Abstract
Objective:
Sleep patterns in children with Autism Spectrum Disorder (ASD) appear to diverge from typical development in the second or third year of life. Little is known, however, about the occurrence of sleep problems in infants who later develop ASD and possible impacts on early brain development. In a longitudinal neuroimaging study of infants at familial high (HR) or low risk (LR) for ASD, parent-reported sleep-onset problems were examined in relation to subcortical brain volumes in the first two years of life.
Methods:
432 infants were included across 3 groups: HR who developed ASD (n=71), HR who did not (n=234), and LR (n=127). Sleep-onset problem scores (derived from an infant temperament measure) were evaluated in relation to longitudinal high-resolution T1 & T2 structural imaging data acquired at 6, 12, and 24 months of age.
Results:
Sleep-onset problems were more common at 6–12 months for infants who later developed ASD. Infant sleep-onset problems were related to hippocampal volume trajectories from 6–24 months only for those HR infants who developed ASD. Brain-sleep relationships were specific to the hippocampus; no significant relationships were found with volume trajectories of other subcortical structures examined (amygdala, caudate, globus pallidus, putamen, or thalamus).
Conclusions:
These findings provide initial evidence that sleep-onset problems in the first year of life precede ASD diagnosis and are associated with altered neurodevelopmental trajectories in HR siblings who go on to develop ASD. If replicated, these findings could provide new insights into a potential role of sleep difficulties in the development of ASD.
Introduction
The majority of the first 12 months of life is spent asleep (1). Critical brain maturation processes are thought to occur during sleep in early development (2; 3)—for example, development of the visual cortex relies upon both sensory stimulation during wakefulness and endogenous stimulation during sleep, which together guide neuronal differentiation and developmentally-regulated synaptic plasticity (4).Children with autism spectrum disorder (ASD) are 2–3 times more likely to have difficulties with initiating or sustaining sleep than children who are typically-developing (TD; 5). The impact of inadequate sleep on child cognitive development, behavior, and family functioning in ASD is evident in the behavioral literature (6–8). However, to our knowledge, no research has examined the impact of poor sleep on brain development in this population.
Reduced sleep duration in ASD vs. TD has been reported as early as 30 months of age (9), and can persist through adulthood (7). Sleep patterns in ASD may diverge even earlier; a recent study of over 1,000 children found that the number of night wakings at 12 months was associated with ASD screening scores at 24 months (10). Emerging evidence suggests that preclinical brain and behavior differences exist during infancy in children later diagnosed with ASD. Atypical sensorimotor development (11) and accelerated brain growth in the first year of life have been shown to precede the consolidation of ASD symptoms (12; 13). Given that a substantial portion of an infant’s time is spent asleep, it is possible that some of the preclinical differences seen in early neurodevelopment in ASD could be related to atypical sleep.
The aim of the current study was to characterize altered sleep patterns and associations with brain development in a sample of infants at familial high risk (HR) and low risk (LR) for ASD. This sample provided an opportunity to characterize sleep difficulties in infants who develop ASD, and between those at high and low risk. The focus of the current report was on the relationship between sleep during infancy and subcortical volume during early development. We chose to focus on subcortical development as altered volume in these structures (i.e., hippocampus, amygdala, thalamus, basal ganglia) has been associated with ASD in numerous studies (reviewed in 13-15) and also with sleep problems. Measures of insomnia severity in adults (i.e., arousal level, sleep latency, quality, and fragmentation) have been associated with decreased hippocampal (16–18) and putamen volumes (18). Existing literature on brain correlates of pediatric sleep problems is limited. Sleep duration during weekdays in TD children was positively associated with bilateral hippocampal volume, after adjusting for age, sex, and intracranial volume (19). A recent prospective neuroimaging study found that trajectories of sleep disturbance from age 2 months to 6 years were associated with smaller total brain volumes by age 7; subcortical volumes were not significantly different after correcting for total volume, however (20). Therefore, whether sleep disturbance in early childhood is associated with morphometric changes in subcortical structures remains an open question—one well-suited for investigation in the context of ASD.
We examined associations between sleep difficulties and developmental trajectories in six subcortical structures that are altered in ASD: the amygdala, hippocampus, caudate, putamen, globus pallidus and thalamus (13–15). Based on available evidence of early sleep differences associated with ASD screener scores (10), we hypothesized that sleep difficulties in the first 12 months of life would occur more often for high-risk infants who go on to develop ASD (HR-ASD), least often for low-risk (LR) infants, and intermediate for HR infants who do not develop ASD (HR-NonASD) (21). We further hypothesized that poor sleep would be related to alterations in subcortical brain morphometry; this hypothesis was non-directional, given the paucity of data regarding the impact of sleep difficulties on brain morphometry in early development. Additional follow-up analyses were conducted to determine whether hemispheric differences, cognitive ability, infant temperament, or the timing of sleep measurement were significant contributors to the sleep-brain relationships we observed.
Methods
Participants
This study includes data from 432 HR and LR infants collected across four clinical sites. Parents provided informed consent for their infant to participate, and all study procedures were approved by the institutional review boards at each site. Infants were assessed at 6, 12 and 24 months with MRI scans, parent-report measures, and standardized measures of cognitive and adaptive functioning. The 24-month visit also included a diagnostic assessment for ASD, which yielded three outcome groups: (1) HR-ASD infants who met criteria for ASD (n = 71) (2) HR-NonASD infants who did not meet criteria for ASD (n = 234); and (3) LR infants who did not meet criteria for ASD (n =127). See Table 1 for participant characteristics by group. Unequal group sizes were anticipated given prior evidence from infant sibling studies of a 20% conversion rate for high-risk siblings.41 Our analysis approach was able to accommodate unequal group variances and thus did not require a balanced design (see Statistical Analysis). Full inclusion/exclusion criteria and details of the behavioral assessment are provided in the Supplement. While there is general consensus that ASD can be reliably diagnosed by 24 months,42 approximately half of the infants in this sample participated in a second diagnostic visit at 36 months; those who met diagnostic criteria at either timepoint were included in the ASD group (see Supplementary Table 1) given prior evidence that high-risk siblings with higher cognitive ability and more subtle ASD symptoms may move between diagnostic categories throughout early childhood.43
Table 1.
Participant characteristics by diagnostic group.
| HR-ASD | HR Non-ASD | LR | Group comparison | ||
|---|---|---|---|---|---|
| Total N | 71 | 234 | 127 | -- | |
| Included scans | |||||
| 6 mo timepoint | 54 | 164 | 110 | X2(4) = 6.3 | P = .2 |
| 12 mo timepoint | 44 | 178 | 95 | ||
| 24 mo timepoint | 52 | 161 | 74 | ||
| Sex | X2(2) = 17 | p = .0002 | |||
| Male | 59 | 133 | 73 | ||
| Female | 12 | 101 | 54 | ||
| Age in months | |||||
| First MRI | 6.6 (.6) | 6.6 (.7) | 6.7 (.7) | F(2,322) = .9 | P = .4 |
| Second MRI | 12.7 (.7) | 12.6 (.7) | 12.7 (.7) | F(2,317) = .5 | p = .6 |
| Third MRI | 24.6 (.7) | 24.7 (.9) | 24.7 (.9) | F(2,288) = .6 | P = .5 |
| ADOS (24 mo) | |||||
| Restricted/ Repetitive | 6.0 (2.6) | 3.0 (2.3) | 2.2 (2.0) | F(2,416) = 68.1 | p < .0001 |
| Social Affect | 5.6 (2.1) | 1.8 (1.1) | 1.7 (1.0) | F(2,416) = 251.2 | p < .0001 |
| Mullen (24 mo) | 81.7 (16.6) | 103.5 (15.8) | 110.9 (16.5) | F(2,418) = 74.3 | p < .0001 |
Values denote Ns or Mean (SD). ADOS: Autism Diagnostic Observation Schedule (calibrated severity scores shown for restricted/repetitive behaviors and social affective symptoms); Mullen scores shown are Early Learning Composite scores, which are normed and standardized (M = 100, SD = 15).
Measurement of Sleep-Onset Problems in Infancy
Although the larger study did not include a standardized assessment of sleep, infant development was characterized at 6 and 12 months of age with the Infant Behavior Questionnaire-Revised (IBQ-R) (22), a measure of temperament with five items related to settling and sleep initiation (IBQ-R items 21, 22, 23, 24 and 25; see Box 1). IBQ-R items were rated for frequency on a 7 point scale ranging from “never” to “always”. After reverse scoring, an average was taken across all five items to generate the Infant Sleep-Onset Problems (ISOP) score. Higher ISOP scores indicated more difficulty with sleep initiation and longer sleep-onset latencies.
Box 1.
Questions from the IBQ-R used to develop the ISOP score. Items on the IBQ-R were scored for frequency on a 7-point scale ranging from “never” to “always”. R indicates that item was reversed-scored, such that a higher total score was indicative of more sleep-onset problems.
| When going to sleep at night, how often did your baby: |
| 21. Fall asleep within 10 minutes? R |
| 22. Have a hard time settling down to sleep? |
| 23. Settle down to sleep easily? R |
| When your baby awoke at night, how often did s/he: |
| 24. Have a hard time going back to sleep? |
| 25. Go back to sleep immediately? R |
ISOP scores were available from 432 infants from 6–12 months; infants included in the analyses had data from the 6-month visit only (n = 46), the 12-month visit only (n = 78), or both (n = 308). ISOP scores from 6–12 months were significantly correlated, with the strongest stability across time demonstrated for the HR-ASD group (r = .54, p < .0001), followed by the LR group (r = .41, p < .0001) and the HR-NonASD group (r = .33, p < .0001). To examine the relationship between sleep-onset problems during infancy and subcortical development in the full sample of children, ISOP scores from the 6- and 12-month assessments were averaged to create a single ISOP score for each participant. Select analyses were also repeated using ISOP scores from the 6-month and 12-month assessments only (see Results).
Validation of the Infant Sleep-Onset Problems (ISOP) Score
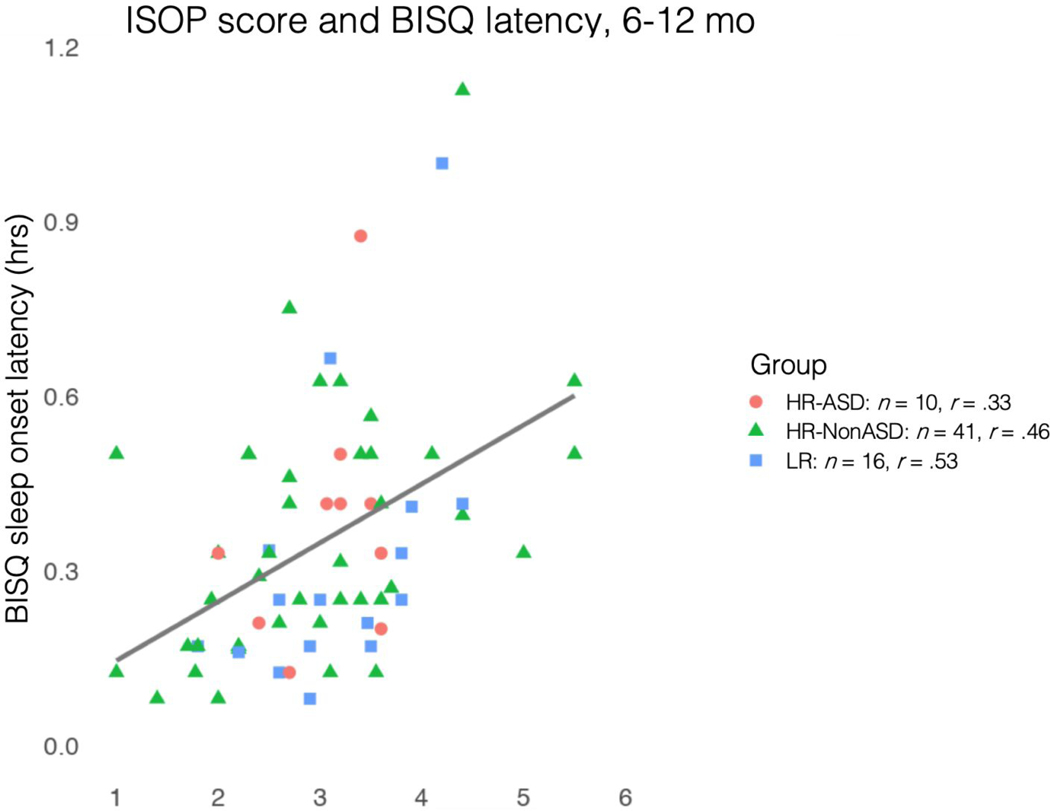
A subset of participants in the larger study were given the Brief Infant Sleep Questionnaire (BISQ; 23) as part of a sub-study conducted at one of the clinical sites (The Children’s Hospital of Philadelphia). Sixty-seven participants (10 HR-ASD, 41 HR-NonASD, 16 LR) had both ISOP and BISQ scores available between 6–12 months. This offered the opportunity to empirically evaluate the use of the IBQ-derived ISOP score for convergent validity by comparing it against a validated infant sleep measure (23). Average sleep latency from the BISQ (time taken to fall asleep at night, in hours) was chosen for comparison to our ISOP score, which measures difficulty settling to sleep at the beginning or middle of the night. The IBQ-based ISOP score was significantly correlated with average sleep latency as measured on the BISQ during the same developmental period (Pearson r = .44 p = .0002; Figure 1). In contrast, it was not correlated with other aspects of sleep measured on the BISQ (e.g., average duration of nocturnal wakefulness; Pearson r = .04 p = .72). Finally, the five IBQ-R sleep items that form the ISOP were internally consistent at both 6 months (Cronbach’s alpha = .76, 95% CI [.74, .78]) and 12 months (Cronbach’s alpha = .76, 95% CI [.74, 78]). Based on this evidence of high internal consistency, and convergent and divergent validity with a previously-validated measure of sleep latency in infancy, we determined that the IBQ-derived ISOP score had sufficient construct validity for the purposes of this study.
Figure 1.
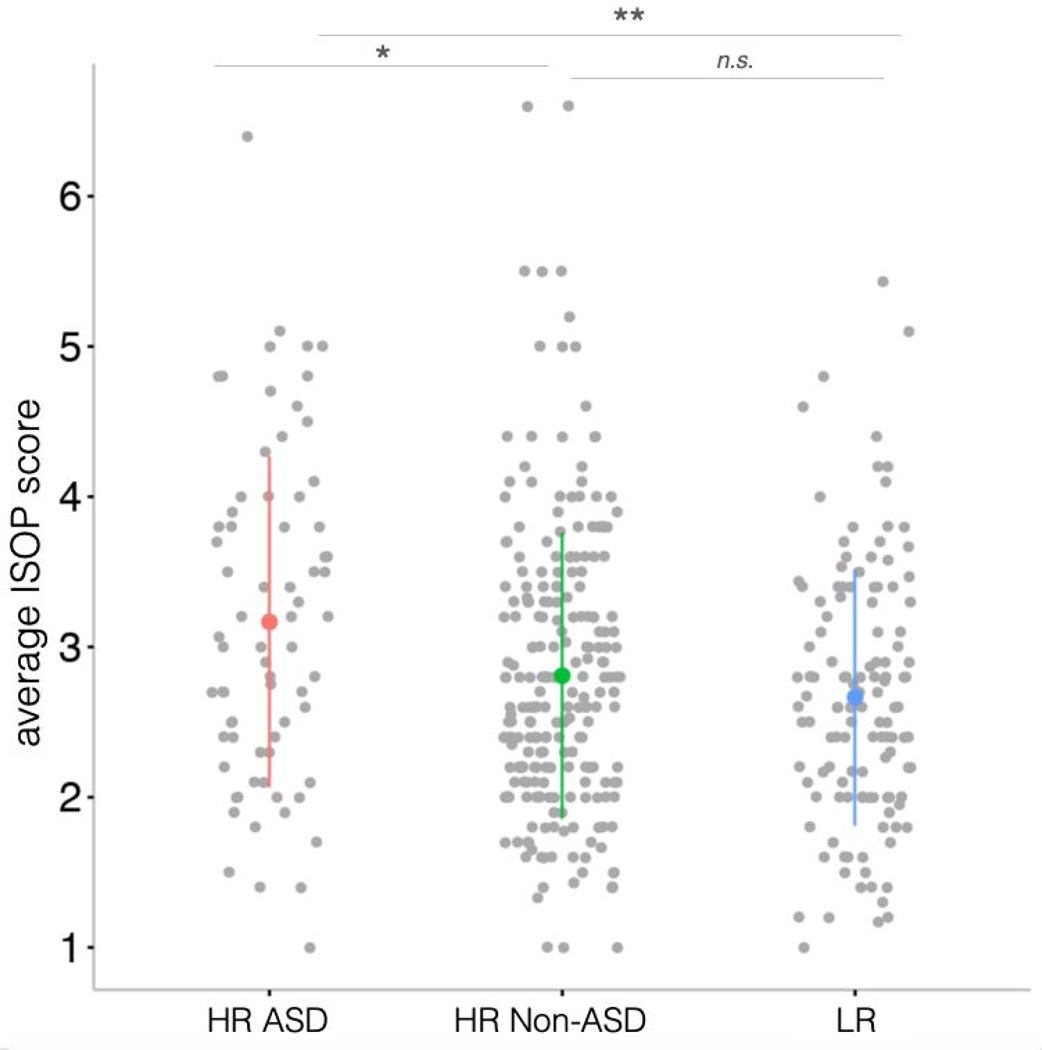
Average ISOP score at 6-12 months by outcome group. A significant difference was found across groups, with infants who developed ASD (HR-ASD) showing more parent-reported sleep-onset problems at 6-12 months than infants who did not develop ASD (with and without familial risk). Colored bars depict standard deviation for each group. ** p < .01, * p < .05, n.s. not significant.
MRI Acquisition and Processing
Imaging data were collected during natural sleep at 6, 12, and 24 months. High-resolution T1- and T2-weighted imaging data (1mm3 voxels) were acquired on identical 3T Siemens TIM Trio scanners equipped with standard 12-channel head coils across all sites. Geometry phantoms were scanned monthly and human phantoms (two adult subjects) were scanned annually to monitor scanner stability at each site across the study period.
T1- and T2-weighted images that passed visual inspection for quality control (99% and 98% of scans, respectively) underwent distortion correction, mutual registration, transformation to stereotactic space, and CSF/brain tissue segmentation. A graph-based, multi-atlas method developed by investigators in the IBIS network was employed to segment the subcortical structures (detailed in Supplement). Atlas templates were derived from 16 cases at each time point (6, 12, and 24 months), which were manually segmented by a single experimenter, used as training images in the multi atlas segmentation, and then applied to all 6,12 and 24 month data sets. All segmentations underwent visual quality inspection by two experimenters (blind to diagnosis, risk status, sex, scan site). Over 98% of scans met quality inspection criteria for segmentation, with no difference in segmentation pass rate between groups. A total of 932 scans, across three groups and three timepoints, were included in the current analysis (150 HR-ASD; 503 HR-NonASD; 279 LR; Table 1).
Statistical Analysis
Categorical differences in ISOP scores were tested via ANOVA, and Pearson correlation coefficients were calculated between ISOP scores and continuous measures of child functioning. Linear mixed-effect models were used to predict bilateral volume trajectories for 6 subcortical structures (hippocampus, amygdala, caudate, globus pallidus, putamen, thalamus). Volumes were summed across hemispheres to reduce the number of comparisons, consistent with previous work showing no laterality effects in subcortical volumes in this sample (24). Individual intercepts were included as a random effect. ISOP score, age, quadratic effect of age (age2), sex, and group interactions with each were included as fixed effects. Total cerebral volume and scan site were also included as covariates. All tests were two-tailed with α = 0.05, and an FDR procedure was used to correct for multiple comparisons.
Any subcortical structure associated with ISOP scores was subjected to additional follow-up analyses to determine the strength and specificity of the finding (additional details in Supplement). Specifically, we tested whether sleep-brain associations 1) were present in both hemispheres, 2) persisted when controlling for cognitive ability, 3) were specific to IBQ-R sleep items (rather than infant temperament more broadly), 4) persisted when including ISOP scores from 6 or 12 months only.
Results
ISOP scores across diagnostic groups
ISOP scores (Figure 2) were compared across the three diagnostic groups and differed significantly (F(2,429)= 6.41, p = .002); a post-hoc Tukey test revealed greater sleep-onset problems for HR-ASD compared to the HR-NonASD (difference .36; 95% CI [.06, .66]; p = .02) and LR groups (difference −.50; 95% CI [−.83, −.17]; p = .001). ISOP scores did not differ significantly between the HR-NonASD and LR infants (difference −.14; 95% CI [−.39, .10]; p = 0.36). A disorder-specific effect was also found when looking at ISOP scores from the 6 month timepoint only (HR-ASD vs. HR-NonASD: t(82.7) = 2.72, p = .008; HR-ASD vs. LR: t(89.0) = 3.0, p = .003; HR-NonASD vs. LR: t(272.3) = .53, p = .60) and 12 month timepoint only (HR-ASD vs. HR-NonASD; t(90.9) = 2.00, p = .04; HR-ASD vs. LR: t(108.4) = 3.0, p = .004; HR-NonASD vs. LR: t(229.4) = 1.62, p = .11). Of note, there was no difference in ISOP scores between male and female infants in the sample (F(1,430) = .10, p = .75).
Figure 2.
Validation of the ISOP score (from the IBQ-R) against a measure of sleep onset latency from a commonly-used parent-report measure of infant sleep, the Brief Infant Sleep Questionnaire (BISQ).
The finding of categorical differences in ISOP Scores motivated exploration of associations between ISOP score and continuous measures of cognitive and adaptive development and ASD symptoms. Correlation results are presented in full in the Supplement (Table 2). At each timepoint (6 mo and 12 mo), ISOP score correlated significantly with expressive language scores on the Mullen (6 mo r = −.14, p = 01; 12 mo r = −.11, p = .03), but none of the other behavioral measures. By 24 months, average ISOP scores were significantly correlated with ADOS social affect severity scores (r = .14, p = .004), Mullen expressive language scores (r = −.11, p = .03), Vineland communication (r = −.13, p = .01), and Vineland socialization scores (r = −.15, p = .003). Across the entire sample, infants with worse sleep from 6–12 months showed weaker social communication skills by 24 months.
Sleep-onset problems in relation to brain volume trajectories from 6–24 mo
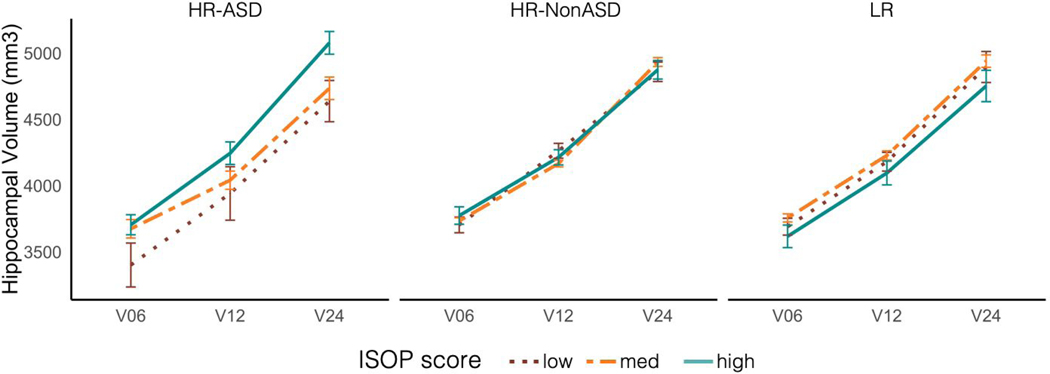
Separate linear mixed-effect models were conducted for each subcortical structure (see Supplementary Table 3). Only hippocampal trajectory was significantly predicted by ISOP score (β = 101.0, t = 3.2, p = .008), and group moderated the association between ISOP score and hippocampal trajectory in infancy (Figure 3). Examination of group-specific parameter estimates revealed that sleep-onset difficulties in infancy were associated with increased hippocampal volume from 6–24 months only for HR siblings who went on to develop ASD (β=104.7; t=2.8, p=.006); no relationship between sleep and hippocampal trajectory was found for the HR-NonASD (β=−11.5; t=.6, p=.6) and LR groups (β=−4.9; t=.2, p=.9).
Figure 3.
Hippocampal volume trajectories from 6-24 months. Categorical differences in ISOP score are shown for visualization purposes only; all analyses treated ISOP score as a continuous variable. Moving left to right, hippocampal volumes for infants with low (1 SD < mean), medium, and high (1 SD > mean) ISOP scores are shown by outcome group: HR-ASD, HR-NonASD, and LR. Higher ISOP scores indicate longer sleep onset latency.
Hemispheric differences
Examining associations in the left and right hemisphere separately revealed that ISOP score was significantly associated with trajectories of both left and right hippocampal volume. Specifically, there was a significant main effect of ISOP score (Left: β = 47.9, t = 3.0, p = .002; Right: β = 53.1, t = 3.1, p = .002), and significant Group x ISOP interactions for each hemisphere (Left: HR-ASD vs. HR-NonASD: β = −49.9, t = 2.7, p = .007; HR-ASD vs LR: β = −54.5, t = 2.5, p = .012; Right: HR-ASD vs. HR-NonASD: β = −59.0, t = 2.3, p = .003; HR-ASD vs LR: β = −54.3, t = 2.3, p = .02).
Hippocampal trajectory and sleep-onset problems, controlling for cognitive ability
The association between ISOP score and hippocampal trajectory persisted after adding 24-month cognitive ability (Mullen) scores to the model. Specifically, there was a significant main effect of ISOP score (β = 100.0, t = 3.1, p = .002), and a significant Group x ISOP interaction (HR-ASD vs. HR-NonASD: β = −106.7, t = 2.9, p = .005; HR-ASD vs LR: β = 111.7, t = 2.5, p = .012). 24-month Mullen scores did not predict additional variance in hippocampal trajectory (β = −0.8, t = .9, p = .37).
Hippocampal trajectory and other IBQ-R subscales
The IBQ-R has 14 subscales (see 22 for details). The five sleep items that comprise the ISOP are part of the Falling Reactivity/Rate of Recovery from Distress scale. To examine the specificity of the sleep-hippocampal finding, the same full linear mixed model predicting bilateral hippocampal trajectory was repeated replacing the ISOP score with the scores from each IBQ-R subscale (including the full 13-item Falling Reactivity/Rate of Recovery scale, each averaged from 6–12 months). None of the other IBQ-R subscales were significantly related to hippocampal volume (see Supplementary Table 4 for detail), including the full Falling Reactivity/Rate of Recovery scale from which the sleep items were derived. This suggests that the relationship with hippocampal trajectory may be specific to the five IBQ-R items comprising the ISOP score, and does not represent a more general relationship with infant behavior or temperament.
Hippocampal trajectory and sleep-onset problems measured at 6 and 12 months
The final follow-up analyses investigated whether the associations between ISOP score and trajectories of hippocampal volume from 6–24 months would persist if restricted to sleep measured at a single timepoint in development (either 6 or 12 months). Models were run substituting 6-month and 12-month ISOP scores for the averaged score, and including cognitive ability (Mullen Early Learning Composite) measured at that same timepoint (6 or 12 months).
The association between hippocampal volume trajectory and sleep persisted when using ISOP scores measured only at a single timepoint (6 months or 12 months). Specifically, in the 6-month model there was a significant main effect of 6-month ISOP (β = 75.9, t = 2.3, p = .02), and a significant Group x 6-month ISOP interaction (HR-ASD vs. HR-NonASD: β = −78.4, t = −2.1, p = .04; HR-ASD vs LR: β = −109.8, t = 2.6, p = .008). 6-month Mullen scores did not predict additional variance in hippocampal trajectory (β = 0.8, t = .6, p = .6).
Similarly, in the 12-month model there was a significant main effect of 12-month ISOP (β = 74.5, t = 2.4, p = .02), and a significant Group x 12-month ISOP interaction for the contrast HR-ASD vs. HR-NonASD (β = −86.9, t = −2.4, p = .02). With 12-month ISOP, the HR-ASD vs LR contrast did not reach significance (β = −43.9, t = −1.1, p = .3). 12-month Mullen scores did not predict additional variance in hippocampal trajectory (β = −.22, t = .20, p = .8).
Discussion
In a large sample of infants at high and low familial risk for ASD, we found that sleep-onset problems were more prevalent at 6–12 months for infants who went on to develop ASD. Difficulties with sleep onset during infancy were associated with weaker social communication skills by 24 months, and were related to hippocampal volume trajectory from 6–24 months only for HR-ASD infants. HR-ASD infants with sleep-onset difficulties at 6–12 months showed increased hippocampal volume trajectories compared to HR-ASD infants with better sleep. The association with sleep-onset problems in infancy was specific to the hippocampus; no significant sleep-brain associations were found for the other subcortical structures examined (amygdala, caudate, globus pallidus, putamen, and thalamus). Additional analyses revealed that the observed association between sleep-onset problems and hippocampal trajectories in the HR-ASD group existed in each hemisphere and persisted after controlling for cognitive ability at 24 months. Furthermore, the association was specific to sleep-onset problems and did not generalize to other aspects of infant temperament measured on the IBQ-R. Finally, sleep-hippocampal associations persisted in models that included sleep and cognitive ability measured at a single timepoint (6 or 12 months).
Our finding that early sleep onset problems are specific to ASD is in keeping with prior infant-sibling studies. Sleep patterns in typically-developing children who have a sibling with ASD are similar to those with no family history (21; 25). Associations with hippocampal trajectories were also specific to the HR-ASD group. In contrast to prior studies with typically-developing older children, adults with insomnia, and sleep-deprived animals (18; 19; 26), sleep-onset problems in HR infants who developed ASD were associated with increased (rather than decreased) hippocampal volume across early development. The reason for this disorder-specific effect and its directionality are unclear, but it may be related to a disrupted coupling of hippocampal and brain size that has been observed in other ASD samples (27). Overgrowth of some structures, but not others, at different stages of development have been frequently reported in ASD (12; 13; 28). Indeed, in the same infant sibling sample as the current study, early cortical overgrowth was associated with later social deficits (12), larger corpora callosa were associated with later restricted/repetitive behavior (29), and larger thalamus, caudate, and amygdala volumes were associated with abnormal language profiles (24). In a departure from this prior work, however, we show evidence of a brain-behavior relationship during infancy that precedes the onset of ASD symptoms. If replicated, these findings suggest that an individual difference in infant behavior (i.e., sleep onset difficulties) could help predict which infants will show abnormal trajectories of hippocampal growth prior to the onset of ASD symptoms.
The demonstration that our findings are specific to the hippocampus is in keeping with a large body of non-ASD work linking sleep to morphometric changes (16–18) and accumulation of metabolic byproducts in the hippocampus (e.g. beta-amyloid; 30). Sleep also plays a role in hippocampally-mediated cognitive processes, including spatial and declarative learning and consolidation (31). Adequate sleep may be required for typical hippocampal maturation: young (but not adolescent) mice who were deprived of REM sleep showed reduced long-term potentiation stability and lower expression of glutamatergic signaling proteins, both suggestive of altered hippocampal development (32). Thus, the hippocampus appears to be a brain region that is particularly sensitive to the effects of disturbed or inadequate sleep. However, it is important to recognize that the sleep-hippocampal associations we observed do not reveal a causal direction. Inadequate sleep could cause changes in the hippocampus that confer vulnerability to disorders of neurodevelopment or neurodegeneration. Alternatively, preexisting neurobiological or genetic differences could result in both hippocampal changes and sleep disturbance during the early stages of a disorder.
A variety of critical neurobiological processes occur during sleep and could underlie our findings. One potential mechanism involves neuroinflammatory processes that are modulated during sleep. Sleep deprivation has been shown to increase neuroinflammation, which affects synaptic plasticity in the hippocampus (33). Sleep disturbance has been associated with inflammatory responses in the hippocampus, but not the cortex, of adult mice, and impaired subsequent performance on a hippocampally-dependent learning and memory task (34). In humans, the data on sleep-inflammation associations are mixed. A recent meta-analysis found that increased levels of inflammatory markers were associated with sleep disturbance and long sleep duration (> 8 hrs/night), but not short sleep duration (< 7 hrs/night; 35). However, evidence of potential involvement of inflammatory responses in the pathogenesis of ASD (36) suggests that investigating sleep and neuroinflammation longitudinally in studies of early brain development (such as this one) is a promising direction for future research.
This study has several limitations, including the use of a novel measure of sleep during infancy. Although our measure of sleep-onset problems (derived from five items on the IBQ-R) showed strong correspondence with BISQ scores and strong internal consistency in a subsample of infants, it is based on parent report, and has not (to our knowledge) been validated against objective measures of sleep in infancy (i.e., actigraphy or polysomnography). In addition, these five items measured only one aspect of sleep (sleep-onset latency), and did not provide information about sleep fragmentation, quality, or overall duration. Moving forward, it will be important to conduct more comprehensive sleep assessments to determine which characteristics of sleep during infancy are most relevant to brain and behavior development. Another important area for future research will be to investigate associations between infant sleep and cortical development, as altered cortical morphometry has been associated with ASD in high-risk siblings12 and chronic sleep disturbance in typically-developing children.20 Given the early stages of this work, and the absence of prior work looking at sleep and neurodevelopment in infants at high risk for ASD, we believe these preliminary findings are of interest and warrant extension to other brain regions and replication with alternative measures of sleep.
If replicated, these observations could provide new insights into a potential role for disturbed sleep in the development of ASD. The high prevalence of ASD and comorbid sleep difficulties in some rare genetic syndromes (37) has led to the suggestion that ASD and sleep problems may have shared etiology (38). Impairments in circadian timing (39), sleep-wake regulation (8), sensory processing (38), and affective functioning (7) may underlie both sleep difficulties and core symptoms of ASD. The efficacy of both pharmacologic (e.g., melatonin) and behavioral sleep interventions (40) for some children with ASD suggests that both neurobiological and psychosocial factors should be considered. Our findings provide initial evidence that sleep difficulties in the first year of life may precede ASD diagnosis and are associated with altered neurodevelopmental trajectories in HR siblings who go on to develop ASD. Future work will reveal the implications of these results for understanding neurodevelopment in ASD and for developing early, targeted interventions for sleep difficulties in infants at high risk for ASD.
Supplementary Material
Acknowledgements:
We gratefully acknowledge the families who participated in this research. This work was supported by a National Institutes of Health Autism Center of Excellence grant (National Institute of Mental Health and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant number HD055741 to JP); Autism Speaks (6020); and the Simons Foundation (140209). Further support was provided by National Institute Of Mental Health (F32MH118689 to K.E.M). The funders had no role in study design, data collection, analysis, data interpretation, or writing of the report.
Footnotes
Disclosures: The authors report no financial relationships with commercial interests.
Contributor Information
Katherine E. MacDuffie, Department of Speech and Hearing Sciences, University of Washington, Seattle.
Mark D. Shen, Department of Psychiatry, University of North Carolina Chapel Hill.
Stephen R. Dager, Department of Radiology, University of Washington, Seattle.
Martin A. Styner, Department of Psychiatry, University of North Carolina Chapel Hill; Biomedical Research Imaging Center, University of North Carolina Chapel Hill.
Sun Hyung Kim, Department of Psychiatry, University of North Carolina Chapel Hill.
Sarah Paterson, Department of Psychology, Temple University, Philadelphia.
Juhi Pandey, Department of Child Psychiatry and Behavioral Sciences, Children’s Hospital of Philadelphia.
Tanya St. John, Department of Speech and Hearing Sciences, University of Washington, Seattle.
Jed T. Elison, Institute of Child Development, University of Minnesota, Minneapolis.
Jason J. Wolff, Department of Educational Psychology, University of Minnesota, Minneapolis.
Meghan R. Swanson, Department of Behavioral and Brain Sciences, University of Texas at Dallas.
Kelly N. Botteron, Department of Child Psychiatry, Washington University School of Medicine in St. Louis.
Lonnie Zwaigenbaum, Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada.
Joseph Piven, Neurodevelopmental Research Center, University of North Carolina Chapel Hill.
Annette M. Estes, Department of Speech and Hearing Sciences, University of Washington, Seattle.
References
- 1.Iglowstein I, Jenni OG, Molinari L, Largo RH: Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics 2003; 111:302–307 [DOI] [PubMed] [Google Scholar]
- 2.Kayser MS, Biron D: Sleep and Development in Genetically Tractable Model Organisms. Genetics 2016; 203:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernier A, Carlson SM, Bordeleau S, Carrier J: Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning. Child Dev 2010; 81:1739–1752 [DOI] [PubMed] [Google Scholar]
- 4.Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA: Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 2002; 110:431–443 [DOI] [PubMed] [Google Scholar]
- 5.Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, Schieve LA,Levy SE: Sleep Problems in 2- to 5-Year-Olds With Autism Spectrum Disorder and Other Developmental Delays. Pediatrics 2019; 143:e20180492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Conduit R, Lockley SW, Rajaratnam SM, Cornish KM: The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord 2014; 6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richdale AL, Schreck KA: Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews 2009; 13:403–411 [DOI] [PubMed] [Google Scholar]
- 8.Cortesi F, Giannotti F, Ivanenko A, Johnson K: Sleep in children with autistic spectrum disorder. Sleep Med. 2010; 11:659–664 [DOI] [PubMed] [Google Scholar]
- 9.Humphreys JS, Gringras P, Blair PS, Scott N, Henderson J, Fleming PJ, Emond AM: Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Archives of Disease in Childhood 2014; 99:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen AKD, Murphy LE, Kocak M, Tylavsky FA, Pagani LS: Prospective Associations Between Infant Sleep at 12 Months and Autism Spectrum Disorder Screening Scores at 24 Months in a Community-Based Birth Cohort. J Clin Psychiatry 2018; 79:16m11127 [DOI] [PubMed] [Google Scholar]
- 11.Estes A, Zwaigenbaum L, Gu H, St John T, Paterson S, Elison JT, Hazlett H, Botteron K, Dager SR, Schultz RT, Kostopoulos P, Evans A, Dawson G, Eliason J, Alvarez S, Piven J, IBIS Network: Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord 2015; 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J, IBIS Network, Clinical Sites, Data Coordinating Center, Image Processing Core, Statistical Analysis: Early brain development in infants at high risk for autism spectrum disorder. Nature 2017; 542:348–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girault JB, Piven J: The neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clinics of North America. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaral DG, Schumann CM, Nordahl CW: Neuroanatomy of autism. Trends in Neurosciences 2008; 31:137–145 [DOI] [PubMed] [Google Scholar]
- 15.Ecker C: The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017; 21:18–28 [DOI] [PubMed] [Google Scholar]
- 16.Noh HJ, Joo EY, Kim ST, Yoon SM, Koo DL, Kim D, Lee G-H, Hong SB: The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol 2012; 8:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N: Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol. Psychiatry 2010; 68:494–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo DL, Shin J-H, Lim J-S, Seong J-K, Joo EY: Changes in subcortical shape and cognitive function in patients with chronic insomnia. Sleep Med. 2017; 35:23–26 [DOI] [PubMed] [Google Scholar]
- 19.Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K, Fukuda H, Kawashima R: Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. NeuroImage 2012; 60:471–475 [DOI] [PubMed] [Google Scholar]
- 20.Kocevska D, Muetzel RL, Luik AI, Luijk MPCM, Jaddoe VW, Verhulst FC, White T, Tiemeier H: The Developmental Course of Sleep Disturbances Across Childhood Relates to Brain Morphology at Age 7: The Generation R Study. Sleep 2017; 40 [DOI] [PubMed] [Google Scholar]
- 21.Schwichtenberg AJ, Young GS, Hutman T, Iosif A-M, Sigman M, Rogers SJ, Ozonoff S: Behavior and sleep problems in children with a family history of autism. Autism Res 2013; 6:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartstein MA, Rothbart MK: Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development 2003; 26:64–86 [Google Scholar]
- 23.Sadeh A: A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 2004; 113:e570–7 [DOI] [PubMed] [Google Scholar]
- 24.Swanson MR, Shen MD, Wolff JJ, Elison JT, Emerson RW, Styner MA, Hazlett HC, Truong K, Watson LR, Paterson S, Marrus N, Botteron KN, Pandey J, Schultz RT, Dager SR, Zwaigenbaum L, Estes AM, Piven J, IBIS Network: Subcortical Brain and Behavior Phenotypes Differentiate Infants With Autism Versus Language Delay. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwichtenberg AJ, Hensle T, in SHCP, 2016: Sibling sleep—What can it tell us about parental sleep reports in the context of autism? psycnet.apa.org [DOI] [PMC free article] [PubMed]
- 26.Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D: Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J. Physiol. (Lond.) 2003; 549:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhardt VP, Iosif A-M, Libero L, Heath B, Rogers SJ, Ferrer E, Nordahl C, Ghetti S, Amaral D, Solomon M: Understanding Hippocampal Development in Young Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redcay E, Courchesne E: When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry 2005; 58:1–9 [DOI] [PubMed] [Google Scholar]
- 29.Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, Botteron KN, Elison JT, Dager SR, Estes AM, Hazlett HC, Schultz RT, Zwaigenbaum L, Piven J, IBIS Network: Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 2015; 138:2046–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shokri-Kojori E, Wang G-J, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND: β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreutzmann JC, Havekes R, Abel T, Meerlo P: Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 2015; 309:173–190 [DOI] [PubMed] [Google Scholar]
- 32.Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, Shaffery JP: Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience 2008; 153:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P: Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol. Dis. 2013; 52:229–236 [DOI] [PubMed] [Google Scholar]
- 34.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SAP, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z: Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol. Dis. 2012; 48:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irwin MR, Olmstead R, Carroll JE: Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016; 80:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokobza C, Van Steenwinckel J, Mani S, Mezger V, Fleiss B, Gressens P: Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019; 85:155–165 [DOI] [PubMed] [Google Scholar]
- 37.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts J, Baker C, Vulto-van Silfhout AT, Schuurs-Hoeijmakers JH, Fichera M, Bosco P, Buono S, Alberti A, Failla P, Peeters H, Steyaert J, Vissers LELM, Francescatto L, Mefford HC, Rosenfeld JA, Bakken T, O’Roak BJ, Pawlus M, Moon R, Shendure J, Amaral DG, Lein E, Rankin J, Romano C, de Vries BBA, Katsanis N, Eichler EE: Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014; 158:263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzone L, Postorino V, Siracusano M, Riccioni A, Curatolo P: The Relationship between Sleep Problems, Neurobiological Alterations, Core Symptoms of Autism Spectrum Disorder, and Psychiatric Comorbidities. J Clin Med 2018; 7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glickman G: Circadian rhythms and sleep in children with autism. Neuroscience & Biobehavioral Reviews 2010; 34:755–768 [DOI] [PubMed] [Google Scholar]
- 40.Malow BA, Byars K, Johnson K, Weiss S, Bernal P, Goldman SE, Panzer R, Coury DL, Glaze DG, Sleep Committee of the Autism Treatment Network: A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics 2012; 130 Suppl 2:S106–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozonoff Sally, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011; 128: e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estes Annette, St John Tanya, and Dager Stephen R.. What to Tell a Parent Who Worries a Young Child Has Autism. JAMA psychiatry 2019; 76: 1092–1093. [DOI] [PubMed] [Google Scholar]
- 43.Brian Jessica, et al. Stability and change in autism spectrum disorder diagnosis from age 3 to middle childhood in a high-risk sibling cohort. Autism 2016; 20: 888–892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.