Abstract
Objectives
To explore the clinicopathological features and prognosis of breast cancer with special histological types.
Materials and methods
The information of breast cancer patients was obtained from the Surveillance, Epidemiology, and End Results (SEER) database (2010–2016). Comparative analyses were performed to explore the difference in clinicopathological characteristics and propensity score matching (PSM) was used to weaken the effects from clinical profiles. Survival analysis was conducted to investigate the prognostic effects from histological types, and the prognostic factors of this group of patients were identified with the univariate COX proportional model.
Results
A total of 242863 breast cancer patients were eligible, of which 230213 individuals were ductal breast cancer (IDC) and 12650 individuals were special breast lesions, respectively. Comparatively, special breast cancer had a lower histological grade, a smaller tumor size, a lower proportion of nodal involvement and distant metastasis, in addition to a higher proportion of triple-negative subtype. The overall prognosis of special histological breast cancer was comparable to IDC, while the survival of HER2 enriched breast cancer was in favor of special breast cancer. With the PSM performance, the prognosis exhibited an inferior profile in the metaplastic breast cancer and was significantly favorable to apocrine, medullary, micropapillary, and papillary breast cancer.
Conclusion
This study revealed that the special histological breast cancer presented distinct clinicopathological characteristics and great heterogeneity in the prognosis among diverse histological subtypes.
Keywords: Breast cancer, Histological types, Clinicopathological characteristics, Prognosis
Highlights
-
•
Breast cancer is a heterogenous disease with diverse histological subtypes.
-
•
Special histological breast cancer exhibits distinct clinicopathological profiles.
-
•
Prognosis of special histological breast cancer is profoundly heterogenous.
-
•
Histological subtype is an independent prognostic indicator of breast cancer.
1. Introduction
Breast cancer is a heterogenous disease, varying from clinical presentation to molecular features, and tends to exhibit potentially distinct prognosis [1]. Among the integrity of histological subtypes for the cancer of the breast, ductal breast cancer (IDC) is the most common histological type, which occupy more than 75% proportion of the entire population [2].
Breast cancer with unusual histological types is a kind of specific breast cancer, which were estimated in a low prevalence and consists of diverse categories with a beyond standard number according to different guidelines, and at least eighteen histological types were currently presented and remained updated with the in-depth understanding and advances in molecular pathology [3]. Previous studies have managed to discuss the clinical features and prognosis of special histological breast cancer [[4], [5], [6], [7]]. However, most of them were studies that merely contained a limited cohort adopted in a single medical institution, of which the characteristics of clinical profiles and prognosis could be discordant, or primarily focused on a specific category of breast lesions. Moreover, because of the relatively lower prevalence of special histological breast cancer, presented studies primarily centered on the breast cancer with common histological types, and this group of unusual patients were not systematically investigated on the basis of sufficient clinical data to present the specific biological features and clinical behaviors [[8], [9], [10], [11], [12], [13], [14], [15]].
Herein, we did this study to portray the clinicopathological characteristics and discuss the underlying prognostic indicators and outcomes, with the aim of shedding light on the treatment and providing potential benefit for the patients with special histological breast cancer in clinical practice.
2. Materials and methods
2.1. Population
A retrospective study was conducted using the Surveillance, Epidemiology, and End Results (SEER) database, of which the breast cancer dataset (2010–2016, November 2018 submission) was retrieved for the following cohort selection and eligibility assessment. The inclusion criteria were as follows: (1) female; (2) breast cancer with confirmation of positive histopathology. Patients were ruled out if the population demographics and clinicopathological information were incomplete. Clinicopathological characteristics adopted into analyses consisted of age at diagnosis, race, histological grade, tumor size, nodal status, distant metastases, postoperative TNM staging, molecular subtypes, surgical performance, the receipt of radiotherapy, and the chemotherapeutic delivery.
2.2. Outcomes
In this study, special histological types of breast cancer (special breast cancer) were defined as the positively confirmed histopathological classifications arising from breast other than ductal or lobular breast carcinoma. According to SEER terminology, molecular subtypes were categorized into four varieties, including hormonal receptor (HR) positive/human epithelial growth factor receptor 2 (HER2) negative (luminal A), HR-negative/HER2-positive (luminal B), HR-negative/HER2-positive (HER2), and HR-negative/HER2-negative (TN). Overall survival (OS) was the interval from the diagnosis of breast cancer to the death caused by any reasons or the last follow-up. The American Joint Committee on Cancer (AJCC) 7th guideline was followed to examine the TNM staging of breast cancer.
2.3. Statistical analysis
The comparative analyses of population demographics and clinicopathological characteristics from IDC and special breast cancer were performed using Pearson Chi-square and Fishers’ exact probability tests for qualitative data and t-test or Wilcoxon rank test for quantitative data on normal and abnormal distribution, respectively. Propensity score matching (PSM) was conducted to calibrate the objective differences in characteristics at baseline of breast cancer groups. Survival outcomes were compared using Kaplan-Meier method with log-rank tests, and the prognostic factors of special breast cancer were identified with the univariate COX proportional model. All statistical analyses were two-sided and conducted by SPSS version 26.0 and R software (3.6.4).
3. Results
A total of 242863 breast cancer patients diagnosed as breast cancer was eligible, of which 230213 individuals were IDC and 12650 individuals were special breast lesions, respectively. There were eight groups of histological breast cancer subtypes identified in this study, including the adenoid cystic, apocrine, medullary, metaplastic, micropapillary, mucinous, papillary, and tubular breast cancer. Population demographics and clinicopathological characteristics of identified participants were presented in Table 1 and Table S1.
3.1. Demographics and clinicopathological characteristics
Substantial differences were detected in the demographics and clinicopathological characteristics. The median age of two groups of patients was 60.99 and 64.24 years (P < 0.0001), and the proportion of the white race was relatively lower in special breast cancer (the white race, 77.7% vs. 79.3%, P < 0.0001). In comparisons with IDC, special breast cancer had a lower histological grade (III-IV, 23.1% vs. 36.4%, P < 0.0001), a smaller tumor size (>5 cm, 8.1% vs. 8.3%, P < 0.0001), a lower nodal involvement (N0, 85.3% vs. 71.0%, P < 0.0001), and a lower rate of de novo stage IV disease (M1, 1.9 vs. 4.0%, P < 0.0001), while the proportion of TN subtype was significantly higher in patients with special breast cancer (TN, 16.2% vs. 12.7%, P < 0.0001). Concerning therapeutic options, the receipt of both systemic and local treatment was consistently lower in the patients with special breast cancer, including radiotherapy (48.1% vs. 50.6%, P < 0.0001), and chemotherapy delivery (26.9% vs. 41.7%, P < 0.0001).
Regarding the specific subtypes, patients with special histological breast cancer tended to present an aged trend than those with IDC. Histological grade of IDC was significantly higher than that of medullary breast cancer (III-IV, 94.5% vs. 36.4%, P < 0.0001) and metaplastic breast cancer (III-IV, 82.7% vs. 36.4%, P < 0.0001), while was relatively lower than that of adenoid cystic breast cancer (III-IV, 13.9% vs. 36.4%, P < 0.0001), mucinous breast cancer (III-IV, 4.0% vs. 36.4%, P < 0.0001), and tubular breast cancer (III-IV, 0.5% vs. 36.4%, P < 0.0001). Tumor size of IDC tended to be increased in comparisons with tubular breast cancer (>5 cm, 0.8% vs. 8.3%, P < 0.0001), yet relatively shrank than that of metaplastic breast cancer (>5 cm, 23.6% vs. 8.3%, P < 0.0001). Nodal involvement of IDC was greatly frequent than that of adenoid cystic breast cancer (N0, 97.0% vs. 71.0%, P < 0.0001), mucinous breast cancer (N0, 92.2% vs. 71.0%, P < 0.0001), papillary breast cancer (N0, 90.0% vs. 71.0%, P < 0.0001), and tubular breast cancer (N0, 96.2% vs. 71.0%, P < 0.0001), while was less common than that of micropapillary breast cancer (N0, 54.6% vs. 71.0%, P < 0.0001). The proportion of TN breast cancer were dramatically higher in adenoid cystic breast cancer (TN, 74.8% vs. 12.7%, P < 0.0001), apocrine breast cancer (TN, 52.4% vs. 12.7%, P < 0.0001), medullary breast cancer (TN, 57.3% vs. 12.7%, P < 0.0001), and metaplastic breast cancer (TN, 68.6% vs. 12.7%, P < 0.0001).
3.2. Prognosis
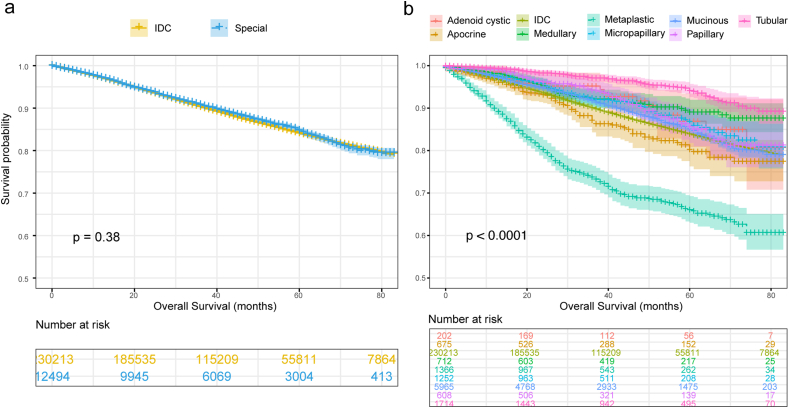
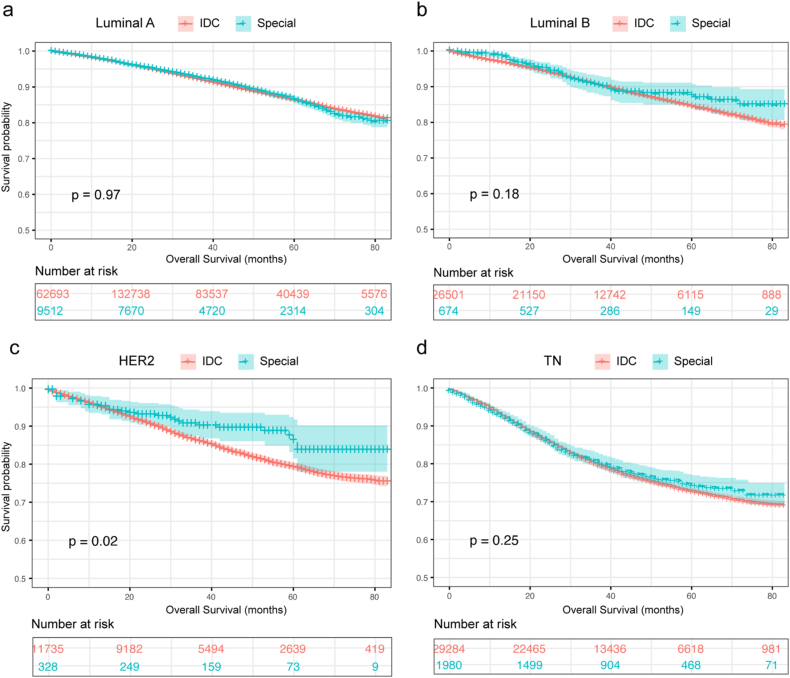
The OS of special breast cancer and IDC was 74.11 m (95%CI, 73.68m–74.53 m) and 73.93 m (95%CI, 73.83m–74.02 m) respectively with no statistical difference (P = 0.38), while a great heterogeneity was detected among the identified histological subtypes of breast cancer (P < 0.0001) (Fig. 1a and b). Regarding the prognosis of molecular types, the OS was significantly improved in special breast cancer with the subtypes of HER2 (mOS, 74.88 m vs. 71.21 m, P = 0.02), however, no statistical difference was revealed between two groups of patients with luminal A, luminal B, and TN subtypes (Fig. 2a–d).
Fig. 1.
Comparative analysis for the prognosis of IDC and special breast cancer with overall survival (OS) (a) and respective OS (b). IDC = ductal breast cancer; Special = special breast cancer.
Fig. 2.
Comparative analysis for the prognosis in regard to molecular subtypes including luminal A (a), luminal B (b), HER2 enriched (c), and triple-negative breast cancer (d). IDC = ductal breast cancer; Special = special histologic breast cancer; TN = triple-negative breast cancer.
Concerning the specific categories, the prognosis of luminal A breast cancer, in comparisons with IDC, was profoundly better with tubular type (mOS, 79.57 m vs. 75.32 m, P < 0.0001), while was worse with metaplastic (mOS, 62.73 m vs. 75.32 m, P < 0.0001) and apocrine category (mOS, 69.95 m vs. 75.32 m, P = 0.005). This result was in consistent with that of luminal B subtype, which the OS of metaplastic breast cancer was apparently reduced in metaplastic breast cancer (mOS, 58.26 m vs. 74.20 m, P = 0.005). Prognosis of IDC was consistently worse in HER2 subtype with mucinous histological type (mOS, 81.42 m vs. 71.27 m, P = 0.025), and in TN subtype with adenoid cystic type (mOS, 76.31 m vs. 67.09 m, P < 0.0001), apocrine type (mOS, 71.82 m vs. 67.09 m, P = 0.003), medullary type (mOS, 75.84 m vs. 67.09 m, P < 0.0001). However, the survival of metaplastic breast cancer with TN subtype was obviously worse than that of IDC (Table S2).
The prognostic indicators were explored on the basis of a univariate COX regression analysis, which suggested that age at diagnosis (P < 0.0001), race (P < 0.0001), histological grade (P < 0.0001), nodal status (P = 0.026), distant metastasis (P < 0.0001), and molecular subtype (P < 0.0001) were the vital factors for the survival of special breast cancer. Besides, therapeutic performance, including surgery (P < 0.0001), radiotherapy (P < 0.0001), chemotherapy (P < 0.0001), exerted a significant influence on the prognosis of patients with special breast cancer (Table 2).
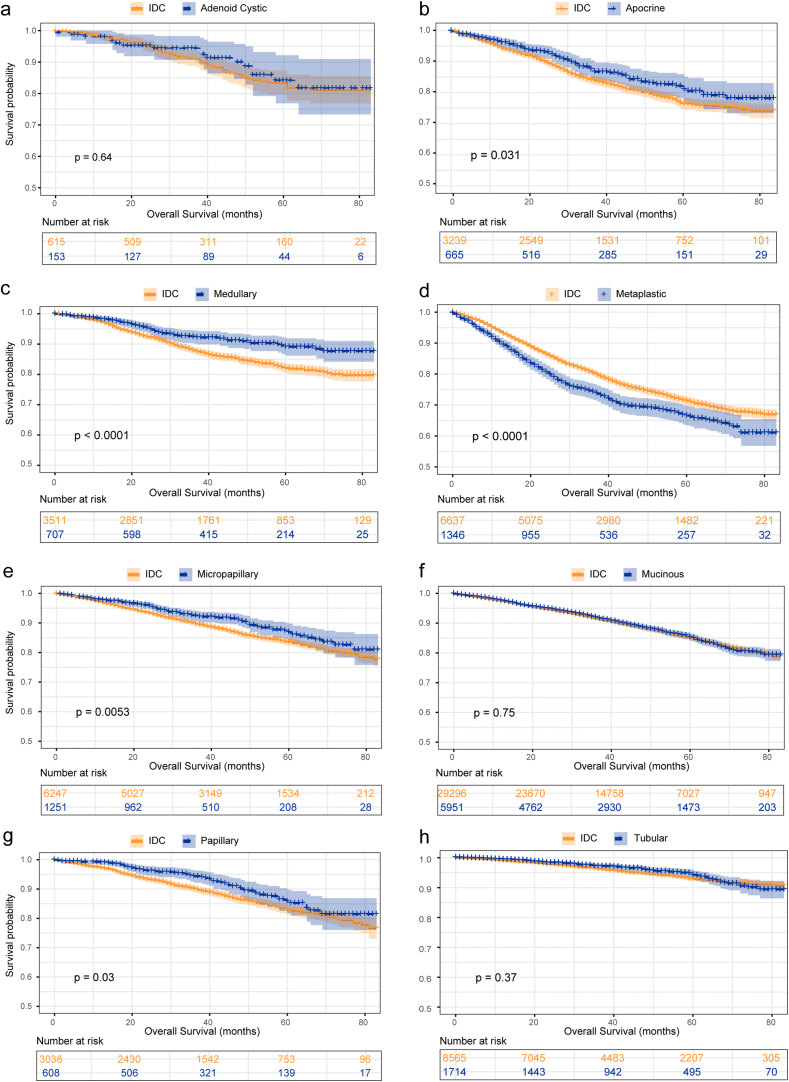
To further eliminate the variations at baseline, we did a PSM in a 1:5 ratio to illustrate the effects from histological types on prognostic outcomes. The curated clinicopathological characteristics and treatment options were demonstrated in Tables S3–10 and were well balanced. The survival of metaplastic breast cancer was significantly shortened in comparisons with IDC (mOS, 62.26 m vs. 66.39 m, P < 0.0001), while apocrine (mOS, 72.40 m vs. 69.90 m, P = 0.031), medullary (mOS, 76.86 m vs. 73.01 m, P < 0.0001), micropapillary (mOS, 75.53 m vs. 73.52 m, P = 0.005), and papillary breast cancer (mOS, 75.77 m vs. 73.52 m, P = 0.030) consistently exhibited prognostic advantages over IDC (Fig. 3a–h). With regard to the prognosis associated with molecular subtypes, an obvious decreased survival was detected in the metaplastic breast cancer with luminal A (mOS, 62.73 m vs. 70.81 m, P < 0.0001) and TN subtype (mOS, 61.70 m vs. 63.90 m, P = 0.037), while an identical tendency with a favorable survival was demonstrated in the medullary breast cancer with luminal A (mOS, 77.65 m vs. 74.69 m, P=0.015) and TN subtype (mOS, 75.74 m vs. 70.56 m, P < 0.0001). Survival outcomes from comparative analysis were presented in Table S11.
Fig. 3.
Comparative analysis for the prognosis of IDC and special breast cancer after 1:5 propensity score matching analysis. IDC = ductal breast cancer.
4. Discussion
To our knowledge, this is the first study that systematically discusses the clinical features and prognosis of breast cancer with special histological types. Our study overall presented the characteristics and survival outcomes of special breast cancer, and comprehensively compared the differences in the features between IDC and special breast lesions.
To explore the distinctions in clinicopathological profiles, we firstly performed a comparative analysis of features from the cohorts with IDC and special breast cancer. On the basis of the identified cohort with a considerable size, our study revealed that profound differences existed through the group of clinicopathological variables between the two cohorts, including age at diagnosis, race, pathological TNM staging, molecular subtypes, and therapeutic options. An aging trend was revealed through the comparisons, which indicated that patients diagnosed as special breast cancer were approximately four years later than that with IDC. Besides, it was obvious that the percentage of the white race was relatively higher in the IDC, while the black and Asian American people held a larger proportion in special breast cancer. Regarding clinicopathological factors, special breast cancer tended to present less aggressiveness of biological behaviors, which exhibited the relatively lower histological grade and TNM staging. However, the proportion of TN breast cancer with special histological subtypes was significantly higher than that of IDC. Concerning treatment options, patients with special histological breast cancer were less accessible to therapeutics, with a lower rate of the performance of both systemic and local treatment, and could be the cause of the prognostic discrepancy.
Our study suggested that the heterogeneity apparently existed in the prognosis of special breast cancer. Overall, there was no apparent discrepancy in the prognosis of IDC and special breast cancer, which was paradoxically inconsistent with the previous understanding of the excellent prognosis from the breast cancer with special histological subtypes [16]. Although there was no significant difference was acquired from overall comparative analysis, substantial heterogeneity was revealed from the following respective comparisons, which promoted us to further explore the potential factors resulting in these discrepancies. To clarify the differences in survival associated with molecular features, we did analyses toward distinct subtypes, of which the results were suggestive of a definite prognostic divergence lying in the HER2 subtype while no difference was revealed in the endocrine-related and TN breast cancer. This finding was inconsistent with the previous studies, which could be the consequences of distinct taxonomy for breast cancer pathology [17]. We also explored the prognostic indicators, of which the results suggested that age at diagnosis, race, histological grade, nodal status, distant metastasis, molecular subtypes, and the delivery of treatment could exert a significant effect on the prognosis of special breast cancer.
With the aim of eliminating the great variations of clinical profiles, accordingly, we performed PSM analyses and the effects, from the specific histological subtypes, on survival were successively discussed. Results demonstrated that the prognosis of the metaplastic breast cancer was comparatively worse than that of IDC, while a favorable survival was detected in the apocrine, medullary, micropapillary, and papillary breast cancer. These results were accordant with the findings retrieved by the previous studies regarding the prognostic outcomes associated with histological types, however, paradoxically, to some extent inconsistent with the believe that the prognosis of invasive breast cancer with special histological types was considered as better than that of special breast cancer, and the distinct survival outcomes estimated from the prior research [4,[18], [19], [20], [21]].
This study confirmed the clinicopathological and prognostic distinctions among histological subtypes, of which the evidence was promising in serving the clinical practice. However, comprehensive and precision treatment for breast cancer should integrate comprehensive features to improve prognosis and provide increasing survival benefits for patients. The research discussing the correlations between histologic subtypes and clinical efficacy has emerged and initially presented the associations with drug response [22,23]. Since the long-term believe that histological types were the consequence of origins from the distinct microanatomical structures of breast tissue was challenged, the hypothesis that the molecular features promoted the diverse differentiation which contributed to the following phenotypes has been gradually illustrated [[24], [25], [26]]. By virtue of high-throughput and microarray-based technologies, the genome and transcriptome features of 11 special histological types of breast cancer have been investigated, of which the findings indicated that the molecular heterogeneity should take into consideration for the amelioration of the histopathological taxonomy for breast cancer [3]. With the identification of novel biomarkers and the development of promising therapeutics, the implications of histological subtypes are supposed to be broadly investigated and further enriched.
There were some inevitable limitations in this study. For starters, the histological subtypes of breast neoplasms included in our research was undergone selected, of which the clinicopathological features were complete, and the eligible classifications might not be identical to the system for the histotype of breast cancer recommended by WHO [27]. Besides, a few prognostic indicators, such as Ki67 index or intravascular invasion, are not recorded in this publicly available database and, consequently, could not adopt into analyses, which could create discrepancies in the rountinary classifications of molecular subtypes like luminal B as well as weaken the power of this study. Last, confirmation of histological type was according to the leading components, of which constituted more than 90% could be diagnosed, and chances were that histological profiles tend to comprise a mixture of histotypes under the circumstance that there was no immunohistochemical panel result recorded in the SEER database. This ‘none-pure’ possibility could latently add bias into our study.
In conclusion, our study suggested that breast cancer with special histological types demonstrated distinct clinicopathological characteristics and heterogeneous prognosis among diverse histological categories. The heterogeneity of breast cancer with distinguished pathological profiles is warranted to be explored in the upcoming practice.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.09.006.
Contributor Information
Jiayu Wang, Email: wangjiayu8778@sina.com.
Binghe Xu, Email: xubingheBM@163.com.
Appendices.
Table 1.
The population demographics and clinicopathological characteristics at baseline of cohort.
| Variables | IDC (N = 230213) |
Special breast cancer (N = 12650) |
P value | ||
|---|---|---|---|---|---|
| No | Percent (%) | No | Percent (%) | ||
| Age | 60.99 | 64.24 | <0.0001 | ||
| Race | <0.0001 | ||||
| White | 181539 | 79.3 | 9772 | 77.7 | |
| Black | 25498 | 11.1 | 1597 | 12.7 | |
| Other | 21970 | 9.6 | 1212 | 9.6 | |
| Grade | <0.0001 | ||||
| Grade1 | 49583 | 21.5 | 5820 | 46.0 | |
| Grade2 | 97036 | 42.2 | 3912 | 30.9 | |
| Grade3 | 82997 | 36.1 | 2844 | 22.5 | |
| Grade4 | 597 | 0.3 | 74 | 0.6 | |
| T stage | <0.0001 | ||||
| T0 | 66 | <0.01 | 5 | <0.01 | |
| T1 | 146043 | 63.4 | 8111 | 64.2 | |
| T2 | 65065 | 28.3 | 3496 | 27.6 | |
| T3 | 10796 | 4.7 | 711 | 5.6 | |
| T4 | 8243 | 3.6 | 327 | 2.5 | |
| N stage | <0.0001 | ||||
| N0 | 163359 | 71.0 | 10787 | 85.3 | |
| N1mi | 6070 | 2.6 | 172 | 1.4 | |
| N1 | 43131 | 18.7 | 1174 | 9.3 | |
| N2 | 10982 | 4.8 | 298 | 2.4 | |
| N3 | 6671 | 2.9 | 219 | 1.7 | |
| M stage | <0.0001 | ||||
| M0 | 221060 | 96.0 | 12406 | 98.1 | |
| M1 | 9153 | 4.0 | 244 | 1.9 | |
| pTNM | <0.0001 | ||||
| I | 126639 | 55.0 | 7657 | 60.5 | |
| II | 72481 | 31.5 | 3983 | 31.5 | |
| III | 21961 | 9.5 | 766 | 6.1 | |
| IV | 9132 | 4.0 | 244 | 1.9 | |
| Subtype | <0.0001 | ||||
| LuminalA | 162693 | 70.7 | 9578 | 75.7 | |
| LuminalB | 26501 | 11.5 | 685 | 5.4 | |
| HER2 | 11735 | 5.1 | 338 | 2.7 | |
| TN | 29284 | 12.7 | 2049 | 16.2 | |
| Surgery | <0.0001 | ||||
| Yes | 217567 | 94.5 | 12200 | 96.4 | |
| No | 12646 | 5.5 | 450 | 3.6 | |
| Radiotherapy | <0.0001 | ||||
| Yes | 116589 | 50.6 | 6085 | 48.1 | |
| No/Unknown | 113624 | 49.4 | 6565 | 51.9 | |
| Chemotherapy | <0.0001 | ||||
| Yes | 96005 | 41.7 | 3408 | 26.9 | |
| No/Unknown | 134208 | 58.3 | 9242 | 73.1 | |
Table 2.
Univariate COX regression analysis of the prognostic factors of special breast cancer.
| Variables | Overall survival |
|
|---|---|---|
| HR (95%CI) | P value | |
| Age | <0.0001 | |
| ≤40 years | Reference | |
| 40–65 years | 1.40 (0.98–2.00) | 0.061 |
| ≥65 years | 3.62 (2.56–5.14) | <0.0001 |
| Race | <0.0001 | |
| White | Reference | |
| Black | 1.29 (1.12–1.49) | <0.0001 |
| Other | 0.70 (0.56–1.87) | 0.001 |
| Grade | <0.0001 | |
| Grade1 | Reference | |
| Grade2 | 1.07 (0.93–1.22) | 0.364 |
| Grade3 | 1.70 (1.48–2.05) | <0.0001 |
| Grade4 | 1.51 (0.96–2.38) | 0.078 |
| T stage | <0.0001 | |
| T0 | Reference | |
| T1 | 8.94 (0.09–11.95) | 0.734 |
| T2 | 9.46 (0.40–11.68) | 0.731 |
| T3 | 14.90 (0.42–22.66) | 0.705 |
| T4 | 18.55 (0.73–33.11) | 0.693 |
| N stage | 0.026 | |
| N0 | Reference | |
| N1mi | 1.09 (0.90–1.33) | 0.375 |
| N1 | 1.10 (0.66–1.84) | 0.713 |
| N2 | 1.44 (1.03–2.00) | 0.033 |
| N3 | 1.28 (0.94–1.73) | 0.119 |
| M stage | <0.0001 | |
| M0 | Reference | |
| M1 | 5.08 (3.32–7.78) | <0.0001 |
| pTNM | <0.0001 | |
| I | Reference | |
| II | 1.68 (1.22–2.31) | 0.001 |
| III | 2.63 (1.71–4.04) | <0.0001 |
| IV | 4.18 (2.96–8.34) | <0.0001 |
| Subtype | <0.0001 | |
| LuminalA | Reference | |
| Luminal | 0.79 (0.60–1.03) | 0.078 |
| HER2 | 0.96 (0.67–1.37) | 0.812 |
| TN | 1.69 (1.46–1.97) | <0.0001 |
| Surgery | <0.0001 | |
| No | Reference | |
| Yes | 0.34 (0.28–0.41) | <0.0001 |
| Radiotherapy | <0.0001 | |
| No/Unknown | Reference | |
| Yes | 0.51 (0.46–0.58) | <0.0001 |
| Chemotherapy | <0.0001 | |
| No/Unknown | Reference | |
| Yes | 0.61 (0.52–0.70) | <0.0001 |
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rakha E., Pareja F. Seminars in cancer biology; 2020. New advances in molecular breast cancer pathology. [DOI] [PubMed] [Google Scholar]
- 2.Yerushalmi R., Hayes M., Gelmon K. Breast carcinoma--rare types: review of the literature. Ann Oncol : official journal of the European Society for Medical Oncology. 2009;20:1763–1770. doi: 10.1093/annonc/mdp245. [DOI] [PubMed] [Google Scholar]
- 3.Weigelt B., Horlings H., Kreike B., Hayes M., Hauptmann M., Wessels L. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 4.Yadav S., Yadav D., Zakalik D. Squamous cell carcinoma of the breast in the United States: incidence, demographics, tumor characteristics, and survival. Breast Canc Res Treat. 2017;164:201–208. doi: 10.1007/s10549-017-4251-3. [DOI] [PubMed] [Google Scholar]
- 5.Lin S., Liu C., Tao Z., Zhang J., Hu X. Clinicopathological characteristics and survival outcomes in breast carcinosarcoma: a SEER population-based study. Breast. 2020;49:157–164. doi: 10.1016/j.breast.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J., Wang X., Wang C. Invasive cystic hypersecretory carcinoma of the breast: a rare variant of breast cancer: a case report and review of the literature. BMC Canc. 2019;19:31. doi: 10.1186/s12885-018-5260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Work M., Andrulis I., John E., Hopper J., Liao Y., Zhang F. Risk factors for uncommon histologic subtypes of breast cancer using centralized pathology review in the Breast Cancer Family Registry. Breast Canc Res Treat. 2012;134:1209–1220. doi: 10.1007/s10549-012-2056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Malone K., Porter P., Weiss N., Tang M., Daling J. Reproductive and anthropometric factors in relation to the risk of lobular and ductal breast carcinoma among women 65-79 years of age. Int J Canc. 2003;107:647–651. doi: 10.1002/ijc.11465. [DOI] [PubMed] [Google Scholar]
- 9.Beaber E., Holt V., Malone K., Porter P., Daling J., Li C. Reproductive factors, age at maximum height, and risk of three histologic types of breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology. 2008;17:3427–3434. doi: 10.1158/1055-9965.EPI-08-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves G., Pirie K., Green J., Bull D., Beral V. Reproductive factors and specific histological types of breast cancer: prospective study and meta-analysis. Br J Canc. 2009;100:538–544. doi: 10.1038/sj.bjc.6604853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrazzo E., Frusone F., Milana F., Sagona A., Gatzemeier W., Barbieri E. Mucinous breast cancer: a narrative review of the literature and a retrospective tertiary single-centre analysis. Breast. 2020;49:87–92. doi: 10.1016/j.breast.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akrami M., Arasteh P., Mokhtari M., Tahmasebi S., Zangouri V., Hosseini S. Does metaplastic breast carcinoma demonstrate a different clinicopathological behavior in our region: the Shiraz Breast Cancer Registry. Breast J. 2019;25:157–159. doi: 10.1111/tbj.13183. [DOI] [PubMed] [Google Scholar]
- 13.Polamraju P., Haque W., Cao K., Verma V., Schwartz M., Klimberg V. Comparison of outcomes between metaplastic and triple-negative breast cancer patients. Breast. 2020;49:8–16. doi: 10.1016/j.breast.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz P., Bendrat K., Sonnenberg M., Trautmann C., Ott G., Heidemann E. Tubular breast cancer. A retrospective study. Anticancer Res. 2014;34:3647–3656. [PubMed] [Google Scholar]
- 15.Kulkarni N., Pezzi C., Greif J., Suzanne Klimberg V., Bailey L., Korourian S. Rare breast cancer: 933 adenoid cystic carcinomas from the National Cancer Data Base. Ann Surg Oncol. 2013;20:2236–2241. doi: 10.1245/s10434-013-2911-z. [DOI] [PubMed] [Google Scholar]
- 16.Page D. Special types of invasive breast cancer, with clinical implications. Am J Surg Pathol. 2003;27:832–835. doi: 10.1097/00000478-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Montagna E., Maisonneuve P., Rotmensz N., Cancello G., Iorfida M., Balduzzi A. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Canc. 2013;13:31–39. doi: 10.1016/j.clbc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Huober J., Gelber S., Goldhirsch A., Coates A., Viale G., Öhlschlegel C. Prognosis of medullary breast cancer: analysis of 13 international breast cancer study group (IBCSG) trials. Ann Oncol : official journal of the European Society for Medical Oncology. 2012;23:2843–2851. doi: 10.1093/annonc/mds105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D., Zhong C., Cheng Y., Zhu N., Tan Y., Zhu L. A competing nomogram to predict survival outcomes in invasive micropapillary breast cancer. J Canc. 2019;10:6801–6812. doi: 10.7150/jca.27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher B., Dignam J., Tan-Chiu E., Anderson S., Fisher E., Wittliff J. Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst. 2001;93:112–120. doi: 10.1093/jnci/93.2.112. [DOI] [PubMed] [Google Scholar]
- 21.Ong C., Campbell B., Thomas S., Greenup R., Plichta J., Rosenberger L. Metaplastic breast cancer treatment and outcomes in 2500 patients: a retrospective analysis of a national oncology database. Ann Surg Oncol. 2018;25:2249–2260. doi: 10.1245/s10434-018-6533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristofanilli M., Gonzalez-Angulo A., Sneige N., Kau S., Broglio K., Theriault R. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2005;23:41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama F., Horii R. Therapeutic strategies for breast cancer based on histological type. Breast Canc. 2009;16:168–172. doi: 10.1007/s12282-009-0126-8. [DOI] [PubMed] [Google Scholar]
- 24.Borri F., Granaglia A. Pathology of triple negative breast cancer. Semin Canc Biol. 2020 doi: 10.1016/j.semcancer.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Jackson H., Fischer J., Zanotelli V., Ali H., Mechera R., Soysal S. The single-cell pathology landscape of breast cancer. Nature. 2020;578:615–620. doi: 10.1038/s41586-019-1876-x. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Oprea-Ilies G., Krishnamurti U. New developments in breast cancer and their impact on daily practice in pathology. Arch Pathol Lab Med. 2017;141:490–498. doi: 10.5858/arpa.2016-0288-SA. [DOI] [PubMed] [Google Scholar]
- 27.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P. Breast cancer. Nature reviews Disease primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.