Significance
Whether and how pDCs cross-prime CD8 T cells in vivo remain controversial despite extensive studies. Using a vaccine model where antigens were only delivered to pDCs, this report demonstrated that pDCs induced cross-priming and durable CD8 T cell immunity in vivo. However, cross-presenting pDCs required cDCs to achieve cross-priming by transferring antigens to cDCs. cDC1s but not cDC2s played a critical role in pDC-mediated cross-priming, despite both subsets acquiring antigens from pDCs similarly. Antigen transfer from pDCs to bystander cDCs was mediated by pDC-derived exosomes (pDCexos), which were generated under various conditions. Importantly, these pDCexos required cDCs to prime CD8 T cells, similarly to cross-presenting pDCs, thus identifying the pDCexo/cDCs pathway as a mechanism for pDCs to achieve cross-priming.
Keywords: plasmacytoid dendritic cells, conventional dendritic cells, antigen transfer, exosomes, cross-priming
Abstract
Although plasmacytoid dendritic cells (pDCs) have been shown to play a critical role in generating viral immunity and promoting tolerance to suppress antitumor immunity, whether and how pDCs cross-prime CD8 T cells in vivo remain controversial. Using a pDC-targeted vaccine model to deliver antigens specifically to pDCs, we have demonstrated that pDC-targeted vaccination led to strong cross-priming and durable CD8 T cell immunity. Surprisingly, cross-presenting pDCs required conventional DCs (cDCs) to achieve cross-priming in vivo by transferring antigens to cDCs. Taking advantage of an in vitro system where only pDCs had access to antigens, we further demonstrated that cross-presenting pDCs were unable to efficiently prime CD8 T cells by themselves, but conferred antigen-naive cDCs the capability of cross-priming CD8 T cells by transferring antigens to cDCs. Although both cDC1s and cDC2s exhibited similar efficiency in acquiring antigens from pDCs, cDC1s but not cDC2s were required for cross-priming upon pDC-targeted vaccination, suggesting that cDC1s played a critical role in pDC-mediated cross-priming independent of their function in antigen presentation. Antigen transfer from pDCs to cDCs was mediated by previously unreported pDC-derived exosomes (pDCexos), that were also produced by pDCs under various conditions. Importantly, all these pDCexos primed naive antigen-specific CD8 T cells only in the presence of bystander cDCs, similarly to cross-presenting pDCs, thus identifying pDCexo-mediated antigen transfer to cDCs as a mechanism for pDCs to achieve cross-priming. In summary, our data suggest that pDCs employ a unique mechanism of pDCexo-mediated antigen transfer to cDCs for cross-priming.
As the initiators of antigen-specific immune responses, dendritic cells (DCs) play a central role in regulating both T cell immunity and tolerance (1). There are two major DC populations, conventional/classical DCs (cDCs) and the plasmacytoid DCs (pDCs). While both cDCs and pDCs could be developed from a common DC progenitor (CDP) and share key transcriptional factors and dependence on Fms-like tyrosine kinase 3 ligand (Flt3-L), pDCs were most noted as a unique DC subset that produced large amount of type I interferons (IFN-I) and have been extensively studied for their function in generating antiviral immunity by sensing viral RNA and DNA by toll-like receptor (TLR)-7 and -9 (2–10). Besides their function in producing IFN-I, recent studies have also highlighted that pDCs exert strong tolerogenic functions to mediate immune tolerance by inducing T cell deletion, CD4 T cell anergy, and Treg differentiation (4, 11, 12). In tumors, pDCs are generally thought to play a tolerogenic role, as accumulation of pDCs in multiple tumors was often associated with poor prognosis (4, 6, 13–17). On the other hand, activation of pDCs has also been reported to induce immunogenic responses and has shown therapeutic efficacy in human cancers, indicating pDC-mediated anti-tumor immunity (14, 18–22). However, how pDCs function in generating CD8 T cell immunity remains poorly understood.
Although initially thought of as the less efficient antigen presenting cells (APCs), pDCs can present antigens to activate both CD4 T cells as well as CD8 T cells through cross-presentation (23, 24). Cross-priming, the activation of naive CD8 T cells following DC-mediated cross-presentation—the process through which exogenous antigens are processed and presented onto MHC class I molecules—plays a major role in generating CD8 T cell immunity against cancers and viruses, upon vaccination, as well as in the induction of immune tolerance (cross-tolerance) (25–28). Recent discovery that type 1 cDCs (cDC1s) cross-primed antigen-specific CD8 T cells in tumors has highlighted the importance of DC-mediated cross-priming in generating CD8 T cell immunity and in determining the efficacy of cancer immunotherapies, including immune checkpoint blockade (ICB) (29–32). However, the function and roles of pDCs in cross-priming have remained understudied and not well understood. In fact, despite pDCs having been shown to be capable of cross-presentation in vitro (33–37), even the involvement of pDCs in cross-priming in vivo is still under debate (26, 38–40). Several studies have suggested that pDCs were either not involved in or incapable of cross-priming in vivo. For example, pDCs did not cross-present viral antigens during either influenza A virus (IAV) or herpes simplex virus 1 (HSV-1) infection (41, 42), and depletion of pDCs did not affect cross-presentation and clearance of viral antigens (43). CpG-stimulated pDCs have been shown to be defective in cross-priming naive CD8 T cells compared to cDCs (44), and pDCs were not able to cross-prime naive CD8 T cells in vivo (45). On the other hand, other reports have suggested that pDCs did cross-prime CD8 T cells in vivo (46–49). Despite the controversy, however, it’s likely that pDCs at least play some roles in regulating cross-priming, as increasing evidence has emerged showing that both pDCs and cDCs are required to achieve optimal cross-priming and CD8 T cell immunity under diverse conditions (50–55). Furthermore, immunotherapies with pDCs either alone or in combination with cDCs have shown promising clinical results (18, 21, 22, 56, 57), although it remains unclear whether pDCs exert their effects directly through their cross-priming or indirectly by regulating other immune cells (i.e., cDCs, T cells, and natural killer [NK] cells) through pDC activation and subsequent production of IFN-I and other cytokines (58, 59). Thus, there is a critical need to better understand whether and how pDCs cross-prime CD8 T cells to advance these promising pDC-based immunotherapies clinically.
Exosomes are small membrane vesicles about 30- to 150-nm diameter in sizes that form within late multivesicular endosomal compartments containing proteins, lipids, and nucleic acids, and play an important role in intercellular communications and material transfer of their cargo (60–63). DC-derived exosomes (DCexos) additionally carry functional MHC class I/II-peptide antigen complexes (pMHCI and pMHCII) and costimulatory molecules and have been shown to prime both CD4 and CD8 T cells, making them attractive candidates as cancer vaccines (64–67). Of note, whether pDCs generate exosomes and how they function in priming CD8 T cells have not been investigated, despite studies showing that pDC functions were regulated by exosomes (68–70).
Here we report that delivering antigens specifically to pDCs by targeting antibodies in vivo resulted in efficient cross-priming of naive CD8 T cells and generation of durable immunity. However, efficient cross-priming of CD8 T cells in vivo required cDCs that were not targeted, as depletion of cDCs abrogated effector differentiation of CD8 T cells. Further analysis revealed that while pDCs transferred antigens to cDCs leading to both pDCs and cDCs expressing MHCI-antigen (pMHCI) complexes on their surfaces, only cDCs but not pDCs effectively primed naive OTI cells ex vivo. Taking advantage of an in vitro culture system where antigens were only accessible to pDCs, we were able to confirm the requirement of bystander cDCs for pDC-mediated cross-priming, showing that cross-presenting pDCs primed naive CD8 T cells by transferring antigens to bystander cDCs. cDC1s but not cDC2s were required for pDC-mediated cross-priming, despite both cDC1s and cDC2s receiving antigen from pDCs similarly, suggesting that cDC1s played a critical role in pDC-mediated cross-priming postantigen presentation. We have further demonstrated that antigen transfer from pDCs to bystander cDCs was mediated through pDC-derived exosomes (pDCexos), which were also produced by pDCs under various conditions. Importantly, these pDCexo primed naive antigen-specific CD8 T cells only in the presence of bystander cDCs, suggesting that a pDCexo-mediated cDC-dependent cross-priming might be employed by pDCs to achieve cross-priming.
Results
Targeting pDCs In Vivo Led to Cross-Priming of Antigen-Specific CD8 T Cells.
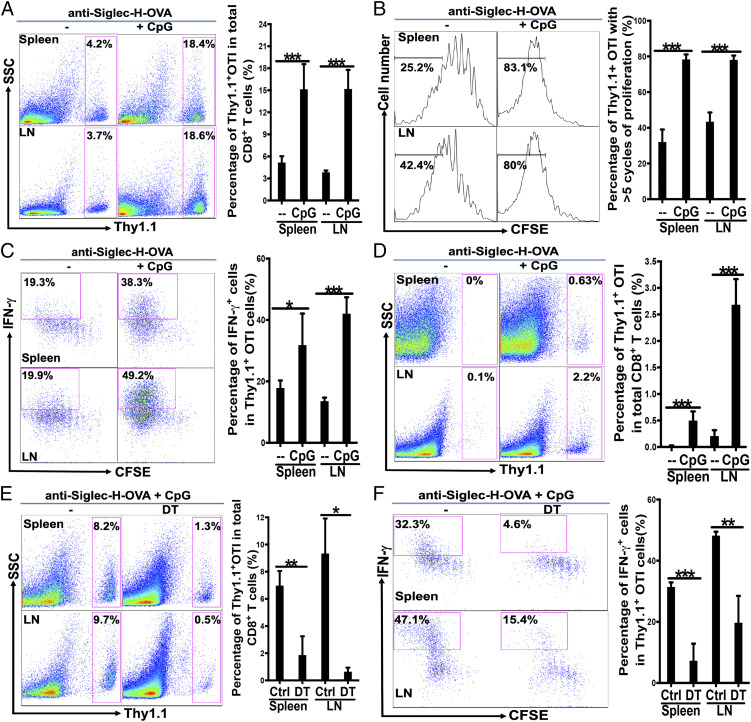
We have previously engineered recombinant anti-Siglec-H and anti-Bst2 antibodies to express antigens of interest (anti-Siglec-H-Ag and anti-Bst2-Ag) and shown that immunization with 10 to 20 μg of anti-Siglec-H-antigen specifically targeted pDCs but not cDCs in vivo, leading to presentation of antigens on MHCII for at least 72 h (71, 72). As immunization with anti-Siglec-H-Ag have been shown to prime CD4 T cell to mediate tolerance either with or without adjuvant (71), we decided to first use pDC-targeting anti-Siglec-H-OVA to examine whether pDCs cross-prime CD8 T cells in vivo, and if so whether pDC-mediated cross-priming similarly leads to CD8 T cell tolerance. Wild-type (WT) mice were immunized with anti-Siglec-H-OVA either with or without TLR9 agonist CpG following adoptive transfer of 5- (6)-carboxyfluorescein diacetate succinimidyl diester (CFSE)-labeled OVA-specific CD8 T (OTI) cells, lymph node (LN) and spleen cells were then examined for CD8 T cell responses. Immunization with anti-Siglec-H-OVA alone led to some proliferation of OTI cells in both LN and spleen (Fig. 1 A and B). However, the addition of CpG greatly enhanced the proliferation of transferred OTI cells, resulting in about a three- to fourfold increase in the percentages of Thy1.1+ OTI cells in spleen and LN, respectively (Fig. 1A). Examination of CFSE dilution further revealed that immunization with anti-Siglec-H-OVA plus CpG resulted in about 80% of Thy1.1 OTI T cells that have undergone more than five cycles of proliferation, compared to 30 to 40% with anti-Siglec-H-OVA alone (Fig. 1B). Thus, while the OVA antigen was cross-presented to CD8 T cells by anti-Siglec-H-OVA alone, addition of an adjuvant CpG substantially improved efficacy of antigen cross-presentation.
Fig. 1.
Targeting pDCs by anti-Siglec-H-OVA plus CpG led to strong OVA-specific CD8 T cell responses that were dependent on cDCs. (A–C) WT mice were immunized with anti-Siglec-H-OVA either alone or with CpG (n = 5), and cross-priming was examined as described in Materials and Methods. (A) The percentages of Thy1.1+ OTI cells out of total CD8 T cells are depicted with representative plots on the Left and bar graph with statistics analysis on the Right. The percentages of Thy1.1+ OTI cells that had undergone more than five cycles of proliferation are depicted in B with representative histograms and bar graph with statistics analysis. The percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are shown in C with representative plots and statistics analysis in bar graph. Student’s t test was performed for A–F and NS = P > 0.05%, *P < 0.05, **P < 0.01, ***P < 0.001. (D) Immunization with pDC-targeting anti-Siglec-H-OVA alone led to tolerance while immunization with anti-Siglec-H-OVA plus CpG led to strong recall responses. WT mice (n = 5) were immunized as in A and recalled at 21 d with OVA in CFA, and recall responses were examined. The percentages of Thy1.1+ OTI cells out of total CD8 T cells are depicted. (E and F) cDCs play a critical role in cross-priming upon immunization with pDC-targeting anti-Siglec-H-OVA plus CpG. CD11c-DTR→WT bone marrow chimeras (n = 3 to 5) were treated with DT or PBS on days −2, 0, and 2, and cross-priming was examined. The percentages of Thy1.1+ OTI cells out of total CD8 T cells are shown in E; and mean and SD of the percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are shown in F. Data shown are representative of at least three independent experiments.
To determine whether the proliferating OTI cells differentiated into effector cells under these conditions, we examined IFN-γ production. In LNs, while immunization with anti-Siglec-H-OVA plus CpG resulted in about 50% IFN-γ-producing Thy1.1+ OTI cells, immunization with anti-Siglec-H-OVA alone produced much less IFN-γ-producing effector cells (<20%, Fig. 1C). Similar results were obtained in spleen (Fig. 1C). Taken together, our data suggested that anti-Siglec-H-OVA plus CpG were capable of efficiently priming naive OTI cells and induced their differentiation into IFN-γ-producing effector cells.
As we previously have shown immunization with anti-Siglec-H-OVA with or without the adjuvant led to CD4 T cell tolerance (71), we asked whether our observed priming of naive CD8 T cells (Fig. 1 A–C) similarly led to CD8 T cell tolerance. WT mice immunized with anti-Siglec-H-OVA alone or with CpG were recalled with OVA in Complete Freund's Adjuvant (CFA) at 21 d. No recall responses were observed in WT mice immunized with anti-Siglec-H-OVA alone (Fig. 1D), suggesting that immunization with anti-Siglec-H-OVA alone led to tolerance. However, strong recall responses were observed in mice immunized with anti-Siglec-H-OVA plus TLR9 agonist CpG as adjuvants (Fig. 1D), suggesting that CD8 T cell tolerance was reversed, resulting in CD8 T cell immunity when activation stimuli were provided, in contrast to our data on CD4 T cell tolerance (71).
cDCs Are Required for Cross-Priming upon Immunization with Anti-Siglec-H-OVA Plus CpG.
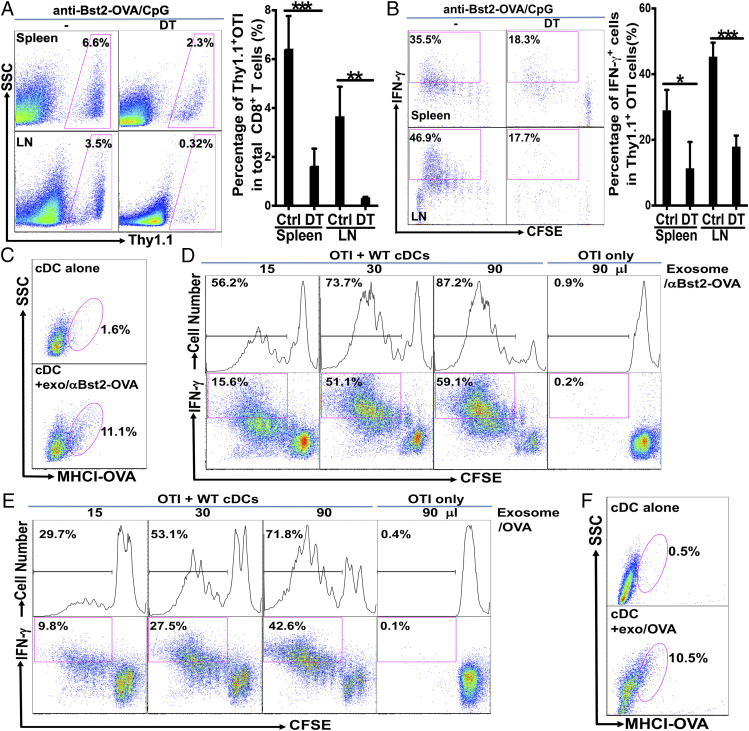
We next asked whether pDCs alone were sufficient for cross-priming. We generated CD11c-DTR→WT bone marrow chimeras by transferring bone marrow cells of CD11c-DTR (CD45.2) mice into lethally irradiated WT (CD45.1) mice, which allow the depletion of cDCs but preserving the majority of pDCs (about 80%) upon treatment with diphtheria toxin (DT) (73). Chimeras were treated with DT or phosphate buffered saline (PBS), and immunized with anti-Siglec-H-OVA plus CpG following adoptive transfer of CFSE-labeled naive OTI cells. While chimeras treated with PBS exhibited strong cross-priming of naive OTI cells with strong proliferation and differentiation into IFN-γ-producing effectors, chimeras depleted of cDCs exhibited substantially reduced proliferation and percentages of IFN-γ-producing effectors (Fig. 1 E and F), suggesting that cDCs might be required for cross-priming by in vivo pDC-targeted vaccine with anti-Siglec-H-OVA plus CpG. However, it should be noted that other non-DCs might also contribute to pDC-mediated cross-priming based on these data, as some macrophages and NK cells have been shown to be depleted with the DT/CD11c-DTR system (73).
pDCs Targeted by Anti-Siglec-H-OVA Transfer Antigens to cDCs to Prime Antigen-Specific CD8 T Cells.
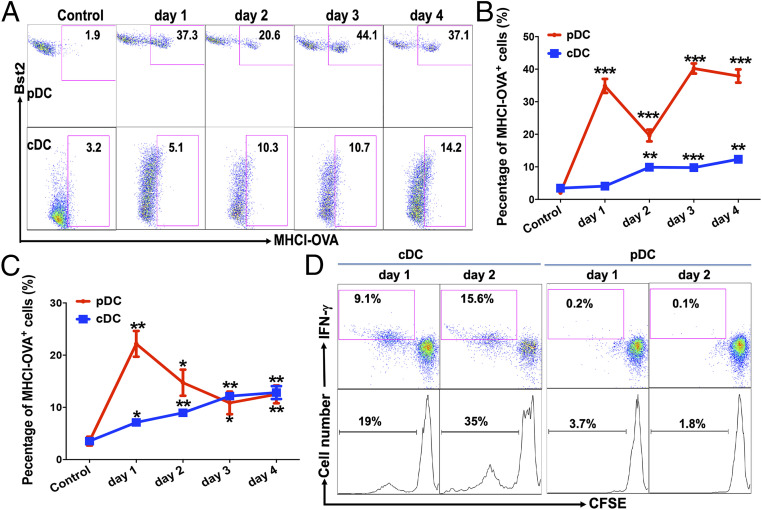
We next asked what the roles pDCs and cDCs played in anti-Siglec-H-OVA-induced cross-priming in vivo. We first examined the expression of MHCI-OVA complexes on DCs upon immunization. WT mice were immunized with anti-Siglec-H-OVA plus CpG, and the expression of MHCI-OVA (H-2Kb-SIINFEKL) complexes on pDCs and cDCs was examined at various times after immunization, taking advantage of the anti-H-2Kb-SIINFEKL antibody that specifically recognizes the SIINFEKL epitope from OVA presented on MHCI (H-2Kb). MHCI-OVA complexes appeared on pDCs in the spleen as early as 1 d after immunization and persisted for at least 4 d (Fig. 2 A and B, Upper). Interestingly, significant expression of MHCI-OVA complexes was detected in nontargeted cDCs with a slightly delayed kinetics (Fig. 2 A and B, Lower). We also observed a similar pattern of MHCI-OVA expression in LN pDCs and cDCs, with the exception that significantly higher MHCI-OVA expression was detected on cDCs on day 1 (Fig. 2C). As our previous study has shown that anti-Siglec-H-Ag specifically delivered antigens to pDCs but not cDCs in vivo (71), OVA antigens observed on nontargeted cDCs in vivo were likely transferred from pDCs instead of mistargeting. Taken together, these data suggest that pDCs targeted by anti-Siglec-H-OVA likely transfer OVA antigens to nontargeted cDCs, leading to both pDCs and cDCs expressing MHCI-OVA complexes on their surface.
Fig. 2.
Targeting pDCs with anti-Siglec-H-OVA plus CpG in vivo led to the expression of functional MHCI-OVA (H-2Kb-SIINFEKL) complexes on cDCs. (A–C) WT mice were immunized with anti-Siglec-H-OVA plus CpG, and spleen and LNs were processed at indicated times (n = 3) and subjected to flow cytometry. pDCs and cDCs were gated as CD11cintermediate Bst2+ and CD11chigh Bst2− cells, respectively. The percentages of H-2Kb-SIINFEKL+ cells out of total pDCs and cDCs in spleens are shown in A with representative flow plots in B. The percentages of H-2Kb-SIINFEKL+ cells out of total pDCs and cDCs in LNs are depicted in C. Each time point was compared to controls. (D) cDCs but not pDCs from immunized WT mice were able to prime naive OTI CD8 T cells. pDCs and cDCs were isolated from pooled spleens of WT mice immunized as in A and then cocultured with naive OTI cells. The percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are shown (Upper), and the percentages of proliferated (CFSElow) OTI cells out of total OTI cells are shown (Lower). Data shown are representative of three or more independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
As cDCs have been shown to be required for anti-Siglec-H-OVA-induced cross-priming of naive antigen-specific CD8 T cells (Fig. 1 E and F), the findings that both pDCs and cDCs presented OVA antigen on MHCI prompted us to examine the contribution of pDCs and cDCs to CD8 T cell priming. WT mice were immunized with anti-Siglec-H-OVA plus CpG, and cDCs and pDCs were isolated from spleens at days 1 and 2 and cocultured with naive OTI cells. Surprisingly, isolated pDCs did not support OTI proliferation (Fig. 2D), despite their high expression of MHCI-OVA (Fig. 2A). Thus, although pDCs could efficiently process and present OVA antigen onto MHCI to form MHCI-OVA complexes on their surface, pDCs alone were not sufficient to prime naive CD8 T cells. On the other hand, cDCs depleted of pDCs by combining Pan DC isolation kit with a mixture of antibodies against pDC-specific receptors (anti-B220, anti-Siglec-H, and anti-Bst2; see Materials and Methods for details) were able to induce OTI proliferation as well as differentiation into IFN-γ-producing effectors (Fig. 2D). Taken together, our data suggested that while pDCs targeted by anti-Siglec-H-OVA in vivo efficiently presented antigens onto MHCI (as detected by anti-H-2Kb-SIINFEKL), cDCs that received antigen from pDCs played a critical role in mediating cross-priming of naive antigen-specific CD8 T cells.
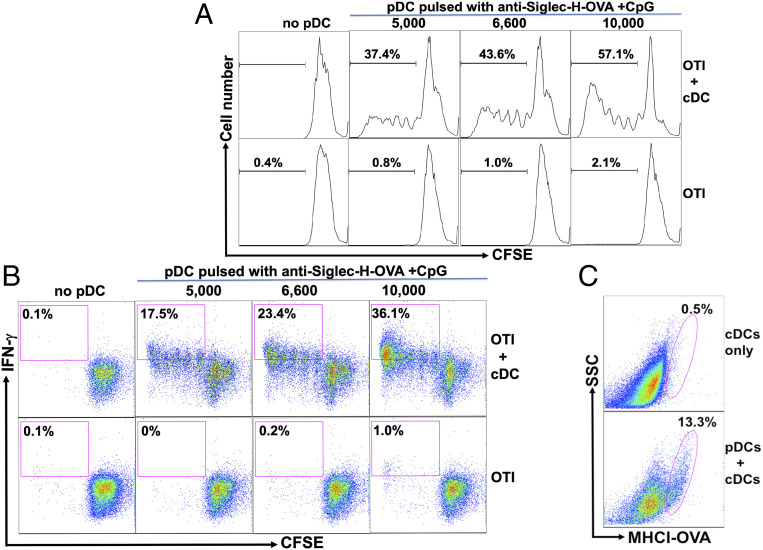
Although our in vivo and ex vivo data strongly suggested that pDCs transferred antigens to cDCs which then primed antigen-specific CD8 T cells, we could not rule out the involvement of other cells/factors due to the complexity of the in vivo system. To further confirm that cross-presenting pDCs transfer antigens to cDCs to mediate cross-priming, we developed an in vitro system where antigens (anti-Siglec-H-OVA) were only accessible to pDCs. WT mice were treated with Flt3L for various days to expand the DC populations, as previous studies have shown that both pDCs and cDCs from Flt3L-treated mice exhibited similar expression profiles as their counterparts in naive mice (74). Isolated pDCs were pulsed with anti-Siglec-H-OVA plus CpG and washed thoroughly before cocultured with OTI in the presence or absence of naive cDCs that had no contact with anti-Siglec-H-OVA throughout the experiments. While pDCs pulsed with anti-Siglec-H-OVA plus CpG efficiently presented OVA antigen on MHCI as detected by flow cytometry (SI Appendix, Fig. S1), these pDCs are not efficient in inducing proliferation of naive OTI cells (Fig. 3 A and B, Lower), consistent with our ex vivo data on pDCs (Fig. 2D). Not surprisingly, no proliferation was observed when OTI cells were cultured with naive cDCs alone (Fig. 3A). However, when pDCs were added to cDCs/OTI cocultures, strong proliferation of OTI cells was observed in a dose-dependent manner (Fig. 3 A, Upper). Importantly, significant percentages of the proliferating OTI cells also differentiated into IFN-γ-producing effectors (Fig. 3 B, Upper), suggesting that while cross-presenting pDCs alone were not sufficient to prime naive antigen-specific CD8 T cells, the cooperation of cross-presenting pDCs and antigen-naive cDCs efficiently primed OTI cells to become IFN-γ-producing effectors.
Fig. 3.
Antigen-loaded pDCs required the help of cDCs to prime antigen-specific CD8 T cell in vitro. (A and B) While pDCs pulsed with anti-Siglec-H-OVA plus CpG failed to prime naive OTI cells by themselves, these pDCs induced strong priming of naive OTI cells in the presence of naive cDCs. pDCs were pulsed with anti-Siglec-H-OVA plus CpG for 4 h and cocultured with naive OTI cells (1 × 105 cells) either with or without bystander splenic cDCs. Percentages of proliferated CFSElow OTI are shown in A and the percentages of proliferated IFN-γ+ cells (CFSElowIFN-γ+) out of total OTI cells are shown in B. (C) pDCs pulsed with anti-Siglec-H-OVA plus CpG transferred antigens to antigen-naive cDCs. pDCs pulsed with anti-Siglec-H-OVA plus CpG were cultured with freshly isolated congenic cDCs (CD45.1 vs. CD45.2), and subjected to flow cytometry. Expression of MHCI-OVA on gated cDCs are shown. Data shown are representative of three or more independent experiments.
These results prompted us to test whether cross-presenting pDCs (SI Appendix, Fig. S1) transfer antigen to naive cDCs, as our in vivo data have suggested. Freshly isolated cDCs (CD45.1 or CD45.2) were cultured either alone or with cross-presenting pDCs (different from cDCs for CD45). As shown in Fig. 3C, coculture of naive cDCs with pulsed pDCs led to significant expression of MHCI-OVA (H-2Kb-SIINFEKL) on cDCs. Thus, our in vitro data have confirmed that cross-presenting pDCs transferred antigens to antigen-naive cDCs and required cDCs to prime naive antigen-specific CD8 T cells.
Batf3-Dependent cDC1s Played a Critical Role in Mediating pDC-Induced Cross-Priming.
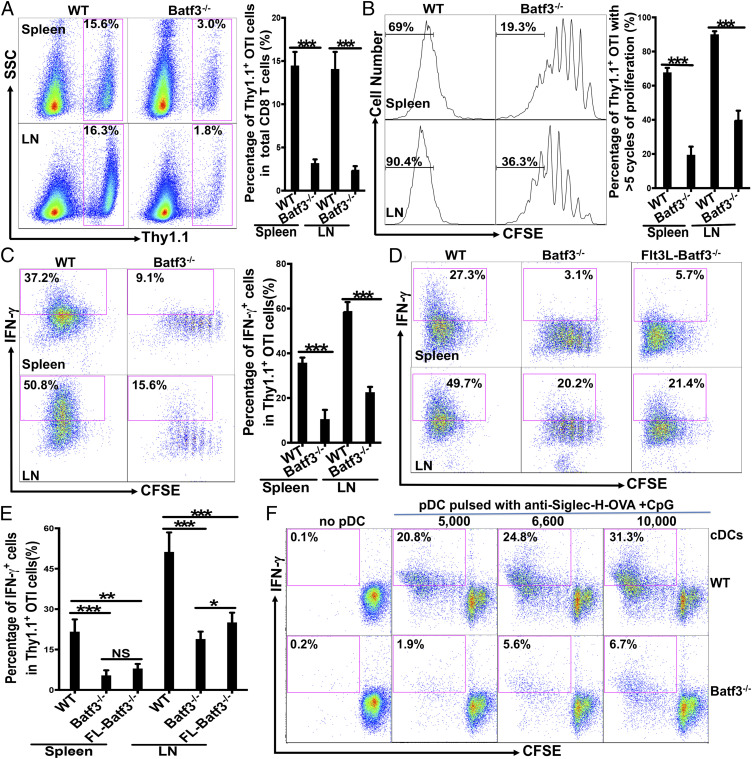
Next we asked which cDC subsets are involved in pDC-mediated cross-priming. As cDC1s have been shown to play a critical role in initiating CD8 T cell immune responses through cross-presentation in vivo (75), we asked whether cDC1s played a critical role in mediating pDCs’ function in cross-priming. We thus used Batf3−/− mice, which lack both CD8+ cDC1s and migratory CD103+ cDC1s (75), to determine whether the loss of cDC1s would affect cross-priming upon pDC-targeted vaccination with anti-Siglec-H-OVA plus CpG. Significantly reduced percentages of the Thy1.1+ OTI cells in total CD8 T cells were observed in Batf3−/− mice compared to WT mice (Fig. 4A), with the OTI cells undergoing fewer rounds of proliferation (Fig. 4B). More strikingly, the percentages of IFN-γ-producing effectors in both spleen and LN of Batf3−/− mice were greatly reduced compared to WT mice (Fig. 4C), suggesting that BATF3-dependent cDC1s (CD8+ and CD103+ cDC1s) play a critical role in cross-priming of antigen-specific CD8 T cells upon pDC-targeted vaccination.
Fig. 4.
Batf3-depedent cDC1s played a critical role in pDC-mediated cross-priming in vivo. (A–C) Batf3−/− mice exhibited significantly reduced cross-priming. WT and Batf3−/− mice (n = 5) were examined for cross-priming as in Fig. 1. The percentages of Thy1.1+ OTI cells out of total CD8 T cells were depicted with representative plots in A, Upper; the percentages of Thy1.1+ OTI cells that had undergone more than five cycles of proliferation are depicted in B; and the percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are depicted in C. (D and E) Increasing the number of DCs in Batf3−/− mice failed to restore cross-priming. WT, Batf3−/−, and Flt3L-treated Batf3−/− mice (n = 4) were examined for cross-priming as in C. The percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are shown for both spleen and LNs in D with representative plots and in E with bar graph for statistics analysis. (F) WT but not Batf3−/− cDCs were able to mediate efficient priming of OTI cells by WT pDCs in vitro. WT pDCs pulsed with anti-Siglec-H-OVA plus CpG were cocultured with naive OTI cells (1 × 105 cells) and 2 × 104 WT or Batf3−/− cDCs as indicated. Percentages of proliferated IFN-γ+ cells (CFSElowIFN-γ+) out of total OTI cells are shown. Data are representative of two or more independent experiments. NS = P > 0.05%, *P < 0.05, **P < 0.01, and ***P < 0.001.
We next asked whether the deficiency in OTI proliferation and differentiation could be restored by increasing the number of other cDCs to compensate for the lack of cDC1s. Batf3−/− mice were treated with Flt3L for 8 to 9 d so that DCs other than cDC1s were expanded. WT, naive Batf3−/− mice, and Flt3L-treated Batf3−/− mice were then examined for cross-priming upon immunization with anti-Siglec-H-OVA plus CpG. Flt3L-treated Batf3−/− mice exhibited significantly increased percentages of both pDCs and cDCs (SI Appendix, Fig. S2A). The proliferation of naive OTI CD8 T cells was partially restored in Flt3L-treated Batf3−/− mice, with the percentage of OTI cells that underwent six or more (more than five) rounds of proliferation increased to levels comparable to WT mice (SI Appendix, Fig. S2 B and C). However, Flt3L treatment failed to restore IFN-γ-producing effectors in Batf3−/− mice (Fig. 4 D and E, WT vs. Flt3L-Batf3−/−), suggesting that increasing other DCs including pDCs and cDC2s, could not compensate for cDC1s’ function in priming naive CD8 T cells to differentiate into effector cells. However, it should be noted that Flt3L treatment itself might lead to defective cross-priming through other mechanisms. For instance, Flt3L-induced increased number/level of pDCs has been shown to result in pDCs being more tolerogenic to negatively regulate T cell functions (76). Similarly, Flt3L treatment has been shown to expand regulatory T cells (77), leading to suppressed CD8 T cell priming.
To directly determine whether cDC1s play a critical role in pDC-mediated cross-priming, we turned to an in vitro coculture system where only pDCs, cDCs, and CD8 T cells were present. Proliferation and effector differentiation of OTI cells were greatly reduced in cocultures with Batf3−/− splenic cDCs compared to WT cDCs, with various numbers of cross-presenting pDCs (SI Appendix, Fig. S2D and Fig. 4F). The deficiency of Batf3−/− splenic cDCs was not due to their inability in receiving OVA antigens from pDCs, as both Batf3−/− splenic cDCs and WT CD8− cDC2s were similar in their expression of MHCI-OVA on their surface upon interaction with pulsed (cross-presenting) pDCs (SI Appendix, Fig. S2E). Furthermore, similar percentages of MHCI-OVA-expressing cells were observed among WT CD8+ cDC1s and CD8− cDC2s populations in WT cDCs (SI Appendix, Fig. S2 E, Upper), suggesting that CD8− cDC2s were equally efficient in acquiring antigens from pDCs compared to CD8+ cDC1s.
Taken together, our data suggest that while cross-presenting pDCs transfer antigens to both cDC1s and cDC2s with similar efficiency, cDC1s were intrinsically more potent in priming naive CD8 T cells for effector differentiation compared to cDC2s and play a critical role in pDC-targeted vaccination-induced cross-priming in vivo.
Antigen-Presenting pDCs Conditioned cDCs to Prime Antigen-Specific CD8 T Cells by a Contact-Independent Mechanism.
Next we asked how pDCs transferred antigens to cDCs. Previous studies have shown that through cell-cell contact, DCs could either acquire preprocessed peptide (antigen) via gap junctions (78, 79), or the preformed MHCI-antigen complexes from neighboring DCs or other cells by “cross-dressing” (80–84). We thus asked whether antigen transfer from pDCs to cDCs was also contact dependent.
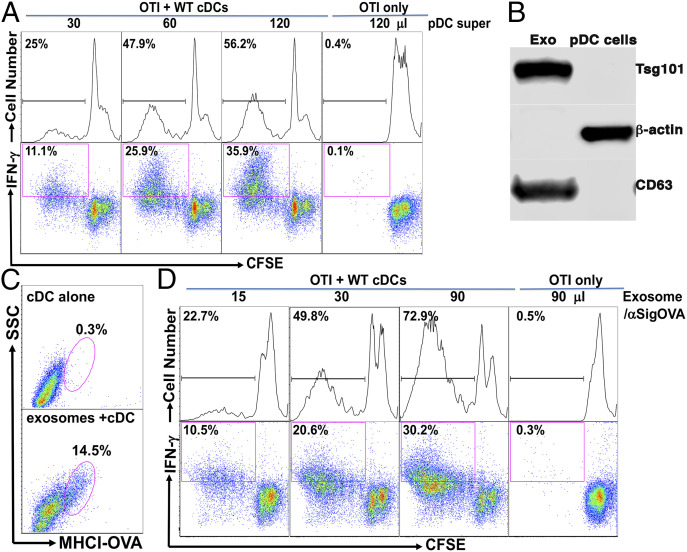
We first examined whether supernatants of pulsed pDCs could substitute pDCs to enable bystander cDCs to prime OTI cells. Isolated pDCs were pulsed with anti-Siglec-H-OVA plus CpG and were extensively washed before plating. Addition of pDC supernatants to cDC/OTI cocultures led to a dose-dependent increase in proliferation, whereas addition of pDC supernatants to OTI cells induced no proliferation (Fig. 5A). Further examination also confirmed that significant percentages of the proliferating OTI cells differentiated into IFN-γ-producing effectors (Fig. 5A), suggesting that pDC-produced soluble factor(s) could confer on cDCs the ability to prime naive CD8 T cells. Transwell experiments also confirmed that pDC-derived soluble factor(s) is responsible for inducing cDC-dependent CD8 T cell priming. cDCs/OTI cocultures were placed in 3 μM Transwell, and pDCs pulsed with anti-Siglec-H-OVA plus CpG were cultured in the outer chamber. Indeed, we observed significant proliferation of OTI cells in Transwell if large numbers of pulsed pDCs were placed in the outer chamber (SI Appendix, Fig. S3A), further confirming that antigen-presenting pDCs induced cDCs to prime CD8 T cells in a contact-independent manner.
Fig. 5.
Antigen-presenting pDCs conditioned cDCs to prime antigen-specific CD8 T cells by a contact-independent mechanism, likely through pDC-derived exosomes. (A) pDC-produced supernatants induced a dose-dependent priming of naive OTI cells that was dependent on the presence of cDCs. pDCs were pulsed with anti-Siglec-H-OVA plus CpG and thoroughly washed before culture. Culture supernatants were added to OTI cells in the presence or absence of naive cDCs as indicated. Percentages of total proliferated cells (CFSElow) and proliferated IFN-γ+ cells (CFSElowIFN-γ+) out of total OTI cells are shown. (B) pDCs-generated exosomes were enriched for exosome markers CD63 and Tsg101. pDCs were cultured with anti-Siglec-H-OVA and CpG, and exosomes were isolated using an Invitrogen kit. Isolated exosomes and total cell lysates were subjected to Western blot for CD63, Tsg101, and β-actin. (C) pDCs-produced exosomes transferred antigens to cDCs. cDCs were cultured either alone or with pDCs-generated exosomes and subjected to flow cytometry. Expression of MHCI-OVA (H-2Kb-SIINFEKL) on cDCs is shown. (D) pDCs-produced exosomes induced a dose-dependent priming of naive OTI cells that is dependent on the presence of cDCs. pDCs-generated exosomes were added to OTI cells in the presence or absence of naive cDCs and subjected to flow cytometry. Percentages of total proliferated cells (CFSElow) and proliferated IFN-γ+ cells (CFSElowIFN-γ+) out of total OTI cells are shown. Data shown are representative of three or more independent experiments.
As our data have suggested pDCs transferred antigens to cDCs to prime CD8 T cells, we asked whether pDC supernatants also transferred antigens to cDCs. Coculture of naive cDCs with pDC supernatants led to significant expression of MHCI-OVA (H-2Kb-SIINFEKL) on cDCs (SI Appendix, Fig. S3B). Taken together, our data suggest that pDCs confer on bystander cDCs the ability to prime CD8 T cells in a cell contact-independent manner, likely by transferring antigens to cDCs through soluble factor(s).
pDC-Generated Exosomes Transferred Antigens to Naive cDCs, Rendering These cDCs Competent for Priming of Antigen-Specific CD8 T Cells.
Next we investigated what soluble factors were employed by pDCs to transfer antigens to cDCs. As DCexos have been shown to transfer MHC/antigen complexes by cross-dressing (85–87), and the size of exosomes (30 to 150 nM in diameter) would allow them easy passage through the 3-μM pores of the Transwells we used in SI Appendix, Fig. S3A, we thus asked whether pDCs generated exosomes and if so, whether pDCexos mediated antigen transfer from pDCs to cDCs. pDCs were cultured in media containing exosome-free fetal bovine serum (FBS) with anti-Siglec-H-OVA plus CpG, and exosomes were purified according to the manufacturer’s manual. Western blot analysis showed that pDCexos were positive for exosome markers CD63, TSG101 (Fig. 5B), confirming that pDCs cultured with anti-Siglec-H-OVA plus CpG indeed generated exosomes. Nanoparticle tracking analysis (NTA) of the isolated exosomes revealed homogenous size distributions with median diameter of 83 ± 1.3 nM and about 1.4 ± 0.2 × 109 exosomes/106 pDCs in one representative preparation.
Next we asked whether pDCexos transferred antigens to cDCs. pDCexos were cultured with freshly isolated cDCs, and the expression of MHCI-OVA (H-2Kb-SIINFEKL) was examined. As shown in Fig. 5C, addition of pDCexos (at 200 μL/1 × 106 pDCs) led to significant up-regulation of MHCI-OVA in bystander cDCs, suggesting that pDCexos indeed transferred OVA antigen to cDCs. We thus asked whether pDCexos could similarly render naive bystander cDCs competent for CD8 T cell priming. Addition of pDCexos to cDC/OTI cocultures led to a dose-dependent increase in OTI proliferation and their differentiation into IFN-γ-producing effectors, whereas addition of pDCexos to OTI cells without bystander cDCs induced no proliferation (Fig. 5D). Thus, our data suggest that pDCs targeted by anti-Siglec-H-OVA employ a mechanism to prime antigen-specific CD8 T cells by transferring antigens to cDCs through exosomes.
In addition to TLR9, pDCs also express TLR7, and stimulation with TLR7 agonist R848 and CpG (1668, type B) have been shown to lead to distinctively different activation-induced pDC populations (5). We thus asked whether targeting pDCs with anti-Siglec-H-OVA plus the TLR7 agonist R848 cross-prime naive CD8 T cells through exosomes. Exosomes were isolated from supernatants of pDCs cultured with anti-Siglec-H-OVA plus R848. Similar to exosomes generated from pDCs cultured with anti-Siglec-H-OVA plus CpG, R848-treated pDC-derived exosomes induced OTI proliferation and their differentiation into IFN-γ-producing effectors only in the presence of bystander cDCs (SI Appendix, Fig. S4A). Thus, pDC-targeted vaccine via Siglec-H with either TLR7 or TLR9 agonists as adjuvant cross-primed naive CD8 T cells through a similar mechanism involving exosomes.
As we had clearly shown that cross-presenting pDCs achieved cross-priming through pDCexos with model antigen ovalbumin, we next asked whether pDCexo-mediated cross-priming also applied to other antigens. We have thus cloned the human melanoma antigen gp100 (hgp100) into anti-Siglec-H-OVA constructs to express anti-Siglec-H-hgp100. Exosomes were similarly isolated from pDCs cultured with anti-Siglec-H-hgp100 plus CpG, and added to gp100-specific CD8 T cells (pme-1) with or without cDCs. While addition of pDCexos to cDCs/pmel-1 led to significant pmel-1 cell proliferation and differentiation into IFN-γ-producing effectors, addition of pDCexos to pmel-1 cells without bystander cDCs led to no proliferation (SI Appendix, Fig. S4B), suggesting that the mechanism of pDCexo-mediated cDC-dependent cross-priming is not limited to OVA antigen.
Taken together, our data suggest that pDCs targeted by anti-Siglec-H-antigens employ a mechanism to prime antigen-specific CD8 T cells by transferring antigens to cDCs through exosomes.
pDCs Treated with Anti-Bst2-OVA or Soluble OVA Protein Similarly Produced Exosomes to Cross-Prime OVA-Specific CD8 T Cells by Transferring Antigens to cDCs.
As Siglec-H exhibits distinct functions in pDCs (48, 88), we asked whether the mechanism that pDCs enable cDCs to prime CD8 T cells by transferring antigens to cDCs through exosomes is unique to targeting pDCs via Siglec-H. We had previously generated another pDC-targeting antibody against pDC receptor Bst2 (72), which differs from pDC-targeted anti-Siglec-H-antigen in inducing CD4 and CD8 T cell immunity (71).
We first asked whether targeting pDCs with anti-Bst2-OVA also required cDCs to cross-prime antigen-specific CD8 T cells. CD11c-DTR→WT bone marrow chimeras were treated with DT and cross-priming was examined as with anti-Siglec-H-OVA. While chimeras treated with PBS exhibited strong cross-priming of naive OTI cells resulting in strong proliferation and differentiation into IFN-γ-producing effectors following immunization of anti-Bst2-OVA plus CpG, both OTI cell proliferation and differentiation into IFN-γ-producing effectors were significantly impaired in DT-treated chimeras (Fig. 6 A and B), suggesting that cDCs are also required for cross-priming by in vivo DC vaccine targeting pDCs with anti-Bst2-OVA.
Fig. 6.
pDCs treated with anti-Bst2-OVA and soluble ovalbumin protein similarly generated exosomes to cross-prime OVA-specific CD8 T cells by transferring antigens to cDCs. (A and B) cDCs play a critical role in cross-priming upon immunization with pDC-targeting anti-Bst2-OVA and CpG. CD11c-DTR→WT bone marrow chimeras (n = 4) were treated with DT or PBS on days −2, 0, and 2, and immunized with anti-Bst2-OVA plus CpG following adoptive transfer of CFSE-labeled naive OTI cells. The percentages of Thy1.1+ OTI cells out of total CD8 T cells are depicted in A, and the percentages of IFN-γ+ cells out of total Thy1.1+ OTI cells are shown in B. (C) cDCs expressed functional MHCI-OVA upon coculture with pDC-derived exosomes. cDCs were cultured alone or with exosomes from pDCs treated with anti-Bst2-OVA plus CpG, and subjected to flow cytometry. Expression of MHCI-OVA (H-2Kb-SIINFEKL) on cDCs is shown. (D) Exosomes from WT pDCs treated with anti-Bst2-OVA plus CpG induced cDC-dependent priming of naive OTI CD8 T cells. WT pDCs were cultured with anti-Bst2-OVA plus CpG and isolated exosomes were added to OTI cells in the presence or absence of naive WT cDCs. Percentages of total proliferated cells (CFSElow) and proliferated IFN-γ+ cells (CFSElowIFN-γ+) out of total OTI cells were shown. (E) Exosomes from WT pDCs treated with OVA protein plus CpG induced cDC-dependent priming of naive OTI CD8 T cells. WT pDCs were cultured with soluble OVA protein plus CpG, and isolated exosomes were cultured with naive OTI cells with or without bystander cDCs. OTI cell proliferation and differentiation are presented as in D. (F) cDCs expressed functional MHCI-OVA upon coculture with exosomes from pDCs treated with ovalbumin protein. cDCs were cultured alone or with exosomes from pDCs treated with soluble OVA protein and subjected to flow cytometry. Expression of MHCI-OVA (H-2Kb-SIINFEKL) on cDCs is shown. Data shown are representative of at least two independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
We next asked whether pDCs targeted by anti-Bst2-OVA employed a similar mechanism involving exosomes to cross-prime OTI CD8 T cells. Addition of exosomes from pDCs cultured with anti-Bst2-OVA plus CpG to naive cDCs led to the expression of MHCI-OVA (H-2Kb-SIINFEKL) on these cDCs (Fig. 6C), suggesting that exosomes from pDCs targeted by anti-Bst2-OVA similarly mediated the transfer of antigens to cDCs. More importantly, exosomes from pDCs cultured with anti-Bst2-OVA plus CpG similarly induced a dose-dependent proliferation and effector differentiation of naive OTI cells in the presence of cDCs (Fig. 6D), suggesting that pDCs targeted with anti-Bst2-antigen (OVA) and anti-Siglec-H-antigen shared the mechanism of transferring antigens via exosomes to cDCs to cross-prime antigen-specific CD8 T cells.
As activated pDCs have been shown to cross-present soluble protein in vivo (46), we asked whether pDCs might produce exosomes for cross-priming with soluble proteins. Not surprisingly, exosomes from pDCs cultured with soluble OVA protein primed naive OTI cells only in the presence of cDCs (Fig. 6E), suggesting that pDCs loaded with soluble proteins might also prime antigen-specific CD8 T cells through a cDC-dependent mechanism mediated by pDCexos. Addition of exosomes from pDCs cultured with OVA protein plus CpG to naive cDCs led to the expression of MHCI-OVA on these cDCs (Fig. 6F), suggesting that exosomes from pDCs loaded with soluble OVA protein could similarly mediate the transfer of antigens to cDCs. Taken together, our studies suggested that pDCs could employ an exosome-mediated and cDC-dependent mechanism for cross-priming under multiple settings.
Discussion
In this report we have identified a cDC-dependent mechanism for pDCs to achieve cross-priming in vivo. Although extensive studies have shown that pDCs play critical roles in viral immunity, autoimmunity, and immune tolerance, conflicting results on whether pDCs played a critical role in cross-priming in vivo have been reported (41, 43, 45–48). Surprisingly, the opposite results seem to be on the priming of CD8 T cells instead of cross-presentation, as both human and murine pDCs have been shown to be capable of cross-presentation (33–37, 46). The involvement of pDCs in cross-presentation in vivo is further supported by recent evidence suggesting that pDCs transported antigens to promote immune tolerance in vivo (74, 89). However, the efforts to delineate the precise role of pDCs in cross-priming in vivo were further complicated as IFN-I produced by pDCs efficiently activates and recruits cDCs, B cells, T cells, and NK cells to indirectly affect CD8 T cell priming (90). In this report, we were able to delineate the contribution of pDCs versus cDCs in in vivo cross-priming and uncover an unexpected mechanism by which cross-presenting pDCs required cDCs to cross-prime CD8 T cells in vivo, taking advantage of a pDC-targeted vaccine model which confines cross-presentation specifically to pDCs. Using an in vitro culture system where we only allowed pDCs the access to antigens, we further demonstrated that cross-presenting pDCs primed naive antigen-specific CD8 T cells only in the presence of bystander cDCs, thus confirming the requirement of cDCs for pDCs to achieve cross-priming. Moreover, the in vitro system allowed us to directly demonstrate that cross-presenting pDCs transferred antigens to bystander cDCs, consistent with our observation in vivo. Taken together, this report uncovers a mechanism that cross-presenting pDCs achieve cross-priming by transferring antigens to cDCs in vitro and in vivo. This mechanism that cross-presenting pDCs require the help of cDCs to prime CD8 T cells by transferring antigens to bystander cDCs has not been previously reported, and likely provides a mechanistic explanation for the reported opposite results regarding whether pDCs cross-prime CD8 T cells in vivo. For instance, the lack of cross-priming by pDCs in cDC-depleted CD11c-DTR mice (45), similar to our observation (Fig. 1), could be explained by the requirement of cDCs instead of pDCs’ inability to cross-prime CD8 T cells in vivo. Our findings additionally provide a mechanism for the synergistic effects of pDCs and cDCs on cross-priming. Of note, recent studies have shown that both pDCs and cDCs are required to achieve optimal cross-priming and CD8 T cell immunity under diverse conditions (50–55), although the underlying mechanisms for the synergy were not well understood. In light of our findings, future studies are warranted to determine whether and how pDC-mediated antigen transfer to cDCs plays a role in enhancing cross-priming and CD8 T cell immunity.
Another interesting finding was that while CD4 T cell tolerance was observed upon immunization with anti-Siglec-H-OVA without or with adjuvants (71), CD8 T cell tolerance was only observed upon immunization with anti-Siglec-H-OVA alone. Addition of the TLR9 agonist CpG, which directly activated both pDCs and cDCs, reversed CD8 T cell tolerance leading to CD8 T cell immunity, suggesting that immunization with anti-Siglec-H-OVA plus adjuvants simultaneously led to CD4 T cell tolerance and CD8 T cell immunity. The observed CD8 T cell immunity is not entirely surprising, as immunization with OVA-conjugated anti-Siglec-H plus CpG has previously been shown to generate CD8 T cell immunity, presumably through cross-presentation (91). Furthermore, pDCs have been shown to play a negative role in CD4 T cell responses but a positive role in CD8 T cell immunity in vivo (48). How pDCs achieved CD8 T cell immunity while maintained CD4 T cell tolerance is not entirely clear. However, neither regulatory T cells nor the maturation/cytokine production of pDCs (by comparing pDCs upon immunization of anti-Siglec-H-OVA and anti-Bst2-OVA plus CpG) has been shown to play a role in CD4 T cell tolerance (71), suggesting that CD4 and CD8 T cell tolerance might be independent instead of systematically regulated. Interestingly, while MHCI-Ag complexes were detected in both pDCs and cDCs, MHCII-Ag complexes were only observed on pDCs but not cDCs (71), suggesting that cDCs were used differently in the priming of CD4 versus CD8 T cells. Further studies are required to elucidate the mechanisms of how pDC-targeted immunization through Siglec-H differentially regulates CD4 and CD8 T cell tolerance/responses.
Among cDCs, Batf3-dependent cDC1s are recognized as the superior APCs in cross-presenting exogenous antigens including tumor antigens to prime CD8 T cells (75, 92, 93). However, cDC2s have also been shown to play a preeminent role in priming CD8 T cells in studies of cross-dressed DCs. For instance, although both cDC1s and cDC2s have been shown to prime CD8 T cells by acquiring antigens through cross-dressing (81), cross-dressed CD8− cDC2s but not CD8+ cDC1s have been shown to play a critical role in CD8 T cell priming in viral infection models (82, 84). As to whether cDC2s were the preferred recipients of antigens was not directly examined, it remains to be determined whether the critical role of cDC2s was due to their enhanced abilities at acquiring antigens and/or priming CD8 T cells. On the other hand, Li et al. have shown that cross-dressed Batf3-dependent cDC1s played a critical role in priming both naive and memory CD8 T cells after DNA and cellular vaccination (83). However, a direct comparison of cDC1s and cDC2s in acquiring antigens was not performed in this study, thus it’s also unclear whether cDC1s’ critical role was due to their capacity in acquiring antigens or priming CD8 T cells. In this report, we were able to show that Batf3-dependent cDC1s have a nonredundant role in priming naive CD8 T cells to differentiate into effector cells. Furthermore, we have also demonstrated that CD8− cDC2s and CD8+ cDC1s were equally efficient in acquiring antigens from pDCs, indicating that cDC1s’ essential role in cross-priming CD8 T cells for effector differentiation is independent of their ability in cross-presentation or receiving antigens from pDCs. Thus, while this study has focused on the expression of MHCI-Ag complexes or Signal 1 on bystander cDCs through antigen transfer, other mechanisms, including maturation (Signal 2), cytokine production such as IL-12 (Signal 3) of cDC1s, and pDC/pDCexo-mediated transfer of other molecules besides antigens, likely contribute to the critical roles cDC1s play in pDC-mediated cross-priming. Indeed, IL-12 production by cDC1s has been shown to play a critical role in cross-priming and CTL responses in a tumor setting (94), although the involvement of cDC1-produced IL-12 in CD8 T cell priming might be context dependent (95, 96). Taken together, our data suggest that cDC1s also exhibit increased capacity in priming CD8 T cells to differentiate into effectors compared to cDC2s postantigen presentation. It’s interesting to note that in Li et al.’s and our studies on vaccination models, cDC1s played a critical role in cross-priming after acquiring antigens (83), while cDC2s were the critical player in viral infection (82, 84), suggesting that cDC1s and cDC2s might have a division of labor in cross-priming depending on different settings. Together, these studies depict a picture that different coordination/cooperation of multiple subsets of DCs are utilized to mount sophisticated immune responses to diverse challenges. Thus, it will be interesting to investigate the roles and regulation of cDC1s and cDC2s in cross-priming with regards to their acquiring antigens and priming CD8 T cells under different conditions, including viral infection, inflammation, and vaccination.
As pDCexos have not been reported, current studies of DCexos are almost exclusively focusing on exosomes from cDCs (65, 97). These DCexos (exosomes from cDCs) have been shown to prime CD8 T cells either directly, or indirectly by transferring antigens to bystander DCs (82, 86, 87, 98), thus providing an alternative mechanism for cDCs to achieve cross-priming. Using a pDC-targeted vaccine model, we have now demonstrated that pDCs produced exosomes, and these pDCexos mediated antigen transfer from pDCs to bystander cDCs. Functionally, pDCexos primed naive antigen-specific CD8 T cells only in the presence of bystander cDCs, similarly to cross-presenting pDCs. Together with our finding that cross-presenting pDCs were unable to efficiently prime naive antigen-specific CD8 T cells by themselves, our data suggested that pDCs employed a unique mechanism of pDCexo-mediated antigen transfer to cDCs to achieve cross-priming. The generation of pDCexos was not limited to the model system we used, which targeted model antigen ovalbumin to Siglec-H on pDCs. We have additionally shown that pDCexos were generated by pDCs under various conditions including with a tumor antigen, with antigen targeted to Bst2 and soluble protein. More importantly, all these pDCexos similarly primed antigen-specific CD8 T cells only in the presence of bystander cDCs, suggesting that the pDCexo/cDCs pathway might also play a role in cross-priming under these settings. However, whether the pDCexo/cDCs pathway plays a critical role in cross-priming when soluble proteins not specifically targeted to pDCs were administrated in vivo is not clear. In this regard, previous studies on pDCs’ function in cross-priming CD8 T cells upon administration of soluble OVA might provide some insight. Using Siglec-H-DTR mice, Takagi et al. have shown that OVA-specific CD8 T cell responses were reduced after pDC depletion, suggesting that pDCs might play a positive role in cross-priming CD8 T cells (48). However, other Siglec-H+ cells such as macrophages instead of pDCs might also be responsible for the reduced cross-priming, and whether pDCs functioned in cross-priming with OVA antigens and whether cDCs were required for pDCs’ function in cross-priming were not directly examined (48). In another study by Mouriès et al., isolated pDCs from mice that were administrated soluble OVA have been shown to prime naive OTI cells ex vivo (46), suggesting that cDCs and the pDCexo/cDCs pathway were not required or involved for pDCs’ function in cross-priming. Thus, whether antigens were specifically targeted through receptors to pDCs or not likely affects whether pDCs cross-present such antigens and employ the pDCexo/cDCs pathway to cross-prime CD8 T cells in vivo. In light of our findings on pDCexos, future work is warranted to directly test whether pDCs transfer different nontargeted antigens through pDCexos to promote cross-priming in vivo. DCexos have garnered much interest for their potential as cell-free therapeutic agents, as being inert vesicles they are more resistant to immunomodulation (i.e., tumor microenvironment) and amenable to the manufacturing process at lower cost compared to DCs (65, 99). Indeed, vaccines with tumor antigen-pulsed DCexos have exhibited better efficacy than DC vaccines in preclinical tumor models (100). In a transplantation model, a recent study has shown that donor DCexos (from cDCs) instead of DCs also played a major role in priming allogenic CD4 and CD8 (cross-priming) T cells (101). However, all three DCexo clinical trials using autologous antigen-loaded DCexos (from cDCs) failed to generate the desired T cell responses (102–104), suggesting that there’s a critical need to better understand how DCexos mediate cross-priming. The identification of pDCexos and their function in cross-priming thus offered a welcome addition to the DCexo repertoire. Further studies of these identified pDCexos are required to determine their function/regulation and potential application.
Materials and Methods
Mice.
C57BL/6, CD11c-DTR, Batf3−/−, and the gp100-specific TCR transgenic Thy1.1+ Pmel-1 mice were purchased from The Jackson Laboratory. CD45.1 mice were purchased from Charles River Laboratories. CD8 TCR transgenic Thy1.1+ Rag1−/− OTI mice were generated as described previously (105), and Thy1.1+ Rag1−/− Pmel-1 mice were generated similarly. For the generation of bone marrow chimeras, lethally irradiated CD45.1 B6 mice were reconstituted with 3 to 5 × 106 bone marrow cells containing CD11-c-DTR bone marrow cells (CD45.2). All procedures on animals followed protocols approved by the Institutional Animal Care and Use Committee at Roswell Park Comprehensive Cancer Center.
Antibodies and Reagents.
Anti-CD11c and anti-mPDCA-1 (Bst2) magnetic microbeads, antibodies to Siglec-H, Bst2, and B220, mouse CD8α+ T cell isolation kit, mouse plasmacytoid DC isolation kit, and mouse pan DC isolation kit were purchased from Miltenyi Biotec. Anti-Siglec-H-OVA and anti-Bst2-OVA have been described previously (71, 72). Recombinant human Flt3-L was purchased from BioXcell. Antibodies to Thy1.1, TCR Vα2, and Vβ5.1/5.2, Vβ13, CD8α, Siglec-H, Bst-2, B220, CD63, CD45.1, CD45.2, CD44, CD62L, CD80, CD86, MHC class II I-Ab, and TNF-α were purchased from Biolegend Inc. Brefeldin A (BFA), antibodies to CD11c, H-2Kb-SIINFEKL, and IFN-γ were purchased from eBioscience. Total exosome isolation kits (from cell culture media) were purchased from Thermo Fisher Scientific. CpG 1668 was purchased from Invitrogen or obtained from Oligo Factory. The 3-μM cell Falcon culture inserts were purchased from Corning. Staining for surface and intracellular antigen expression was performed as previously described (106). In brief, cells from spleen and pooled draining LNs were stimulated for 5 h with OTI or hgp10025–33 peptide (1 to 4 μg/mL, AnaSpec Inc.) in the presence of BFA (5 μg/mL), stained for cell surface protein expression followed by fixation and permeabilization, and staining for intracellular antigens like IFN-γ. Where indicated, OTI CD8 T cells were labeled with CFSE and checked by flow cytometry before transfer. We used a Fortessa (BD Biosciences) with subsequent data analysis using FlowJo (Tree Star).
Isolation and Purification of TCR Transgenic CD8 T Cells and DCs.
Naive OTI and Pmel-1 Thy1.1+ CD8 T cells were isolated from pooled LNs of Thy1.1+ Rag1−/− OTI and Thy1.1+ Rag1−/− Pmel-1 mice, respectively, and routinely checked with Thy1.1, CD44, and CD62L for purity and activation. The percentages of Thy1.1+ cells were around 85 to 95%. For cross-priming assays, either in vivo or in vitro, naive OTI Thy1.1+ CD8 T cells were labeled with 5 μM CFSE (Molecular Probes) in 5% FCS PBS at room temperature for 15 min and washed three times before injection or plating. To expand DCs, mice were injected with Flt3-L for 6 to 15 d. To purify total DCs from spleen, we used either anti-CD11c-conjugated beads or pan-DC isolation kit and columns (Miltenyi Biotech) following the manufacturer’s protocols. For isolation of cDCs depleted of pDCs, we have modified the protocol to use anti-Siglec-H-biotin, anti-B220-biotin, and either anti-Bst2-biotin or anti-Bst2 microbeads together with pan-DC isolation kit. The isolated cDCs generally contain <2% of pDCs. To purify pDCs, mice were generally treated with Flt3-L for 12 to 15 d when the percentages of cDCs start to turn lower. Flt3L-treated mouse spleen cells were first incubated with small amounts of anti-CD11c microbeads to deplete cDCs, followed by pDC isolation with anti-Bst2 microbeads according to the manufacturer’s protocols. The isolated pDCs generally contain <5% of CD11chigh cDCs.
Immunization and DT Treatment.
For immunization (vaccination), anti-Siglec-H-OVA and anti-Bst2-OVA (10 to 20 μg/per mouse) were injected i.v. into the tail veins in 200 μL PBS, and CpG (50 to 100 μg/per mouse) was injected s.c. at two sites on hind legs in 200 μL PBS. For studying DC vaccination-induced CD8+ T cell responses, 0.5 to 2 × 106 naive OTI Thy1.1+ CD8+ T cells were adoptively transferred i.v. into tail veins 1 d before immunization. Mice were examined 3 to 4 d after immunization for primary CD8 T cell response: briefly, LN and spleen cells were stimulated for 5 h in the presence of BFA and OTI peptide, stained for surface Thy1.1, CD8, and intracellular cytokines (i.e., IFN-γ), and evaluated by flow cytometry. For recalled CD8 T cell response, immunized mice were challenged at day 21 after immunization, with chicken ovalbumin (Worthington Biochemical) and CFA (Sigma-Aldrich) emulsion that was injected s.c. into four legs in 200 μL, and spleen and LN cells were examined for CD8 T cells response as above 5 d after challenge. For DT experiments with CD11c-DTR→WT bone marrow chimeras, chimeric mice were treated with DT or PBS on days −2, 0, and 2, and immunized on day 0.
Cross-Priming Assays and DC-T Cell Cocultures.
For in vivo cross-priming assay, mice were immunized with anti-Siglec-H-OVA, or anti-Bst2-OVA plus CpG, followed by adoptive transfer of 0.5 to 2 × 106 CFSE-labeled naive Thy1.1+ OTI cells. LN and spleen cells were isolated 3 to 4 d after immunization, stimulated for 5 h in the presence of BFA and OTI peptide, stained for surface Thy1.1, CD8, and intracellular antigens like IFN-γ, and subjected to flow cytometry to evaluate proliferation by CFSE dilution and effector differentiation by IFN-γ production. For cross-priming of Flt3-l-treated Batf3−/− mice, Batf3−/− mice were treated with Flt3-L every day for the duration of the experiments, immunized with anti-Siglec-H-OVA plus CpG on day 9 following OTI adoptive transfer.
For in vitro cross-priming, 1 × 105 naive OTI cells were cultured in u-bottom 96-well plates, and 2 × 104 cDCs were added when indicated. For pulsed pDCs, isolated pDCs were pulsed with anti-Siglec-H-OVA, or anti-Bst2-OVA plus CpG for 4 h, thoroughly washed and added to OTI as indicated. For pDC supernatants, pulsed pDCs were cultured for 2 to 3 d and supernatants were obtained by centrifugation. For pDC-derived exosomes, exosomes from 1 × 106 pDCs (about 1.4 ± 0.2 × 109 exosomes) were resuspended in 200 μL of cultured media and added to OTI with or without cDCs as indicated. The various cocultures were stimulated for 5 h in the presence of BFA and antigens and subjected to flow cytometry to evaluate proliferation by CFSE dilution and effector differentiation by IFN-γ production.
Isolation and Analysis of Exosomes.
pDCs were cultured in RPMI media with exosome-depleted FBS, and exosomes were obtained by using Total Exosome Isolation Kit according to the manufacturer’s protocols. NTA of the isolated exosomes was carried out with Zetaview (Particle Metrix, Gmbh). For Western blotting, pDC-produced exosomes were lysed and subjected to Western blot analysis with anti-β-actin, anti-CD63, and anti-Tsg101 according to the manufacturer’s protocols.
Statistical Analysis.
The statistical significance of experimental results was evaluated with Excel or GraphPad Prism 8 using two-tailed unpaired two-sample Student’s t test. P values less than 0.05 were considered significant and are denoted as NS > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Yi Zhang, Amy Kemper, and Zheng Gang Zhang for technical support. This work was supported by a bridge grant from the Roswell Park Comprehensive Cancer Center, an award from the Roswell Park Alliance Foundation, National Cancer Institute Cancer Center Support grants 5P30 CA016056 and R01CA198105 (to A.J.), and an internal grant from the Henry Ford Health System.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002345117/-/DCSupplemental.
Data Availability.
All data for this paper are included in the manuscript and SI Appendix.
References
- 1.Steinman R. M., Some interfaces of dendritic cell biology. APMIS 111, 675–697 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Colonna M., Trinchieri G., Liu Y. J., Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Gilliet M., Cao W., Liu Y. J., Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Swiecki M., Colonna M., The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alculumbre S. G. et al., Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol. 19, 63–75 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Mitchell D., Chintala S., Dey M., Plasmacytoid dendritic cell in immunity and cancer. J. Neuroimmunol. 322, 63–73 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Anderson D. A. 3rd, Murphy K. M., Briseño C. G., Development, diversity, and function of dendritic cells in mouse and human. Cold Spring Harb. Perspect. Biol. 10, a028613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X., Chapman N. M., Chi H., Emerging roles of cellular metabolism in regulating dendritic cell subsets and function. Front. Cell Dev. Biol. 6, 152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alculumbre S. et al., Plasmacytoid pre-dendritic cells (pDC): From molecular pathways to function and disease association. Semin. Cell Dev. Biol. 86, 24–35 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Musumeci A., Lutz K., Winheim E., Krug A. B., What makes a pDC: Recent advances in understanding plasmacytoid DC development and heterogeneity. Front. Immunol. 10, 1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goubier A. et al., Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guéry L., Hugues S., Tolerogenic and activatory plasmacytoid dendritic cells in autoimmunity. Front. Immunol. 4, 59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treilleux I. et al., Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 10, 7466–7474 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Demoulin S., Herfs M., Delvenne P., Hubert P., Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: Insight into the molecular mechanisms. J. Leukoc. Biol. 93, 343–352 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Aspord C., Leccia M. T., Charles J., Plumas J., Melanoma hijacks plasmacytoid dendritic cells to promote its own progression. OncoImmunology 3, e27402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Wu J., Zhu S., Liu Y. J., Chen J., Disease-associated plasmacytoid dendritic cells. Front. Immunol. 8, 1268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saadeh D., Kurban M., Abbas O., Plasmacytoid dendritic cell role in cutaneous malignancies. J. Dermatol. Sci. 83, 3–9 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Tel J. et al., Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 73, 1063–1075 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Lombardi V. C., Khaiboullina S. F., Rizvanov A. A., Plasmacytoid dendritic cells, a role in neoplastic prevention and progression. Eur. J. Clin. Invest. 45 (suppl. 1), 1–8 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Veglia F., Gabrilovich D. I., Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 45, 43–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westdorp H. et al., Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunother. Cancer 7, 302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bol K. F. et al., The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J. Immunother. Cancer 7, 109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colonna M., Cella M., Crosspresentation: Plasmacytoid dendritic cells are in the business. Immunity 27, 419–421 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Villadangos J. A., Young L., Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Melief C. J., Cancer immunotherapy by dendritic cells. Immunity 29, 372–383 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Joffre O. P., Segura E., Savina A., Amigorena S., Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Kurts C., Robinson B. W., Knolle P. A., Cross-priming in health and disease. Nat. Rev. Immunol. 10, 403–414 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Paulete A. R. et al., Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol. 28 (suppl. 12), xii44-xii55 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Broz M. L. et al., Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Canc. Cell 26, 638–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spranger S., Bao R., Gajewski T. F., Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Salmon H. et al., Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Paulete A. R. et al., Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 6, 71–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffel G. et al., Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 27, 481–492 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Di Pucchio T. et al., Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 9, 551–557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klechevsky E. et al., Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 116, 1685–1697 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segura E., Durand M., Amigorena S., Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 210, 1035–1047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberkampf M. et al., Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat. Commun. 9, 2241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nierkens S., Tel J., Janssen E., Adema G. J., Antigen cross-presentation by dendritic cell subsets: One general or all sergeants? Trends Immunol. 34, 361–370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adiko A. C., Babdor J., Gutiérrez-Martínez E., Guermonprez P., Saveanu L., Intracellular transport routes for MHC I and their relevance for antigen cross-presentation. Front. Immunol. 6, 335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Embgenbroich M., Burgdorf S., Current concepts of antigen cross-presentation. Front. Immunol. 9, 1643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemann E. A., Sjaastad L. E., Langlois R. A., Legge K. L., Plasmacytoid dendritic cells require direct infection to sustain the pulmonary influenza A virus-specific CD8 T cell response. J. Virol. 90, 2830–2837 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H. K. et al., Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 206, 359–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GeurtsvanKessel C. H. et al., Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J. Exp. Med. 205, 1621–1634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salio M., Palmowski M. J., Atzberger A., Hermans I. F., Cerundolo V., CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199, 567–579 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapoznikov A. et al., Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 204, 1923–1933 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouriès J. et al., Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood 112, 3713–3722 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama M. et al., Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood 113, 2088–2095 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Takagi H. et al., Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity 35, 958–971 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Moffat J. M. et al., Targeting antigen to bone marrow stromal cell-2 expressed by conventional and plasmacytoid dendritic cells elicits efficient antigen presentation. Eur. J. Immunol. 43, 595–605 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Yoneyama H. et al., Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202, 425–435 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou Y. et al., Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J. Immunol. 178, 1534–1541 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Liu C. et al., Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J. Clin. Invest. 118, 1165–1175 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers G. L. et al., Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood 129, 3184–3195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brewitz A. et al., CD8+ T cells orchestrate pDC-XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 46, 205–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lozza L. et al., Crosstalk between human DC subsets promotes antibacterial activity and CD8+ T-cell stimulation in response to bacille Calmette-Guérin. Eur. J. Immunol. 44, 80–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aspord C. et al., HLA-A(*)0201(+) plasmacytoid dendritic cells provide a cell-based immunotherapy for melanoma patients. J. Invest. Dermatol. 132, 2395–2406 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Wimmers F., Schreibelt G., Sköld A. E., Figdor C. G., De Vries I. J., Paradigm shift in dendritic cell-based immunotherapy: From in vitro generated monocyte-derived DCs to naturally circulating DC subsets. Front. Immunol. 5, 165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas M. et al.; IMPULSE study team , Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: Results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann. Oncol. 29, 2076–2084 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nierkens S. et al., Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 71, 6428–6437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Théry C., Zitvogel L., Amigorena S., Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Colombo M., Raposo G., Théry C., Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Kalluri R., The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jella K. K. et al., Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel) 6, 69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., Yin Y., Lai R. C., Lim S. K., Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 5, 518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitt J. M. et al., Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest. 126, 1224–1232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindenbergh M. F. S., Stoorvogel W., Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu. Rev. Immunol. 36, 435–459 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Kowal J., Tkach M., Dendritic cell extracellular vesicles. Int. Rev. Cell Mol. Biol. 349, 213–249 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Bastos-Amador P. et al., Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. J. Leukoc. Biol. 91, 751–758 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Bracamonte-Baran W. et al., Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc. Natl. Acad. Sci. U.S.A. 114, 1099–1104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salvi V. et al., Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 3, e98204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loschko J. et al., Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J. Immunol. 187, 6346–6356 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Loschko J. et al., Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective T cell-mediated immunity. J. Immunol. 186, 6718–6725 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Bar-On L., Jung S., Defining in vivo dendritic cell functions using CD11c-DTR transgenic mice. Methods Mol. Biol. 595, 429–442 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Hadeiba H. et al., Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity 36, 438–450 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hildner K. et al., Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hassan M. et al., Flt3L treatment of bone marrow donors increases graft plasmacytoid dendritic cell content and improves allogeneic transplantation outcomes. Biol. Blood Marrow Transplant. 25, 1075–1084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein O. et al., Flt3 ligand expands CD4+ FoxP3+ regulatory T cells in human subjects. Eur. J. Immunol. 43, 533–539 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Neijssen J. et al., Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Mendoza-Naranjo A. et al., Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 178, 6949–6957 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Dolan B. P., Gibbs K. D. Jr., Ostrand-Rosenberg S., Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J. Immunol. 177, 6018–6024 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Smyth L. A. et al., The relative efficiency of acquisition of MHC:peptide complexes and cross-presentation depends on dendritic cell type. J. Immunol. 181, 3212–3220 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Wakim L. M., Bevan M. J., Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471, 629–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L. et al., Cross-dressed CD8α+/CD103+ dendritic cells prime CD8+ T cells following vaccination. Proc. Natl. Acad. Sci. U.S.A. 109, 12716–12721 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smyth L. A. et al., Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J. Immunol. 189, 2274–2282 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Théry C. et al., Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3, 1156–1162 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Nakayama M., Antigen presentation by MHC-dressed cells. Front. Immunol. 5, 672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campana S., De Pasquale C., Carrega P., Ferlazzo G., Bonaccorsi I., Cross-dressing: An alternative mechanism for antigen presentation. Immunol. Lett. 168, 349–354 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Blasius A. L., Colonna M., Sampling and signaling in plasmacytoid dendritic cells: The potential roles of Siglec-H. Trends Immunol. 27, 255–260 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Ennamorati M. et al., Intestinal microbes influence development of thymic lymphocytes in early life. Proc. Natl. Acad. Sci. U.S.A. 117, 2570–2578 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guéry L. et al., Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 74, 6430–6440 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Zhang J. et al., Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 107, 3600–3608 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Bandola-Simon J., Roche P. A., Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol. 113, 31–37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chrisikos T. T. et al., Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol. Immunol. 110, 24–39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garris C. S. et al., Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity 49, 1148–1161.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui W., Joshi N. S., Jiang A., Kaech S. M., Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27, 2177–2187 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Theisen D. J. et al., WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362, 694–699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian H., Li W., Dendritic cell-derived exosomes for cancer immunotherapy: Hope and challenges. Ann. Transl. Med. 5, 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.André F. et al., Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172, 2126–2136 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Bell B. M., Kirk I. D., Hiltbrunner S., Gabrielsson S., Bultema J. J., Designer exosomes as next-generation cancer immunotherapy. Nanomedicine (Lond.) 12, 163–169 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Zitvogel L. et al., Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 4, 594–600 (1998). [DOI] [PubMed] [Google Scholar]
- 101.Liu Q. et al., Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Invest. 126, 2805–2820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Escudier B. et al., Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 3, 10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morse M. A. et al., A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 3, 9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Besse B. et al., Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology 5, e1071008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang X., et al. , β-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8+ T cells. J. Leukoc. Biol. 95 (1), 179–190, 10.1189/jlb.0613330 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Jiang A. et al., Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 27, 610–624 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for this paper are included in the manuscript and SI Appendix.