Highlights
-
•
Dementia may occur in the early stages of HD and with independence of disease burden.
-
•
More severe posterior-cortical atrophy is associated with dementia in HD.
-
•
Neuropsychological alterations of dementia in HD extends beyond executive dysfunction.
-
•
CAG-independent neuropathological mechanisms may contribute to dementia in HD.
Keywords: Huntington’s disease, Dementia, Neuropsychology, Mild cognitive impairment, Movement disorders
Abstract
Background
Huntington’s disease (HD) is a fatal genetic neurodegenerative disorder with no effective treatment currently available. Progressive basal ganglia and whole-brain atrophy and concurrent cognitive deterioration are prototypical aspects of HD. However, the specific patterns of brain atrophy underlying cognitive impairment of different severity in HD are poorly understood. The aim of this study was to investigate the specific structural brain correlates of major cognitive deficits in HD and to explore its association with neuropsychological indicators.
Participants
Thirty-five symptomatic early-to-mild HD patients and 15 healthy controls (HC) with available T1-MRI imaging were included in this study.
Methods
In this cross-sectional study, HD patients were classified as patients with (HD-Dem) and without (HD-ND) major cognitive impairment in the range of dementia. This classification was based on previously validated PD-CRS cutoff scores for HD. Differences in brain atrophy across groups were studied by means of grey-matter volume voxel-based morphometry (GMV-VBM) and cortical thickness (Cth). Voxelwise and vertexwise general linear models were used to assess the group comparisons, controlling for the effects of age, sex, education, CAG repeat length and severity of motor symptoms. Clusters surviving p < 0.05 and family-wise error (FWE) correction were considered statistically significant. In order to characterize the impact on cognitive performance of the observed brain differences across groups, GMV and Cth values in the set of significant regions were computed and correlated with specific neuropsychological tests.
Results
All groups had similar sociodemographic profiles, and the HD groups did not significantly differ in terms of CAG repeat length. Compared to HC, both HD groups exhibited significant atrophy in multiple subcortical and parietal brain regions. However, compared to HC and HD-ND groups, HD-Dem patients showed a more prominent pattern of reduced GMV and cortical thinning. Importantly, this thinning was restricted to regions of the parietal-temporal and occipital cortices. Furthermore, these brain alterations were further associated with poorer cognitive performance in tasks assessing frontal-executive and attention domains as well as memory, language and constructional abilities.
Conclusions
Major cognitive impairment in the range of dementia in HD is associated with brain and cognitive alterations exceeding the prototypical frontal-executive deficits commonly recognized in HD. The observed posterior-cortical damage identified by MRI and its association with memory, language, and visuoconstructive dysfunction suggest a strong involvement of extra-striatal atrophy in the onset of severe cognitive dysfunction in HD patients. Critically, major cognitive impairment in this sample was not associated with CAG repeat length, age or education. This finding could support a possible involvement of additional neuropathological mechanisms aggravating cognitive deterioration in HD.
1. Introduction
Huntington’s disease (HD) is a monogenic, autosomal dominant, neurodegenerative disease caused by the an abnormal CAG expansion on the HTT gene (Walker, 2007). Its inevitably progressive course is characterized by progressive motor abnormalities and neuropsychiatric symptoms, early cognitive deterioration, and the development of dementia (Ross et al., 2014, Peavy et al., 2010).
The key neuropathological hallmark of HD is progressive atrophy of the basal ganglia, which may be detected up to 15 years before motor symptoms appear and the diagnosis is made. Compelling evidence, however, shows that HD affects not only basal ganglia but the whole-brain(Rosas et al., 2008). Cross-sectional and longitudinal studies have illustrated that even in the premanifest stage of the disease, grey matter volume (GMV) reductions, cortical thinning (Cth), and reduced brain metabolism affect multiple extra-striatal regions. These regions comprise extensive territories of the parietal, temporal and occipital lobes that also contribute to the clinical expression of the disease(Rosas et al., 2008, Rosas et al., 2005, Nopoulos et al., 2010, Tabrizi et al., 2009, Coppen et al., 2018, Rub et al., 2015, Kuwert et al., 1990).
From a neurocognitive perspective, HD has been described as a prototypical frontal-subcortical dementia due to striatal, but also thalamic degeneration(Kassubek et al., 2005), with frontal-executive disturbances, attention deficits, and processing speed reduction as the most consistently affected cognitive domains(Snowden, 2017). Involvement of the caudate nucleus and putamen in cognitive aspects of HD is characteristic(Tabrizi et al., 2009, Dogan et al., 2013). However, many studies in HD have shown that as the neuropathological changes extend to multiple extra-striatal regions, cognitive domains other than frontal-executive functions become affected. These include alterations in visuomotor integration(Gomez-Anson et al., 2009, Say et al., 2011), episodic and autobiographical memory(Carmichael et al., 2019, Harris et al., 2019), visual perception(Martinez-Horta et al., 2019, Coppen et al., 2019), mental rotation(Wolf et al., 2014, Labuschagne et al., 2016) and language production and organization(Hinzen et al., 2018, Chan et al., 2019, Chenery et al., 2002, Podoll et al., 1988), suggesting that the cognitive phenotype of HD cannot be attributed solely to frontal-striatal dysfunction.
Longitudinal studies have demonstrated that CAG repeat length plays a key role in the linear decline of cognitive capacity throughout disease progression(Tabrizi et al., 2012, Paulsen et al., 2014, Consortium, 2015, Langbehn et al., 2019). However, age-at-onset (AAO) and the rate of progression of cognitive deterioration varies greatly among individuals even when age, education level and CAG repeat length are equivalent(Consortium, 2015, Braisch et al., 2019). This heterogeneity suggests that, besides CAG repeat length, other mechanisms such as environmental and genetic variables also contribute to the neuropathological and clinical progression of cognitive deterioration in HD. It has recently been shown, for example, that genetic variability in chromosome 15, contributes to age at onset of motor and cognitive symptoms (Consortium, 2015). Other mechanisms, such as MAPT haplotypes and their role in the expression of TAU, have also been associated with cognitive progression in HD (Vuono et al., 2015).
Studies addressing the mechanisms involved in the differential expression of cognitive deterioration in HD are scarce, probably due to the lack of specific diagnostic criteria to differentiate HD patients with mild cognitive impairment from those with severe or major cognitive impairment in the range of dementia, and even from those with normal cognition (Mestre et al., 2018). The specific patterns of brain alterations underlying major cognitive impairment in HD thus remain elusive, partly because of the difficulty in appropriately grouping patients according to cognitive status. To overcome this limitation, we recently validated the Parkinson’s Disease – Cognitive Rating Scale (PD-CRS) as a screening tool for global cognition in HD(Martinez-Horta et al., 2020). We showed that the HD-specific PD-CRS cutoff scores have a good capacity to discriminate between categories of cognitive status in patients with HD. Although this approach may have limitations, it distinguishes patients with normal or mild global cognitive defects from those with severe cognitive impairment in the range of dementia.
In the present study, our main aim was to explore specific structural brain differences underlying major cognitive impairment in the range of dementia in HD and to explore their association with poorer cognitive performance.
2. Methods
2.1. Participants
We included thirty-five symptomatic gene-mutation carriers with CAG repeat length > 38 regularly attending the outpatient HD-Clinic of the Movement Disorders Unit at Hospital de la Santa Creu i Sant Pau in Barcelona, and fifteen age- and education-matched healthy controls (HC). HC were non-consanguineous relatives of the HD participants. None of the HCs had a history of neurologic, psychiatric or uncompensated systemic diseases. Similarly, all HD participants were free of any neurological disorder other than HD and had no history of brain surgery, traumatic brain injury, epilepsy, drug abuse, or uncompensated systemic disease.
Written informed consent was obtained from all participants and all procedures were performed in accordance with the standards of the Ethics Committee at Hospital de la Santa Creu i Sant Pau in Barcelona and in accordance with the 1964 Declaration of Helsinki and its later amendments.
2.2. Assessments
HD patients were classified as with severe or without severe cognitive impairment using the HD-specific PD-CRS cutoff scores(Martinez-Horta et al., 2020). These cutoff scores were determined in HD population in a previous study using as gold standard the presence of major cognitive and functional impairment. Thus, in accordance with this previous study we used the PD-CRS total score ≤ 64 to classify patients as with major cognitive impairment in the range of dementia(Martinez-Horta et al., 2020). This instrument assesses nine subtests: immediate and delayed verbal memory, confrontation naming, attention, working memory, unprompted draw of a clock, copy of a clock, alternating verbal fluency, and action verbal fluency. The total score is computed by summing all the raw scores obtained in each subtest, but specific scores can be grouped to compute a “frontal-subcortical” score and a “posterior-cortical” score(Pagonabarraga et al., 2008). In the present study, the PD-CRS total score was used to group patients according to their cognitive status. Accordingly, patients who obtained a PD-CRS total score > 64 were included in the “non-demented” group (HD-ND) whereas patients who obtained a PD-CRS total score ≤ 64 were considered as with major cognitive impairment in the range of dementia (HD-Dem). The non-demented group included a slight proportion of participants with mild cognitive deficits whereas in the demented group all participants exhibited severe cognitive deficits. Accordingly, this approach allowed us to compare participants with severe cognitive impairment vs those with minor or absent cognitive impairment according to the PD-CRS. Additionally, we administered the Mini-Mental State Examination (MMSE) screening test for comparative analyses with the classification done with the PD-CRS.
We also administered the composite cognitive score of the Unified Huntington’s Disease Rating Scale (UHDRS cogscore) and other measures commonly used in cognitive assessment protocols in HD (Landwehrmeyer et al., 2017). Accordingly, assessments included the Symbol Digit Modalities Test (SDMT), the phonetic fluency test with letters F, A and S, the Stroop color-naming, word-reading and interference tests, the semantic fluency test (animals) and parts A and B of the Trail Making Test (TMT). Raw scores for all these measures were adjusted for age and education level and then converted to z-scores. A global composite z-score was also calculated combining all these measures.
Screening for global cognitive status was also performed in all HC using the PD-CRS and the MMSE. In this group, all participants obtained a PD-CRS > 81 (mean = 106.7 ± 10.7) and a MMSE > 27 (mean = 29.3 ± 0.8), indicating absence of cognitive impairment.
For the HD participants, a neurologist specialized in HD (JPP) rated the severity of motor symptoms using the Unified Huntington’s Disease Rating Scale – Total motor score (UHDRS-TMS)(Hs, 1996). All HD participants obtained a diagnostic confidence level = 4, indicating that motor abnormalities were unequivocal signs of HD with a confidence level of 99%. All HD patients were classified as having early stage or mild disease according to a total functional capacity score (TFC) > 6(Shoulson and Fahn, 1979). The disease burden score (DBS), an index assumed to reflect lifelong exposure to mutant huntingtin, was calculated using the following formula based on age and CAG repeat length: DBS = age × (CAG-35.5)(Penney et al., 1997). We also recorded socio-demographic and clinical data, including age, sex, education and global cognitive functioning.
2.3. Neuroimaging acquisition and preprocessing
Volumetric MRI was available for all participants. 3D-T1 images were acquired on a 3 T Philips Achieva using an MP-RAGE sequence (TR/TE = 6.74/3.14 ms, flip-angle = 8°, field of view = 23 cm, matrix = 256x256 and slice thickness = 1 mm).
We applied voxel-based morphometry (VBM) and cortical thickness (Cth) neuroimaging preprocessing procedures. A standard VBM pipeline using the Statistical Parametrical Mapping software package (SPM12, http://www.fil.ion.ucl.ac.uk/spm) was performed(Martinez-Horta et al., 2019). Briefly, GMV tissue probability maps were computed from T1-MRI scans. These maps were then normalized to the Montreal Neurological Institute (MNI) space by applying the DARTEL algorithm. The resulting normalized GMV maps were then smoothed using an isotropic spatial filter of 8x8x8mm full-width at half-maximum (FWHM) to reduce inter-individual variability.
Cth analysis was performed using the FreeSurfer 6.0 software package (https://surfer.nmr.mgh.harvard.edu). The specific methods used for cortical reconstruction of T1-MRI brain images have been fully described elsewhere(Fischl and Dale, 2000). In short, optimized surface deformation models following intensity gradients accurately identify white matter and gray matter boundaries in the cerebral cortex, from which cortical thickness is computed at each vertex of the resulting surface. The resulting cortical surfaces are normalized to average space and smoothed using a Gaussian kernel of 15 mm FWHM.
2.4. Statistical analysis
Socio-demographic and clinical variables were subjected to ANOVA between the three groups. Post-hoc independent t-test comparisons were performed between the three groups for continuous variables and χ2 for categorical variables. To calculate the effect size of the differences observed between cognitive groups we used Cohen’s d coefficient (d values: 0–0.2, small effect size; 0.6, moderate effect size; ≥ 0.8, large effect size).
Voxelwise and vertexwise measures derived from VBM and Cth analyses were introduced into a generalized lineal model (GLM) to compare these measures across groups, using age, sex, education, CAG repeat length and UHDRS-TMS as covariates. The following pairwise group comparisons were performed: HC > HD-ND, HC > HD-Dem, and HD-ND > HD-Dem. The set of clusters surviving p < 0.05 and family-wise error (FWE) correction for multiple-comparison (cluster-level Bonferroni correction for VBM and Monte-Carlo simulation with 10,000 repeats for Cth) were considered statistically significant.
To investigate the clinical translation of the imaging findings, we computed quantitative volumetric and mean Cth information at the identified clusters where we observed significant differences across groups. Using linear regression analysis, we then studied the association of these imaging measures with the different cognitive variables within an exploratory analysis, controlling again for the effect of potential confounders such as age, education, CAG repeat length and UHDRS-TMS, for which a p-value < 0.05 was considered significant.
3. Results
3.1. Clinical and sociodemographic data
The sample consisted of 35 HD patients (mean age = 51.8 ± 12; mean CAG = 43.7 ± 2.8; mean years of education = 11.5 ± 4.5; mean TFC = 11.2 ± 2) and 15 HC (mean age = 45.8 ± 8; mean years of education = 12.3 ± 1.6). According to the PD-CRS score, n = 20 were included in the HD-ND group (mean PD-CRS total score = 84.3 ± 15; mean age = 50.8 ± 11; mean CAG = 43.3 ± 3) and n = 15 in the HD-Dem group (mean PD-CRS total score = 51.2 ± 10; mean age = 53.2 ± 14; mean CAG = 44 ± 3). As summarized in table 1, no significant differences were found between HC, HD-ND and HD-Dem groups regarding age and education. In the HD groups, no significant differences were found between HD-ND and HD-Dem regarding age, gender, education, CAG repeat length or DBS, but the HD-Dem group showed a significantly higher UHDRS-TMS, lower TFC, lower UHDRS cogscore, lower PD-CRS total score, and lower MMSE score. In the HD-ND group, the mean MMSE score was 27.85 ± 1.8 (no impairment) while in the HD-Dem group it was 21.6 ± 2.6 (below the general < 24 cutoff for dementia).
Table 1.
Clinical and Sociodemographic characteristics of the sample.
| Controls | HD | HD-ND | HD-Dem | P | |
|---|---|---|---|---|---|
| Age | 45.9 ± 8 | 51.8 ± 12 | 50.85 ± 11 | 53.2 ± 14 | a0.096; b0.156; c0.102; d0.590 |
| Gender (f/m) | 4/11 | 23/12 | 12/8 | 11/4 | a0.012; b0.052; c0.013; d0.324 |
| Education | 12.3 ± 2 | 11.5 ± 4 | 11.2 ± 5 | 12 ± 4 | a0.376; b0.346; c0.778; d0.615 |
| CAG | – | 43.7 ± 3 | 43.3 ± 3 | 44 ± 3 | d0.424 |
| DBS1 | – | 492 ± 84 | 469 ± 80 | 523 ± 83 | d0.061 |
| UHDRS-TMS2 | – | 28.5 ± 17 | 22.5 ± 18 | 37.2 ± 11 | d < 0.01 |
| TFC3 | – | 11.2 ± 2 | 12.1 ± 1 | 10 ± 2 | d < 0.01 |
| PD-CRS total score | 106.7 ± 10.7 | 70.17 ± 21 | 84.3 ± 15 | 51.2 ± 10 | a < 0.001; b < 0.001; c < 0.001; d < 0.001 |
| UHDRS cogscore | – | 170.2 ± 78 | 212 ± 72 | 114 ± 47 | d < 0.001 |
| MMSE | 29.3 ± 0.8 | 25.2 ± 3.8 | 27.8 ± 1.8 | 21.6 ± 2.6 | a < 0.001; b<0.005; c < 0.001; d < 0.001 |
Disease burden score; 2Unified Huntington’s disease rating scale – Total motor score; 3Total functional capacity.
HC vs HD
HC vs HD-ND
HC vs HD-Dem
HD-ND vs HD-Dem
3.2. Neuropsychological performance in HD groups
The HD-ND group obtained a mean PD-CRS total score of 84.3 ± 15 which, according to PD-CRS criteria, situates this group in the range between cognitive normality and mild cognitive deficits. Looking at the prevalence of cases scoring above the proposed cutoff score for mild cognitive impairment in the HD-ND group (PD-CRS ≤ 81), we saw that 45% of the patients scored for mild cognitive impairment while 55% scored for cognitive normality. None of the defects, however, were severe enough to fulfill the criteria for dementia per specific PD-CRS criteria in HD.
As reported in table 2, after adjustment for age and education, all the measures obtained through the UHDRS cogscore and through the other Enroll-HD assessments were significantly different between groups in all the cases with the exception of the phonetic verbal fluency. In the HD-ND group, all the obtained z-scores were in the lower range of normality, but above the critical cutoff of −1.5 SD with the exception of the phonetic verbal fluency (-1.6 ± 0.9). In this group the mean composite z-score was −1.2 ± 0.9 with 35% of cases scoring below −1.5 SD. Conversely, in the HD-Dem group all measures scored below −2 SD and the mean composite z-score was −2.3 ± 0.7 with 73.3% of cases scoring below −1.5 SD. In congruence with the range of PD-CRS scores obtained by the HD-ND group, performance below −1.5 SD was found in more than half of the non-demented cases for the Stroop color-naming, Stroop word-reading, Stroop interference, and for the phonetic verbal fluency (FAS). In the HD-Dem group, performance below −1.5 SD was found in>80% of cases in most of the tasks Table 3.
Table 2.
Performance in neuropsychological measures.
| HD-ND | HD-Dem | P | d | ||
|---|---|---|---|---|---|
| PD-CRS Total | 84.3 ± 16 | 51.2 ± 10 | <0.001 | 1.543 | |
| PD-CRS frontal-subcortical | 57.2 ± 14 | 28.9 ± 7 | <0.001 | 1.560 | |
| PD-CRS posterior-cortical | 27.1 ± 3 | 22.2 ± 6 | <0.005 | 0.993 | |
| Immediate verbal memory | 8.1 ± 1 | 5.5 ± 2 | <0.001 | 1.168 | |
| Naming | 17.6 ± 2 | 14.5 ± 4 | <0.005 | 0.946 | |
| Sustained attention | 6.7 ± 2 | 2.2 ± 2 | <0.001 | 1.418 | |
| Working memory | 5.1 ± 1 | 3 ± 2 | <0.005 | 1.084 | |
| Clock drawing | 8.3 ± 2 | 6.2 ± 3 | <0.01 | 0.866 | |
| Clock copying | 9.4 ± 1 | 7.6 ± 2 | <0.005 | 0.957 | |
| Delayed verbal memory | 6.2 ± 2 | 3.3 ± 2 | <0.001 | 1.203 | |
| Alternating verbal fluency | 9.6 ± 5 | 3.7 ± 2 | <0.001 | 1.202 | |
| Action verbal fluency | 12.9 ± 6 | 5.5 ± 1 | <0.001 | 1.251 | |
| SDMT | 31.8 ± 15 | 15.9 ± 6 | <0.001 | 1.391 | |
| Z-score | −1.1 ± 1.3 | −2.2 ± 1 | <0.05 | 1.039 | |
| % cases Z-score < -1.5 SD | 35% | 66.7% | χ2 = 0.65 | ||
| Stroop color-naming | 51.2 ± 16 | 28.2 ± 12 | <0.001 | 1.626 | |
| Z-score | −1.3 ± 1.1 | −2.6 ± 0.5 | <0.001 | 1.272 | |
| % cases Z-score < -1.5 SD | 55% | 93.3% | χ2 < 0.05 | ||
| Stroop word-reading | 76.6 ± 25 | 41.3 ± 20 | <0.001 | 1.559 | |
| Z-score | −1.3 ± 1.3 | −2.4 ± 0.8 | <0.01 | 1.143 | |
| % cases Z-score < -1.5 SD | 50% | 80% | χ2 = 0.07 | ||
| Stroop interference | 28 ± 11 | 14.5 ± 8 | <0.001 | 1.493 | |
| Z-score | −1.3 ± 1 | −2.2 ± 0.7 | <0.005 | 0.948 | |
| % cases Z-score < -1.5 SD | 50% | 80% | χ2 = 0.07 | ||
| FAS | 24.2 ± 13 | 14.5 ± 11 | <0.05 | 0.805 | |
| Z-score | −1.6 ± 0.9 | −2.1 ± 1 | 0.116 | 0.561 | |
| % cases Z-score < -1.5 SD | 60% | 80% | χ2 = 0.18 | ||
| Semantic fluency | 14.8 ± 4 | 9 ± 3 | <0.001 | 1.640 | |
| Z-score | −1.3 ± 0.8 | −2.3 ± 0.5 | <0.005 | 0.994 | |
| % cases Z-score < -1.5 SD | 30% | 80% | χ2 < 0.005 | ||
| TMT-A | 57.2 ± 32 | 118.8 ± 62 | <0.005 | 1.248 | |
| Z-score | −0.7 ± 1.4 | −2.1 ± 1 | <0.005 | 1.416 | |
| % cases Z-score < -1.5 SD | 0% | 100% | χ2 < 0.001 | ||
| TMT-B | 149.6 ± 80 | 221.8 ± 34 | <0.01 | 1.174 | |
| Z-score | −1 ± 1.5 | −2.3 ± 0.8 | <0.01 | 1.250 | |
| % cases Z-score < -1.5 SD | 40% | 80% | χ2 = 0.05 | ||
| Composite Z-score | −1.2 ± 0.9 | −2.3 ± 0.7 | <0.005 | 1.078 | |
| % cases Z-score < -1.5 SD | 35% | 73.3% | χ2 = 0.05 |
Table 3.
Cluster description table of the VBM-GMV analyses.
| MNI coordinates (x, y, z) | Cluster size | T value | ||
|---|---|---|---|---|
| HD-ND > HD-Dem | ||||
| Right insula | 48–8 5 | 2298 | 5.22 | |
| Right posterior insula | 36–27 17 | 3.79 | ||
| Right superior temporal gyrus | 45–24 3 | 3.54 | ||
| Left superior temporal gyrus | −56–6 −2 | 2568 | 4.31 | |
| Left supramarginal gyrus | −45–26 24 | 4.10 | ||
| Left posterior insula | −36–23 23 | 4.82 | ||
| HC > HD-ND | ||||
| Left insula | −31 9 10 | 6473 | 7 | |
| Left inferior oPFC | −41 38–9 | 6.33 | ||
| Left caudate/putamen | −12 3 20 | 6.13 | ||
| Right insula | 31 14 8 | 6871 | 6.17 | |
| Right caudate/putamen | 9 14–1 | 6.15 | ||
| Right rolandic operculum | 36–15 20 | 5.75 | ||
| Right inferior oPFC | 30 30–8 | 5.69 | ||
| Right mid-occipital | 36–68 26 | 1115 | 5.15 | |
| Right parietal superior | 26–69 51 | 4.23 | ||
| Right mid-frontal | 33 59 15 | 1330 | 4.84 | |
| Right superior oPFC | 29 51–3 | 4.54 | ||
| Right mid- oPFC | 53 42–6 | 4.24 | ||
| HC > HD-Dem | ||||
| Bilateral caudate/putamen/insula | −20 14 6 | 37,308 | 8.18 | |
| Right superior occipital | 21–90 17 | 3970 | 6.21 | |
| Right lingual gyrus | 17–86 −11 | 6 | ||
| Right inferior occipital gyrus | 30–87 −6 | 5.81 | ||
| Right precentral gyrus | 45 0 35 | 857 | 5.18 | |
| Left frontal superior medial | −6 24 42 | 2957 | 4.89 | |
| SMA | −9 21 51 | 4.02 | ||
| Left superior frontal | −17 44 38 | 4.77 | ||
FWE corrected (p < 0.05)
Group comparisons between HD-ND and HD-Dem groups showed that HD-Dem scores were significantly lower on all the cognitive tests. We found a large effect size in all groups according to d Cohen’s coefficient > 0.8.
3.3. GMV differences between groups
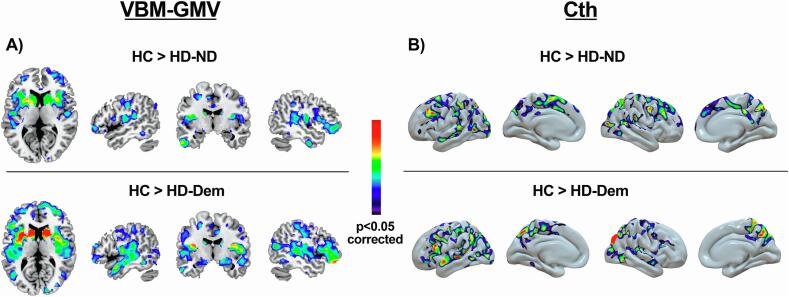
The voxelwise group comparison revealed significant GMV differences when comparing the HD-ND and HD-Dem groups with the HC group, and also when comparing the HD-ND group with the HD-Dem group. Specifically, compared to the HC group, HD-ND showed significantly lower GMV in large cortico-subcortical clusters. The set of significant clusters included the bilateral caudate nucleus and putamen, the bilateral insula, the bilateral inferior orbital prefrontal cortex (oPFC), and also the right rolandic operculum, the right mid-occipital gyrus, the right superior parietal gyrus, and the right mid-frontal and mid- and superior oPFC. Comparing HC and HD-Dem, we found marked GMV differences bilaterally in the whole basal ganglia and in the insular cortex, in the left superior and medial frontal cortex, and in several posterior-cortical clusters, including the right superior and inferior occipital gyrus and the right lingual gyrus.
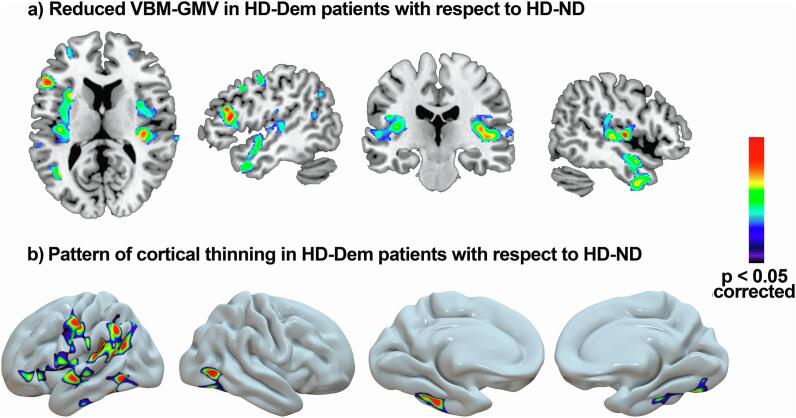
When we compared the HD-ND and HD-Dem group, the latter showed significantly reduced GMV in parietal-temporal regions, specifically in the bilateral anterior and posterior insular cortex, the superior temporal gyrus, and the left supramarginal gyrus (Fig. 1).
Fig. 1.
Regions showing lower GMV (A) and lower Cth (B) in the HD-ND and in the HD-Dem groups compared to the HC group. No regions showed a significant increase in grey matter volume. For depicting purposes, the image is shown at p < 0.001.
3.4. Cth differences between groups
The vertexwise comparison between HC and HD-ND groups showed a pattern of cortical thinning bilaterally in multiple fronto-temporal and parieto-occipital regions in the HD-ND group. These regions were the rostral mid-frontal and superior frontal gyrus, the orbital PFC, the superior temporal gyrus, the supramarginal, inferior and superior parietal gyrus, the lateral occipital gyrus, the precuneus, the precentral gyrus, and the pars triangularis. Compared to the HC group, the HD-Dem group showed a similar but more significant thinning pattern than that in the HD-ND group. Differences were also found in the post-central gyrus, the inferior and the mid-temporal gyrus, and the fusiform gyrus.
Comparing the HD-ND and HD-Dem groups we found that the HD-Dem group was characterized by a pattern of cortical thinning predominantly affecting fronto-temporal and parietal regions of the left hemisphere and temporo-occipital regions of the right hemisphere. In the left hemisphere, significant differences involved the inferior temporal and the fusiform gyrus, the supramarginal and inferior parietal gyrus, the precentral and postcentral gyrus, the caudal and rostral mid-frontal gyrus, the insula, the pars triangularis, and the rostral middle frontal gyrus. Conversely, in the right hemisphere, differences were eminently circumscribed to the temporal pole, the superior and inferior temporal gyrus, the fusiform gyrus and the lateral occipital gyrus (Fig. 2).
Fig. 2.
Regions showing lower GMV (A) and lower Cth (B) in the HD-Dem group than in the HD-ND group. No regions showed a significant increase in grey matter volume. For depicting purposes, the image is shown at p < 0.005.
3.5. Cognitive-imaging regression analysis:
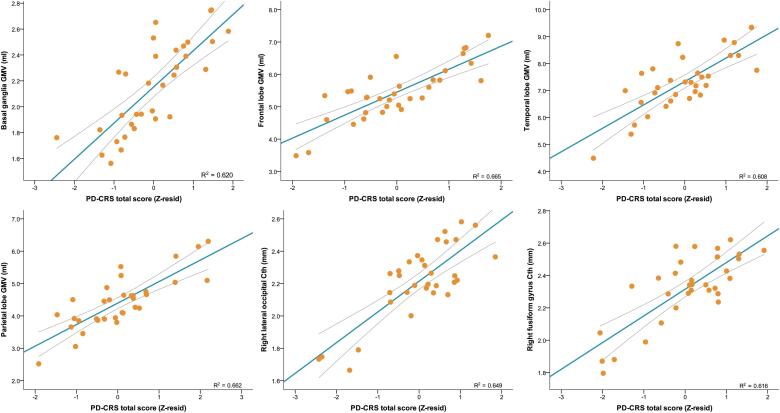
Linear regression analysis showed that after adjusting for age, education, CAG repeat length and UHDRS-TMS, GMV at multiple clusters obtained in the VBM analysis contributed strongly to global cognitive performance. PD-CRS total score performance was strongly associated with GMV in the basal ganglia (β = 0.456; P = 0.017), the frontal lobes (β = 0.586; P = 0.005), the temporal lobes (β = 0.587; P = 0.004), the insular cortex (β = 0.621; P = 0.002), the mid cingulate (β = 0.431; P = 0.036), and the occipital lobe (β = 0.441; P = 0.043). PD-CRS performance was also associated with GMV in the parietal lobe (β = 0.373; P = 0.043). However, this association was also influenced by the UHDRS-TMS (β = -0.390; P = 0.038) (Fig. 3).
Fig. 3.
Linear regression analysis depicting correlations between GMV/Cth clusters and PD-CRS total score.
Following the same linear regression analysis approach, we looked at specific clusters of GMV and their association with PD-CRS total score and performance in each subtest as well as with performance in all the other cognitive measures administered. Given the high number of correlation analyses performed between each cluster and each PD-CRS subtest, these results are reported in supplementary data. The PD-CRS total score was associated with GMV in the left (β = 0.491; P = 0.013) and right putamen (β = 0.556; P = 0.006), with the left (β = 0.523; P = 0.011) and right inferior oPFC (β = 0.531; P = 0.009), with the left (β = 0.602; P = 0.003) and right insular cortex (β = 0.632; P = 0.002), with the left (β = 0.666; P = 0.001) and right superior temporal gyrus (β = 0.495; P = 0.011), with the left (β = 0.563; P = 0.009) and right inferior temporal gyrus (β = 0.548; P = 0.010), with the right cuneus (β = 0.508; P = 0.019), with the left mid oPFC (β = 0.515; P = 0.020), with the right mid-temporal pole (β = 0.473; P = 0.014), with the left (β = 0.648; P = 0.002) and right mid-frontal gyrus (β = 0.579; P = 0.007), with the left (β = 0.591; P = 0.003) and right-mid temporal gyrus (β = 0.567; P = 0.006), with the right inferior parietal lobe (β = 0.482; P = 0.007), with the right rectus gyrus (β = 0.573; P = 0.006), with the right superior oPFC (β = 0.501; P = 0.019), and with the left (β = 0.520; P = 0.013 and right medial superior PFC (β = 0.545; P = 0.011).
Focusing in the other cognitive measures, we observed that SDMT performance correlated with GMV in the left mid-temporal gyrus (β = 0.515; P < 0.005), the left superior temporal gyrus (β = 0.409; P < 0.05) and the inferior frontal gyrus (β = 0.450; P < 0.05). Semantic fluency correlated strongly with GMV in the right insular cortex (β = 0.493; P < 0.01), the left superior temporal gyrus (β = 0.500; P < 0.01), the right inferior temporal gyrus (β = 0.489; P < 0.01), the bilateral mid-temporal pole (β = 0.522; P < 0.005), and the right mid-frontal gyrus (β = 0.463; P < 0.05).
In the Cth analyses, multiple regions overlapping the VBM-GMV findings were strongly associated with cognitive performance by means of the PD-CRS total score. The most significant clusters were found at the level of posterior-cortical regions, specifically at the fusiform gyrus in the temporo-occipital region (β = 0.491; P = 0.008). The PD-CRS posterior-cortical score was associated with cortical thickness in the right lateral occipital (β = 0.392; P < 0.05) and in the right fusiform gyrus (β = 0.512; P < 0.01). Focusing on PD-CRS subtests, immediate verbal memory was associated with the left inferior temporal cortex (β = 0.435; P < 0.05). Confrontation naming (β = 0.465; P < 0.05), sustained attention, and (β = 0.396; P < 0.05), and action verbal fluency (β = 0.446; P < 0.05) where associated with the right fusiform gyrus. Working memory was also associated with the left supramarginal gyrus (β = 0.535; P < 0.005), and the left fusiform gyrus (β = 0.541; P < 0.005). Focusing on the cogscore and related measures, total cogscore was not associated with Cth. The Stroop color-naming was associated with the superior temporal gyrus (β = 0.395; P < 0.05), and with the right lateral occipital gyrus (β = 0.449; P < 0.05), and the semantic verbal fluency was associated with the right fusiform gyrus (β = 0.407; P < 0.05).
4. Discussion:
In the present study, major cognitive impairment in the range of dementia -as determined using the specific PD-CRS cutoff scores for HD- is associated with brain and cognitive differences that exceed the prototypical pattern of frontal-executive, attention and processing speed deficits attributed to frontal-striatal atrophy, and critically involve more severe atrophy of posterior-cortical brain regions.
Our results highlight that severe cognitive impairment in the range of dementia may occur in the early stages of HD and with relative independence of the CAG repeat length, DBS, age and education. Notably, this more severe form of cognitive impairment is associated with a widespread pattern of cortical thinning and whole-brain atrophy that involves multiple cortical and subcortical clusters. The imaging data, highlights that, as previously reported, GMV atrophy is mostly ascribed to the basal ganglia, frontal and occipital lobe, and Cth involves the temporal and parieto-occipital regions(Rosas et al., 2008, Rosas et al., 2005, Tabrizi et al., 2009). Importantly, group comparisons showed that a more prominent decrease of GMV and Cth is present in those participants exhibiting severe cognitive deterioration even when controlling for the effect of age, CAG, education of UHDRS-TMS. Accordingly, more advanced disease stage or higher DBS cannot explain these differences.
Our findings are in accordance with previous works showing that the development of cognitive deterioration in HD cannot be solely attributed to basal ganglia atrophy(Rosas et al., 2008, Nopoulos et al., 2010, Tabrizi et al., 2009, Coppen et al., 2018, Podoll et al., 1988, Say et al., 2011, Carmichael et al., 2019, Harris et al., 2019, Martinez-Horta et al., 2019, Wolf et al., 2014, Labuschagne et al., 2016, Hinzen et al., 2018, Chan et al., 2019). Structures of the basal ganglia, such as the caudate nucleus and putamen, but also the insular cortex, the PFC and the occipital and parietal cortex, strongly differentiated non-demented HD patients from healthy controls. However, the most representative brain changes differentiating patients with major cognitive impairment from non-demented HD patients were found at the level of parieto-temporal regions, including, bilaterally, the anterior and posterior insular cortex, the superior temporal gyrus, and the left supramarginal gyrus. In terms of cortical thinning, our results pointed in the same direction, supporting the critical participation of decreased cortical thinning in fronto-temporal and parietal regions of the left hemisphere and in temporo-occipital regions of the right hemisphere in the more severe forms of cognitive deterioration in HD.
The involvement of cortical atrophy in HD, especially in parietal and occipital regions, is a well-known finding supported by several imaging studies(Rosas et al., 2008, Rosas et al., 2005, Kuwert et al., 1990, Tabrizi et al., 2009, Coppen et al., 2018). However, the functional translation of these cortical changes is partially understood. In this sense, our data supports for the first time that more aggressive cortical atrophy is, at least, critically associated with the presentation of a significantly more sever profile of multi-domain cognitive and functional affectation in HD.
As expected, both the measures of GMV and cortical thinning correlated with multiple cognitive variables. However, among the various measures obtained for cognitive performance, those focusing on executive functions, memory, processing speed, language and constructional abilities were those better characterizing patients with dementia. These data suggest that the neurocognitive profile differs between HD patients with and those without major cognitive impairment in the range of dementia according to the PD-CRS classification. Whereas HD patients with normal-to-mild cognitive deficits exhibit a prototypical frontal-executive dysfunction profile, HD patients with major cognitive impairment exhibit a cortical-subcortical profile with deficits extending beyond executive functions and involving amnestic difficulties, constructional apraxia, confrontation naming deficits and reduced semantic abilities. However, these findings emerged performing comparisons with the subtests comprising the PD-CRS, it is, the instrument used to classify patients. Thus, further studies should explore the specific neuropsychological correlates of major cognitive impairment and related brain differences in HD using additional comprehensive neuropsychological assessment.
Previous studies have highlighted the participation of extra-striatal GMV atrophy and cortical thinning in the clinical expression of HD(Rosas et al., 2008, Rosas et al., 2005, Nopoulos et al., 2010, Tabrizi et al., 2009). When addressing cognitive aspects, these previous studies focused on specific measures of verbal fluency and psychomotor processing speed but not on multiple measures of global cognitive performance. Although they performed correlational analysis between brain structure and cognitive performance, they did not compared patients according to the severity of cognitive deterioration. Nevertheless, cortical thickness of the pre-central gyrus, the superior temporal gyrus, the superior frontal gyrus, the lingual gyrus, the precuneus and the cuneus were found to be associated with verbal fluency performance. Cortical thickness of the pre-central gyrus, the bilateral paracentral lobule and the occipital cortex were also associated with psychomotor processing speed(Rosas et al., 2008, Nopoulos et al., 2010). Regarding the impact of cortical changes over functional capacity, the most significant associations were found between TFC and the motor cortex, the superior parietal, and the cuneus(Rosas et al., 2008). Similarly, other studies highlighted the involvement of multiple areas related to executive functions and sensorimotor and visuospatial processing in cognitive performance in HD(Garcia-Gorro et al., 2019). Interestingly, more recent data also point to hippocampal-dependent memory deficits in HD that cannot be solely explained as a function of degeneration in the basal ganglia(Carmichael et al., 2019, Harris et al., 2019).
Keeping in mind that the two HD groups were matched for age, gender, education, CAG and disease burden and that healthy controls were also matched in terms of age, and education, our findings suggest that mechanisms other than only those promoted as a function of CAG repeat length, DBS, age or education level contribute to a differential course and expression of cognitive deterioration in HD. Although mutant huntingtin aggregation is the primary mechanism leading to HD(Langbehn et al., 2019, Penney et al., 1997, Wild et al., 2015), multiple mechanisms are also known to participate in the neuropathology of HD(Rub et al., 2016). However, how these additional mechanisms contribute to variability in the phenotypic expression of HD is not fully elucidated. Of these mechanisms, inflammatory, autoimmune activity and TAU pathology have been suggested to play a cardinal role in neurodegeneration and clinical expression of HD(Rocha et al., 2016). The question regarding the possible participation of these mechanisms in the acceleration of cognitive deterioration and related neurodegeneration in HD is only partially understood. TAU pathology and the related MAPT H2 haplotype have been associated with the rate of cognitive decline in HD(Vuono et al., 2015, Fernandez-Nogales et al., 2014). Whether these mechanisms contribute to the extent of brain differences observed in our sample is as yet unknown. In this sense, further studies must clarify the role of TAU pathology and other mechanisms on neurodegeneration and related cognitive deficits in HD. Moreover, how and when these mechanisms start promoting clinical and brain changes and why they affect people with equivalent CAG and DBS in a different manner merits further in-depth research. In any case, the present study adds novel evidence on the heterogeneity of HD, and supports the need identifying mechanisms participating in this clinical heterogeneity. This is of major importance taking into account that ongoing and imminent clinical trials on HD are focused on huntingtin-lowering strategies but did not take into account other potential factors contributing to disease severity(Tabrizi et al., 2019).
In any case, pinpointing the pattern of brain changes and the cognitive profile of major cognitive impairment in the range of dementia in HD has valuable implications that merit further research in bigger samples and in a longitudinal design.
The main limitation of the present study is the lack of a gold-standard to classify patients according to cognitive status, but the method we used to classify patients has recently been shown to be reliable in this population(Martinez-Horta et al., 2020). Despite this limitation, it is reasonable to assume that the measure we used for classification differentiates patients with normal cognitive function or mild cognitive defects from those with severe cognitive alterations. However, the concept of dementia used in the present work must be taken cautiously because of the absence of HD-specific clinical diagnostic criteria for dementia. Moreover, the use of a functional assessment other than the TFC, specifically addressing cognitive-related functional deficits, must be considered in further studies. Other obvious limitations to take in consideration are the small sample size and the cross-sectional design of the study. Accordingly, will be required to confirm our findings in further studies addressing this question in a bigger sample and in a longitudinal setting.
5. Conclusions
Overall, significant patterns of cortical thinning and reduced GMV in parieto-temporal and occipital regions are associated with more severe cognitive deterioration in HD. The addition of cortical-instrumental, semantic and amnestic-like deficits to the prototypical frontal-executive neurocognitive profile of HD is a differentiating characteristic of major cognitive impairment in the range of dementia in HD. Early detection of brain changes and cognitive parameters associated with severe forms of cognitive deterioration in HD may contribute to the early identification of individuals at greater risk of a more rapid and aggressive course of cognitive impairment. Identifying the mechanisms that contribute to this more aggressive cognitive deterioration should be of major relevance in the planning of future clinical trials.
CRediT authorship contribution statement
Saul Martinez-Horta: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - original draft, Writing - review & editing. Frederic Sampedro: Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Andrea Horta-Barba: Data curation, Formal analysis, Project administration, Writing - review & editing. Jesús Perez-Perez: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration. Javier Pagonabarraga: Conceptualization, Resources, Writing - original draft, Writing - review & editing. Beatriz Gomez-Anson: Methodology, Writing - original draft, Writing - review & editing. Jaime Kulisevsky: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors wish to thank all those at the Hospital de la Santa Creu i Sant Pau involved in the study. The authors also wish to extend their gratitude to the study participants and their families.
Funding
The present study was partially funded by a Spanish Government Grant (PI17/001885) from the ISCIII (Spain), Fondos FEDER (Spain) and by the “Human Biology Project Grant” of the Huntington’s Disease Society of America (USA).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102415.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Walker F.O. Huntington's disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H., Scahill R.I., Leavitt B.R., Stout J.C., Paulsen J.S., Reilmann R., Unschuld P.G., Wexler A., Margolis R.L., Tabrizi S.J. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nature reviews. Neurology. 2014;10(4):204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- Peavy G.M., Jacobson M.W., Goldstein J.L., Hamilton J.M., Kane A., Gamst A.C., Lessig S.L., Lee J.C., Corey-Bloom J. Cognitive and functional decline in Huntington's disease: dementia criteria revisited. Movement disorders : official journal of the Movement Disorder Society. 2010;25(9):1163–1169. doi: 10.1002/mds.22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Salat D.H., Lee S.Y., Zaleta A.K., Pappu V., Fischl B., Greve D., Hevelone N., Hersch S.M. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain : a journal of neurology. 2008;131(Pt 4):1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P.C., Aylward E.H., Ross C.A., Johnson H.J., Magnotta V.A., Juhl A.R., Pierson R.K., Mills J., Langbehn D.R., Paulsen J.S. Cerebral cortex structure in prodromal Huntington disease. Neurobiology of disease. 2010;40(3):544–554. doi: 10.1016/j.nbd.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Hevelone N.D., Zaleta A.K., Greve D.N., Salat D.H., Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J., Langbehn D.R., Leavitt B.R., Roos R.A., Durr A., Craufurd D., Kennard C., Hicks S.L., Fox N.C., Scahill R.I., Borowsky B., Tobin A.J., Rosas H.D., Johnson H., Reilmann R., Landwehrmeyer B., Stout J.C. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. The Lancet. Neurology. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen E.M., Grond J.V., Hafkemeijer A., Barkey Wolf J.J.H., Roos R.A.C. Structural and functional changes of the visual cortex in early Huntington's disease. Hum. Brain Mapp. 2018;39(12):4776–4786. doi: 10.1002/hbm.24322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub U., Seidel K., Vonsattel J.P., Lange H.W., Eisenmenger W., Gotz M., Del Turco D., Bouzrou M., Korf H.W., Heinsen H. Huntington's Disease (HD): Neurodegeneration of Brodmann's Primary Visual Area 17 (BA17) Brain Pathol. 2015;25(6):701–711. doi: 10.1111/bpa.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwert T., Lange H.W., Langen K.J., Herzog H., Aulich A., Feinendegen L.E. Cortical and subcortical glucose consumption measured by PET in patients with Huntington's disease. Brain : a journal of neurology. 1990;113(Pt 5):1405–1423. doi: 10.1093/brain/113.5.1405. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Juengling F.D., Ecker D., Landwehrmeyer G.B. Thalamic atrophy in Huntington's disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb. Cortex. 2005;15(6):846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Snowden J.S. The Neuropsychology of Huntington's Disease. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2017;32(7):876–887. doi: 10.1093/arclin/acx086. [DOI] [PubMed] [Google Scholar]
- Dogan I., Eickhoff S.B., Schulz J.B., Shah N.J., Laird A.R., Fox P.T., Reetz K. Consistent neurodegeneration and its association with clinical progression in Huntington's disease: a coordinate-based meta-analysis. Neuro-degenerative diseases. 2013;12(1):23–35. doi: 10.1159/000339528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Anson B., Alegret M., Munoz E., Monte G.C., Alayrach E., Sanchez A., Boada M., Tolosa E. Prefrontal cortex volume reduction on MRI in preclinical Huntington's disease relates to visuomotor performance and CAG number. Parkinsonism & related disorders. 2009;15(3):213–219. doi: 10.1016/j.parkreldis.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Say M.J., Jones R., Scahill R.I., Dumas E.M., Coleman A., Santos R.C., Justo D., Campbell J.C., Queller S., Shores E.A., Tabrizi S.J., Stout J.C. Visuomotor integration deficits precede clinical onset in Huntington's disease. Neuropsychologia. 2011;49(2):264–270. doi: 10.1016/j.neuropsychologia.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Carmichael A.M., Irish M., Glikmann-Johnston Y., Singh P., Stout J.C. Pervasive autobiographical memory impairments in Huntington's disease. Neuropsychologia. 2019;127:123–130. doi: 10.1016/j.neuropsychologia.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Harris K.L., Armstrong M., Swain R., Erzinclioglu S., Das T., Burgess N., Barker R.A., Mason S.L. Huntington's disease patients display progressive deficits in hippocampal-dependent cognition during a task of spatial memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2019;119:417–427. doi: 10.1016/j.cortex.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S., Horta-Barba A., Perez-Perez J., Antoran M., Pagonabarraga J., Sampedro F., Kulisevsky J. Impaired face-like object recognition in premanifest Huntington's disease. Cortex; a journal devoted to the study of the nervous system and behavior. 2019;123:162–172. doi: 10.1016/j.cortex.2019.10.015. [DOI] [PubMed] [Google Scholar]
- Coppen E.M., Jacobs M., van der Zwaan K.F., Middelkoop H.A.M., Roos R.A.C. Visual Object Perception in Premanifest and Early Manifest Huntington's Disease. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2019;34(8):1320–1328. doi: 10.1093/arclin/acz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.C., Sambataro F., Vasic N., Baldas E.M., Ratheiser I., Bernhard Landwehrmeyer G., Depping M.S., Thomann P.A., Sprengelmeyer R., Sussmuth S.D., Orth M. Visual system integrity and cognition in early Huntington's disease. The European journal of neuroscience. 2014;40(2):2417–2426. doi: 10.1111/ejn.12575. [DOI] [PubMed] [Google Scholar]
- Labuschagne I., Cassidy A.M., Scahill R.I., Johnson E.B., Rees E., O'Regan A., Queller S., Frost C., Leavitt B.R., Durr A., Roos R., Owen G., Borowsky B., Tabrizi S.J., Stout J.C. Visuospatial Processing Deficits Linked to Posterior Brain Regions in Premanifest and Early Stage Huntington's Disease. Journal of the International Neuropsychological Society : JINS. 2016;22(6):595–608. doi: 10.1017/S1355617716000321. [DOI] [PubMed] [Google Scholar]
- Hinzen W., Rossello J., Morey C., Camara E., Garcia-Gorro C., Salvador R., de Diego-Balaguer R. A systematic linguistic profile of spontaneous narrative speech in pre-symptomatic and early stage Huntington's disease. Cortex; a journal devoted to the study of the nervous system and behavior. 2018;100:71–83. doi: 10.1016/j.cortex.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.C.S., Stout J.C., Vogel A.P. Speech in prodromal and symptomatic Huntington's disease as a model of measuring onset and progression in dominantly inherited neurodegenerative diseases. Neurosci. Biobehav. Rev. 2019;107:450–460. doi: 10.1016/j.neubiorev.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Chenery H.J., Copland D.A., Murdoch B.E. Complex language functions and subcortical mechanisms: evidence from Huntington's disease and patients with non-thalamic subcortical lesions. International journal of language & communication disorders. 2002;37(4):459–474. doi: 10.1080/1368282021000007730. [DOI] [PubMed] [Google Scholar]
- Podoll K., Caspary P., Lange H.W., Noth J. Language functions in Huntington's disease. Brain : a journal of neurology. 1988;111(Pt 6):1475–1503. doi: 10.1093/brain/111.6.1475. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J., Reilmann R., Roos R.A., Durr A., Leavitt B., Owen G., Jones R., Johnson H., Craufurd D., Hicks S.L., Kennard C., Landwehrmeyer B., Stout J.C., Borowsky B., Scahill R.I., Frost C., Langbehn D.R. Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. The Lancet. Neurology. 2012;11(1):42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- Paulsen J.S., Long J.D., Johnson H.J., Aylward E.H., Ross C.A., Williams J.K., Nance M.A., Erwin C.J., Westervelt H.J., Harrington D.L., Bockholt H.J., Zhang Y., McCusker E.A., Chiu E.M., Panegyres P.K. Clinical and Biomarker Changes in Premanifest Huntington Disease Show Trial Feasibility: A Decade of the PREDICT-HD Study. Front. Aging Neurosci. 2014;6:78. doi: 10.3389/fnagi.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium G.M.o.H.s.D.G.-H. Identification of Genetic Factors that Modify Clinical Onset of Huntington's Disease. Cell. 2015;162(3):516–526. doi: 10.1016/j.cell.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn D.R., Stout J.C., Gregory S., Mills J.A., Durr A., Leavitt B.R., Roos R.A.C., Long J.D., Owen G., Johnson H.J., Borowsky B., Craufurd D., Reilmann R., Landwehrmeyer G.B., Scahill R.I., Tabrizi S.J. Association of CAG Repeats With Long-term Progression in Huntington Disease. JAMA neurology. 2019 doi: 10.1001/jamaneurol.2019.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisch U., Muche R., Rothenbacher D., Landwehrmeyer G.B., Long J.D., Orth M. Identification of symbol digit modality test score extremes in Huntington's disease, American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2019;180(3):232–245. doi: 10.1002/ajmg.b.32719. [DOI] [PubMed] [Google Scholar]
- Vuono R., Winder-Rhodes S., de Silva R., Cisbani G., Drouin-Ouellet J., Spillantini M.G., Cicchetti F., Barker R.A. The role of tau in the pathological process and clinical expression of Huntington's disease. Brain : a journal of neurology. 2015;138(Pt 7):1907–1918. doi: 10.1093/brain/awv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre T.A., Bachoud-Levi A.C., Marinus J., Stout J.C., Paulsen J.S., Como P., Duff K., Sampaio C., Goetz C.G., Cubo E., Stebbins G.T., Martinez-Martin P. Rating scales for cognition in Huntington's disease: Critique and recommendations. Movement disorders : official journal of the Movement Disorder Society. 2018;33(2):187–195. doi: 10.1002/mds.27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Horta S., Horta-Barba A., Perez-Perez J., Sampedro F., de Lucia N., De Michele G., Kehrer S., Priller J., Migliore S., Squitieri F., Castaldo A., Mariotti C., Mananes V., Lopez-Sendon J.L., Rodriguez N., Martinez-Descals A., Garcia-Ruiz P., Julio F., Januario C., Delussi M., de Tommaso M., Noguera S., Ruiz-Idiago J., Sitek E.J., Nuzzi A., Pagonabarraga J., Kulisevsky J. Utility of the Parkinson's disease-Cognitive Rating Scale for the screening of global cognitive status in Huntington's disease. J. Neurol. 2020 doi: 10.1007/s00415-020-09730-6. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J., Kulisevsky J., Llebaria G., Garcia-Sanchez C., Pascual-Sedano B., Gironell A. Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23(7):998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer G.B., Fitzer-Attas C.J., Giuliano J.D., Goncalves N., Anderson K.E., Cardoso F., Ferreira J.J., Mestre T.A., Stout J.C., Sampaio C. Data Analytics from Enroll-HD, a Global Clinical Research Platform for Huntington's Disease. Movement disorders clinical practice. 2017;4(2):212–224. doi: 10.1002/mdc3.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hs G. Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group, Movement disorders : official journal of the Movement Disorder Society. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Shoulson I., Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- Penney J.B., Jr., Vonsattel J.P., MacDonald M.E., Gusella J.F., Myers R.H. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann. Neurol. 1997;41(5):689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S., Moreu A., Perez-Perez J., Sampedro F., Horta-Barba A., Pagonabarraga J., Gomez-Anson B., Lozano-Martinez G.A., Lopez-Mora D.A., Camacho V., Fernandez-Leon A., Carrio I., Kulisevsky J. The impact of bilingualism on brain structure and function in Huntington's disease. Parkinsonism & related disorders. 2019;60:92–97. doi: 10.1016/j.parkreldis.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. PNAS. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gorro C., Llera A., Martinez-Horta S., Perez-Perez J., Kulisevsky J., Rodriguez-Dechicha N., Vaquer I., Subira S., Calopa M., Munoz E., Santacruz P., Ruiz-Idiago J., Mareca C., Beckmann C.F., de Diego-Balaguer R., Camara E. Specific patterns of brain alterations underlie distinct clinical profiles in Huntington's disease. NeuroImage. Clinical. 2019;23 doi: 10.1016/j.nicl.2019.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild E.J., Boggio R., Langbehn D., Robertson N., Haider S., Miller J.R., Zetterberg H., Leavitt B.R., Kuhn R., Tabrizi S.J., Macdonald D., Weiss A. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington's disease patients. J. Clin. Investig. 2015;125(5):1979–1986. doi: 10.1172/JCI80743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub U., Seidel K., Heinsen H., Vonsattel J.P., den Dunnen W.F., Korf H.W. Huntington's disease (HD): the neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol. 2016;26(6):726–740. doi: 10.1111/bpa.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N.P., Ribeiro F.M., Furr-Stimming E., Teixeira A.L. Neuroimmunology of Huntington's Disease: Revisiting Evidence from Human Studies. Mediators Inflamm. 2016;2016:8653132. doi: 10.1155/2016/8653132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Nogales M., Cabrera J.R., Santos-Galindo M., Hoozemans J.J., Ferrer I., Rozemuller A.J., Hernandez F., Avila J., Lucas J.J. Huntington's disease is a four-repeat tauopathy with tau nuclear rods. Nat. Med. 2014;20(8):881–885. doi: 10.1038/nm.3617. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J., Leavitt B.R., Landwehrmeyer G.B., Wild E.J., Saft C., Barker R.A., Blair N.F., Craufurd D., Priller J., Rickards H., Rosser A., Kordasiewicz H.B., Czech C., Swayze E.E., Norris D.A., Baumann T., Gerlach I., Schobel S.A., Paz E., Smith A.V., Bennett C.F., Lane R.M. Targeting Huntingtin Expression in Patients with Huntington's Disease. The New England journal of medicine. 2019;380(24):2307–2316. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.