Abstract
West Nile virus (WNV) is a single-stranded RNA arbovirus of Flavivirus genus that is endemic to the United States and known to cause neuroinvasive disease. Diagnosis is confirmed by the presence of WNV-specific IgM antibodies within serum or cerebrospinal fluid (CSF). Radiologically, it presents as hyperintense T2 signal within deep brain structures (ie, thalami and mid-brain) with or without cerebral peduncle and substantia nigra involvement. On diffusion-weighted imaging, restricted diffusion is reported in basal ganglia and disseminated throughout the white matter. In this report, we describe the imaging findings for 2 cases of WNV from our institution; a 56-year-old female and a 34-year-old female. Increased vigilance for WNV is warranted, particularly in immunosuppressed patients presenting with a clinical picture of viral meningoencephalitis despite initial negative magnetic resonance imaging or CSF analysis. A high suspicion for WNV disease should prompt repeat imaging or laboratory workup.
Keywords: West Nile virus, Meningoencephalitis
Abbreviations: WNV, West Nile virus; PCR, polymerase chain reaction; MRI, magnetic resonance imaging; IVIG, intravenous immunoglobulin; WNND, West Nile neuroinvasive disease
Background
West Nile virus (WNV) is an RNA virus belonging to the Flaviviridae family transmitted by the Culex mosquito, endemic to East Africa, Europe, West Asia, and North America [1]. Although the majority of infected patients develop a mild self-resolving form of illness, severe meningoencephilitis may occur in a minority of cases with the very young, elderly, and immunocompromised at greatest risk [1,2]. Although the virus can affect any part of the neural axis, there is a predilection for the brainstem, cerebellum, and anterior horn cells of the spinal cord [1]. Neuroinvasive variants of WNV are seen in less than 1% of cases, but the mortality may be up to 10% [3].
The neurological manifestations of WNV includes: (1) Neuromuscular involvement, that is, poliomyelitis-like syndrome resulting in asymmetric flaccid paralysis of one or more limbs, (2) encephalitis, meningitis or encephalomeningitis. These clinical syndromes, however, can have significantly overlapping features and mimic other conditions such as Guillain-Barré s Syndrome [1,4,5]. In rare cases, spinal cord involvement has been reported with WNV infection [3]. The diagnosis is confirmed with presence of WNV-specific IgM antibodies either from serum or cerebrospinal fluid (CSF).
Radiologically, it presents as hyperintense T2 signal within deep brain structures (ie, basal ganglia especially thalami and mid-brain) with or without cerebral peduncle and substantia nigra involvement. On diffusion weighted imaging, restricted diffusion is reported in basal ganglia and disseminated throughout the white matter [6].
In this report we describe imaging findings in 2 cases of West Nile encephalitis that we from our institution with a range of characteristic MRI findings with the goal of increasing radiologic and clinical diagnosis of the condition.
Case 1
A 56-year-old African-American woman with past medical history significant for multiple sclerosis and chronic kidney disease status postrenal and pancreatic transplant 10 years prior on mycophenolate and cyclosporine, presented in September with worsening left lower extremity weakness. Her hospital course was complicated by the fever of unknown origin with a sudden decline in mentation and severe brainstem dysfunction, requiring mechanical ventilation. Meningitis was suspected and empiric antibiotic therapy was initiated. Initial serum and CSF analysis was nonconclusive and a viral panel was negative. Initial MRI brain demonstrated nonspecific white matter changes.
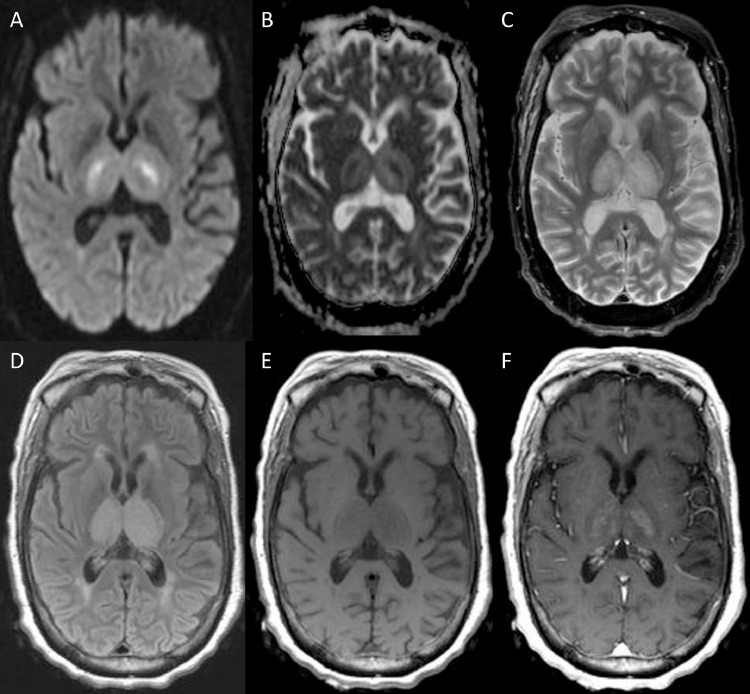
One-week follow-up CSF analysis demonstrated elevated white blood cell count with lymphocytic pleocytosis. Repeat magnetic resonance imaging (MRI) of the brain demonstrated new symmetric T2-hyperintense signal in the bilateral thalami, with associated diffusion restriction and mild abnormal contrast enhancement (Fig. 1). In addition to viral encephalitis, the possibility of deep cerebral venous thrombosis was considered, however a subsequent magnetic resonance venogram was negative. One-week follow-up WNV serology ultimately returned positive with elevated IgM antibodies definitively establishing the diagnosis of WNV. Unfortunately, the patient's condition progressively deteriorated and she was discharged home with hospice.
Fig. 1.
One-week follow-up magnetic resonance imaging (MRI) of the brain for Case 1, obtained 10 days after onset of symptoms. Diffusion Weighted Imaging demonstrates symmetric restricted diffusion (hyperintense signal) in bilateral thalami (Fig. 1A), with corresponding hypointense signal on apparent diffusion coefficient (ADC) (Fig. 1B), T2-weighted (Fig. 1C) and FLAIR sequences (Fig. 1D) demonstrates corresponding hyperintense signal in thalami.T1-weighted precontrast (Fig. 1E) and postcontrast (Fig. 1F) demonstrates abnormal contrast enhancement in the bilateral thalami.
Case 2
A 34-year-old Caucasian female with past medical history significant for hypertension and end stage renal disease, status post renal transplantation presented in late September with light headedness, abdominal pain, weakness, and fever. The patient's medication regimen included Mycophenolate mofetil, Prednisone, Tacrolimus, Valganciclovir, Trimethoprim, and Sulfamethoxazole. On the fifth day of admission, her mental status rapidly declined with diminished alertness and increasing lethargy, eventually requiring intubation for hypoxic respiratory failure. Initial CSF analysis was unremarkable, including a negative viral panel. WNV for next generation sequencing was sent to an outside laboratory. Neurological exam further declined as the she became responsive to painful stimuli only and developed a left gaze palsy. Repeat CSF analysis performed 8 days after initial CSF sampling revealed an elevated cell count with lymphocytic pleocytosis, elevated protein, low-normal glucose, and a negative viral panel.
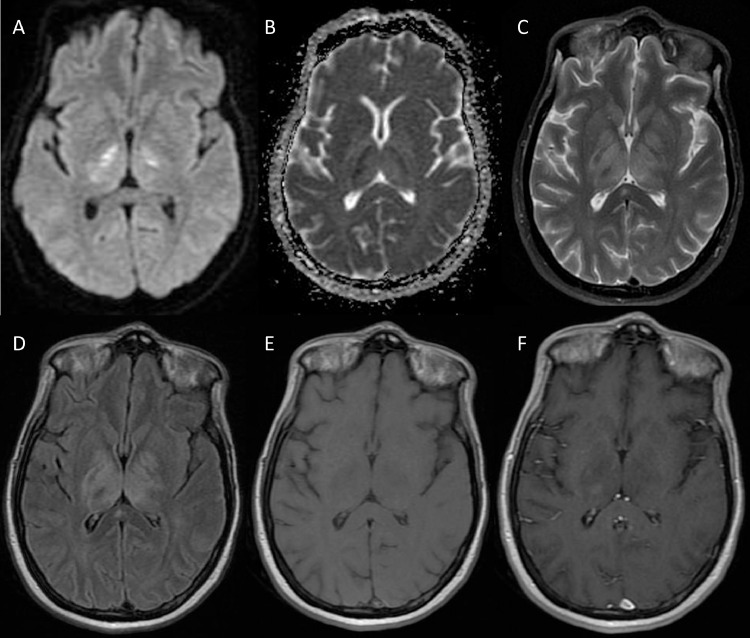
Initial brain MRI revealed only chronic microvascular ischemic changes. Repeat imaging 9 days later demonstrated diffusion restriction and T2W and FLAIR hyperintense signal with expansile changes within the bilateral centrum semiovale, corona radiata, deep gray nuclei, deep white matter tracts, midbrain, bilateral superior cerebellar peduncles, and left cerebellar hemisphere. Additionally, there was faint postcontrast enhancement within the right thalamus (Fig. 2).
Fig. 2.
MRI for Case 2, obtained 14 days after onset of symptoms. Diffusion weighted imaging demonstrates restricted diffusion (hyperintense signal) in bilateral medial lentiform nuclei and lateral thalami (Fig. 2A), with corresponding hypointense signal on ADC (Fig. 2B); T2-weighted (Fig. 2C) and FLAIR sequences (Fig. 2D) demonstrate corresponding hyperintense signal in bilateral medial lentiform nuclei, lateral thalami, posterior limbs of the internal capsules and callosal splenium; T1-weighted precontrast (Fig. 2E) and postcontrast (Fig. 2F) demonstrates abnormal right thalamic enhancement.
WNV polymerase chain reaction and next generation sequencing returned positive, with negative CSF IgG and IgM. Despite initiation of intravenous immunoglobulin (IVIG) therapy, the patient's neurologic status continued to deteriorate including absent gag and corneal reflexes, very mild oculocephalic reflex, absent extremity motor response, and hyporeflexia. A tracheostomy was performed, and she was eventually discharged to a long-term acute care facility.
Discussion
Most cases of WNV are either asymptomatic or mild and present with low-grade fever, headache, rash, myalgia, and malaise lasting 3 to 10 days following an incubation period of 3 to 14 days [1,2]. Despite representing a minority of infections, severe neuroinvasive disease may occur, particularly in immunocompromised individuals, and result in a wide spectrum of clinical features leading to irreversible cognitive impairment, coma, or death [2,5].
Increased vigilance for WNV is warranted during the late summer months when most outbreaks of WNV in the United States occur [2,7]. Findings suspicious for CNS involvement of WNV should prompt evaluation of CSF for WNV IgM antibodies on MAC-ELISA to establish the diagnosis [8,9]. The use of polymerase chain reaction is generally reserved for immunocompromised individuals who may lack sufficient antibody formation against the virus [6,10]. When these methods fail to identify an etiology in immunocompromised patients, but WNV encephalitis is highly suspected, genomic sequencing of RNA from the CSF may identify the viral pathogen as was performed in Case 2 [11].
Neuroradiological findings during active West Nile Virus neuroinvasive disease (WNND) vary, but often correlate with clinical manifestations of the disease. CT of the brain is typically normal [2]. MRI abnormalities usually appear within several weeks after onset of illness. Onset of MRI features may be delayed in immunocompromised patients [2,12]. Patients with meningitis secondary to WNV may show meningeal involvement in the form of leptomeningeal enhancement [2,12]. Early signs of West Nile encephalitis likely first appear as diffusion restriction, followed by hyperintense signal abnormality on T2 weighted and FLAIR sequences most commonly involving the bilateral thalami, basal ganglia, posterior limb of the internal capsule, midbrain, and pons [2,12,13]. Less commonly, high signal T2W, and FLAIR abnormalities are seen involving the cerebral white matter, cerebellar peduncles, and cerebellar hemispheres [13]. Diffusion restriction may be the first MR feature to normalize on follow-up examinations [14]. Moreover, the presence of diffusion restriction only may portend a favorable neurologic prognosis when compared to those with abnormalities on diffusion-weighted imaging and T2/FLAIR [12].
Our cases demonstrated symmetric thalamic, basal ganglia, and brainstem involvement. The collective presence of these features is important to differentiate from other diagnoses via imaging and clinical history. Bilateral thalamic lesions have been described in a variety of neoplastic, metabolic, infectious, and vascular conditions [7]. Wernicke's encephalopathy for example, commonly presents with dorsomedial thalamic FLAIR hyperintensity, but is often distinguished by concomitant presence of FLAIR hyperintensity of the mammillary bodies and periaqueductal gray as well as history of nutritional deficiency or alcoholism [7,15]. Thrombosis of deep cerebral veins may also exhibit expansile thalami with hyperintense T2/FLAIR signal as seen in Case 1, but is differentiated by lack of signal intensity within deep venous sinuses on magnetic resonancevenography [7].
Intraspinal WNND involvement may be seen as focal T2 abnormalities, typically within the ventral horns, and manifest as acute flaccid paralysis syndrome. Such lesions have been reported within the thoracolumbar spine as well as conus medullaris and cauda equina and mimic transverse myelitis [2].
An estimated 48% of patients with WNND die within 90 days of disease onset [16]. Of those who survive to discharge, roughly 10% die within the convalescent phase [16]. Individuals, who survive beyond the convalescent phase, are more likely to suffer from long-term cognitive dysfunction. Follow-up MRI often shows permanent cortical thinning and regional atrophy compared to age and gender controls [17].
The imaging and clinical features of WNV encephalitis are considerably variable and overlapping with other conditions ranging from acute and chronic demyelinating disorders to other viral illnesses. Increased vigilance for WNV is warranted, particularly in immunosuppressed patients presenting with a clinical picture of viral meningoencephalitis despite initial negative MRI or CSF analysis. A high suspicion for WNV disease may prompt repeat imaging or more in-depth laboratory workup such as with next generation sequencing to obtain the diagnosis. The following MRI features should raise suspicion for WNV infection: hyperintense T2 signal within deep brain structures (ie, basal ganglia especially thalami and mid-brain) with or without cerebral peduncle and substantia nigra involvement and diffusion restriction in basal ganglia.
Footnotes
Patient consent statement: This work contains no identifiable information or images of included patients. The work was deemed IRB exempt by the Wayne State University IRB Review Board.
Conflict of interest: No Conflicts of interest to declare.
References
- 1.DeBiasi RL. West Nile virus neuroinvasive disease. Curr infect Dis Rep. 2011;13:350–359. doi: 10.1007/s11908-011-0193-9. [DOI] [PubMed] [Google Scholar]
- 2.Zak IT, Altinok D, Merline JR, Chander S, Kish KK. West Nile virus infection. Am J Roentgenol. 2005;184(3):957–961. doi: 10.2214/ajr.184.3.01840957. [DOI] [PubMed] [Google Scholar]
- 3.Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606‐623. doi: 10.3390/v6020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 5.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin A, Van Gerpen JA. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 6.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AB, Smirniotopoulos JG, Rushing EJ, Goldstein SJ. Bilateral thalamic lesions. AJR Am J Roentgenol. 2009;192(2):W53–W62. doi: 10.2214/AJR.08.1585. [DOI] [PubMed] [Google Scholar]
- 8.Debiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2(5):264‐275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long MT, Jeter W, Hernandez J, Sellon DC, Gosche D, Gillis K. Diagnostic performance of the equine IgM capture ELISA for serodiagnosis of West Nile virus infection. J Vet Intern Med. 2006;20(3):608–613. doi: 10.1892/0891-6640(2006)20[608:dpotei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Arboviral Diseases, Neuroinvasive and Non-neuroinvasive 2015 Case Definition. https://www.cdc.gov/westnile/symptoms/index.html
- 11.Wilson MR, Zimmermann LL, Crawford ED, Sample HA, Soni PR, Baker AN. Acute West Nile virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant. 2017;17(3):803–808. doi: 10.1111/ajt.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26(2):289–297. [PMC free article] [PubMed] [Google Scholar]
- 13.Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26(8):1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 14.Nouranifar RK, Ali M, Nath J. The earliest manifestation of focal encephalitis on diffusion-weighted MRI. Clin Imaging. 2003;27(5):316–320. doi: 10.1016/s0899-7071(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Galluci M, Carollo C. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171–176. doi: 10.3174/ajnr.A1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philpott D, Nolan MS, Evert N, Mayes B, Hesalroad D, Fronken E. Acute and delayed deaths after West Nile virus infection, Texas, USA, 2002–2012. Emerg Infect Dis. 2019;25(2):256–264. doi: 10.3201/eid2502.181250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray KO, Nolan MS, Ronca SE, Datta S, Govindarajan K, Narayana PA. The neurocognitive and MRI outcomes of West Nile virus infection: preliminary analysis using an external control group. Front Neurol. 2018;9:111. doi: 10.3389/fneur.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]