Significance
Vascular dysfunction, as it develops either during normal aging or vascular disease, remains a major medical problem. The amyloid Medin, which is derived from its precursor protein MFG-E8 (through unknown mechanisms), forms insoluble aggregates in the vasculature of virtually anybody over 50 years of age, and it has been hypothesized that Medin aggregation could contribute to age-associated vascular decline; however, mechanistic analyses have so far been lacking. Our data now demonstrate that reminiscent of humans, mice also develop Medin deposits in an age-dependent manner. Importantly, mice that genetically lack Medin show reduced vascular dysfunction in the aged brain. Therefore, the prevention of Medin accumulation should be investigated as a novel therapeutic approach to preserve vascular health in the aging population.
Keywords: Medin, MFG-E8, cerebrovascular dysfunction, aging, amyloid
Abstract
Medin is the most common amyloid known in humans, as it can be found in blood vessels of the upper body in virtually everybody over 50 years of age. However, it remains unknown whether deposition of Medin plays a causal role in age-related vascular dysfunction. We now report that aggregates of Medin also develop in the aorta and brain vasculature of wild-type mice in an age-dependent manner. Strikingly, genetic deficiency of the Medin precursor protein, MFG-E8, eliminates not only vascular aggregates but also prevents age-associated decline of cerebrovascular function in mice. Given the prevalence of Medin aggregates in the general population and its role in vascular dysfunction with aging, targeting Medin may become a novel approach to sustain healthy aging.
Amyloids comprise about 36 identified proteins, which under physiological conditions can convert to insoluble aggregates that are often associated with pathological alterations in the amyloid-containing tissue (1). The most common human amyloid described so far is Medin (also known as AMed), which has been found in ∼97% of Caucasians above 50 y of age (2), with Medin deposition found predominantly in the thoracic aorta and other arteries of the upper body (3). Medin has been described as a 50-amino acid-long internal fragment of the protein Milk fat globule-EGF factor-8 (MFG-E8) (4), which itself is best known for its role in the phagocytosis of apoptotic cells but is also required for neovascularization, explaining its localization in blood vessels (5). Under which conditions and how Medin is cleaved from MFG-E8 remains unknown, but its exceedingly high prevalence in the aging population begs the question whether Medin—similar to other amyloids—is associated with tissue dysfunction (6). Of note, previous research suggests that age-associated structural and functional alterations of the arteries contribute to cardiovascular diseases (7), and a role of Medin in promoting age-related vascular dysfunction has been hypothesized based on analyses of human autopsy and postmortem aorta samples (8–10). Most recently, evidence of increased Medin levels in patients with vascular dementia compared to cognitively unimpaired individuals was also reported (11). However, mechanistic studies and therefore conclusive evidence for a detrimental role of Medin deposition are so far lacking. This is largely because studies on human tissue lack appropriate controls (due to the presence of Medin deposits in virtually all aged human samples) and could therefore only be correlative in nature, and because no animal model for Medin deposition has so far been described that would enable mechanistic analyses.
Therefore, we analyzed here whether Medin deposition also occurs in the vasculature of aging mice. Indeed, we find extracellular Medin aggregates in C57BL/6J mice, with deposition developing in an age-dependent manner. Notably, Medin aggregates are absent in genetically engineered mice that lack the Medin-containing C2 domain of its precursor protein MFG-E8. Moreover, in these Medin-deficient mice, age-associated vascular dysfunction in cerebral arteries is virtually eliminated. Thus, our data provide direct evidence for a pathological role of this highly prevalent human amyloid.
Results
MFG-E8/Medin Aggregates Form in the Mouse Aorta with Age.
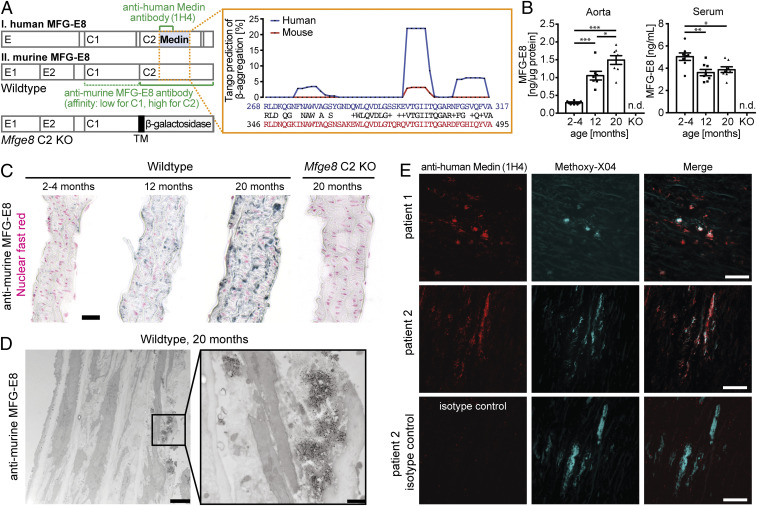
In humans, Medin aggregates are detectable in blood vessels of the upper body in virtually everyone above the age of 50 (2). While two prior studies in rats and monkeys have reported an increase of aortic levels of the Medin precursor protein MFG-E8 with age (12, 13), it has so far not been investigated whether Medin aggregates are found in other species than humans. Of note, murine and human Medin show 78% amino acid sequence homology (Needleman–Wunsch Global Alignment, BLAST) with the most aggregation-prone region showing high conservation (Fig. 1A) (14, 15). This indicates that mice may be a suitable model to study Medin pathophysiology, although the aggregation propensity of murine Medin may be more limited than its human form (based on TANGO analysis; Fig. 1A).
Fig. 1.
MFG-E8–positive aggregates accumulate in the mouse aorta with age. (A, Left) Schematic structure of human and murine MFG-E8 showing the major protein domains and highlighting protein regions recognized by the two antibodies used throughout this study (green). The structure of the truncated Mfge8 gene in the C2 domain knockout mice is shown at the bottom, indicating the introduction of a β-galactosidase reporter gene fused to a transmembrane domain (TM), which traps the gene product inside the cell. (Right) Amino acid sequence comparison of the reported human Medin sequence with the homologous murine sequence; TANGO prediction of aggregation-prone peptides within the Medin sequence, with high conservation but lower aggregation propensity in the mouse (Top). (B) Protein levels in WT mouse aorta and serum in young adult (2–4 mo old; naorta = 3/3, nserum = 5/3 female/male), adult (12-mo-old, naorta = 3/4, nserum = 3/5 female/male) and aged (20-mo-old, naorta = 3/5, nserum = 3/5 female/male) animals and Mfge8 C2 knockout tissue (n = 1/1 female/male). Data shown are means ± SEM, with one-way ANOVA for aorta: F (2,18) = 29.97, P < 0.0001; serum: F (2,21) = 6.85, P = 0.005; *P < 0.05, **P < 0.01, ***P < 0.001 for post hoc Tukey test. n.d.: not detectable. (C) Representative immunohistochemical staining for MFG-E8 (black) and cell nuclei (red) of 5-µm sections of the mouse aorta in WT and Mfge8 C2 KO animals. (D) Immuno-EM for MFG-E8 of aged WT mouse aorta (n = 2 female mice; 24-mo-old); no aggregates were found in young adult (n = 2 male; 3-mo-old) or aged Mfge8 C2 KO (n = 1/1 male/female; 21–23 mo old) animals. (E) Staining of human aorta sections (5 µm) with anti-human Medin antibody (clone 1H4) or an isotype-control antibody (Bottom) and the amyloid-binding dye Methoxy-X04. (Scale bars: C and E, 25 µm; D, Left, 2500 nm; D, Right, 500 nm.)
To assess whether Medin is deposited in the mouse aorta, we collected aorta samples from mice at the age of 2–4, 12, and 20 mo. First, we tested whether levels of MFG-E8 increase with age (using a commercial ELISA). Indeed, aorta homogenates showed a significant increase in MFG-E8 protein levels in 12-mo-old compared to 2- to 4-mo-old mice and increased even further in 20-mo-old animals (Fig. 1B). Interestingly, at the same time that levels of MFG-E8 increased in the aorta, they decreased in the serum, demonstrating that these changes were not due to blood contamination in the tissue. Immunostaining with a polyclonal antibody against murine MFG-E8, which we found to have high affinity for the C2 and lower affinity for the C1 domain (SI Appendix, Fig. S1A and Fig. 1A), confirmed that aortic MFG-E8 staining increases with age (Fig. 1C). Interestingly, MFG-E8 staining revealed irregularly shaped lumps along elastic fibers, reminiscent of findings in human tissue where Medin is found in “nodules and thin streaks” in the aortic media and is closely associated with elastic fibers (2, 16). We also confirmed these previous results in human tissue using a new monoclonal anti-human Medin antibody (1H4) (Fig. 1E). While prior studies could not demonstrate that antibodies were specifically detecting Medin, we here ascertained staining specificity by analyzing tissue from mice that lack the Medin-containing C2 domain of MFG-E8 (Mfge8 C2 knockout [KO]) (Fig. 1A). In these functional knockout mice, the C2 domain is replaced with a β-galactosidase reporter gene fused to a transmembrane domain. While this does not prevent gene expression, it effectively traps the truncated MFG-E8/β-galactosidase fusion protein inside the cell, leading to a complete absence of secreted MFG-E8 in Mfge8 C2 KO animals (as previously confirmed in the aorta and for different cell types; refs. 5, 17, 18 and cp. SI Appendix, Fig. S4 for brain tissue). In line with the high affinity of the polyclonal anti-MFG-E8 antibody for the C2 domain (SI Appendix, Fig. S1A), staining was absent in the aorta of Mfge8 C2 KO mice (Fig. 1C) and, accordingly, no signal was found in the ELISA for MFG-E8 (Fig. 1B).
To determine whether the observed staining patterns in wild-type (WT) mice were indeed due to extracellular aggregates, we next performed immuno-electron microscopy (EM) of aortas from young adult and aged WT mice as well as aged Mfge8 C2 KO mice. As expected from our immunohistochemical analyses, only aged WT animals demonstrated immunolabeling, which was found localized on extracellular aggregates (Fig. 1D). While these aggregates appeared amorphous (rather than fibrillar) under EM, these findings demonstrate that MFG-E8 (or its fragments) forms extracellular aggregates in an age-specific manner in the mouse aorta.
In humans, aortic Medin deposits can be stained with amyloid-binding dyes such as Methoxy-X04, which we also confirmed here (Fig. 1E and see SI Appendix, Table S1 for sample information). In apparent contrast, aggregates in the mouse aorta failed to stain with Methoxy-X04 or Congo Red (SI Appendix, Fig. S2). However, in our hands, not all Medin-positive aggregates (stained with anti-human Medin antibody 1H4) in human tissue were stained by amyloid-binding dyes (Fig. 1E; n = 3 patients, SI Appendix, Table S1 and Fig. S2). Thus, similar to our observations in mice, some Medin deposits in human tissue do not display the characteristic β-sheet structure of other amyloids, possibly representing an earlier aggregation stage.
Aortic Deposits in Mice Show Biochemical Characteristics of Protein Aggregates.
Given the amorphous appearance of aortic aggregates in aged mice by EM, we wanted to determine whether these deposits shared biochemical features of other aggregated proteins, i.e., protease resistance and insolubility in aqueous media. Therefore, we used a previously published amyloid purification protocol (19) to determine if the aged aorta would contain aggregated proteins. We first tested this protocol using brains of either aged APP23 or APP Dutch transgenic animals. APP23 mice are a model of Alzheimer’s disease pathology with widespread parenchymal and vascular amyloid-β deposition (20) while aged APP Dutch animals show amyloid-β deposition restricted to cerebral blood vessels (21). In brief, homogenates were lysed and subjected to iodixanol gradient centrifugation. Samples were then digested with benzonase and proteinase-K (PK) and were subsequently ultracentrifuged to recover the remaining protease-resistant, insoluble material (Fig. 2A). As expected, this procedure yielded enriched monomeric and oligomeric amyloid-β species from the brains of both aged APP23 and APP Dutch animals (SI Appendix, Fig. S1B).
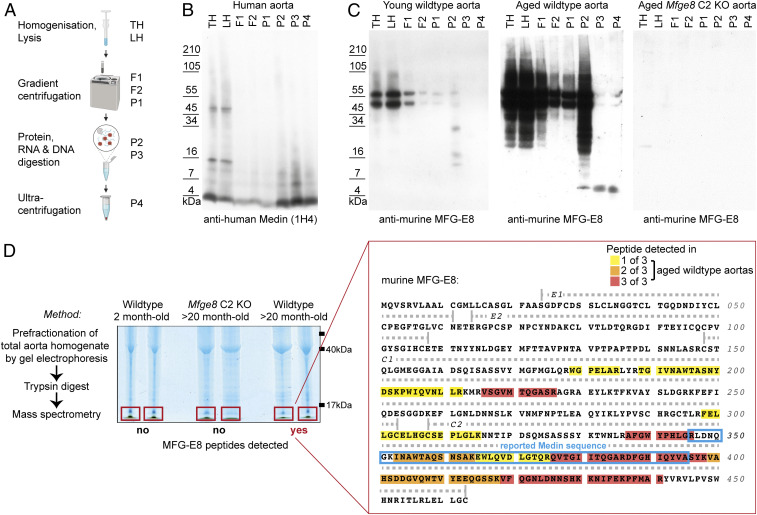
Fig. 2.
Aortic deposits in mice show biochemical characteristics of protein aggregates and are enriched in Medin-containing fragments of MFG-E8. (A) Graphical summary of the purification procedure for the enrichment of protein aggregates from tissue homogenates. (B) Analysis of human aorta homogenates (LH, lysed homogenate; TH, total homogenate) reveals enrichment of Medin-positive bands, corresponding to monomeric (∼4 kDa) and oligomeric (∼8/12 kDa) species. (C) In mouse aorta samples (pools of 16 aortas were used as input), full-length MFG-E8 is degraded and a ∼5-kDa fragment is enriched only from aged WT but not young WT or aged Mfge8 C2 KO aortas. (D) Mass spectrometry (MS) analysis of total aorta homogenate of young adult WT (2-mo-old; n = 1/1 female/male) and aged (20-mo-old) Mfge8 C2 KO (n = 2 females) and WT (n = 1/2 female/male) mice. Following prefractionation and in-gel digestions, gel sections containing small proteins (<17 kDa) were excised and subjected to MS analysis. No MFG-E8 peptides were found in samples from young WT or aged Mfge8 C2 KO animals while aged WT aortas contained ≤17 kDa fragments of MFG-E8, which were most consistently found in the C2 domain (color-code indicates detection in different number of samples; yellow/orange/red = 1/2/3 of 3 samples). The reported human Medin sequence is highlighted with a blue frame.
Next, we analyzed fresh-frozen aorta samples from human patients, which showed Medin staining by immunohistochemistry (cp. Fig. 1E). In human aorta samples, Western blotting of the different fractions obtained from the amyloid purification protocol using the monoclonal anti-human Medin antibody (1H4) revealed protein bands corresponding to full-length MFG-E8 but also bands indicating monomeric and possibly oligomeric Medin species (approximately 4, 8, and 12 kDa). After benzonase (P2) and proteinase K (P3) digestion, full-length MFG-E8 and other proteins were degraded, while the protease-resistant material showed distinct bands with molecular mass corresponding to monomeric and oligomeric Medin species as well as a higher molecular mass smear, possibly reflecting higher order aggregates (Fig. 2B).
Analyzing mouse samples next, Western blotting demonstrated full-length MFG-E8 in the total aorta homogenate (TH) of both young and aged WT mice (Fig. 2C; samples are pools of 16 mouse aortas), with significantly stronger signals in the aorta homogenate of aged mice, reflecting our ELISA measurements (Fig. 1B). In contrast, the purification protocol only enriched a protease-resistant MFG-E8–positive fragment from the aorta of aged WT mice, while neither young WT nor aged Mfge8 C2 KO aortas yielded MFG-E8 species in the final aggregate-containing fractions (Fig. 2C). Notably, the protease-resistant MFG-E8 fragment in the aged mouse aorta showed a molecular mass of approximately 5 kDa, corresponding to the reported size of Medin (4). These results demonstrate that MFG-E8 fragments, which similar to other protein aggregates are protease-resistant as well as insoluble in aqueous buffers and show a molecular mass that corresponds to the reported size of Medin, accumulate in the aging mouse aorta.
Identification of a Medin-Like Fragment in the Aging Mouse Aorta.
To ascertain that the ∼5-kDa band observed by Western blotting was indeed a Medin-containing peptide, we used a shotgun mass spectrometry approach (Fig. 2D). Briefly, aorta homogenates were prefractionated by protein gel electrophoresis, and only proteins smaller than ∼17 kDa were excised to exclude full-length MFG-E8. First, we verified that we could detect Medin peptides in aggregated material by analyzing recombinant human Medin that had been aggregated in vitro. Indeed, following gel electrophoresis and excision of a monomeric as well as an oligomeric band, almost the entire Medin sequence (with the exception of short N- and C-terminal peptides) could be detected by mass spectrometry (SI Appendix, Fig. S3).
Next, we compared murine aorta samples (again without further purification procedures) from young and aged WT and aged Mfge8 C2 KO mice. Here, low molecular mass peptides of MFG-E8 (≤17 kDa) were detectable only in aged WT aortas. Strikingly, the majority of these peptides could be assigned to the C2 domain and were enriched in the sequence corresponding to the originally reported human Medin (Fig. 2D), also consistent with our epitope mapping of the anti-murine MFG-E8 antibody (Fig. 1A and SI Appendix, Fig. S1A). Thus, our mass spectrometry results demonstrate that—similar to observations in human patients—Medin-containing fragments are accumulating in the aging mouse aorta, further corroborating our analyses using immunohistochemistry and biochemical purification approaches.
MFG-E8 and Medin in the Aging Brain.
In humans, Medin deposition has not only been observed in the thoracic aorta but also other larger arteries of the upper body, including basilar and temporal arteries (3, 22, 23) and most recently also arterioles in the brain parenchyma (11). To confirm Medin deposition in cerebral blood vessels, we stained brain sections of aged patients without any major brain diseases (male and female, 80–86 y old; see SI Appendix, Table S2 for details) with the anti-human Medin antibody, 1H4. Strikingly, Medin deposits could be seen within and outside the smooth muscle cell layer in leptomeningeal vessels, larger parenchymal vessels, and even smaller capillaries, where they also showed aggregate-like morphology (Fig. 3A). Of note, these deposits did not show immunoreactivity for amyloid-β nor were they positive for the amyloid-binding dye Methoxy-X04 (Fig. 3 A, Bottom), in contrast to our findings in the human aorta, where both Methoxy-X04–positive and –negative aggregates were found (Fig. 1E).
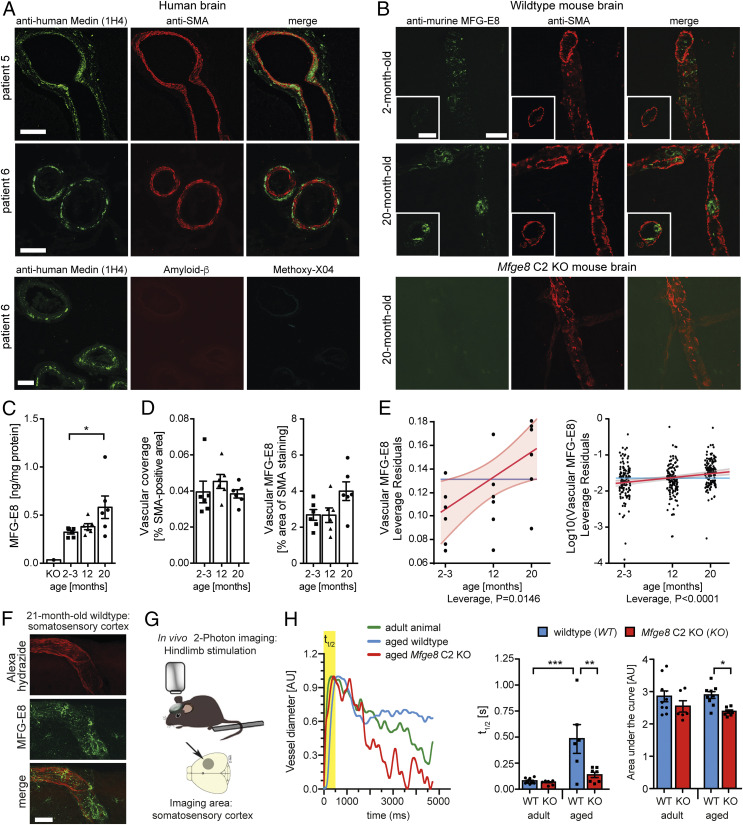
Fig. 3.
MFG-E8/Medin deposition causes age-associated cerebrovascular dysfunction. (A) In brain sections of healthy human patients (n = 3 patients analyzed), extensive vascular staining of aggregate-like structures is seen with a monoclonal anti-human Medin antibody (1H4). Aggregates are largely localized in the tunica media and the parenchymal side of the vessels. (Bottom) Medin-positive deposits do not stain with the amyloid-dye Methoxy-X04 and are negative for amyloid-β. (B) In aged (20-mo-old) but not young (2-mo-old) WT mice, aggregate-like structures are also present in the brain vasculature, where they are found mostly in the tunica media and the luminal side of blood vessels. (C) Quantification of total MFG-E8 protein levels with age in the WT mouse brain (n = 3/3 females/males per group, significant Kruskal–Wallis test: KW statistic = 5.93, P = 0.047, followed by Dunn’s post hoc comparison, *P < 0.05). (D) Quantification of the overall vascular density (area of smooth muscle actin [SMA] staining) and vascular MFG-E8 staining (% MFG-E8 staining within SMA-positive area) in brain sections with age in WT mice (n = 3/3 females/males per group). Data shown are means ± SEM. (E) Regression analysis for the impact of age on cerebrovascular accumulation of MFG-E8/Medin (effect leverage plot, where a least squares line [red] and confidence bands [shaded red] are fitted). Analysis for the mean vascular MFG-E8 staining per animal (Left) and per brain section (Right). (F) Representative confocal z-stack of an artery from the middle cerebral artery territory in the somatosensory cortex (horizontal brain section). (G) Schematic illustration of in vivo analysis of vascular function in the brain of living mice using two-photon imaging of functional hyperemia. (H) Representative traces (Left) and quantification of the change in diameter of individual arterioles in adult WT (n = 4; 5/6/6/6-mo-old male) or Mfge8 C2 KO (n = 3; 6/6/8-mo-old male) and aged WT (n = 4; 21/22/22/22-mo-old male) or Mfge8 C2 KO (n = 3; 19/22/27-mo-old male) animals (Right), with t1/2 reflecting the speed of vessel dilation, and the area under the curve reflecting the speed of constriction (1–3 arterioles per animal). Arterial dilation and constriction are significantly improved in aged Mfge8 C2 KO compared to aged WT animals. Two-way ANOVA; t1/2: significant main effects of age*genotype/genotype/age: F (1,25) = 7.763/9.369/16.15, P = 0.01/0.0052/0.0005; Area under the curve: significant main effect of genotype, F (1,28) = 8.62, P = 0.0066; *P < 0.05, **P < 0.01, ***P < 0.001 for post hoc Bonferroni comparisons. Data shown are means ± SEM (data points are measurements from individual blood vessels). (Scale bars: A, 50 µm; B and F, 25 µm.)
Next, we analyzed brain tissue from mice to determine whether cerebral blood vessels would display age-related Medin deposition. Indeed, immunohistochemical staining showed MFG-E8–positive blood vessels in the mouse brain, with more intense and aggregate-like staining being observed in aged animals (Fig. 3B). However, these deposits were again not stained by the classical amyloid-binding dyes Methoxy-X04 and Congo Red (SI Appendix, Fig. S2). Nevertheless, an age-related increase in cerebral MFG-E8 protein levels could be observed in WT mice by ELISA (Fig. 3C), reflecting our findings in the aorta (Fig. 1). Moreover, semiautomated quantification of smooth muscle actin and MFG-E8 staining in serial brain sections revealed no difference in total vascular coverage but showed an increase of cerebrovascular MFG-E8 staining with age (Fig. 3 D and E). Notably, in brain sections from aged Mfge8 C2 KO animals, we could detect intracellular MFG-E8-positive puncta both in parenchymal as well as vascular cells, reflecting expression and intracellular retention of the truncated MFG-E8/β-galactosidase fusion protein (SI Appendix, Fig. S4A). Accordingly, in brain homogenates from aged Mfge8 C2 KO animals, the fusion protein appeared as a distinct band ∼200 kDa (in line with previous reports; ref. 18), while the band of full-length MFG-E8 observed in WT animals was completely absent (SI Appendix, Fig. S4B). Thus, our results indicate that in WT mice, the aorta as well as cerebral blood vessels show age-associated deposition of MFG-E8 (fragments) that are likely to contain Medin.
Lack of MFG-E8 Rescues Age-Associated Vascular Dysfunction.
We wondered whether Medin deposition could contribute to the decline in vascular function, which occurs with age both in mice and humans (24–26). Because age-related changes in regional cerebral blood flow are not detectable by positron emission tomography (PET) or MRI measurements in WT animals (27), we assessed cerebral vascular function by two-photon in vivo imaging, which has been shown to detect age-associated cerebrovascular alterations in mice (28). Here, we analyzed the function of cerebral arterioles in living animals, focusing on the middle cerebral artery territory in the sensorimotor cortex, where we also observed Medin deposits in aging mice (Fig. 3F). We triggered increases in blood flow to the hindlimb region by evoking neuronal activity using mechanical hindlimb stimulation (Fig. 3G), a mechanism called functional hyperemia (29, 30). Indeed, in response to hindlimb stimulation, adult animals (6.4 ± 1.1 mo old) showed a rapid dilation of arteries followed by a slower constriction; this response was indistinguishable between adult WT and Mfge8 C2 KO animals (Fig. 3H). Importantly, in aged WT animals (22.1 ± 2.4 mo old) dilation of the imaged arteries was significantly slower than in adult animals, in line with increased vascular arterial stiffness in aging animals and humans (31, 32). In contrast, both dilation and constriction were significantly improved in aged Mfge8 C2 KO compared to WT animals (Fig. 3H). Thus, our data demonstrate that the lack of Medin improves vascular function in aging animals.
It has been suggested that vascular amyloid may lead to blood vessel dysfunction through toxic effects on smooth muscle cells (23, 33). However, in contrast to previous reports (33), we did not observe overt loss of smooth muscle cells in cerebrovascular regions with Medin deposits (Fig. 3A). Moreover, neither global endothelial cell volume (based on CD31 staining) nor astrocytic endfeet coverage of cerebral blood vessels (based on Aquaporin-4 staining), which is crucial for appropriate neurovascular coupling (25, 30), were affected by aging or in WT versus Mfge8 C2 KO animals (SI Appendix, Fig. S5). While we cannot exclude that the lack of MFG-E8 affected other compartments of the brain, our data strongly suggest that aggregation of Medin (or larger MFG-E8 fragments) contributes to age-related vascular dysfunction in WT mice, possibly through affecting vascular elasticity (34).
Discussion
Our data presented here indicate that the most common human amyloid, Medin (or Medin-containing fragments of MFG-E8), also shows age-associated aggregation in WT mice and demonstrate a pathogenic role of its accumulation in driving age-associated vascular dysfunction. Strikingly, Medin deposits form in aging mice despite their relatively limited lifespan, reflecting the previous finding that Medin deposits can be found in the vast majority of people over 50 y of age (2). However, in mice, Medin aggregates do not stain with classical amyloid dyes (Congo Red, Methoxy-X04) and appear amorphous by EM, possibly reflecting their lower (predicted) aggregation propensity (Fig. 1A) or indicating an early stage of fibril formation and/or maturation. Interestingly, it has been reported that in human aortic tissue the amount of Medin in its amyloid state is significantly lower in patients with aortic aneurysm or dissection, while nonfibrillar Medin deposits are significantly higher in the diseased aorta (10) and correlate with reduced aortic elasticity (9). Thus, nonfibrillar forms of Medin could in fact be more pathogenic, and notably, we find that Medin deposits in the human brain vasculature do not stain with amyloid dyes. It has been reported that amyloids can exist as structurally distinct “strains” (with different affinity for common amyloid dyes), which propagate through prion-like mechanisms, i.e., through templated misfolding of the native peptide (e.g., refs. 35, 36). Thus, it is conceivable that different strains of Medin form in the mouse and human vasculature and become (locally) amplified, providing a possible explanation for the differences in amyloid dye affinity observed in our analyses. Further relating to the role of amyloids in vascular dysfunction, it has been shown that soluble amyloid-β monomers and oligomers significantly impair vascular function in Alzheimer’s disease (e.g., refs. 37–39), and it will therefore be important to determine which specific types of Medin aggregates are responsible for inducing vascular dysfunction in the aging brain.
Although Medin is the most common human amyloid described so far, little is known about its contribution to disease. Correlative analyses of human tissue suggest that MFG-E8 and Medin may contribute to vasculitis and thoracic aneurysms and dissection (9, 10) as well as vascular dementia (11). Mechanistically, it has been hypothesized that Medin amyloid leads to cell toxicity through promoting inflammatory and oxidative stress, thereby altering the arterial wall structure and predisposing arteries to age-related vascular dysfunction and disease (40–43). However, these studies were unable to determine whether Medin aggregates were cause or consequence of pathological vascular changes. Our observation that Medin deposition occurs in murine blood vessels with age and is absent in Mfge8 C2 KO animals allowed us to examine in living mice whether this process contributes to vascular dysfunction. Our results now provide direct evidence that Medin aggregation within (cerebral) blood vessels may be causal for vascular dysfunction. Whether this occurs independently or is interlinked with previously reported mechanisms of age-related cerebrovascular dysfunction (including increases in reactive oxygen species; ref. 26) requires further investigation. Nevertheless, our findings indicate that vascular function can be improved by preventing Medin aggregation. Given the high prevalence of cardiovascular, cerebrovascular, and neurodegenerative diseases in the aging population (44, 45), preservation of vascular function remains a major challenge in medical research (46–48). Targeting Medin aggregation should therefore be investigated as a novel therapeutic option to promote healthy aging of the vasculature. Notably, the NMR structure of the Medin-containing C2 domain of human MFG-E8 has been determined (Protein Data Bank ID code 2L9L; ref. 49); this may allow for rational drug design to prevent Medin aggregation through kinetic stabilization of its native structure, as exemplified by recent approaches to prevent transthyretin amyloidosis (50).
Finally, a potential interaction between Medin and amyloids in the brain is also of interest, because amyloidosis is prevalent in many neurodegenerative diseases. Previous experiments showed that Medin can act as a heterologous seed for the aggregation of serum amyloid A (51), but whether it interacts with other extracellular amyloids and in particular amyloid-β, which deposits both in the parenchyma as well as in blood vessels of the brain, remains unknown. Despite their structural similarities, amyloids do not necessarily coaggregate and can even show cross-inhibitory effects (52, 53). Therefore, understanding if and how Medin may contribute to age-related amyloidosis in the brain will be investigated in future studies.
Materials and Methods
Human Tissue.
Ascending aortic tissue samples were obtained from patients undergoing elective aneurysmal repair at Liverpool Heart and Chest Hospital (SI Appendix, Table S1). This study was ethically approved by Liverpool Bio-Innovation Hub (project approval reference 15–06 and 18–07). One case (patient 2) was obtained from informed consent postmortem collection through the Leeds GIFT scheme. Ethical approval for this patient was conferred by National Research Ethics Service Committee East of England-Cambridge South (approval reference 11/EE/0528). Human brain tissue (SI Appendix, Table S2) was obtained from the Queen Square Brain Bank for Neurological Disorders (University College London Institute of Neurology, London, UK; approval protocol no. EXTMTA5/16).
This study was also approved by the ethical committee of the Medical Faculty, University of Tübingen, Germany (protocol no. 354/2016BO2). Informed consent was obtained from all participants.
Mice.
Male and female C57BL/6J and C57BL/6J-Mfge8 Gt(KST227)Byg mice (5) (generously provided by Clotilde Théry, INSERM U932, Institute Curie, Paris, France), were bred in-house under specific pathogen-free conditions. All experiments were performed in accordance with German veterinary office regulations (Baden-Württemberg and Hessen) and were approved by the local authorities for animal experimentation (Regierungspräsidium) of Tübingen, Germany (approval nos: N03/14, N02/15, N07/16, §4MIT v. 05.03.2018, §4MIT v. 18.08.2016) and Frankfurt, Germany (protocol no. FR-1001).
Tissue Collection and Analyses.
For brain and aorta preparation, mice were deeply anesthetized and transcardially perfused with phosphate-buffered saline and processed for biochemical analyses and immunostaining as described in SI Appendix, Materials and Methods.
Two-Photon Imaging of Vascular Function.
Cranial window surgeries were carried out as previously described in detail (54–56), using hindlimb stimulation to elicit functional hyperemia in the middle cerebral artery territory of the sensorimotor cortex, as described in detail in SI Appendix, Materials and Methods.
Statistics.
Statistical analysis was performed using Prism 6 and JMP software (version 14.2.0) as indicated in the figure legends and as described in detail in SI Appendix, Materials and Methods. All data shown are means ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Jörg Odenthal and Carina Leibssle for assistance with animal maintenance and care, Franziska Klose and Karsten Boldt (Core Facility for Medical Bioanalytics, University of Tübingen) for technical help, and Anke Biczysko for expert technical assistance with EM, and all other members of our labs. This work was supported by German Research Foundation (DFG) Grants NE 1951/2-1 and -2 (to J.J.N.) and British Heart Foundation Grant FS/12/61/29877 (to J.M.). Work in the J.K.H. laboratory is supported by DFG Grants HE 6867/4-1 and 3-1. The graphics in Fig. 2A and SI Appendix, Fig. S1 were generated using biorender.com.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011133117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Benson M. D. et al., Amyloid nomenclature 2018: Recommendations by the international society of amyloidosis (ISA) nomenclature committee. Amyloid 25, 215–219 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Mucchiano G., Cornwell G. G. 3rd, Westermark P., Senile aortic amyloid. Evidence for two distinct forms of localized deposits. Am. J. Pathol. 140, 871–877 (1992). [PMC free article] [PubMed] [Google Scholar]
- 3.Peng S. et al., Medin and medin-amyloid in ageing inflamed and non-inflamed temporal arteries. J. Pathol. 196, 91–96 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Häggqvist B. et al., Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc. Natl. Acad. Sci. U.S.A. 96, 8669–8674 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestre J.-S. et al., Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 11, 499–506 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg D., Jucker M., The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najjar S. S., Scuteri A., Lakatta E. G., Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension 46, 454–462 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Muckle T. J., Giant cell inflammation compared with amyloidosis of the internal elastic lamina in temporal arteries. Arthritis Rheum. 31, 1186–1189 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Davies H. A. et al., Idiopathic degenerative thoracic aneurysms are associated with increased aortic medial amyloid. Amyloid 26, 148–155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng S. et al., Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab. Invest. 87, 1195–1205 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Karamanova N. et al., Endothelial immune activation by medin: Potential role in cerebrovascular disease and reversal by monosialoganglioside-containing nanoliposomes. J. Am. Heart Assoc. 9, e014810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z. et al., Milk fat globule protein epidermal growth factor-8: A pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ. Res. 104, 1337–1346 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M. et al., MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell 11, 500–508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Escamilla A.-M., Rousseau F., Schymkowitz J., Serrano L., Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22, 1302–1306 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Larsson A. et al., Unwinding fibril formation of medin, the peptide of the most common form of human amyloid. Biochem. Biophys. Res. Commun. 361, 822–828 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Kholová I., Niessen H. W. M., Amyloid in the cardiovascular system: A review. J. Clin. Pathol. 58, 125–133, 10.1136/jcp.2004.017293 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atabai K. et al., Mfge8 is critical for mammary gland remodeling during involution. Mol. Biol. Cell 16, 5528–5537 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandrot E. F. et al., Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. U.S.A. 104, 12005–12010 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stöhr J. et al., Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. U.S.A. 109, 11025–11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturchler-Pierrat C. et al., Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U.S.A. 94, 13287–13292 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzig M. C. et al., Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci. 7, 954–960 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Peng S., “Medin amyloid in human arteries and its association with arterial diseases,” Dissertation, Uppsala Universitet, Uppsala, Sweden (2006).
- 23.Peng S., Glennert J., Westermark P., Medin-amyloid: A recently characterized age-associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid 12, 96–102 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Tarantini S., Tran C. H. T., Gordon G. R., Ungvari Z., Csiszar A., Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 94, 52–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iadecola C., Nedergaard M., Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Kang H.-M., Sohn I., Jung J., Jeong J.-W., Park C., Age-related changes in pial arterial structure and blood flow in mice. Neurobiol. Aging 37, 161–170 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Maier F. C. et al., Longitudinal PET-MRI reveals β-amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion. Nat. Med. 20, 1485–1492 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Park L., Anrather J., Girouard H., Zhou P., Iadecola C., Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow Metab. 27, 1908–1918 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Shih A. Y., et al. , Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cereb. Blood Flow Metab. 32, 1277–1309 (2012).Correction in: J. Cereb. Blood Flow Metab. 33, 319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attwell D. et al., Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajdu M. A., Heistad D. D., Siems J. E., Baumbach G. L., Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ. Res. 66, 1747–1754 (1990). [DOI] [PubMed] [Google Scholar]
- 32.Paini A. et al., Carotid and aortic stiffness: Determinants of discrepancies. Hypertension 47, 371–376 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Winkler D. T. et al., Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J. Neurosci. 21, 1619–1627 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson A. et al., Lactadherin binds to elastin–A starting point for medin amyloid formation? Amyloid 13, 78–85 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Condello C. et al., Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, E782–E791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jucker M., Walker L. C., Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 21, 1341–1349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nortley R. et al., Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suo Z. et al., Soluble Alzheimers β-amyloid constricts the cerebral vasculature in vivo. Neurosci. Lett. 257, 77–80 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Niwa K. et al., Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc. Natl. Acad. Sci. U.S.A. 97, 9735–9740 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madine J., Middleton D. A., Comparison of aggregation enhancement and inhibition as strategies for reducing the cytotoxicity of the aortic amyloid polypeptide medin. Eur. Biophys. J. 39, 1281–1288 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Chiang H., Chu P., Lee T., MFG-E8 mediates arterial aging by promoting the proinflammatory phenotype of vascular smooth muscle cells. J. Biomed. Sci. 26, 61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M., Wang H. H., Lakatta E. G., Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling. Curr. Vasc. Pharmacol. 11, 768–776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migrino R. Q. et al., Amyloidogenic medin induces endothelial dysfunction and vascular inflammation through the receptor for advanced glycation endproducts. Cardiovasc. Res. 113, 1389–1402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen S. W., Wong C. H. Y., Aging- and vascular-related pathologies. Microcirculation 26, e12463 (2019). [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization , “Global status report on noncommunicable diseases 2014” (World Health Organization, Geneva, Switzerland, 2014). [Google Scholar]
- 46.Claassen J. A. H. R., Cognitive decline and dementia: Are we getting to the vascular heart of the matter? Hypertension 65, 505–506 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Pase M. P. et al., Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham third generation cohort study. Hypertension 67, 513–519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Torre J. C., Cerebral hemodynamics and vascular risk factors: Setting the stage for Alzheimer’s disease. J. Alzheimers Dis. 32, 553–567 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Ye H. et al., NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim. Biophys. Acta 1828, 1083–1093 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Alhamadsheh M. M. et al., Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci. Transl. Med. 3, 97ra81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson A., Malmström S., Westermark P., Signs of cross-seeding: Aortic medin amyloid as a trigger for protein AA deposition. Amyloid 18, 229–234 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Bachhuber T. et al., Inhibition of amyloid-β plaque formation by α-synuclein. Nat. Med. 21, 802–807 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Coomaraswamy J. et al., Modeling familial Danish dementia in mice supports the concept of the amyloid hypothesis of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 107, 7969–7974 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hefendehl J. K. et al., Long-term in vivo imaging of β-amyloid plaque appearance and growth in a mouse model of cerebral β-amyloidosis. J. Neurosci. 31, 624–629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hefendehl J. K. et al., Repeatable target localization for long-term in vivo imaging of mice with 2-photon microscopy. J. Neurosci. Methods 205, 357–363 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Füger P. et al., Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 20, 1371–1376 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.