Abstract
Background:
Achillea wilhelmsii C. Koch hydroalcoholic extract (AWHE) is proven to induce cell death. Previous studies suggested that AWHE is an effective inhibitor against the proliferation of prostate cancer cells. The present study aimed to evaluate possible alterations of cell death-associated genes and determine the growth inhibitory activity of AWHE on HeLa cervical cancer cells.
Methods:
The antiproliferative activity of AWHE was tested using the tetrazolium dye-based colorimetric assay (MTT assay). The mRNA levels of Vascular endothelial growth factor (VEGF), caspase-3, and Breast Cancer Susceptibility gene 1 (BRCA1) were measured using the real-time Polymerase Chain Reaction method. The in-cell levels of phosphorylated H2AX were determined using the in-cell ELISA method. The data were analyzed using the non-parametric ANOVA and Friedman tests. P<0.05 was considered statistically significant.
Results:
Based on the MTT assay, The half-maximal inhibitory concentration and 81.99 µg/mL, respectively. The mRNA levels of BRCA1 increased after 12 and 24 hours of treatment (P<0.001), while the mRNA levels of VEGF significantly decreased after 12 hours (P=0.003) and 24 hours (P=0.001). Caspase-3 expression was increased in the HeLa cells after 6 and 12 hours (P<0.001) whereas γ-H2AX levels significantly increased after 24 and 48 hours of treatment (P<0.001).
Conclusion:
AWHE possesses growth inhibitory activity by altering the expression of cell death-associated genes. Using extracts from herbal plants may provide alternative strategies to be deployed in the fight against cancer.
Keywords: Achillea , Uterine cervical neoplasms , Cell death , DNA repair
What’s Known
Cervical cancer is a major health concern and one of the most significant types of cancer in women worldwide.
Achillea wilhelmsii C. Koch is a plant with components capable of triggering multiple signaling pathways, including cell death.
What’s New
Achillea wilhelmsii C. Koch possessed pro-apoptotic effects and growth inhibitory activity of malignant cervical cells.
Achillea wilhelmsii C. Koch reduced the expression of cell death-associated genes while enhancing phosphorylation of H2AX, as a highly sensitive marker of unrepaired DNA damage.
Introduction
Uterine cervical neoplasms, commonly known as cervical cancer (CC), is a major health concern and the fourth leading cause of cancer death among women worldwide. 1 The World health organization has reported that CC represented 6.6% of all female malignancies in 2018 with an estimated 570,000 new cases per year. 2 Many types of human papillomavirus (HPV) are believed to be the leading cause of CC. 3 The diagnosis of CC is primarily by Papanicolaou (Pap) smear and microscopic observation of visible lesions. 4 The treatment of pre-cancerous lesions includes cold knife conization, loop electrosurgical excision procedure (LEEP), freezing, cauterization, and laser surgery. However, to date, the main treatment strategies for the invasive stage of CC are chemotherapy and radiotherapy. 5 Recently, molecular biomarkers such as serum squamous cell carcinoma antigen (SCC-Ag), serum fragments of cytokeratin (CYFRA), carcinoma embryonic antigen (CEA), serum soluble CD44 (sCD44), and HPV-DNA screening tests have been suggested as practical diagnostic and prognostic CC markers. 6
Since certain anti-cancer compounds induce cellular stresses that lead to DNA lesion formation, the linkage between cell death, cell invasion, and DNA repair in response to genotoxic agents has become an exciting area in the field of cancer research. 7 Several DNA repair pathways usually repair DNA double-strand breaks (DSBs). If unrepaired, the accumulation of these lesions will trigger cell death mechanisms. 8 Normally, the programmed cell death (PCD) regulates the development and proliferation of tumor cells while the dysfunction of this process leads to cell growth and invasion. 9 Apoptosis, necrotic cell death, and autophagy are the three essential types of PCD. 10 Apoptosis protects neoplastic cells from oxidative stress and hypoxia. 11 This mechanism has two primary pathways, the extrinsic and the intrinsic pathway, and the caspase enzymes are the key regulatory proteins in both pathways. 12 Caspase-3 is a member of the caspase family that activates caspases-6 and -7; necessary for the formation of apoptotic bodies. 13 Previous experiments have shown that low expression of caspase-3 gene may have a facilitative role in the transformation and development of CC. 14
Deficiencies in DNA repair mechanisms can lead to cancer development. 15 A previous study has shown that defects in DNA repair may lead to chromosomal aberrations, leading to malignant transformation of cells. 16 The breast cancer susceptibility gene 1 (BRCA1) is hyperphosphorylated in response to DNA damage and thus activates the repair of DSBs which leads to the initiation of homologous recombination (HR) pathway in response to certain chemicals. 17 The effects of BRCA1 phosphorylation on the HR pathway are not known. BRCA1 is suggested to have a dual regulatory role by maintaining genome integrity and controlling the homology-directed DNA repair. 18 Moreover, to examine the efficiency of the DNA damage repair, the phosphorylated histone H2AX variant (γ-H2AX) has been introduced as a reliable marker. H2AX phosphorylation is required for the recruitment of DNA damage response associated factors at the damaged sites. Hence, determining γ-H2AX levels could determine the efficacy of anti-tumor treatment and to anticipate the sensitivity of different cancer cells to DNA damaging chemicals. 19
Angiogenesis, mediated by several proteins, including vascular endothelial growth factor (VEGF), is the fundamental pathway in CC progression and mortality since tumor cells use new blood and lymphatic vessels for metastatic spread, proliferation, and supply of oxygen. 20 , 21 This endothelial factor is a glycoprotein overexpressed in highly metastatic cells. 22 The VEGF overexpression is associated with suppression of apoptotic cell death while affecting DNA repair mechanisms. 23
Due to many irreversible side effects, the use of chemotherapy or radiotherapy is limited as the primary cancer treatment. Therefore, identifying a new therapeutic approach is necessary while the use of medicinal herbs can be a putative option for this purpose. 24 For example, many natural health products have been suggested as VEGF inhibitors, including Viscum album (European mistletoe), Silybum marianum (milk thistle), and Camellia sinensis (green tea). 25 Moreover, Achillea falcata and Acacia nilotica have shown remarkable anti-proliferative and pro-apoptotic effects on various types of cancer cells. 26 Achillea wilhelmsii, an herbal plant belonging to the family of Asteraceae, contains several components such as flavonoids, alkaloids, and cineol, which have been reported to possess cell death-inducing effects and antioxidant properties. 27 Anti-cancer effects of Achillea wilhelmsii C. Koch hydroalcoholic extract (AWHE) on breast and prostate cancers have been reported in previous studies, but little is known about its putative effects on other types of cancer cells.
The present study aimed to evaluate the anti-proliferating and cell death-inducing potential of AWHE on human HeLa cervical cancer cells as well as measuring a very sensitive DNA damage marker. We hypothesized that these signaling pathways could be effective alternatives to conventional chemotherapeutic procedures in CC therapy.
Materials and Methods
The Ethics Committee of Zahedan University of Medical Sciences (Zahedan, Iran) approved the study protocol (code: IR.ZAUMS.REC.1396.375).
Chemicals
Fetal bovine serum (FBS) was purchased from Gibco (Rockville, MD, USA). 3-(4,5-dimethylhiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were procured from Sigma (St. Louis, USA). Trypan blue, antibiotic-antimycotic solution, trypsin, phosphate-buffered saline (PBS), and RPMI 1640 culture medium were procured from INOCLON (G. Innovative Biotech Co, Iran). cDNA synthesis kit and SYBR Green PCR Master Mix were purchased from TaKaRa Bio Inc. (Dalian, China) and Ampliqon A/S (Odense, Denmark), respectively.
Plant Material and Preparation of the Hydroalcoholic Extract
Aerial parts and roots of Achillea wilhelmsii C. Koch were collected during summer 2018 from the southeast regions of Iran. Later, the plant was taxonomically authenticated by the Herbarium of the Department of Biology at the University of Sistan and Balouchestan, Zahedan, Iran (herbarium number: 2345). After drying and grinding, extraction was performed with 70% ethanol solvent (350 mL) using a Soxhlet extractor (50 °C, 5 h). Following filtering of the solution through Whatman 41 filter paper, the hydroalcoholic extract was dried at 45 °C using a centrifugal evaporator (MAXI DRY-LYO, Denmark) to remove the remaining solvent. Then, 100 mg of AWHE was weighed and dissolved in 1 mL of DMSO and kept at -20 °C until further use.
Cell Culture and Treatments
HeLa cervical cancer cells were obtained from the Cell Repository of the Research Institute of Biotechnology, Ferdowsi University of Mashhad (Mashhad, Iran). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 culture medium supplemented with 10% FBS and an antibiotic-antimycotic solution containing streptomycin (105 µg/mL), penicillin (100 U/mL), and amphotericin B (2.5 mg/L); incubated under standard cell culture conditions (at 37 °C humidified air containing 95% atmospheric air and 5% CO2). For all measurements, an equal volume of FBS-containing medium (<1% DMSO as solvent) was used as the control.
MTT Cytotoxic Assay
The MTT assay determines the cytotoxicity of an antiproliferative agent at different exposure periods. The cells were seeded in a 96-well plate (4500 cells per well). While reaching 80% confluency, 200 µL of AWHE (with increasing concentrations dissolved in the culture medium, ranging from 0 to 400 µg/mL) was added to each well and incubated for 24, 48, and 72 hours. Then, the cells were again incubated for 4 hours with 0.8 mg/mL of MTT (dissolved in serum-free RPMI 1640 medium). Washing with PBS (1 mL) was followed by the addition of DMSO (1 mL) and gentle shaking for 15 minutes to dissolve the formazan crystals. The absorbance was recorded at 570 nm using a microplate reader (Stat Fax 2100, USA). After 24 to 72 hours of treatment, the half-maximal inhibitory concentration (IC50) values of AWHE were calculated using PRISM 6.0 (GraphPad Software, CA, USA) 28 and concentration-response curves were plotted.
Gene Expression Assay
Primer Design
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), VEGF, BRCA1, and caspase-3 gene sequences were obtained from the National Center for Biotechnology Information database. Using Primer3 software, forward (F) and reverse (R) primer sequences for all four genes were designed (table 1) to ensure amplification of the transcript variants 1, 2, 4 and 7 of GAPDH, transcript variants 1 to 10 of VEGF, transcript variants 1 to 4 of BRCA1, and transcript variants 1, 2, 3, 5 and 6 of caspase-3. GAPDH was used as a housekeeping gene, which believed to have stable expression and selected as the internal control.
Table 1.
Primer sequences of vglyceraldehyde 3-phosphate dehydrogenase, vascular endothelial growth factor, breast cancer susceptibility gene, and caspase-3
| Genes | Sequence |
|---|---|
| GAPDH (forward) | CATGTAGTTGAGGTCAATGAAGG |
| GAPDH (reverse) | GAGCCACATCGCTCAGACAC |
| VEGF (forward) | GAAGGAGGAGGGCAGAATCATCAC |
| VEGF (reverse) | CACAGGATGGCTTGAAGATGTACTC |
| BRCA1 (forward) | ACAGCTGTGTGGTGCTTCTGTG |
| BRCA1 (reverse) | CATTGTCCTCTGTCCAGGCATC |
| caspase-3 (forward) | AGAACTGGACTGTGGCATTGA |
| caspase-3 (reverse) | GCTTGTCGGCATACTGTTTCA |
GAPDH: Glyceraldehyde 3-phosphate dehydrogenase, VEGF: Vascular Endothelial Growth Factor, BRCA1: Breast Cancer Susceptibility gene 1
Total RNA Extraction and cDNA Synthesis
Cells were seeded (25×104 cells/well) and incubated for 20 hours before treatment with AWHE (100 µg/mL) in a time-dependent manner (at 0, 6, 12, and 24 hours). Then, the cells were trypsinized and washed with 1 mL cold PBS. According to the manufacturer’s protocol, 1 mL RNX (SinaClon, Tehran, Iran) was added to the cell pellets, and total RNA was extracted. The precipitated RNA was resuspended in 50 μL Diethyl Pyrocarbonate water and the quantity of the extracted RNA was evaluated using Ultraviolet spectrophotometric reading at the wavelengths of 260 nm and 280 nm (260/280 ratio). According to manufacturer’s instructions for cDNA synthesis, a mixture of reagents including template RNA (~4 μg), 2.5 μL RNase-free dH2O, 0.5 μL of Oligo dT primer, and 0.5 μL of random 6 mers were incubated for 5 minutes at 65 °C. Then, 0.5 μL of PrimeScriptTM RT enzyme Mix I and 2 μL of 5X PrimeScriptTM buffer were added.
Polymerase chain reaction (PCR) was performed in 10 μL volumes as follows: 15 minutes at 37 °C and 5 seconds at 85 °C followed by holding at 4 °C.
Real-time PCR Analysis
The real-time PCR was used to determine VEGF, BRCA1, and caspase-3 mRNA levels according to the manufacturer’s protocol using an ABI sequence detection system (Applied Biosystems, Foster City, CA, USA). Briefly, 1 μL of each forward and reverse primers, 10 μL of SYBR Green EXTaq II (2X), 2 μL of cDNA, and 6 μL of distilled water were mixed and reactions were set up under the following conditions: denaturation at 96 °C for 9 minutes, 35 cycles at 96 °C for 45 seconds, and 58 °C for 30 seconds followed by an extension at 72 °C for 45 seconds. Fold change between samples was measured using the 2-ΔΔCt method.
Quantification of H2AX Phosphorylation (γ-H2AX)
γ-H2AX levels were measured using a human phospho-histone H2AX (S139) in-cell ELISA kit according to manufacturer’s protocol at 0 (control), 6, 12, 24, and 48 hours of treatment with 100 µg/mL of AWHE. Briefly, the capture antibody was coated onto a 96-well microplate, and after 24 hours of incubation at 37 °C, the microplates were washed 3 times. Later, 300 μL of block buffer was added to each well and incubated again at 37 °C for 90 minutes. Following aspiration, the cell lysate standard was added to each well. The plate was subsequently sealed and incubated for 2 hours at 37 °C. In the next step, aspiration/washing was repeated, and 100 μL of the diluted detection antibody was added to each well before incubation for 2 hours at 25 °C. Afterward, 100 μL of the diluted streptavidin-HRP A was added to microwells and after 20 minutes of dark incubation at 37 °C and repeated washings, 50 μL of stop solution was added and the optical density of each well was measured (directly at 450 nm) using the microplate reader. The fold change was calculated by dividing the percentage of absorbance of the treated cells by that of the untreated (control) cells.
Statistical Analysis
All experiments were performed in triplicate. The non-parametric ANOVA and Friedman tests were applied to analyze the data using SPSS software version 22.0 (SPSS Inc., USA) and the GraphPad Prism 6.0 Software. P<0.05 was considered statistically significant.
Results
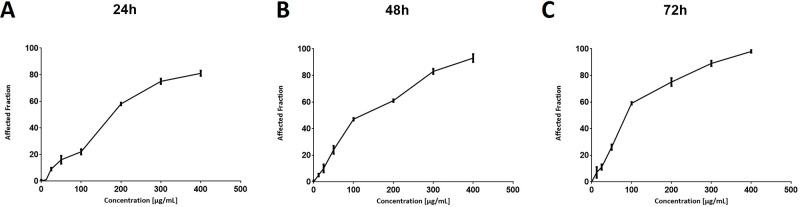
A significant growth-suppressing effect of AWHE was observed in HeLa cells after treatment for 24 (P=0.0038), 48 (P=0.0032), and 72 (P=0.006) hours. Based on affected fractions, concentration-response curves were plotted to represent the inhibitory effect of AWHE in both concentration- and timedependent manner (figure 1). The IC50 values after 24, 48, and 72 hours of AWHE treatment were 168.1, 100.1, and 81.99 µg/mL, respectively.
Figure 1.
Cytotoxic effects of hydroalcoholic extract of Achillea wilhelmsii C. Koch hydroalcoholic extract were evaluated after (A) 24, (B) 48, and (C) 72 hours of treatment as determined by tetrazolium dye-based colorimetric assay (MTT assay) in Hela cell line
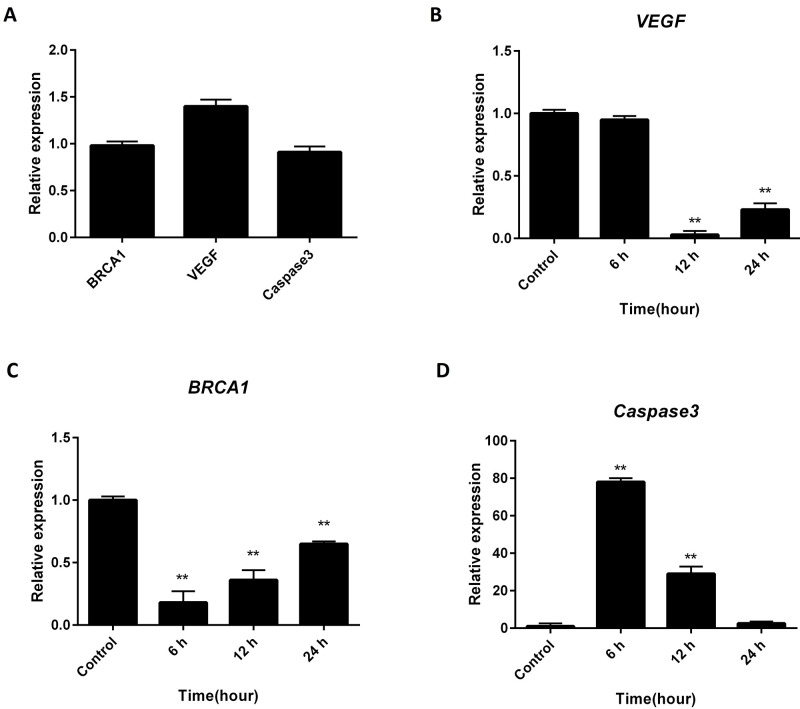
Real-time PCR analysis revealed that baseline expression of caspase-3 was lower than the other two genes, with VEGF having significantly higher mRNA levels compared with BRCA1 and caspase-3 (figure 2A), suggesting that HeLa cells could be highly metastatic. The mRNA levels of VEGF, BRCA1, and caspase-3 were evaluated in a time-dependent manner (at 0, 6, 12, and 24 hours) as Hela cells were treated with 100 µg/mL of AWHE (figures 2B, 2C, and 2D). Although mRNA levels of VEGF were not affected after six hours (P=0.19), they decreased significantly after 12 hours by 97% (P=0.03) and after 24 hours by 73% (P=0.01) compared with the adjusted untreated cells. However, the changes in BRCA1 and caspase-3 expression levels followed different patterns. The mRNA levels of BRCA1 significantly decreased by 82% after six hours of AWHE treatment, but it increased after 12 hours by 64% and 24 hours by 35% compared with the control (P<0.001). Similarly, caspase-3 gene expression increased drastically (about 78 folds) after six hours of exposure to 100 µg/mL of AWHE (P<0.001) and returned to basal expression levels after 24 hours; exhibiting no significant differences compared to the start of exposure (P=0.4).
Figure 2.
Real-time Polymerase Chain Reaction assay was used to assess the mRNA level of genes related to cell death pathways following AWHE treatment in HeLa cell line. (A) Comparison of the baseline expression levels of target genes. Relative expression of (B) Vascular Endothelial Growth Factor (VEGF), (C) Breast Cancer Susceptibility gene 1 (BRCA1), and (D) caspase-3. **P<0.05 compared to adjacent untreated control.
As shown in figure 3, following 6 and 12 hours’ treatment, the γ-H2AX levels were not affected compared to the untreated cells (P=0.26 and P=0.1, respectively), but these values significantly increased to about 5.5- and 4.2-fold after 24 and 48 hours of treatment, respectively (P<0.001). Overall, the results showed that AWHE was capable of decreasing BRCA1 expression and enhancing phosphorylation of H2AX, as a marker of unrepaired DNA.
Figure 3.
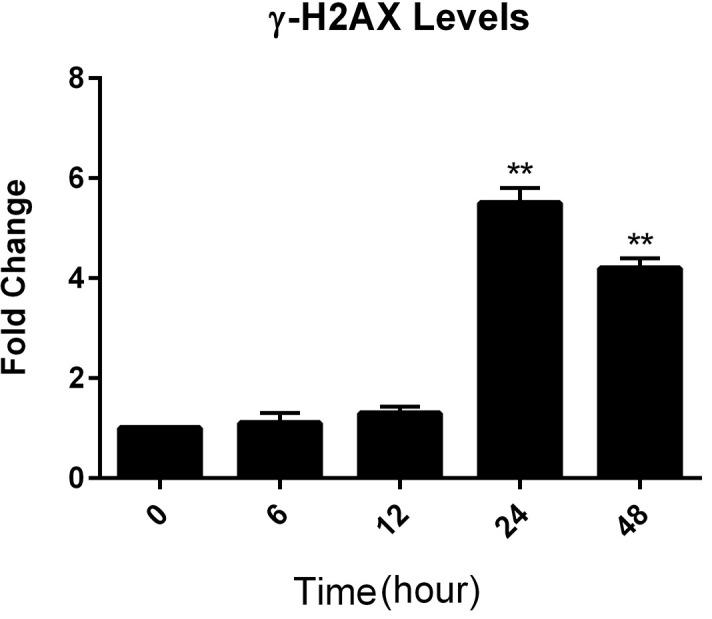
Analysis of in-cell levels of γ-H2AX following 0, 6, 12, 24, and 48 hours of treatment. Enhanced H2AX phosphorylation was observed in HeLa cells after 24 and 48 hours of exposure to 100 µg/mL of Achillea wilhelmsii C. Koch hydroalcoholic extract. **P<0.05 compared to untreated control.
Discussion
To the best of our knowledge, this is the first study aimed to evaluate cell death-inducing capacity and the anticancer potential of Achillea wilhelmsii on human cervical cancer cells. We found that AWHE was able to induce cell death in a concentration- and time-dependent manner in HeLa cells (by MTT test), which is consistent with a previous study that evaluated the anti-tumor effects of this plant on other cancer cell lines. 29 In contrast, another study reported that AWHE induced apoptosis in HeLa cells with concentrations equal to 100 µg/mL after 48 hours of treatment whereas this plant was reported to demonstrate the appropriate inhibitory effect at 150 µg/mL concentration (IC50) on PC-3 cell line following the same treatment period; indicating that HeLa cells were more sensitive to AWHE compared with the metastatic PC-3 cell line.27 As anticipated, the analysis of baseline expression of BRCA1, VEGF, and caspase-3 revealed that HeLa cells express higher levels of VEGF compared with the other two genes, but the mRNA levels of VEGF were not significantly higher than that of the other two genes. We found that the mRNA levels of VEGF decreased significantly after 12 and 24 hours of treatment with AWHE, whereas caspase-3 gene expression increased drastically after six hours of exposure to concentrations equal to IC50 values of AWHE. These findings were again consistent with the findings of a previous study reporting that octacosanol isolated from the plant Tinospora cordifolia could significantly down-regulate VEGF expression in Ehrlich ascites tumor cells. 30 No studies have reported possible changes in the expression levels of factors related to cell invasion in gynecological-related cancers. In a study by Galavi and colleagues, 25 μg/mL of AWHE significantly increased caspase-3 activity in MDA-Mb-468 breast cancer cells after 12 hours of exposure. 31 Likewise, an in vitro study on the apoptotic-inducing effects of Achillea teretifolia Willd extracts on prostate cancer cells reported that the methanol extract of this plant significantly up-regulated the Bax and caspase-3 genes, whereas the expression of Bcl-2 as an anti-apoptotic protein was down-regulated. This confirms the hypothesis that the extracts of Achillea species are potent apoptosis-inducing agents by modulating the Bax/Bcl-2 ratio and inducing cell cycle independent cell death. 32 However, in the present study, we only determined the expression levels of caspase-3 as a factor involved in both apoptotic pathways. Since this hydroalcoholic extract might induce unexpected alterations in mRNA levels of pro- and anti-apoptotic factors, we could not fully confirm the efficacy of AWHE. Apart from changes in the mRNA levels of VEGF and caspase-3, a significant down-regulation of BRCA1 was noticed, especially at 6 and 12 hours of AWHE treatment; perturbing the possibility of a BRCA-dependent DSB repair pathway. Both BRCA1 and BRCA2 proteins have been implicated in the HR pathway. Individuals who carry biallelic mutations in BRCA1 or BRCA2 are potentially susceptible to any type of cancer other than breast and ovarian cancers. 33 This hypothesis was later tested by measuring the quantity of in-cell levels of γ-H2AX. We observed statistically significant enhancements of H2AX phosphorylation after 24 and 48 hours of AWHE treatment. Based on our findings, we propose that BRCA1 down-regulation and H2AX phosphorylation might be indicative of the effect of AWHE in suppressing DNA repair pathway, resulting in the accumulation of unrepaired DSBs. Increased H2AX phosphorylation by herbal plants was reported earlier by Montano and colleagues, showing that 320 μg/mL of Nerium oleander leaves extract caused DNA damage in lung-derived tumor cells. 34 No study has reported the possible changes in γ-H2AX levels in response to AWHE treatment.
Increased consumption of plant-based compounds has emerged as the most cost-effective approach to reduce the risk of malignancies. Flavonoids, among natural products within these types of plants, are regarded as naturally occurring polyphenols with several subclasses, including flavonols, isoflavones, flavanones, anthocyanins, and proanthocyanidins. These chemicals are proven to have high anti-tumor activity while activating signal pathways in cancer cells, leading to cell death and decreased proliferation. 35 A. wilhelmsii is a plant rich in saponin, including anti-inflammatory, antispasmodic, antidiabetic, anticancer, and cytotoxic activities. 36 By measuring the chemicals of A. wilhelmsii ethanolic extracts, Bali and colleagues exhibited a high total phenolic content which was higher in methanol extract compared to that of the aqueous extract. Besides, significant up-regulation of the Bax and caspase-3 genes were observed in a time- and concentration-dependent manner. Bali and colleagues also reported that the use of methanolic and aqueous extracts of A. wilhelmsii revealed no cytotoxic effects. This was in full agreement with our findings since our results showed significant anti-proliferative effects of AWHE on HeLa cells. A previous study by Sharififar and colleagues reported the immunomodulatory potential of aqueous extract of A. wilhelmsii. Based on their observations, the aqueous extract of A. wilhelmsii exerted a stimulatory effect on both humoral and cellular immune functions in mice. 37 Another study reported that A. wilhelmsii contains flavonoids and monoterpenes such as 1,8-cineole and α-pinene that can induce apoptotic cell death. 38 Recently, novel anti-tumor therapies are based on simultaneous inhibition of both apoptosis and metastasis pathways as an alternative to CC conventional treatment. 39 Furthermore, a previous study showed that AWHE has antioxidant and pro-apoptotic properties; 40 providing a rationale for using this plant in cancer cells other than breast- and prostate-derived tumors. However, there is still little information about other effects of AWHE on cancer cells, especially CC.
One of the limitations of the present study was that we did not investigate the pattern of DNA fragmentation as a marker of direct DNA damage. Moreover, we only determined the possible anti-tumor effects of hydroalcoholic extract of AWHE, while we could have used either the aqueous extract or ethanolic extract of this plant. Although we measured the mRNA levels of BRCA1, VEGF, and caspase-3 genes, assessment of their protein levels could have been advantageous. Evaluation of caspase-3 enzymatic activity besides performing flow-cytometric analysis and TUNEL assay could give a better understanding of the type of cell death predominantly triggered while exposing HeLa cervical cancer cells to AWHE.
Conclusion
AWHE possesses growth inhibitory activity by altering the expression of cell death-associated genes. Using extracts from herbal plants may provide alternative strategies to be deployed in the fight against cancer. Further studies should focus on determining the expression of genes involved in other DNA repair pathways or necrotic cell death.
Acknowledgement
The present study was financially supported by a grant from Zahedan University of Medical Sciences, Zahedan, Iran (grant number: 8656).
Conflict of Interest: None declared.
References
- 1.Miglierini P, Malhaire JP, Goasduff G, Miranda O, Pradier O. Cervix cancer brachytherapy: high dose rate. Cancer Radiother. 2014;18:452–7. doi: 10.1016/j.canrad.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Mohanty G, Ghosh SN. Risk factors for cancer of cervix, status of screening and methods for its detection. Arch Gynecol Obstet. 2015;291:247–9. doi: 10.1007/s00404-014-3492-1. [DOI] [PubMed] [Google Scholar]
- 3.Denny L. Control of cancer of the cervix in low- and middle-income countries. Ann Surg Oncol. 2015;22:728–33. doi: 10.1245/s10434-014-4344-8. [DOI] [PubMed] [Google Scholar]
- 4.Narayan K, Lin MY. Staging for cervix cancer: Role of radiology, surgery and clinical assessment. Best Pract Res Clin Obstet Gynaecol. 2015;29:833–44. doi: 10.1016/j.bpobgyn.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Lynge E, Rygaard C, Baillet MV, Dugue PA, Sander BB, Bonde J, et al. Cervical cancer screening at crossroads. APMIS. 2014;122:667–73. doi: 10.1111/apm.12279. [DOI] [PubMed] [Google Scholar]
- 6.Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015;445:7–11. doi: 10.1016/j.cca.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–48. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12:1528–34. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S, Yoshida T, Tsujioka M, Arakawa S. Autophagic cell death and cancer. Int J Mol Sci. 2014;15:3145–53. doi: 10.3390/ijms15023145. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–44. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 13.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Gan M, Zhang G, Zhou T, Yan M, Wang S. Expression of survivin, caspase-3 and p53 in cervical cancer assessed by tissue microarray: correlation with clinicopathology and prognosis. Eur J Gynaecol Oncol. 2010;31:662–6. [PubMed] [Google Scholar]
- 15.Sarasin A, Stary A. Human cancer and DNA repair-deficient diseases. Cancer Detect Prev. 1997;21:406–11. [PubMed] [Google Scholar]
- 16.Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6:157. doi: 10.3389/fgene.2015.00157. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–71. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:708–18. doi: 10.1128/mcb.24.2.708-718.2004. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podhorecka M, Skladanowski A, Bozko P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/920161. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskander RN, Tewari KS. Targeting angiogenesis in advanced cervical cancer. Ther Adv Med Oncol. 2014;6:280–92. doi: 10.1177/1758834014543794. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–9. doi: 10.2147/vhrm.2006.2.3.213. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herve MA, Buteau-Lozano H, Vassy R, Bieche I, Velasco G, Pla M, et al. Overexpression of vascular endothelial growth factor 189 in breast cancer cells leads to delayed tumor uptake with dilated intratumoral vessels. Am J Pathol. 2008;172:167–78. doi: 10.2353/ajpath.2008.070181. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastukh V, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, et al. An oxidative DNA “damage” and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1367–75. doi: 10.1152/ajplung.00236.2015. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–7. doi: 10.5681/apb.2014.062. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr Oncol. 2006;13:99–107. doi: 10.3747/co.v13i3.88. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Q, Zang YH. Antiproliferative and apoptotic effects of the ethanolic herbal extract of Achillea falcata in human cervical cancer cells are mediated via cell cycle arrest and mitochondrial membrane potential loss. J BUON. 2015;20:1487–96. [PubMed] [Google Scholar]
- 27.Ashtiani M, Nabatchian F, Galavi HR, Saravani R, Farajian-Mashhadi F, Salimi S. Effect of Achillea wilhelmsii extract on expression of the human telomerase reverse transcriptase mRNA in the PC3 prostate cancer cell line. Biomed Rep. 2017;7:251–6. doi: 10.3892/br.2017.956. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern B, Li W, Bono C, Lee LF, Kraynov E. Receptor occupancy and blocking of STAT5 signaling by an anti-IL-7 receptor alpha antibody in cynomolgus monkeys. Cytometry B Clin Cytom. 2016;90:191–8. doi: 10.1002/cyto.b.21247. [DOI] [PubMed] [Google Scholar]
- 29.Saravani R, Galavi HR, Shahraki A. Inhibition of Phosphodiesterase 5 and Increasing the Level of Cyclic Guanosine 3’,5’ Monophosphate by Hydroalcoholic Achillea wilhelmsii C. Koch Extract in Human Breast Cancer Cell Lines MCF-7 and MDA-Mb-468. Breast Cancer (Auckl) . 2017;11:1178223417690178. doi: 10.1177/1178223417690178. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-<kappa>B and its DNA binding activity. Eur J Pharmacol. 2008;588:141–50. doi: 10.1016/j.ejphar.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Galavi HR, Saravani R, Shahraki A, Ashtiani M. Anti-proliferative and apoptosis inducing potential of hydroalcoholic Achillea wilhelmsii C. Koch extract on human breast adenocarcinoma cell lines MCF-7 and MDA-Mb-468. Pak J Pharm Sci. 2016;29:2397–403. [PubMed] [Google Scholar]
- 32.Bali EB, Acik L, Elci P, Sarper M, Avcu F, Vural M. In vitro anti-oxidant, cytotoxic and pro-apoptotic effects of Achillea teretifolia Willd extracts on human prostate cancer cell lines. Pharmacogn Mag. 2015;11:S308–15. doi: 10.4103/0973-1296.166060. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–75. doi: 10.1002/cncr.29041. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon-Montano JM, Burgos-Moron E, Orta ML, Mateos S, Lopez-Lazaro M. A hydroalcoholic extract from the leaves of Nerium oleander inhibits glycolysis and induces selective killing of lung cancer cells. Planta Med. 2013;79:1017–23. doi: 10.1055/s-0032-1328715. [DOI] [PubMed] [Google Scholar]
- 35.George VC, Dellaire G, Rupasinghe HPV. Plant flavonoids in cancer chemoprevention: role in genome stability. J Nutr Biochem. 2017;45:1–14. doi: 10.1016/j.jnutbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Ali N, Shah SW, Shah I, Ahmed G, Ghias M, Khan I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Complement Altern Med. 2011;11:106. doi: 10.1186/1472-6882-11-106. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. Indian J Exp Biol . 2009 ; 47 :668–71. [PubMed] [Google Scholar]
- 38.Asadi-Samani M, Kooti W, Aslani E, Shirzad H. A Systematic Review of Iran’s Medicinal Plants With Anticancer Effects. J Evid Based Complementary Altern Med. 2016;21:143–53. doi: 10.1177/2156587215600873. [DOI] [PubMed] [Google Scholar]
- 39.Burikhanov R, Hebbar N, Noothi SK, Shukla N, Sledziona J, Araujo N, et al. Chloroquine-Inducible Par-4 Secretion Is Essential for Tumor Cell Apoptosis and Inhibition of Metastasis. Cell Rep. 2017;18:508–19. doi: 10.1016/j.celrep.2016.12.051. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bashi DS, Fazly Bazzaz BS, Sahebkar A, Karimkhani MM, Ahmadi A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm Biol. 2012;50:1168–76. doi: 10.3109/13880209.2012.662235. [DOI] [PubMed] [Google Scholar]