Significance
Genome fluidity is directly associated with the emergence and evolution of many bacterial pathogens. Horizontally acquired genetic elements, which harbor fitness traits, are open source for the bacterial evolution. The genome of Vibrio cholerae is equipped with multiple mobile genetic elements (MGEs), which are crucial for disease development and survival. The present study engineered the genome of a well characterized clinical V. cholerae strain N16961 and reported (i) stability of MGEs in cholera pathogen, (ii) interactions between the core genome and MGEs, and (iii) role of MGEs in the modulation of cellular proteome. The engineered strain could be a potential candidate for understanding evolution of cholera pathogen and development of new therapeutic interventions.
Keywords: genomic islands, mobile genetic elements, site-specific integration, proteome, cholera
Abstract
Bacterial species are hosts to horizontally acquired mobile genetic elements (MGEs), which encode virulence, toxin, antimicrobial resistance, and other metabolic functions. The bipartite genome of Vibrio cholerae harbors sporadic and conserved MGEs that contribute in the disease development and survival of the pathogens. For a comprehensive understanding of dynamics of MGEs in the bacterial genome, we engineered the genome of V. cholerae and examined in vitro and in vivo stability of genomic islands (GIs), integrative conjugative elements (ICEs), and prophages. Recombinant vectors carrying the integration module of these GIs, ICE and CTXΦ, helped us to understand the efficiency of integrations of MGEs in the V. cholerae chromosome. We have deleted more than 250 acquired genes from 6 different loci in the V. cholerae chromosome and showed contribution of CTX prophage in the essentiality of SOS response master regulator LexA, which is otherwise not essential for viability in other bacteria, including Escherichia coli. In addition, we observed that the core genome-encoded RecA helps CTXΦ to bypass V. cholerae immunity and allow it to replicate in the host bacterium in the presence of similar prophage in the chromosome. Finally, our proteomics analysis reveals the importance of MGEs in modulating the levels of cellular proteome. This study engineered the genome of V. cholerae to remove all of the GIs, ICEs, and prophages and revealed important interactions between core and acquired genomes.
Bacterial genomes are highly heterogeneous, ranging from 500 to 10,000 kb in size, composed of single or multiple circular or linear chromosomes (Chr) with or without extra chromosomal genetic elements (1). The size and content of the bacterial genome are widely influenced by its lifestyle and living environments. Comprehensive analysis of genome sequences has shown the presence of number of horizontally acquired mobile genetic elements (MGEs) in the genomes of free living and facultative pathogens that contribute to virulence (2), colonization (3), invasions (4), antimicrobial resistance (5), xenobiotic degradation (6), and metabolic processes (7). Genomic islands (GIs), the most significant MGEs in the bacterial genome, are typically 10- to 200-kb discrete DNA segments that have distinct base composition compared to the whole genome, are distributed sporadically, encode mobility attributes, and are often linked with tRNA genes and flanked by direct repeat sequences (8). GIs are widely distributed in the genomes of both pathogenic and nonpathogenic bacterial species, are crucial for genome plasticity, and play important roles in the adaptation and evolution by encoding functions that do not exist in the core genome (9). GIs integrate to the host chromosome by site-specific recombination, can excise to form circular intermediates, and move into the genome of other bacteria via horizontal gene transfer (HGT) (10, 11). HGT is not restricted within the bacterial kingdom; it has become evident that MGEs present in the bacterial genomes can also move to the plant and animal genomes via lateral gene transfer (12).
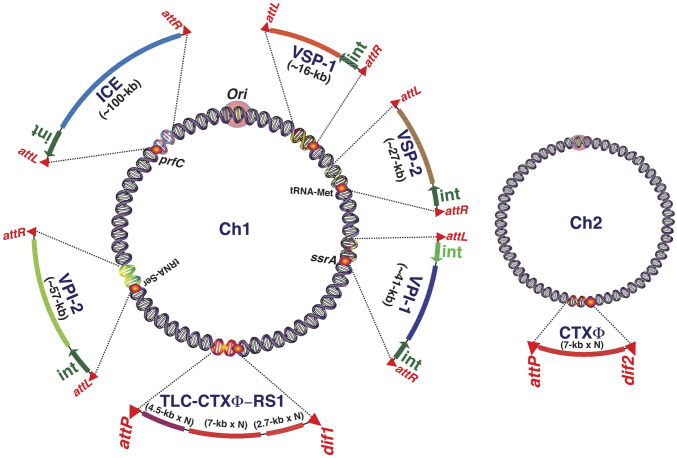
Vibrio cholerae, the Gram-negative bacterial pathogen is responsible for the severe acute diarrheal disease cholera, harbors two nonhomologous circular chromosomes, chromosome 1 (Chr1) and chromosome 2 (Chr2) (13). Both the chromosomes are fortified with multiple prophages and GIs (Fig. 1). Two key virulence factors of V. cholerae, a pilus colonization factor toxin-coregulated pilus (TCP) and a potent enterotoxin cholera toxin (CT), which helps the pathogen to colonize host intestinal epithelial cell surface and manifest the disease cholera, are part of the horizontally acquired Vibrio pathogenicity island-1 (VPI-1) and CTXΦ genome, respectively (2, 3). Genomic variability and clonal divergence of clinical and environmental V. cholerae isolates are mainly determined by the GIs (14–16). Following entry into host cytoplasm, GIs integrate into a specific site of chromosome using DNA recombinases (17, 18). The chromosome dimer resolution sites (dif1 and dif2) of V. cholerae harbored several prophages known as integrative mobile elements exploiting Xer recombination (IMEX). CTXΦ and other vibriophages like RS1Φ, TLCΦ, and VGJΦ are well-known IMEXs (18). Of the several GIs, the VPI-1 is ubiquitous in the genome of all of the epidemic-causing V. cholerae isolates. VPI-1 integrates site-specifically into the transfer-messenger (tm) RNA (ssrA) locus of Chr1 (19). Vibrio pathogenicity island-2 (VPI-2), the GI that encodes functions for scavenging (nanH), transport (dctPQM), and catabolism (nanA, nanE, nanK, and nagA) of sialic acid, integrates into the tRNA-serine locus of Chr1 (20). The other GI, Vibrio seventh pandemic island-1 (VSP-1), encodes dinucleotide cyclase, the enzyme essential for producing intracellular signaling molecule cAMP-GMP, and integrates into the site between VC0174 and VC0186 in the strain N16961 (20). Vibrio seventh pandemic island-2 (VSP-2) is comparatively less stable and encodes RNase H1, DNA repair protein, methyl-accepting chemotaxis proteins, and type IV pilus and integrates into tRNA-methionine locus (13). Both the islands, VSP-1 and VSP-2, are consistently found in the genomes of seventh pandemic V. cholerae O1 El Tor and O139 strains, but are majorly absent in the genome of the first six pandemic strains recorded between 1817 and 1923 (21). SXT, a self-transmissible integrative conjugative element (ICE) that carries multiple antibiotic-resistance genes and confers resistance to sulfamethoxazole, trimethoprim, and streptomycin, was initially identified at the prfC locus in the genome of O139 V. cholerae (22). Among prophages integrated at the dif loci, CTX prophage is ubiquitously present in all of the epidemic strains. The toxin-linked cryptic phage (TLCΦ) and satellite phage RS1 are sporadic even in the clinical isolates (23). Except CTXΦ and RS1Φ, integrations of all of the GIs and SXT present in V. cholerae are reversible (20, 24). Although the excision frequency of GIs and SXT in V. cholerae is known, no study has been conducted to determine the frequency of loss of GIs under in vitro (test tube experiment) and in vivo (animal model) conditions. No effort so far has been made to construct derivatives of a clinical V. cholerae strain devoid of GIs, SXT, and/or prophages to understand the importance of each of these genetic elements in the adaptation and evolution of the cholera pathogen. More importantly, no study has been conducted to understand the importance of GIs in the essentiality of gene functions encoded by the core genome. Until now, we also have limited knowledge regarding how GIs in V. cholerae modulate the level of different cellular proteins.
Fig. 1.
Distribution of genomic islands (GIs), prophages, and integrative conjugative element (ICE) in the genome of V. cholerae clinical isolates. Each of the genetic elements except prophages is linked with different mobility functions like integrase and repeat sequences. GC contents of the acquired genetic elements are also distinct compared to the core genome. VPI-1, Vibrio pathogenicity island-1; VPI-2, Vibrio pathogenicity island-2; VSP-1, Vibrio seventh pandemic island-1; VSP-2, Vibrio seventh pandemic island-2; int, integrase.
In this study, we have engineered the genome of V. cholerae reference strain N16961 and its derivatives to monitor in vitro and in vivo stability of the GIs. We selectively or simultaneously deleted all of the GIs and prophages from the genome of N16961 and studied their integration and excision efficiency in the presence and absence of other GIs. We have also demonstrated how CTX prophage determines the essentiality of SOS master regulator LexA in V. cholerae, which is otherwise not essential in other bacterial species, including Escherichia coli. We have identified how chromosomally encoded function helps CTXΦ to bypass host immunity and replicate in the bacterial cell in the presence of similar prophages in the V. cholerae genome. Finally, we have analyzed whole-cell proteome of V. cholerae in the presence and absence of each of the GIs to unveil their contribution in modulating cellular metabolic processes. The knowledge generated from the present study will be of immense importance to understand the dynamics and importance of GIs in the adaptation and evolution of cholera pathogen and could be translated to develop V. cholerae-specific therapeutics to control cholera and other enteric diseases.
Results
Development of Reporter Strains to Monitor the Frequency of Loss of GIs in V. cholerae.
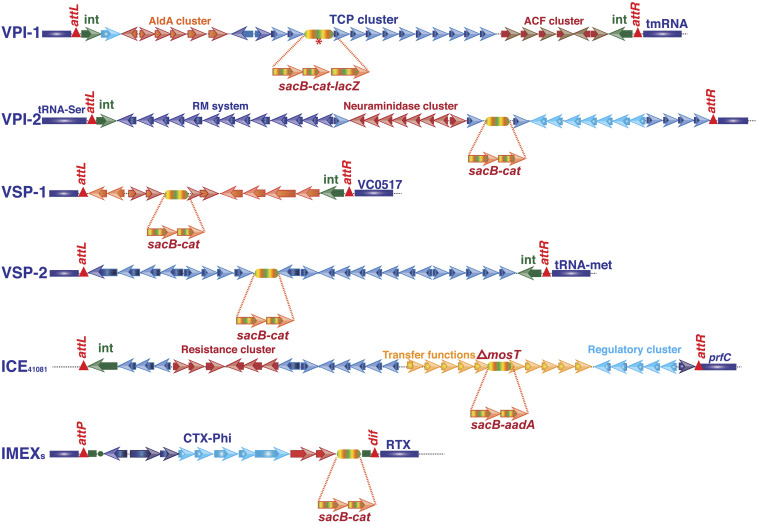
Previous works that studied the excision phenomenon of GIs in V. cholerae adopted the PCR assay (19, 20). However, this assay is not proficient in monitoring the loss of GIs and unable to isolate bacterial cells from a heterogeneous population that have lost the GI from their genome. In this study, we engineered the genome of different V. cholerae strains to monitor the frequency of loss of different GIs under in vitro and in vivo growth conditions. Each GI (VPI-1, VPI-2, VSP-1, and VSP-2) and the prophages were tagged with multiple selectable (cat), counter-selectable (sacB), and chromogenic (lacZ) alleles in the clinical V. cholerae strain N16961 and its derivatives (Fig. 2). Since N16961 genome is devoid of SXT, we tagged this element with similar selectable and counter-selectable marker in a V. cholerae O1 El Tor clinical isolate 41081 (Table 1). The allele sacB-cat or sacB-cat-lacZ or sacB-aadA was introduced into the GIs or SXT by sequential allelic exchange method using derivatives of suicide vector pKAS32 harboring selectable marker bla and counter-selectable marker rpsL in the plasmid backbone (SI Appendix, Table S1). First cross-over intermediate strain carrying vector backbone (pKAS32) and reporter allele (sacB-cat or sacB-cat-lacZ or sacB-aadA) was confirmed by antibiotic resistance (CamR or SpcR and AmpR) and susceptibility to streptomycin and sucrose. The desired reporter strains SB30 (VPI-1::sacB-cat), SB26 (VPI-2::sacB-cat), SB23 (VSP-1::sacB-cat), DD1(VSP-2::sacB-cat), BB2 (CTXΦ::sacB-cat), and JV3 (SXT::sacB-aadA) were confirmed by antibiotic resistance, sucrose susceptibility, colony color, PCR assay, and DNA sequencing (Fig. 3). Since integration of CTXΦ and RS1Φ are irreversible, the TLC-CTX-RS1 prophage array in the genome of N16961 was first replaced by spectinomycin resistance cassette by allelic exchange method. Subsequently, CTXΦ::sacB-cat element was introduced in the lacZ-dif1 allele by site-specific recombination to develop the recombinant bacterial strain BB2 (Table 1). Antibiotic resistance phenotype, PCR assay, and blue-white screening confirmed the desired strain BB2.
Fig. 2.
Schematic representation of the genetic organization of the genomic islands (GIs), integrative conjugative element (ICE), and prophages and location of reporter genes in the tagged genome. Selectable (cat), counter-selectable (sacB), and chromogenic (lacZ) alleles were introduced in the V. cholerae genome, adopting an allelic exchange method. Asterisk indicates that Vibrio pathogenicity island-1 (VPI-1) was tagged with sacB-cat as well as sacB-cat-lacZ alleles.
Table 1.
Relevant genotype and phenotype of wild-type and genetically modified Vibrio cholerae strains used in the study
| Strain | Genotype and phenotype | Reference |
| N16961 | Wild type, O1 El Tor, Stpr | 13 |
| O395 | Wild type, O1 classical, Stpr | 5 |
| VCE232 | Wild type, O4 environmental | 5 |
| 41081 | O1 El Tor, SXT+ve, NAr, Funr, Plmr | MVIDH, India |
| MO10 | Wild type, O139 serogroup, Stpr, SXT+ | 22 |
| BS1 | N16961ΔTLCΦ-CTXΦ-dif1::lacZ-dif1Δdif2, Stpr, Spcr | 25 |
| BS11 | BS1ΔrecA, Stpr, Spcr | 25 |
| AP7 | N16961ΔrecA, Stpr | This study |
| AP8 | VCE232ΔrecA::aph, Kanr | This study |
| AP10 | O395ΔrecA::aph, Stpr, Kanr | This study |
| SB23 | N16961vsp1::sacB-cat, Stpr, Camr, Sucs | This study |
| SB25 | N16961Δvsp1, Stpr | This study |
| DD1 | N16961vsp2::sacB-cat, Stpr, Camr | This study |
| DD2 | N16961Δvsp2, Stpr | This study |
| SB30 | N16961vpi1::sacB-cat, Stpr, Camr, Sucs | This study |
| SB31 | N16961Δvpi1, Stpr | This study |
| SB26 | N16961vpi2::sacB-cat, Stpr, Camr, Sucs | This study |
| SB27 | N16961Δvpi2, Stpr | This study |
| SB32 | N16961Δvsp1 vsp2::sacB-cat, Stpr, Camr, Sucs | This study |
| SB33 | N16961Δvsp1 Δvsp2, Stpr | This study |
| SB42 | N16961Δvpi2, ∆lacZ, Stpr | This study |
| SB43 | N16961Δvpi1, ∆lacZ, Stpr | This study |
| SB44 | N16961Δvsp1 Δvsp2 ∆lacZ, Stpr | This study |
| SB51 | N16961Δvpi2∆lacZvpi-1::sacB-cat-lacZ, Stpr, Camr, Sucs | This study |
| SB52 | N16961 ∆vpi-1 ∆vpi-2 ∆lacZ, Stpr | This study |
| SB45 | N16961Δvsp1Δvsp2∆lacZvpi1::sacB-cat-lacZ, Stpr, Camr, Sucs | This study |
| SB47 | N16961Δvsp1Δvsp2 ∆vpi1 ∆lacZ, Stpr | This study |
| SB46 | N16961Δvsp1Δvsp2∆lacZvpi-2::sacB-cat-lacZ, Stpr, Camr, Sucs | This study |
| SB48 | N16961 ∆vsp1 ∆vsp2∆ vpi-2 ∆lacZ, Stpr | This study |
| SB49 | N16961Δvsp1Δvsp2∆vpi1∆lacZvpi2::sacB-cat-lacZ, Stpr, Camr, Sucs | This study |
| SB50 | N16961Δvsp1Δvsp2 ∆vpi1∆vpi2 ∆lacZ, Stpr | This study |
| SB53 | N16961Δvpi1Δvpi2Δvsp1Δvsp2ΔlacZΔTLCΦ-RS1-CTXΦ-dif1::aadA, Stpr, Specr | This study |
| SB58 | N16961Δvsp1::pBD60, Stpr, Camr | This study |
| SB59 | N16961Δvsp2:: pBD60, Stpr, Camr | This study |
| SB56 | N16961::cat, Stpr, Camr | This study |
| SB60 | N16961 Δvsp1 Δvsp2::cat, Stpr, Camr | This study |
| SB62 | O395::sh ble, Stpr, Zeor | This study |
| SB63 | N16961 ∆vpi-1 ∆vpi-2::pBD60, Stpr, Camr | This study |
| BB1 | N16961Δvpi1Δvpi2Δvsp1Δvsp2ΔlacZΔTLCΦ-RS1-CTXΦ-dif1::lacZ-dif1 | This study |
| BB2 | N16961Δvpi1Δvpi2Δvsp1Δvsp2ΔlacZΔTLCΦ-RS1-CTXΦ-dif1::lacZ-dif1- CTXΦ-sacB-cat | This study |
| BS20 | BS1ΔlexA, Stpr, Spcr, Kanr | 26 |
| BD14 | BS20 lexA(Ind −) | 26 |
| JV2 | 41081SXT:ΔstrAB, Stpr NAr, Funr, Plmr | This study |
| JV3 | 41081SXT:ΔstrAB::sacB-aadA, Stpr NAr, Funr, Plmr, Specr | This study |
| JV4 | 41081ΔSXT, Stpr, NAr, Funr, Plmr | This study |
| JV5 | BS1ΔlexAΔrecA, Stpr, Spcr, Kanr | This study |
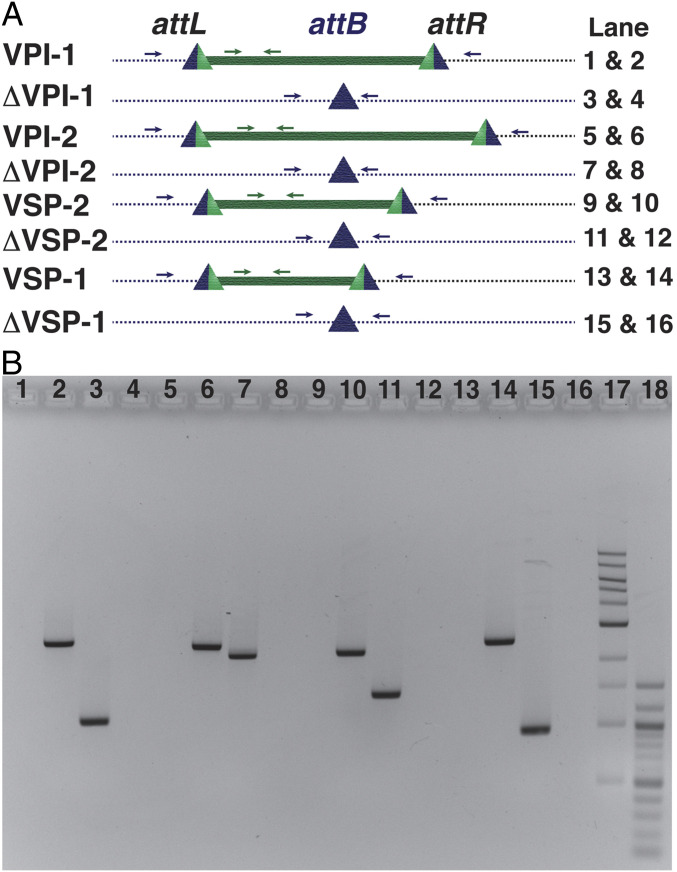
Fig. 3.
Genetic organization of chromosomal integration sites (attB) before and after excision of all of four genomic islands from the genome of N16961. (A) attL and attR sites were formed due to site-specific recombination between genomic island attachment site (attP) and chromosomal attachment site (attB). Lane numbers 17 and 18 contained 1-kb and 100-bp DNA ladders, respectively. (B) Polymerase chain reaction (PCR) analysis of N16961 and its derivatives in the presence and absence of genomic islands (GIs). Amplified PCR products were resolved in an agarose gel. Lane number and corresponding amplicon are mentioned in A. Blue arrows indicate primers that bind to the upstream and downstream of GI, and green arrows indicate primers that bind to the GI.
Determination of In Vitro and In Vivo Frequency of Loss of GIs, Integrative Conjugative Element, and CTX Prophage.
To monitor the stability of all of the four GIs, CTX prophage, and SXT element under in vitro growth conditions, the reporter strains engineered in this study (SB30, SB26, SB23, DD1, BB2, and JV3) were grown in nutritionally rich medium (Luria–Bertani broth; LB) for 12 h at 37 °C. Cultures of the reporter strains were plated onto Luria agar (LA) plates supplemented with sucrose (15%) and streptomycin (100 μg/mL). Colonies grown on the selection plates were monitored for their sensitivity to chloramphenicol or spectinomycin depending on the resistance cassette used to tag the GIs, CTX prophage, and SXT element. The excised colonies were further subjected to PCR confirmation by amplifying a chromosomal attachment region of each of the GIs (Fig. 3). Attachment sequences (attB) for each GI and SXT were confirmed by DNA sequencing. Total number of bacterial cells in an overnight-grown bacterial culture was determined by plating the same culture on an LA plate supplemented with streptomycin. We observed different frequency of loss for different GIs (Table 2). Highest frequency of loss under in vitro growth conditions was observed for the VPI-1, while no loss was detected for CTX prophage (Table 2).
Table 2.
In vitro and in vivo loss frequency of different genomic islands
| Genomic Islands/ICE/Prophages | Bacterial strain | Loss frequency, mean ± SEM | |
| In vitro | In vivo* | ||
| VPI-1::sacB-cat | SB30 | 3.26 × 10−4 (±0.00007) | 1.03 × 10−9 |
| VPI-2::sacB-cat | SB26 | 1.40 × 10−4 (±0.00005) | 1.20 × 10−8 |
| VSP-1::sacB-cat | SB23 | 4.21 × 10−5 (±0.00001) | 2.11 × 10−9 |
| VSP-2::sacB-cat | DD1 | 1.41 × 10−4 (±0.00001) | 1.08 × 10−9 |
| SXT::sacB-aadA | JV3 | 1.53 × 10−4 (±0.00005) | ND |
| CTXϕ:: sacB-cat | BS37 | <10−10 | <10−10 |
In vivo rabbit ileal loop experiment was done in duplicate set.
To monitor the frequencies of loss of different GIs under in vivo growth conditions, we adopted the ligated rabbit ileal loop experimental model. Reporter strains harboring flagged GIs in their genome were inoculated into a ligated ileal loop, and the frequencies of loss of each GI were measured after 12 h by plating cells onto selection plates supplemented with sucrose (15%) and streptomycin (100 μg/mL). Like the in vitro experiment, total numbers of bacterial cells in the ligated ileal loop fluid were quantified by plating the fluid on the LA plate supplemented with streptomycin. The highest frequency of loss was observed for the VPI-2 compared to any other GIs (Table 2). No loss was observed for the CTX prophage (Table 2). Reporter strains carrying deletion of GIs isolated from ligated ileal loops were confirmed by restreaking the colonies onto the Vibrio-specific selective medium TCBS and LA supplemented with streptomycin (resistant) or chloramphenicol (sensitive). Interestingly, we observed that the frequency of loss of all of the GIs from the V. cholerae genome was very high under in vitro condition compared to in vivo-grown cells (Table 2).
V. cholerae Strains Devoid of Any Genomic Islands and Prophages Are Viable and Can Multiply both in Rich and Minimal Media.
We have done extensive genome engineering of N16961 to remove all its GIs and prophages from its genome. First, we tagged the GIs with sacB-cat or sacB-cat-lacZ and then isolated V. cholerae cells that lost the tagged GI by plating the cells on selection medium supplemented with sucrose (Table 1). To isolate strains lacking different combinations of MGE like ΔVPI-1 (SB31), ΔVPI-2 (SB27), ΔVSP-1 (SB25), ΔVSP-2 (DD2), ΔTLCΦ-CTXΦ-RS1Φ (BS1), ΔVPI-1ΔVPI-2 (SB52), ΔVSP-1ΔVSP-2 (SB44), ΔVSP-1ΔVSP-2ΔVPI-1 (SB47), ΔVSP-1ΔVSP-2ΔVPI-2 (SB48), ΔVSP-1ΔVSP-2ΔVPI-1ΔVPI-2 (SB50), and ΔVSP-1ΔVSP-2ΔVPI-1ΔVPI-2ΔTLCΦ-CTXΦ-RS1Φ::lacZ-dif1 (BB1) strain (Table 1), we utilized conditional toxicity of sacB to the host bacteria.
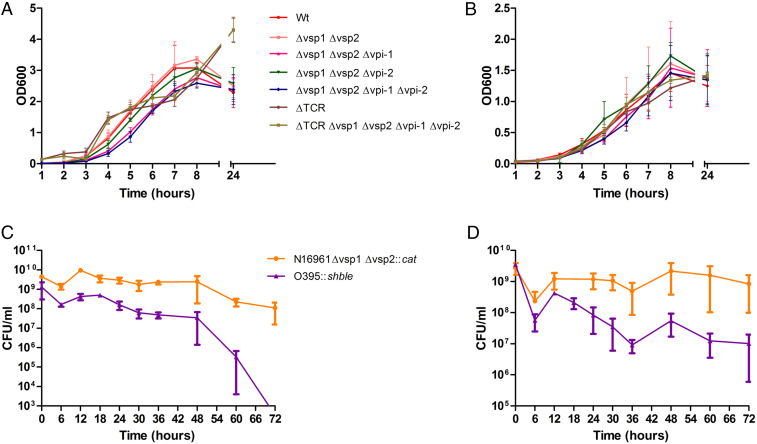
We measured the growth rate of N16961 and its derivatives with or without GIs and prophages in a nutritionally rich (LB) or minimal (M9) medium (nutritionally poor). V. cholerae cells devoid of any GIs and prophages were able to grow in both media (Fig. 4 A and B). However, growth of SB50 (Δvsp-1Δvsp-2∆vpi-1∆vpi-2) was significantly decreased (P < 0.01) during 5, 6, and 7 h of growth when compared with its parent strain N16961 (Fig. 4A). We also observed significant reduction (P < 0.05) in growth of the engineered strain SB47 (Δvsp-1Δvsp-2 ∆vpi-1) during 5, 6, and 7 h of incubation. Interestingly, BB1, the derivative of SB50 carrying dif1 in the Chr1 but devoid of all of the four GIs and prophages in the chromosome, showed a similar growth profile as the WT strain N16961 (Fig. 4 A and B). We have not observed any major changes in growth profile either in M9 or LB between N16961 and BB1 strains (Fig. 4 A and B). It has previously been reported that the V. cholerae O1 El Tor strain N16961 has a significant growth advantage over the classical biotype strain O395 in stationary-phase culture when both strains were cocultured in LB under optimal growth conditions (27). The major difference between El Tor and classical biotypes with respect to GI is the absence of both VSP-1 and VSP-2 in the latter. We investigated whether O1 classical strain O395 can compete with O1 El Tor strain SB60 devoid of VSP-1 and VSP-2 islands. To differentiate the SB60 and O395 strains in the coculture, we introduced two different resistance gene cassettes, cat and sh ble, in the dif1 site of El Tor and classical strains, respectively. The mixed culture was grown in LB or M9 medium under optimal growth conditions, and the number of bacterial cells was measured at different time points (Fig. 4 C and D). Our finding indicates that O395 classical biotype can compete with the VSP-1 and VSP-2–deleted El Tor biotype when they were cocultured in M9 medium. However, the VSP-1– and VSP-2–deleted El Tor strains can outcompete O395 in the LB medium. This finding indicates that the acquisition of VSP-1 and VSP-2 in the genome of V. cholerae provided certain metabolic traits that possibly helped the seventh pandemic El Tor biotype to outcompete the sixth pandemic-causing classical biotype. However, additional role of oriC functions might also help the El biotype to outcompete the classical biotype in nutrient-rich environment even in the absence of such metabolic traits (28). It has previously been reported that the oriC of the classical biotype carries a single base mutation (T→G) in its conserved AT-rich 13-mer R repeat region compared to the oriC of El Tor biotype (28). It has been demonstrated that the minichromosome copy number and its transformational efficiency are substantially reduced due to substitution mutation in the oriC of the classical biotype (28). This might be one of the reasons for less competency of classical biotype while coculturing with the El Tor biotype in the nutrient-rich medium.
Fig. 4.
Growth curve of wild type and genetically engineered V. cholerae strains in rich (LB) and nutritionally defined culture medium (M9). The El Tor strain N16961 and its derivatives were grown individually in monocultures in LB (A) and M9 (B), and the growth of the bacterial cells was monitored by measuring the optical density of the culture at 600 nm (OD600) in a spectrophotometer at different time points. Growth competition between ΔVSP-1ΔVSP-2 N16961 and O395 was also done in rich (C) and minimal medium (D) in a cocultured medium by inoculating similar number of cells together (1:1). CFU count of each strain was determined by plating the culture in rich medium (LA) supplemented with specific antibiotic. TCR = TLCΦ-CTXΦ-RS1Φ.
Integration Efficiency of Different GIs, SXT, and CTXΦ in the Presence and Absence of Acquired Genome.
Studies on integration efficiency of GIs are complicated because of their large size and nonreplicative nature of their excised circular form. Therefore, to avoid this complexity, we amplified the integrative module of GIs, SXT, and CTXΦ from its excised circular form, which includes attachment site (attP) with or without recombinases (Fig. 5). The integration modules were cloned in a conditionally replicative conjugative vector (SI Appendix, Table S1). Recombinant vector containing integration module of GIs, SXT, or CTXΦ was introduced by conjugation into V. cholerae strains carrying native chromosomal attachment site (attB) with or without other GIs. Integration of integrative module in the chromosome of V. cholerae was determined based on the antibiotic (chloramphenicol or zeocin) resistance profile of recipient cells. Integration of a GI at specific chromosomal locus was further confirmed by PCR assay and blue-white colorimetric assay. Conjugation of different recombinant vectors carrying nonreplicative integration module of different GIs, SXT, and CTXΦ gave rise to different numbers of transconjugants (Table 3). Among nonreplicative GIs and SXT, the highest number of transconjugants was obtained with the recombinant vector carrying the integration module of SXT (Table 3). Absence of GIs and prophages increased the integration efficiency of SXT. A minimal number of transconjugants was obtained with recombinant vector carrying the integration module of VSP-1 (Table 3). However, when compared with the integration efficiency of GIs and SXT with the replicative and integrative module of CTXΦ, the highest number of transconjugants was obtained with the phage genome (Table 3). To differentiate between V. cholerae cells carrying the integrative or replicative form of the RS2 module of CTXΦ, we fused the chromosomal attachment site dif1 in the coding region of the lacZ gene amplified from E. coli. The lacZ-dif1 allele produced functional β-galactosidase and generated blue colonies in the presence of chromogenic substance X-gal in the selection plate. We then deleted endogenous lacZ gene from V. cholerae cells, and the TLC-CTX-RS1 prophage array was replaced with lacZ-dif1 allele (SI Appendix, Fig. S3). The reporter strain carrying the replicative RS2 module of CTXΦ turned blue in the presence of X-gal on the selection plate, while the strain carrying integrative RS2 in the dif1 in the chromosomal lacZ-dif1 allele produced white colonies due to inactivation of lacZ gene and abolishing β-galactosidase production (SI Appendix, Fig. S3). The number of transconjugants with same recombinant vector in the presence and absence of other GIs in the host cells showed different outcomes (Table 3). The integration efficiencies of CTXΦ and its derivatives were measured in the absence of TLCΦ and other prophages. The integration module of SXT and VPI-1 can integrate almost with equal efficiency in the presence and absence of other GIs in the V. cholerae genome. Integration efficiency of VPI-2 was reduced in the absence of other GIs. Interestingly, integration efficiency of VSP-1 and VSP-2 was increased in the absence of GIs (Table 3).
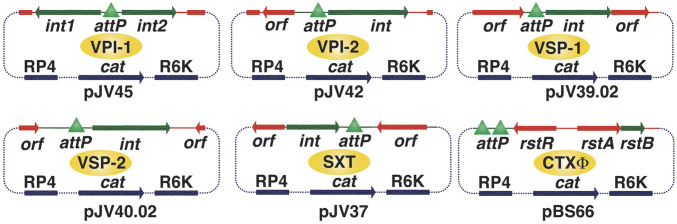
Fig. 5.
Genetic tools engineered to measure the integration efficiency of different GIs, ICE (SXT), and CTXΦ. The integration module of all of the elements was amplified from their excised circular form, which include attachment site (attP) with or without tyrosine recombinases. The original vector (pSW23T) was unable to replicate in V. cholerae due to its conditional ori (R6K) function.
Table 3.
Integration frequencies of different genomic islands, CTXΦ and SXT element in the presence and absence of acquired genetic elements
| Genetic element | Bacterial strain | Integration frequency (SD) |
| SXT | N16961 | 1.56 × 10−1 (0.0814) |
| BB1 | 3.13 × 10−1 (0.1808) | |
| VPI-1 | SB31 | 1.3 × 10−3 (0.0005) |
| BB1 | 1.3 × 10−3 (0.0005) | |
| VPI-2 | SB27 | 3.6 × 10−3 (0.0020) |
| BB1 | 2.6 × 10−4 (0.0002) | |
| VSP-I | SB25 | 9.3 × 10−6 (1.1547E-06) |
| BB1 | 5 × 10−5 (1.90351E-05) | |
| VSP-II | DD2 | 8.9 × 10−3 (0.0077) |
| BB1 | 1.08 × 10−2 (0.0060) | |
| CTXΦ | BS1 | 2.2 × 10−3 (0.0012) |
| BB1 | 2.9 × 10−2 (0.046) |
We have also measured the integration efficiency of the nonreplicative RS2 module of CTXΦ in the dif1 site of Chr1 in V. cholerae reporter strain BS1 and BB1. We observed similar integration efficiency of nonreplicative RS2 module in the presence and absence of different GIs. The integration event of nonreplicative RS2 module was site-specific (≥99.3%) and irreversible, both in the presence and absence of other GIs (Table 3).
Cross-Talk between Horizontally Acquired Genetic Elements and Core Genome.
Cross-talk between GIs and prophages in V. cholerae is a well-known phenomenon (29, 30). However, the influence of acquired functions in the functionality of genes present in the core genome is not known. Understanding the molecular basis of essentiality of LexA in V. cholerae is of special interest because of its contribution in the biology of horizontally acquired MGEs, especially in the replication, integration, and production of extrachromosomal CTXΦ from prophages and excision and dissemination of SXT. We resolved the mystery of LexA essentiality in V. cholerae by deleting the lexA gene from the chromosome in the engineered V. cholerae strain BB1 deleted with GIs and prophages (Table 1). We utilized a recombinant vector pAP17 containing kanamycin resistance cassette (aph3) flanked by the upstream and downstream regions of lexA to delete the lexA gene by allelic exchange method. To identify the key genetic element responsible for essentiality of the lexA gene in V. cholerae genome, we selected single or multiple GI or prophage-deleted strains (Table 1) followed by an attempt to delete lexA in each of these engineered strains. Surprisingly, such screening allowed us to discover that the lexA gene is not essential in TLC-CTX-RS1 prophage-deleted V. cholerae cells, but it is still essential in single or multiple GI-negative strain (Table 4). To determine whether TLC-CTX-RS1 array or any particular one of these phages is responsible for the essentiality of lexA, each of them was sequentially deleted, followed by an attempt to delete lexA in those engineered strains. Interestingly, it was identified that lexA is not essential only in CTX prophage-deleted cells (Table 4). CTX prophage is composed of RS2 and core region. While RS2 carries rstR-rstA-rstB genes, core segment carries seven different genes including CT-coding ctxAB genes. On the other hand, the RS1Φ is identical to the RS2 except it carries an extra gene, called rstC. The rstA, rstB, and rstR gene products are essential for phage replication, integration, and transcription regulation, respectively. Since the expression of rstR, rstA, and rstB is under the control of LexA from PrstA promoter (31), we therefore analyzed the function of each of these proteins to understand their contribution, if any, in the essentiality of lexA gene in V. cholerae. RstB is a single-stranded DNA binding protein, which helps in integration of CTXΦ and the RS1Φ at the chromosomal dif site. RstR is a transcriptional regulator, which represses the transcription of rstA, rstB, and rstC from the PrstA promoter (SI Appendix, Fig. S3). RstA is a Rep protein, which introduces DNA nicks in the orictx sequence and helps CTXΦ to generate extrachromosomal-phage genome from the prophage. We hypothesized that, in the absence of LexA-mediated repression, there could be excess production of RstA from the CTX and RS1 prophages, which in turn generates frequent nicks in the CTX and RS1 prophage DNA, leading to lethal chromosomal breaks. To test this hypothesis, we tried to integrate CTXΦ or CTXΦ-RS1Φ in tandem in V. cholerae ΔlexA ΔCTXΔRS1 mutant but failed to integrate either multiple copies of CTXΦs or CTXΦ-RS1Φ in tandem. In sharp contrast, integration of multiple copies of CTXΦs or CTXΦ-RS1Φ in tandem in the dif1 site of the V. cholerae ΔCTXΔRS1 but lexA-positive strain was easily obtained (SI Appendix, Fig. S1). Since RecA is intricately associated with LexA stability and thus prophage gene expression, we engineered a ΔlexAΔrecA double-mutant strain JV5 and investigated the CTX prophage number in the dif1 site after introducing the phage by conjugation. Like ΔlexA strain, the single-copy CTX prophage was also dominant in ΔlexAΔrecA double mutant (SI Appendix, Fig. S1). To provide further evidence that the multiple copy of functional rstA is associated with the essentiality of LexA in V. cholerae, we performed site-directed mutagenesis to replace the catalytic tyrosine residue of the RIYNK motif of RstA protein with phenylalanine residue. Due to this point mutation, the engineered CTXΦ encoding catalytically inactive RstAY237F mutant protein was unable to initiate rolling circle replication in V. cholerae. However, a single copy of CTXΦ carrying nonfunctional RstA was stably lysogenized both in the presence and absence of LexA. These results thus strongly suggest that, when functional rstA gene is present in V. cholerae genome, deletion of lexA is lethal to the bacterium. Monitoring of phenotypes (antibiotic resistance) as well as genotypes (PCR assay and sequencing) confirmed the authenticity of these V. cholerae mutants.
Table 4.
Essentiality of lexA for the viability of Vibrio cholerae in the presence and absence of genomic islands and prophages
| Strain | Genotype | Frequency of deletion of lexA | lexA essentiality |
| N16961 | Wild type | <1.0 × 10−10 | Yes |
| SB31 | ΔVPI-1 | <1.14 × 10−9 | Yes |
| SB27 | ΔVPI-2 | <1.03 × 10−9 | Yes |
| SB25 | ΔVSP-1 | <2.91 × 10−9 | Yes |
| DD2 | ΔVSP-2 | <2.1 × 10−9 | Yes |
| SB50 | ΔVPI-1ΔVPI-2ΔVSP-1ΔVSP-2 | <4.0 × 10−9 | Yes |
| B33 | ΔTLCΔRS1 | <3.92 × 10−10 | Yes |
| SB53 | ΔVPI-1ΔVPI-2ΔVSP-1ΔVSP-2 ΔTLCΔCTXΔRS1 | 4.93 × 10−7 | No |
| BS1 | ΔTLCΔCTXΔRS1 | 2.59 × 10−5 | No |
Frequency of lexA deletion was measured from at least two independent experiments.
Core Genome-Encoded RecA Helps CTXΦ to Overcome Host Immunity and Reinfect V. cholerae.
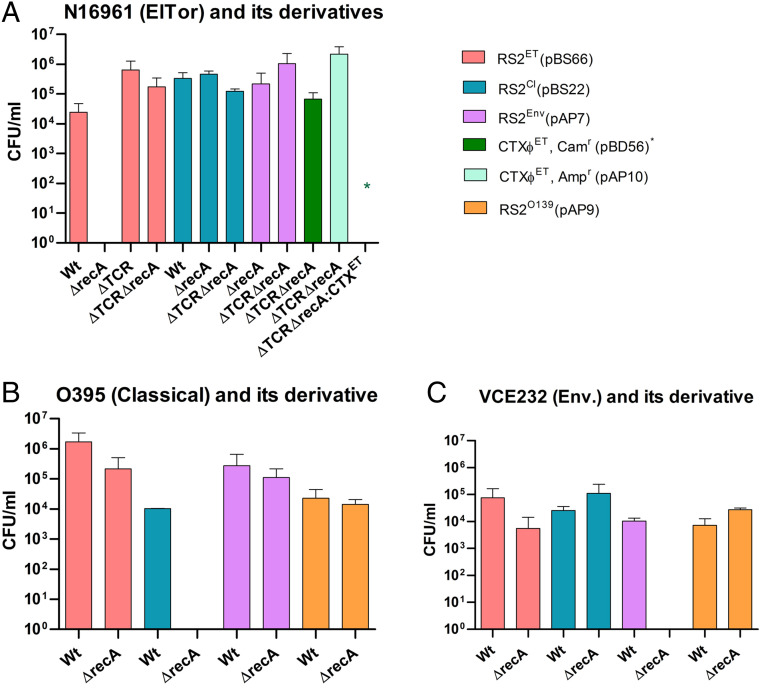
Other than phage-encoded RstA, chromosomally encoded UvrD helicase and LexA also play an important role in CTXΦ replication (30). It was reported that RstR, the CTXΦ encoded transcriptional factor, binds to the rstA promoter (PrstA) and represses its expression from the prophage genome (31). However, complete repression occurs by cooperative interaction with LexA, the master regulator of bacterial SOS response. The importance of RecA, the second crucial protein for the bacterial SOS response, in the replication of CTXΦ in V. cholerae has not been explored. We measured the replication efficiency of El Tor, classical, and environmental types of CTXΦs in the presence and absence of RecA in different V. cholerae strains (Fig. 6). The genome of N16961 harbors a CTXΦET prophage in its large chromosome. We introduced the replication and integration module of CTXΦET (RS2ET) and CTXΦCl (RS2Cl) into RecA-positive N16961 and isolated transconjugants on a selection plate supplemented with chloramphenicol. We obtained 2.44 × 104 and 3.32 × 105 transconjugants carrying RS2ET (pBS66) and RS2Cl (pBS22), respectively (Fig. 6). We had also introduced complete CTXΦET, RS2ET, RS2Cl, RS2Env, and RS1Φ into a reporter V. cholerae strain BS1 lacking the CTXΦ prophage in its genome, which has a functional RecA. It is noteworthy to mention that similar numbers of transconjugants were obtained for each of the phages introduced (Fig. 6). When we introduced each of these phages in the BS1 derivative harboring CTXΦET prophage at the dif1 loci, here also we obtained a similar number of transconjugants carrying replicative and integrative phage genomes or RS modules. The results indicate that reinfection by CTXΦ is possible even in the presence of biotype-specific rstR and functional recA genes in the host genome (Fig. 6).
Fig. 6.
Analysis of the replication efficiency of different CTXΦ in the presence and absence of RecA function. The bars represent numbers of CFUs carrying replicative module of CTXΦ. Replicative modules were transferred to the host bacteria by 3 h conjugation. Transconjugants were selected based on the antibiotic-resistance phenotypes. Two-way RM-ANOVA was used to infer statistical significance of the differences of CFU numbers. Error bars indicate SDs. Wt, wild type; ET, El Tor (A); Cl, classical (B); Env, environmental (C); TCR, TLC-CTX-RS1 prophage. * = CTXΦETCam.
Since the function of RecA is intricately associated with the SOS response and modulates LexA autoproteolytic activity, we extended our study to decipher the role of RecA in CTXΦ replication. To do this, all of the genetic elements, namely CTXΦET, RS2ET, RS2Cl, and RS2Env, were introduced into the recA-deleted BS1 strain, and the numbers of transconjugants on the selection plates were compared. Similar numbers of transconjugants carrying replicative and integrative phage genomes or RS modules were obtained in the presence of functional RecA (SI Appendix, Table S2). We then deleted the recA gene in N16961 and introduced CTXΦET, RS2ET, RS2Cl, and RS2Env to monitor replication efficiency of different phage-derived elements. To our surprise, while no transconjugant was obtained for the CTXΦET or RS2ET element in the absence of recA, transconjugants for RS2Cl (4.6 × 105) or RS2Env (5 × 104) element were obtainable using the same V. cholerae mutant strain (Fig. 6). We further confirmed this phenotype using the N16961-derived BS11 strain, which lacks recA and all of the prophages. Once CTXΦET was introduced, it also showed immunity against reinfection with the similar type of CTXΦ (Fig. 6). We also observed similar phenotypes in classical (O395) and environmental (VCE232) V. cholerae strains when we introduced CTXΦCl or CTXΦEnv replication and integration module to the strains lacking recA gene but containing CTXΦCl or CTXΦEnv in the chromosome, respectively (Fig. 6 and SI Appendix, Table S2). In both cases, CTXΦ can replicate in the absence of recA but presence of different biotypes of CTX prophages in the chromosome. We also ensured that the transmission process is not reduced in the absence of recA gene function by using a replicative conjugative plasmid pFX497 (SI Appendix, Table S1) as a control, which gave a similar number of transconjugants in the presence or absence of recA. However, RecA function was not linked with the integration efficiency of CTXΦ, since it is able to integrate at the dif site with almost equal efficiency in presence or absence of RecA function (SI Appendix, Table S2). The findings of the present study demonstrate that RecA helps CTXΦ replication in the presence of preexisting CTX prophage in the host chromosome.
Contribution of GIs in Modulating Cellular Proteome.
For identification, quantification, and comparison of whole-cell proteomes of wild type V. cholerae strain N16961 and its derivative BB1 devoid of GIs and prophages, we performed experiments using a high-throughput liquid chromatography (LC) system (Eksigent 2D) coupled with Triple TOF 5600 mass spectrometer (AB Sciex). We have identified a total of 1,864 proteins in both strains (Dataset S1). Based on the proteome profile of N16961 and BB1, we categorized the protein pool into three classes: (i) proteins with lower abundance (>1.5 log fold changes) or absent in BB1, (ii) proteins with higher abundance (>1.5 log fold changes) in BB1, and (iii) proteins with similar abundance in both strains. Proteins that are not detectable in BB1 but found in the N16961 strain were analyzed to find their coding sequences. We have identified similar numbers of proteins in N16961 (n = 1,765) and BB1 (n = 1,724) strains. A total of 141 proteins were detected only in N16961, of which 41 proteins are encoded by the genes present in Chr2. More than 10 proteins are encoded by the 4 GIs, VPI-1 (n = 1), VPI-2 (n = 4), VSP-1 (n = 3), VSP-2 (n = 1), and CTXΦ (n = 2), which were detected in N16961 but not in BB1. We observed that 131 proteins were differentially expressed (log fold change 1.5 and P value <0.05) between N16961 and BB1 strains. Out of 131 differentially expressed proteins in BB1, 59 and 72 proteins were up- and down-regulated, respectively. Proteins down-regulated in BB1 belong to uncharacterized proteins (n = 8), outer membrane proteins (n = 2), flagellar biosynthetic proteins (n = 2), aspartate modifying enzymes (n = 2), and several others. Enzymes involved in succinate metabolic pathways (n = 3), RNA degradation (n = 2), and ABC transporters (n = 2) are up-regulated in the absence of MGEs. Both small- and large-subunit ribosomal proteins are up- and down-regulated in the absence of MGEs. We observed that 61 proteins encoded by the Chr2 were detectable either in N16961 (n = 25) or BB1 (n = 36).
Discussion
Acquisition of virulence functions among enteric pathogens through lateral gene transfer, loss of redundant and nonessential genes among intracellular pathogens, and antigenic drift among mucosal pathogens are well recognized evidences in the postgenomic era (32). A hallmark of evolution of the cholera pathogen is its high proficiency in genetic exchange and genomic plasticity. V. cholerae is naturally competent, and different pathways, including transformation, conjugation, and transduction can facilitate HGT in the pathogen. Comparative analysis of genome sequences of thousands of V. cholerae isolates collected over a period of 170 y revealed its genome plasticity and continuous evolution due to acquisition or loss of specific genomic segments, specifically bacteriophages, ICEs, GIs, and plasmids (14, 21, 33). MGEs carrying toxin, virulence factors, antimicrobial resistance function, metabolic enzymes, and other fitness factors are ubiquitously present in the genome of toxigenic V. cholerae strains. The genus Vibrio and other members of Vibrionaceae commonly harbor two nonhomologous circular chromosomes, Chr1 and Chr2 (34). However, a recent report indicates that V. cholerae might carry a single fused chromosome instead of two chromosomes (35). Exploration of the bipartite and single chromosome-containing genomes of clinical V. cholerae isolates identified four non–self-mobilizing genomic islands (VPI-1, VPI-2, VSP-1, VSP-2) and one self-mobilizing ICE and multiple prophages (CTXΦ, RS1Φ, TLCΦ) in most of the seventh pandemic isolates. CTXΦ, VPI-1, and VPI-2 are ubiquitously present in all of the epidemic isolates, whereas VSP-1 and VSP-2 islands are commonly present in the seventh pandemic O1 and O139 isolates (14, 33). Except IMEXs, all of the GIs and ICEs reported in V. cholerae encode their own tyrosine recombinases as predominant integrases and integrate reversibly in a specific chromosomal locus by site-specific recombination (19, 20). Changes in the bacterial genome sequences due to accumulation of spontaneous point mutations are of the order of 10−9 to 10−7 nucleotides per year (36). Analysis of multiple genomes of V. cholerae isolated from the same epidemic or even from the same patient found that genomic contents of the isolates may differ from less than 10 to more than 100 genes within each patient (37). Presence or absence of strain-specific DNA segments is found in the specific loci of either chromosome of V. cholerae.
Compared to acquisition, deletion events of genomic segments are highly variable in V. cholerae and other bacterial species, including E. coli (38). The genomic diversities among clinical and nontoxigenic environmental V. cholerae strains are also very high (39). It has been found that, like clinical isolates, the genomes of the environmental V. cholerae strains may also be equipped with several GIs, prophages, and plasmids. The GIs of V. cholerae can excise from the chromosome using tyrosine recombinases and form circular intermediate (20). The circular intermediate of GIs can be lost during cell division, or it may reintegrate in the same locus by site-specific recombination. Although it is well established that the major drivers for genomic plasticity in V. cholerae and other enteric pathogens are GIs and other MGEs, their frequency of loss under in vitro and in vivo conditions have not been well studied. In addition, cross-talk among GIs and between GI and prophages are known, but the influence of GI-encoded functions in the essentiality of genes located in the core genome has not been reported. Nevertheless, the molecular basis of host immunity to prevent reinfections of CTXΦ in V. cholerae is yet to be understood. In the present study, we have done extensive genome engineering of clinical V. cholerae isolates N16961 and 41081 and generated multiple (n = 12) reporter strains to study the in vitro and in vivo stability and integration efficiency of GIs, ICE, and prophages. Each element was tagged with selectable (cat/aadA), counter-selectable (sacB), and chromogenic (lacZ) markers to measure the frequency of loss of tagged MGEs under in vitro and in vivo conditions and to isolate different derivatives that lost the GIs and ICE. The frequency of loss of GIs differs widely from 3.26 × 10−4 to 4.21 × 10−5. GIs that are highly conserved in O1 El Tor, classical, and O139 clinical isolates and essential for virulence are more stable compared to the GIs present in most of the seventh pandemic isolates. Several factors, including mode of integration, expression level of recombinases, and recombination directionality factors, can influence the excision frequencies (40), which is crucial for determining the frequency of loss. It is important to note that, while integration of any GI is reversible, none of these GIs is able to replicate in its excised circular form. After excision, GI may reintegrate into chromosome; otherwise, the excised form cannot be distributed in dividing daughter cells. In sharp contrast, this is not the case for ICE, which has the capacity to excise, replicate, and reintegrate. The frequency of loss of ICE is surprisingly low because of its replication ability in the excised form. Since the integration of CTXΦ is irreversible, we were unable to find any isolate that has lost the prophages.
The simultaneous removal of all of the GIs and prophages from the chromosome of N16961 helped us to understand the cross-talk between core and acquired genomes and importance of horizontally acquired genetic elements in the adaptation of V. cholerae. The core genome encodes all of the essential functions of bacteria and is highly conserved within and between bacterial species. The core genome of V. cholerae harbors 343 essential genes, while the core genome of E. coli houses 287 genes essential for growth in nutrient-rich medium (41, 42). Exclusive essentiality of few genes or domains of genes, including rctB, dam, parA2, and parB2 in V. cholerae, are linked with the replication and segregation of bipartite genome of the cholera pathogen. The SOS master regulator LexA is essential in V. cholerae but dispensable in E. coli and other bacteria like Salmonella enterica and Shigella dysenteriae. The reason for the essentiality of LexA for viability of V. cholerae cells was not known previously. In this study, we conclusively established that the catalytic function of RstA, a Rep protein essential for the replication of CTXΦ, is the main player to make LexA essential for viability in the toxigenic V. cholerae strains. Since CTXΦ and RS1Φ are not present in the genome of E. coli, S. enterica, S. dysenteriae, and other bacterial species, deletion of lexA from the genome of these bacteria is feasible. This indicates that, other than encoding CT, tandem copies of CTX prophages also contribute to the fitness of V. cholerae by preventing loss or inactivation of such an important molecule in the genome of cholera pathogen. On the other hand, V. cholerae cells help CTXΦ for tandem integration, possibly through multiple events of infections, by providing RecA function. RecA may also help CTXΦ replication by inducing autoproteolysis of LexA in the presence of ssDNA and limiting the availability of RstR, leading to the derepression of the essential phage replication protein RstA (SI Appendix, Fig. S3). The cross-biotype CTXΦ infections and their efficient replication in the presence and absence of RecA further support our hypothesis. The major differences in genome sequences between different CTXΦs are mainly located in the rstR gene (43). The nucleotide sequences of rstACl and rstBCl are very similar (94% identical) to their El Tor homologs, and the amino acid sequences of these proteins are 99% identical. In contrast, the nucleotide sequences of the rstR genes are highly divergent between the two biotypes and other serogroups of V. cholerae. The RstREl and RstRCl share only 24% identical and 32% similar amino acid sequences (43). Such heterogeneity among regulatory proteins and complex interactions between the bacterial core genome and phage-encoded functions possibly facilitates CTXΦ to select compatible host cells for survival and multiplication.
Other than providing toxin and antimicrobial resistance functions, the acquired genome of V. cholerae, including GIs, ICE, and prophages, also contributes in modulating host cellular proteome. Core genome-encoded gene functions like LexA can modulate the expression of different genes present in the horizontally acquired genetic elements (44). Chromosomally encoded tyrosine recombinases (XerC and XerD) also contribute substantially in the biology of IMEXs (18). Cross-talk between different acquired genetic elements has also been demonstrated in V. cholerae (40). Several VPI-1–encoded proteins modulate the expression of ctxAB genes in the genome of CTXΦ under optimal growth conditions and also in the host-associated infection period. Our findings on the differential expression of cellular proteins in the presence and absence of acquired genome provided further insights about their cooperative interactions. In this study, using whole-genome proteome analysis, we have generated further information by identifying an additional pool of proteins (n = 72) that are down-regulated in the absence of GIs and prophages. We observed that core genome-encoded proteins like outer membrane proteins (n = 2), flagellar biosynthetic proteins (n = 2), amino acid modifying enzymes (n = 2), and several others are down-regulated in the absence of acquired genetic elements. Since several proteins are involved in various metabolic functions, changes in their expression patterns may also have effects on the cellular metabolome and the fitness of V. cholerae.
Conclusion
Our study provides deep insights into the stability of GIs and other MGEs in the genome of V. cholerae. These findings will help us to understand how the cholera pathogen has evolved by capturing genetic elements equipped with toxin genes, virulence traits, AMR genes, and other metabolic functions. The findings suggest that the degree of stability of GIs and other MGEs in the genome is directly linked with its importance in pathogenicity and fitness potential of V. cholerae. Since CTXΦ encodes the key virulence factor CT, its stability was very high in the genome of V. cholerae. The GIs that are important for disease severity and sustainability of V. cholerae but not essential for manifesting the disease cholera are less stable compared to CTXΦ. Since gene content variation in the flexible gene pool accumulates more quickly than accumulation of point mutations in the core genome, MGEs and HGT have major contributions in the rapid evolution and emergence of enteric bacterial pathogens. The functions encoded by the core and acquired genome evolved mutually to modulate their essentiality, level of cellular metabolites, fitness of the pathogen, epidemiology, etiology, and sustainability of the V. cholerae in host intestine and natural environment. Our findings regarding the essentiality of LexA in toxigenic V. cholerae and contribution of RecA in reinfection of CTXΦ established how the core and flexible genome work together to maintain the sustainability of functions that are crucial for virulence, genome repair, and dynamics of GIs and other MGEs. A better understanding of the functions of the genetic contents of MGEs present in the genome of V. cholerae and their contribution in the modulation of the proteome would certainly be helpful to forecast evolution and the emergence of enteric pathogens.
Methods
Bacterial Strains, Plasmids, and Culture Conditions.
Bacterial strains and plasmids used in this study and their relevant genotypes are listed in Table 1 and SI Appendix, Table S1, respectively. E. coli and V. cholerae cells were grown in Luria broth (LB) with shaking (180 rpm) at 37 °C or Luria agar (LA) unless stated otherwise. Sucrose sensitivity assay was performed by plating the bacteria onto LA supplemented with 15% sucrose and incubating at 24 °C. TCBS (thiosulfate citrate bile salts sucrose) agar was used as a selective medium for V. cholerae. Antibiotics were used at the following concentrations: ampicillin (100 μg/mL), streptomycin (100 μg/mL), zeocin (25 μg/mL), kanamycin (40 μg/mL), spectinomycin (50 μg/mL), and chloramphenicol (30 μg/mL for E. coli and 2 μg/mL for V. cholerae). Blue-white screening was performed by adding 20 μg/mL of X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) on the selection plate. For long-term storage, bacterial strains were maintained at −80 °C in LB containing 20% glycerol (vol/vol).
Transformation and Conjugation.
For chemical transformation, rubidium chloride-treated E. coli cells were used and subjected to heat shock at 42 °C for 60 s. Conjugation was performed between diaminopimelic acid (DAP) auxotroph E. coli β2163 donor cells carrying desired plasmid and V. cholerae as recipient cells. Exponentially grown donor and recipient cells were mixed together at a 1:2 ratio, inoculated onto a 0.22-μm sterile filter paper placed on an LA plate supplemented with 0.3 mM DAP, and incubated at 37 °C overnight. Transconjugants were selected based on antibiotic-resistance traits of conjugative plasmids and DAP auxotrophy of donor cells.
Genomic Island Loss Assays.
V. cholerae strains carrying genomic island tagged with sacB-cat or sacB-cat-lacZ or sacB-aadA were grown to stationary phase in LB at 37 °C with shaking overnight and then plated on LA (15% sucrose) plates supplemented with or without 20 μg/mL of X-gal and incubated for 16 h at 24 °C. About 300 colonies were restreaked on separate LA plates supplemented with chloramphenicol/streptomycin/spectinomycin or sucrose. Subsets of the colonies, which showed sensitivity to chloramphenicol or spectinomycin and resistance to streptomycin and sucrose, were selected for further confirmation by PCR amplification of the genomic island region to be excised.
Genetic Manipulations.
The recombinant vectors were developed by using restriction enzymes and T4 DNA ligase (NEB). Desired DNA fragments were PCR-amplified by using high-fidelity Taq DNA polymerase (NEB). E. coli FCV14 was used as host bacterium to select and amplify recombinant vectors. All gene deletions and replacements were performed via allelic exchange method using the derivatives of suicide vector pKAS32 or pDS132 as described previously (26). For delivering vectors to the V. cholerae, we adopted a conjugation method. E. coli β2163 was used as a donor strain. For all gene deletions, around 500- to 700-base pair (bp) homologous regions upstream and downstream of the respective ORF were PCR-amplified using specific primer combinations (SI Appendix, Table S3). The amplified products were purified before restriction enzyme digestion and introduced into similarly digested suicide vector by DNA ligation.
For removing the entire genomic islands (GIs) from V. cholerae genome, first, the GIs were tagged with sacB-cat, sacB-aadA, or sacB-cat-lacZ alleles. Briefly, a portion of the GI was PCR-amplified and then cloned into pSW23T (SI Appendix, Table S1). The recombinant vectors were used as a template for inverse PCR by which a portion GI was replaced with sacB-cat, sacB-aadA, or sacB-cat-lacZ was cloned. Finally, the recombinant vectors carrying sacB-cat, sacB-aadA, or sacB-cat-lacZ alleles flanked by the small segments of GIs were used to tag the GIs by the sequential allelic exchange method as described previously (45).
The GI flagged strains became resistant to chloramphenicol/spectinomycin but sensitive to sucrose. For the removal of the entire GI, the strains carrying flagged GI were grown in LB without any antibiotics. The V. cholerae strain that lost its GI was isolated simply by plating the sacB-cat, sacB-aadA, or sacB-cat-lacZ flagged strain on to LA plates supplemented with 15% sucrose with or without X-gal. The loss of GIs from the bacterium was confirmed by monitoring sensitivity to chloramphenicol/spectinomycin and also by PCR assay.
Rabbit Ileal Loop Assay.
Rabbit ileal loop assay was performed in young New Zealand White rabbits (1.5 to 2.0 kg) as described earlier (46). Prior to surgery, rabbits were fasted for 24 h, but water was given ad libitum. V. cholerae cells were grown in LB up to mid-log phase, followed by washing with sterile phosphate-buffered saline (PBS). Rabbits were anesthetized, laparotomy was performed, and the ileum was ligated into discrete loops, each measuring ∼10 cm. Each loop was inoculated with 108 CFU of V. cholerae strain (N16961, BS1, SB31, or SB55) in PBS. The loop containing only PBS was used as a negative control. The intestine was returned to the peritoneum, and the abdomen was sutured. Rabbits were euthanized after 18 h, and the abdomen was opened, followed by measurement of loop length and accumulated fluid volume in each loop. Fluid accumulation (FA) ratio was calculated by the following formula: volume of fluid accumulated (milliliters)/length of intestine (centimeters). FA ratio of greater than 1.0 was considered as a strong positive response, while FA ratio of less than 0.2 indicated negative response. All the animal experiments were conducted following the standard operating procedure as outlined by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India. The animal experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC) of National Institute of Cholera and Enteric Diseases (NICED) with Registration No. 68//Rebi/S/1999/CPCSEA valid 17/7/2024, Approval No.: NICED/CPCSEA/68/GO/(25/294)/2019-IAEC/SM/1.
To measure the in vivo stability of the pathogenicity islands of V. cholerae, the sacB-cat–tagged strains (SB23, SB26, SB30, and DD1) were inoculated into rabbit ileum, and then the animal passaged strains were plated onto both TCBS agar and LA supplemented with 15% sucrose and streptomycin (100 μg/mL).
Whole-Cell Proteome Analysis.
Overnight-grown V. cholerae strains N16961 and BB1 were used for total protein extraction and trypsin digestion. We used an LC system (Eksigent 2D) coupled with a Triple TOF 5600+ mass spectrometer (AB Sciex) for proteome analysis. The V. cholerae-specific library was created by bRP-C18-HPLC followed by LC-MS/MS for label-free quantitation in SWATH-MS workflow. In brief, the peptides from the cell lysate of both the V. cholerae strains were fractionated using an Agilent 1200 HPLC system on a high-pH reverse-phase Zorbax 300 Extend-C18 column (Agilent Technology). The eluted 48 fractions were pooled to the final 12 fractions. Each fraction was desalted and subjected to 90-min gradient LC-MS/MS run in data-dependent acquisition (DDA) mode to generate a V. cholerae spectral library. For quantitative analysis, each sample was acquired in triplicate in data-independent acquisition (DIA) mode and the instrument was configured as described by Gillet et al. (47). MaxQuant version 1.6 was used for peptide and protein identification, and a Spectronaut pulsar (Biognosys) with default settings was used in SWATH processing and quantification. Proteins with a ratio of >1.5 were considered differentially expressed. In all cases, P < 0.05 (t test) was considered significant in protein quantification.
Supplementary Material
Acknowledgments
We thank Dr. Raghunath Chatterjee, Human Genetics Unit, Indian Statistical Institute, Kolkata, India, for critical reading of the manuscript. The authors acknowledge Mr. Naveen Kumar and other lab members for technical support. This study received financial support from the Department of Biotechnology (DBT), Government of India (Grant No. BT/MB/THSTI/HMC-SFC/2011). A.P. and J.V. received support from the DBT, Government of India. P.K. received research fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006283117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Casjens S., The diverse and dynamic structure of bacterial genomes. Annu. Rev. Genet. 32, 339–377 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Waldor M. K., Mekalanos J. J., Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Karaolis D. K., Somara S., Maneval D. R. Jr., Johnson J. A., Kaper J. B., A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature 399, 375–379 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Frost L. S., Leplae R., Summers A. O., Toussaint A., Mobile genetic elements: The agents of open source evolution. Nat. Rev. Microbiol. 3, 722–732 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Verma J. et al., Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 116, 6226–6231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spanogiannopoulos P., Bess E. N., Carmody R. N., Turnbaugh P. J., The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 14, 273–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuel B. S. et al., Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. U.S.A. 104, 10643–10648 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrindt U., Hochhut B., Hentschel U., Hacker J., Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2, 414–424 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Langille M. G., Hsiao W. W., Brinkman F. S., Detecting genomic islands using bioinformatics approaches. Nat. Rev. Microbiol. 8, 373–382 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Boyd E. F., Almagro-Moreno S., Parent M. A., Genomic islands are dynamic, ancient integrative elements in bacterial evolution. Trends Microbiol. 17, 47–53 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Das B., Insights into TLCΦ lysogeny: A twist in the mechanism of IMEX integration. Proc. Natl. Acad. Sci. U.S.A. 116, 18159–18161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker J., Hentschel U., Dobrindt U., Prokaryotic chromosomes and disease. Science 301, 790–793 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Heidelberg J. F. et al., DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406, 477–483 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weill F. X. et al., Genomic history of the seventh pandemic of cholera in Africa. Science 358, 785–789 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Domman D. et al., Integrated view of Vibrio cholerae in the Americas. Science 358, 789–793 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Mutreja A. et al., Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477, 462–465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grindley N. D., Whiteson K. L., Rice P. A., Mechanisms of site-specific recombination. Annu. Rev. Biochem. 75, 567–605 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Das B., Martínez E., Midonet C., Barre F. X., Integrative mobile elements exploiting Xer recombination. Trends Microbiol. 21, 23–30 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Rajanna C. et al., The vibrio pathogenicity island of epidemic Vibrio cholerae forms precise extrachromosomal circular excision products. J. Bacteriol. 185, 6893–6901 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy R. A., Boyd E. F., Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J. Bacteriol. 190, 636–647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devault A. M. et al., Second-pandemic strain of Vibrio cholerae from the Philadelphia cholera outbreak of 1849. N. Engl. J. Med. 370, 334–340 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Waldor M. K., Tschäpe H., Mekalanos J. J., A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J. Bacteriol. 178, 4157–4165 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grim C. J. et al., Genome sequence of hybrid Vibrio cholerae O1 MJ-1236, B-33, and CIRS101 and comparative genomics with V. cholerae. J. Bacteriol. 192, 3524–3533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Val M. E. et al., The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol. Cell 19, 559–566 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Das B., Bischerour J., Val M. E., Barre F. X., Molecular keys of the tropism of integration of the cholera toxin phage. Proc. Natl. Acad. Sci. U.S.A. 107, 4377–4382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pant A. et al., Effect of LexA on chromosomal integration of CTXϕ in Vibrio cholerae. J. Bacteriol. 198, 268–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradhan S., Baidya A. K., Ghosh A., Paul K., Chowdhury R., The El Tor biotype of Vibrio cholerae exhibits a growth advantage in the stationary phase in mixed cultures with the classical biotype. J. Bacteriol. 192, 955–963 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha A., Haralalka S., Bhadra R. K., A naturally occurring point mutation in the 13-mer R repeat affects the oriC function of the large chromosome of Vibrio cholerae O1 classical biotype. Arch. Microbiol. 182, 421–427 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Faruque S. M., Mekalanos J. J., Pathogenicity islands and phages in Vibrio cholerae evolution. Trends Microbiol. 11, 505–510 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Martínez E., Campos-Gómez J., Barre F. X., CTXϕ: Exploring new alternatives in host factor-mediated filamentous phage replications. Bacteriophage 6, e1128512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimsey H. H., Waldor M. K., Vibrio cholerae LexA coordinates CTX prophage gene expression. J. Bacteriol. 191, 6788–6795 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochman H., Davalos L. M., The nature and dynamics of bacterial genomes. Science 311, 1730–1733 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Weill F. X. et al., Genomic insights into the 2016-2017 cholera epidemic in Yemen. Nature 565, 230–233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada K., Iida T., Kita-Tsukamoto K., Honda T., Vibrios commonly possess two chromosomes. J. Bacteriol. 187, 752–757 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruhn M. et al., Functionality of two origins of replication in Vibrio cholerae strains with a single chromosome. Front. Microbiol. 9, 2932 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroeder J. W., Yeesin P., Simmons L. A., Wang J. D., Sources of spontaneous mutagenesis in bacteria. Crit. Rev. Biochem. Mol. Biol. 53, 29–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levade I. et al., Vibrio cholerae genomic diversity within and between patients. Microb. Genom. 3, e000142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochma H., Jones I. B., Evolutionary dynamics of full genome content in Escherichia coli. EMBO J. 19, 6637–6643 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faruque S. M. et al., Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. U.S.A. 101, 2123–2128 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter M. R., Rozovsky S., Boyd E. F., Pathogenicity island cross talk mediated by recombination directionality factors facilitates excision from the chromosome. J. Bacteriol. 198, 766–776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao M. C. et al., High-resolution definition of the Vibrio cholerae essential gene set with hidden Markov model-based analyses of transposon-insertion sequencing data. Nucleic Acids Res. 41, 9033–9048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato J., Hashimoto M., Construction of consecutive deletions of the Escherichia coli chromosome. Mol. Syst. Biol. 3, 132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimsey H. H., Waldor M. K., CTXphi immunity: Application in the development of cholera vaccines. Proc. Natl. Acad. Sci. U.S.A. 95, 7035–7039 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krin E. et al., Expansion of the SOS regulon of Vibrio cholerae through extensive transcriptome analysis and experimental validation. BMC Genomics 19, 373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A., Bag S., Das B., Novel genetic tool to study the stability of genomic islands. Recent Pat. Biotechnol. 12, 200–207 (2018). [DOI] [PubMed] [Google Scholar]
- 46.De S. N., Chatterje D. N., An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J. Pathol. Bacteriol. 66, 559–562 (1953). [DOI] [PubMed] [Google Scholar]
- 47.Gillet L. C. et al., Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics 11, O111.016717 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.