Abstract
The Flint water crisis raised questions about the factors resulting in unacceptable soluble lead concentrations in the city’s drinking water. Although water treatment strategies, failure to follow regulations, and unethical behavior were all factors, knowledge deficits at the intersection of several scientific fields also contributed to the crisis. Pursuit of opportunities to address unresolved scientific questions can help avert future lead poisoning disasters. Such advances will enable scientifically based, data-driven risk assessments that inform decisions involving drinking water systems. In this way, managers and decision makers can anticipate, monitor, and prevent future lead in water crises.
Keywords: lead, drinking water, corrosion, thermodynamics, public health
There continues to be great societal interest regarding lead contamination of drinking water from leaded water distribution systems. Lead poisoning from degrading lead pipes is a pervasive problem involving a legacy set of materials that will continue to occur until a course of action with stronger scientific foundations is taken. Since wholescale eradication of lead from drinking water systems is improbable, another approach is needed that targets deficiencies in our understanding of lead release and eliminates those deficiencies through research and education. Mechanisms of drinking water corrosion need to be reinvestigated within the scientific community, with a focus on the science governing lead release and consumption.
How Flint Happened, How it Happens Today, and How to Stop it Tomorrow
Lead (Pb) is regulated by the US Environmental Protection Agency (EPA) because consumption has been linked to detrimental health effects such as neurological damage (1) and adverse pregnancy outcomes (2). A recent lead poisoning crisis caught global attention when catastrophically high levels of soluble Pb were recorded in the drinking water of Flint, MI (3, 4)—far above the 15-μg/L Pb2+ (7.2 × 10−8 M Pb2+) action level set by the EPA’s Lead and Copper Rule (LCR) (5). The Flint water crisis prompted state and federal investigations to identify the causes of the Pb release and Pb corrosion. Financial distress, a switch in treated source water, a failure to follow federal corrosion control laws, lax regulatory oversight, high risk created by corrosive drinking water chemistry, and an inability to enforce the LCR dating back at least to a prior Pb crisis in Washington, DC drinking water in 2001 to 2004 were all identified as contributing factors (4, 5). The scientific triggers for the higher lead release were 1) very high incidences of lead service lines, lead solder, galvanized iron pipe, and leaded brass in the water distribution system; 2) increased water acidity; 3) increased chloride to sulfate mass ratio from use of ferric chloride coagulation and the Flint River’s natural salinity; and 4) a failure to continue orthophosphate water treatment for corrosion control even though it had been successfully used for decades (3). Corrosion control treatments utilizing phosphate or its alternatives are intended to reduce water lead levels by a combination of reduced release rates from chemical/electrochemical dissolution, reduced solubility of adherent Pb scales lining Pb service lines, and reduced likelihood of particulates sloughing off of interior pipe walls into the water stream (6–8). The more corrosive water (a result of improper treatment of Flint River water) and failure to continue orthophosphate corrosion control in Flint triggered extensive iron and lead corrosion as well as lead release, creating “red water” complaints, rapid loss of disinfectant residuals, and an outbreak of Legionnaires disease that killed 12 people. The levels of lead in the blood and biomass of the city residents in the absence of phosphate were significantly elevated above levels measured when the water was treated with phosphate (9). Ultimately, the switch intended to save $200 million over 25 y (10) triggered one of the highest profile federal emergencies in the United States, resulting in $450 million in aid from state and federal governments to date, billions in lawsuits, and criminal indictments. The failure to implement corrosion control treatment at a cost of $100/d (11) was far exceeded by the cost of using the untreated Flint River for 18 mo.
This is only a more recent example of a long series of drinking water lead poisoning disasters (3, 4, 12–14). With the legacy use of Pb plumbing in the 1960s and 1970s (and even through 1986 in Chicago), there is every reason to assert that Pb exposure may continue to occur, and in the aftermath of Flint, there is an unshakable perception of a problem undermining trust in drinking water and government (15, 16). In the Washington, DC 2001 to 2004 water crisis, there was a lack of understanding of the effect of chlorine disinfectant on protective tetravalent lead oxide stability (2, 12, 17). The switch to chloramine disinfectant (electro-) chemically destabilized the protective tetravalent lead oxide scale, resulting in unsafe soluble Pb accumulation in the water (17). Serious lead contamination concerns in public schools have occurred in Los Angeles, Baltimore, and Seattle (18). As urban infrastructure continues to age and worldwide access to drinking water becomes increasingly threatened (19), the corrosion of drinking water distribution systems becomes more important than ever (20). All of this underscores the urgent purpose of this perspective: to advocate, inspire, highlight the high impact of, and call for improved scientific inquiry to unmask the many factors governing soluble Pb release and accumulation during corrosion processes. Central to this understanding are thermodynamic and kinetic principles that govern lead release and accumulation in drinking water by electrochemical oxidation. The associated needs, gaps, and opportunities are highlighted herein. The role that citizens, water utilities, funding agencies, and water quality technicians can play to encourage and support scientific advancements in our understanding of lead corrosion in drinking water systems is discussed throughout.
Improvements in Understanding the Risk of Lead Release to Enable Data-Driven Risk Assessment and Management
Today’s methods of testing drinking water for soluble Pb release are not predictive; they involve point-of-use water sampling and forensic analysis of corrosion products on old corroded pipes as evidence. If the LCR limits are exceeded by water sampling, the exact root causes are not clear. In contrast, a more complete scientific analysis of processes governing the oxidation and the subsequent “fate” of soluble Pb in drinking water systems can yield large benefits for society.
Current standards of water sampling, while compliant with the LCR, are insufficient to determine the risk of lead contamination nor its driving forces and dependencies. They are reactionary and nonsystematic while providing little help for the “next time” based on “lessons learned.” Moreover, proper sampling protocols are often ignored (if not gamed) (17, 21) or are simply nonconservative in conveying the amount of ingestible Pb present in drinking water. The LCR acknowledges that any given system will have a distribution of Pb concentration in the drinking water ranging from high to low depending on the samples gathered for collection. Simply put, the LCR requires that most of the Pb content, not all of it, falls below the 15-µg/L action level. For example, an LCR-compliant city with a 90th percentile Pb level of 10 µg/L could still have 1% of homes that contain 70 µg/L Pb and 0.1% that contain 1,717 µg/L Pb (21). Hence, current practice does not enable data-driven risk assessment and preventative decision making; it very simply indicates a problem after it happens! Without a proper corrosion control strategy bolstered by scientific understanding, sampling alone is inefficient and wishful. Unless something changes, these disasters will keep occurring. A lessons learned approach is also an inadequate way to predict soluble Pb release under future circumstances, as it requires extrapolations in order to forecast behavior at some other location outside the existing database of knowledge. Furthermore, there is no direct way to differentiate all of the potential sources and origins of Pb contamination in drinking water systems: municipal service lines, household plumbing, solder joints, and Pb-containing brass fixtures (22) (as well as faucets and water meters) (23).
A predictive framework for lead release based on governing thermokinetic factors is needed to properly assess the risk of future lead corrosion crises and anticipate the effect(s) of changes in water chemistry. Indeed, perhaps the most significant factor in the Flint and DC water crises was the lack of scientific knowledge about the interplay between water chemistry and Pb corrosion, which could have enabled data-driven decision making. The scientific understanding of drinking water corrosiveness has improved since Pb plumbing was utilized in modern water distribution infrastructure (7, 24, 25), but the capabilities needed to predict Pb release lag far behind. Moreover, what is known by the scientific community is not readily conveyed to technicians at the many municipal water authorities, let alone policy makers (20). Proactive management relies on better, or more complete, foundational science. It requires accurate thermodynamic and kinetic data and predicative modeling to manage the risk of water conditions that might allow unacceptable lead release. To accomplish this, models incorporating thermodynamic principles and improved kinetic understanding to fully grasp the effects of water chemistry, episodic use of certain chemicals, stagnation, and flow on the release of soluble Pb and its accumulation must be developed. Technicians need such capabilities distilled into well-grounded and user-friendly tools to assess current risks and understand how Pb corrosion and release are affected by their water treatment decisions (i.e., to enable anticipation and management). An understanding of factors governing corrosion kinetics, scale formation and protection, and particulate stability and release is acutely needed to accomplish this goal. A major improvement upon the existing practices would be for water utilities and water policy stewards to qualify any proposed changes to water distribution systems with the EPA before changes are made. A risk-based cost–benefit assessment of proposed changes could then be made.
Communicating Complexity with Thermodynamic Diagrams
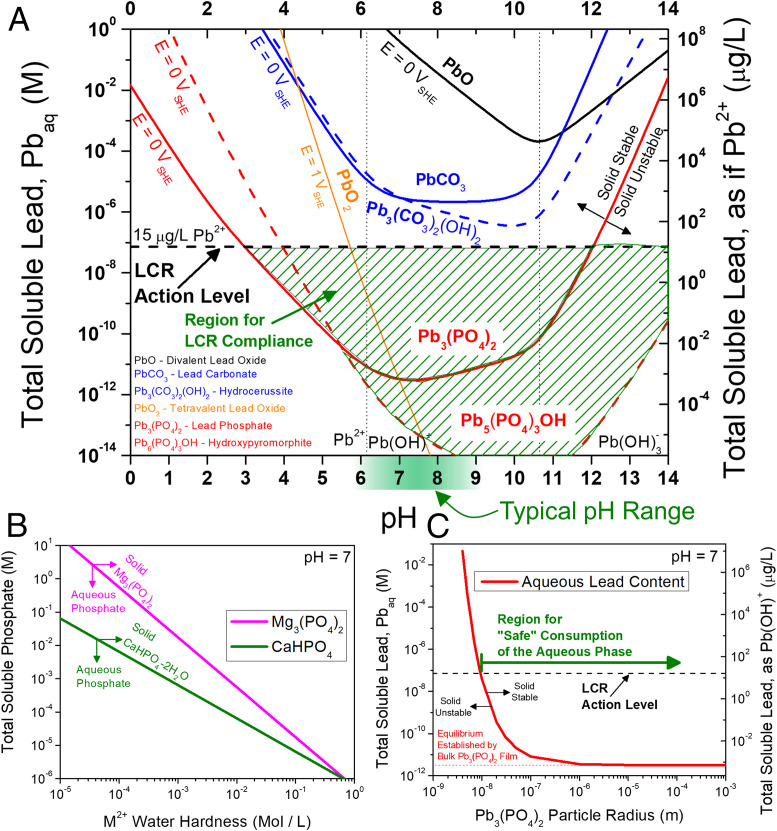
Lead release is governed by a combination of thermodynamics and kinetic processes. Crucial to understanding lead corrosion is lead solubility in drinking water and the stability of various so-called protective and inhibiting oxidized lead compounds as a function of the myriad of water chemistry and material variables. Commonly considered factors that affect Pb corrosion are pH and the concentration of species like chloride (Cl−), sulfate (SO42−), carbonate (CO32−), phosphate (PO43−), magnesium (Mg), and/or calcium (Ca) (24). They influence the stability of compounds by binding with free Pb2+ and/or Pb4+ in solution, reacting with Pb2+ and/or Pb4+ to precipitate solid phases, or depositing Mg and/or Ca scales on the pipe surface. To highlight one example of the merits of thermodynamic principles, consider the information conveyed in chemical stability diagrams (8, 26) (Fig. 1A), which are predominance diagrams that map the stability of solid Pb compounds vs. soluble Pb as a function of pH and ion concentrations. They report what Pb-based molecular compounds are predicted to form on the surface of a Pb pipe in drinking water and critically, what the equilibrium concentration of dissolved soluble Pb in solution is for a given compound. Here, diagrams are calculated in terms of the total soluble lead concentration [which includes aqueous Pb2+, Pb(OH)a2−a, PbClb2−b, Pb4+, etc.] since this is bioavailable lead ingested by human consumption (notwithstanding solid, particulate forms of lead, to be discussed in Remaining Knowledge Gaps Explained by Thermodynamic and Kinetic Understandings). That concentration is crucial in ascertaining whether thermodynamic corrosion control is advisable relative to the LCR action level. Such a diagram would have illuminated the possible viability, concerns, and remedies associated with many of the historic lead in water crises.
Fig. 1.
Thermodynamic diagrams exhibiting the types of calculations that describe the Pb–drinking water system. (A) Chemical stability diagram highlighting the relative stabilities of various Pb-based compounds as a function of pH and total soluble Pb concentration. The EPA action limit of 15 μg/L Pb2+ is included for reference (horizontal dashed line). Diagrams were constructed for a representative drinking water chemistry where the concentrations of carbonate and chloride are 1 mM each, and an inhibitor concentration of [PO43−] = 0.1 mM utilizing thermodynamic data in Lange’s Handbook of Chemistry (37) except for the hydroxylated carbonate and phosphate compounds (dashed lines), which were taken from the American Water Works Association (8). (B) The effect of water hardness in limiting available phosphate (aqueous) in drinking water. (C) The effect of decreasing particle size on destabilizing the solid phase assuming a surface energy of 3 J/m2 for Pb3(PO4)2 particles in water.
Pipeline corrosion can result in the release of ingestible Pb from chemical and/or electrochemical dissolution (whether in the form of soluble species or particulate solids). While chemical thermodynamics cannot predict metallic corrosion or Pb-compound chemical dissolution rates, it can predict under what conditions a thermodynamically stable film forms on the surface. These films might act as kinetic barriers to hinder corrosion by covering lead pipes and functioning as sinks to sequester soluble Pb. In the case of Pb in drinking water, sulfate, carbonate, and phosphate films have been suggested to beneficially affect the corrosion rate in one of these ways (6, 7, 24, 27). However, stable film development requires a certain equilibrium chemistry to exist. All of these Pb-based films have thermodynamic dependencies on pH and soluble Pb concentration, as in the case of Pb3(PO4)2 governed by the following reaction:
This equilibrium is depicted in Fig. 1A by the solid red line. With this in mind, it is possible that a Pb-based film (which may limit the corrosion rate to a degree) may be in equilibrium with a toxic concentration of soluble Pb at equilibrium when stable. This occurs when the equilibrium concentration of soluble Pb exceeds the concentration of soluble Pb allowed by the LCR (i.e., the pH-independent tolerable value defined by the EPA as shown by the dashed horizontal line [LCR] in Fig. 1). For a given drinking water chemistry (such as pH, chloride content, etc.), the stability of various Pb-based films will require different concentrations of soluble Pb to maintain equilibrium as illustrated in Fig. 1A, a chemical stability diagram constructed considering a representative drinking water chemistry. As shown, only the Pb-phosphate films (Fig. 1A, red lines) can fix the total concentration of soluble Pb below the EPA action level (black horizontal dashed line) over a range of pH from 3 to 14+ depending on the nature of the phosphate film (the green shaded region indicates range of “compliant” water achieved by phosphate-based films). A freely corroding Pb pipe will oxidize to release Pb ions into the water. In phosphate-treated water, a Pb-phosphate film will form that establishes a soluble Pb concentration as a function of pH (the solid and dashed red lines for each Pb-phosphate film). In the absence of phosphate (or alternative inhibitor), the next most stable product is a Pb-carbonate film, which establishes a much higher soluble Pb concentration as a function of pH (the solid and dashed blue lines for each Pb-carbonate film). A normal operational range of tap water pH is 6.5 to 8.5, well within this compliant region (compliant here means below the EPA action level; in reality, no amount of lead consumption is acceptable)—but only in the presence of phosphate. In other words, it is thermodynamically impossible to satisfy the EPA LCR without an inhibitor (like phosphate, silicate, etc.) that establishes an equilibrium soluble Pb concentration below 15 μg/L.
Relying solely on alkalinity (carbonate), hardness (Mg2+ and/or Ca2+ content), or pH will not satisfy the LCR. This fact defies what often has been promulgated in past Pb literature based on the failure to adequately or properly consider these thermodynamic principles (27). Simply having a Pb scale is not enough (16). The scale must thermodynamically limit the soluble Pb concentration as low as possible, or at least below the LCR action level. The difference is striking: without phosphate, the lowest amount of dissolved lead that could be established in equilibrium (with lead carbonate) is 2.2 × 10−6 M [493 µg/L as Pb(OH)+], 30× higher than the action level. With phosphate, that concentration is 3.2 × 10−12 M [7.2 × 10−4 as Pb(OH)+] or 1,000,000× lower than possible with carbonate alone and four orders of magnitude lower than the action level. This difference, while easily conveyed by the chemical stability diagram, was evidently not considered preceding the Flint water crisis. This is not surprising, considering that the role of phosphate as a toxicological inhibitor (meaning an inhibitor that can remove soluble Pb ions) has been overlooked in the literature. Clearly, more information on a broader set of compounds is necessary, including their size, crystallographic, and epitaxial details that might change equilibrium soluble Pb concentrations (as will be discussed vis-à-vis Fig. 1C in Remaining Knowledge Gaps Explained by Thermodynamic and Kinetic Understandings). Other avenues of exploration include selection of new inhibitor chemicals that form low-solubility Pb-based films (like phosphate does) and surface engineering with surfactants that fine-tune Pb-based film morphologies to optimize protective coverage on the pipe wall.
Can Kinetics Prevail When Thermodynamics Fail?
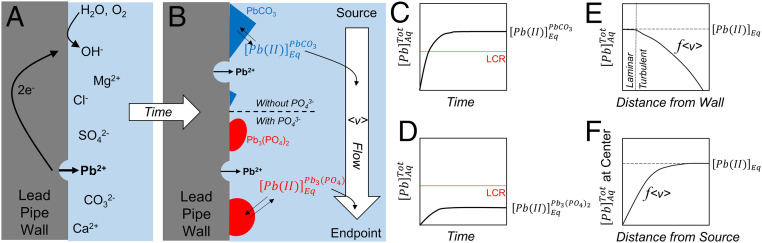
When the compounds forming a scale cannot provide a low equilibrium soluble Pb concentration, another remedy might be to dilute or wash the soluble lead away—in the same vein as the well-known quip “control pollution by dilution” or “dilution is the solution.” Fig. 2 schematically shows a Pb pipe corrosion cell, the progression of simultaneous scale formation by precipitation or direct electrocrystallization, and in parallel, soluble Pb release both with and without phosphate (or another scale former such as lead carbonate) at short (Fig. 2A) and long (Fig. 2B) times. The schematic highlights the need for improved kinetic understanding of a myriad of factors including the surface science associated with scaling, nucleation, and growth. Scale growth, interfacial reaction rates, and crystallographic effects are not well understood. The mechanism by which the scale inhibits soluble Pb accumulation requires detailed understanding of all of these processes. Corrosion scale nucleation and growth will readily influence the mass transfer kinetics of Pb release from the pipe wall into the bulk drinking water. Models show how interfacial Pb release (as governed by the pipe wall scale) will contribute to the accumulation of Pb in the bulk drinking water (8).
Fig. 2.
Schematic illustration of the kinetic processes controlling lead service line scale formation and Pb release at (A) short times and (B) long times. The total soluble Pb concentration increases with time toward the equilibrium concentration for that particular compound, which may be (C) above the LCR or (D) below the LCR, depending on the compound. The effect of flow is important too, as it influences the mass transport of Pb away from (E) the pipe wall to the pipe centerline and also (F) downstream to the endpoint device.
Pb accumulation in drinking water also depends on water flow and pipe geometry (8). Fig. 2 C and D shows the chemical trajectory of soluble Pb concentration vs. time in a phosphate-free (Fig. 2C) and phosphate-inhibited (Fig. 2D) system. The interfacial corrosion reactions increase the concentration of soluble Pb near the surface until an equilibrium concentration is achieved. The equilibrium concentration of soluble Pb may be below the LCR action level [as with Pb3(PO4)2] or above it (as with PbCO3) as indicated by Fig. 1A. In the case of lead carbonate, only kinetic inhibition of Pb accumulation has any chance of maintaining the soluble Pb concentration below the LCR action level. This is not recognized by those who proffer this solution. In this situation, the only way to avoid the accumulation of toxic levels of soluble Pb is if the rate of clean water replenishing the system outpaces soluble Pb production and accumulation because the equilibrium soluble lead content is too high (as with high throughput of replenishing water and slow Pb release kinetics) (Fig. 2E). This approach is not without its pitfalls. A stagnant solution will eventually equilibrate to the equilibrium soluble Pb concentration even in the bulk (pipe center), whereas at high flow velocities (ν), the concentration of soluble Pb in the bulk pipe solution may be negligible (comparable to the chemistry of the clean source water). Even then, after enough contact with Pb pipe surfaces as the water travels from source to sink, clean water can accumulate soluble Pb levels up to the equilibrium concentration, as in Fig. 2F. Water that contacts a relatively short distance of lead pipe is expected to have a lower concentration of soluble Pb compared with water that contacts a relatively large distance of lead pipe. This points to the need for better understanding of both thermodynamic (Fig. 1) and kinetic (Fig. 2) factors that govern soluble Pb accumulation.
Periodic sampling of drinking water for soluble Pb is essentially a snapshot in time of the global water quality averaged over the pipe diameter as a slug of water at that particular moment or a “shot in the dark” with the hope that the soluble Pb concentration is below the action level specified by the LCR without really understanding why. In reality, the soluble Pb concentration can vary wildly over orders of magnitude, and while most of it will fall below the EPA action level, there is clearly still a portion that resides above this level (21). This uncomfortably large variation is a result of many influential variables that have not been properly assessed that govern lead release. Clearly, higher-fidelity kinetic models must be developed that address all of the thermodynamic and kinetic processes and controlling factors represented in Fig. 2 with the goal of predicting a reasonable estimate of soluble Pb concentration as a function of all of the variables mentioned and others. Similar models already exist to predict FeCO3 scaling in oil and gas piping, for instance.
An Important Lesson from Flint
Turning back to Flint, let us review what really went wrong. Phosphate inhibitor was deliberately omitted from Flint’s water treatment after a switch in its water supply to the Flint River in April of 2014, resulting in more corrosive drinking water (3, 17, 28). That decision was graver than anyone knew at the time. By the following April, the lead concentration in the drinking water was recorded to be 217 to 13,200 µg/L (far above the 15-µg/L action level) (3, 4). With the aid of chemical thermodynamics and dissemination of intuitive thermodynamic and kinetic frameworks that are easy for technicians and water engineers to use (even possibly via smart device applications), anyone who does not specialize in corrosion science or in corrosion control could have been informed about the risks of not inhibiting the drinking water. In the DC water crisis (12, 17), the switch from chlorine to chloramine disinfectant lowered the surface electrode potential to a range where tetravalent lead oxide is unstable; tetravalent lead oxide is quite stable at elevated potentials such as 1 V vs. the standard hydrogen electrode (orange line in Fig. 1A). Information such as conveyed in these diagrams can provide a valuable resource for educating municipal water authorities and local, state, and federal governments (17) and can help policy makers make informed decisions concerning inhibitor selection and drinking water chemistries. Indeed, the more scientific tools that are available to municipal water engineers and the decision makers they report to, the better as this subject presents a multifaceted and complex problem. Elevated electrode potential, for example, while possibly beneficial with respect to lead release, would likely exacerbate localized corrosion of steel or copper. The relative importance of electrode potential on each of these aspects of drinking water corrosion is not clearly understood but should be.
Remaining Knowledge Gaps Explained by Thermodynamic and Kinetic Understandings
The Effect of Water Hardness.
This perspective summarizes basic thermochemical and kinetic corrosion frameworks for assessing lead content in drinking water, showing where knowledge is lacking and opportunities for improvement exist. Additional information can be conveyed by these thermochemical and kinetic frameworks informed by more fundamental science than is available in the literature to date. Often, the effect of water hardness (Mg2+ and Ca2+ content) is reduced simply to the precipitation of Mg and/or Ca scales on the pipe walls—with limited benefit, as the science of Pb release mediated by these scales is not well known. Moreover, the effect of hardness beyond the scale formation of Mg2+ vs. Ca2+ is ignored. While Mg and/or Ca scale precipitation might be beneficial, the presence of Mg2+ or Ca2+ in water could be detrimental if phosphate inhibitor is sequestered out of solution by the formation of Mg and/or Ca phosphate compounds. Fig. 1B shows that as hardness increases, the total concentration of available phosphate inhibitor decreases; it is removed from solution to form Mg and/or Ca phosphate. In this way, a hard municipal water would have a lower capacity for phosphate inhibitor dosing than a soft water. This is problematic since the concentration of phosphate inhibitor in solution has been shown to affect the corrosion kinetics of Pb oxidation, with higher doses resulting in a lower measured oxidation rate (29). Furthermore, the conditions and propensity to which Ca2+ binds phosphate are different for Mg2+, which has also not been considered previously. To the contrary, water hardness is usually reported such that Mg and Ca contents are treated equivalently without discrimination (e.g., total Mg and Ca content is commonly expressed as an equivalent milligrams per liter of CaCO3). In general, phosphate concentrations are often intended to be low enough to avoid Mg and/or Ca scale precipitation and require limiting to modest concentrations to the point where opportunities might be missed to achieve corrosion benefits. Calculations such as those depicted in Fig. 1B inform the optimal window for phosphate inhibitor dosing.
Particulate Lead.
Soluble Pb content in drinking water is not the only source of ingestible Pb. Small particulates of solid (nonaqueous) Pb-containing species can form in water distribution systems (3, 30). Lead scales or particles can be dislodged from pipe walls and pass through to endpoint water outlets where they can be ingested if proper filtration is not in place. The pH within the human stomach is low enough (below four) to drastically increase the solubility of even Pb3(PO4)2 (Fig. 1A) (30). So, solid forms of Pb are ingested, dissolved in the stomach, and made bioavailable to the rest of the body—with severe repercussions. For this reason, filtration systems are often deployed to mechanically capture these Pb-containing particulates. Pb particles can frequently be so large that they are not completely dissolved by the LCR sample preparation procedure (particles larger than 12 μm in diameter are commonly observed) (30), thereby resulting in a report of Pb content in the water that is lower than what would actually be bioavailable if/when ingested. The size and distribution of these particles may vary, but the effect of size on the stability of the particle can be calculated (to the best of our knowledge, it has not been calculated in the literature previously). As a solid particle becomes smaller, a greater concentration of equilibrium soluble Pb is needed to maintain solid phase stability (Fig. 1C). Below the micrometer scale, solid Pb-based particles are increasingly unstable compared with soluble Pb. Indeed, if the bulk concentration of soluble Pb is assumed to be fixed by the macrosized phosphate scale (at ∼10−12 M), then all particle diameters smaller than 100 nm are thermodynamically unstable and dissolve to soluble Pb. This provides a lower-bound particle size with which to design filtration systems for particulate Pb capture. Additionally, surfactant engineering is motivated to 1) alter the surface energy of Pb3(PO4)2 to extend particle stability to larger and more easily filtered sizes and 2) maximize scales adherence to resist formation of dislodged Pb-containing particulates. Currently, insufficient understanding exists regarding the properties of these Pb particles.
Clarion Call for Renewed Commitment to Lead Corrosion Science and Engineering
While there is some Pb corrosion knowledge, much more needs to be uncovered as soon as possible given 1) our aging infrastructure, 2) changes in water sourcing as water scarcity increases, and 3) prohibitively high cost of lead service line replacement especially when relegated to homeowners (20). For example, the Trenton (New Jersey) Water Works Lead Service Line Replacement Program incentivizes homeowners to replace their lead service lines by limiting homeowner expenses to $1,000. The program covers the rest of the cost for replacement, which typically runs between $3,000 to $7,000. While this program will benefit many, it may still be cost prohibitive to many low-income urban residents. Pb plumbing is still very prevalent in the developed Western water distribution infrastructure, which creates greater risk that pipeline corrosion will result in Pb poisoning. Even pipeline leaks that do not directly result in Pb release (as with steel rupture, for example) increase the risk of Pb exposure due to interruption in the water supply chain during the time taken to repair the break (31). Indeed, when a seemingly small change is made in water chemistry or distribution patterns, Pb can be released (4, 17). Moreover, external factors such as temperature, weather, and rising seas may influence the corrosiveness of drinking water (17). The only sure way to safeguard against Pb poisoning would be to replace such service lines (both public and domestic) altogether. This would be both costly and likely ineffective. While removing Pb plumbing is of obvious merit, many sources of Pb can remain. Pb-based solder is used to connect piping, and Pb is present in trace amounts in Cu-rich alloys and disproportionately, preferentially leaches out into drinking water in greater quantities than predicted by the content of Pb in the alloy (22). There is a significant amount of Pb trapped in the oxides on iron pipes as well (3, 31–33). Unless all galvanized steel pipes used in the same system with Pb pipe are also replaced, these oxides remain as potential sources of Pb. In the interim, as water service lines composed of Pb are slowly removed, release can be worsened. Partial replacement of a Pb pipe with copper pipe can establish a galvanic corrosion cell when Pb and copper are in close proximity (33–36). In this galvanic cell, Pb corrosion is accelerated to higher rates than in the absence of copper (or steel as well) (33–36). Moreover, while total replacement of Pb service lines is a wonderful goal, finding all sources of Pb can be difficult. Following a hypothetical analysis by Triantafyllidou and Edwards (21), just 1 foot of leftover Pb service line contains enough Pb that if 1% of it dissolves into the volume of water consumed by an average US family in 1 y, that water would be contaminated above the LCR action level.
The source of soluble Pb in a water distribution system is difficult to elucidate since water is only sampled at an endpoint device after having traveled from the source to the sink in contact with any number of possible sources. Especially lacking are holistic kinetic models that incorporate the rate of Pb release from all possible sources considering various operating conditions. One promising strategy to elucidate the relative contributions of different Pb sources is to leverage source tracing techniques via metal stable isotope analysis. A representative water recirculating rig composed of the various types of Pb sources (Pb pipe, Pb alloyed brass pipe, Pb-containing galvanized steel pipe, Pb-based soldered joint, etc.) can be constructed in which the specific isotopic fingerprint from each Pb source is known. Each Pb source can be characterized before rig construction to determine the natural variances in Pb isotope ratio (204Pb, 206Pb, 207Pb, 208Pb) among the different sources. The relative contributions of the different Pb isotopes in the water as a function of various operating conditions would be traceable to the sources of Pb release in the system. This strategy would enhance our understanding of how Pb is released from Pb pipe and also from other not-so-obvious sources of Pb, which is dearly lacking.
The general aim of replacing all sources of lead in drinking water systems does not circumnavigate the remaining knowledge gaps in our understanding of lead release—knowledge gaps that contributed to the crisis we find ourselves in today. If these knowledge gaps persist, then lead release incidents will continue, even (especially) as lead is replaced.
An opportunity exists to tackle the threat of lead in drinking water. Policy makers can help by working to rejuvenate our aging water distribution infrastructure; find effective ways to fund promising research to fill our existing knowledge gaps; and develop technologies to monitor, anticipate, and manage water quality. Industries can also work to create affordable smart sensor technology that can autonomously report water properties such as pH, temperature, electrode potential, hardness, conductivity, phosphate content, and maybe even soluble Pb content. Part per billion real time lead sampling technology is lacking.
Such technology can then be made available to the public to empower the advent of a citizen science revolution in drinking water research. Citizen scientists of Flint, MI played an important role in bringing the water crisis to light (3, 4). Citizen scientists could outfit their plumbing with smart sensor technology linked through cell phones to provide invaluable data to water utilities to enable live mapping of water conditions. Such data could be harvested broadly and stored on the cloud. Several institutions have already successfully implemented crowdsourcing citizen science platforms such as the National Weather Service (e.g., Cooperative Observer Program), the National Oceanic and Atmospheric Administration (e.g., Track the Tides), NASA (e.g., Sungrazer Project), and even the EPA (e.g., Air Sensor Toolbox). Crowdsourcing of public data from citizen scientists would greatly increase the amount of data available on water quality and enable data analytics and probabilistic studies linking water qualities to corrosive conditions that can forewarn of lead poisoning events.
There have been many calls to action in the wake of the Flint, MI water crisis (17, 28) and many noted engineering, scientific, and ethical failures by state governments and municipal water utilities resulting in a loss of trust between citizens and their government. A better-informed society can prevent such disasters from happening in the future through improved risk assessment, anticipation, and management of factors affecting Pb release. We all have a part to play in averting future Pb poisoning disasters. Further elucidation of the scientific factors associated with Pb release is warranted as a key part in the overall strategy to improve the quality of life for all, independent of socioeconomic circumstance. The following recommendations address some of the needs, gaps, and opportunities discussed herein.
Promote artificial intelligence and machine learning (informed by probabilistic data interpretation, data analytics, and risk management) to identify relationships between water and pipe conditions and high soluble Pb levels in drinking water. A predictive risk management-based numerical ranking method approach that incorporates a set of risk factors associated with Pb release dependent on variability of conditions could be implemented in place of the existing reactionary “go no-go” threshold mandated by the EPA. The exact selection, ranking, and weighing of the risk factors are currently unknown but would be informed by historic case studies, subject matter experts, and continued research discoveries.
Improve the scientific understanding of phosphate as an inhibitor to Pb release to address its function both as a corrosion inhibitor (reduced corrosion rates) and as a toxicological inhibitor (reduced access to soluble Pb) (Fig. 1). As discussed above, the surface science of phosphate film growth can be better explored, and the role of phosphate as a toxicological inhibitor has been hitherto underappreciated. New and efficacious inhibitors with even better protection attributes should also be considered.
Develop thermodynamic and kinetic models that can forecast what Pb-based compounds and soluble Pb levels exists for a given water condition. The kinetic model should factor in sources and sinks of soluble Pb, water chemistry, release rates, and kinetic laws such that accumulation of soluble Pb in water can be forecasted over time. Thermochemical data on Pb-based compounds should be bolstered by first-principles calculations to reduce the inherent uncertainties present from source to source. Free access of these data fosters wide use by scientists, engineers, and water authorities.
Support scientific research that seeks to better understand lead corrosion in drinking water systems. Two examples discussed herein are 1) the role of drinking water hardness in limiting the upper capacity for phosphate treatment and 2) the effect of particle size on the stability of solid Pb-based particles and the implications for water treatment and filtration. These and other topics provide fertile ground for scientific inquiry into causes and treatments for lead corrosion in drinking water systems.
Establish more robust techniques to detect and assess Pb corrosion (29) and the fate of soluble Pb, perhaps by exploiting sensors and cyber–physical technologies. After they are developed, techniques can be deployed in the field by citizen scientists with great benefit. Create an integrated, standardized national database of harvested water chemistry, corrosion control, and physical features in water systems along with corresponding soluble Pb levels.
Acknowledgments
We acknowledge Prof. Marc Edwards for providing insights, proofreading early drafts, and suggesting selected references. R.J.S. also acknowledges his current affiliation with the US Naval Academy, which was uninvolved in the publication of this article. Consequently, the views expressed in this perspective do not represent the views of the US Naval Academy or the US Government. The perspective shared in this article is not financed by any funding institution. R.J.S. and J.R.S. were supported logistically by the Center for Electrochemical Science and Engineering at the University of Virginia.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.A.C. is a guest editor invited by the Editorial Board.
Data Availability.
Data used in the construction of the thermodynamic plots and spreadsheet data have been deposited in the Open Science Framework (https://osf.io/yqv5h/). Thermodynamic data were retrieved from Lange’s Handbook of Chemistry (37) and select referenced literature sources.
References
- 1.Canfield R. L., et al. , Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 348, 1517–1526 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards M., Fetal death and reduced birth rates associated with exposure to lead-contaminated drinking water. Environ. Sci. Technol. 48, 739–746 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Pieper K. J., Tang M., Edwards M. A., Flint water crisis caused by interrupted corrosion control: Investigating “ground zero” home. Environ. Sci. Technol. 51, 2007–2014 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Pieper K. J., et al. , Evaluating water lead levels during the Flint water crisis. Environ. Sci. Technol. 52, 8124–8132 (2018). [DOI] [PubMed] [Google Scholar]
- 5.US Environmental Protection Agency, Control of lead and copper. Electronic Code of Federal Regulations (2020). https://www.ecfr.gov/cgi-bin/text-idx?SID=9c5415b2fe8eb76878a169c14454171f&mc=true&node=sp40.25.141.i&rgn=div6. Accessed 14 August 2020.
- 6.Edwards M., McNeill L. S., Effect of phosphate inhibitors on lead release from pipes. J. Am. Water Works Assoc. 94, 79–90 (2002). [Google Scholar]
- 7.Schock M. R., Understanding corrosion control strategies for lead. J. Am. Water Works Assoc. 81, 88–100 (1989). [Google Scholar]
- 8.AWWA , Internal Corrosion of Water Distribution Systems (American Water Works Association, ed. 2, 1996). [Google Scholar]
- 9.Roy S., Tang M., Edwards M. A., Lead release to potable water during the Flint, Michigan water crisis as revealed by routine biosolids monitoring data. Water Res. 160, 475–483 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Flint , “Resolution to purchase capacity from Karegnondi water authority” (Flint Resolution 2013EM041, Flint, MI, 2013).
- 11.Monahan K., Rappleye H., Gosk S., Sandler T., Internal email: Michigan “blowing off” Flint over lead in water. NBC, 6 January 2016. https://www.nbcnews.com/storyline/flint-water-crisis/internal-email-michigan-blowing-flint-over-lead-water-n491481. Accessed 14 August 2020.
- 12.Edwards M., Triantafyllidou S., Best D., Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001–2004. Environ. Sci. Technol. 43, 1618–1623 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Troesken W., The Great Lead Water Pipe Disaster (MIT Press, 2006). [Google Scholar]
- 14.Ganim S., For 10 years, a chemical not EPA approved was in their drinking water. CNN, 28 November 2018. https://www.cnn.com/2018/11/11/health/denmark-sc-water-chemical-not-epa-approved/index.html. Accessed 1 April 2019.
- 15.Rabin R., The lead industry and lead water pipes “A Modest Campaign.” Am. J. Public Health 98, 1584–1592 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renner R., Plumb crazy. Science 315, 1669 (2007). [Google Scholar]
- 17.Roy S., Edwards M. A., Preventing another lead (Pb) in drinking water crisis: Lessons from the Washington D.C. and Flint MI contamination events. Curr. Opin. Environ. Sci. Health 7, 34–44 (2019). [Google Scholar]
- 18.Lambrinidou Y., Triantafyllidou S., Edwards M., Failing our children: Lead in U.S. school drinking water. New Solut. 20, 25–47 (2010). [DOI] [PubMed] [Google Scholar]
- 19.National Academy of Engineering , Grand Challenges for Engineering (National Academy of Engineering, 2008). [Google Scholar]
- 20.Siegel S. M., Troubled Water: What’s Wrong with What We Drink (St. Martin’s Publishing Group, 2019). [Google Scholar]
- 21.Triantafyllidou S., Edwards M., Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Crit. Rev. Environ. Sci. Technol. 42, 1297–1352 (2012). [Google Scholar]
- 22.Paige J. I., Covino B. S., Leachability of lead from selected copper-base alloys. Corrosion 48, 1040–1046 (1992). [Google Scholar]
- 23.Vaccari D. A., How not to get the lead out—Lead service line replacement will not solve our drinking water crisis. Curr. Pollut. Rep. 2, 200–202 (2016). [Google Scholar]
- 24.Nguyen C. K., Clark B. N., Stone K. R., Edwards M. A., Role of chloride, sulfate, and alkalinity on galvanic lead corrosion. Corrosion 67, 065005-1–065005-9 (2011). [Google Scholar]
- 25.Zhou P., Hutchison M. J., Scully J. R., Ogle K., The anodic dissolution of copper alloys: Pure copper in synthetic tap water. Electrochim. Acta 191, 548–557 (2016). [Google Scholar]
- 26.Santucci R. J., McMahon M. E., Scully J. R., Utilization of chemical stability diagrams for improved understanding of electrochemical systems: Evolution of solution chemistry towards equilibrium. npj Mat. Degrad. 2, 1 (2018). [Google Scholar]
- 27.Cramer S. D., Covino B. S., ASM Handbook Volume 13b Corrosion: Materials (ASM International, 1990). [Google Scholar]
- 28.Scully J. R., The corrosion crisis in Flint, Michigan: A call for improvements in technology. Bridge (Wash. D.C.) 46, 19–29 (2016). [Google Scholar]
- 29.Nalley L. K., Rafla V. N., Santucci R. J., Scully J. R., Method to rapidly characterize reduced lead corrosion in phosphate inhibited drinking water. Corrosion 75, 147–151 (2018). [Google Scholar]
- 30.Triantafyllidou S., Parks J., Edwards M., Lead particles in potable water. J. Am. Water Works Assoc. 99, 107–117 (2007). [Google Scholar]
- 31.Little B. J., Lee J. S., Gerke T. L., The relationship between iron oxides/oxyhydroxides and toxic metal ions in drinking water distribution systems—a review. Corrosion 73, 138–143 (2016). [Google Scholar]
- 32.Masters S., Edwards M., Increased lead in water associated with iron corrosion. Environ. Eng. Sci. 32, 361–369 (2015). [Google Scholar]
- 33.Clair J. St., Cartier C., Triantafyllidou S., Clark B., Edwards M., Long-term behavior of simulated partial lead service line replacements. Environ. Eng. Sci. 33, 53–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J., Gan F., Triantafyllidou S., Nguyen C. K., Edwards M. A., Copper-induced metal release from lead pipe into drinking water. Corrosion 68, 1037–1048 (2012). [Google Scholar]
- 35.Cartier C., et al. , Impact of treatment on Pb release from full and partially replaced harvested Lead Service Lines (LSLs). Water Res. 47, 661–671 (2013). [DOI] [PubMed] [Google Scholar]
- 36.St. Clair J., Stamopoulos C., Edwards M., Technical note: Increased distance between galvanic lead:copper pipe connections decreases lead release. Corrosion 68, 779–783 (2012). [Google Scholar]
- 37.Speight J. G., Lange’s Handbook of Chemistry (McGraw-Hill Education, ed. 17, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the construction of the thermodynamic plots and spreadsheet data have been deposited in the Open Science Framework (https://osf.io/yqv5h/). Thermodynamic data were retrieved from Lange’s Handbook of Chemistry (37) and select referenced literature sources.