Abstract
Background
Sepsis remains a leading cause of death worldwide despite advances in management strategies. Preclinical and observational studies have found mortality benefit with high-dose vitamin C in sepsis. Our study aims to prospectively evaluate the effect of intravenous hydrocortisone, vitamin C [ascorbic acid (AA)], and thiamine (HAT) administration in reducing inpatient all-cause mortality among patients with septic shock.
Materials and methods
Our single-center, prospective, open-label, randomized controlled trial recruited patients with admitting diagnosis of septic shock and assigned eligible patients (1:1) into either intervention (HAT) or control group (routine). The HAT group received intravenous combination of vitamin C (1.5 g every 6 hours), thiamine (200 mg every 12 hours), and hydrocortisone (50 mg every 6 hours) within 6 hours of onset of septic shock admission. The treatment was continued for at least 4 days, in addition to the routine standard of care provided to the control group. Thiamine and hydrocortisone use in control arm was not restricted. Vitamin C levels were estimated at baseline and at the end of the 4 days of treatment for both groups. The primary outcome evaluated was mortality during inpatient stay.
Results
Among 90 patients enrolled, 88 patients completed the study protocol. The baseline characteristics between the HAT (n = 45) and the routine (n = 43) groups were comparable. The all-cause mortality in the HAT cohort was 57% (26/45) compared to 53% (23/43) in the routine care group (p = 0.4, OR 1.19, 95% CI 0.51–2.76). The time to reversal of septic shock was significantly lower in the HAT (34.58 ± 22.63 hours) in comparison to the routine care (45.42 ± 24.4 hours) (p = 0.03, mean difference −10.84, 95% CI −20.8 to −0.87). No significant difference was observed between the HAT and the routine care with respect to changes in sequential organ failure assessment (SOFA) scores at 72 hours (2.23 ± 2.4 vs 1.38 ± 3.1), the use of mechanical ventilation (48% vs 46%), and mean Vasoactive Inotropic Score (7.77 ± 12.12 vs 8.86 ± 12.5).
Conclusion
Intravenous administration of vitamin C, thiamine, and hydrocortisone did not significantly improve the inpatient all-cause mortality among patients with septic shock.
Clinical significance
HAT protocol does not reduce hospital mortality but decreases time to shock reversal in septic shock.
How to cite this article
Mohamed ZU, Prasannan P, Moni M, Edathadathil F, Prasanna P, Menon A, et al. Vitamin C Therapy for Routine Care in Septic Shock (ViCTOR) Trial: Effect of Intravenous Vitamin C, Thiamine, and Hydrocortisone Administration on Inpatient Mortality among Patients with Septic Shock. Indian J Crit Care Med 2020;24(8):653–661.
Keywords: Ascorbic acid, HAT protocol, Mortality, Sepsis, Septic shock, Vitamin C
Introduction
Sepsis remains as a life-threatening medical condition of dysregulated immune response with a mortality rate exceeding 50% in septic shock. More than 5 million deaths globally could be attributed to sepsis.1,2 Given the high burden of sepsis worldwide, the search continues for effective targeted therapies in improving sepsis outcomes, especially in developing countries with a focus on inexpensive, safe, and accessible interventions.3,4 Besides the short-term mortality, septic patients suffer from numerous short- and long-term complications, with a reduced quality of life and an increased risk of death following acute event.5
Sepsis is the result of a complex interaction between the host response and infecting organism resulting in severe organ dysfunction.6 Widespread distribution of proinflammatory mediators plays an important role in the pathogenesis and high morbidity and mortality associated with sepsis. High cellular production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) contributes to oxidative stress, leading to consequent tissue and organ damage. Vitamin C is an essential water-soluble nutrient which plays a role in mediating inflammation through antioxidant activities and is also important in the synthesis of cortisol, catecholamines, and vasopressin, which are key mediators in the disease process.7 It is understood to synergistically act with steroids to protect against and reverse vascular endothelial dysfunction caused by the endotoxin released during sepsis. The high dose of vitamin C is also thought to increase the risk of kidney damage by leading to the formation of oxalate crystals. Oxidation of the oxalate to carbon dioxide occurs by a series of steps that include the enzyme glyoxylate aminotransferase which requires thiamine pyrophosphate as a coenzyme.
Recent evidence-based research has suggested the beneficial impact of vitamin C administration on patients with severe sepsis and septic shock. Preliminary studies indicated positive effect on multiple organ failure and endothelial injury.8,9 The administration of thiamine and hydrocortisone has been independently reported to improve sepsis outcomes, such as lactate clearance and shock reversal.10,11 Reduction of sequential organ failure assessment (SOFA) scores, time to shock resolution, and proinflammatory biomarkers have been reported with Vit C administration.8,12 VITAMINS trial did not observe any improvement in time alive and vasopressor-free duration.13 Ambiguity existed in relation to the beneficial effects of vitamin C as several recent randomized clinical trials failed to demonstrate a positive impact on clinical outcomes, warranting further research. Accordingly, our randomized clinical trial investigated the effect of hydrocortisone, vitamin C [ascorbic acid (AA)], and thiamine (HAT) intravenous administration on improving all-cause mortality among patient with septic shock.
Materials and Methods
Study Design and Setting
This Vitamin C Therapy Or Routine care in septic shock (ViCTOR) Trial is an investigator initiated, prospective, single-site, open-label, randomized controlled trial conducted at an academic tertiary care center in South India. The study was approved by the Institutional Research Ethics Committee (MD/MS2017/56) and registered with CTRI: (CTRI/2018/07/014787). Patient enrolment into the study commenced from June 2018 and continued till August 2019. Informed consent was obtained from patients or legal surrogate prior to study recruitment.
Study Participants
All patients admitted to the participating intensive care units (ICUs) of the tertiary referral teaching hospital were identified by an alert from the primary physician/nurse. Adult non-pregnant patients with septic shock (Surviving Sepsis Campaign 2016 guidelines14) and within 6 hours of initiation of inotropic support were eligible for recruitment. Patients with burns, limitations of care due to terminal illness or acute liver failure were excluded from the study.
Randomization
Once consent was obtained, patients were randomly assigned in 1:1 ratio into either the HAT or the routine group. Patient enrolment proceeded according to a preformed randomization list generated through an online randomization software, which employed random permuted block sizes of length 6 to ensure balance over time.
Study Intervention
Following an informed consent, the randomized patients in the HAT cohort received intravenous hydrocortisone (50 mg every 6 hours), vitamin C (AA) (1.5 g every 6 hours), and thiamine (200 mg every 12 hours) for 4 days, with the first doses of the drugs administered within 6 hours of onset of septic shock/admission, with routine care. Vitamin C was infused over 60 minutes using a photolysis preventing infusion set. Routine group received the standard of care for septic shock. The use of hydrocortisone and vitamin supplements in the control group was at the treating physician's discretion.
Data Collection
The data collected included basic demographics — Age, sex, admitting diagnosis, comorbidities, complete blood count, renal and liver function profile, procalcitonin, details of vasopressor use (hourly dosage) PaO2/FiO2, Glasgow Coma Scale (GCS), need for mechanical ventilation, length of ICU and hospital stay, acute kidney injury (AKI), blood culture, lactate, fluids administered, and SOFA score. ∆SOFA was calculated as the absolute difference between the SOFA scores at admission (ASOFA) and at 72 hours. Initial fluid resuscitation was performed with normal saline as per surviving sepsis campaign guidelines. New onset of AKI was defined as the increase in serum creatinine to ≥1.5 times the baseline value after initiation of the HAT protocol.15 The Inotrope Score (IS) was calculated by adding dopamine dose (μg/kg/minute) + dobutamine dose (μg/kg/minute) + 100 × epinephrine dose (μg/kg/minute). The Vasoactive Inotropic Score (VIS) is IS + 10 × milrinone dose (μg/kg/minute) + 10,000 × vasopressin dose (units/kg/minute) + 100 × norepinephrine dose (μg/kg/minute).16 The vitamin C level at baseline (prior to therapy) and after 4 days was collected for both groups. Data collected were compiled in a spreadsheet by a trained medical staff and data of 30 randomly selected patients were independently cross-checked and verified for data fidelity by another investigator.
Outcome Measures
The primary outcome evaluated was inpatient all-cause mortality between the HAT and the routine groups. The secondary endpoints assessed in our study included time to shock reversal [time to shock reversal is defined as the amount of time (in hours) required to withdraw all vasoactive medication supports from recruitment in study] change in SOFA score over 72 hours, need for mechanical ventilation (institution of invasive mechanical ventilation for respiratory support), incidence of new onset of AKI, ICU, and hospital length of stay (LOS).
Study Measurements
AA Measurements in Plasma
Two blood samples were collected per patient for vitamin C assay from both the study groups. The initial sample was collected before the first dose of vitamin C was administered and the final sample between 2 and 6 hours after the last dose of vitamin C on day 4. Concentration of AA in plasma was determined using a validated high-performance liquid chromatography mass spectrometry (HPLC-MS/MS) method.17 Protein precipitation extraction (PPE) technique was used to separate AA from the complex biological matrix. All the sample treatment and extraction of AA from plasma were carried out under monochromatic light and water bath (2–8°C). Ascorbic acid 13C6 was used as an internal standard for this analysis.
Samples were treated with dithiothreitol and metaphosphoric acid solution to increase the stability of AA in blood/plasma. For extracting AA from plasma, thawed 50 μL plasma aliquots added with 20 μL of internal standard solution and 1 mL of 2% formic acid each were centrifuged and 300 μL of the supernatant was added with 300 μL of 2 mM of ammonium formate solution. Final samples were transferred to autosampler of HPLC for analysis. Ascorbic acid was detected in the electrospray ionization (ESI) negative ion mode. Eight point calibration curves were constructed in drug-free plasma for AA, concentration ranging from 0.5 to 80 μg/mL. The complete protocol in detail for AA estimation in blood/plasma is available in Appendix I.
Study Sample Size Determination
Based on the mortality rates in control and interventional groups reported by Marik et al., a minimum sample size of 84 patients was calculated using nMaster© software with an estimated risk difference of 32% at 80% power and alpha error of 0.05.18
Statistical Analysis
Descriptive statistics were used to summarize the key outcome measures. The baseline characteristics and treatment outcomes were compared between the routine care and the HAT groups. Continuous variables were analyzed using Student's t or Mann–Whitney U tests and categorical variables were compared using Chi-square or Fisher's exact tests. Outcomes observed to be statistically significant across the study arms were reanalyzed by adjusting for corticosteroid therapy.19 Kaplan–Meier plots were used to evaluate the survival of patients in each group. A log-rank test was run to determine if there were differences in the survival distribution between the HAT and the routine groups. Statistical software SPSS 20.0 version (IBM, USA) was used for the analysis. A two-sided p value of <0.05 was considered statistically significant.
Results
Patient Characteristics
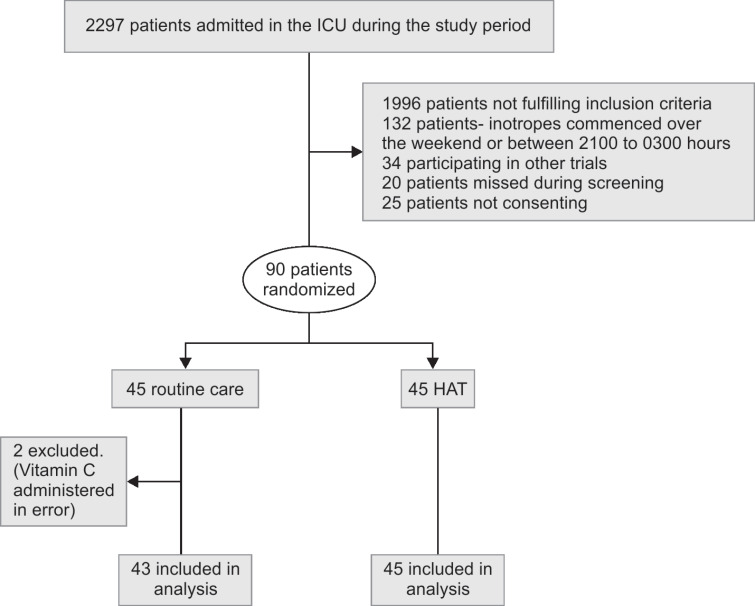
The CONSORT (consolidated standards of reporting trials) diagram of patient enrolment is provided in Flowchart 1. A total of 2,297 patients were screened among the two participating ICUs in the hospital, between June 2018 and August 2019. Three hundred and one patients were found to be eligible and 90 were randomized for the study after consent, with 45 in each group. Two patients were excluded from the routine care group for final per-protocol analysis as they received vitamin C inadvertently. Baseline characteristics on ICU admission were comparable between the groups and are enumerated in Table 1. Pneumonia (42%) was the major focus of infection for sepsis in our study cohort. Primary outcomes and secondary outcomes were assessed for the study cohort according to per-protocol analysis and intention-to-treat (ITT) analysis.
Flowchart 1.
Patient recruitment flowchart
Table 1.
Baseline characteristics of study cohort
| Characteristics | Routine (43) | HAT (45) |
|---|---|---|
| Age, mean (range) | 59.37 ± 15.01 | 58.69 ± 14.89 |
| Male | 32 (74) | 31 (69) |
| Weight | 66.35 ± 12.5 | 63.8 ± 12.4 |
| Hypertension | 21 (48) | 21 (46) |
| Diabetes mellitus | 25 (58) | 22 (48) |
| CAD | 10 (20) | 4 (8) |
| CKD | 11 (25) | 6 (14) |
| CLD | 13 (30) | 12 (26) |
| COPD | 2 (4) | 1 (2) |
| CVA | 2 (4) | 1 (2) |
| Malignancy | 10 (20) | 12 (25) |
| Immunosuppression | 6 (13) | 4 (8) |
| Pneumonia | 16 (37) | 21 (46) |
| Urosepsis | 9 (21) | 12 (26) |
| Abdomen | 14 (32) | 17 (37) |
| Skin and soft tissue sepsis | 6 (14) | 2 (4) |
| Multidrug resistance | 23 (47) | 26 (53) |
| PaO2/FiO2 | 292.2 ± 201.4 | 311.1 ± 255.5 |
| HR (/minute) | 100 ± 22.9 | 102.8 ± 23.4 |
| RR (/minute) | 23.14 ± 5.9 | 23.02 ± 6.3 |
| GCS | 15 (2–15) | 15 (2–15) |
| CRP (mg/dL) | 143.57 ± 99.0 | 134.6 ± 22.1 |
| Procalcitonin (ng/mL) | 26.84 ± 34.18 | 16.38 ± 28.21 |
| SOFA | 10.89 ± 3.82 | 11.22 ± 2.99 |
| Bilirubin (mg/dL) | 4.69 ± 6.5 | 3.5 ± 6.7 |
| Creatinine (mg/dL) | 2.5 ± 1.9 | 2.8 ± 1.6 |
| INR | 1.84 ± 1.3 | 1.7 ± 0.8 |
| Lactate (mmol/L) | 3.99 ± 3.24 | 3.42 ± 2.20 |
Study Outcomes
Primary Endpoint
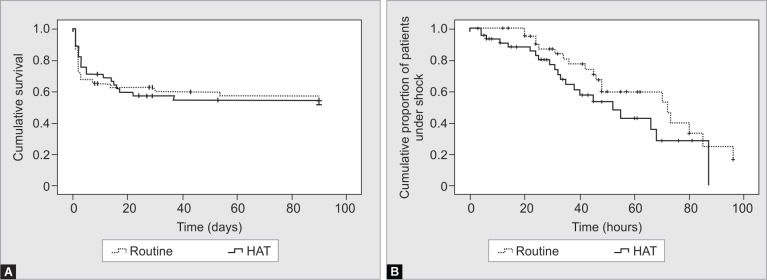
The primary endpoint of all-cause mortality was 53% (23/43) and 57% (26/45) in the routine care and the HAT groups, respectively, and was not significantly different (Table 2). The survival distributions as per Kaplan–Meier analysis did not exhibit a significant difference between the HAT and the routine study groups (log-rank test: p = 0.4) (Fig. 1A).
Table 2.
Major outcomes of study cohort
| Characteristics | n = 88 | Routine (43) | HAT (45) | p value |
|---|---|---|---|---|
| Mortality (%) | 49 (55) | 23 (53) | 26 (57) | 0.4 |
| Time to reversal of septic shock (hours) | 39.8 ± 23.9 | 45.42 ± 24.4 | 34.58 ± 22.63 | 0.03 |
| Mean vasoactive inotropic score on admission | 8.39 ± 10.47 | 7.89 ± 10.66 | 8.86 ± 10.38 | 0.66 |
| Highest vasoactive inotropic score | 45.9 ± 71.5 | 45.4 ± 64.1 | 46.3 ± 78.7 | 0.93 |
| Mean vasoactive inotropic score | 8.3 ± 12.4 | 8.86 ± 12.5 | 7.77 ± 12.12 | 0.6 |
| Mechanical ventilation | 44 (50%) | 20 (46.5) | 22 (48.8) | 0.4 |
| Ascorbic acid levels | ||||
| Before regimen | 1.8 ± 2.07 | 1.49 ± 1.39 | 2.1 ± 2.61 | 0.15 |
| After regimen | 51.8 ± 91.1 | 2.2 ± 4.1 | 94.2 ± 107.42 | <0.001 |
| SOFA@ 72 hours | 9.11 ± 3.7 | 9.3 ± 3.8 | 8.9 ± 3.6 | 0.7 |
| ∆SOFA | 1.83 ± 2.7 | 1.38 ± 3.1 | 2.23 ± 2.4 | 0.22 |
| New onset of AKI | 12 (14) | 5 (12) | 7 (15) | 0.4 |
| Procalcitonin on day 3 | 12.38 ± 21.4 | 18.97 ± 27.90 | 6.73 ± 11.5 | 0.03 |
| Procalcitonin clearance at 72 hours | 682.8 ± 2288.72 | 152.7 ± 478.2 | 1118.3 ± 3012.9 | 0.1 |
| Lactate on day 3 | 1.65 ± 0.91 | 1.79 ± 1.11 | 1.63 ± 0.85 | 0.9 |
| Lactate clearance at 24 hours | 5.98 ± 58.04 | 10.38 ± 44.14 | 2.1 ± 68.26 | 0.5 |
| ICU LOS | 10.49 ± 11.78 | 8.44 ± 8.16 | 12.44 ± 14.2 | 0.1 |
| Hospital LOS | 26.38 ± 25.0 | 20.9 ± 15.01 | 31.58 ± 31.06 | 0.043 |
Figs 1A and B.
(A) Kaplan–Meier survival distributions for the two study groups with respect to mortality; (B) Kaplan–Meier survival distributions for the two study groups with respect to septic shock reversal
Secondary Endpoints
The mean time to shock reversal was significantly lower in the HAT group (34.58 ± 22.63 hours) in comparison to the routine group (45.42 ± 24.4) (p = 0.03, mean difference −10.84, 95% CI −20.8 to −0.87). However, no significant difference was observed between the HAT and the routine groups in survival distributions with respect to mean time to shock reversal as per Kaplan–Meier plots (log rank test: p = 0.05) (Fig. 1B). The time to shock reversal, which was found to be significant (p = 0.03) between the HAT and the control arms, was reanalyzed after adjustment to steroid use. Hours to shock reversal remained significant (p = 0.04) after regression methods where steroid use was considered as a covariate. Shock reversal within 12 hours of enrolment was significantly higher in the HAT group (n = 14, 31%) as compared to the control group (n = 4, 9%) (p = 0.01). Similar results were observed for shock reversal at 36 hours (p < 0.001).
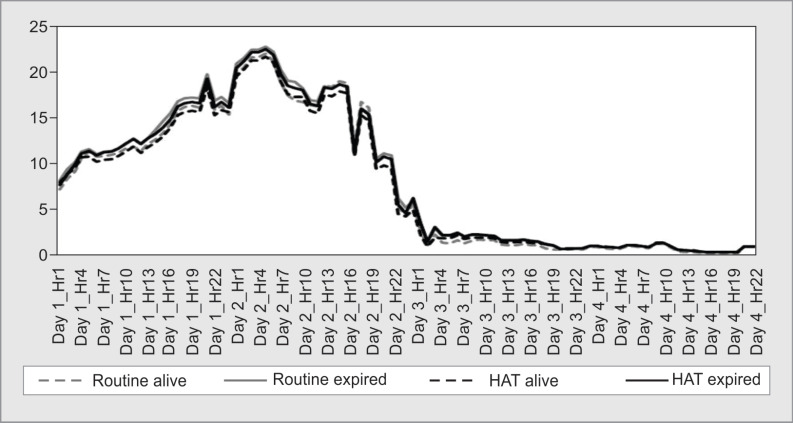
Average length of hospital stay was significantly higher in the HAT group (20.9 ± 15.01:31.58 ± 31.06, p = 0.04). After adjusting for outliers, the average LOS between the study groups did not significantly differ (p = 0.19). Baseline procalcitonin at ICU admission (p = 0.18) and procalcitonin clearance (p = 0.1) did not significantly differ between the study groups. Other secondary outcomes including change in SOFA over 72 hours, length of ICU stay, duration of mechanical ventilation, and the highest VIS score did not exhibit a significant difference between the study groups. New onset of AKI was observed to be 7 (15%) in the HAT and 5 (12%) in the routine groups (Table 2). The mean VIS score among the groups over the 4 days were comparable and is charted in Figure 2.
Fig. 2.
Distribution of mean Vasoactive Inotropic Scores (VIS) of study groups over the treatment period
SOFA Score Subgroup Analysis
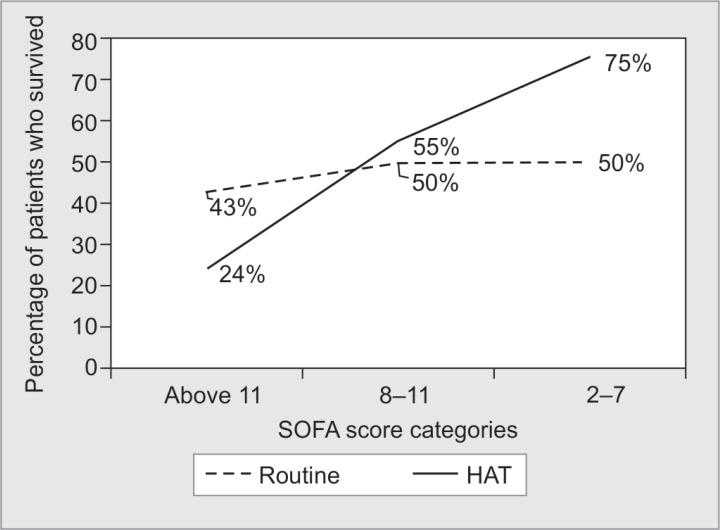
SOFA score on admission was 10.89 ± 3.82 in the routine group and 11.2 ± 2.99 in the HAT group. Distribution of patients across the SOFA score categories (2–7, 8–11, >11) were also comparable between the two groups (p = 0.3). A subgroup analysis revealed that the survival proportions of patients were significantly different between the HAT and the routine groups with respect to SOFA score categories (p = 0.005). The proportion of patients belonging to SOFA category of 2–7 in the HAT group (75%) who survived was higher than patients belonging to the same SOFA category in the routine group (50%). An increasing survival trend was observed for patients in the HAT group in comparison to the routine group as SOFA score severity decreased (Fig. 3).
Fig. 3.
Distribution of survived patient proportions among SOFA score categories in study groups
Microbiological Profile
The microbiological profile of the routine group and the HAT group is provided in Table 3. The proportion of multidrug resistant (MDR) bacteria in the routine and the HAT groups was 55.58 and 55.5%, respectively. Gram-negative organisms, mainly Klebsiella (35%) and E. coli (18%), predominated the positive cultures. Of note, the proportion of cultures positive for Klebsiella (68%) (p = 0.01) and Candida (64%) (p = 0.28) was observed to be higher in the HAT group.
Table 3.
Microbiological profile
| Characteristics | n = 88 (%) | MDR (%) | Control arm (43) | Vitamin C arm (45) |
|---|---|---|---|---|
| Organisms | ||||
| Klebsiella pneumonia | 31 (35) | 24(77) | 10 (32) | 21 (68) |
| E. coli | 16 (18) | 12 (75) | 7 (44) | 8 (57) |
| Candida | 11 (13) | 7 (64) | 4 (36) | 7 (64) |
| Enterococcus | 6 (8) | 4 (67) | 3 (50) | 3 (50) |
| Pseudomonas | 7 (8) | 5 (71) | 3 (43) | 4 (57) |
| Acinetobacter | 3 (3) | 3 (100) | 2 (67) | 1 (33) |
AA Levels
Mean baseline values of vitamin C did not significantly differ between the study groups, with 1.49 ± 1.39 and 2.1 ± 2.61 μg/mL in the routine and the HAT groups, respectively (p = 0.6). Posttreatment regimen, the vitamin C levels measured 2.2 ± 4.1 and 94.2 ± 107.42 μg/mL in the routine and the HAT groups, respectively (p < 0.001). Outliers (>90th percentile) and inconsistent AA measurements reported for nine patients were removed from the final analysis. No adverse events attributable to HAT therapy were recorded during the study.
ITT Analysis
A total of 90 patients randomized in the study into 45 patients each in the routine and the HAT groups were evaluated for primary and secondary outcomes as per ITT. Inpatient mortality was reported to be 49% (25) and 51% (26) in the routine and the HAT groups, respectively, and no significant difference was observed. The mean time to shock reversal was significantly higher in the routine group (45.42 ± 23.8) in comparison to the HAT group (34.58 ± 22.63) (p = 0.03). The major secondary outcomes including the change in SOFA scores over 72 hours, length of ICU stay, and the highest VIS score did not exhibit a significant difference between the study groups.
Discussion
In our randomized controlled trial, we found that the HAT protocol (combination of hydrocortisone, AA, and thiamine) within 6 hours of diagnosis of septic shock, did not reduce all-cause in-hospital mortality in patients with septic shock. Our results are consistent with other recently published randomized controlled trials that explored the use of HAT protocol in sepsis and septic shock.12,13,20,21
The ViCTOR trial was performed at a tertiary care teaching center in South India. A comparison of other five randomized controlled trials that investigated the use of AA (and its combination) in sepsis or septic shock is presented in Table 4. The trial which had looked at similar outcomes was VITAMINS trial but they had initiated the HAT protocol later in the course of sepsis after a mean time of 23 hours of commencement of inotropes, which was perceived as the reason for the lack of benefit of the HAT protocol. The delay in the initiation of the HAT protocol was addressed in our protocol in which HAT was strictly initiated within 6 hours but clear mortality benefit could not be demonstrated. The lack of mortality benefit might be due to the fact that our HAT cohort was sicker as evidenced by higher mean ASOFA scores (11.22 ± 2.99) compared to the study cohort described in Marik et al. (mean ASOFA 8.3) and VITAMINS trial (mean ASOFA 8.4). However, our cohort was sicker than the cohort of VITAMINS trial as evidenced by the higher ASOFA with a considerable proportion of them falling into ASOFA >11 category (33%). Our cohort had high prevalence of Gram-negative and MDR organisms. Our mortality is consistent with those published from the subcontinent in patients with septic shock.22 There was higher incidence of culture positivity for Klebsiella and Candida in the HAT group which could have played a role in tilting the balance of the survival rates in the HAT group. Similarly, the LOS was observed to be significantly higher in the HAT cohort when compared to the routine group. However, no significant difference was observed in LOS between the HAT and the routine groups when adjusted for outliers in LOS for the whole cohort by removing outliers greater than 90th percentile (p = 0.19) and 1.5 times interquartile range (IQR) (p = 0.49). The mean SOFA scores at admission were also higher in the HAT cohort (11.22 ± 2.99) relative to the routine group (10.89 ± 3.82) without statistical significance (p = 0.64) and higher SOFA scores are reported to be associated with prolonged LOS.23,24
Table 4.
Comparison of mortality and major outcomes across RCTs on use of ascorbic acid
| Outcomes | ViCTOR | CITRIS-ALI | Vitamins | Wani2020 | HYVCTTSSS | ORANGES |
|---|---|---|---|---|---|---|
| Country | India | USA | Australia, NZ, Brazil | India | China | USA |
| Primary outcome | All-cause mortality | Change in mSOFA @96 hours | Duration of time alive and free of vasopressor administration up to day 7 | In-hospital mortality | 28-day all-cause mortality | Resolution of shock and change in SOFA |
| Sample size | 43:45 septic shock | 83:84 | 107:109 septic shock | 50:50 sepsis and septic shock | 40:40 sepsis and septic shock | 69:68 septic and septic shock |
| SOFA (enrolment) | 10.9 ± 3.8:11.2 ± 3 | 10.3 ± 3.1:9.8 ± 3.2 | 8.4 ± 2.7:8.6 ± 2.7 | 9.36 ± 3.66:9.22 ± 3.54 | 10.1 + 4.0:9.6 + 4.5 | 7.9 ± 3.5:8.3 ± 3 |
| Mortality (%) | 49:51:00 | 46.3:29.8* | 24.5:28.6 | 42:40:00 | 35:27.5% | 19:16 |
| Shock reversal (hours) | 45.4 ± 24.4:34.58 ± 22.63* | 96.13 ± 40.50:75.72 ± 30.29* | 58.5 [28–104]:46 [23.8–102.5]* | 53 ± 38:27 ± 22* | ||
| AKI (%) | 47:53:00 | 72.1:69.2 | 75:79 | |||
| ∆SOFA | 1.38 ± 3.1:2.23 ± 2.4 | 3.5:3 (96 hours) | −1 (−3 to 0):−2 (−4 to 0)* | 6.62 ± 3.94:5.64 ± 3.55 | 1.8+3.0:3.5+3.3* | 1.93 ± 3.5:2.9 ± 3.3 |
| LOS (ICU) (days) | 8.44 ± 8.16:12.44 ± 14.2 | 7.5[4–11.8]:7.5[4–12.8] | 4.66 ± 3.45:4.76 ± 4.3 | |||
| LOS (H) (days) | 20.9 ± 15.01:31.58 ± 31.06* | 12.3 (6.2–26.1):12.3 (6.2–26.0) | 10.70 ± 6.39:11.82 ± 7.36 | 11 ± 6.2:11.5 ± 6.8 | ||
| Mechanical ventilation (%) | 46.5:48.8 | 62.5:61.7 | 6:06 | |||
| Comment | 62% control received steroid | 68–72% in septic shock | Control:Hydrocort 50 mg every 6 hours | 75% in septic shock at enrolment. 41% in control arm received steroids |
Implies statistical significance
The mean time for shock reversal showed significant reduction in the HAT group as compared to the routine group. The early shock reversal observed in the HAT group was observed to be significant even after adjustment to use of corticosteroid therapy. Although these could not be translated into a mortality benefit, early time to shock reversal could make the HAT protocol a potential adjunct therapy in sepsis to provide a window of hemodynamic stability for the patient.
Hypovitaminosis is defined as an AA level of 23 μmol/L and severe AA deficiency defined as an AA level 11.3 μmol/L.12 The measured vitamin C levels in the ORANGES trial, CITRIS-ALI and by Marik et al. were 21.7 ± 14.8, 17.9 ± 2.4, and 14.7 ± 11.8 μmol/L, respectively.12,18,25 Our analysis of vitamin C levels in these patients presents an interesting picture of baseline hypovitaminosis (Vit C in the control group was 1.49 ± 1.39 and in the HAT group was 2.1 ± 2.61 μg/dL).
We see that although HAT protocol seems to be safe and is helpful in reducing the inflammatory markers and provides early shock reversal, mortality benefit was observed only in a post hoc subset of patients with a lower SOFA score. Chang and colleagues showed in their prespecified subgroup analysis that the HAT protocol may produce a mortality benefit when administered within 48 hours of diagnosis of sepsis.21 There are signals from our study, ORANGES trial and from Chang and colleagues (HYVCTTSSS trial) that if the HAT protocol is initiated early, before circulatory and other organ failure ensues, it could be a useful adjunct therapy by reducing circulatory failure. However, this needs to be independently verified.
No adverse event attributable to vitamin C therapy was recorded in our study. New onset of AKI in the cohort had no significant association between the HAT and the routine groups. The drugs and dose we used for the trial intervention is the one used by Marik et al. in his study.18 Whether a different dosage can produce a different effect cannot be determined.
Conclusion
We conclude that intravenous vitamin C, thiamine, and hydrocortisone in the dose and duration used did not affect hospital mortality in patients with septic shock. Vitamin C therapy seems to be safe. There is evidence of reduction in the inflammatory process and therefore, the effect of earlier commencement of HAT protocol in reducing the mortality in sepsis needs further evaluation. Attributable mortality to septic shock was not determined for our cohort.
Clinical Significance
HAT protocol does not reduce hospital mortality but decreases time to shock reversal in septic shock.
Acknowledgments
We are thankful to Dr Subhash Todi and the Indian Society of Critical Care Medicine for providing the complete funding to conduct the study. We would also like to acknowledge Mr Sabyasachi Patri, Senior Consultant and Founder, BioIntegrity Consulting.
Appendix I
Ascorbic Acid Measurements in Plasma
Concentration of AA in plasma was determined using a validated HPLC-MS/MS method. The method was developed and validated by our CRO partner, Biointegrity Consulting. Protein precipitation extraction (PPE) technique was used to separate AA from the complex biological matrix. All the sample treatment and extraction of AA from plasma were carried out under monochromatic light and water bath (2–8°C). Ascorbic acid 13C6 was used as an internal standard for this analysis.
Sample Treatment
To increase the stability of AA in blood/plasma, 5 μL of 1M of dithiothreitol was added to the blood per 1 mL and plasma was separated by using refrigerated centrifuge (4,000 rpm for 10 minutes at 5°C). To the obtained plasma, 100 μL of 1% metaphosphoric acid solution was added to per 1 mL of plasma. 1 M of dithiothreitol and 1% metaphosphoric acid solution were also added to calibration curve samples and quality control samples in same proportion as added in the biological samples after collection and stored in freezer (−80°C).
Extraction of AA from Plasma
Samples were retrieved from deep freezer (−80°C) and allowed to thaw at room temperature. Samples were vortexed for 30 seconds and centrifuged for 5 minutes. Twenty microliters of internal standard solution were added in 50 μL of each plasma sample and 1 mL of 2% formic acid in acetonitrile solution was added in each sample. Samples were vortexed for 5 minutes, followed by centrifuged for 15 minutes at 4,000 rpm at 5°C. From the centrifuged solution, 300 μL of supernatant was taken and added to 300 μL of 2 mM of ammonium formate solution, pH 3.05 taken in prelabeled HPLC vials. Final samples were vortexed for 10 seconds and transferred to autosampler of HPLC for analysis.
Sample Analysis
The LC-MS/MS instrument includes HPLC from Schimadzu and MS/MS (API-4000 model) from Applied Biosystems Sciex, USA. The HPLC system is equipped with autosampler with thermos at 5°C. The column used was Thermo Scientific, Syncronis C18, 150 × 4.6 mm, 5 μm, heated at 30°C. The mobile phase was a mixture of acetonitrile and 2 mm ammonium formate solution (pH 3.00) in ratio of 70:30 (v/v). Injection volume was 2 μL and runtime was 4.20 minutes. Ascorbic acid was detected in the electrospray ionization (ESI) negative ion mode. Eight point calibration curves were constructed in drug-free plasma for AA, concentration ranging from 0.5 to 80 μg/mL.
Kutnink MA, Skala JH, Sauberlich HE, Omaye ST. Simultaneous determination of AA, isoascorbic acid (erythorbic acid) and uric acid in human plasma by high-performance liquid chromatography with amperometric detection. Journal of Liquid Chromatography 1985;8(1):31–46. DOI: 10.1080/01483918508067061.
Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, et al. Ascorbic acid dynamics in the seriously ill and injued. The Journal of Surgical Research 2003;109(2):144–148.
Footnotes
Source of support: Indian Society of Critical Care Medicine
Conflict of interest: None
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Teng J, Pourmand A, Mazer-Amirshahi M. Vitamin C: the next step in sepsis management? J Crit Care. 2018;43:230–234. doi: 10.1016/j.jcrc.2017.09.031. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308. doi: 10.1001/jama.2014.2637. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettilä V. Long-term outcome and quality-adjusted life years after severe sepsis*. Crit Care Med. 2009;37(4):1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Prim. 2016;2(1):16045. doi: 10.1038/nrdp.2016.45. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belsky JB, Wira CR, Jacob V, Sather JE, Lee PJ. A review of micronutrients in sepsis: the role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr Res Rev. 2018;31(2):281–290. doi: 10.1017/S0954422418000124. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12(1):32. doi: 10.1186/1479-5876-12-32. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka H. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration. Arch Surg. 2000;135(3):326. doi: 10.1001/archsurg.135.3.326. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock*. Crit Care Med. 2018;46(11):1747–1752. doi: 10.1097/CCM.0000000000003311. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797–808. doi: 10.1056/NEJMoa1705835. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis. Chest. 2020;158(1):164–173. doi: 10.1016/j.chest.2020.02.049. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock. JAMA. 2020;323(5):423. doi: 10.1001/jama.2019.22176. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (kidney disease improving global outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29(3):299–307. doi: 10.5935/1678-9741.20140049. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery. Pediatr Crit Care Med. 2014;15(6):529–537. doi: 10.1097/PCC.0000000000000153. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. 2003;109(2):144–148. doi: 10.1016/S0022-4804(02)00083-5. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock. Chest. 2017;151(6):1229–1238. doi: 10.1016/j.chest.2016.11.036. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15(1):139. doi: 10.1186/1745-6215-15-139. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wani SJ, Mufti SA, Jan RA, Shah S, Qadri SM, Khan UH, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Auckl) 2020;52(4):271–278. doi: 10.1080/23744235.2020.1718200. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, et al. Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock (HYVCTTSSS): a randomized controlled clinical trial. Chest. 2020;158(1):174–182. doi: 10.1016/j.chest.2020.02.065. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Bhattacharya M, Todi S. Epidemiology of adult-population sepsis in india: a single center 5 year experience. Indian J Crit Care Med. 2017;21(9):573–577. doi: 10.4103/ijccm.IJCCM_240_17. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh C-C, Chen Y-A, Hsu C-C, Chen JH, Chen WL, Huang CC, et al. Quick-SOFA score ≥ 2 predicts prolonged hospital stay in geriatric patients with influenza infection. Am J Emerg Med. 2020;38(4):780–784. doi: 10.1016/j.ajem.2019.06.041. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Milić M, Goranović T, Holjevac JK. Correlation of APACHE II and SOFA scores with length of stay in various surgical intensive care units. http://www.ncbi.nlm.nih.gov/pubmed/19860111. Coll Antropol. 2009;33(3):831–835. [PubMed] [Google Scholar]
- 25.Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure. JAMA. 2019;322(13):1261. doi: 10.1001/jama.2019.11825. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]