Significance
Mast cell (MC)-associated disorders exhibit a sex bias, with females at increased risk. While attention has been directed to adult sex hormones as a mechanism for disease risk between the sexes, epidemiological evidence clearly shows that these same sex biases also exist in prepubertal children, thus challenging this concept. Here, we show that perinatal, but not adult gonadal, androgens play a protective role in MC-mediated anaphylaxis severity into adulthood. We propose that perinatal androgens mediate their protective effects via programming of bone marrow MC precursors to exhibit reduced granule histamine and release. These findings shift attention to perinatal life as a critical period for potential interventions to mitigate MC-associated disease risk across the lifespan in males and females.
Keywords: mast cell, sex differences, immune, inflammation, histamine
Abstract
Mast cell (MC)-associated diseases, including allergy/anaphylaxis and neuroinflammatory pain disorders, exhibit a sex bias, with females at increase risk. While much attention has been directed toward adult sex hormones as drivers of sex differences, that female sex bias in MC-associated diseases is evident in prepubertal children, suggesting early-life origins of sex differences which have yet to be explored. Utilizing rodent models of MC-mediated anaphylaxis, our data here reveal that, 1) compared with females, males exhibit significantly reduced severity of MC-mediated anaphylactic responses that emerge prior to puberty and persist into adulthood, 2) reduced severity of MC-mediated anaphylaxis in males is linked with the naturally high level of perinatal androgens and can be recapitulated in females by perinatal exposure to testosterone proprionate, 3) perinatal androgen exposure guides bone marrow MC progenitors toward a masculinized tissue MC phenotype characterized by decreased concentration of prestored MC granule mediators (e.g., histamine, serotonin, and proteases) and reduced mediator release upon degranulation, and 4) engraftment of MC-deficient KitW-sh/W-sh mice with adult male, female, or perinatally androgenized female MCs results in MC-mediated anaphylaxis response that reflects the MC sex and not host sex. Together, these data present evidence that sex differences in MC phenotype and resulting disease severity are established in early life by perinatal androgens. Thus, factors affecting levels of perinatal androgens could have a significant impact on MC development and MC-associated disease risk across the life span.
The concept of immunological differences between the sexes is well established, with women, in general, mounting stronger immune responses compared with males. While it is thought that heightened immune responses may predispose females to a number of inflammatory, autoimmune, and chronic pain disorders (1–7), a higher immune response could be beneficial, as women often have reduced rates of viral, bacterial, and parasitic infections and mount more effective responses to vaccines (8, 9). Adult sex hormones, including estrogen and testosterone, have received the most attention as potential drivers of sex differences in immunological disorders; however, there is considerable evidence that female sex bias in immune disorders exists in prepubertal children, including conditions such as autoimmune disease, chronic pain disorders, and certain allergic disorders (5, 10–15). Further, female children, like adult females, are less vulnerable to infections and death and mount greater immune responses to vaccination compared to male children (16, 17). Despite the growing number of studies reporting sex biases in disease risk, the biological mechanisms underlying sex differences in immune disorders remain poorly defined.
Mast cells (MCs) are innate immune cells located throughout the body and are known best for their proinflammatory roles in allergic disorders and anaphylaxis. Positioned strategically at host−environment interfaces (e.g., skin, gut mucosa, airways, meninges), and in close proximity to blood vessels and neurons, MCs orchestrate innate and adaptive immune responses to allergens, antigens, and pathogens (18). Further, MCs possess the unique ability to store large amounts of preformed proinflammatory mediators (e.g., histamine, serotonin proteases, etc.) in their cytoplasmic granules which are released and which can initiate rapid and robust physiologic effects on the vasculature, epithelium, and nervous systems. While rapid and robust initiation of immune response by MCs is critical in host defense, excessive and systemic activation of MCs can result in chronic inflammation or potentially result in fatal anaphylaxis. MCs have been shown to contribute to pathophysiology associated with autoimmune diseases and chronic pain conditions such as irritable bowel syndrome and migraine (19, 20). It has also become increasingly recognized that MC-associated disorders exhibit sex biases, with females at increased risk (1–7). Further, as discussed above, sex differences in these disorders exist in prepubertal children (5, 10–15), suggesting that sex differences may emerge early in life. However, the origin and mechanisms of sex differences in MC diseases have not been previously investigated.
In our previous work, we demonstrated sex differences in adult MCs, namely, that female MCs express a vastly different RNA transcriptome than males, and exhibit an increased capacity to store mediators such as histamine and proteases within their secretory granules (21). Further, we showed that enhanced mediator content in female MCs coincided with greater psychological stress- and IgE-mediated serum histamine levels, anaphylaxis, and intestinal permeability compared with adult males (21). Likewise, other have reported sexually dimorphic patterns in cytokine induction in bone marrow-derived MC (BMMCs), which were proposed to mediate sex differences in an experimental autoimmune encephalitis model (22), supporting the sexually dimorphic nature of the MC. However, a large gap in knowledge exists regarding mechanisms driving sex differences in MCs, particularly those that contribute to the emergence of sex differences prior to puberty. Here we demonstrate, using rodent models, that sex differences in MC-mediated anaphylactic responses emerge prior to puberty and that males exhibited reduced histamine levels and anaphylaxis severity compared with females, which persists into adulthood. Further, we show that the reduced anaphylactic severity in males is associated with exposure to high levels of perinatal androgens and subsequent programming of bone marrow MC progenitors toward a masculinized MC phenotype which, in turn, attenuates anaphylaxis in juvenile and adult animals.
Results
Sexually Dimorphic Anaphylaxis Responses Are Not Dependent on Adult Gonadal Hormones.
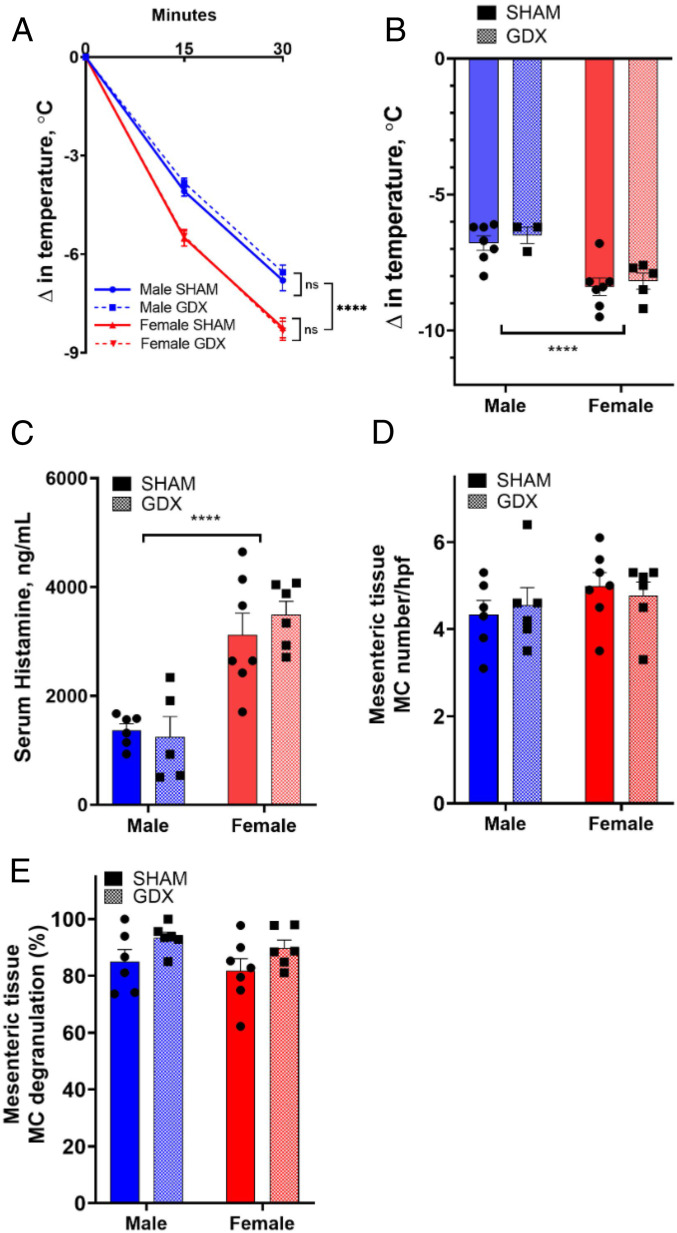
We have previously demonstrated, in an MC-dependent model of IgE-mediated passive systemic anaphylaxis (PSA), that adult female mice exhibited greater serum histamine levels and more severe anaphylaxis (hypothermia and clinical scores) compared with adult males (21). Because adult gonadal hormones have been shown to play a role in sex differences in many disease models, we first established the contributions of adult gonadal androgens in the sexually dimorphic responses observed in a model of IgE-mediated PSA. Gonadectomized (GDX) and sham-operated (SHAM) male and female mice were sensitized overnight with IgE directed against DNP (dinitrophenyl) and then challenged 24 h later with DNP-HSA (human serum albumin) as an antigen to induce anaphylaxis. As anticipated, SHAM females exhibited more severe hypothermic responses compared to SHAM males (Fig. 1 A and B). Adult gonadectomy had no effect on these sex differences, with comparable hypothermic responses between SHAM and GDX females, and, likewise, for SHAM and GDX males (Fig. 1 A and B). Serum levels of histamine, the major MC mediator responsible for PSA-induced increased vascular permeability and hypothermia, were higher 30 min post DNP in both female SHAM and GDX mice compared with respective males (SHAM female vs. SHAM male: 2.28-fold, GDX female vs. GDX male: 2.81-fold; Fig. 1C), overall indicating that gonadectomizing did not influence the magnitude of the hypothermia response regardless of the sex. Quantification of tissue MCs determined by toluidine blue staining confirmed that adult gonadectomizing did not influence the number or degranulation status of tissue MCs (Fig. 1 D and E). Together, these experiments show that sex differences in IgE-mediated MC histamine release and anaphylaxis are not dependent on adult gonadal hormones.
Fig. 1.
MC-mediated anaphylaxis is sexually dimorphic without the presence of adult gonadal hormones. Male and female mice were GDX or SHAM at 12 wk of age. Mice (13 wk to 14 wk old) were sensitized with DNP-specific IgE (10 μg i.p.) and challenged the following day with DNP-HSA (500 μg i.p.; n = 5 to 7 per group). (A) Male and female GDX mice had sexually dimorphic hypothermia responses that were not different from male and female SHAM mice. Repeated measures two-way ANOVA with Tukey’s multiple comparisons test. (B) Peak temperature change was greater in female mice regardless of group. Two-way ANOVA with Sidak’s multiple comparisons test. (C) Serum histamine levels 30 min after anaphylaxis were greater in female mice, regardless of GDX status, compared with male cohorts. No difference in serum histamine levels was found between GDX and SHAM groups within their sex. Two-way ANOVA with Sidak’s multiple comparisons test. (D) Gonadectomizing did not affect the number of MCs in mesenteric tissue MCs in male or female mice, and MC number was not different between the sexes. Two-way ANOVA with Sidak’s multiple comparisons test. (E) Gonadectomizing had no overall effect on tissue MC degranulation measured as the percentage of degranulated MCs 30 min after induction of anaphylaxis. Two-way ANOVA with Sidak’s multiple comparisons test. MCs were counted in 10 randomly chosen fields (200× magnification) per mouse. Unpaired Student’s t test. Data represent mean ± SEM; ns: nonsignificant; ****P < 0.0001.
Sex Differences in MC-Mediated Anaphylaxis Emerge prior to Puberty.
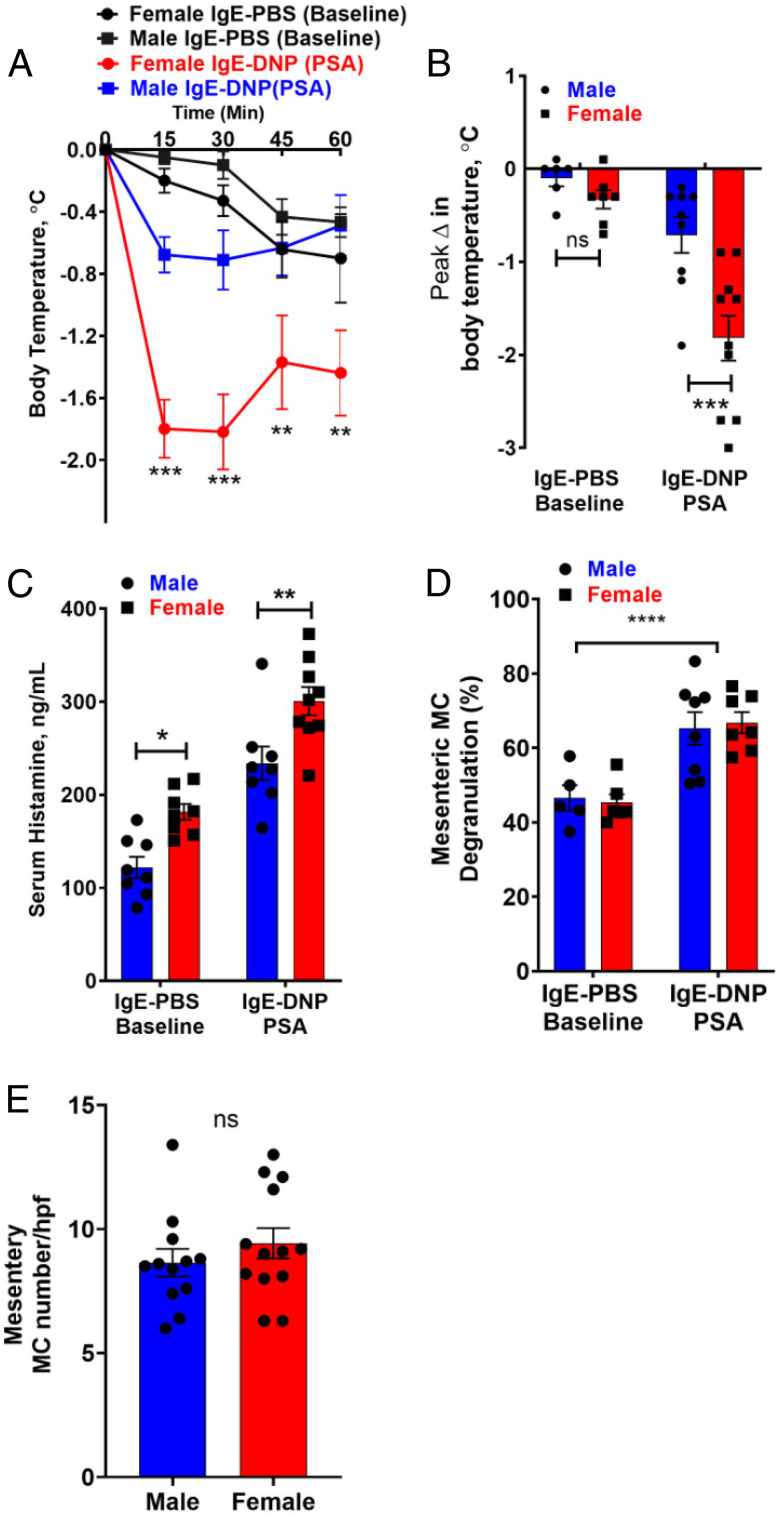
Given that adult gonadal hormones alone were shown not to be major determinants of sex differences in MC-mediated anaphylaxis, we focused our attention on potential early mechanisms of sexual differentiation. First, we investigated whether sex differences emerged prior to puberty, by comparing MC-mediated anaphylaxis in 14-d-old male and female mice, which represents a prepubertal stage in C57BL/6 mice (23). In general, PSA-induced hypothermic responses were lesser in magnitude in neonatal mice compared with adults (Fig. 1), which could be due to the lower concentrations of serum histamine observed in prepubertal mice, compared with adult mice. Nonetheless, sex differences were observed in prepubertal mice, with female mice exhibiting more severe PSA-induced hypothermic responses (Fig. 2 A and B; 30 min: female, Δ −1.82 °C; male, Δ −0.71 °C) and greater serum histamine levels compared with prepubertal males (Fig. 2C). We conducted additional experiments in 14-d-old MC-deficient KitW-sh/W-sh (Sash) mice to confirm that histamine and hypothermia responses in prepubertal mice were MC dependent. IgE-sensitized prepubertal MC-deficient Sash mice exhibited low basal levels of serum histamine that did not elevate with PSA induction with DNP injection (SI Appendix, Fig. S1A), thus confirming that MCs are the main contributors to histamine and hypothermia responses observed in prepubertal mice. Consistent with our previous findings in adult mice (21), prepubertal male and female mice had similar numbers of intestinal mesenteric tissue MCs (Fig. 2D). The percentage of degranulated in mesenteric tissue MCs was increased following DNP-induced anaphylaxis; however, the level of activation between females and males was similar (Fig. 2E). Overall, these data demonstrate evidence that sex differences in MC-mediated histamine release and anaphylaxis emerge prior to puberty and independent of adult sex hormones.
Fig. 2.
Prepubertal mice exhibit sexually dimorphic MC-mediated anaphylaxis. Male and female prepubertal mice (14 d of age) were sensitized with DNP-specific IgE (10 μg i.p.) and challenged the following day with DNP-HSA (500 μg i.p., n = 8 to 10 per sex) or PBS (n = 6 to 8 per sex). (A) Prepubertal male and female mice exhibited a decrease in body temperature in response to PSA. Prepubertal female mice had more severe hypothermia responses starting at 15 min and lasting for the remainder of the experiment. RM two-way ANOVA with Sidak’s multiple comparisons test. (B) Prepubertal female mice had greater drop in temperature after DNP injection compared to prepubertal male mice. Two-way ANOVA with Sidak’s multiple comparisons test. (C) PSA increased serum histamine levels in both prepubertal male and female mice. Prepubertal female mice exhibited higher serum histamine levels 15 min after DNP or PBS injection compared to prepubertal males. Two-way ANOVA with Sidak’s multiple comparisons test. (D) DNP injection increased the percentage of degranulated MCs to a similar level in male and female prepubertal mice; n = 7 or 8 per sex. No difference in MC degranulation was observed between the sexes in IgE-PBS treated groups; n = 5 or 6 per sex. (E) MC number per high powered field (hpf) in intestinal mesenteric windows was similar in prepubertal male and female mice; n = 12 or 13 per sex; unpaired Student’s t test. Experimental animals within each treatment were derived from three independent animal cohorts and dams to establish reproducibility; data represent means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
An additional finding worth noting in this experiment was that female mice had higher serum histamine levels compared with males when measured at baseline (prior to DNP injection) (Fig. 2C). The elevated serum histamine in females may be due to the short period of isolation stress when mice were removed from their group-housed cages and placed in individual cages for the PSA induction and monitoring. To confirm this, we measured serum histamine levels from prepubertal mice immediately upon removal from their home cage, which eliminated sex differences in basal histamine (SI Appendix, Fig. S1B), which, together, supports that elevated basal serum histamine in female mice was likely stress induced, which is supported by our previous studies showing the effects of stress on MC activation and histamine release (24).
Perinatal Androgens in Males as Critical Early-Life Modulators of Sex Differences in MC Histamine Levels and Anaphylaxis.
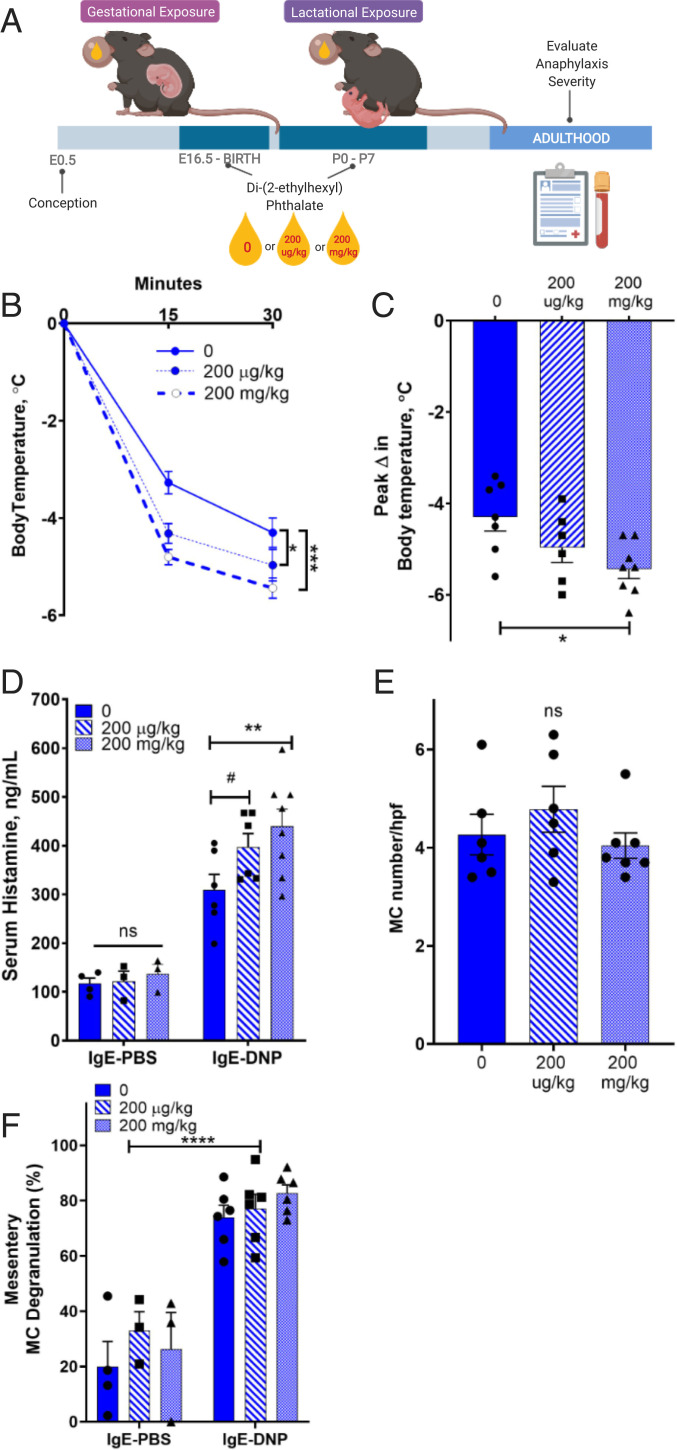
The above experiments in 14-d-old mice demonstrate that sex differences in MC histamine and anaphylaxis support that mechanisms of sex differences have early-life origins. The perinatal androgen surge in prenatal and early postnatal life, which occurs during the “organizational period of sexual development” in males, is a critical organizer of many sex-specific traits such as the genitalia and brain (25). Unlike estrogens, androgens show striking sex differences during perinatal development, with markedly higher levels in males than in females. Therefore, we hypothesized that perinatal androgens in males were critical to development of sexually dimorphic anaphylaxis responses. To test this hypothesis, we inhibited perinatal androgen production in males by exposing pregnant dams to low and high doses of di-(2-ethylhexyl) phthalate (DEHP), a pervasive environmental endocrine disruptor that has well-established antiandrogen effects although it decreases testosterone production in Leydig cells of the testes (26, 27). Administration of DEHP to dams was continued postpartum (postnatal d0 to d7), resulting in lactational exposure and thus also inhibiting the postnatal androgen surge in male offspring (Fig. 3A). To validate the antiandrogen effects of DEHP, we measured anogenital distance (AGD), an androgen-sensitive measurement in rodents and humans (28, 29), which showed that DEHP-exposed male offspring had significantly decreased AGDs measured at prepubertal, pubertal, and adult time points (SI Appendix, Fig. S2A), thus confirming the DEHP-mediated inhibition of androgen levels during early development as demonstrated previously (30). Perinatal DEHP-exposed males also exhibited reduced testis weights, further confirming decreased testosterone action (SI Appendix, Fig. S2B). In agreement with our hypothesis, adult males previously exposed to DEHP in the perinatal organizational period exhibited heightened MC-mediated anaphylaxis as determined by increased hypothermia responses (Fig. 3 B and C) and elevated serum histamine levels (Fig. 3D) compared with control (non-DHEP exposed) males. Perinatal DEHP exposure did not influence tissue MC numbers (Fig. 3E) or the percentage of degranulated tissue MCs (Fig. 3F). DEHP-exposed female offspring had reduced AGDs (SI Appendix, Fig. S3A), which has been reported previously (30). While not statistically significant, hypothermia response was numerically greater in DHEP exposed females (SI Appendix, Fig. S3 B–F), which, along with the AGD measures, suggests that overall androgen exposure, perhaps indirectly through male littermates, could also impact female MC-dependent measures. Together, these results show that inhibition of the perinatal androgen surge in males with DHEP results in heightened severity of MC-mediated anaphylaxis in adulthood, thus supporting a protective role of perinatal androgens in males in MC-mediated anaphylaxis in adulthood. These findings may also provide insight into the link between DEHP exposure and increased allergic disease and asthma in people (31).
Fig. 3.
Perinatal exposure to the antiandrogen, DEHP in males increases the severity of MC-mediated anaphylaxis into adulthood. (A) Schematic of DHEP exposure. Created with BioRender.com. Male (8 to 10 wk old) perinatally DEHP-treated or CO-treated mice were sensitized with DNP-specific IgE (5 μg i.p.) and challenged the following day with DNP-HSA (500 μg i.p., n = 6 to 8) or PBS (n = 3 or 4 per group). (B) In response to IgE-DNP, male mice exposed to perinatal DEHP had greater serum histamine levels compared to CM; two-way ANOVA with Sidak’s multiple comparisons test. (C) Male mice exposed to DEHP perinatally had more severe hypothermia responses compared to controls in response to PSA. RM two-way ANOVA with Dunnett’s multiple comparisons test. (D) Peak temperature change was greatest after high-dose perinatal DEHP exposure. One-way ANOVA with Dunnett’s multiple comparisons test. (E) Perinatal DEHP exposure did not change intestinal mesenteric MC number; one-way ANOVA with Tukey’s multiple comparisons test. (F) PSA increased MC degranulation to similar levels in all groups; two-way ANOVA with Sidak’s multiple comparisons test. Data represent means ± SEM. Experimental animals within each treatment were derived from six dams and conducted in two independent cohorts to establish reproducibility; #P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Does Perinatal Androgenization of Female Offspring Reduce the Severity of Adult MC-Mediated Anaphylaxis?
The above studies provide evidence that normal exposure to high levels of perinatal androgens in males is protective against MC-mediated anaphylaxis in adulthood. To further explore this concept, we tested the hypothesis that exposure of females to high levels of androgens during the perinatal period would protect female mice from anaphylaxis by reducing MC histamine levels and hypothermia responses. To recapitulate the male perinatal androgen surge, testosterone propionate (TP) was administered to pregnant dams (in utero) and to neonatal pups to mimic the prenatal and postnatal androgen surges in males (Fig. 4A). The effectiveness of the androgenization protocol was confirmed by measuring AGD, body weight, and visceral fat volume. Androgenized females (AF) had greater AGDs, heavier body weights, and greater visceral fat volume compared to control females (CF), thus confirming the successful androgenization protocol (SI Appendix, Fig. S4 A, E, and F). Male offspring from perinatally TP-exposed litters (androgenized male offspring [AM]) had decreased postpubertal body weights, AGD, and testis weight, which is in agreement with previous findings in the literature (32) and proposed to be a result of induction of a negative feedback system in response to “supraphysiologic” levels of testosterone (33) (SI Appendix, S4 B–D). Together, these data confirm that androgenization with TP during the perinatal period was effective in masculinizing female mice. All mice were GDZ in adulthood to remove the confounding effects of perinatal androgen treatment on subsequent gonadal hormone production. Perinatally AF exhibited significantly reduced severity of MC-mediated anaphylaxis as demonstrated by significantly reduced hypothermia responses (Fig. 4 B and C) and lower serum histamine levels (Fig. 4D) compared with CF following induction of IgE-mediated anaphylaxis. The levels of MC histamine and hypothermia responses in AF mice were reduced to the level of control males (CM), reflecting a complete sex reversal with regard to these measurements. AM also exhibited significantly reduced hypothermia response compared with CM mice (Fig. 4 B and C). A numerical but nonsignificant reduction in serum histamine level was observed in AM compared with CM (Fig. 4D). To determine whether perinatal androgenization reduced preformed MC mediators other than histamine, we also measured serum MC protease 1 (MCPT-1) levels, which were also reduced in AF group (Fig. 4E). Quantification of the number and percent of degranulated MCs intestinal mesenteric windows (Fig. 4 F and G) from adult mice demonstrated that perinatal androgenization or sex did not significantly change the number of tissue MCs or their ability to degranulate. Together, these experiments demonstrate that perinatal androgen exposure results in attenuated MC-mediated anaphylaxis into adulthood, by reducing MC mediator (e.g., histamine and MCPT-1) levels and hypothermic responses.
Fig. 4.
Perinatal androgen exposure in females masculinizes MC-mediated anaphylactic responses into adulthood. (A) Schematic of perinatal androgenization protocol created with BioRender.com. Adult (8 wk old) male and female mice that were perinatally TP-treated or SO-treated were sensitized with DNP-specific IgE (10 μg i.p.) and challenged the following day with DNP-HSA (500 μg i.p., n = 6 to 9 per group) or PBS (n = 3 per sex). (B) Control (CF) mice had more severe hypothermia responses compared to AF mice. CM and AF mice had similar hypothermia responses. Androgenized male (AM) mice had reduced hypothermia compared to all other groups. RM two-way ANOVA with Tukey’s multiple comparisons test. (C) Peak temperature change was greatest in CF mice. One-way ANOVA with Tukey’s multiple comparisons test. (D) Serum histamine levels were increased in all groups 30 min after DNP injection. Perinatal testosterone exposure greatly reduced histamine levels in female mice to the level of CM. Two-way ANOVA with Sidak’s multiple comparisons test. (E) Serum MCPT-1 levels were increased in all groups 30 min after DNP injection. Serum MCPT-1 levels were below the limit of detection in mice treated with PBS. Perinatal androgenization greatly reduced MCPT-1 levels in female mice to the level of CM. (F) One-way ANOVA with Tukey’s multiple comparisons test. MC number in intestinal mesenteric windows was not different between groups; n = 5 to 7 per group; one-way ANOVA with Tukey’s multiple comparisons test. (G) The percentage of mesenteric MCs that exhibited a degranulated appearance was increased following induction of PSA in intestinal mesentery, but there were no differences between control and androgenized mice. Two-way ANOVA with Sidak’s multiple comparisons test. (H) Meningeal MC number was similar between groups; n = 7 to 10 per group. One-way ANOVA with Tukey’s multiple comparisons test. (I) PSA increased MC degranulation in the meninges to similar levels in all groups. Two-way ANOVA with Sidak’s multiple comparisons test. Data represent means ± SEM. Experimental animals within each treatment were derived from at least seven dams and studies conducted in at least three independent cohorts to establish reproducibility. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Tissue MCs from Testicular Feminization Mutant Rats and Perinatally Androgenized Mice Exhibit Reduced Cellular Histamine Content.
The above experiments show that perinatal androgen exposure is a major factor in sex differences in MC-mediated anaphylaxis into adulthood. However, why CM and AF mice exhibited reduced MC histamine levels and reduced severity of anaphylaxis despite having similar numbers and levels of tissue MC activation is unclear. Given these findings and our previously published in vitro studies that showed that male and female MCs had similar levels of intracellular Ca2+ signaling, we investigated the hypothesis that perinatal androgens exposures alter the mediator concentration stored within MC granules. To test this hypothesis, we first measured total cellular histamine concentration in peritoneal tissue MCs obtained from testicular feminization mutant rats (Tfm), which lack functional androgen receptors throughout life, thus rendering them insensitive to androgens (34). We used Tfm rats, as opposed to Tfm mice, as they offer certain advantages in that Tfm rats do not exhibit elevated testosterone levels as mice do, which could potentially confound results as a result of aromatization of testosterone into estrogen. Further, isolation of tissue MCs from rats allows for the collection of a sufficient number of tissue MCs to evaluate individual animals without having to pool samples between multiple animals as in mice. We found that rat peritoneal MCs (pMCs) obtained from wild-type (WT) male rats had significantly lower histamine concentrations compared with pMCs obtained from WT female rats (SI Appendix, Fig. S5A), thus confirming the sex differences in tissue MC phenotype. Moreover, pMCs from Tfm male rats exhibited a feminized MC phenotype with increased histamine concentration compared with WT male littermates (SI Appendix, Fig. S5A). To confirm this result in tissue MC from mice, we measured histamine content in pooled pMCs adult CM, CF, and AF mice, which showed that CM pMCs had lower histamine concentrations compared with CF pMCs and that AF pMCs had reduced histamine concentrations compared with CF (SI Appendix, Fig. S5B). Together, these experiments demonstrate that exposure to high perinatal androgen levels results in tissue MCs with a significantly reduced MC histamine concentration. Further, the Tfm model results indicate that the action of perinatal androgens on reducing tissue MC histamine content are, in part, mediated via androgen receptors.
Perinatal Androgens Program Bone Marrow MC Stem Cells toward Reduced MC Mediator Concentration.
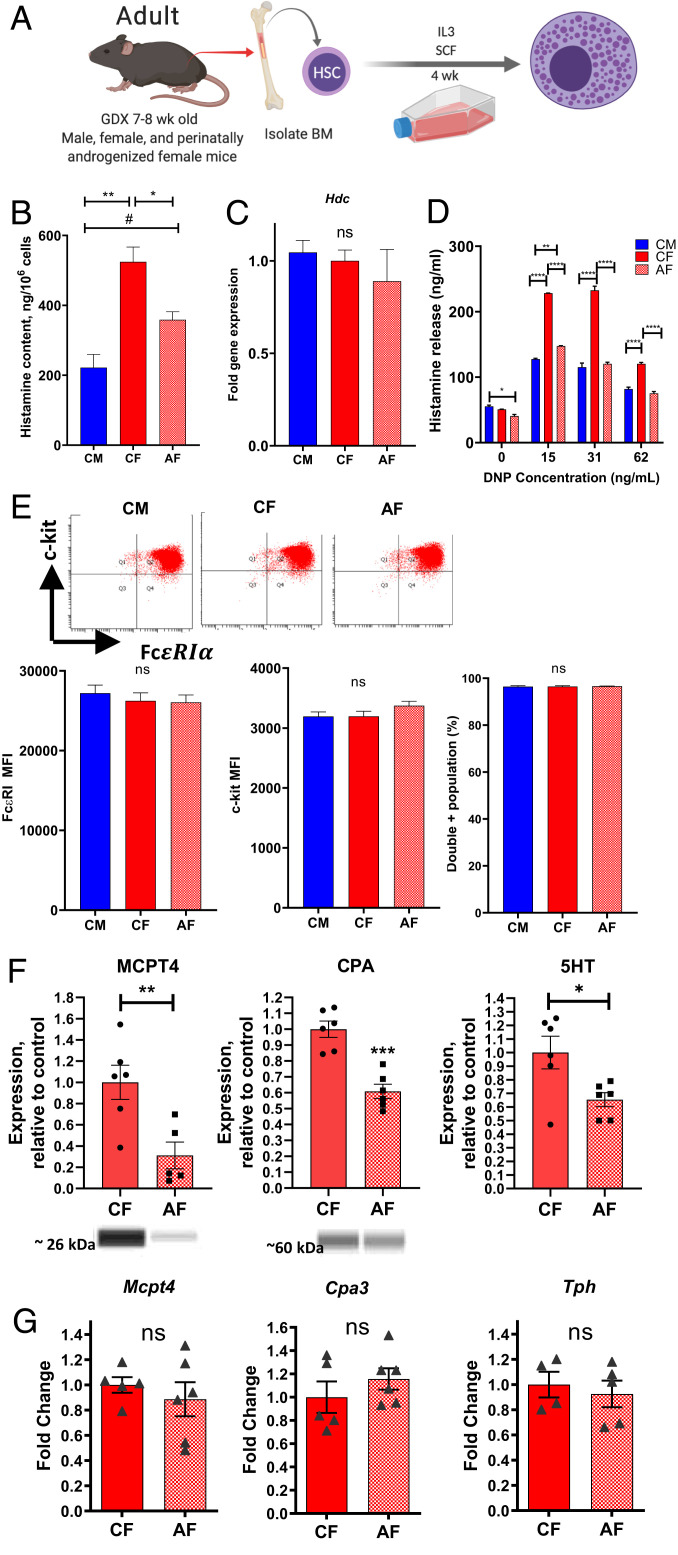
Adult MCs are derived from the bone marrow and circulate until they reach peripheral tissue sites, where they mature and acquire MC-specific characteristics including histamine and protease synthesis and subsequent storage into secretory granules. Our previous studies conducted with cultured BMMCs obtained from adult female and male mice revealed sexually dimorphic MC granule phenotype, indicating that BMMC progenitors were preprogrammed to exhibit sex differences in MC mediator concentrations (21). Therefore, we hypothesized that exposure to perinatal androgens results in the programming of bone marrow MC precursors toward reduced MC mediator concentration into adulthood. Pregnant dams and pups were administered TP as described previously. BM progenitor cells were harvested from femurs from adult CM, CF, and AF offspring, and BMMCs were derived in culture in the presence of SCF (stem cell factor) and IL3-containing media as described in Methods (Fig. 5A). In agreement with our hypothesis and pMC experiments, BMMCs from AF mice had significantly reduced concentration of histamine as measured in cell pellets under baseline conditions, compared with CF BMMCs. (Fig. 5B). Relative gene expression for the rate-limiting enzyme in histamine synthesis, histidine decarboxylase (Hdc), was similar between CF and AF BMMCs, suggesting that reduced cellular histamine content was not due to down-regulated histamine synthesis (Fig. 5C). As a result, histamine levels in BMMC supernatants following induction of IgE-DNP-mediated degranulation were significantly reduced in AF BMMCs (Fig. 5D). BMMCs derived from CM, CF, and AF mice had similar expression levels of the MC surface markers, c-kit, and FcεRI receptors, indicating that the reduced cellular and released histamine concentrations from AF BMMCs were not associated with alterations in c-kit or IgE receptor expression (Fig. 5E). Because we showed that prepubertal androgenized mice exhibited reduced in vivo MC histamine level and anaphylaxis, we also measured histamine levels and release in BMMCs derived from prepubertal mice (SI Appendix, Fig. S6A). Like adult BMMCs, prepubertal BMMCs derived from AF mice exhibited reduced cellular histamine concentrations (SI Appendix, Fig. S6B) and histamine release following IgE-DNP stimulation (SI Appendix, Fig. S6C), compared with those from CF mice. When comparing adult BMMCs with prepubertal BMMCs, it appeared that the effect of androgenization on BMMC histamine content was more pronounced in prepubertal mice, as it reduced cellular histamine levels to that of CM, indicating a more complete reversal of sex differences. The reason for this finding is unclear, but puberty is also a sensitive period for gonadal hormones and thus may also play an additional role in modifying sex differences in MC phenotype (35).
Fig. 5.
Perinatal androgen exposure programs bone marrow MC progenitors for reduced MC granule mediator concentrations in adulthood. (A) Schematic depicting derivation of BMMCs from femoral bone marrow of 7- to 8-wk-old GDX CM, CF, and AF mice. Created with BioRender.com. (B) Total histamine concentration was reduced in perinatally AF BMMCs compared to CF BMMCs. (C) Hdc gene expression in BMMCs. One-way ANOVA with Tukey’s multiple comparisons test. (D) BMMCs were sensitized with monoclonal anti-DNP IgE (0.5 µg/mL) overnight and later stimulated with 0, 15, 31, and 62 ng/mL DNP-HSA for 1 h. Supernatants from AF BMMCs had reduced concentrations of histamine after FcεRI stimulus compared with CF BMMCs, and had similar responses to CM BMMCs, with the exception of 15 ng/mL DNP stimulus. Two-way ANOVA with Tukey’s multiple comparisons test. (E) BMMCs were stained with fluorescent-conjugated c-kit (Pacific Blue) and FcεRI (PE) antibodies, and representative images of two-color flow cytometric analysis of FcεRI (horizontal axis) and c-kit (vertical axis) receptor expression from CM, CF, and AF adult BMMCs are shown. Flow cytometry geometric means (bar graphs) for c-kit, FcεRI, and c-kit/FcεRI double positive cell percentages showed no difference between BMMC groups. One-way ANOVA with Tukey’s multiple comparisons test. (F) Androgenized BMMCS exhibited reduced levels of MC proteases MCPT4 and CPA (Western blot analysis), and 5HT (ELISA) in BMMC pellets. (G) Relative mRNA expression for Mcpt4, Cpa3, and Tph in BMMC pellets was not different across experimental groups. One-way ANOVA with Tukey’s multiple comparisons test. Data are representative of experiments from five or six independent BMMC cultures in each experimental group to establish reproducibility. #P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
While we have focused predominantly on histamine, as it is considered the major MC granule mediator involved in the acute clinical onset of anaphylaxis, we also wanted to know whether exposure to perinatal androgens resulted in reduced levels of other MC granule mediators. Western blot and enzyme-linked immunosorbent assay (ELISA) analysis of BMMC cell pellets for MC protease 4 (MCPT4), carboxypeptidase (CPA), and serotonin (5HT) showed significantly reduced concentrations of these mediators in AF BMMCs compared with CF controls (Fig. 5F). Like Hdc gene expression, corresponding messenger RNA (mRNA) levels for the MCPT4 (Mcpt4) and CPA (Cpa3), or the major rate-limiting enzyme in 5HT synthesis (tryptophan hydroxylase; Tph), were not different between CF and AF BMMCs (Fig. 5G). Together, these results demonstrate that biological sex and perinatal androgens induce a long-term programming of bone marrow MC progenitors toward a masculinized MC phenotype which is characterized by reduced cellular concentration of multiple prestored MC granule mediators, in turn, leading to reduced levels of released mediators upon degranulation.
Biological Sex via Perinatal Androgens Programming of MC Phenotype Determines Anaphylaxis Severity.
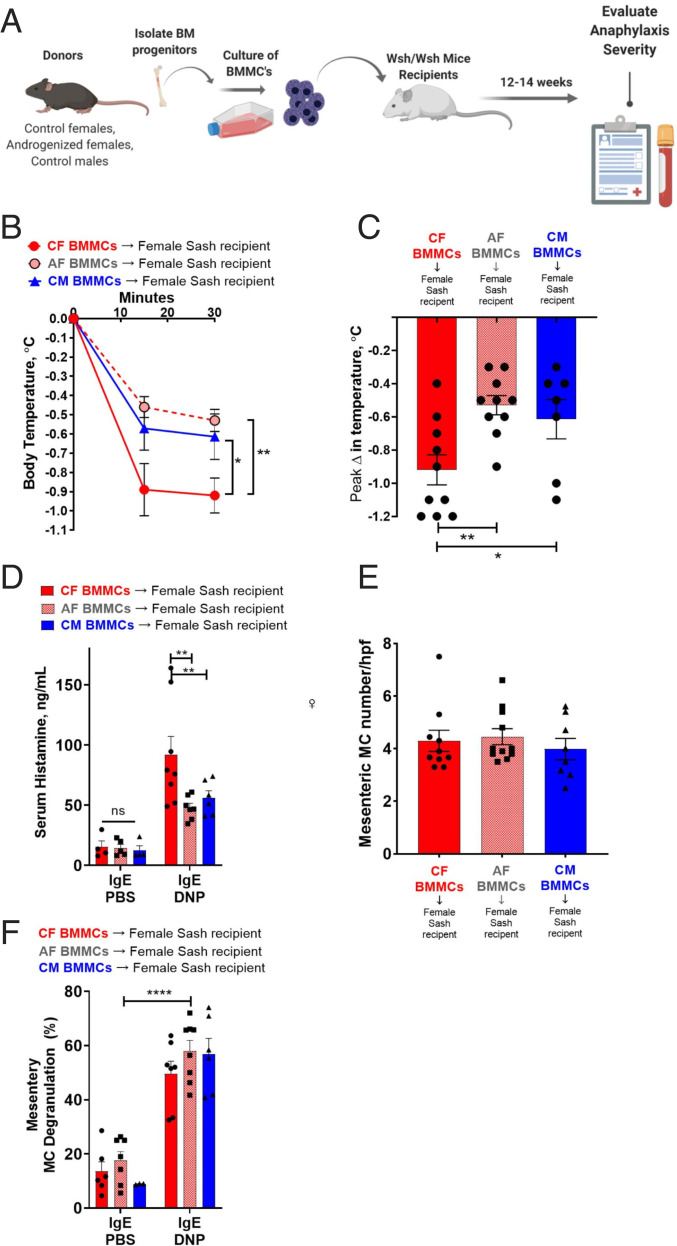
Given that perinatal androgen exposure in females resulted in a programming of MC bone marrow progenitors toward a masculinized phenotype characterized by reduced granule mediator concentration, we next tested the in vivo contribution of this programming event and MC phenotype to MC activity and anaphylaxis severity. Toward this end, we utilized the MC knock-in approach where female and male MC-deficient Sash mice were engrafted with BMMCs derived from bone marrows of adult mice from the same or opposite sex (Fig. 6A). Twelve weeks after BMMC engraftment, IgE-mediated MC activation and hypothermia responses were evaluated. In general, IgE-mediated, BMMC-engrafted Sash mice exhibited significant hypothermia and elevations in serum histamine following induction of PSA with IgE-DNP. The magnitude of the responses in the engrafted Sash mice were lesser compared with WT mice (Figs. 1–3), which was expected due to the inability to fully reconstitute tissue MC levels to that of WT mice. Nonetheless, the significant PSA responses indicated a successful tissue MC engraftment. Female Sash mice engrafted with female (CF) BMMCs exhibited more severe PSA-induced hypothermia (Fig. 6 B and C) and greater serum histamine levels (Fig. 6D) compared with Sash mice engrafted with male (CM) BMMCs, thus recapitulating the sex differences observed in WT female and male mice. Moreover, female Sash mice engrafted with BMMCs from perinatally AF exhibited attenuated hypothermic responses (Fig. 6 B and C) and serum histamine levels (Fig. 6D) compared with CF-engrafted Sash mice. Engraftment rates of BMMCs (Fig. 6E) and mesenteric tissue MC degranulation percent (Fig. 6F) were similar across experimental groups, confirming that differences between experimental groups were not due to different BMMC engraftment efficiencies.
Fig. 6.
MC-deficient KitW-sh/W-sh mice engrafted with BMMCs from adult male, female, and perinatally AF exhibited serum histamine and hypothermia responses that represented the BMMC phenotype and not host sex. (A) Schematic depicting engraftment protocol. Created with BioRender.com. Adult female MC-deficient KitW-sh/W-sh mice were engrafted with BMMCs derived from adult GDX male, female, or perinatally AF mice (i.p., 1 × 107 BMMCs). Adult male MC-deficient KitW-sh/W-sh mice were engrafted with BMMCs derived from adult GDX male or female mice (i.p., 1 × 107 BMMCs). Eight to twelve weeks after engraftment, mice were exposed to PSA for 30 min. (B) Female KitW-sh/W-sh mice engrafted with female BMMCs had more severe hypothermic responses to PSA compared to male and perinatally AF BMMCs. Engrafted mice exposed to IgE-PBS treatment had no change in temperature; n = 7 to 10 per group; RM two-way ANOVA with Dunnett’s multiple comparisons test. (C) Female KitW-sh/W-sh mice engrafted with female BMMCs had greatest change in peak temperature after PSA. One-way ANOVA with Dunnett’s multiple comparisons test. (D) KitW-sh/W-sh mice engrafted with BMMCs had increased serum histamine levels after DNP injection compared to PBS injection. Female KitW-sh/W-sh mice engrafted with female BMMCs had greater serum histamine levels after PSA compared to female KitW-sh/W-sh mice engrafted with AF BMMCs and female KitW-sh/W-sh mice engrafted with CM BMMCs; IgE-PBS; n = 3 or 4 per group, IgE-DNP; n = 6 to 8 per group; two-way ANOVA with Dunnett’s multiple comparisons test. (E) MC degranulation percentage was similar in all groups at baseline and after PSA; IgE-PBS; n = 4 or 5 per group, IgE-DNP; n = 5 or 6 per group; two-way ANOVA with Dunnett’s multiple comparisons test. (F) intestinal mesenteric MC numbers were similar in female KitW-sh/W-sh mice engrafted with BMMCs; n = 8 to 11 per group; one-way ANOVA with Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ****P < 0.0001.
Like BMMC-engrafted females, Sash males engrafted with BMMCs from CF had more severe hypothermic responses (SI Appendix, Fig. S7A) and greater serum histamine levels (SI Appendix, Fig. S7B) compared with mice engrafted with BMMCs from CM. Male and female Sash mice exhibited low levels of serum histamine after PSA (SI Appendix, Fig. S7C), confirming that responses observed in the engrafted Sash mice were MC dependent. Together, these data demonstrate that the sexual phenotype of the MC, but not tissue sex-specific host factors, drive sex differences observed in IgE-mediated anaphylaxis.
Discussion
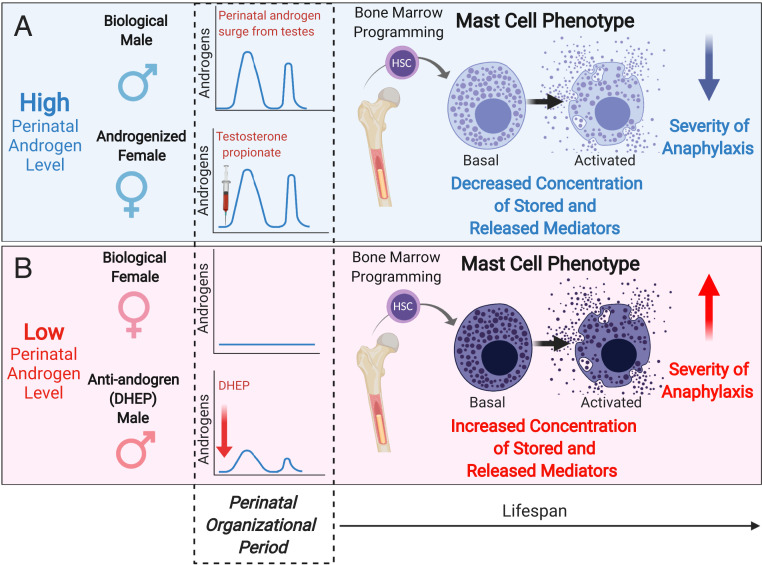
Classical MC-associated disorders, such as allergy and anaphylaxis, and a number of diseases where MCs are thought to mediate the pathophysiology, such as autoimmune disease and functional bowel disorders (e.g., irritable bowel syndrome), exhibit a striking sex bias, with females at increased risk (1–5, 10–14). While adult sex hormones have received most of the attention as a mechanism driving sex differences, that sex-biased diseases also occur in prepubertal children challenges this notion and suggests that sex differences in MC-associated diseases have early-life origins, a concept which has been largely unexplored. Here we demonstrate that sex differences in MC-mediated anaphylaxis emerge prior to puberty and are determined by the perinatal androgen levels during the critical perinatal period of sexual organization. Perinatal androgens exposure leads to programmed, phenotypic changes in BMMC progenitors and tissue MCs, characterized by reduced MC granule mediator levels and reduced severity of MC-related disease pathophysiology compared with females (Fig. 7).
Fig. 7.
A model for the role of perinatal androgen levels in sexual dimorphism of MC phenotype and disease severity throughout the lifespan. (A) In the presence of high perinatal androgen levels (e.g., normal perinatal androgen surge in males or androgenization of females with TP), bone marrow MC progenitors develop into tissue MCs that have decreased storage of preformed granule mediators (e.g., histamine, proteases, serotonin), resulting in lower amounts of released mediators upon activation and decreased the severity of anaphylaxis from neonatal period into adulthood. (B) In the absence of high perinatal androgens (e.g., biological female or exposure to antiandrogen compounds such as phthalates [DHEP] in males), bone marrow MCs develop into tissue MCs that have increased concentrations of MC granule mediator, resulting in higher amounts of released mediators upon activation thus enhancing the severity of anaphylaxis. Created with BioRender.com.
Adult Gonadal Sex Hormones Are Not Critical Determinants of Sex Differences in MC-Mediated Anaphylaxis in Mice.
The mechanism of sex differences in MC-associated disorders is poorly understood, and the few studies on this topic have focused attention on adult sex hormones. It is known that human and rodent MCs express receptors for estrogen, progesterone, and androgen receptors (36–39) and that progesterone and estrogens, but not testosterone, have been shown to induce mild degranulation responses in cultured MCs (36, 38, 39). We and others have demonstrated that the number of MCs change can change significantly throughout the stages of the estrous cycle (21, 38, 40). Also, Hox et al. (41) showed that administration of estrogen to adult GDX female mice enhanced the hypothermia response induced by IgE-mediated anaphylaxis. However, in the present study, we showed that gonadectomizing in adult females and male mice did not significantly impact the level of serum histamine, tissue MC activation, or the severity of hypothermia and thus did not eliminate sex differences. Taking the literature and our work together, this suggests that, while adult sex hormones have the potential to modulate MC activity, they are not major determinants in the sexually dimorphic responses in MC-mediated anaphylaxis.
Sex Differences in MC-Mediated Anaphylaxis Emerge prior to Puberty and Are Driven by Perinatal Androgens.
In the present study, we show that sex differences in histamine release from MCs and hypothermia were evident in 14-d-old prepubertal mice, with females exhibiting greater responses. Along with the lack of effect of adult gonadectomizing described above, these findings are in agreement with the human literature showing sex biases in MC-associated immune disease in prepubertal children and support the concept that sex differences in MC responses and anaphylaxis have early-life origins.
A critical early-life driver of sexual differentiation in males is androgen produced by the fetal/neonatal testes. Both prenatal and early postnatal androgens drive both masculinization and defeminization of tissues, including the reproductive organs and the brain. This is distinctly different from females, who seem to develop their sexual phenotype prepubertally without the help of high levels of gonadal steroids. In the present study, interfering with the normal perinatal androgen production in males with perinatal exposure (in utero and postnatal) to the antiandrogenic chemical DEHP resulted in heightened MC histamine release and increased severity of MC-mediated anaphylaxis in adult male offspring. These studies confirmed the importance of perinatal androgens in shaping later-life MC responses in general but may also have specific implications for early-life phthalate exposures in people. In utero phthalate exposure has been correlated with allergic disease in humans and in rodent models (31, 42). While in vitro studies showed that cultured human and rodent MCs lines degranulate in response to DEHP exposure (43, 44), our studies demonstrate that exposure to DEHP during a critical hormone-sensitive perinatal period interferes with normal androgen production which alters the developmental programming of MC responses, resulting in long-lasting effects. These findings have important public health implications in that they support a mechanistic link between early-life DEHP exposure and allergic and inflammatory diseases in humans.
In support of perinatal androgens as major players in the early-life origins of sex differences, we show here that recapitulating the male perinatal androgen surge in females via in utero and neonatal exposure to TP resulted in masculinized MCs responses in offspring demonstrated by greatly reduced MC-mediated histamine levels, and dampened the severity of anaphylaxis into adulthood. Interestingly, perinatal TP also led to minor but significant reductions in IgE-mediated hypothermia responses in males, suggesting that the normal physiologic levels of androgens produced by the male testes may not be sufficient to fully masculinize MC responses.
Perinatal Androgens Program the Phenotype of BMMC Progenitors and Tissue MCs to Have Reduced Storage of MC Granule-Associated Mediators.
While manipulation of perinatal androgens levels had a significant impact on the MC histamine levels and MC-mediated anaphylaxis, the number or degree of activation of tissue MCs was not different in adult mice. Lenz et al. (45) reported that newborn male rats had higher numbers and activated MCs in the preoptic area of the brain compared with females, which was linked to permanent effects on adult sexual behavior. While we did not measure early-life sex or androgen-sensitive changes in peripheral tissue MCs in the present study, based on the study of Lenz et al. (45), it possible that androgen manipulations may have an early influence on tissue MC numbers and activation, and thus are important to consider in future investigations. Given the lack of differences in MC number and activation observed in prepubertal and adult mice in the present study, this suggested that perinatal androgens could be influencing the content of MC mediators to be released upon subsequent stimulation. In agreement with this hypothesis, we showed that Tfm male rats with dysfunctional androgen receptors exhibit a feminized MC phenotype with increased amounts of cellular histamine. We also showed the opposite effect with pMCs from perinatally androgenized mice exhibiting reduced cellular histamine content. Results from these studies raised the question of how exposure to perinatal androgens during a relatively short critical period resulted in long-lasting effects on MC mediator release and severity of anaphylaxis into adulthood. The derivation of MCs from pluripotent hematopoietic stem cells obtained from adult female mice that were previously androgenized in the perinatal period had significantly reduced MC mediator storage, thus exhibiting the in vivo tissue phenotype which indicated that androgen exposure during the perinatal critical period can impart long-lasting programming effects on the BM myeloid system on MC precursors which give rise to sex-specific MC phenotype in the tissue environment. The significance of these programmed changes in MC phenotype was demonstrated in the current study showing that engraftment of BMMCs from adult male, female, and perinatally androgenized mice into MC-deficient mice resulted in MC-mediated anaphylaxis responses that mimicked the MC sex and androgenized phenotype. Thus, these studies demonstrate that sex differences and perinatal androgen influences on MC phenotype, as opposed to host sex or tissue environment and response to mediators, are a major driver of in vivo sex differences. Given that stem cells from the common myeloid lineage give rise to a variety of other innate immune cells, we speculate that exposure to perinatal androgens could potentially modulate sex differences in these other hematopoetically derived immune cell populations. Other components of the immune system have been shown to be permanently altered by androgens during the perinatal period. For example, early-life androgen exposure alters female lymphocyte characteristics such as self-antigen recognition, overall number, cytokine responses, and antibody responses toward male phenotypes (46–49). Previous studies also have demonstrated that neonatal female mice exposed to testosterone had reduced incidence and severity of disease in adulthood for autoimmune models of systemic lupus erythematosus and diabetes (50−51). Such changes in susceptibility may also be related to androgens and their masculinizing effects on other immune cells (48, 49).
In summary, our data establish a critical role of perinatal androgens in programming BMMC progenitors toward reduced MC mediator concentration, storage, and release, which contributes to the reduced MC-associated anaphylaxis in males, compared with females. Future investigations aimed at understanding of the mechanisms by which perinatal androgens impact MC development and lifetime disease risk could unveil new therapeutic targets for decreasing MC disease susceptibility. Also, given the equally beneficial roles that MCs play, such as in host defense, immune modulation, and wound healing, deciphering these same mechanisms may also provide novel targets for boosting MC-mediated immune responses under certain conditions for benefit.
Methods
Animals.
C57BL/6J (stock no. 000664) and KitW-sh/W-sh (stock no. 012861) mice derived from founding colony breeders (The Jackson Laboratory) were housed under specific pathogen-free conditions in facilities accredited by the Association for Assessment and Accreditation for Laboratory Care International. Mice were group-housed with littermates in light- and temperature-controlled rooms and provided ad libitum access to water and a standard commercial rodent chow diet. Adult male Long Evans rats carrying Tfm of the androgen receptor and littermate WT male and female rats derived from founding colony breeders (Charles River, MA) were bred in the animal facilities of Michigan State University and genotyped using PCR as described (34). Rats were group-housed and provided ad libitum access to water and rodent chow.
Gonadectomy Protocol.
Where indicated, animals were anesthetized under isoflurane anesthesia and GDX or SHAM at 7 wk to 12 wk of age, using sterile techniques. Briefly, under deep surgical anesthesia, male mice were castrated via a single midscrotal incision, and the testes were removed using a cautery pen (Gemini Cautery System, Braintree Scientific Inc.). Hemostasis was verified, and the skin incision was sutured closed. In female mice, two flank incisions were performed, and the ovaries were located and exteriorized through the muscle wall. Ovaries were removed using a cautery pen, and hemostasis was verified before suturing both the muscle and skin layers closed. In SHAM mice, the same procedures were performed without the removal of the testes or ovaries. Ketoprofen (Zoetis) analgesia was given at the onset of the procedure and continued the following 2 d. GDX mice were allowed to recover and used in indicated experiments 1 wk to 2 wk after surgical procedure. Uterine weight was evaluated 1 wk after ovariectomy or sham operations as a bioassay to assess quality of ovariectomy and reduced estrogen levels in GDX female mice (52).
PSA and Serum Histamine and MCPT-1 Measurement.
PSA was performed as described previously (21, 24), and the detailed protocol is available in SI Appendix. Serum histamine and MCPT-1 levels were measured with specific ELISA kits (Oxford Biomedical Research and Invitrogen, respectively).
Perinatal Androgenization Protocol.
Virgin female mice were paired with males, and mating was confirmed by the presence of a copulatory plug (gestational day 0.5). Pregnant female mice were injected with TP (100 μg in 0.05 mL of sesame oil [SO]) or vehicle (0.05 mL of SO; subcutaneously on the dorsum of the back) beginning on gestational day 16.5 until parturition. TP (100 µg in 0.05 mL of SO) or vehicle injections (0.05 mL of SO), administered via the intraperitoneal (i.p.) route, were continued in pups on alternate days from P1 to P7. Together, the objective of the prenatal and postnatal androgenization periods was to recapitulate the perinatal androgen surge and defeminize female mice as described (26, 27). Because perinatal hormone treatment can alter adult hormone profiles by influencing the hypothalamic−pituitary−gonadal axis, perinatally androgenized male and female mice, along with their SO-treated counterparts, were GDX in adulthood prior to use in experiments to remove this confounding effect (25).
Perinatal DEHP Exposure Protocol.
Virgin female mice were paired with males, and mating was confirmed by the presence of a copulatory plug (gestational day 0.5). Daily from gestational day 16.5 until the litter was 7 d of age, dams consumed a Cocoa Puff covered in corn oil containing either low or high doses of the antiandrogenic chemical DEHP (200 μg/kg BW (body weight) or 200 mg/kg BW; Sigma-Aldrich) or corn oil alone as previously described (44). Females were observed to ensure the entire Cocoa Puff was consumed. Oral DEHP exposure was chosen to mimic dietary exposure, which is the most common route for humans (53). The low dose of DEHP was selected to mimic a high-risk environmental exposure in humans, and a high dose of DEHP was chosen to compare to other studies using similar concentrations (53). High and low doses of oral DEHP have been shown to decrease testicular testosterone production (54, 55). Given that the antiandrogenic action of DEHP results in interference with the normal cascade of androgen-dependent outcomes, AGD and testis weight were measured to evaluate the efficacy of DEHP exposure. Mice subjected to DEHP exposure underwent PSA as described above.
Body Weight, Organ Volume and Weight, and AGD Measurement.
Body weights were measured to reflect prepubertal, pubertal, and adult time points. At the same time points, AGD, as defined by the distance from the anus to the base of the genital tubercle, was measured in millimeters, using digital calipers. AGD is normally twice as long in males as in females and is determined by androgen action during early development (56). Further, AGD can be perturbed by early-life manipulations of androgens, including TP and DEHP exposure. AGD was normalized to the cubed root of body weight to account for body size effects (56). Visceral fat volume was determined in anesthetized mice using the Quantum GX microCT Imaging System (Perkin-Elmer). Paired testicles were collected and weighed for absolute and relative (percent of body weight) weight to evaluate the effect of TP or DEHP exposure.
BMMC Engraftment in KitW-sh/W-sh (Sash) Mice.
Female KitW-sh/W-sh mice were injected intraperitoneally with 1 × 107 BMMCs (4 wk in culture; suspended in 100 μL of sterile phosphate-buffered saline [PBS]) derived from 8-wk-old GDX CF, perinatally AF, and CM mice. The same experiment was performed in male KitW-sh/W-sh mice with BMMCs derived from 8-wk-old GDX CM and CF mice. Six to twelve weeks after engraftment, mice were subjected to the PSA protocol described above. Rectal temperatures were monitored, and mice were killed 30 min after DNP injection for blood collection by cardiac puncture for later histamine analysis. Small-intestinal mesenteric windows and meninges were harvested, fixed in Carnoy’s fixative, and stained with toluidine blue to evaluate the degree of engraftment. Ten nonoverlapping fields were counted per animal, and the average number of MCs and the percent degranulated were calculated for each group of animals. MCs were not present in the meninges of any KitW-sh/W-sh repleted with BMMCs, demonstrating a local reconstitution of MCs, as expected (57). Spleens of nonrepleted and repleted KitW-sh/W-sh mice along with C57BL/6 WT controls were collected and weighed for analysis.
Tissue MC Histological Analysis.
Small-intestinal mesentery windows were whole-mounted on glass slides, fixed with Carnoy’s fixative, and stained with toluidine blue (0.5%, 0.5 pH) to assess MC number and degranulation status. Calvariums were fixed with Carnoy’s fixative, and the meninges were separated and floated onto glass slides. Meninges were dried and stained with toluidine blue (0.5%, 0.5 pH) for 30 min to assess MC number and degranulation status. Intact and degranulated tissue MCs were counted under an optical microscope (Leica DM750) at 200× magnification in a blinded manner. Ten nonoverlapping fields were counted per animal, and the average number of MCs as well as the percent degranulated were calculated for each tissue and within each group. Degranulated tissue MCs were defined as MCs showing release of their cellular granules.
Isolation of pMCs.
The pMCs were collected from adult Long Evans rats (WT males, WT females, and Tfm males) by performing peritoneal lavage with 10 mL of Hanks’ balanced salt solution (1×) with (ethylenedinitrilo)tetraacetic acid (1 mM) followed by a 70% Percoll gradient as previously described (58). Purity of separation was confirmed to be >95%, by staining with toluidine blue. The pMCs were counted using trypan blue exclusion, and equal numbers of cells were lysed using RIPA (radioimmunoprecipitation assay) buffer supplemented with phosphatase and protease inhibitors, followed by sonication (Sonic Dismembrator Model 100, Fisher Scientific), for later histamine measurement.
Generation of Murine BMMCs.
Bone marrow progenitor cells were harvested from femurs of mice and cultured in IL-3 (5 ng/mL) and SCF (5 ng/mL) containing media, and phenotypically characterized with c-Kit and FcεRI by flow cytometry as described previously (21, 24) and in SI Appendix.
BMMC Stimulation and Mediator Measurement.
BMMCs (2 × 106 cells per mL) were sensitized with 0.5 µg/mL mouse monoclonal anti-DNP IgE (SPE-7, Sigma-Aldrich) overnight and then stimulated with various doses of DNP-HSA (Sigma-Aldrich) for 1 h. Supernatant was collected and kept at −80 °C until further analysis. For measurement of cellular mediator concentrations, BMMCs were lysed using RIPA buffer with protease inhibitors, sonicated, and stored at −80 °C. Histamine levels and in cell supernatants and lysates were quantified using a competitive histamine ELISA (Oxford Biomedical Research), and values were normalized. Serotonin concentration in lysed BMMC supernatants was determined using ELISA kit (Serotonin ELISA kit, ADI-900-175, Enzo Life Sciences Inc.) according to manufacturer’s directions. Samples were diluted 200×, and serotonin concentrations (nanograms per milliliter) were standardized to 1 × 106 cells.
Western Blot Analysis of BMMCs.
Protein was isolated from BMMCs using RIPA buffer with protease (Product no. 11697498001, Roche) and phosphatase (PI78426, Thermo-Fisher) inhibitors, and protein was quantified using the Pierce BCA Protein Assay Kit (Thermo-Fisher). Protein expression was assessed using the Protein Simple Wes™ capillary electrophoresis system (Protein Simple). Briefly, samples were diluted to 0.324 μg/μL in provided 1× sample buffer and 1× fluorescent molecular weight marker/reducing agent (Protein Simple). Samples were then vortexed, heat-denatured for 5 min at 95 °C, and loaded into the Wes™ assay plate (Protein Simple). Primary antibodies were loaded at 1:50 for Carboxypeptidase A (ab173283, Abcam) and at 1:10 for Chymase 1 (18189-1-AP, Proteintech) and Mast Cell Protease 4 (ab92368, Abcam). Blocking solution, horseradish-peroxidase conjugated secondary antibodies, and chemiluminescent substrate were loaded per kit instructions (Separation Module SM-W004, Protein Simple). The assay plate was then loaded into the Wes™ machine for automated electrophoresis (375 V for 40 min). Protein expression was quantified via densitometry of protein bands performed using ImageJ software (NIH) and calibrated to 1 × 106 cells. Final data were presented as expression value relative to the average of the control (nonandrogenized BMMCs).
RT-PCR.
RNA was isolated from nonstimulated BMMC pellets using TRIzol (Life Technologies) extraction protocol and purified with MicroRNeasy kit (Qiagen) following the manufacturer’s instructions. RNA was reverse transcribed into complementary DNA (cDNA) with Maxima First Strand cDNA Synthesis Kit for RT-qPCR with dsDNase reverse transcription kit (Thermo Fisher Scientific) following the manufacturer’s guidelines. Transcript levels were amplified using SYBR green (CFX connect, BioRad) and a defined set of primers (SI Appendix, Table). Glyceraldehyde-3-phosphate dehydrogenase served as normalization control. Results were quantified with the ΔΔCt method. All reactions were performed in triplicate.
Statistical Analysis.
In vivo and in vitro studies are presented as means ± SEM from a representative experiment with number of animals per group indicated in figure legends. Experimental results were repeated in a minimum of two independent experiments. Details of specific statistical analyses are included in the corresponding figure legends. All statistical analyses and calculations were performed with GraphPad Prism 8 software (GraphPad).
Study Approval.
All experimental procedures were reviewed and approved by Michigan State University’s Institutional Animal Care and Use Committee (Protocol 02/18-023-01).
Supplementary Material
Acknowledgments
This study was supported by NIH Grants NIH R01 HD072968 (to A.J.M. and C.L.J.), NIH R21 AI140413 (to A.J.M. and C.L.J.), and NIH F30 OD025354 (to E.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.A.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915075117/-/DCSupplemental.
Data Availability Statement.
All study data are included in the article and SI Appendix.
References
- 1.Acker W. W. et al., Prevalence of food allergies and intolerances documented in electronic health records. J. Allergy Clin. Immunol. 140, 1587–1591.e1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loewendorf A. I. et al., Roads less traveled: Sexual dimorphism and mast cell contributions to migraine pathology. Front. Immunol. 7, 140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovell R. M., Ford A. C., Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Simpson C. R., Newton J., Hippisley-Cox J., Sheikh A., Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. J. R. Soc. Med. 101, 558–563 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Perez A., Aponte Z., Vidaurre C. F., Rodriguez L. A., Anaphylaxis epidemiology in patients with and patients without asthma: A United Kingdom database review. J. Allergy Clin. Immunol. 125, 1098–1104.e1 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Ortona E. et al., Sex-based differences in autoimmune diseases. Ann. Ist. Super. Sanita 52, 205–212 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Webb L. M., Lieberman P., Anaphylaxis: A review of 601 cases. Ann. Allergy Asthma Immunol. 97, 39–43 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Klein S. L., Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 26, 247–264 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Klein S. L., Flanagan K. L., Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Abu-Arafeh I., Razak S., Sivaraman B., Graham C., Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Dev. Med. Child Neurol. 52, 1088–1097 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Korterink J. J., Diederen K., Benninga M. A., Tabbers M. M., Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS One 10, e0126982 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman M. et al., Changing trends in sex specific prevalence rates for childhood asthma, eczema, and hay fever. Pediatr. Pulmonol. 42, 60–65 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Chiaroni-Clarke R. C., Munro J. E., Ellis J. A., Sex bias in paediatric autoimmune disease–Not just about sex hormones? J. Autoimmun. 69, 12–23 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Remes S. T. et al., Prevalence of allergic rhinitis and atopic dermatitis among children in four regions of Finland. Allergy 53, 682–689 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Ballardini N. et al., Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: A report from the BAMSE birth cohort. Br. J. Dermatol. 168, 588–594 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Alkema L., Chao F., You D., Pedersen J., Sawyer C. C., National, regional, and global sex ratios of infant, child, and under-5 mortality and identification of countries with outlying ratios: A systematic assessment. Lancet Glob. Health 2, e521–e530 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Flanagan K. L., Fink A. L., Plebanski M., Klein S. L., Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 33, 577–599 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Abraham S. N., St John A. L., Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjea D., Martinov T., Mast cells: Versatile gatekeepers of pain. Mol. Immunol. 63, 38–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benoist C., Mathis D., Mast cells in autoimmune disease. Nature 420, 875–878 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Mackey E. et al., Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol. Sex Differ. 7, 60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russi A. E., Ebel M. E., Yang Y., Brown M. A., Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl. Acad. Sci. U.S.A. 115, E1520–E1529 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson J. F., Karelus K., Felicio L. S., Johnson T. E., Genetic influences on the timing of puberty in mice. Biol. Reprod. 42, 649–655 (1990). [DOI] [PubMed] [Google Scholar]
- 24.D’Costa S. et al., Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J. Allergy Clin. Immunol. 143, 1865–1877.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy M. M., Arnold A. P., Ball G. F., Blaustein J. D., De Vries G. J., Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 32, 2241–2247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armoskus C., Mota T., Moreira D., Tsai H. W., Effects of prenatal testosterone exposure on sexually dimorphic gene expression in the neonatal mouse cortex and hippocampus. J. Steroids Horm. Sci. 5, 1000139 (2014). [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll J. C. et al., Sex differences in β-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 1366, 233–245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swan S. H. et al.; Study for Future Families Research Team , Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113, 1056–1061 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster P. M., Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29, 140–147, NaN–185 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Macleod D. J. et al., Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 33, 279–287 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Ait Bamai Y. et al., Effects of prenatal di(2-ethylhexyl) phthalate exposure on childhood allergies and infectious diseases: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 618, 1408–1415 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Quinnies K. M., Harris E. P., Snyder R. W., Sumner S. S., Rissman E. F., Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One 12, e0171977 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf C. J., Hotchkiss A., Ostby J. S., LeBlanc G. A., Gray L. E. Jr., Effects of prenatal testosterone propionate on the sexual development of male and female rats: A dose-response study. Toxicol. Sci. 65, 71–86 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Fernandez R. et al., A molecular method for classifying the genotypes obtained in a breeding colony from testicular feminized (Tfm) rats. Horm. Metab. Res. 35, 197–200 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Sisk C. L., Zehr J. L., Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 26, 163–174 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Chen W. et al., Human mast cells express androgen receptors but treatment with testosterone exerts no influence on IgE-independent mast cell degranulation elicited by neuromuscular blocking agents. Exp. Dermatol. 19, 302–304 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Jing H., Wang Z., Chen Y., Effect of oestradiol on mast cell number and histamine level in the mammary glands of rat. Anat. Histol. Embryol. 41, 170–176 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Jensen F. et al., Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One 5, e14409 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaitsu M. et al., Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol. Immunol. 44, 1977–1985 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boes T., Levy D., Influence of sex, estrous cycle, and estrogen on intracranial dural mast cells. Cephalalgia 32, 924–931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hox V. et al., Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J. Allergy Clin. Immunol. 135, 729–736.e5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimber I., Dearman R. J., An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 271, 73–82 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Lee J., Oh P. S., Lim K. T., Allergy-related cytokines (IL-4 and TNF-α) are induced by Di(2-ethylhexyl) phthalate and attenuated by plant-originated glycoprotein (75 kDa) in HMC-1 cells. Environ. Toxicol. 26, 364–372 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Nakamura R., Teshima R., Sawada Ji., Effect of dialkyl phthalates on the degranulation and Ca2+ response of RBL-2H3 mast cells. Immunol. Lett. 80, 119–124 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Lenz K. M. et al., Mast cells in the developing brain determine adult sexual behavior. J. Neurosci. 38, 8044–8059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dörner G., Eckert R., Hinz G., Androgen-dependent sexual dimorphism of the immune system. Endokrinologie 76, 112–114 (1980). [PubMed] [Google Scholar]
- 47.Weinstein Y., Ran S., Segal S., Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 132, 656–661 (1984). [PubMed] [Google Scholar]
- 48.Müller W. et al., Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc. Biol. Sci. 272, 1971–1977 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konstadoulakis M. M. et al., Effect of testosterone administration, pre- and postnatally, on the immune system of rats. Horm. Metab. Res. 27, 275–278 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K., Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J. Exp. Med. 147, 1568–1583 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nugent B. M. et al., Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 18, 690–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokov A. F., Ko D., Richardson A., The effect of gonadectomy and estradiol on sensitivity to oxidative stress. Endocr. Res. 34, 43–58 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kavlock R. et al., NTP center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 16, 529–653 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Parks L. G. et al., The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 58, 339–349 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Fiandanese N. et al., Maternal exposure to a mixture of di(2-ethylhexyl) phthalate (DEHP) and polychlorinated biphenyls (PCBs) causes reproductive dysfunction in adult male mouse offspring. Reprod. Toxicol. 65, 123–132 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Gallavan R. H. Jr., Holson J. F., Stump D. G., Knapp J. F., Reynolds V. L., Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod. Toxicol. 13, 383–390 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Grimbaldeston M. A. et al., Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen B. M., Swindle E. J., Iwaki S., Gilfillan A. M., Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr. Protoc. Immunol. 74, Unit 3.23.1−3.23.13 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.