Abstract
Background
Mitochondrial succinate accumulation has been suggested as key event for ischemia reperfusion injury in mice. No specific data are however available on behavior of liver mitochondria during ex situ machine perfusion in clinical transplant models.
Methods
We investigated mitochondrial metabolism of isolated perfused rat livers before transplantation. Livers were exposed to warm and cold ischemia to simulate donation after circulatory death (DCD) and organ transport. Subsequently, livers were perfused with oxygenated Belzer-MPS for 1h, at hypothermic or normothermic conditions. Various experiments were performed with supplemented succinate and/or mitochondrial inhibitors. The perfusate, liver tissues, and isolated mitochondria were analyzed by mass-spectroscopy and fluorimetry. Additionally, rat DCD livers were transplanted after 1h hypothermic or normothermic oxygenated perfusion. In parallel, perfusate samples were analysed during HOPE-treatment of human DCD livers before transplantation.
Findings
Succinate exposure during rat liver perfusion triggered a dose-dependent release of mitochondrial Flavin-Mononucleotide (FMN) and NADH in perfusates under normothermic conditions. In contrast, perfusate FMN was 3-8 fold lower under hypothermic conditions, suggesting less mitochondrial injury during cold re-oxygenation compared to normothermic conditions. HOPE-treatment induced a mitochondrial reprogramming with uploading of the nucleotide pool and effective succinate metabolism. This resulted in a clear superiority after liver transplantation compared to normothermic perfusion. Finally, the degree of mitochondrial injury during HOPE of human DCD livers, quantified by perfusate FMN and NADH, was predictive for liver function.
Interpretation
Mitochondrial injury determines outcome of transplanted rodent and human livers. Hypothermic oxygenated perfusion improves mitochondrial function, and allows viability assessment of liver grafts before implantation.
Funding
detailed information can be found in Acknowledgments.
Keywords: FMN, Complex I, Hypothermic oxygenated perfusion, Normothermic oxygenated perfusion, Liver transplantation
Abbreviations: AMP, Adenosine monophosphate; ADP, Adenosine diphosphate; ALT, Alanine aminotransferase; AS, Anastomotic strictures; AST, Aspartate aminotransferase; ATP, adenosine triphosphate; CD-68, Cluster of differentiation 68; CS, cold storage; DAMP`s, Danger associated molecular pattern`s; DBD, Donation after brain death; DBQ, Decylubiquinone; DCD, Donation after circulatory death; D-HOPE, Dual hypothermic oxygenated perfusion; DWIT, Donor warm ischemia time; EAD, Early allograft dysfunction; ECD, Extended criteria donor; ECMO, Extracorporeal membrane oxygenation; FAD, Flavin adenine dinucleotide; FMN, flavin mononucleotide; GSH, Glutathione; HA, Hepatic artery; HAR, Hexaammineruthenium; HAT, Hepatic artery thrombosis; HMGB-1, High mobility group box-1 protein; HMP, Hypothermic machine perfusion; HOPE, Hypothermic oxygenated perfusion; H&E, Hematoxylin and eosin; IC, Ischemic cholangiopathy; ICAM-1, Intercellular adhesion molecule-1; ICU, Intensive care unit; IMP, Inosine monophosphate; KC`s, Kupffer cells; LDH, Lactate dehydrogenase; LT, Liver transplantation; MELD, Model of end stage liver disease; MPS, Machine perfusion solution; MPT pore, Mitochondria permeability transition pore; NAD/NADH, Nicotine adenine dinucleotide (oxidized/ reduced); NAS, Non-anastomotic strictures; NRP, Normothermic regional perfusion; NMP, Normothermic machine perfusion; OAA, Oxaloacetate; OLT, Orthotopic liver transplantation; PNF, Primary non function; PV, portal vein; RET, Reverse electron flow; ROS, Reactive oxygen species; SDH, Succinate dehydrogenase; SEC, Sinusoidal endothelial cells; TLR-4, Toll-like-receptor-4; 8-OHdG, 8-hydroxy-2-deoxy guanosine
Graphical Abstract
Research in Context.
Evidence before this study
Recent work has highlighted that succinate accumulation during ischemia is an inevitable part of every organ procurement and transplantation, causing relevant injury during ischemia-reperfusion by electron overflow at mitochondrial complex I. While this scenario is well defined for re-oxygenation under normothermic conditions, little is known on mitochondrial behavior during cold oxygenation. Here we uncover in a translational project a novel mechanism, by which oxygenated reperfusion in the cold leads to significantly less mitochondrial injury in livers, despite succinate provocation, when compared to normothermic conditions.
Added value of this study
First, we show in rodent livers, that the release of mitochondrial flavin mononucleotide (FMN) during reoxygenation after ischemia under normothermic conditions depends on mitochondrial succinate. Secondly, reperfusion (reoxygenation) under cold conditions (HOPE) induces also mitochondrial FMN release, dependent on succinate levels, but to a significant less degree. Third, HOPE-treatment (cold oxygenation) induces a metabolic switch in mitochondria through an improved enzymatic activity of complex I, II and IV. Such findings correlate with a decrease of lactate, succinate, and purine derivates in liver tissues after HOPE, and are further paralleled by an increase of mitochondrial ATP and a reduced NADH/NAD ratio. Fourth, HOPE treated livers disclose consecutively less oxidative injury and less downstream inflammation during subsequent transplantation. Finally, the extent of mitochondrial FMN and NADH release during HOPE in human livers, and the linked nucleotide breakdown as a consequence of mitochondrial function, is a signature for liver graft injury, and serves as a predictor for human liver graft function before implantation.
Implications of the available evidence
We report a novel mechanism of preventing liver ischemia reperfusion injury, which allows simultaneously monitoring of liver graft injury while restoring mitochondrial function. We expect a high clinical impact due to the urgent clinical need for safe utilization of injured livers.
Alt-text: Unlabelled box
Introduction
Current discoveries in the field of hepatic ischemia-reperfusion (I/R) point to mitochondrial-derived oxidative stress as key mechanism of tissue damage [1], [2], [3]. Optimization strategies for the treatment of ischemic organs may, therefore, depend on effective protection from mitochondrial injury. Ex situ perfusion of organs for transplantation is a new and promising strategy to modify and improve cellular conditions before implantation. Two main perfusion methods for livers and kidneys have been developed and are currently under discussion in clinical practice [4]. The first approach aims to simulate thein vivo conditions of organ perfusion, at normothermic temperatures with blood and nutrients at physiological perfusion pressure and flow [5]. In contrast, an alternative technique involves hypothermic organ perfusion (4–12°C), with a blood-free preservation solution at non-physiologic perfusion pressure and flows [6]. The effect of both perfusion techniques on mitochondrial recovery of ischemic livers is currently unknown.
Here, we unravel the role of the respiratory electron chain during ex situ perfusion of ischemic livers under cold and warm conditions. We used a standardized ex situ rat liver perfusion model with synchronous fluorometric assessment of newly discovered markers of mitochondrial injury. We performed a metabolic analysis of liver tissues, intact mitochondria and acellular perfusate to assess mitochondrial function.
Collectively, our data suggest that, unlike normothermic oxygenated perfusion, hypothermic oxygenated perfusion is protective against liver ischemia-reperfusion injury. This phenomenon is triggered by a unique response of mitochondria to ischemia-reperfusion under hypothermic conditions. We have translated our findings in the human setting of liver transplantation, and developed a technique to monitor liver function by a combination of two metabolic markers, both detectable during cold perfusion and enabling us to evaluate the quality of donor livers and predict graft implantation success already before transplantation.
Methods
I. Rat liver experiments
Animals
Male Brown Norway rats (250–320 g) were used for all rat experiments. Animals were maintained on laboratory diet and water according to the Swiss Animal Health Care law. All experiments were approved by the cantonal veterinary office Zurich (01/2012, 218/2014). Anesthesia during liver procurement was maintained with isoflurane. Additional rat liver transplant experiments were authorized by the Italian animal authority (Direzione generale della sanita animale e dei farmaci veterinary) according to the protocol No 90/2020-PR. Experiments were done in the laboratory linked to the animal facility of the University Hospital Zurich and the Centro di Stabulazione degli animali da laboratorio (Ce.S.A.L.), Firenze.
Study design and experimental groups
All experiments were carried out with a donation after circulatory death (DCD) animal model (30 min in situ warm ischemia) with subsequent cold ischemia for 4 h. Ex situ reperfusion was performed for 1h either normothermically (normothermic oxygenated perfusion, NT) at 37°C with Belzer MPS, or hypothermically (hypothermic oxygenated perfusion, HOPE) at 10°C with Belzer MPS. We added succinate every 10 min by repeated bolus injection, in the presence or absence of mitochondrial inhibitors during perfusion. We chose the following experimental groups with random assignment for NT or HOPE:
-
1.
Belzer MPS without succinate
-
2.
Belzer MPS + 2.5 mM succinate
-
3.
Belzer MPS + 25 mM succinate
-
4.
Belzer MPS + 250 mM succinate
Experiment 2 was repeated with either additional malonate (20 mM), rotenone (0.035 mM), or cyanide (20 mM) in the perfusate. Additional experiments were performed with adding ethanol (200 mM) in the presence or absence of 4-methylpyrazol (100 mM) during HOPE.
Additional experiments were also performed for NT with perfusion with diluted blood, and with NT performed after HOPE. In some experiments we performed also an intermittent perfusion stop (ischemia) for 10 min with subsequent reperfusion.
In a next step, we assessed metabolic and mitochondrial function in DCD rat livers before and after HOPE, without adding succinate during perfusion.
The following groups were investigated:
Baseline: fresh resected liver without warm ischemia and minimal (0.5 h) cold ischemia
DCD + CS: 30 min in situ warm ischemia + 4 h cold storage
DCD + CS + 1 h HOPE: 30 min in situ warm ischemia + 4 h cold storage + 1 h HOPE
All experimental groups consisted of at least 6 experiments per groups.
Liver procurement and cold storage
Following midline incision, cardiac arrest was induced by opening of the diaphragm without previous heparinization of donor animals. After 30 min of in situ warm ischemia, livers were flushed in situ with 10 ml cold heparinized Institute George Lopez (IGL)-1 solution via the portal vein. Livers were then excised (weight 9.8 ± 0.6 g) and placed in precooled IGL-1 solution at 4°C. Afterwards, livers tissues were either exposed to 4h cold storage alone (for tissue analysis and mitochondrial isolation) or to 4 h cold storage followed by ex situ perfusion under different conditions (see above). For machine perfusion, all livers received a stent in the portal vein.
Hypothermic oxygenated perfusion (HOPE)
Livers to be perfused at 10°C were connected to the precooled perfusion device after initial cold storage. Machine perfusion was maintained for 1 h under computerized pressure control (≤3 mmHg), resulting in flow rates between 1 and 2 ml/minutes. Active oxygenation was provided by a hollow fiber oxygenator (Hugo Sachs Electronic), delivering a perfusate pO2 of 60–80 kPa. We used 100 ml recirculating UW gluconate solution (Belzer MPS) as perfusate. The perfusion box and perfusate were both maintained at 10°C by an open bath thermostat (Huber, Germany).
Normothermic oxygenated perfusion
Livers to be perfused at 37°C were connected to the prewarmed perfusion device after initial cold storage. Machine perfusion was maintained for 1 h under computerized pressure control (12 mm Hg), resulting in flow rates between 15–18 ml/min. Active oxygenation was provided as in hypothermic perfusion experiments. We used 100 ml recirculating UW gluconate solution (Belzer MPS) or diluted blood as perfusate. The perfusion box and perfusate were both maintained at 37°C by an open bath thermostat (Huber, Germany).
Additional normothermic perfusion experiments were performed using diluted heparinized blood (hematocrit 14–16) instead of Belzer MPS. The perfusate was substituted with nutrients according to earlier reports [7].
Transplant experiments
We performed orthotopic liver transplantation in controls (non-ischemic livers, baseline), cold storage DCD livers (30 min in situ warm ischemia + 4 h cold storage), in HOPE treated DCD livers (30 min in situ warm ischemia + 4 h cold storage + 1 h HOPE), and in normothermically perfused DCD livers (30 min in situ warm ischemia + 4 h cold storage + 1 h normothermic perfusion with diluted blood). Liver transplantation was performed as previously reported [7]. Liver tissue and blood samples were taken after 24 h.
Endpoints
1. Quantification of FMN
Real time release of flavin mononucleotide (FMN) in the perfusate was determined by fluorescence spectroscopy. A light probe was connected to a halogen light source to emit monochrome light at a wavelength of 450 nm on the recirculating perfusate (Fig. 1A-B). A spectrometric receiver probe with sufficiently high resolution (e.g. 4.6 nm) was used to quantify the proportion of emitted fluorescent light by the FMN molecules. The fluorescence emission maximum of FMN was measured between 500–600 nm (Avaspec HS2048XL-EVO, Avantes).
Fig. 1.
Perfusion circuit and online fluorescence of FMN
The perfusion circuit used for all rat liver perfusions included a temperature control device, flow and pressure sensors, the perfusion chamber, where the liver was placed with another temperature sensor, the sampling port, an injection port for the application of succinate or mitochondrial blockers, and a flow cell at the outflow of the liver to quantify the fluorescence (a). Detection of the emitted light was quantified between 500–600 nm, while excitation was performed at a wavelength of 450 nm. Normo- or hypothermic oxygenated perfusion was performed with a computer adjusted pressure-controlled system to automatically limit perfusion pressure at 12 mm Hg (normothermic) or at 3 mm Hg (hypothermic), and to allow the liver to autoregulate the flow, which becomes subsequently detected by a flow sensor.
Real time response with an increasing fluorescence is demonstrated in accordance with succinate supplementation during normothermic perfusion, while under hypothermic oxygenated conditions, much lower perfusate fluorescence FMN release was detected despite succinate trigger (b).
Flavin mononucleotide (FMN) was also determined by a lab-based fluorescence assessment from perfusate samples. All samples containing blood cells were primarily centrifugated before assessment. Additionally, we confirmed these measurements by targeted liquid chromatography-mass spectroscopy (LC-MS). Results from different types of measurements (fluorescence spectroscopy and LC-MS were correlated for each sample. Fluorescence measurements were calibrated with standards to confirm increasing concentrations correlate with increasing positivity at the respective wavelength.
2. Quantification of NADH
Mitochondrial respiration rate during cold machine perfusion was measured by the amount of oxidized NADH (NAD+), determined spectroscopically and also by fluorescence in perfusates samples (excitation wavelength 360 nm, emission peak 460 nm). In addition, perfusate NAD+ and NADH values were determined by targeted Liquid Chromatography-mass spectroscopy (LC-MS). Results from different types of measurements (fluorescence spectroscopy and LC-MS were correlated. The spectroscope was calibrated with NADH standards to confirm increasing concentrations correlate with increasing positivity at the respective wavelength.
3. Quantification of 8-OHdG and HMGB-1 (in Perfusate)
Oxidative damage of DNA was detected in plasma by 8-hydroxy-2-deoxy Guanosine (8-OHdG) ELISA (Abnova; KA0444) and nuclear injury was measured by release of high mobility group box protein – 1 (HMGB-1; IBL International GmbH; ST51011).
4. Histology and Immunohistochemistry
The following staining procedures were performed after 1h perfusion in each experimental group: 8-OHdG (AA1005.1), Haematoxylin-Eosin (H&E) - staining for necrosis, Toll-Like-Receptor-4 –staining (TLR-4; LS-B2070, RRID:AB_1277478), CD 68 – staining (macrosialin; MCA341R, RRID:AB_2291300), ICAM (Santa Cruz, RRID:AB_631159). Histological assessments were determined by manual counting in 20 random visual fields (2.5x or 10x). All histological analyses were performed in a blinded fashion with respect to the experimental groups.
5. Metabolite extraction and profiling by targeted liquid chromatography-mass spectrometry (LC-MS/MS)
About 30 mg of N2 shock frozen liver tissues and 30 µl of perfusate samples (per experimental group and run) were used for metabolite profiling. Homogenization for liver tissues was performed with a FastPrep (60 s, 4 m x s−1) in a buffer containing 1.5 ml methanol and 0.1% ammonium acetate. The protein pellet gained after brief centrifugation was used in a bicinchoninic acid (BSA) based protein assay for normalization among samples. An internal standard (IS) mixture, containing chloramphenicol and C13-labeled L-glutamine, L-arginine, L-proline, L-valine, and uracil was added to each supernatant (10 µM final concentration).
For metabolite extraction in perfusates, proteins were precipitated by the addition of two volumes of acetonitrile, vortexing for 5 min and centrifugation (16.000) at 4C° for 10 min. Again, the protein pellets were used for normalization and an (IS) was added, as above. Metabolites in the supernatants were extracted with methyl tert-butyl ether (MTBE), methanol, and water [8].
Subsequent separation of the metabolites was performed on an LC instrument (1290 series UHPLC; Agilent, Santa Clara, CA), online coupled to a triple quadrupole hybrid ion trap mass spectrometer QTrap 6500 (Sciex, Foster City, CA), based on multiple reaction monitoring (MRM) method, as reported previously [9]. Three transitions per selected metabolite were measured.
Relative quantification was performed using MultiQuantTM software v.2.1.1 (Sciex, Foster City, CA). Peak integrations were reviewed manually and peak intensities were normalized, first against the internal standards, and subsequently against protein abundances obtained from the BCA assay. The first transition of each metabolite was used for relative quantification between samples.
Nicotine adenine dinucleotide (NAD), succinic acid, inosine, inosine monophosphate (IMP), adenosine monophosphate (AMP), adenosine diphosphate (ADP), adenosine trisphosphate (ATP), hypoxanthine, xanthine, fumaric acid, lactate, uric acid, and flavin mononucleotide (FMN) were determined by targeted LC-MS/MS.
6. Mitochondrial analysis, complex I, II, IV activity
Liver mitochondria membranes were isolated from fresh rat liver samples, e.g. DCD livers before and after HOPE or NT, immediately frozen in liquid nitrogen at -80°C. Healthy control livers, without exposure to ischemia, served as baseline. We performed, first, a metabolic analysis of isolated mitochondria, to determine purine, pyrimidine nucleotides and FMN in relation to mitochondrial succinate. Next, we investigated mitochondrial function of complex I, II, and IV as explained in detail in [24]. Complex I NADH-dependent activities were measured spectrophotometrically using Molecular Device plate reader (Varian Cary 4000 or plate-reader Molecular Devices) at 25°C as a decrease in absorption at 340 nm with 120 μM NADH in standard assay medium (0.25 M sucrose, 50 mM Tris-HCl pH 8.5, 0.2 mM EDTA, 15 μg/ml alamethicin, 2 mM MgCl2) and containing 15–20 μg protein/ml of membranes. Permeabilisation with alamethicin stimulates any NADH-dependent activities around 30%, indicating that our preparation contains 30% of still intact mitochondria membranes even after freezing/thawing cycle. In order to estimate relative content of Complex I, measurements of NADH: HAR oxidoreductase were carried out in the standard assay medium with 1 mM HAR. Rotenone sensitive (>95%) NADH:decylubiquionone reductase activity of Complex I alone was measured in standard assay medium supplemented with cyanide (2 mM) and 31 µM decylubiquinone (DBQ). Ferrocytochrome c oxidase activity of Complex IV was measured in assay medium (125 mM KCl, 14 mM NaCl, 20 mM HEPES, 0.2 mM EGTA pH 7.2) with 0.025% n-dodecyl β-d-maltoside (DDM) and 55 μM of reduced ferrocytochrome c [10]. The enzymatic activity was 100% sensitive to 0.5 mM cyanide. Succinate dehydrogenase activity was determined spectrophotometrically with Q1 (15 μM) as electron acceptor in standard medium supplemented with 20 mM succinate and 1 mM cyanide. All obtained mitochondrial preparations catalyze succinate oxidation (not shown) with a pronounced lag-phase with initial rate is 30–60% of the final linear steady-state rate (not shown). Prior to activity measurements membranes were incubated with malonate to replace OAA from the binding site (30 min × 30C).
II. Human liver perfusion
Perfusate samples were obtained during hypothermic oxygenated perfusion (HOPE) of human liver grafts donated after circulatory death (DCD). Following standard cold storage with IGL-1, livers underwent a pressure controlled hypothermic perfusion at 10 ° with highly oxygenated perfusate (PO2 60–80 kPa) for 1–2 h. Perfusion was performed at 10°C using Belzer MPS (Bridge to Life) as perfusate and the Liver Assist device (Organ Assist). During perfusion, multiple perfusate samples were obtained and analyzed by fluorimetry (perfusate 1:4 diluted) and LC-MS. The results were correlated to clinical parameters after liver transplantation, including graft survival. HOPE perfusates were also assessed for nicotine adenine dinucleotide (NAD), succinic acid, inosine, inosine monophosphate (IMP), hypoxanthine, xanthine, uric acid, N-acetyl-L-glutamic acid and flavin mononucleotide (FMN) through LC-MS.
Quantification and Statistical analysis
All activities were measured in duplicates or quadruplicates at 25°C. All activities are shown in μmol substrate/min/mg protein. All data are presented using the median and interquartile range (IQR) for continuous variables. The non-parametric Mann-Whitney U-test was used to determine whether significant differences existed between 2 groups. A p value of <0.05 was deemed statistically significant. Pearson's parametric correlation was used to assess the relationship between to metric variables.
Receiver-operating characteristic (ROC) curves were used to determine the threshold of highest sensitivity and specificity in human perfusate FMN samples for liver graft loss.
All data were analyzed using IBM® SPSS® v.24.0 (Armonk, NY: IBM Corp.) and GraphPad Prism, version 8.0 (San Diego, CA, USA).
Quality control and ethical approval
Completeness, plausibility, and validity of the data were independently verified (by A.Sc., X.M., A.St., M.M., P.M., A.G., D.M. and P.D.). The study approval from the cantonal ethics commission was obtained prior to study initiation (ZH-Nr. 2015-0200). Animal experiments were approved by the cantonal veterinary office Zurich (01/2012, 218/2014) and protocol No 90/2020-PR.
Role of the Funding source
We confirm that all funders played no role in study design, data collection, data analysis, interpretation, or writing of the report.
Results
Mitochondrial response during normothermic and hypothermic liver reperfusion
Oxygenation of ischemic tissue results in oxidative stress, generated by an electron leak at mitochondrial complex I [1,11]. The primary driver of such mitochondrial-derived reactive oxygen species (ROS) is an ischemic accumulation of the tricarboxylic acid (TCA) cycle metabolite succinate [3,[11], [12], [13]]. Data obtained from ischemic brain mitochondria have further suggested, that the flavin mononucleotide (FMN) site of complex I is responsible for the production of ROS via reverse electron transfer (RET), and that accumulation of succinate triggers release of FMN from mitochondrial complex I [2,14,15].
We intended, therefore, to investigate, if mitochondrial FMN release occurs also in livers during I/R, and how this phenomenon depends on temperature. We used an isolated perfused rat liver model, with circulating acellular buffer (Belzer Machine Perfusion Solution (MPS)), the currently most used machine perfusate for all abdominal organs [16,17]. We chose a clinical related model with livers injured by a combination of warm in situ ischemia, simulating donation after circulatory death (DCD), and subsequent cold storage, simulating conventional organ transport before transplantation. Furthermore, we added succinate to the circulating perfusate and analyzed succinate uptake in liver mitochondria at different temperatures, as well as related mitochondrial injury.
For this purpose, we applied a continuous real-time fluorimetry of the perfusate, using an excitation wavelength at 360 nm (NADH) and 450 nm (FMN), and emission spectra between 400 and 600 nm (Fig. 1, Supplemental Figure 1d). High levels of perfusate oxygen (pO2 60–80 kPa) compensated the lack of red blood cells [18], [19], [20].
Normothermic reoxygenation of DCD livers causes mitochondrial injury
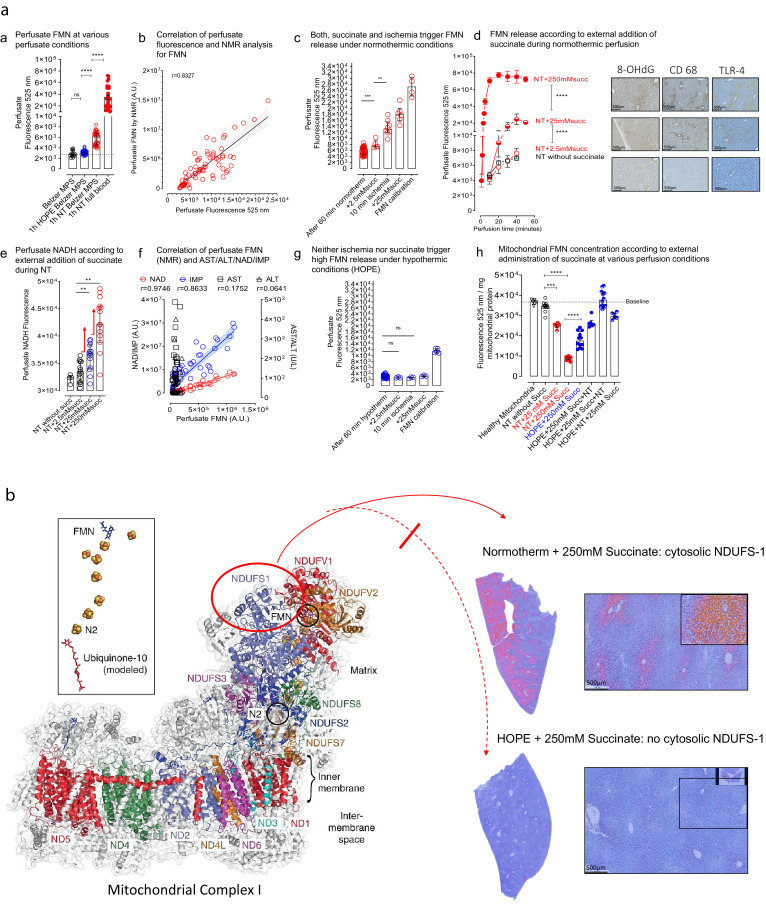
In a first step, we showed, that cumulative mitochondrial succinate uptake from perfusate was not different between hypo- and normothermic perfusion (Supplementary Fig. 1a). Due to limited cellular membrane permeability for succinate, however, mitochondrial succinate increase was approximately 200-fold less than added perfusate succinate (Supplementary Fig. 1b). Next, we demonstrated that acellular perfusion of rodent DCD livers at 37°C, without including mitochondrial substrates, resulted in a significant release of FMN in the perfusate (Fig. 2a), detectable by both, fluorimetry and NMR analysis (Rho = 0.8327) (Fig. 2b). Notably, perfusate FMN increased further under normothermic perfusion with blood instead of Belzer MPS (Fig. 2a). We observed also, that the presence of succinate in the circulating perfusate at normothermic (37°C) conditions, triggered a dose-dependent FMN increase (Fig. 2 c, d), similar as intermittent exposure of livers to 10 min ischemia (Fig. 2c, Supplementary Fig. 1d). Furthermore, perfusate FMN correlated well with perfusate NAD/NADH and IMP (Fig. 2e, f), but not with the release of cytosolic enzymes, e.g. AST and ALT (Fig. 2f), indicating the absence of cell rupture. In addition, mitochondrial FMN and nucleotide content were inversely related to perfusate values (Fig. 2h, Supplementary Fig. 1c, e, f), pointing to mitochondrial origin of FMN release.
Fig. 2.
a: Detection of mitochondrial FMN and metabolite release in perfusates: Release of FMN occurred at various perfusate conditions, including hypothermic or normothermic acellular perfusion, and by normothermic perfusion with whole blood, which resulted in highest FMN release (n = 20) (a). Perfusate FMN, detected by fluorescence, was validated by NMR analysis (Rho = 0.8327) (measured in all rodent samples n > 55) (b). Administration of succinate or intermittent ischemia/reperfusion triggered relevant FMN release during normothermic perfusion. (n = 6–7 for all groups other than: 60 min normotherm alone: n = 19 and n = 4 in FMN calibration) (c). Release of FMN increased in accordance with the amount of administered succinate (2.5 mM < 25 mM < 250 mM succinate; n = 6–7 for all groups) (d). Perfusate NADH quantification through spectroscopy demonstrated increasing concentrations according to administered succinate (n = 15 for all groups other than: NT without succinate, n = 5) (e). Perfusate FMN concentrations correlated also well with NAD (Rho = 0.9746) and IMP (Rho = 0.8633), in contrast to release of cytosolic liver enzymes (AST, ALT) (n = 50, human samples) (f). Neither succinate application or intermittent ischemia/reperfusion was found to trigger high FMN release during hypothermic oxygenated perfusion (n = 6 other than calibration group, n = 4 and end of 60 min hypotherm: n = 18) (g). Mitochondrial FMN concentrations were inversely correlated to perfusate FMN, with low intramitochondrial FMN after normothermic perfusion. In contrast, mitochondria were significantly protected from FMN loss during hypothermic oxygenated perfusion despite succinate application (n = 6–12) (h) (Mann-Whitney U-test was used for statistical analysis). Fig. 2b: Imaging of mitochondrial complex I injury: cytosolic staining of released NDUFS-1: With high liver injury, NDUFS-1, a subunit of complex I dissociates from complex 1 and is released into the cytosol. Liver tissues were stained for NDUFS-1 in different groups. Normothermic perfusion with 250 mM Succinate led to high injuries of the mitochondrial complex I as shown by a high number of NDUFS-1 positive hepatocytes in the staining, when compared to hypothermic perfusion, where almost no NDUFS-1-positive cytosols were seen.
Next, high perfusate succinate concentrations under normothermic conditions induced oxidative stress, assessed by an increased concentration of 8-OHdG and HMGB in the perfusate, and with downstream activation of Kupffer cells, visualized by specific immunostaining for 8-OHdG, and toll-like receptor-4 activation (TLR-4) (Fig. 3a–d).
Fig. 3.
Downstream inflammation under conditions with maximal succinate administration in perfusates: Histological images of various cellular compounds in livers perfused under normothermic or hypothermic conditions with exposure to perfusate succinate. Parenchymal and non-parenchymal liver cells were protected from oxidative injury and activation under hypothermic conditions. In contrast, reperfusion under normothermic conditions induced oxidative injury, toll like receptor- and Kupffer cell activation (n = 6 and 8, and 2 HPF/experiment) (a). These findings were paralleled by higher perfusate NADH concentrations (n = 15, duplicates) (b), higher oxidated DNA (n = 6 and 8) (C), and higher release of danger signals (HMGB-1) during normothermic perfusion, when compared to hypothermic conditions (n = 6) (d) (Mann-Whitney U-test was used for statistical analysis).
Third, we showed that succinate in the presence of malonate, a competitive inhibitor of the carboxylate binding site of complex II, increased once more NADH and FMN release (Fig. 4a, c), in contrast to inhibition of complex IV by cyanide or inhibition of complex I by rotenone (Fig. 4c). Succinate plus malonate resulted in increased purine salvage, detectable by high IMP simultaneously with low hypoxanthine and xanthine (Fig. 4e–h).
Fig. 4.
Assessment of mitochondrial function during normo- and hypothermic perfusion in the presence of mitochondrial inhibitors: Various mitochondrial inhibitors were administered during liver perfusion together with succinate. Inhibition of complex II by malonate triggered increase of perfusate NADH during normothermic (a) and during hypothermic conditions (b). However, complex II inhibition caused only under normothermic conditions relevant FMN release (c), in contrast to hypothermic perfusion (HOPE) (d). During HOPE, high FMN release occurred with a combination of complex I and complex II inhibitors, or by excess of NADH donors (d). Administration of increasing succinate, or administration of a combination of succinate and malonate, led to increasing NAD in perfusates under normothermic conditions (e). Succinate plus malonate resulted also in increased purine salvage, detectable by high IMP (f) simultaneously with low hypoxanthine and xanthine (n =8–10, single measurements or duplicates) (g, h) (Mann-Whitney U-test was used for statistical analysis).
Hypothermic reoxygenation of DCD livers is less harmful
In another set of experiments, we investigated whether FMN released from mitochondria occurred also during hypothermic oxygenated perfusion (HOPE) at 10°C. However, in contrast to the normothermic perfusion experiments above, we observed a 3 to 8-fold lower FMN release with and without succinate exposure (Fig. 2g, 4d). Consistently, the NADH content in the perfusate was significantly less, compared to normothermic conditions, despite an excess of succinate during HOPE (Fig. 3b, 4b), and mitochondrial concentrations of FMN remained high (Fig. 2h). In addition, downstream activation of Kupffer and endothelial cells were not detectable during HOPE (Fig. 3), indicating less overall inflammation. Of note, neither inhibition of complex I, II or complex IV resulted in increased FMN (Fig. 4d). Increased mitochondrial injury was further visualized by staining of released NDUFS-1, e.g. one of the core subunits of the N-module of complex I, which accommodate FMN and the iron clusters N1a, N3, N1b, N4, and N5. Cytosolic NDUFS-1 was detectable after normothermic perfusion with succinate in contrast to hypothermic perfusion (Fig. 2b).
Importantly, while succinate alone failed to provoke high release of FMN under hypothermic oxygenated conditions, inhibition of complex I and II together caused FMN release (Fig. 4d), as well as adding of NADH donors (ethanol). In contrast, adding ethanol in the presence of methylpyrazol, e.g. inhibiting the NADH producing ethanol dehydrogenase, prevented FMN release (Fig. 4d). Such experiments support the hypothesis, that FMN release is mainly induced in the cold by an increase of reduction state of complex I and increase of NADH/NAD+ ratio [2,21].
In summary, under conditions of normothermic reperfusion in the presence of high intra-mitochondrial succinate concentrations, complex I-bound FMN dissociates due to over-reduction of the enzyme. A reductive dissociation of FMNH2 would be followed by a rapid non-enzymatic oxidation to FMN by oxygen with ROS formation [22]. As a consequence, mitochondrial oxidative injury occurs under normothermic reperfusion conditions, and is further aggravated by malonate (Fig. 5a). In contrast, FMN release and oxidative stress decreases significantly under hypothermic reperfusion conditions, despite succinate provoke and despite the presence of malonate (Fig. 5b). Of note, the extent of mitochondrial FMN release during I/R is easily detectable in the perfusate during oxygenated liver perfusion, regardless of the perfusion temperature applied.
Fig. 5.
Mechanism of reoxygenation under normo- and hypothermic conditions: Under conditions of normothermic reperfusion in the presence of increased intra-mitochondrial succinate concentrations ①, complex I-bound FMN dissociates due to over-reduction of NADH dehydrogenase②. A reductive dissociation of FMNH2 would be followed by a rapid oxidation to FMN by oxygen with ROS formation③ and significant mitochondrial dysfunction, leading to ATP breakdown ④. As a consequence, mitochondrial oxidative injury occurs under normothermic reperfusion conditions, and is further aggravated by malonate (a). In contrast, FMN release and oxidative stress decreases significantly under hypothermic reperfusion conditions, despite succinate provoke and despite the presence of malonate (b).
Metabolic reprogramming of ischemic rat liver mitochondria by HOPE treatment
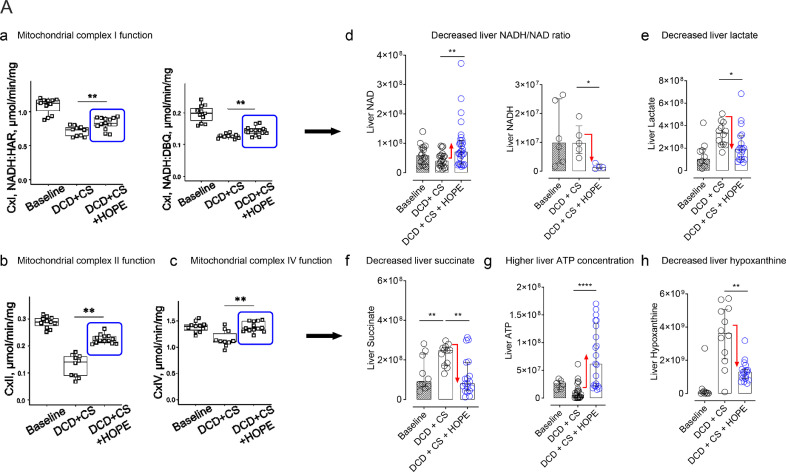
After demonstrating low mitochondrial injury during cold reoxygenation of rat DCD livers, we analyzed mitochondrial function in isolated mitochondria after cold storage, and after applying HOPE subsequently to cold storage. We found, first, a clear decrease in both NADH: hexaammineruthenium (HAR) reductase and NADH: decylubiquinone (DBQ) reductase activity of complex I in conventional stored DCD livers (Fig. 6a; a). In contrast, HOPE treatment of cold-stored DCD livers significantly improved the enzymatic activity of complex I (Fig. 6a; a). Secondly, HOPE also increased the activity of complex II and complex IV (Fig. 6a; b–c). These results correlated well with a significant decrease of lactate, succinate, and hypoxanthine levels in liver tissues by HOPE, as well as in an increase of liver ATP (Fig. 6a), and a significant decrease of the NADH/NAD ratio.
Fig. 6.
a: Mitochondrial complex I–IV function without and with hypothermic oxygenated perfusion (HOPE): Hypothermic oxygenated perfusion (HOPE), applied in DCD livers after cold ischemia, improved complex I, II and IV function (n = 11–12)(a, b, c). Subsequently, this resulted in decreased NADH/NAD tissue ratios (d), while lactate and succinate were metabolized (e, f), and liver ATP was uploaded (g) by purine salvage pathways (h). Fig. 6b: Mitochondrial reprogramming by hypothermic oxygenated perfusion (HOPE): Isolated mitochondria were analyzed by metabolite extraction and targeted liquid chromatography mass spectroscopy in four experimental groups, e.g. in normothermic perfusion (NT) of rat DCD livers, without or with high perfusate succinate (250 mM), and in HOPE treated rat DCD livers, with application of 250 mM succinate already in the HOPE perfusate, followed by normothermic perfusion. Despite the presence of high intramitochondrial succinate load during HOPE treatment, mitochondria maintained functioning with preserved FMN and NAD, metabolized succinate, and uploaded purine nucleotides, in contrast to untreated livers (n = 10–12, except NADH: n = 6) (Mann-Whitney U-test was used for statistical analysis). Fig. 6c: DCD liver injury & ATP 24 h after liver transplantation: Significantly lower liver injury was found after transplantation of HOPE treated livers compared to normothermic perfusion, as shown by liver transaminases, measured in the recipient 24 h after DCD liver transplantation (n = 5–7) (a). Such findings were paralleled by higher cellular energy after HOPE treatment, when compared to normothermic perfusion, measured through ATP content (n = 6–8) (b). The liver histology confirmed these findings with more signs of injury after normothermic perfusion and transplantation, compared to HOPE (n = 6, duplicates of 2HPF per experiment per group) (c) (Mann-Whitney U-test was used for statistical analysis).
We confirmed these results in additional experiments on a mitochondrial level, with application of high doses (250 mM) of perfusate succinate during HOPE, followed by normothermic reperfusion. HOPE treatment enabled mitochondria to completely fuel down succinate and restore nucleotides, while preventing FMN loss and downstream inflammation, in contrast to untreated livers (Fig. 6b).
We conclude, that HOPE treatment for one hour in rat DCD livers is sufficient to induce mitochondrial reprogramming towards more oxidized state and to upload the adenine nucleotide pool. This effect is caused by the improved function of mitochondrial respiratory complexes.
Liver transplant function improved after HOPE compared to normothermic oxygenated perfusion
To further confirm our findings, we analyzed liver function, liver tissue ATP and histology after transplantation of unperfused and perfused DCD rat livers (30 min warm in situ ischemia + 4 h cold storage), e.g. after cold storage alone, after endischemic normothermic oxygenated perfusion with diluted blood for 1 h, and after endischemic hypothermic oxygenated perfusion with Belzer MPS for 1 h. The results showed in accordance to earlier reports [7], that normothermic perfusion improved ATP and decreased hepatocellular injury compared to cold storage. HOPE treatment resulted however in further upload of ATP and best protection compared to cold storage or endischemic normothermic perfusion (Fig. 6c).
Flavin and NAD/NADH in liver perfusate serves as viability marker in human liver transplantation
Based on previous experimental research, we have been using cold oxygenated machine liver perfusion (HOPE) since 2012 in human extended criteria donor livers before transplantation, to optimize graft quality [16]. Currently, approximately 120 HOPE-treated human livers have been implanted in Zurich, with an overall 5-year graft survival, censored for tumor related recipient death, of more than 90% [16]. Within a translational aspect of the present study we analyzed the perfusate for FMN and metabolite release of fifty human livers, treated by HOPE before implantation. Corresponding to the experiments in rodent DCD livers above, we found an initial FMN release within the first 10–30 min of HOPE treatment in human livers (Fig. 7a), with significant differences comparing DCD and DBD grafts (Fig. 7b). In addition, the quantity of perfusate FMN during HOPE correlated well with graft function and clinical outcome after graft implantation. For example, perfusate analysis of livers, which developed irreversible graft injury, e.g. primary non-function (PNF) or irreversible cholangiopathy, revealed extremely high FMN values within the first 30 minutes of HOPE perfusion, which was therefore predictive for graft loss (c-statistic 0.94, Fig. 7c). A comparison of metabolites in perfusates of failed (n = 7) vs survived grafts (n = 43) showed 8-fold higher FMN values and 6-fold higher NAD values in grafts which were lost (Fig. 7d–f). All purine metabolites (IMP, inosine, xanthine, hypoxanthine, uric acid) were also significantly different.
Fig. 7.
Human perfusate analysis during HOPE: Human livers donated after circulatory death (DCD), or donated after brain death (DBD), underwent hypothermic oxygenated perfusion (HOPE) for 1–2h after cold storage prior to liver implantation. Perfusate fluorescence was measured during HOPE in 50 livers. Corresponding to experimental data in rodents, human livers released FMN during HOPE (a). The amount of perfusate FMN within the first 30 min correlated well with graft outcome after transplantation, with a discriminative threshold of 8 × 103 arbitrary units of perfusate FMN fluorescence, e.g. 100 ng FMN/ml circulating perfusate = 0.2 μg FMN release/g liver (3l perfusate, 1.5 kg liver). Accordingly, high FMN perfusate concentrations resulted in a very high percentage of graft loss after transplantation (6/9, 67%), in contrast to good outcome at low FMN perfusate values (1/41, 2.4 %) (a). FMN release during HOPE also discriminated highly between DCD and DBD livers (b), and FMN quantification at 30 min of hypothermic oxygenated perfusion revealed an excellent c-statistic (0.9441) by ROC curve analysis (c). Mitochondrial metabolites were further quantified in the HOPE perfusate of human livers. The highest fold increase, comparing lost vs survived grafts, was found for perfusate NAD and FMN (d). Predictive discrimination was achieved through either quantification of perfusate FMN within the first 30 min (area under curve, AUC 1-30 min) or for single perfusate FMN measurements at 30 min (n = 50 human livers) (e). NADH quantification at 30 min of HOPE treatment in human livers confirmed similar significant differences between lost and survived grafts after liver transplantation (7 grafts were lost, measurement done in triplicates) (f) (Mann-Whitney U-test was used for statistical analysis). Perfusate levels of human DCD livers with high injury (graft loss) were in the range of rat livers perfusions with high doses of succinate, underlining the clinical relevance of the model (g).
We conclude, that the extent of nucleotide breakdown as a consequence of mitochondrial dysfunction is a signature for graft injury, and predictive for human liver graft function. FMN and NAD/NADH release appear as novel perfusate markers for assessing mitochondrial metabolism and cumulative graft injury already during donor liver machine perfusion.
Discussion
The results of this study point to a uniform mechanism of mitochondrial-based liver I/R injury. First, we show that succinate accumulation and flavin mononucleotide (FMN) release from mitochondria are initial events during liver reperfusion, leading to a multifactorial injury including mitochondrial ROS release, DAMP signaling, and activation of non-parenchymal liver cells. Second, we demonstrate that exposure of mitochondria to a combination of succinate and oxygen leads to significantly less mitochondrial FMN dissociation under cold compared to normothermic conditions. Third, we show, that hypothermic oxygenation of mitochondria induces a metabolic reprogramming within one hour, with a significant decrease of succinate and uploading of the intracellular nucleotide content. Next, we translated our results to human and demonstrate that FMN release and NADH oxidation during HOPE serves as an easily detectable novel parameter to predict liver function prior to implantation.
Our data underline recent findings that accumulation and mitochondrial oxidation of succinate causes extensive damage in transplanted organs, and should therefore primarily be targeted by new therapeutic methods to improve outcome [23]. Mitochondrial complex I catalyzes the first step of the mitochondrial electron transfer in the respiratory chain. It contains 8 iron-sulfur clusters as well as non-covalently bound FMN, and is solely responsible for the oxidation of NADH, generated during catabolism (glycolysis, TCA cycle, etc.). It is also the major contributor to ATP synthesis in mitochondria [12]. Under physiologic conditions, FMN is tightly bound to the enzyme. However, FMN loss from complex I is possible when complex I is fully reduced, i.e. when the electron transport chain is halted during ischemia [15,16,24,25].
Recent reports have suggested, that mitochondrial RET can occur between complex II and I early upon reperfusion of ischemic tissue, due to ischemic accumulation of succinate [26], [27], [28]. In addition, adenine nucleotides are degraded during I/R, caused by progressive hydrolysis of ATP to AMP during ischemia, with a further breakdown by the purine nucleotide cycle [29,30], generating hypoxanthine and inosine. During normothermic reperfusion, the restoration of adenine nucleotide levels is delayed by approximately 45 min, due to the relatively slow rate of the purine salvage pathway, resulting in low levels of ADP during the early reperfusion phase [29,31,32]. Simultaneously, accumulated succinate is rapidly oxidized by complex II under conditions of ADP deficit, resulting in hyperpolarization of the membrane, and therefore supporting RET at complex I [33]. In steady-state conditions this leads to a complete reduction of the NAD+ pool and is also associated with the highest rate of ROS formation in the mitochondrial matrix [11,33].
As demonstrated in brain mitochondria, an over-reduction of complex I induce FMN dissociation from the enzyme, causing its inactivation [14,15,24]. A similar loss of mitochondrial FMN during ischemia has been described during cardiac I/R in a dog model [25]. Here we confirm, that the presence of succinate triggers significant mitochondrial FMN release and NADH accumulation in rat and human DCD livers during normothermic oxygenated perfusion, together with oxidative injury and downstream activation of toll-like receptors of non-parenchymal liver cells. However, FMN release was not preventable in our model by malonate and appeared therefore not RET-induced. We interpreted this as direct evidence for complex I damage with mitochondria operating preferably in Mode 1 according to Murphy [1], underlining an early oxidative injury of complex I, for example by modification of critical thiol residues (-SH) [34].
In contrast, oxygenation of liver tissue under hypothermic conditions protected mitochondria strongly from FMN release, despite excessive succinate load, while the addition of NADH donors provoked FMN release also during hypothermic oxygenation. In addition, the mitochondrial complex I and II activity even improved during 1 h HOPE.
Such remarkable differences in FMN release under hypothermic versus normothermic perfusion conditions is related to a unique mitochondrial response under cold conditions. A slow-down of the mitochondrial activity in the cold, e.g. by decreased proton motive force during hypothermic oxygenation after ischemia, or by delayed D-A transition of complex I may prevent over-reduction of FMN and subsequently lessen FMNH2 release. Based on this, the central protective mechanism of cold oxygenation after ischemia depend on effective oxidation of accumulated succinate while avoiding mitochondrial injury [35]. Likewise, protective effects of HOPE in rodent DCD hearts were clearly related to succinate metabolization [36].
Of note, a reversible downregulation of the electron transport has already been reported in all forms of mammalian hypometabolism during natural hibernation [37,38], as well as after pharmacological induced suspended animation [39]. We may speculate, that inducing a hypometabolic state before normothermic reperfusion is eventually related to an ancestral behavior of mitochondrial proteins in the cold, similarly to the protection from oxidative stress during arousal after torpor [38,40]. For example, arctic ground squirrels tolerate hypoxia during arousal without subsequent development of relevant ischemia-reperfusion injury. Likewise, during hibernation, arterial oxygen saturation is significantly higher compared to euthermia [41], and the ATP level is well preserved [42], similarly to hypothermic oxygenated perfusion [30,[43], [44], [45], [46], [47], [48], [49]]. These findings were paralleled by the determination of the key role of complex I-derived oxidative stress in aging comparing different species with a short or long life span.
A limitation of our model is the lack of a direct proof of FMN loss by mitochondrial complex I. However, based on the comparison of mitochondrial and perfusate FMN, which was inversely related, we indirectly concluded mitochondrial origin of FMN release. Notably, FMN sources in mammalian cells are limited, with only 6 out of 76 flavoproteins containing non-covalently bound FMN, which potentially can be released. Of those, only NADH dehydrogenase, e.g. complex I, is located intra-mitochondrial, thus underlining our hypothesis.
Another shortcoming is the use of un-physiologic high succinate concentrations in our perfusion model, e.g. 2.5, 25, 250 mM. However, perfusate succinate application was entirely pH-adjusted, and was used as a tool to induce a highly standardized increased level of mitochondrial succinate. Due to poor membrane permeability of succinate, the concentrations of perfusate succinate needed to be high enough for provoking more than 10 fold changes in mitochondrial succinate concentrations, which have been recently reported to occur under liver ischemia [3].
We believe that our results have a direct clinical impact because transferring organs into a hibernation-like status could be a novel protective concept [34,40]. This approach is however in contrast to an upfront normothermic liver perfusion strategy [50], [51], [52], [53], [54]. Our data demonstrate instead, that in situ and ex situ normothermic perfusion is likely to induce inflammatory pathways through mitochondrial-mediated injury, depending on the level of succinate accumulation, consistent with recent findings on ex situ normothermic perfusion of other solid organs, for example of ischemic hearts, lungs, and kidneys [55]. This injury occurs within the first few minutes of normothermic reperfusion and is unlikely to be completely preventable by inhibitors of RET. Instead, an effective upregulation of mitochondrial repair mechanism, as seen in vivo, would be required, implicating long-term normothermic perfusion strategies, e.g. perfusion for several days or weeks [56].
Importantly, mitochondrial injury can also occur during cold oxygenated perfusion, yet at much lower levels. The addition of mitochondria-targeted anti-oxidative scavengers to the machine perfusate would, therefore, be a further topic of research [57]. The unique figure of mitochondrial response at hypothermic temperatures allows, however, fueling of accumulated succinate during cold oxygenated perfusion, without significant injury to complex I. Simultaneously, the cellular ATP pool is uploaded by continuous fueling proton pumps and ATP synthase. Liver cells are therefore recharged with nucleotides without significant injury. Importantly, eventual complex I injury, as displayed by FMN- and NADH release, during hypothermic oxygenated perfusion can be quantified before using the graft for transplantation. This measurement is elegantly performed in a real-time fashion from perfusates obtained during machine liver perfusion. Remarkably, such a concept originates from an earlier publication 50 years ago on the fluorometric assessment of mitochondrial redox changes [58].
In summary, we demonstrate, that perfusion of the ischemic liver with a hypothermic oxygenated solution (HOPE) prevents the impairment of the mitochondrial electron transport chain and protects from subsequent reperfusion injury. This result is in stark contrast to reperfusion under normothermic conditions. Short-term, cold oxygenation for mitochondrial conditioning may, therefore, be considered for ischemic solid human organs prior to ex situ normothermic perfusion or implantation.
Contributors
Conceptualization, A.Sc. and P.D; Methodology, A.Sc., X.M., A.St., M.M., A.G, D.M, and P.D.; Investigation, A.Sc., X.M., A.St., M.M., P.K., O.dR., P.M., A.G., D.M. and P.D.; Writing – Original Draft, A.Sc. and P.D.; Writing – Review & Editing, A.Sc., X.M., M.M., P.M., A.G., D.M. and P.D.; Funding Acquisition, A.Sc., A.G., D.M. and P.D.; Resources, P.M., A.G., D.M., and P.D.; Supervision, P.M., A.G. and P.D. All authors read and approved the final version of the manuscript.
Declaration of Interests
This study was conducted at the University Hospital Zurich (USZ). Raw data and laboratory analysis results were extracted at the laboratory of the Department of Visceral Surgery and Transplantation of the USZ. Data analysis was carried out at the laboratory of the Department of Visceral Surgery and Transplantation of the USZ. The authors have declared that no competing interests exist. The study was principally funded by a main research grant from the swiss national foundation (SNF), awarded to Professor Philipp Dutkowski (Ref: 32003B-140776/1, 3200B-153012/1, 31IC30-166909). Mitochondrial and tissue analysis was further supported by the Max Planck Society (Dr. David Meierhofer) and the NIH grant: R01NS112381 awarded to Dr. Alexander Galkin. There are no patents involved or affecting this study.
Acknowledgments
Acknowledgments
Funding was provided by the Swiss National Science Foundation grant no 32003B-140776/1, 3200B-153012/1, 320030-189055/1, and 31IC30-166909 to P.D. This work was further supported by the Max Planck Society to D.M. and the NIH grant: 1R01NS112381 to A.G. We thank Beata Lukaszewska-McGreal for metabolomic sample preparation.
Data Sharing Statement
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, figure 7, and supplemental material).
What other documents will be available? None available.
When will data be available (start and end dates)? Beginning 1 year after publication until 3 years.
With whom? Investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose.
For what types of analyses? To achieve aims in the approved proposal.
By what mechanism will data be made available? Proposals may be submitted up to 36 months following article publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.103014.
Appendix. Supplementary materials
References
- 1.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J [Internet] 2009;417(1):1–13. doi: 10.1042/BJ20081386. http://www.ncbi.nlm.nih.gov/pubmed/19061483%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2605959%5Cnhttp://biochemj.org/lookup/doi/10.1042/BJ20081386 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim M, Stepanova A, Niatsetskaya Z, Sosunov S, Arndt S, Murphy MP. Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature [Internet] 2014;515(V):431–435. doi: 10.1038/nature13909. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Raemdonck D, Neyrinck A, Rega F, Devos T, Pirenne J. Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transpl. 2013;18(1):24–33. doi: 10.1097/MOT.0b013e32835c494f. [DOI] [PubMed] [Google Scholar]
- 5.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL. A randomized trial of normothermic preservation in liver transplantation. Nature [Internet] 2018 doi: 10.1038/s41586-018-0047-9. http://www.nature.com/articles/s41586-018-0047-9 Available from: [DOI] [PubMed] [Google Scholar]
- 6.Schlegel A, Rougemont O De, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013 Feb;58(2):278–286. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel A, Kron P, Graf R, Dutkowski P, Clavien PA. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61(6):1267–1275. doi: 10.1016/j.jhep.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Matyash V, Liebisch G, Kurzchalia T V, Shevchenko A, Schwudke D. Lipid extraction by methyl-terf-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008 doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gielisch I, Meierhofer D. Metabolome and proteome profiling of complex i deficiency induced by rotenone. J Proteome Res. 2015 doi: 10.1021/pr500894v. [DOI] [PubMed] [Google Scholar]
- 10.Vanneste W, Ysebaert-Vanneste M, Mason H. The decline of molecular activity of cytochrome oxidase during purification. J Biol Chem. 1974 Dec 10;249(23):7390–7401. [PubMed] [Google Scholar]
- 11.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabolism. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Dröse S, Brandt U, Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. et Biophys. Acta - Proteins Proteom. 2014;1844:1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Siebels I, Dröse S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta - Bioenerg. 2013;1827(10):1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Stepanova A, Kahl A, Konrad C, Ten V, Starkov AS, Galkin A. Reverse electron transfer results in a loss of flavin from mitochondrial complex I: Potential mechanism for brain ischemia reperfusion injury. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17730242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepanova A, Sosunov S, Niatsetskaya Z, Konrad C, Starkov A, Manfredi G. Redox-dependent loss of flavin by mitochondrial complex i in brain ischemia/reperfusion injury. Antioxid Redox Signal. 2019 Sep 20;31(9):608–622. doi: 10.1089/ars.2018.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlegel AA, Muller X, Kalisvaart M, Muellhaupt B, Perera M, Isaac J. Outcomes of liver transplantations from donation after circulatory death (DCD) treated by hypothermic oxygenated perfusion (HOPE) before implantation. J Hepatol. 2019;70(1):50–57. doi: 10.1016/j.jhep.2018.10.005. Jan. [DOI] [PubMed] [Google Scholar]
- 17.Jochmans I, Akhtar MZ, Nasralla D, Kocabayoglu P, Boffa C, Kaisar M. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant. 2016;16(9):2545–2555. doi: 10.1111/ajt.13778. [DOI] [PubMed] [Google Scholar]
- 18.Lüer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23(9):944–950. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoyer DP, Gallinat A, Swoboda S, Wohlschlaeger J, Rauen U, Paul A. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation. 2014;98(9):944–950. doi: 10.1097/TP.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 20.Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol [Internet] 2016;10(7):841–859. doi: 10.1586/17474124.2016.1149062. http://www.scopus.com/inward/record.url?eid=2-s2.0-84961203071&partnerID=tZOtx3y1 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Pell VR, Chouchani ET, Murphy MP, Brookes PS, Krieg T. Moving forwards by blocking back-flow the yin and yang of MI therapy. Circul Res. 2016;118:898–906. doi: 10.1161/CIRCRESAHA.115.306569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994 [PubMed] [Google Scholar]
- 23.Martin J, Costa A, Gruszczyk A, Beach T, Allen F, Prag H. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat Metab. 2019;1(10):966–974. doi: 10.1038/s42255-019-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahl A, Stepanova A, Konrad C, Anderson C, Manfredi G, Zhou P. Critical role of flavin and glutathione in complex i-mediated bioenergetic failure in brain ischemia/reperfusion injury. Stroke. 2018 doi: 10.1161/STROKEAHA.117.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouslin W, Ranganathan S. Impaired function of mitochondrial electron transfer complex I in canine myocardial ischemia: Loss of flavin mononucleotide. J Mol Cell Cardiol. 1983 doi: 10.1016/0022-2828(83)90329-2. [DOI] [PubMed] [Google Scholar]
- 26.Grover SD, Wedding RT. Kinetic ramifications of the association-dissociation behavior of NAD malic enzyme : a possible regulatory mechanism. Plant Physiol. 1982 doi: 10.1104/pp.70.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison DK, Fasching M, Fontana-Ayoub M, Gnaiger E. Cytochrome redox states and respiratory control in mouse and beef heart mitochondria at steady-state levels of hypoxia. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00146.2015. [DOI] [PubMed] [Google Scholar]
- 28.Kinugasa H, Whelan KA, Tanaka K, Natsuizaka M, Long A, Guo A. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene. 2015 doi: 10.1038/onc.2014.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinugasa Y, Ogino K, Furuse Y, Shiomi T, Tsutsui H, Yamamoto T. Allopurinol improves cardiac dysfunction after ischemia-reperfusion via reduction of oxidative stress in isolated perfused rat hearts. Circ J. 2003 doi: 10.1253/circj.67.781. [DOI] [PubMed] [Google Scholar]
- 30.Harrison GJ, Willis RJ, Headrick JP. Extracellular adenosine levels and cellular energy metabolism in ischemically preconditioned rat heart. Cardiovasc Res. 1998 doi: 10.1016/s0008-6363(98)00123-0. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay JG. ATP-induced oxidation of the a32+CO compound in pigeon heart mitochondria. Arch Biochem Biophys. 1974 doi: 10.1016/0003-9861(74)90532-3. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay JG, Wilson DF. Apparent Adenosine Triphosphate Induced Ligand Change in Cytochrome as of Pigeon Heart Mitochondria. Biochemistry. 1972 doi: 10.1021/bi00774a031. [DOI] [PubMed] [Google Scholar]
- 33.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature [Internet] 2014;515(7527):431–435. doi: 10.1038/nature13909. http://www.nature.com/doifinder/10.1038/nature13909 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jani A, Epperson E, Martin J, Pacic A, Ljubanovic D, Martin SL. Renal protection from prolonged cold ischemia and warm reperfusion in hibernating squirrels. Transplantation. 2011 doi: 10.1097/TP.0b013e3182366401. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann J, Otarashvili G, Meszaros A, Ebner S, Weissenbacher A, Cardini B. Restoring mitochondrial function while avoiding redox stress: The key to preventing ischemia/reperfusion injury in machine perfused liver grafts? Int J Mol Sci. 2020 doi: 10.3390/ijms21093132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyss R, Méndez Carmona N, Arnold M, Segiser A, Mueller M, Dutkowski P. Hypothermic, oxygenated perfusion (HOPE) provides cardioprotection via succinate oxidation prior to normothermic perfusion in a rat model of donation after circulatory death (DCD) Am J Transpl. 2020 doi: 10.1111/ajt.16258. Aug 12. [DOI] [PubMed] [Google Scholar]
- 37.Mathers KE, McFarlane S V, Zhao L, Staples JF. Regulation of mitochondrial metabolism during hibernation by reversible suppression of electron transport system enzymes. J Comp Physiol B Biochem Syst Environ Physiol. 2017 doi: 10.1007/s00360-016-1022-0. [DOI] [PubMed] [Google Scholar]
- 38.Staples JF. Metabolic suppression in mammalian hibernation: the role of mitochondria. J Exp Biol. 2014 doi: 10.1242/jeb.092973. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann C, Nussbaum B, Calzia E, Radermacher P, Wepler M. Gaseous mediators and mitochondrial function: The future of pharmacologically induced suspended animation? Front Physiol. 2017 doi: 10.3389/fphys.2017.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol [Internet] 2005;288(3):G473–G480. doi: 10.1152/ajpgi.00223.2004. http://www.ncbi.nlm.nih.gov/pubmed/15701622 Available from: [DOI] [PubMed] [Google Scholar]
- 41.Quintin J, Ma Q, Zhang Z, Barnes BM, Podgoreanu M V. Organ protective mechanisms common to extremes of physiology: a window through hibernation biology. Integr Comparat Biol. 2014 doi: 10.1093/icb/icu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown JCL, Staples JF. Substrate-specific changes in mitochondrial respiration in skeletal and cardiac muscle of hibernating thirteen-lined ground squirrels. J Comp Physiol B Biochem Syst Environ Physiol. 2014 doi: 10.1007/s00360-013-0799-3. [DOI] [PubMed] [Google Scholar]
- 43.Burlage L, Hessels L, van Rijn R, Matton A, Fujiyoshi M, van den Berg A. Opposite acute potassium and sodium shifts during transplantation of hypothermic machine perfused donor livers. Am J Transpl. 2018 doi: 10.1111/ajt.15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stegemann J, Minor T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology. 2009;58(3):331–336. doi: 10.1016/j.cryobiol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Boteon Y, Laing R, Schlegel A, Wallace L, Smith A, Attard J. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl. 2018 doi: 10.1002/lt.25315. Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vries Y, Matton A, Nijsten M, Werner M, van den Berg A, de Boer M. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transpl. 2018 doi: 10.1111/ajt.15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S, Lee SH, Korneszczuk K, Culberson CR, Southard JH, Berthiaume F. Improved preservation of warm ischemic livers by hypothermic machine perfusion with supplemented University of Wisconsin solution. J Invest Surg [Internet] 2008;21(2):83–91. doi: 10.1080/08941930701883657. http://www.ncbi.nlm.nih.gov/pubmed/18340625 Available from: [DOI] [PubMed] [Google Scholar]
- 48.Westerkamp A, Karimian N, Matton A, Mahboub P, van Rijn R, Wiersema-Buist J. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825–835. doi: 10.1097/TP.0000000000001081. Apr. [DOI] [PubMed] [Google Scholar]
- 49.Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013 Nov;59(5):984–991. doi: 10.1016/j.jhep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Jassem W, Xystrakis E, Ghnewa Y, Yuksel M, Pop O, Martinez-Llordella M. Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology. 2018 doi: 10.1002/hep.30475. [DOI] [PubMed] [Google Scholar]
- 51.Quintini C, Liu Q. Disrupting the field of organ preservation: normothermic preservation in liver transplantation. Transplantation. 2018;102(11):1783–1785. doi: 10.1097/TP.0000000000002410. Nov. [DOI] [PubMed] [Google Scholar]
- 52.Schneeberger S. Life of a liver awaiting transplantation. Nature. 2018;557(7703):40–41. doi: 10.1038/d41586-018-04458-w. [DOI] [PubMed] [Google Scholar]
- 53.Ceresa CDL, Nasralla D, Jassem W. Normothermic machine preservation of the liver: state of the art. Curr Transpl Rep. 2018 doi: 10.1007/s40472-018-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasralla D. Liver transplant “game changing” treatment approved. BBC News. 2019 January 16 [Google Scholar]
- 55.Hosgood SA, Moore T, Kleverlaan T, Adams T, Nicholson ML. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J Transl Med. 2017;15(1) doi: 10.1186/s12967-017-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eshmuminov D, Becker D, Hefti M, Borrego L, Schuler M, Hagedorn C. Ex-vivo liver perfusion with preservation of full hepatic functions for one week in a swine model. Nat Biotechnol. 2019 in press. [Google Scholar]
- 57.Dare AJ, Logan A, Prime TA, Rogatti S, Goddard M, Bolton EM. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J Hear Lung Transpl. 2015 doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholz R, Thurman RG, Williamson JR, Chance B, Bücher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.