Abstract
OBJECTIVE
There is a paucity of data evaluating recent changes in clinical and prescriber characteristics of patients initiating sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1RA).
RESEARCH DESIGN AND METHODS
U.S.-based administrative claims data (July 2013 to June 2018) were used to identify initiators of SGLT2i and GLP-1RA.
RESULTS
Over 5 years, empagliflozin initiation (as a proportion of SGLT2i) increased by 57.1% (P < 0.001 for trend), while canagliflozin initiation declined by 75.1% (P < 0.001). Empagliflozin was the only agent within SGLT2i with an increase in the proportion of patients with myocardial infarction, stroke, or heart failure (collectively called CVD-HF) (P < 0.001). Liraglutide initiation (as a proportion of total GLP-1RA) declined by 32.1% (P < 0.001), and dulaglutide initiation increased by 34.1% (P < 0.001); the proportion of patients with CVD-HF increased the most in liraglutide initiators (5.1% increase; P < 0.001). Most prescribers were internists or endocrinologists; cardiologist prescribing remained low (<1%).
CONCLUSIONS
For SGLT2i, shifts in preference for empagliflozin followed changes in drug labels and guidelines, while for GLP-1RA, other factors such as price or ease of administration may have led to a preference for dulaglutide over liraglutide.
Introduction
Patients diagnosed with type 2 diabetes are at increased risk of cardiovascular disease (CVD), the leading cause of mortality in this population (1,2). In recent years, two glucose-lowering drug classes, sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1RA), demonstrated a benefit on cardiovascular end points in patients with established CVD or high cardiovascular risk (3–5).
Drug labels for two SGLT2i (empagliflozin [2016] and canagliflozin [2016]) and one GLP-1RA (liraglutide [2017]) were changed to include an indication for reductions in cardiovascular events among patients with established CVD. In 2018, the American Diabetes Association’s Standards of Medical Care in Diabetes recommended the addition of SGLT2i (particularly empagliflozin) or GLP-1RA (particularly liraglutide) as second-line therapy after metformin in patients with established CVD. In an effort to increase awareness of these therapies among cardiologists, in the same year the American College of Cardiology released a report on minimizing cardiovascular risk in patients with diabetes (6).
This study aimed to characterize the changes in prescribing patterns of these two medication classes in relation to the release of new evidence and labeling changes. We hypothesized that among SGLT2i and GLP-1RA initiators: 1) the proportion of patients with evidence of CVD would increase, particularly among those initiating empagliflozin or liraglutide; 2) the use of empagliflozin and liraglutide would increase over other agents within their class; and 3) the proportion of prescriptions by cardiologists would increase, especially in patients with co-occurring CVD.
Research Design and Methods
Study Population
The study used Optum Clinformatics Data Mart insurance data between July 2013 and June 2018. We identified two cohorts of patients initiating either SGLT2i (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1RA (albiglutide, exenatide, liraglutide, dulaglutide, or semaglutide subcutaneous) using similar inclusion criteria. The date of medication initiation was designated as the index date. Patients were required to be new users (no dispensing of the drug class in 12 months prior to index) and were excluded if they were younger than 18 years, did not have a diagnosis of type 2 diabetes, or had a diagnosis of type 1 diabetes or gestational diabetes mellitus, cancer, or HIV, or nursing home or hospice stay.
The study was approved by the Brigham and Women’s Institutional Review Board.
Patient Characteristics
The 5-year period was segmented into 10 equal intervals of 6 months; patients were assigned to one of these intervals based on their index date. Patients could contribute to both SGLT2i and GLP-1RA cohorts if the inclusion criteria were met.
Within each time interval, we estimated the proportion of patients with diagnosis of myocardial infarction (MI), stroke, or heart failure (HF), collectively called CVD-HF. National Provider Identifiers were used to group prescribers by medical specialty: internal or family medicine (internists); endocrinology, cardiology, other physician specialty; and nurse practitioners or physician assistants.
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). Changes in baseline characteristics were reported for the overall cohorts and within a subgroup of patients with CVD-HF. To account for changes in coding practices due to implementation of ICD-10 during this period, a cohort of patients initiating other second-line noninsulin therapies (dipeptidyl peptidase 4 inhibitors, sulfonylureas, or thiazolidinediones) was created to serve as a time-trend control group.
Results
Patients initiating SGLT2i (n = 90,096) (Supplementary Table 1) were more likely to be male (56.5%), with a mean age (SD) of 55.7 (10.1) years (Supplementary Table 1). The proportion of patients with CVD-HF increased by 3.4% (8.8% to 12.2%; P < 0.001 for trend). As a proportion of overall prescriptions, prescriptions originating from endocrinologists declined by 12.0% (but increased in absolute number of prescriptions; P < 0.001), those from internists were unchanged (P = 0.58), and those from cardiologists remained low but increased marginally (P < 0.001). Findings were similar in the subgroup of patients with CVD-HF initiating SGLT2i (Supplementary Table 2).
Patients initiating GLP-1RA (n = 71,348) (Supplementary Table 3) were less likely to be male (48.3%), with a mean (SD) age of 56.0 (11.5) years. The proportion of CVD-HF increased by 3.9% (10.5% to 14.4%; P < 0.001). Prescriptions from endocrinologists declined as a proportion (but increased in absolute terms; P < 0.001) and those from internists remained consistent (>55%; P = 0.12), while cardiologist prescribing remained low (<0.5%). Findings were similar in a subgroup of patients with CVD-HF initiating GLP-1RA (Supplementary Table 4).
The proportion of CVD-HF diagnosis in patients initiating other second-line glucose-lowering therapies (n = 307,646) (Supplementary Table 5) increased by 1.5% (P = 0.06) in this group.
Trends in Use of Individual Agents
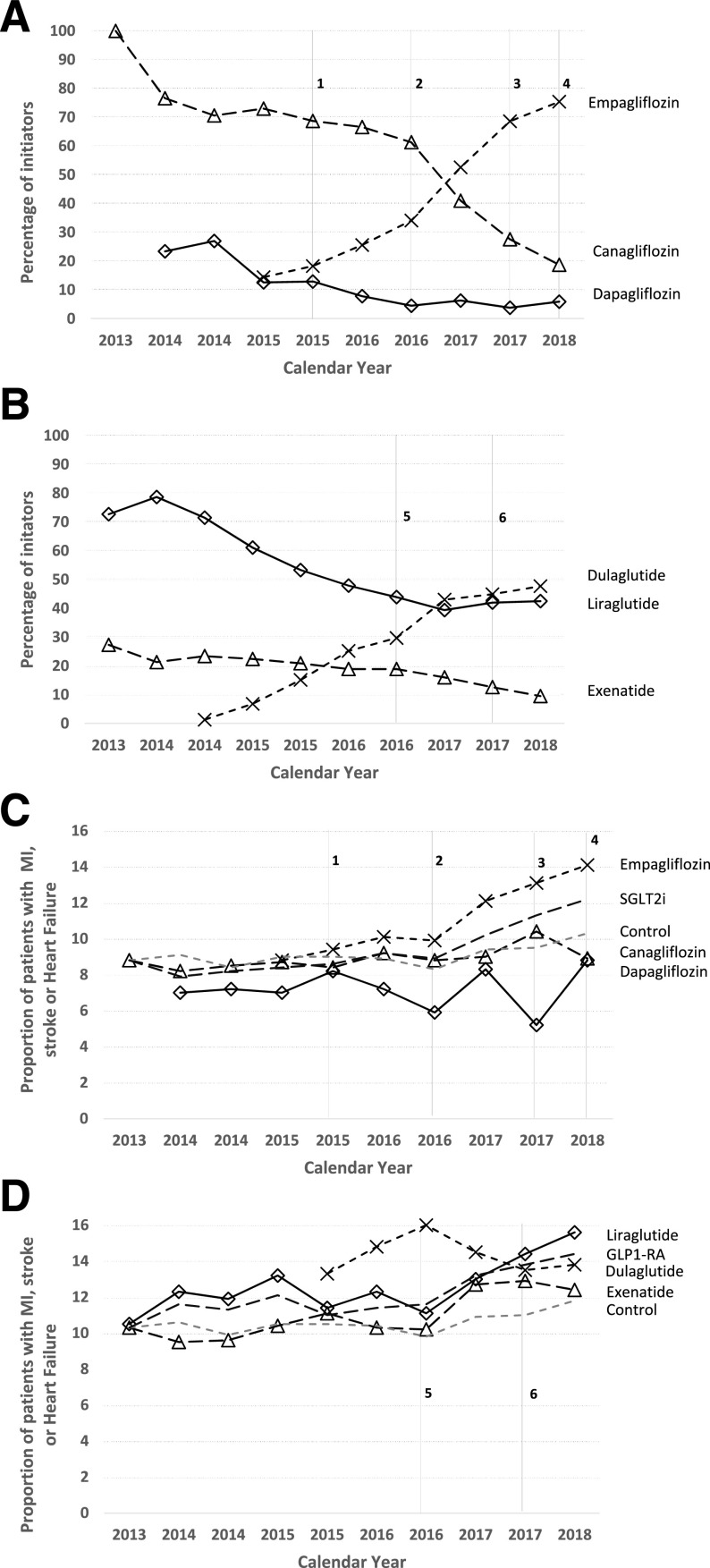
Canagliflozin initiation declined by 75.1% over the study period (100.0% to 24.9% of SGLT2i initiation; P < 0.001), while empagliflozin initiations increased by 51.7% (13.9% to 65.6% of SGLT2i initiations; P < 0.001) (Fig. 1A). Liraglutide initiation declined by 32.1% (72.4% to 40.3% of GLP-1RA initiations; P < 0.001), while dulaglutide initiation increased by 43.8% (5.0% to 48.8% of GLP-1RA initiations; P < 0.001) (Fig. 1B). Initiation trends were similar in the subgroup of patients with CVD-HF (Supplementary Fig. 1).
Figure 1.
Changes in prescribing patterns and cardiovascular characteristics of individual SGLT2i and GLP-1RA medications, 2013–2018. A and B describe the proportion of patients initiating individual SGLT2i between July 2013 and June 2018. C and D describe the proportion of patients with a diagnosis of MI, stroke, or HF initiating individual SGLT2i or GLP-1RA. The dashed gray lines indicate the changes in these characteristics for patients initiating other second-line glucose-lowering therapy. To allow for more direct comparisons (as patients in the control group were significantly older), we anchored these changes to be similar to canagliflozin (8.8%) for SGLT2i and the average of liraglutide and exenatide (10.4%) for GLP-1RA. 1: EMPA-REG is published, showing benefits in the composite of CVD death, nonfatal stroke, and MI. 2: Empagliflozin receives a new Food and Drug Administration indication for reduction in CVD death in patients with established CVD. 3: CANVAS is published, showing a benefit in the composite of CVD death, nonfatal stroke, and MI, but the trial also reported an increase in the risk of amputations, resulting in a black-box warning for canagliflozin. 4: 2018 American Diabetes Association guidelines recommend an SGLT2i (with a focus on empagliflozin) as second-line therapy in patients with established CVD. 5: LEADER is published, showing benefits in CVD death, nonfatal stroke, and MI for liraglutide. SUSTAIN-6 is also published, showing benefit in CVD death, nonfatal stroke, and MI. 6: Liraglutide receives a new indication for reduction in cardiovascular events in patients with established CVD, and Exenatide Study of Cardiovascular Event Lowering (EXSCEL) is published, showing no cardiovascular benefit for exenatide long-acting release.
Among empagliflozin initiators (Fig. 1C), the proportion of patients with CVD-HF increased by 5.3% (8.8% to 14.1%; P < 0.001), with the largest change seen after changes to the drug label. By contrast, CVD-HF did not meaningfully change among patients initiating canagliflozin (P = 0.065), dapagliflozin (P = 0.87), or other medications (P = 0.060). CVD-HF proportion increased by 5.1% among liraglutide initiators (Fig. 1D) (10.5% to 15.6%; P = 0.018), 2.1% for exenatide (10.3% to 12.4%; P = 0.003), and 0.5% for dulaglutide (13.3% to 13.8%; P = 0.77).
Conclusions
This study examined patients initiating SGLT2i and GLP-1RA between 2013 and 2018 and found that among SGLT2i, empagliflozin became the most prescribed agent driven by an increasing proportion of patients with a diagnosis of CVD-HF. Within GLP-1RA, dulaglutide initiations surpassed liraglutide; however, liraglutide initiators were more likely to have a diagnosis of CVD-HF. For both classes, internists and endocrinologists were the primary prescribers; cardiologist prescribing remained minimal even in the subgroup of patients with established CVD-HF.
Changes in the drug label for canagliflozin (boxed warning for amputation) and empagliflozin (indication for reductions in cardiovascular events and death) in 2016 likely contributed to a rapid change in prescribing preference for empagliflozin. By contrast, due to the reduced frequency of administration and possible formulary preferences, dulaglutide initiations surpassed liraglutide, the only GLP-1RA with evidence of cardiovascular benefit at the time. These findings are similar to a study at a tertiary academic medical center, which noted higher use of evidence-based therapies (empagliflozin and liraglutide) (7,8). This difference might be explained by more heterogenous nationwide prescribing patterns compared with a single center. The study found low rates of cardiologist prescribing even in the subgroup of patients with existing CVD. As patients with co-occurring diabetes and CVD are likely to see their cardiologist, these encounters may provide an additional opportunity to optimize their treatment (9).
Limitations are noted. First, study findings are generalizable to patients with commercial insurance or those in Medicare Advantage plans. Second, we did not have access to formulary information, which could have driven some of the observed trends. Third, as the study period included data through June 2018, we were unable to evaluate the trends in the use of newly approved medications like oral semaglutide.
This study shows that by preferring empagliflozin, prescribers have largely reacted in accordance with the available evidence and drug labels, while other factors such as lower price, frequency of administration, or prior authorizations may have led prescribers to select dulaglutide over liraglutide.
Supplementary Material
Article Information
Funding. This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School (Boston, MA).
The sponsor had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Duality of Interest. C.V.D. is supported through the New Jersey Alliance for Clinical and Translational Science (UL1-TR-003017). S.S. is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Bayer, Vertex Pharmaceuticals, and Boehringer Ingelheim unrelated to the topic of this study and is a consultant to World Health Information Science Consultants, LLC, and Aetion, Inc., a software manufacturer in which he owns equity. D.J.W. reports serving on a data monitoring committee for Novo Nordisk. E.P. is supported by a career development grant (K08-AG-055670) from the National Institute on Aging and is coinvestigator of investigator-initiated grants to the Brigham and Women’s Hospital from GlaxoSmithKline and Boehringer Ingelheim, not directly related to the topic of the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.V.D. contributed to the development of the hypotheses, conducted the analyses, interpreted the data, and drafted, reviewed, and edited the manuscript. S.S., D.J.W., and E.P. contributed to the development of the hypothesis, interpreted the data, and reviewed and edited the manuscript. G.B. conducted the analysis, interpreted the data, and reviewed the manuscript. C.V.D. and E.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1943/-/DC1.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34 [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 4.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaduganathan M, Patel RB, Singh A, et al. Prescription of glucagon-like peptide-1 receptor agonists by cardiologists. J Am Coll Cardiol 2019;73:1596–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–3372 [DOI] [PubMed] [Google Scholar]
- 9.Gunawan F, Partridge C, Kosiborod M, Inzucchi S. SUN-149 cardiologist vs. endocrinologist encounters in patients with T2D and CVD: potential implications for glucose-lowering therapy use and education. J Endocr Soc 2019;3:SUN-149 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.