Abstract
Homeostasis in the cardiovascular system is maintained by physiological functions of the Renin Angiotensin Aldosterone System (RAAS). In pathophysiological conditions, over activation of RAAS leads to an increase in the concentration of Angiotensin II (AngII) and over activation of Angiotensin Type 1 Receptor (AT1R), resulting in vasoconstriction, sodium retention and change in myocyte growth. It causes cardiac remodeling in the heart which results in left ventricular hypertrophy, dilation and dysfunction, eventually leading to Heart Failure (HF). Inhibition of RAAS using angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) has shown to significantly reduce morbidity and mortality due to HF. ACEi have been shown to have higher drug withdrawal rates due to discomfort when compared to ARBs; therefore, ARBs are the preferred choice of physicians for the treatment of HF in combination with other anti-hypertensive agents. Currently, eight ARBs have been approved by FDA and are clinically used. Even though they bind to the same site of AT1R displacing AngII binding but clinical outcomes are significantly different. In this review, we described the clinical significance of each ARB in the treatment of HF and their clinical outcome.
Keywords: AT1R, AngII, angiotensin receptor blockers, heart failure, cardiovascular diseases, hypertension, GPCRs
1. INTRODUCTION
Renin angiotensin aldosterone system (RAAS) is a major hormonal system in the body which regulates blood pressure and sodium homeostasis [1, 2]. Its major components are – angiotensinogen (Agt), renin, angiotensin converting enzyme (ACE) and angiotensin type 1 and 2 receptors (AT1R and AT2R) [3]. Agt is a globular protein (α2-globulin) from the serpin family, primarily produced in the liver and released into the circulation [4, 5]. The juxtaglomerular cells in the kidneys convert a proenzyme called pro-renin into a fully active enzyme renin which is released into the circulation when renal blood flow is reduced. Renin converts angiotensinogen to angiotensin I (AngI), a deca-peptide (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu) inactive hormone precursor by cleaving on the N-terminal side of the protein. AngI is then further converted into the active octa-peptide (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu) hormone angiotensin II (AngII). AngII is produced when ACE, primarily found in lung vascular endothelial cells, cleaves two residues on the C-terminal side of AngI [6, 7]. The active peptide hormone AngII binds to AT1R and induces a conformational change to recruit hetero-trimeric G-proteins (Gα-β-ϒ). After G-proteins fall from the receptor, G-protein couple receptor kinases called GRKs phosphorylate the receptor and recruit β-arrestin. After recruitment of β-arrestin by the receptor, it initiates receptor desensitization [8]. Simultaneously, the hetero-trimeric G-protein subunits are split into two parts, Gα and Gβϒ. Gβϒ remains in the plasma membrane and Gα activates an enzyme called phospholipase C (PLC) in the cytosol. PLC cleaves a phospholipid Phosphatidylinositol 4, 5-bisphosphate (PIP2), which is a minor component of cell membrane into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG remains bound to the membrane but IP3 binds to the IP3 receptor (a component of Ca2+ channel) in the smooth endoplasmic reticulum and releases Ca2+ which is required for vasoconstriction, hence blood pressure (BP) is elevated [6–8].
In pathophysiological conditions, there is chronic activation of AT1R by AngII. This leads to over activation of the receptor and subsequently results in high BP, sodium and water retention, neurohumoral activation, and increased vascular ROS production [9–12]. Chronic activation of the receptor also leads to cardiac hypertrophy which causes thickening of the cardiac wall. This phenomenon leads to the development of diastolic and systolic dysfunction which are associated with cardiac morbidity and mortality. Enhanced over-activation of the receptor also leads to increase in ROS production and decrease in NO generation leading to endothelial dysfunction [13, 14]. The combination of these two abnormalities leads to decrease in cardiac output [13, 14]. It causes activation of neurohormonal stimulation, resulting in an increase of circulating catecholamines and AngII, inducing a cascade of events leading to impairment of downstream signaling outcomes including loss of tissue contraction in heart [15]. This increases pre- and post-load in the heart leading to heart failure. Therefore, inhibiting RAAS or blocking AT1R is proposed to control these abnormalities. Angiotensin-converting enzyme inhibitors (ACEi) have limitations when compared with ARBs, this includes – ACEi causing side effects in some patients like dry cough and leading to prejunctional nor-epinephrine release, ARBs are highly specific to AT1R and the beneficial physiological functions of AT2R are unopposed [16, 17].
In this review, we will discuss the importance of ARBs in heart failure. Until now, eight ARBs have been approved by the FDA. They are azilsartan, candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan and valsartan. These ARBs are all highly specific to AT1R and show poor binding to AT2R.
2. STRUCTURE OF AT1R
AT1R is a seven transmembrane G-protein couple receptor. The structure of AT1R was first solved with an antagonist ZD7511 (Pdb id: 4yay) [18] and the clinically used ARB olmesartan (Pdb id: 4zud) [19]. Singh et al., 2017, extensively studied the binding pattern of clinically used ARBs using molecular docking and dynamic simulations and found that all ARBs bind to the same site and reported that two acid moieties are required for binding to AT1R [18, 20]. The major residues involved in ARBs binding are Tyr35, Trp84, Tyr87, Tyr92, Arg167 and Tyr282 (Fig. 1). The mutation of these residues completely abolished the binding of ARBs and AngII. Radiolabelled binding experiments testing ARBs binding to membranes were isolated from HEK-293 AT1R expressing cells and demonstrated that telmisartan has the highest binding affinity [20]. Recently, an active state structure of AT1R bound with SI-AngII (Pdb id: 6do1) was also solved [21]. This study reports that the orthosteric site becomes smaller when bound with SI-AngII and the position of helix-8 embedded in the membrane is different from the ZD7511 structure and it projects into cytosol [21]. The molecular mechanism of how AngII activates the receptor has also been studied recently using molecular dynamic simulations [22].
Fig. (1).
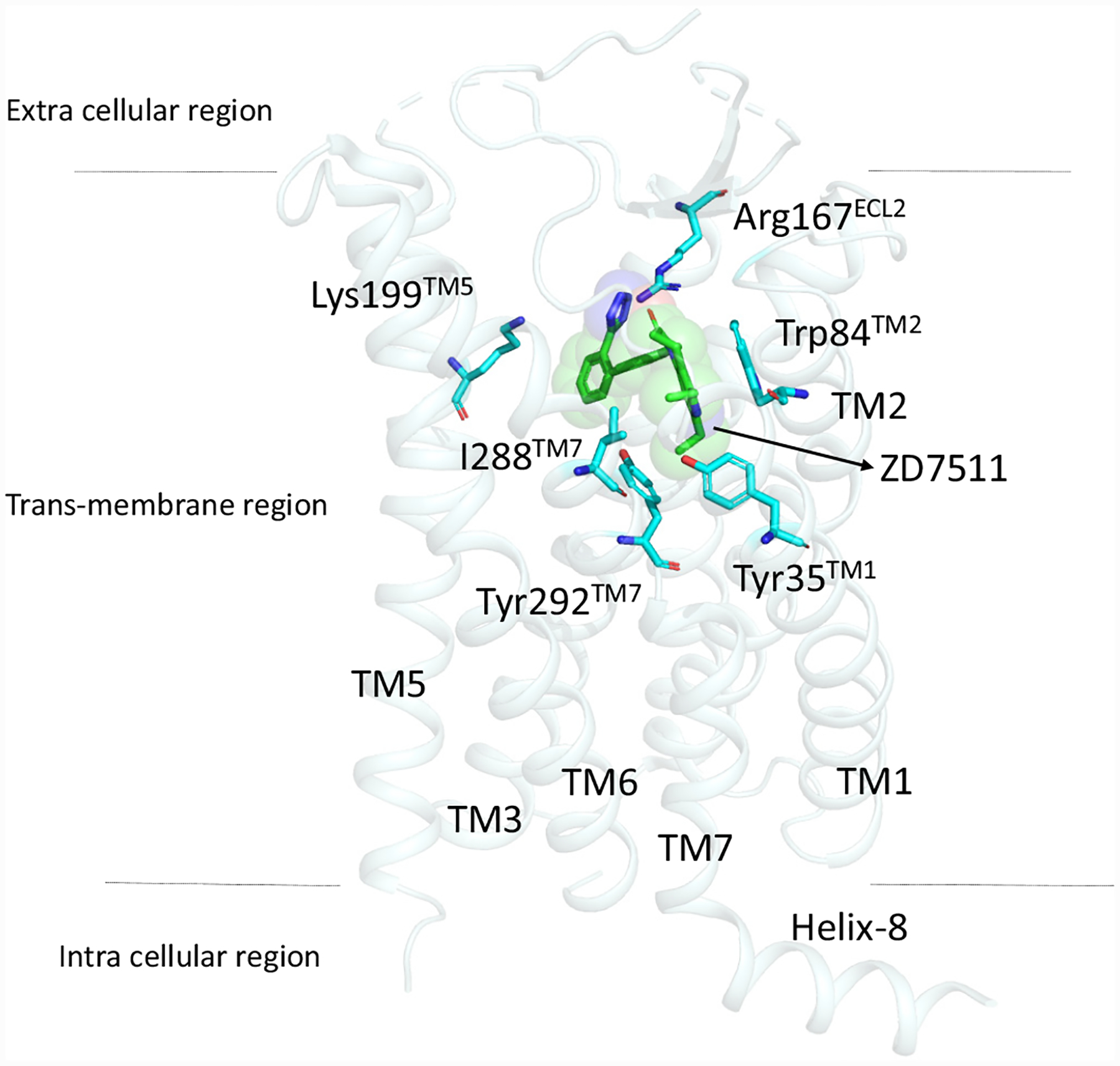
Binding mode of ZD7511 in the orthosteric site of AT1R with residues critical for all ARBs binding. All the ARBs share similar binding residues (detail see reference 20).
3. ANGIOTENSIN RECEPTOR BLOCKERS (ABRs)
ARBs are broadly classified into two types based on their chemical structures (Fig. 2). They are either bi-phenyl tetrazole analogues or non-biphenyl tetrazole analogues. The bi-phenyl tetrazole analogues are azilsartan, candesartan, irbesartan, losartan, olmesartan and valsartan and non-biphenyl tetrazole analogues are eprosartan and telmisartan [20]. Even though all ARBs bind to the same receptor, each has different clinical outcomes. The binding affinity of ARBs with AT1R is in ascending order as follows: telmisartan, candesartan, olmesartan, irbesartan, azilsartan, eprosartan, losartan and valsartan [20].
Fig. (2).
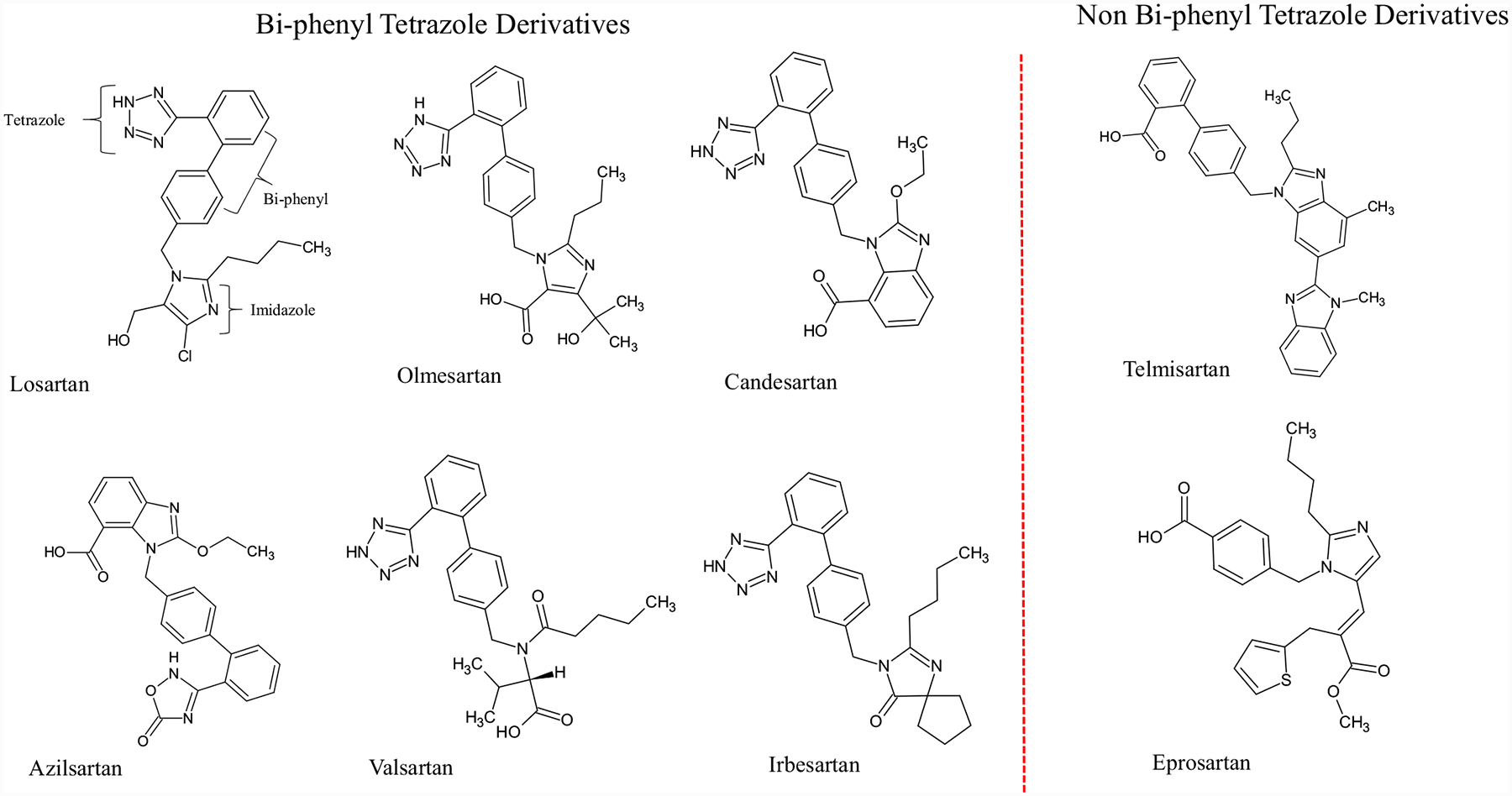
Chemical structures of eight FDA approved clinically used ARBs.
3.1. Losartan
Losartan was the first discovered ARB and it is a prodrug [23]. Losartan is quickly metabolized into Exp3174 by the liver enzyme CYP2C9. E3174 is responsible for the long-lasting effect of losartan and selective blockage of AT1R [24]. ACEi were frequently used but not considered the first choice of physicians for heart failure treatment due to their severe side effects. Therefore, ELITE (Evaluation of Losartan in Elderly) study was performed to study the effect of losartan in heart failure patients due to its similar mechanism of action. The result shows an unexpected survival benefit in elderly heart-failure patients, compared with ACEi (captopril). This led to the ELITE II study with a bigger sample size and found that losartan was not superior to captopril in elderly heart-failure patients but was better tolerated, suggesting that losartan is a good option [25]. However, Svanstrom et al., (2012) reported that a low dose of losartan is associated with higher mortality rate in heart failure patients [26]. Losartan in low or high doses has additional beneficial hemodynamic effects which are observed after 12 weeks of therapy [27].
3.2. Azilsartan
Azilsartan was approved by the FDA in 2011 and used for oral administration as Azilsartan Medoxomil to control high BP [28]. It has an inverse agonistic property and therefore it is clinically significant. It has been shown to better control ambulatory or clinical BP when compared with olmesartan or valsartan [29]. Heart failure patients with high BP show improved diastolic function with azilsartan treatment [30]. Nakamura et al., (2013) observed that azilsartan suppressed cardiac remodeling in post-myocardial infarction (MI) patients without lowering BP [31]. Azilsartan is shown to be better in controlling BP in pre-diabetic and type 2 diabetes mellitus patients suggesting that azilsartan could be an important drug that could reduce morbidity and mortality related to cardiovascular diseases associated with diabetes [32–34].
3.3. Candesartan
Candesartan Cilexetil is orally administered and quickly metabolized as candesartan and absorbed in the gastrointestinal tract [24]. It is shown to significantly reduce mortality due to chronic heart failure in low left ventricular ejection fraction (LVEF) trail with candesartan therapy in combination with other antihypertensive drugs [35–39]. A systematic review on Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) studies reveals that candesartan is a safe and effective option for patients with systolic heart failure [40]. Candesartan therapy has improved heart failure (HF) with mid-range ejection fraction [40].
3.4. Eprosartan
Eprosartan is a well-tolerated AT1R blocker which is very effective in controlling BP as well as providing secondary prevention of cerebrovascular events [24]. It can be used to correct hypercoagulatory syndrome in chronic kidney disease. It has an inhibitory effect on sympathetic nervous activity compared to other ARBs [41] suggesting that it may have beneficial effects in post-MI patients where RAAS is upregulated. Eprosartan better blocked the development of cardiac hypertrophy in rats with aortocaval fistula than the ACEi (enalapril) [42]. Studies have also shown that administration of eprosartan reduces catecholamine release in animal models [43]. It also reduces cardiac hypertrophy which is an index of heart failure, protects renal structural integrity and prevents end-organ failure due to high BP and ultimately reduces mortality [44, 45]. Suzuki et al., (2003) reported that the administration of eprosartan prevents left ventricular dystrophy in dogs with heart failure [46].
3.5. Irbesartan
Irbesartan is taken orally and it is very effective in controlling BP. In addition to its BP-lowering effect, it can also activate PPARϒ which has been shown to improve insulin intolerance and aid patients who suffer from metabolic abnormalities such as atherosclerosis [24]. Therefore, it may have indirect effects on heart failure associated with other metabolic disorders. Several studies including I-PRESERVE (Irbesartan in Heart Failure With Preserved Ejection Fraction Study) have been performed to study the effect of irbesartan in ejection fraction (EF) in heart failure patients and found that there was no significant improvement [47, 48]. Furthermore, no significant effect was seen in the improvement of morbidity and mortality in HF patients [47]. However, irbesartan treatment significantly reduced aldosterone hormone compared to a benazepril treatment group of older patients [49]. It has also been reported that irbesartan has a cardio-protective effect in post-MI patients who have renal dysfunction [50]. Irbesartan in combination with metoprolol and hydrochlorothiazide with non-invasive ventilator in the emergency treatment of severe HF patients improved cardiac function and restored respiratory function [51].
3.6. Olmesartan
Olmesartan is an orally administered drug which is very effective in controlling BP. It has shown to improve insulin sensitivity and produce anti-atherogenic and anti-inflammatory effects in patients with diabetic nephropathy [52]. It has also shown to have beneficial effects in HF patients with preserved left ventricular ejection fraction in combination therapy with beta blockers [53] but no benefits were observed in olmesartan monotherapy in a SUPPORT TRIAL [54]. Animal experiments have shown that olmesartan therapy could be beneficial in combating inflammation, oxidative stress, apoptosis in signaling pathways associated with HF [55, 56]. Long term therapy of olmesartan led to improved clinical outcomes, and it may also produce cerebro- and cardio-vascular events by changing the atheroma volume [57]. SUPPORT TRIAL also observed that triple combination therapy with ACEi and beta-blockers is significantly associated with increased cardiac events [54]. Padwal et al., (2014) observed that olmesartan therapy may contribute to worsened diabetes in patients with a history of cardio-vascular diseases and chronic kidney diseases and suggest caution in olmesartan use [58].
3.7. Telmisartan
Telmisartan is among the widely used anti-hypertensive agents which can effectively control BP [24]. It has the longest plasma half-life, highest lipophilicity and strongest binding to the receptor [20, 24]. A recent meta-analysis and systematic review have shown that telmisartan therapy can improve insulin resistance when compared to other ARBs [59, 60]. This property of telmisartan is known to activate PPARϒ and mediate insulin sensitization and it also has renal anti-inflammatory and anti-oxidant effects [61]. It can also reduce the CVD risks in patients with atherothrombotic disease or diabetes with end-organ damage. It was also reported that telmisartan monotherapy was equivalent to ramipril in patients with vascular disease or high-risk diabetes and was associated with less angioedema but the combination of two drugs was associated with adverse events without any benefits [62] however it significantly reduced all-cause mortality, cardiovascular death, and hospital admission for decompensated heart failure in hemodialysis patients with chronic HF and left ventricular ejection fraction [63]. Therefore, telmisartan is better tolerated and could be regarded as a potential treatment for patients with vascular disease or high-risk diabetes, if ACEi is not tolerated [64]. The Telmisartan Randomised Assessment Study in ACE intolerant subjects with cardiovascular Disease (TRANSCEND) found that MI may be further reduced by telmisartan in hypertensive patients [65]. In rats, treatment with telmisartan is associated with significantly improved left ventricular function and it ameliorated the progression of cardiac remodeling with chronic HF after experimental autoimmune myocarditis [66]. In other studies, telmisartan has shown to decrease the serum copeptin level and alleviated inflammatory reactions, leading to improved heart function in chronic HF patients [67]. On the other hand, it can also increase the serum Adiponectin (APN) levels and significantly reverse the left ventricular remodeling while reducing the blood pressure [68]. It is more effective in reducing Left Ventricular Remodeling and kidney disorders than enalapril treatment for patients with coronary artery disease with diabetic nephropathy [69].
3.8. Valsartan
Valsartan has been shown to be very effective in controlling high BP and it is well-tolerated [24]. In rats, valsartan has been shown to inhibit Hypoxia Inducible Factor (HIF)-1α mediated gene activation and decreases the pathogenic factors in diabetic nephropathy, preventing renal damage [70]. Val-HeFT result has shown significant reduction in morbidity and mortality in the total patient population, reduced the risk of hospitalization [71], and reduced HF-related hospitalization in addition to valsartan-prescribed HF therapy [72]. A significant reduction in aldosterone concentrations in pigs was also observed when using valsartan and benazepril combination therapy [73]. Valsartan therapy was effective in decreasing the frequency of atherosclerotic events such as fetal and non-fatal myocardial infarction, angina, revascularization and stroke, similar to ACEi [74]. However, in elderly patients, hypotension, renal dysfunction and hyperkalemia were more common [74, 75]. Combination therapy results in severe adverse event and no improvement in survival or morbidity were observed in VALIANT trail [74]. Therefore, valsartan could be an alternative to ACEi intolerant patients but shows no improvement in combined therapy with ACEi in post-MI patients. However, extensive studies are currently undergoing testing for combination therapy of valsartan with sacubitril for the treatment of heart failure and have shown some promise [76, 77].
CONCLUSION
Inhibition of RAAS in HF patients significantly decreased morbidity and mortality. ACEi remains the first choice of therapy [78]. ACEi are favored over ARBs due to a study which showed that ACEi can prevent coronary artery diseases in addition to controlling BP [79–81]. ACEi have higher withdrawal due to intolerance. ARBs have significantly lower withdrawal rates than ACEi and most of the ARBs are nearly as effective as ACEi in the treatment of HF. Hence, choosing ARBs as the first line of therapy for the pathophysiology underlying HF significantly reduces morbidity and mortality. All ARBs are very effective at blocking AT1R and reducing blood pressure; however there are notable differences in their efficacy against tissue pathogenesis and related clinical outcomes. Even though all ARBs are as effective as ACEi in the treatment of HF. Out of eight FDA approved ARBs, irbesartan and telmisartan have shown PPARϒ agonistic properties resulting in improved insulin intolerance. Olmesartan treatment also improves insulin sensitivity and produces anti-atherogenic and anti-inflammatory effects in patients with diabetic nephropathy. All the ARBs demonstrate beneficial effects similar to ACEi in the treatment of HF except lower doses of losartan which leads to increased mortality in HF patients. Valsartan in combination with sacubitril therapy proved to be a promising therapy for HF. Eprosartan has an effect on the sympathetic nervous system when compared to other ARBs and it is also able to reduce catecholamine release in animal models. Therefore, eprosartan therapy may have an additional beneficial effect in the treatment of heart failure. However, eprosartan has the shortest bioavailability (< 6 hours) when compared to other ARBs. Large number of studies that show beneficial effects on animals have been reported but there are limited studies on humans. Hence, more human studies are warranted. Recently, crystal structures of AT1R in inactive and active state structures have been solved. Using these crystal structures and cheminformatics tools, exploring structures similar to eprosartan with an increase in bioavailability and affinity may enhance the treatment of HF.
ACKNOWLEDGEMENTS
We thank Russell Desnoyer for his comments and suggestions on this manuscript.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Hall JE. Control of blood pressure by the renin-angiotensin-aldosterone system. Clin Cardiol 1991; 14(8)(Suppl. 4): IV6–IV21. [ 10.1002/clc.4960141802] [DOI] [PubMed] [Google Scholar]
- [2].Yim HE, Yoo KH. Renin-Angiotensin system - considerations for hypertension and kidney. Electrolyte Blood Press 2008; 6(1): 42–50. [ 10.5049/EBP.2008.6.1.42] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 2003; 24(3): 261–71. [ 10.1210/er.2003-0001] [DOI] [PubMed] [Google Scholar]
- [4].Streatfeild-James RM, Williamson D, Pike RN, Tewksbury D, Carrell RW, Coughlin PB. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett 1998; 436(2): 267–70. [ 10.1016/S0014-5793(98)01145-4] [DOI] [PubMed] [Google Scholar]
- [5].Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res 2016; 39(7): 492–500. [ 10.1038/hr.2016.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karnik SS, Unal H, Kemp JR, et al. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol Rev 2015; 67(4): 754–819. [ 10.1124/pr.114.010454] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karnik SS, Singh KD, Tirupula K, Unal H. Significance of angiotensin 1–7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22. Br J Pharmacol 2017; 174(9): 737–53. [ 10.1111/bph.13742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2005; 85(4): 1159–204. [ 10.1152/physrev.00003.2005] [DOI] [PubMed] [Google Scholar]
- [9].Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 2009; 93(3): 569–82. [ 10.1016/j.mcna.2009.02.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ainscough JF, Drinkhill MJ, Sedo A, et al. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 2009; 81(3): 592–600. [ 10.1093/cvr/cvn230] [DOI] [PubMed] [Google Scholar]
- [11].Su Q, Huo CJ, Li HB, et al. Renin-angiotensin system acting on reactive oxygen species in paraventricular nucleus induces sympathetic activation via AT1R/PKCγ/Rac1 pathway in salt-induced hypertension. Sci Rep 2017; 7: 43107 [ 10.1038/srep43107] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramchandran R, Takezako T, Saad Y, et al. Angiotensinergic stimulation of vascular endothelium in mice causes hypotension, bradycardia, and attenuated angiotensin response. Proc Natl Acad Sci USA 2006; 103(50): 19087–92. [ 10.1073/pnas.0602715103] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 2018; 100: 1–19. [ 10.1016/j.vph.2017.05.005] [DOI] [PubMed] [Google Scholar]
- [14].Ogita H, Liao J. Endothelial function and oxidative stress. Endothelium 2004; 11(2): 123–32. [ 10.1080/10623320490482664] [DOI] [PubMed] [Google Scholar]
- [15].Peach MJ, Cline WH Jr, Watts DT. Release of adrenal catecholamines by angiotensin. II. Circ Res 1966; 19(3): 571–5. [ 10.1161/01.RES.19.3.571] [DOI] [PubMed] [Google Scholar]
- [16].Parish RC, Miller LJ. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf 1992; 7(1): 14–31. [ 10.2165/00002018-199207010-00004] [DOI] [PubMed] [Google Scholar]
- [17].Gavras H, Gavras I. Angiotensin converting enzyme inhibitors. Properties and side effects. Hypertension 1988; 11(3 Pt 2): II37–41. [ 10.1161/01.HYP.11.3_Pt_2.II37] [DOI] [PubMed] [Google Scholar]
- [18].Zhang H, Unal H, Gati C, et al. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell 2015; 161(4): 833–44. [ 10.1016/j.cell.2015.04.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang H, Unal H, Desnoyer R, et al. Structural basis for ligand recognition and functional selectivity at angiotensin receptor. J Biol Chem 2015; 290(49): 29127–39. [ 10.1074/jbc.M115.689000] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh KD, Unal H, Desnoyer R, Karnik SS. Divergent spatiotemporal interaction of angiotensin receptor blocking drugs with angiotensin type 1 receptor. J Chem Inf Model 2018; 58(1): 182–93. [ 10.1021/acs.jcim.7b00424] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wingler LM, McMahon C, Staus DP, Lefkowitz RJ, Kruse AC. distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell 2019; 176: 479–90 e12 [ 10.1016/j.cell.2018.12.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singh KD, Unal H, Desnoyer R, Karnik SS. Mechanism of hormone peptide activation of a GPCR: Angiotensin II activated state of AT1R initiated by van der waals attraction. J Chem Inf Model 2019; 59(1): 373–85. [ 10.1021/acs.jcim.8b00583] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wong PC, Price WA Jr, Chiu AT, et al. Nonpeptide angiotensin II receptor antagonists. XI. Pharmacology of EXP3174: an active metabolite of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 1990; 255(1): 211–7. [PubMed] [Google Scholar]
- [24].Arumugam S, Sreedhar R, Thandavarayan RA, et al. Angiotensin receptor blockers: Focus on cardiac and renal injury. Trends Cardiovasc Med 2016; 26(3): 221–8. [ 10.1016/j.tcm.2015.06.004] [DOI] [PubMed] [Google Scholar]
- [25].Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355(9215): 1582–7. [ 10.1016/S0140-6736(00)02213-3] [DOI] [PubMed] [Google Scholar]
- [26].Svanström H, Pasternak B, Hviid A. Association of treatment with losartan vs candesartan and mortality among patients with heart failure. JAMA 2012; 307(14): 1506–12. [ 10.1001/jama.2012.452] [DOI] [PubMed] [Google Scholar]
- [27].Crozier I, Ikram H, Awan N, et al. Losartan hemodynamic study group. Losartan in heart failure. Hemodynamic effects and tolerability. Circulation 1995; 91(3): 691–7. [ 10.1161/01.CIR.91.3.691] [DOI] [PubMed] [Google Scholar]
- [28].Lam S Azilsartan: a newly approved angiotensin II receptor blocker. Cardiol Rev 2011; 19(6): 300–4. [ 10.1097/CRD.0b013e31822e9ba3] [DOI] [PubMed] [Google Scholar]
- [29].White WB, Weber MA, Sica D, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension 2011; 57(3): 413–20. [ 10.1161/HYPERTENSIONAHA.110.163402] [DOI] [PubMed] [Google Scholar]
- [30].Sakamoto M, Asakura M, Nakano A, et al. Azilsartan, but not candesartan improves left ventricular diastolic function in patients with hypertension and heart failure. Int J Gerontol 2015; 9: 201–5. [ 10.1016/j.ijge.2015.06.003] [DOI] [Google Scholar]
- [31].Nakamura Y, Suzuki S, Saitoh S, Takeishi Y. New angiotensin II type 1 receptor blocker, azilsartan, attenuates cardiac remodeling after myocardial infarction. Biol Pharm Bull 2013; 36(8): 1326–31. [ 10.1248/bpb.b13-00194] [DOI] [PubMed] [Google Scholar]
- [32].White WB, Cuadra RH, Lloyd E, Bakris GL, Kupfer S. Effects of azilsartan medoxomil compared with olmesartan and valsartan on ambulatory and clinic blood pressure in patients with type 2 diabetes and prediabetes. J Hypertens 2016; 34(4): 788–97. [ 10.1097/HJH.0000000000000839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Georgiopoulos G, Katsi V, Oikonomou D, et al. Azilsartan as a potent antihypertensive drug with possible pleiotropic cardiometabolic effects: A review study. Front Pharmacol 2016; 7: 235 [ 10.3389/fphar.2016.00235] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Takagi H, Mizuno Y, Niwa M, Goto SN, Umemoto T. A meta-analysis of randomized controlled trials of azilsartan therapy for blood pressure reduction. Hypertens Res 2014; 37(5): 432–7. [ 10.1038/hr.2013.142] [DOI] [PubMed] [Google Scholar]
- [35].Young JB, Dunlap ME, Pfeffer MA, et al. Candesartan in heart failure assessment of reduction in mortality and morbidity (charm) investigators and committees. mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the charm low-left ventricular ejection fraction trials. Circulation 2004; 110(17): 2618–26. [ 10.1161/01.CIR.0000146819.43235.A9] [DOI] [PubMed] [Google Scholar]
- [36].Yusuf S, Pfeffer MA, Swedberg K, et al. Charm investigators and committees. effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the charm-preserved trial. Lancet 2003; 362(9386): 777–81. [ 10.1016/S0140-6736(03)14285-7] [DOI] [PubMed] [Google Scholar]
- [37].Granger CB, McMurray JJ, Yusuf S, et al. CHARM investigators and committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362(9386): 772–6. [ 10.1016/S0140-6736(03)14284-5] [DOI] [PubMed] [Google Scholar]
- [38].Pitt B Candesartan reduced mortality and hospital admissions in chronic heart failure. ACP J Club 2004; 140(2): 32–3. [ 10.1136/ebm.9.2.44] [DOI] [PubMed] [Google Scholar]
- [39].Ripley TL, Chonlahan JS, Germany RE. Candesartan in heart failure. Clin Interv Aging 2006; 1(4): 357–66. [ 10.2147/ciia.2006.1.4.357] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lund LH. Heart failure with mid-range ejection fraction: Lessons from CHARM. Card Fail Rev 2018; 4(2): 70–2. [ 10.15420/cfr.2018.11.2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ohlstein EH, Brooks DP, Feuerstein GZ, Ruffolo RR Jr. Inhibition of sympathetic outflow by the angiotensin II receptor antagonist, eprosartan, but not by losartan, valsartan or irbesartan: relationship to differences in prejunctional angiotensin II receptor blockade. Pharmacology 1997; 55(5): 244–51. [ 10.1159/000139534] [DOI] [PubMed] [Google Scholar]
- [42].Brodsky S, Gurbanov K, Abassi Z, et al. Effects of eprosartan on renal function and cardiac hypertrophy in rats with experimental heart failure. Hypertension 1998; 32(4): 746–52. [ 10.1161/01.HYP.32.4.746] [DOI] [PubMed] [Google Scholar]
- [43].Hollenberg NK. Potential of the angiotensin II receptor 1 blocker eprosartan in the management of patients with hypertension or heart failure. Curr Hypertens Rep 2001; 3(Suppl. 1): S25–8. [ 10.1007/s11906-001-0068-9] [DOI] [PubMed] [Google Scholar]
- [44].Barone FC, Coatney RW, Chandra S, et al. Eprosartan reduces cardiac hypertrophy, protects heart and kidney, and prevents early mortality in severely hypertensive stroke-prone rats. Cardiovasc Res 2001; 50(3): 525–37. [ 10.1016/S0008-6363(01)00257-7] [DOI] [PubMed] [Google Scholar]
- [45].Behr TM, Willette RN, Coatney RW, et al. Eprosartan improves cardiac performance, reduces cardiac hypertrophy and mortality and downregulates myocardial monocyte chemoattractant protein-1 and inflammation in hypertensive heart disease. J Hypertens 2004; 22(3): 583–92. [ 10.1097/00004872-200403000-00022] [DOI] [PubMed] [Google Scholar]
- [46].Suzuki G, Mishima T, Tanhehco EJ, et al. Effects of the AT1-receptor antagonist eprosartan on the progression of left ventricular dysfunction in dogs with heart failure. Br J Pharmacol 2003; 138(2): 301–9. [ 10.1038/sj.bjp.0705032] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359(23): 2456–67. [ 10.1056/NEJMoa0805450] [DOI] [PubMed] [Google Scholar]
- [48].Rector TS, Carson PE, Anand IS, et al. I-PRESERVE Trial Investigators. Assessment of long-term effects of irbesartan on heart failure with preserved ejection fraction as measured by the minnesota living with heart failure questionnaire in the irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial. Circ Heart Fail 2012; 5(2): 217–25. [ 10.1161/CIRCHEARTFAILURE.111.964221] [DOI] [PubMed] [Google Scholar]
- [49].Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf 2015; 38(1): 33–54. [ 10.1007/s40264-014-0239-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Watanabe R, Suzuki J, Wakayama K, et al. Angiotensin II receptor blocker irbesartan attenuates cardiac dysfunction induced by myocardial infarction in the presence of renal failure. Hypertens Res 2016; 39(4): 237–44. [ 10.1038/hr.2015.141] [DOI] [PubMed] [Google Scholar]
- [51].Zhang F, Zhou G, Guo L, Lu F, Zhou G. Comparison of clinical efficacy of metoprolol combined with irbesartan and hydrochlorothiazide and non-invasive ventilator in the emergency treatment of patients with severe heart failure. Exp Ther Med 2018; 16(6): 5059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Utsumi K, Yasuda F, Watanabe Y, et al. Effects of olmesartan and imidapril on the plasma adiponectin, P-selectin, and MDA-LDL levels of diabetic nephropathy patients. Clin Chim Acta 2012; 413(1–2): 348–9. [ 10.1016/j.cca.2011.09.024] [DOI] [PubMed] [Google Scholar]
- [53].Miura M, Sakata Y, Miyata S, et al. SUPPORT Trial Investigators. Influence of Left Ventricular Ejection Fraction on the Effects of Supplemental Use of Angiotensin Receptor Blocker Olmesartan in Hypertensive Patients With Heart Failure. Circ J 2016; 80(10): 2155–64. [ 10.1253/circj.CJ-16-0577] [DOI] [PubMed] [Google Scholar]
- [54].Sakata Y, Shiba N, Takahashi J, et al. SUPPORT Trial Investigators. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: the supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. Eur Heart J 2015; 36(15): 915–23. [ 10.1093/eurheartj/ehu504] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sukumaran V, Veeraveedu PT, Gurusamy N, et al. Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1–7 mas receptor. Mol Cell Endocrinol 2012; 351(2): 208–19. [ 10.1016/j.mce.2011.12.010] [DOI] [PubMed] [Google Scholar]
- [56].Sukumaran V, Watanabe K, Veeraveedu PT, et al. Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiac inflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci 2011; 7(2): 154–67. [ 10.7150/ijbs.7.154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hirohata A, Yamamoto K, Miyoshi T, et al. Four-year clinical outcomes of the OLIVUS-Ex (impact of Olmesartan on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) extension trial. Atherosclerosis 2012; 220(1): 134–8. [ 10.1016/j.atherosclerosis.2011.10.013] [DOI] [PubMed] [Google Scholar]
- [58].Padwal R, Lin M, Etminan M, Eurich DT. Comparative effectiveness of olmesartan and other angiotensin receptor blockers in diabetes mellitus: retrospective cohort study. Hypertension 2014; 63(5): 977–83. [ 10.1161/HYPERTENSIONAHA.113.02855] [DOI] [PubMed] [Google Scholar]
- [59].Wang Y, Qiao S, Han DW, et al. Telmisartan Improves Insulin Resistance: A Meta-Analysis. Am J Ther 2018; 25(6): e642–51. [ 10.1097/MJT.0000000000000733] [DOI] [PubMed] [Google Scholar]
- [60].Suksomboon N, Poolsup N, Prasit T. Systematic review of the effect of telmisartan on insulin sensitivity in hypertensive patients with insulin resistance or diabetes. J Clin Pharm Ther 2012; 37(3): 319–27. [ 10.1111/j.1365-2710.2011.01295.x] [DOI] [PubMed] [Google Scholar]
- [61].Verdecchia P, Angeli F, Gentile G, Mazzotta G, Reboldi G. Telmisartan for the reduction of cardiovascular morbidity and mortality. Expert Rev Clin Pharmacol 2011; 4(2): 151–61. [ 10.1586/ecp.10.141] [DOI] [PubMed] [Google Scholar]
- [62].Vidt DG. Telmisartan, ramipril, or both in patients at high risk for vascular events. Curr Hypertens Rep 2008; 10(5): 343–4. [ 10.1007/s11906-008-0064-4] [DOI] [PubMed] [Google Scholar]
- [63].Cice G, Di Benedetto A, D’Isa S, et al. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol 2010; 56(21): 1701–8. [ 10.1016/j.jacc.2010.03.105] [DOI] [PubMed] [Google Scholar]
- [64].Yusuf S, Teo K, Anderson C, et al. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372(9644): 1174–83. [ 10.1016/S0140-6736(08)61242-8] [DOI] [PubMed] [Google Scholar]
- [65].Foulquier S, Böhm M, Schmieder R, et al. Impact of telmisartan on cardiovascular outcome in hypertensive patients at high risk: a Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease subanalysis. J Hypertens 2014; 32(6): 1334–41. [ 10.1097/HJH.0000000000000154] [DOI] [PubMed] [Google Scholar]
- [66].Sukumaran V, Watanabe K, Veeraveedu PT, et al. Telmisartan, an angiotensin-II receptor blocker ameliorates cardiac remodeling in rats with dilated cardiomyopathy. Hypertens Res 2010; 33(7): 695–702. [ 10.1038/hr.2010.67] [DOI] [PubMed] [Google Scholar]
- [67].Jianghua Z, Shijuan L, Moshui C, et al. The effects of telmisartan on serum level of copeptin in patients with chronic heart failure. Heart 2012; 98(Suppl. 2): E1–E319. [ 10.1136/heartjnl-2012-302920v.10] [DOI] [Google Scholar]
- [68].Lin S, Shi Y, Wei Q, et al. Effects of telmisartan on serum adiponectin and left ventricular remodeling in patients with essential hypertension. Biomed Res 2018; 29(7): 1455–8. [Google Scholar]
- [69].Hou Y, Zhang F, Liu Z, Su S, Wu X, Wang Z. Effect of telmisartan and enalapril on ventricular remodeling and kidney prognosis of patients with coronary artery disease complicated with diabetic nephropathy. Exp Ther Med 2017; 13(1): 131–4. [ 10.3892/etm.2016.3933] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tang L, Yi R, Yang B, Li H, Chen H, Liu Z. Valsartan inhibited HIF-1α pathway and attenuated renal interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res Clin Pract 2012; 97(1): 125–31. [ 10.1016/j.diabres.2012.01.037] [DOI] [PubMed] [Google Scholar]
- [71].Cohn JN, Tognoni G. Valsartan heart failure trial investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23): 1667–75. [ 10.1056/NEJMoa010713] [DOI] [PubMed] [Google Scholar]
- [72].Carson P, Tognoni G, Cohn JN. Effect of Valsartan on hospitalization: results from Val-HeFT. J Card Fail 2003; 9(3): 164–71. [ 10.1054/jcaf.2003.22] [DOI] [PubMed] [Google Scholar]
- [73].Webb RL, de Gasparo M. Role of the angiotensin II receptor blocker valsartan in heart failure. Exp Clin Cardiol 2001; 6(4): 215–21. [PMC free article] [PubMed] [Google Scholar]
- [74].Pfeffer MA, McMurray J, Leizorovicz A, et al. Valsartan in acute myocardial infarction trial (VALIANT): rationale and design. Am Heart J 2000; 140(5): 727–50. [ 10.1067/mhj.2000.108832] [DOI] [PubMed] [Google Scholar]
- [75].Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in acute myocardial infarction trial investigators. valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20): 1893–906. [ 10.1056/NEJMoa032292] [DOI] [PubMed] [Google Scholar]
- [76].Kaplinsky E Sacubitril/valsartan in heart failure: latest evidence and place in therapy. Ther Adv Chronic Dis 2016; 7(6): 278–90. [ 10.1177/2040622316665350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Silva-Cardoso J, Brás D, Canário-Almeida F, et al. Neurohormonal modulation: The new paradigm of pharmacological treatment of heart failure. Rev Port Cardiol 2019; 38(3): 175–85. [ 10.1016/j.repc.2018.10.011] [DOI] [PubMed] [Google Scholar]
- [78].Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1): 51–60. [ 10.1016/j.mayocp.2015.10.019] [DOI] [PubMed] [Google Scholar]
- [79].Turnbull F, Neal B, Pfeffer M, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens 2007; 25(5): 951–8. [ 10.1097/HJH.0b013e3280bad9b4] [DOI] [PubMed] [Google Scholar]
- [80].Lindholm LH, Carlberg B. The new Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014): a giant undertaking. Hypertens Res 2014; 37: 391–2. [DOI] [PubMed] [Google Scholar]
- [81].Guidelines for secondary prevention of myocardial infarction. Circ J 2011; 77: 231–48. [DOI] [PubMed] [Google Scholar]


