Key Points
Question
What is the evidence on the susceptibility to and transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among children and adolescents compared with adults?
Findings
In this systematic review and meta-analysis including 32 studies, children and adolescents younger than 20 years had 44% lower odds of secondary infection with SARS-CoV-2 compared with adults 20 years and older; this finding was most marked in those younger than 10 to 14 years. Data were insufficient to conclude whether transmission of SARS-CoV-2 by children is lower than by adults.
Meaning
Preliminary evidence suggests that children have a lower susceptibility to SARS-CoV-2 infection compared with adults, but the role that children and adolescents play in transmission of this virus remains unclear.
This systematic review and meta-analysis evaluates current evidence on the susceptibility to and transmission of severe acute respiratory syndrome coronavirus 2 among children and adolescents compared with adults.
Abstract
Importance
The degree to which children and adolescents are infected by and transmit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unclear. The role of children and adolescents in transmission of SARS-CoV-2 is dependent on susceptibility, symptoms, viral load, social contact patterns, and behavior.
Objective
To systematically review the susceptibility to and transmission of SARS-CoV-2 among children and adolescents compared with adults.
Data Sources
PubMed and medRxiv were searched from database inception to July 28, 2020, and a total of 13 926 studies were identified, with additional studies identified through hand searching of cited references and professional contacts.
Study Selection
Studies that provided data on the prevalence of SARS-CoV-2 in children and adolescents (younger than 20 years) compared with adults (20 years and older) derived from contact tracing or population screening were included. Single-household studies were excluded.
Data Extraction and Synthesis
PRISMA guidelines for abstracting data were followed, which was performed independently by 2 reviewers. Quality was assessed using a critical appraisal checklist for prevalence studies. Random-effects meta-analysis was undertaken.
Main Outcomes and Measures
Secondary infection rate (contact-tracing studies) or prevalence or seroprevalence (population screening studies) among children and adolescents compared with adults.
Results
A total of 32 studies comprising 41 640 children and adolescents and 268 945 adults met inclusion criteria, including 18 contact-tracing studies and 14 population screening studies. The pooled odds ratio of being an infected contact in children compared with adults was 0.56 (95% CI, 0.37-0.85), with substantial heterogeneity (I2 = 94.6%). Three school-based contact-tracing studies found minimal transmission from child or teacher index cases. Findings from population screening studies were heterogenous and were not suitable for meta-analysis. Most studies were consistent with lower seroprevalence in children compared with adults, although seroprevalence in adolescents appeared similar to adults.
Conclusions and Relevance
In this meta-analysis, there is preliminary evidence that children and adolescents have lower susceptibility to SARS-CoV-2, with an odds ratio of 0.56 for being an infected contact compared with adults. There is weak evidence that children and adolescents play a lesser role than adults in transmission of SARS-CoV-2 at a population level. This study provides no information on the infectivity of children.
Introduction
The degree to which children and adolescents younger than 20 years are infected by and transmit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an unanswered question.1,2,3 These data are vital to inform national plans for relaxing social distancing measures, including reopening schools.
Children and adolescents account for 1% to 3% of reported coronavirus disease 2019 (COVID-19) cases across countries4,5,6,7,8 and an even smaller proportion of severe cases and deaths.5,9 Children appear more likely to have asymptomatic infection than adults, and analyses based on symptom-based series underestimate infections in children. The role that children and adolescents play in transmission of SARS-CoV-2 is dependent on their risk of exposure, their probability of being infected on exposure (susceptibility), the extent to which they develop symptoms on infection, the extent to which they develop a viral load sufficiently high to transmit, and their propensity for making potentially infectious contact with others, dependent on numbers of social contacts across age groups and behavior during those contacts.
Different study types may provide useful information on susceptibility and transmission in children compared with adults, yet each is open to bias. Contact-tracing studies with systematic follow-up of all contacts to estimate secondary attack rates in children and adults can provide strong evidence on differential susceptibility. Findings from some contact-tracing studies suggest that children have lower SARS-CoV-2 secondary attack rates than adults,10 although others have found no difference by age.11 One study from South Korea12 has suggested adolescents but not children may have higher secondary attack rates, although a separate analysis of child cases from the same population identified minimal transmission from these individuals.13
Population screening studies may identify infection through viral RNA detection or antibodies indicating prior infection. However, the prevalence of SARS-CoV-2 in children in a population is not a direct indicator of susceptibility or transmission, as the expected prevalence depends on exposure, susceptibility, proportions of children in the population, mixing rates among children and between adults and children, and timing of social distancing interventions that disrupt mixing.
A number of authors have concluded that children and adolescents may be less susceptible to SARS-CoV-2,2,14 although there are multiple sources of bias in each study type, which can complicate straightforward analysis. In contact-tracing studies, testing of only symptomatic contacts will introduce significant bias, as will seroprevalence studies drawn from clinical contact studies (eg, primary care) or residual laboratory sera. Many studies undertaken quickly during the pandemic are underpowered to identify age differences.
We undertook a systematic review and meta-analysis of published and unpublished literature to assess child and adolescent susceptibility to SARS-CoV-2 compared with adults. We limited this review to contact-tracing studies and population-based studies, as these are likely to be most informative and least open to bias.
Methods
Our review question was “What is the susceptibility to SARS-CoV-2 of children and adolescents compared with adults?” We undertook a rapid systematic review and included contact-tracing studies or prevalence studies in published or preprint form as well as data from a national public health website reporting government statistics and studies. Studies were required to provide data on proven SARS-CoV-2 infection (by polymerase chain reaction or serology) and report either rate of secondary infections in children and adolescents compared with adults or infection prevalence or seroprevalence in children and adolescents separate from adults. We excluded reports of single household or single institution outbreaks; studies of hospitalized patients, clinical studies, and cohorts defined by symptoms; studies of unconfirmed cases, ie, cases based on self-report or symptoms, including contact-tracing studies where only symptomatic contacts were traced; modeling studies or reviews, unless these reported new data; and prevalence studies with ascertainment based on clinical contact and seroprevalence studies of residual sera, as these are likely to underrepresent children. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Where studies were drawn from populations that overlapped, we excluded studies where the time periods overlapped but included studies where time periods did not overlap. We did not include seroprevalence studies only in children in this review, as these did not allow comparison with adults.
We searched 2 electronic databases, PubMed and the medical preprint server medRxiv, on May 16, 2020, and updated this on July 28, 2020. We used the following search terms in PubMed: (“COVID-19”[tw] OR “2019-nCoV”[tw] OR “SARS-CoV-2”[tw]) AND ((child* OR infant*) OR (“transmission”[tw] OR “transmission” [mh]) OR (“Disease Susceptibility”[tw] OR “susceptibility”(mh)) OR (“epidemiology”[tw] OR “epidemiology” [mh]) OR (“contact tracing”[tw] or “communicable disease contact tracing”[mh])). In medRxiv, we undertook separate searches for “child and covid-19,” “covid-19 and epidemiology,” “covid-19 and susceptibility,” and “covid-19 transmission,” as more complex Boolean searches are not available.
Figure 1 shows the PRISMA flow diagram. One researcher (R. M. V.) screened studies based on titles and abstracts to identify potentially eligible studies for full-text review. Full-text studies were then reviewed by 2 researchers for eligibility (R. M. V. and O. T. M. or C. W.), and data were extracted independently by 2 researchers (R. M. V. and either O. T. M. or C. W.). We hand searched cited references in all potentially eligible studies for additional studies and identified additional studies through authors’ professional networks. Data were extracted on country, study type, study context with regards social distancing measures and school closures at the time of the study, case definition, testing method, sampling method, and infection rates in adults and children.
Figure 1. PRISMA Flow Diagram for Search.
Methodological quality of included studies was assessed independently by 3 authors (R. V. M., O. T. M., and C. W.) based on a critical appraisal checklist for prevalence studies.15 We assessed risk of bias using 2 additional criteria: whether symptomatic contacts (in contact-tracing studies) or individuals (in population screening studies) were more likely to participate than asymptomatic ones and whether the obtained sample was more than 75% of the intended sample. Studies were categorized as high quality if they met all quality criteria and had low risk of bias on both criteria; medium quality if they had low risk of bias on 1 or more criteria and met 5 or more of 7 quality criteria; low quality if they met less than 5 quality criteria; or uncertain quality if multiple domains could not be scored.
Contact-tracing studies and population prevalence studies were considered separately. Random-effects meta-analysis with restricted maximum likelihood estimation was undertaken using the meta commands in Stata version 16 (StataCorp). Odds ratios (ORs) were used as the primary metric for contact-tracing studies. Prevalence ratios were used as the primary metric in population-based studies. We planned subgroup analyses using restricted maximum likelihood based on quality of study and age of children and adolescents. P values were calculated using χ2 tests. Significance was set at a P value less than .05, and all P values were 2-tailed.
Results
The PubMed search resulted in 3465 studies and the medRxiv search resulted in 10 461 studies, of which 113 and 90 studies, respectively, were examined in full, and 16 studies included (Figure 1). We identified a further 6 studies through reference checking and 10 studies through professional networks. In total, 32 studies comprising 41 640 children and adolescents and 268 945 adults were included (Table),7,10,12,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 with quality and bias assessments shown in eTable 1 in the Supplement and weblinks for included studies shown in eTable 2 in the Supplement. A total of 18 studies were contact-tracing studies (CTSs),7,10,12,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31 with 3 based in schools,29,30,31 and 14 studies were population screening studies.7,32,33,34,35,36,37,38,39,40,41,42,43,44,45 Two were high quality,33,35 22 were medium quality,10,12,16,21,22,24,25,26,27,29,30,31,32,34,36,38,40,41,42,43,44,45 7 were low quality,17,18,19,20,23,28,39 and 1 was uncertain quality.7,37
Table. Characteristics of Included Studies.
| Source | Status | Location | Recruitment of index cases | Recruitment and isolation of contacts | Contact type | No. of clusters, index cases, and contacts | Case definition and virus testing | Child/adolescent age; adult age |
|---|---|---|---|---|---|---|---|---|
| Contact-tracing studies | ||||||||
| Zhang et al,10 2020 | Published and peer reviewed | Hunan province, China | All confirmed cases identified by Hunan Center for Disease Control between January 16 and March 1, 2020 | Close contacts were identified through contact tracing of confirmed cases and placed under medical observation for 14 d. A close contact was defined as an individual who had unprotected close contact (within 1 m) with a confirmed case or an asymptomatic infection within 2 d before their symptom onset or sample collection. | All contact types | 114 Clusters representing 136 index cases and 7193 contact cases. 1 Index case (0.7%) was <15 y. | Positive findings on RT-PCR. All close contacts were tested in accordance with local policy regardless of symptoms. Percentage of contacts tested was not stated. | 0-14 y; ≥15 y |
| Li et al,16 2020 | Published and peer reviewed | Hubei province, China (hospitals in Zaoyang City and Chibi City) | Index cases identified from 2 hospitals from January 1 to February 13, 2020. Index cases were excluded if members of their family had links to Wuhan province. Not clear if all cases from hospital were sampled or just a subset. | All household contacts were quarantined immediately for 14 d by the local government and monitored daily. | Household contacts | 105 Index patients with their households and all family contacts (n = 392). The proportion of index cases who were children was not reported. | Positive findings on RT-PCR. Nasopharyngeal swab samples were collected at the beginning and the middle of quarantine. All contacts were tested 2 to 4 times. | 0-17 y; ≥18 y |
| Cheng et al,17 2020 | Published and peer reviewed | Taiwan | The initial 100 confirmed cases in Taiwan between January 15 and March 18, 2020. | Close contacts were identified through epidemiological investigation and defined as a person who did not wear appropriate personal protection equipment while having face-to-face contact with a confirmed case for more than 15 min during the investigation period (defined by epidemiological investigation and typically up to 4 d prior to symptom onset or test date for asymptomatic cases). All close contacts were quarantined at home for 14 d after their last exposure to the index case. | All contact types | 100 Index cases and 2761 close contacts. The youngest index case was aged 11 y, although the proportion of index cases that were children was not reported. | Positive findings on RT-PCR. Routine testing for household and health care worker contacts (30.7%). Other contacts (69.3%) were only tested if symptomatic. | 0-19 y; ≥20 y |
| Wang et al,18 2020 | Published and peer reviewed | Wuhan province, China | Patients hospitalized in Union Hospital (n = 85) on February 13 and 14, 2020. Not clear if all cases from hospital were sampled or just a subset. | Household contacts of the hospitalized patients, observed for 14 d. | Household contacts | 85 Households corresponding to the 85 patients were enrolled and 155 household contacts. | Positive findings on RT-PCR and throat swabs. Process for testing household members was not stated, but 33% of household contacts were not tested for SARS-CoV-2. | Child age not defined. |
| Mizumoto et al,19 2020 | Preprint | Japan | Cases that were domestically acquired and confirmed by RT-PCR by March 7, 2020. | Contacts of index cases, definition, and method of ascertainment not given. No details on isolation of contacts. | Not stated. The total number of contacts (8 per index case) suggests these are likely all contacts. | 313 Cases and their 2496 close contacts. | Positive findings on RT-PCR. Process and eligibility for testing of contacts not described. | 0-19 y; ≥20 y |
| Wang et al,20 2020 | Published and peer reviewed | Beijing, China | All laboratory-confirmed (ie, RT-PCR–confirmed) cases in Beijing up to February 21, 2020, recruited through Beijing Center for Disease Control. | From February 28 to March 8, 2020, all household members of index cases were observed for 14 d. Testing and quarantine of contacts not clearly defined. | Household contacts | 124 of 137 Eligible families participated. No primary cases were <18 y. | Index and secondary cases defined as positive findings on RT-PCR. Proportion of PCR testing of secondary contacts was not stated. | 0-17 y; ≥18 y |
| Park et al,12 2020 | Published and peer reviewed | South Korea | All laboratory-confirmed cases in Korea registered with the Korea Center for Disease Control from January 20 to May 13, 2020. | All contacts of index cases registered with Korea Center for Disease Control through a comprehensive national contact-tracing system and observed for a mean of 10 d. | Household and nonhousehold contacts. Only data on household contacts were included in this review. | Studied 59 073 contacts (10 592 were household contacts) of 5706 index cases. Only index cases who reported 1 or more contacts were included; however, only 52% of 10 962 national cases reported in the period were included. | Household and health care worker contacts routinely tested by RT-PCR. Other contacts only tested if symptomatic. | 0-19 y; ≥20 y |
| Dattner et al,21 2020 | Preprint | Israel | Identification of all households in the city of Bnei Brak, Israel, where all household members had been tested by PCR and 1 or more members had positive findings. Households identified through the Israeli COVID-19 database until May 2, 2020, were included. | All household members included. A total of 51% of population were <20 y. | Household | 637 Houses comprising 3353 people, of whom 1510 were positive for SARS-CoV-2. All eligible households were included. Figures 2, 3, and 4 were derived from supplied estimated probabilities of children or adults being the index. | Positive findings on RT-PCR testing of all household members, including index cases and contacts. | 0-19 y; ≥20 y |
| Hu et al,22 2020 | Preprint | Hunan province, China | All cases with contact details were identified from the notifiable infectious diseases reporting system in Hunan province from January 16 to April 2, 2020. | Contacts were quarantined for 14 d and tested with PCR at least once during quarantine. After February 7, 2020, all contacts were tested, but only symptomatic contacts were tested before (approximately 50% of contacts tested). | All contacts | 1178 Cases and their 15 648 contacts. | Positive findings on RT-PCR. | 0-19 y; ≥20 y |
| Laxminarayan et al,23 2020 | Preprint | Tamil Nadu and Andhra Pradesh, India | Index cases identified from state registries and contacts traced by public health agencies in each state from March 5 to June 4, 2020, in Tamil Nadu, India, and from March 5 to May 29, 2020, in Andhra Pradesh, India. | Contacts traced by public health agencies and tested between 5 and 15 d of exposure. Insufficient detail provided. Note that there were twice as many close contacts per index case <18 y compared with >18 y. | All contacts | 4206 Confirmed cases and 64 031 contacts. Note only 4206 cases included of 33 584 total cases (13%), with no detail given on nonrecruitment. | Positive findings on RT-PCR of all contacts regardless of symptoms. | 0-17 y; ≥18 y |
| Liu et al,24 2020 | Published and peer reviewed | Guandong province, China | All cases identified by intensive regional surveillance by local center for disease control from January 15 to March 15, 2020. | Contacts traced and monitored with PCR from throat swabs taken every few days for 14 d; 84% of contacts were quarantined in centralized stations. | All contacts | 1361 Cases reported and 11 868 contacts traced and quarantined. Analysis included 11 580 contacts (98%). | Positive findings on RT- PCR from throat swabs. | 0-19 y; ≥20 y |
| Rosenberg et al,25 2020 | Published and peer reviewed | New York State excluding New York City | Identified and studied 229 initially confirmed cases by PCR from March 2 to 12, 2020. | Active contact tracing by county and state health departments. All household contacts were eligible for PCR testing. Contacts tested 0 to 10 d after index case (43% were tested on day 0, ie, initial index diagnosis day). | Household | 229 Index cases and 343 household contacts. | Positive findings on RT-PCR. All household contacts were eligible for PCR testing; however, not stated what proportion were tested. | 0-17 y; ≥18 y |
| Yousaf et al,26 2020 | Published and peer reviewed | Milwaukee, Wisconsin, and Salt Lake City, Utah | All PCR-positive cases from 2 cities were identified through routine public health surveillance and recruited between March 22 and April 22, 2020. | Active contact tracing by public health departments. All contacts were observed for 14 d with 2 swab tests (RT-PCR) on day 0 and day 14, plus if symptomatic. | Household | 195 of 198 Contacts participated (98.5%). Numbers of index cases not stated. | Positive findings on RT-PCR. All household contacts tested. | 0-17 y; ≥18 y |
| Chaw et al,27 2020 | Preprint | Brunei | All 71 initial cases in Brunei, which arose following a religious event, with cases detected after March 9, 2020. | Detailed contact tracing by Ministry of Health, with RT-PCR testing of all reported contacts. All contacts were quarantined for 14 d and retested if symptomatic. | All contacts | 71 Index cases and 1755 close contacts. All contacts participated. | Positive findings on RT-PCR. | 0-19 y; ≥20 y |
| van der Hoek et al,7,28 2020 | Published and peer reviewed | The Netherlands | National surveillance data from 2 Dutch systems: (1) OSIRIS, a registry of all laboratory-confirmed cases and (2) HPZone. Data on contact tracing from 23 of 25 Dutch municipalities. | Data included up to April 2, 2020. Contact tracing was undertaken for all cases registered in HPZone. Contact infection status identified through linkage to the main national surveillance database, suggesting that only symptomatic secondary cases were included. | All contacts | 231 Cases and 709 close contacts. Proportion of contacts tested not stated. | Positive findings on RT-PCR. | <19 y |
| School contact-tracing studies | ||||||||
| Macartney et al,29 2020 | Published and peer reviewed | New South Wales, Australia | COVID-19 cases in 25 educational settings (15 schools and 10 early learning centers) for which a person (student or staff) with proven COVID-19 (positive findings on PCR) had attended while infectious. Identified through state Notifiable Conditions Information Management System. Schools remained open but students dismissed from March 23, 2020 (<5% student attendance). Note that school attendance remained high at the time that secondary cases were identified in schools, and early-years settings did not close. | January 25 to April 9, 2020. All close contacts (a person who has been in face-to-face contact for at least 15 min or in the same room for at least 40 min with a case while infectious) were followed up. All close contacts observed and tested if symptomatic during the 14-d isolation period. 7 Settings had testing of all contacts 5 to 10 d after last contact plus serology after day 21. | Contacts in educational setting only | 27 Primary cases (12 student and 15 staff cases) and their 1448 school-related and early learning–related close contacts from 25 educational settings. 12 High school cases (8 students; 4 staff) from 10 schools had a total of 695 contacts (598 students; 97 staff). The 5 primary school cases (1 student; 4 staff) from 5 schools had a total of 218 contacts (179 student; 39 staff). 1448 Contacts identified; 663 (43.5%) were tested (PCR or serology or both). | Positive findings on RT-PCR or serology (specific IgG, IgA, IgM detection using indirect immunofluorescence). Swabs taken from 542 of 1448 contacts (37.4%). Serology was performed in 208 of 1448 contacts (14.3%). | 6 wk-18 y; ≥20 y |
| Heavey et al,30 2020 | Published and peer reviewed | Ireland | Screened Ireland national surveillance to identify all PCR-positive cases in children or adults who attended school settings from March 1 to March 12, 2020. | Contacts traced and advised to quarantine at home for 14 d. Tested (PCR) only if symptomatic. | All contacts, including school contacts | 6 Index cases identified (3 adult; 3 <18 y). 1155 Contacts identified (924 children; 101 adults). | Positive findings on RT-PCR testing if symptomatic. | 0-17 y; ≥18 y |
| Yung et al,31 2020 | Published and peer reviewed | Singapore | 3 Potential SARS-CoV-2 seeding incidents in educational settings in Singapore identified from national surveillance during February and March 2020. | Close school contacts (eg, classmates) quarantined for 14 d. Contacts in 1 school and 1 preschool were tested only if symptomatic; these schools were not closed. Contacts in 1 preschool were tested by PCR after an outbreak causing school closure. | School contacts only | 3 PCR-positive child index cases were identified from 2 preschools and 1 secondary school. 188 Contacts were studied, of whom 119 (63%) were tested. | Positive findings on RT-PCR. | 1-16 y |
| Population screening studies | ||||||||
| Gudbjartsson et al,32 2020 | Published and peer reviewed | Iceland | First infection diagnosed on February 28, 2020; containment measures put in place. Primary schools open but some secondary schools closed and moderate restrictions on social contacts from March 13 to April 6, 2020. | National population screening. Open invitation for 87% of participants through online portal but with collection of sample from 1 location (Reyjkavik) and random invitation for a subsample (13%). Children <10 y made up 6.4% of sample. Participation in the study was primarily by request of participants rather than by random sampling, which may have introduced biases in participation. | NA | Only population screening sample reported here. | Positive findings on RT-PCR on nasopharyngeal and oropharyngeal samples. | 0-9 y; ≥10 y |
| Lavezzo et al,33 2020 | Published and peer reviewed | Vo, Veneto region, Italy | Quarantined community in an area of Italy that was affected early and severely in the epidemic; area was locked down starting February 23, 2020, for 2 weeks. Study undertaken close to the imposition of very strict social distancing measures in the region. | All age groups were homogeneously sampled, with age-specific percentages ranging from 70.8% to 91.6%. 2 Surveys undertaken; first survey only included here (overall response rate 85.9%). Those <21 y made up 17% of sample and had a participation rate of 94% (0-10 y) and 95% (11-20 y). | NA | We present data only from this first survey, although the article also reports a second survey undertaken during lockdown. | Positive findings on RT-PCR on nasopharyngeal samples. | 0-20 y; ≥21 y |
| Public Health Agency of Sweden,34 2020 | Online report | Sweden | First death reported in Stockholm on March 11, 2020. Voluntary social distancing measures recommended starting March 16, 2020, with secondary schools recommended to teach virtually. Primary schools and early-years settings remained open throughout. | 2 Nationally representative surveys undertaken by the Public Health Agency of Sweden. Participants invited by email: 2571 of 4480 (57%) participated in April and 2957 of 4487 (66%) in May. Children 0-15 y made up 18.9% of the April sample and 17.2% of the May sample. Participants performed home self-sampling using nasopharyngeal swabs. | NA | NA | Positive findings on RT-PCR on nasopharyngeal samples. | 0-15 y; ≥16 y |
| Office for National Statistics,35 2020 | Online report | England | Strict national social distancing measures enacted March 20, 2020, with gradual easing of lockdown starting May 25, 2020. | Representative sample of 35 801 individuals in England. Those aged 2-19 y made up 17% of the population. Cases were identified by home self-sampling using nasopharyngeal swabs, with carers swabbing young children. 79% of Invited participants provided 1 or more swabs. | NA | Repeated surveys carried out each week. Data shown here are the cumulative prevalence of those ever positive between April 26 and June 27, 2020. | Positive findings on RT-PCR on nasopharyngeal samples. | 2-19 y; ≥20 y |
| Pollán et al,36 2020 | Online report | Spain | Strict social distancing was imposed on March 14, 2020. Some restrictions were lifted on April 27, 2020, and further restrictions lifted on May 11, 2020. | Undertaken by Spanish Ministry of Health. National representative sample obtained from random sampling of households in municipalities across Spain. Of 102 803 approached, 61 075 participants provided point of care samples and 51 958 included in both immunoassay and point of care. Those aged 0-19 y (n = 11 464) made up 23% of the point of care sample and 12.6% of the immunoassay sample. | NA | We used the point-of-care data here owing to the sample being representative of the child population, unlike the immunoassay test. | Point-of-care test: rapid immunochromatography IgG (Zhejiang Orient Gene Biotech) and SARS-CoV-2 immunoassay (Abbott Laboratories). Comparison of the rapid test IgG with SARS-CoV-2 serology in 16 953 of the study sample found 97.3% agreement between tests. | 0-19 y; ≥20 y |
| National Institute for Public Health and the Environment,7,37 2020 | Online report | The Netherlands | Social distancing measures introduced gradually from March 11, 2020. Schools closed from March 15, 2020. | Undertaken by the Netherlands National Institute for Public Health and the Environment. Population-based sampling was undertaken in a random sample of a randomly chosen subset of municipalities across the Netherlands. Total sample of 2096. Those <20 y made up 20% of sample. | NA | Data provided by author F. v. d. K. | Positive findings on serology (IgG). | 0-19 y; ≥20 y |
| Hallal et al,38 2020 | Preprint | Brazil | First cases reported February 27, 2020, with local/state lockdowns during March and April 2020. Some states began to relax measures in April 2020. | Nationwide seroprevalence survey in 133 sentinel cities in 26 Brazilian states. Randomly selected households visited and finger-prick rapid serology test used. Total sample was 24 995 with household response rate (55%). Children heavily underrepresented—2.2% were aged 0-9 y and 9.1% were aged 10-19 y. | NA | NA | Rapid lateral flow test used in our analysis (Wondfo SARS-CoV-2). | 0-19 y |
| Shakiba et al,39 2020 | Preprint | Iran | Population-based seroprevalence study in 5 counties in Guilan province, Iran, in April 2020—previously very high virus prevalence. | Multistage cluster random sampling approach and telephone recruitment of head of household. 196 of 632 Approached households participated (31%) with 528 participants. | NA | NA | VivaDiag COVID-19 IgM/IgG serology. | 0-17 y; ≥18 y |
| Biggs et al,40 2020 | Published and peer reviewed | Georgia | Study undertaken by US Centers for Disease Control and Prevention to coincide with end of shelter-in-place orders (April 3 to 30, 2020). | Survey of a random sample of households in 2 metropolitan Atlanta countries. 696 Persons from 394 of 1675 households (23.5%) participated. Children <18 y were 6.9% of sample compared with 22.4% of population. | NA | NA | Total antibody measured using VITROS 3600 Immunodiagnostic System (Ortho Clinical Diagnostics). | 0-17 y; ≥18 y |
| Stringhini et al,41 2020 | Published and peer reviewed | Geneva canton, Switzerland | First case on February 26, 2020. Schools closed on March 16, 2020, and strict social distancing measures introduced March 20, 2020. Seroprevalence initiated using a population-based sample in canton. | Population based but not fully random sample within canton (region). 1300 Randomly selected adults approached each week for 5 weeks and invited to bring all household members ≥5 y for serology. Only nonsymptomatic individuals studied. A total of 2766 of 5492 (50.4%) agreed to participate, and data presented here for first 1360. Total of 16.4% of sample aged 0-19 y, similar to population. | NA | Indeterminate cases were treated as having negative findings in calculating data for the meta-analysis. | ELISA to spike protein (Euroimmun). | 5-19 y; ≥20 y |
| Nawa et al,42 2020 | Preprint | Utsunomiya, Japan | First cases in Japan starting January 15, 2020. All schools closed February 27, 2020. Survey conducted between the first and second spikes of infection in the city. | Population-based seroprevalence survey: a random sample of 1000 households approached. A total of 742 of 2290 persons (32%) participated. 13% Were <18 y, similar to population. | NA | NA | IgG (Shenzhen YHLO Biotech). | 0-17 y; ≥18 y |
| Pagani et al,43 2020 | Preprint | Lombardy, Italy | The town of Castiglione d’Adda, 4550 inhabitants had high numbers of infections from early in the pandemic. Local lockdown occurred from February 23, 2020. | Entire population (all ages) invited to participate; recruited 4174 of 4550 inhabitants (92%) who had rapid capillary testing, of whom a random sample of 562 (stratified for age and sex) had formal serology by venipuncture. Those <19 y made up 12% of the rapid and formal serology samples. | NA | 22% of Population showed overall positivity (22.2% on rapid test, 22.6% on formal serology). Rapid test used in meta-analyses here, as findings from formal serology were highly similar. | Rapid capillary testing: lateral-flow immunochromatographic test (Prima Lab); serology: CLIA, IgG anti–SARS-CoV-2 (Abbott Laboratories). | 0-19 y; ≥20 y |
| Weis et al,44 2020 | Preprint | Thuringia, Germany | Seroprevalence survey in the previously quarantined community Neustadt-am-Rennsteig, from 6 weeks after a SARS-CoV-2 outbreak (March 22, 2020). Local lockdown initiated. | All community households invited. A total of 626 of 883 (71% of community) enrolled. Focus on child participation and blood collection to be representative. Children 1-17 y were 9.5% of the sample; 620 gave blood and 600 participants had all 6 serological tests performed. | NA | NA | Serology by 6 quantification methods: 2 ELISA and 4 immunoassay: EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics); SARS-CoV-2 IgG ELISA kit (Euroimmun); SARS-CoV-2 S1/S2 IgG CLIA kit (DiaSorin); 2019-nCoV IgG kit (Snibe Co); SARS-CoV-2 IgG CMIA kit (Abbott Laboratories); and Elecsys Anti-SARS-CoV-2 kit (Roche). | 1-17 y; ≥18 y |
| Streeck et al,45 2020 | Preprint | Gangelt, Germany | Carnival held on February 15, 2020. Strict local social distancing measures introduced on February 28, 2020, due to local outbreak and deaths. | A random sample of 600 households was invited to participate, and 1007 individuals from 405 households participated. 919 Provided serology data. A total of 6.0% of sample was made up of those aged 5-14 y. | NA | A total of 62% of the 88 participants who could not be assessed were children not assessed for technical reasons. | Positive findings on serology (IgG). | 5-14 y; ≥15 y |
Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; NA, not applicable; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Contact-Tracing Studies
A total of 6 studies were from mainland China,10,16,18,20,22,24 2 from the US,25,26 and 1 each from Taiwan,17 Japan,19 South Korea,12 Israel,21 the Netherlands,7,28 Brunei,27 and India,23 with 3 CTSs based in schools from Australia,29 Ireland,30 and Singapore.31 Lower secondary attack rates in children and adolescents compared with adults were reported by 11 studies: 5 from provinces of China, including Hunan,10,22 Hubei,16,18 and Beijing,20 and 6 from other countries, including Taiwan,17 Japan,19 the US,25,26 Israel,21 and the Netherlands,7,28 although confidence intervals were wide in some studies.
No significant differences in secondary attack rates by age were reported in 3 studies from Guangdong province, China,24 Brunei,27 and the states of Tamil Nadu and Andhra Pradesh in India,23 with 1 study from South Korea12 reporting high secondary attack rates in those younger than 19 years. In 3 of these studies,12,23,27 secondary attack rates in younger children were low compared with adults, but those among teenagers were as high as or higher than adults.
We undertook a random-effects meta-analysis of secondary attack rates in children and adolescents compared with adults, with data included from 14 studies.10,12,16,17,18,19,20,21,23,24,25,26,27,28 We combined data on children and adolescents younger than 20 years and compared it with an adult group 20 years and older; thus, ORs and prevalence rates for adults may differ from those reported in studies. The pooled OR estimate for all contact-tracing studies of being a child with secondary infection compared with being an adult was 0.56 (95% CI, 0.37-0.85), with high heterogeneity (I2 = 94.6%) (Figure 2).10,12,16,17,18,19,20,21,23,24,25,26,27,28
Figure 2. Pooled Estimate of Odds of Being an Infected Contact Among Children and Adolescents Compared With Adults for All Contact-Tracing Studies.

Children and adolescents included those younger than 20 years, and adults included those 20 years and older. OR indicates odds ratio.
We undertook a meta-analysis of 8 CTSs grouped by age of child (Figure 3)10,12,21,23,24,25,27,28; the ages differed across studies, and children were defined as those younger than 10 to 14 years, adolescents as those older than 10 to 12 years, and adults as those 20 years and older. The pooled OR for children was 0.52 (95% CI, 0.33-0.82), significantly lower than adults (1 [reference]). For adolescents, this was nonsignificant (OR, 1.23; 95% CI, 0.64-2.36). χ2 Test suggested this group difference was significant (χ2 = 4.54; P = .03). When only the 8 high-quality and medium-quality studies with low risk of bias were examined,10,12,16,21,24,25,26,27 this finding was no longer significant (OR, 0.68; 95% CI, 0.41-1.11); however, the difference in estimates between low-quality studies and high-quality and medium-quality studies was not significant (eFigure 1 in the Supplement).
Figure 3. Pooled Estimate of Odds of Being an Infected Contact Among Children and Among Adolescents Compared With Adults for Contact-Tracing Studies.
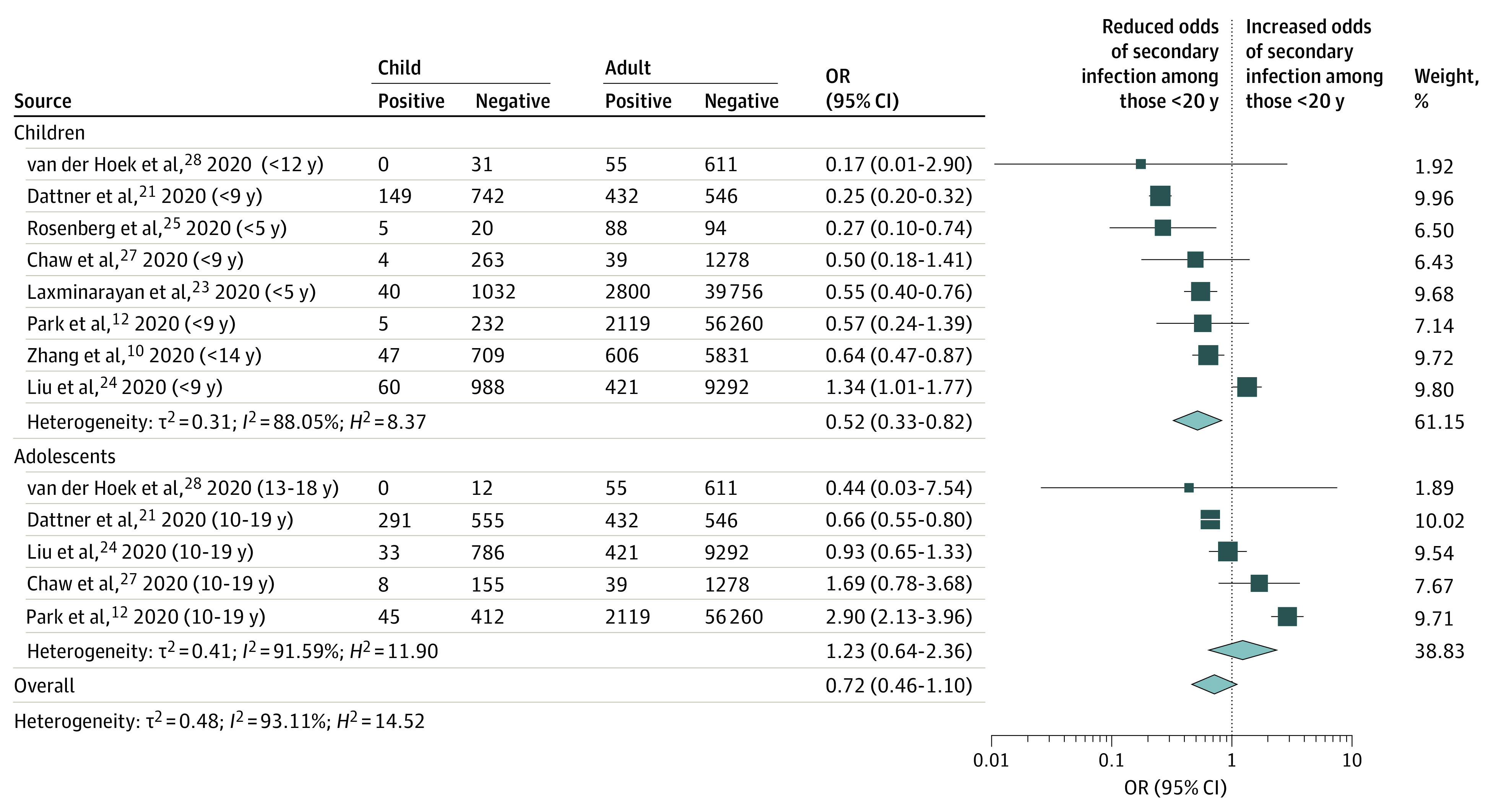
Children included those younger than 10 years, adolescents included those aged 10 to 19 years, and adults included those 20 years and older. OR indicates odds ratio.
We hypothesized that CTSs including only household contacts might provide a clearer indication of the relative susceptibility to infection of children vs adults because all contacts within households might be assumed to receive a similar exposure to infection from index cases. A post hoc analysis by type of contacts (eFigure 2 in the Supplement) showed that studies of household contacts had a lower pooled OR (OR, 0.41; 95% CI, 0.22-0.76)) than studies of all contacts (OR, 0.91; 95% CI, 0.69-1.21; between-group variance: χ21 = 5.31; P = .02).
Three studies undertook contact tracing in schools.29,30,31 A statewide population-based CTS in educational settings in Australia before and during school closures29 found that 27 primary cases (56% staff) across 25 schools or early-years nurseries resulted in 18 secondary cases in 4 settings, including an outbreak of 13 cases in 1 early-years setting initiated by a staff member, with no evidence of child-to-adult transmission. The secondary attack rate was 1.2% (18 of 1448) overall, 0.4% (5 of 1411) when excluding the early-years outbreak, and 2.8% (18 of 633) in those tested. Other national CTSs undertaken in schools in Ireland30 and Singapore31 before schools closed identified very few secondary cases in schools.
Population Screening Studies
Data from prevalence studies for children and adolescents compared with adults are shown in Figure 4.7,32,33,34,35,36,38,39,40,41,42,43,44,45 We did not undertake a meta-analysis of population screening studies given the important differences in the populations, epidemic time points, and methodologies involved.
Figure 4. Ratios of the Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children and Adolescents Compared With Adults in Population Screening Studies.

Four studies reported virus prevalence.32,33,34,35 National prevalence studies from Iceland32 and Sweden34 undertaken while primary schools were open showed lower prevalence among children and adolescents than adults, as did a municipal study from Italy33 undertaken just before lockdown while schools were open. However, a nationally representative survey from England covering lockdown and the subsequent month identified no significant differences by age.35
A total of 10 studies reported seroprevalence,7,36,38,39,40,41,42,43,44,45 3 being nationally representative.7,36,38 A lower seroprevalence was identified in children and in some adolescents compared with adults in a number of studies, including a nationally representative study in Spain (ENE-COVID),36 a Dutch nationally representative study (PIENTER Corona study),7,37 and city or regional studies from Iran,39 the US,40 Switzerland,41 and Japan,42 although no difference by age was found in a survey in 133 sentinel cities in 26 Brazilian states.38 Two community-based studies following localized outbreaks found lower seroprevalence among children and adolescents than adults in Lombardy, Italy,43 and Thuringia, Germany,44 with a second German postoutbreak study45 finding no overall association with age. Examination of seroprevalence findings in children separate from adolescents (eFigure 3 in the Supplement) suggested that seroprevalence was lower among children younger than 10 years than adults but not lower among adolescents aged 10 to 19 years than adults, although this was not formally tested.
Discussion
We identified 32 studies from 21 countries that met our eligibility criteria and provided information on susceptibility to and transmission of SARS-CoV-2 in children and adolescents compared with adults. We excluded studies and study types open to very significant bias, yet studies were predominantly of medium and low quality, with only 2 high-quality studies.34,35 Most studies were from middle-income and high-income countries in East Asia and Europe.
We found preliminary evidence from 15 contact-tracing studies that children and adolescents have lower susceptibility to SARS-CoV-2 infection than adults, with a pooled OR of 0.56 (95% CI, 0.37-0.85). This estimate was little changed when only medium-quality or high-quality studies were examined, although power was reduced and significance was attenuated. Only 1 study13 found a higher odds of infection in those younger than 20 years than adults, although this finding was confined to those aged 10 to 19 years. When studies were categorized by age, lower susceptibility appeared to be confined to those younger than 10 to 14 years, who had 48% lower odds of infection compared with those 20 years and older. The age bands of the studies were not aligned, making direct comparisons challenging.
Data from population screening studies were heterogenous and were not suitable for meta-analysis. Findings consistent with lower seroprevalence in those younger than 20 years compared with adults were reported by 2 national studies,7,36 1 regional study,40 and all of the municipal postoutbreak studies,43,44,45 although confidence intervals were wide in some cases. Two virus prevalence studies similarly reported lower infection rates in those younger than 20 years. In contrast, other studies reported no age-related differences. No studies reported higher prevalence in children and adolescents. Examination of seroprevalence findings in children separate from adolescents showed that most studies were consistent with lower seroprevalence in children compared with adults, although seroprevalence in adolescents appeared similar to adults in all studies.
The findings from the CTSs and prevalence studies are largely consistent in suggesting that those younger than 10 to 14 years are less susceptible to SARS-CoV-2 infection than those 20 years and older, resulting in lower prevalence and seroprevalence. Data specifically on adolescents are sparse but consistent with susceptibility and prevalence rates of adults. Our findings on susceptibility are similar to a modeling analysis by Davies et al,46 which estimated that those younger than 20 years were approximately half as susceptible to SARS-CoV-2 as adults.
We found few data that were informative on the onward transmission of SARS-CoV-2 from children to others. Data from a large Australian school contact-tracing study29 suggest that, at a population level, children and adolescents might play only a limited role in the transmission of this virus. This is consistent with the data on susceptibility noted above, ie, suggesting that lower rates of secondary infection mean that children and adolescents have less opportunity for onward transmission. There is evidence of transmission from children to others in households and in schools, and there have been reported outbreaks in schools.47,48 Other very small studies in Ireland30 and Singapore31 have found low numbers of secondary cases resulting from infected children attending school. This is consistent with a national South Korean study,13 which found the secondary attack rate from children to household members was extremely low. The available studies suggest children and adolescents play a lesser role in transmission of SARS-CoV-2, which is in marked contrast to pandemic influenza.49
Limitations
Our study has a number of limitations. We remain early in the COVID-19 pandemic, and data continue to evolve. It is possible that unknown factors related to age, eg, transience of infection or waning of immunity, bias findings in ways we do not yet understand. Some studies were low quality, and nearly all included studies were open to bias. The secondary infection rate in some CTSs was low, and this may represent an underestimate of the unmitigated household attack rate of SARS-CoV-2, as transmission chains were cut short because of strict control measures.50 Most of the CTSs were undertaken when strict social distancing measures had been introduced, eg, closures of schools and workplaces and restriction of travel. This would have reduced contacts outside the home, especially contacts between children, but it may have increased contacts between children and adults by increasing the household contact rate. The number of contacts nominated and traced for those younger than 20 years was low compared with adults in some studies,12,23 which may have introduced bias. We identified 3 CTSs from Guangdong province11,51,52 that were excluded as they overlapped with findings from Liu et al24; however, findings were unchanged if these studies were included. We included 2 recent large CTSs from India23 and South Korea12; however, numbers of children and data quality appeared low, making firm conclusions difficult.
For population screening studies, the numbers of children tested was small in most of the studies and was frequently less than the 15% to 25% of the population that are younger than 18 years in most countries. This likely reflects lower recruitment of children and may be a source of bias, although the direction of this bias is unclear. Age differentials in sensitivity of swab or antibody tests may also confound findings. Interpreting the observed prevalence and seroprevalence studies requires thorough quantification of social mixing and transmission between age groups and how that changed during lockdowns and social distancing interventions.
Conclusions
There is preliminary evidence that those younger than 10 to 14 years have lower susceptibility to SARS-CoV-2 infection than adults, with adolescents appearing to have similar susceptibility to adults. There is some weak evidence that children and adolescents play a limited role in transmission of SARS-CoV-2; however, this is not directly addressed by our study.
We remain early in our knowledge of SARS-CoV-2, and further data are urgently needed, particularly from low-income settings. These include further large, high-quality contact-tracing studies with repeated swabbing and high-quality virus detection and seroprevalence studies. Studies that investigate secondary infections from child or adolescent index cases compared with secondary infections from adult index cases are particularly needed to assess transmission. Monitoring of infection rates and contact-tracing studies within child care and school settings will also be important. A range of serological studies are planned in many countries, and these need to be sufficiently powered to assess differences in seroprevalence across different age groups and include repeated sampling at different time periods as social distancing restrictions are lifted. We will continue to update this review, including further data as available and updating preliminary data from some included studies.
eTable 1. Quality and bias assessments of included studies.
eTable 2. Web links for included studies.
eFigure 1. Pooled estimate of odds of being an infected contact in children compared with adults in medium-quality compared with low-quality contact-tracing studies.
eFigure 2. Pooled estimate of odds of being an infected contact in children compared with adults in studies including all close contacts compared with household-only contact-tracing studies.
eFigure 3. Ratio of the prevalence of SARS-CoV-2 infection in children and young people by age group compared with adults in population-screening studies.
References
- 1.Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371-372. doi: 10.1016/j.jmii.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munro APS, Faust SN. Children are not COVID-19 super spreaders: time to go back to school. Arch Dis Child. 2020;105(7):618-619. doi: 10.1136/archdischild-2020-319474 [DOI] [PubMed] [Google Scholar]
- 3.Brurberg KG The Role of Children in the Transmission of SARS-CoV-2 (COVID-19), 1st Update—A Rapid Review. Norwegian Institute of Public Health; 2020. [Google Scholar]
- 4.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Article in Chinese. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145-151. [DOI] [PubMed] [Google Scholar]
- 5.Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation protocol. medRxiv. Preprint posted online April 28, 2020. doi: 10.1101/2020.04.23.20076042 [DOI]
- 6.CDC COVID-19 Response Team Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426. doi: 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Public Health and the Environment Children and schools. Accessed July 20, 2020. https://www.rivm.nl/en/novel-coronavirus-covid-19/children-and-covid-19
- 8.COVID-19 National Incident Room Surveillance Team COVID-19, Australia: epidemiology report 13 (reporting week to 23:59 AEST 26 April 2020). Commun Dis Intell. Published online May 1, 2020. doi: 10.33321/cdi.2020.44.35 [DOI] [PubMed] [Google Scholar]
- 9.Ricardo F, Ajelli M, Andrianou X, et al. Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic. medRxiv. Preprint posted online April 11, 2020. doi: 10.1101/2020.04.08.20056861 [DOI] [PMC free article] [PubMed]
- 10.Zhang J, Litvinova M, Liang Y, et al. . Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368(6498):1481-1486. doi: 10.1126/science.abb8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi Q, Wu Y, Mei S, et al. . Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911-919. doi: 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YJ, Choe YJ, Park O, et al. ; COVID-19 National Emergency Response Center, Epidemiology and Case Management Team . Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10). doi: 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Choe YJ, Lee J, et al. . Role of children in household transmission of COVID-19. Arch Dis Child. Published online August 7, 2020. doi: 10.1136/archdischild-2020-319910 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xu W, Dozier M, He Y, Kirolos A, Theodoratou E; UNCOVER . The role of children in transmission of SARS-CoV-2: a rapid review. J Glob Health. 2020;10(1):011101. doi: 10.7189/jogh.10.011101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joanna Briggs Institute Checklist for prevalence studies. Accessed June 8, 2020. https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf
- 16.Li W, Zhang B, Lu J, et al. . The characteristics of household transmission of COVID-19. Clin Infect Dis. Published online April 17, 2020. doi: 10.1093/cid/ciaa450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH; Taiwan COVID-19 Outbreak Investigation Team . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156-1163. doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Ma W, Zheng X, Wu G, Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81(1):179-182. doi: 10.1016/j.jinf.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizumoto K, Omori R, Nishiura H Age specificity of cases and attack rate of novel coronavirus disease (COVID-19). medRxiv. Preprint posted online March 13, 2020. doi: 10.1101/2020.03.09.20033142 [DOI]
- 20.Wang Y, Tian H, Zhang L, et al. . Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5(5):e002794. doi: 10.1136/bmjgh-2020-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: Using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. medRxiv. Preprint posted online June 5, 2020. doi: 10.1101/2020.06.03.20121145 [DOI] [PMC free article] [PubMed]
- 22.Hu S, Wang W, Wang Y, et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. medRxiv. Preprint posted online August 7, 2020. doi: 10.1101/2020.07.23.20160317 [DOI] [PMC free article] [PubMed]
- 23.Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. medRxiv. Preprint posted online July 17, 2020. doi: 10.1101/2020.07.14.20153643 [DOI] [PMC free article] [PubMed]
- 24.Liu T, Liang W, Zhong H, et al. . Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg Microbes Infect. 2020;9(1):1546-1553. doi: 10.1080/22221751.2020.1787799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg ES, Dufort EM, Blog DS, et al. ; New York State Coronavirus 2019 Response Team . COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—March 2020. Clin Infect Dis. Published online May 8, 2020. doi: 10.1093/cid/ciaa549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousaf AR, Duca LM, Chu V, et al. . A prospective cohort study in non-hospitalized household contacts with SARS-CoV-2 infection: symptom profiles and symptom change over time. Clin Infect Dis. Published online July 28, 2020. doi: 10.1093/cid/ciaa1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J SARS-CoV-2 transmission in different settings: analysis of cases and close contacts from the Tablighi cluster in Brunei Darussalam. medRxiv. Preprint posted online July 10, 2020. doi: 10.1101/2020.05.04.20090043 [DOI]
- 28.van der Hoek W, Backer JA, Bodewes R, et al. . The role of children in the transmission of SARS-CoV-2. Article in Dutch. Ned Tijdschr Geneeskd. 2020;164:D5140. [PubMed] [Google Scholar]
- 29.Macartney K, Quinn HE, Pillsbury AJ, et al. ; NSW COVID-19 Schools Study Team . Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. Published online August 3, 2020. doi: 10.1016/S2352-4642(20)30251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heavey L, Casey G, Kelly C, Kelly D, McDarby G. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill. 2020;25(21):2000903. doi: 10.2807/1560-7917.ES.2020.25.21.2000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yung CF, Kam KQ, Nadua KD, et al. . Novel coronavirus 2019 transmission risk in educational settings. Clin Infect Dis. Published online June 25, 2020. doi: 10.1093/cid/ciaa794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudbjartsson DF, Helgason A, Jonsson H, et al. . Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302-2315. doi: 10.1056/NEJMoa2006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavezzo E, Franchin E, Ciavarella C, et al. ; Imperial College COVID-19 Response Team . Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584(7821):425-429. doi: 10.1038/s41586-020-2488-1 [DOI] [PubMed] [Google Scholar]
- 34.Public Health Agency of Sweden Förekomsten av covid-19 i Sverige 21-24 april och 25-28 maj 2020. Accessed July 28, 2020. https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/f/forekomsten-av-covid-19-i-sverige-21-24-april-och-25-28-maj-2020/
- 35.Office for National Statistics Coronavirus (COVID-19) infection survey pilot: England and Wales, 11 September 2020. Accessed July 28, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/11september2020
- 36.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. ; ENE-COVID Study Group . Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Public Health and the Environment PIENTER Corona study. Accessed September 16, 2020. https://www.rivm.nl/en/pienter-corona-study
- 38.Hallal P, Hartwig F, Horta B, et al. Remarkable variability in SARS-CoV-2 antibodies across Brazilian regions: nationwide serological household survey in 27 states. medRxiv. Preprint posted online May 30, 2020. doi: 10.1101/2020.05.30.20117531 [DOI]
- 39.Shakiba M, Hashemi Nazari SS, Mehrabian F, Rezvani SM, Ghasempour Z, Heidarzadeh A Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. Preprint posted online May 1, 2020. doi: 10.1101/2020.04.26.20079244 [DOI]
- 40.Biggs HM, Harris JB, Breakwell L, et al. ; CDC Field Surveyor Team . Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia counties, April 28-May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):965-970. doi: 10.15585/mmwr.mm6929e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringhini S, Wisniak A, Piumatti G, et al. . Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313-319. doi: 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawa N, Kuramochi J, Sonoda S, et al. Seroprevalence of SARS-CoV-2 IgG Antibodies in Utsunomiya City, Greater Tokyo, after first pandemic in 2020 (U-CORONA): a household- and population-based study. medRxiv. Preprint posted online July 26, 2020. doi: 10.1101/2020.07.20.20155945 [DOI]
- 43.Pagani G, Conti F, Giacomelli A, et al. Seroprevalence of SARS-CoV-2 IgG significantly varies with age: results from a mass population screening (SARS-2-SCREEN-CdA). medRxiv. Preprint posted online August 28, 2020. doi: 10.1101/2020.06.24.20138875 [DOI] [PMC free article] [PubMed]
- 44.Weis S, Scherag A, Baier M, et al. Seroprevalence of SARS-CoV-2 antibodies in an entirely PCR-sampled and quarantined community after a COVID-19 outbreak—the CoNAN study. medRxiv. Preprint posted online July 17, 2020. doi: 10.1101/2020.07.15.20154112 [DOI]
- 45.Streeck H, Schulte B, Kuemmerer B, et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. medRxiv. Preprint posted online June 2, 2020. doi: 10.1101/2020.05.04.20090076 [DOI] [PMC free article] [PubMed]
- 46.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM; CMMID COVID-19 Working Group . Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. doi: 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 47.Torres JP, Piñera C, De La Maza V, et al. . SARS-CoV-2 antibody prevalence in blood in a large school community subject to a Covid-19 outbreak: a cross-sectional study. Clin Infect Dis. Published only July 10, 2020. doi: 10.1093/cid/ciaa955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontanet A, Tondeur L, Madec Y, et al. Cluster of COVID-19 in northern France: a retrospective closed cohort study. medRxiv. Preprint posted online April 23, 2020. doi: 10.1101/2020.04.18.20071134 [DOI]
- 49.Zhu Y, Bloxham CJ, Hulme KD, et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections. medRxiv. Preprint posted online March 30, 2020. doi: 10.1101/2020.03.26.20044826 [DOI]
- 50.Sun K, Viboud C. Impact of contact tracing on SARS-CoV-2 transmission. Lancet Infect Dis. 2020;20(8):876-877. doi: 10.1016/S1473-3099(20)30357-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jing QL, Liu MJ, Zhang ZB, et al. . Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. Published online June 17, 2020. doi: 10.1016/S1473-3099(20)30471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Huang Y, Tu C, et al. . Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. Published online May 11, 2020. doi: 10.1093/cid/ciaa557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Quality and bias assessments of included studies.
eTable 2. Web links for included studies.
eFigure 1. Pooled estimate of odds of being an infected contact in children compared with adults in medium-quality compared with low-quality contact-tracing studies.
eFigure 2. Pooled estimate of odds of being an infected contact in children compared with adults in studies including all close contacts compared with household-only contact-tracing studies.
eFigure 3. Ratio of the prevalence of SARS-CoV-2 infection in children and young people by age group compared with adults in population-screening studies.



