easily applicable during cell production according to good manufacturing practice and may also inform the development of more specific, treatment-specific potency assays [8]. Moreover, the techniques reported by Danielyan, Schäfer and colleagues achieve similar results than previously described lentiviral approaches [9], but without the necessity for technically challenging or even permanent cell modification. These features may ultimately foster clinical translation of the approach.
Future research shall address some relevant open questions. For instance, it should be carefully analysed whether the increased migratory abilities are sufficient to increase migratory efficacy over the relatively long distances to or within the human brain. Large animal models, which are increasingly used in neurovascular and neurodegenerative research [10], may be helpful in this regard. Large animal brain size and anatomy are closer to the human one. Moreover, the larger volume and migration distances in the human brain present additional challenges for cell tracking approaches. Thus, research on more effective labelling and sensitive detection techniques for swiftly migrating cells are recommended. Finally, long-term investigations should confirm the excellent safety profile of transiently modified MSCs and NSCs.
In summary, the presented work represents a major step in the direction of practical implementation of stem cell therapies for CNS disorders in humans and may inform the development of functionally improved cell products for that purpose.
Declaration of Interests
The authors do not declare any conflict of interests.
Acknowledgements
No external funding was used for this work. We apologize to the numerous colleagues whose relevant work could not be cited due to space restrictions.
References
- 1.Cui LL, Golubczyk D, Tolppanen AM, Boltze J, Jolkkonen J. Cell therapy for ischemic stroke: are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann Neurol. 2019;86(1):5–16. doi: 10.1002/ana.25493. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 2.Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui LL, Wagner DC. The dark side of the force – constraints and complications of cell therapies for stroke. Front Neurol. 2015;6:155. doi: 10.3389/fneur.2015.00155. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11. doi: 10.1186/scrt544. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danielyan L, Schwab M, Siegel G, Brawek B, Garaschuk O, Asavapanumas N. Cell motility and migration as determinants of stem cell efficacy. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schäfer R, Schwab M, Siegel G, von Ameln-Mayerhofer A, Buadze M, Lourhmati A. Modulating endothelial adhesion and migration impacts stem cell therapies efficacy. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi-Taura A, Okinaka Y, Takeuchi Y, Ogawa Y, Maeda M, Kataoka Y. Bone marrow mononuclear cells activate angiogenesis via gap junction-mediated cell-cell interaction. Stroke. 2020;51(4):1279–1289. doi: 10.1161/STROKEAHA.119.028072. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 7.Savitz SI, Baron JC, Fisher M, STAIR X Consortium Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke. 2019;50(4):1026–1031. doi: 10.1161/STROKEAHA.118.023927. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 8.Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI. Stem cells as an emerging paradigm in stroke 4: advancing and accelerating preclinical research. Stroke. 2019;5090(11):3299–3306. doi: 10.1161/STROKEAHA.119.025436. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 9.Cui LL, Nitzsche F, Pryazhnikov E, Tibeykina M, Tolppanen L, Rytkönen J. Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017;48(10):2895–2900. doi: 10.1161/STROKEAHA.117.017809. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 10.Herrmann AM, Meckel S, Gounis MJ, Kringe L, Motschall E, Mülling C. Large animals in neurointerventional research: a systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations. J Cereb Blood Flow Metab. 2019;39(3):375–394. doi: 10.1177/0271678X19827446. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]